Recombinant Vaccine-Induced Protection against the Highly Pathogenic Simian Immunodeficiency Virus SIVmac251: Dependence on Route of Challenge Exposure (original) (raw)
Abstract
Vaccine protection from infection and/or disease induced by highly pathogenic simian immunodeficiency virus (SIV) strain SIVmac251 in the rhesus macaque model is a challenging task. Thus far, the only approach that has been reported to protect a fraction of macaques from infection following intravenous challenge with SIVmac251 was the use of a live attenuated SIV vaccine. In the present study, the gag, pol, and env genes of SIVK6W were expressed in the NYVAC vector, a genetically engineered derivative of the vaccinia virus Copenhagen strain that displays a highly attenuated phenotype in humans. In addition, the genes for the α and β chains of interleukin-12 (IL-12), as well as the IL-2 gene, were expressed in separate NYVAC vectors and inoculated intramuscularly, in conjunction with or separate from the NYVAC-SIV vaccine, in 40 macaques. The overall cytotoxic T-lymphocyte (CTL) response was greater, at the expense of proliferative and humoral responses, in animals immunized with NYVAC-SIV and NYVAC–IL-12 than in animals immunized with the NYVAC-SIV vaccine alone. At the end of the immunization regimen, half of the animals were challenged with SIVmac251 by the intravenous route and the other half were exposed to SIVmac251 intrarectally. Significantly, five of the eleven vaccinees exposed mucosally to SIVmac251 showed a transient peak of viremia 1 week after viral challenge and subsequently appeared to clear viral infection. In contrast, all 12 animals inoculated intravenously became infected, but 5 to 6 months after viral challenge, 4 animals were able to control viral expression and appeared to progress to disease more slowly than control animals. Protection did not appear to be associated with any of the measured immunological parameters. Further modulation of immune responses by coadministration of NYVAC-cytokine recombinants did not appear to influence the outcome of viral challenge. The fact that the NYVAC-SIV recombinant vaccine appears to be effective per se in the animal model that best mirrors human AIDS supports the idea that the development of a highly attenuated poxvirus-based vaccine candidate can be a valuable approach to significantly decrease the spread of human immunodeficiency virus (HIV) infection by the mucosal route.
Simian immunodeficiency virus (SIV) strain SIVmac251 pathogenicity in rhesus macaques mirrors several aspects of human AIDS (15). Vaccine protection against an intravenous (i.v.) SIVmac251 infection has been extremely difficult to achieve despite the fact that various approaches (41, 42) have been tried. So far, the approach that has induced the best protection against an SIVmac251 i.v. challenge is vaccination with the genetically attenuated SIVmac251 molecular clone with accessory genes including nef deleted (49). However, the protection from SIV disease was achieved at the expense of establishing a chronic infection with the attenuated virus, which has been demonstrated to cause disease in neonatal macaques (5, 50). Therefore, it is highly desirable that an alternative effective vaccine candidate, for use in humans, should mimic the protective attributes of the attenuated SIV vaccine without the dangers of chronic infection or disease.
In several developed countries, use of the human immunodeficiency virus (HIV) blood test and alteration in behavioral practices have substantially decreased the rate of hematogenous HIV transmission, leaving mucosal transmission as the primary route of exposure to HIV throughout the world (22). Thus, vaccine approaches that decrease mucosal transmission without necessarily protecting against i.v. infection could have an impact on the HIV epidemic.
Poxvirus-based HIV recombinants have been (13, 24, 40) and continue to be evaluated as vaccine candidates (37). Due to safety concerns surrounding the use of vaccinia virus vaccine strains and the fact that immunosuppression was a contraindication for vaccination with vaccinia virus, the highly attenuated novel poxvirus vector strains ALVAC, NYVAC, and MVA (1, 2, 4, 18, 32, 34, 36) have drawn considerable attention. However, to date, only NYVAC- and ALVAC-based recombinants expressing immunogens from various heterologous pathogens have been evaluated in humans.
Both NYVAC- and ALVAC-based vaccine candidates that have been assessed in phase I trials have demonstrated excellent safety profiles (8, 38). The replication-incompetent phenotype of ALVAC in nonavian species and the reduced immune responses in vaccinia virus-experienced individuals inoculated with vaccinia virus-based recombinants (11, 21) have provided the impetus for prioritizing ALVAC-based HIV vaccine candidates in clinical trials. In fact, an ALVAC-based recombinant expressing HIV-1MN gp120 and the Gag-protease is currently being assessed in a phase II trial using a prime/boost regimen with rgp 120 (16).
Previous studies with macaques have demonstrated the efficacy of highly attenuated poxvirus vectors, such as NYVAC and ALVAC, in protecting macaques from a nonpathogenic HIV-2 intravenous challenge (1, 2, 4, 18, 35). In those studies, the length of the immunization regimen appeared to be important in that a shortening of the length of the immunization regimen from 17 months to 6 months resulted in the loss of protection (1, 35). In another pilot study, a NYVAC-SIVenv vaccine did not protect macaques following i.v. exposure to SIVmac251 but enabled long-term survival of a few animals, one of which remains disease free 5 years after challenge with SIVmac251 (2). The data from those studies showed that protection from infection or disease did not correlate with the presence of neutralizing antibodies, suggesting that cell-mediated immunity may play a key role in protection. However, in those studies, protection from mucosal challenge was not assessed.
To address issues related to mucosal challenge exposure and to further investigate the role of cell-mediated immunity in protection, an immunization protocol with a NYVAC-SIV construct expressing gag-pol and env from SIVK6W (17) was designed. The protocol was used to evaluate protection from SIVmac251 challenge by the intrarectal (i.r.) and i.v. routes. Additionally, two arms of this protocol evaluated the local coexpression of human interleukin-12 (IL-12), a cytokine with pleiotropic effects which is considered to induce strong type I cellular immune responses (3, 27, 43), thereby affecting vaccine efficacy. The outcome of this study indicates that vaccination with NYVAC-SIV alone was able to protect macaques from an SIVmac251 mucosal challenge exposure and appears to slow disease progression following i.v. SIVmac251 inoculation. Further, the results illustrate the ability to modulate measurable immune responses by cytokine administration without an effect on vaccine efficacy.
This study not only provides data relating to general vaccinology in the HIV arena but also provides supporting safety and immunogenicity data for a potential phase I evaluation to compare this approach with other vector-based approaches in humans.
In addition, this study supports the concept that it is possible to protect macaques from disease or infection depending on the route of exposure with a vaccine based on a live vector previously proven safe in human volunteers. It also underlines the importance of further testing this vaccine approach in animal models where pathogenicity can be scored as a parameter of vaccine success.
MATERIALS AND METHODS
Generation of NYVAC recombinant viruses.
All recombinant viruses were engineered by inserting poxvirus expression cassettes into the NYVAC vector strain by standard in vitro recombination procedures (44). For vP1071, which coexpressed the SIVK6W env and gag-pol components (17), an env expression cassette under the control of the vaccinia virus H6 (early/late) promoter (37) and a gag-pol expression cassette regulated by the vaccinia virus early and intermediate I3L promoters were inserted into the NYVAC tk and 14L loci, respectively (20). The expression of env and gag was confirmed by immunoprecipitation analysis of vP1071-infected Vero cells with a pooled serum sample from HIV-2-seropositive individuals, as described previously (45). Recombinant virus vP1241 was engineered by inserting a vaccinia virus H6-regulated human IL-2 (10) expression cassette into the NYVAC tk locus. Expression of human IL-2 was confirmed by assaying media from vP1241-infected cells for functional IL-2 by the proliferation method using IL-2-dependent CTLL-2 murine cells and a human IL-2 standard (14). Recombinant virus vP1264 was engineered by coinserting the human IL-12 p35 and p40 subunits (48) into the NYVAC tk locus; p35 was under the control of the vaccinia virus (early) E3L promoter (20), and p40 was under the control of the entomopoxvirus (early) 42-kDa promoter (unpublished data). The expression of human IL-12 was confirmed by assaying media from vP1264-infected cells for functional IL-12 by promoting the proliferation of phytohemagglutinin (PHA)-stimulated, IL-2-activated human peripheral blood mononuclear cells (PBMC) (19).
Study design.
Forty macaques were divided into five groups of 8 animals each. The various groups receiving intramuscular inoculation at 0, 1, 6, and 12 months were designated as follows: group B (NYVAC-SIV), group C (a combination of NYVAC-SIV and NYVAC–IL-12), and group D (NYVAC-SIV plus NYVAC–IL-12 and NYVAC–IL-2). The vaccine control groups were inoculated at the same time intervals with either NYVAC–IL-12 (group A) or NYVAC–IL-12 plus NYVAC–IL-2 (group E) in the absence of NYVAC-SIV (Table 1). An additional group of two animals (group F) received the nonrecombinant NYVAC virus. Recombinant and nonrecombinant vectors were given at a dose of 107 PFU (22) at each immunization. Six months following the final administration (month 18), half of the animals in groups A to E were challenged by the i.v. route with 10 infectious units (IU) of SIVmac251 (49). The remaining half of each group, including animals in group F, were challenged i.r. with 20 50% mucosal infectious doses (MID50) of SIVmac251 (32H) (12) 9 months after the last immunization (month 21).
TABLE 1.
Immunization regimen and viral challenge
| Group | Vaccine(s)a | Animals subjected to SIVmac251 challengeb with: | |
|---|---|---|---|
| 10 IU i.v. | 20 MID50 i.r. | ||
| A | NYVAC–IL-12 | 279, 281, 282, 283 | 17604, 17633, 17697, 17701 |
| B | NYVAC-SIV | 268, 269, 270, 278 | 17427,c 17521, 17595, 17085 |
| C | NYVAC-SIV + NYVAC–IL-12 | 272, 273, 274, 275 | 17590, 17601, 17602, 17608 |
| D | NYVAC-SIV + NYVAC–IL-12 + NYVAC–IL-2 | 271, 276, 277, 280 | 17549, 17554, 17557, 17578 |
| E | NYVAC–IL-12 + NYVAC–IL-2 | 284, 285, 286, 287 | 17072, 17291, 17294, 17389 |
| F | NYVAC | 16794, 17547 |
Neutralizing antibody.
Three different neutralization assays using different target cell lines and different viral stocks were performed.
Assay with H9 cells.
Plasma collected from experimental animals was diluted 1:10 in RPMI medium–10% fetal bovine serum (R10) and inactivated at 57°C for 30 min. The inactivated plasma (20 μl) was then serially diluted 1:3 in 96-well plates. SIVK1W viral stock (20 μl), previously titrated on H9 cells, was added to each well, and the wells were incubated for 1 h at 4°C. H9 indicator cells were used as targets for infection. Cells were incubated in R10–Polybrene (2 μg/ml; Sigma) for 20 min, washed twice, and added to 200 μl of diluted plasma-virus mixture at 4 × 104 cells per well for 1 h at 37°C. From each well, 15 μl was transferred to new 96-well plates, and these plates were incubated in R10 at 37°C for 5 days. Virus infection was determined by immunostaining for SIV p27 on cells with a primary murine immunoglobulin G1 (IgG1) antibody (ABI, Rockville, Md.) and, as the secondary antibody, a goat anti-mouse IgG–fluorescein isothiocyanate conjugate (Tago Inc., Burlingame, Calif.). End point titers are reported as the reciprocal of dilution at which infectivity levels were 60% of control values after normalization of the data to control infectivity levels.
Assay with CEM × 174 cells.
Detection of neutralizing antibodies was also performed with a stock of primary SIVmac251 derived directly from the animal challenge stock by a single expansion in rhesus macaque PBMC. Neutralization was assessed by a CEM × 174 cell-killing assay as described previously (33). Briefly, cell-free virus (40 μl containing 750 50% tissue culture infective doses) was incubated with 10 μl of plasma samples in triplicate for 1 h at 30°C. Plasma samples had been heat inactivated at 56°C for 1 h prior to the assay. Twenty microliters was then added to 230 μl of CEM × 174 cells (105 cells) in 96-well culture plates. Cell densities were reduced, and the medium was replaced on days 4 and 8. Viable cells were quantified by neutral red staining on day 10, at which time approximately 80% cell killing was observed in virus control wells (no antibodies). The percentage of cells that remained viable was determined by calculating the difference in absorption at 540 nm (_A_540) between test wells (cells plus plasma sample plus virus) and virus control wells (cells plus virus) and dividing this result by the difference in absorption between cell control wells (cells only) and virus control wells. Neutralization was considered positive when 50% of cells were protected from virus-induced killing.
Assay with human PBMC.
Additional assessments of neutralizing antibodies were made with PHA-stimulated human PBMC (PHA-PBMC), as described previously (34). Here, 40 μl of cell-free virus (750 50% tissue culture infective doses) was incubated with 10 μl of heat-inactivated plasma samples in triplicate for 1 h at 37°C. Twenty microliters was then added to 230 μl of PHA-PBMC (4 × 105 cells in IL-2-containing growth medium) in 96-well culture plates, and the plates were incubated overnight. The cells were washed three times with 250 μl of IL-2-containing growth medium to remove the virus inoculum and antibodies. Washed cells were resuspended in 250 μl of IL-2 growth medium and incubated in fresh 96-well plates for 8 days. SIV p27 in culture supernatants was quantified with a commercial immunoassay as described by the supplier (Organon Teknika Corp., Durham, N.C.). Neutralization was considered positive when p27 production was reduced ≥80% relative to control wells that contained no antibodies.
Cytotoxic T-lymphocyte (CTL) assay.
Effector cells were isolated from whole blood drawn with EDTA over lymphocyte separation medium (Organon Teknika Corp). PBMC (5 × 106) were stimulated in vitro in RPMI complete medium (10% fetal bovine serum penicillin-streptomycin-amphotericin B (Fungizone; GIBCO BRL, Life Technologies, Gaithersburg, Md.) in the presence of concanavalin A (Sigma-Aldrich, St. Louis, Mo.) at 5 μg/ml for 6 days. On day 1 of stimulation, natural human IL-2 (Boehringer Mannheim Corp., Indianapolis, Ind.) at 10 U/ml was added. The cells were then cultured for an additional 5 days. On day 5, autologous transformed B cells for each macaque were infected with vaccinia virus or the ALVAC recombinant at a multiplicity of infection of 10:1. After 1 h, these stimulator cells were washed twice and incubated overnight with 51Cr (10 μCi/ml; 106 cells) (DuPont-New England Nuclear, Boston, Mass.). After being washed three times, both experimental (ALVAC-infected) and control (vaccinia virus-infected) target cells were mixed with autologous effector cells at 40:1, 20:1, and 10:1 ratios for 6 h at 37°C. Spontaneous-release wells contained target cells with no effector cells. Maximum-release wells contained target cells with Tween 20 for lysis. Supernatant from all wells was harvested with a Skatron Instruments (Sterling, Va.) harvest system. Released 51Cr content was read on a gamma counter (Wallac, LKB Diagnostics Inc., Gaithersburg, Md.). Specific release was calculated as {[experimental counts per minute (cpm) − spontaneous cpm]/[maximum cpm − spontaneous cpm]} × 100%. Values are reported as the weighted averages of specific killing (experimental specific release minus control specific release) at each of the three effector-to-target cell ratios, i.e. the sum of the values for percent specific killing at the 40:1, 20:1, and 10:1 ratios/(1 + 0.5 + 0.25).
IL-2 production (T-helper-cell response).
PBMC were cultured in RPMI 1640 (GIBCO, Grand Island, N.Y.) at 37°C in a moist 7% CO2 atmosphere. PBMC were either unstimulated or stimulated with live influenza A virus propagated in chicken eggs (1:500), 10 μg of concanavalin A (Sigma) per ml, PHA (1:80; GIBCO BRL), or SIV native gp120 (5 μg/ml; ABL, Rockville, Md.). For IL-2 production, 3 × 105 PBMC were cultured in 96-well flat-bottom plates (Costar, Cambridge, Mass.) for 6 days in the presence of human anti-IL-2 receptor antibody (anti-Tac; gift from T. A. Waldmann, National Cancer Institute) diluted 1:50 and 2% human AB serum. Supernatants were frozen and stored at −20°C until assayed for IL-2 content. The IL-2 assays consisted of culturing 6 × 103 IL-2-dependent CTLL cells per well in 96-well flat-bottom plates in the presence of three twofold dilutions of supernatants from unstimulated or antigen-stimulated cultures. After 24 h, the cultures were pulsed with 1 μCi of [3H]thymidine (Dupont-New England Nuclear); they were harvested after 18 h with a 96-well harvester (Skatron) and measured by using an LKB β-plate spectrometer (Pharmacia LKB Biotechnology, Piscataway, N.J.). Experimental samples were compared with serial dilutions of recombinant IL-2 (Boehringer Mannheim) and analyzed in parallel, and the results were expressed in terms of milliunits per milliliter.
Virus isolation and determination of viral load in the plasma and lymph nodes.
Virus isolation was carried out by coculturing 2 × 106 to 3 × 106 macaque PBMC with 106 CEM × 174 cells or human PBMC. Rhesus macaque PBMC were cocultured at 1- to 2-week intervals with CEM × 174 cells or PHA-activated human PBMC. Culture supernatants were tested for virus expression with SIV p27 antigen capture kits (Coulter, Hialea, Fla.). Virus isolation was scored as positive if two or more successive antigen capture assays were positive.
SIVmac251 RNA in plasma was quantitated by nucleic acid sequence-based amplification (39). Briefly, RNA was isolated from plasma by the extraction method of Boom et al. (6) and was subjected to isothermal enzymatic amplification with primers which target SIV gag sequences and quantified by electrochemiluminescence chemistry by using an internal standard which was coextracted and coamplified along with the wild-type SIV RNA present in the plasma sample. Quantitation was valid down to approximately 5 × 103 RNA copies/input volume. The virus load in the lymph nodes was measured by in situ reverse transcription (RT)-PCR as previously described.
Statistical analysis.
Comparisons of proportions between two groups were made with Fisher’s exact test for 2-by-2 tables and the Cochran-Armitage trend test for 2-by-3 tables. Differences in plasma viremia levels were assessed with the Wilcoxon rank sum and Kruskal-Wallis tests. Survival analyses were performed with the likelihood ratio test of the Cox proportional-hazards model. All P values reported are two tailed.
RESULTS
Immune responses in the immunized animals before viral challenge.
Neutralizing antibody titers, proliferative responses to SIVmac251 gp120, and CTL activity against gag, pol, and env were measured in the peripheral blood of the immunized animals after each immunization (see Study Design paragraph in Materials and Methods).
The titers of neutralizing antibodies against a laboratory-adapted SIVK1W strain, closely related to the SIVK6W strain (17), whose genes were used in the generation of the NYVAC-SIV recombinant vaccine candidate, were measured in H9 cells, as indicated in Materials and Methods. Neutralizing antibody titers, measured in this assay, were derived from a mean value of titers in four animals in each arm of the immunization protocol (Fig. 1A). Neutralizing antibody titers were not detectable before the third immunization in any of the animal groups but were observed after the third immunization in the animal groups that received the NYVAC-SIV vaccine, irrespective of the presence of cytokines. The mean neutralizing antibody titers were approximately threefold higher in animals vaccinated with NYVAC-SIV in the absence of IL-12 or IL-12 plus IL-2. In all groups, the fourth immunization failed to boost neutralizing antibody titers to levels higher than those obtained by the third immunization (Fig. 1A). The decrease in neutralizing antibodies in the animals that received IL-12 or IL-12 plus IL-2 correlated with an overall decrease of antibody titers against SIV antigens seen in enzyme-linked immunosorbent assays (ELISA) (data not shown). Sera of the animals from the different groups were also tested against the primary SIVmac251 i.v.-challenge stock, which has been shown to be very difficult to neutralize (49). Significant titers of neutralizing antibodies against the viral challenge stock were not detected prior to viral challenge in either the CEM × 174 (Table 2) or the human PHA-PBMC assays (data not shown). Altogether, these data suggest that the addition of IL-12 or IL-12 plus IL-2 at the time of immunization may have reduced the humoral immune response against the vaccine.
FIG. 1.
Immunological parameters measured for vaccinated control animals. (A) Mean titers of neutralizing antibodies (on H9 cells), measured for four animals from each group, at different time points before viral challenge. The vertical bar represents the standard error within each column. Under each column, the week in which the samples were collected is indicated. The months (6 and 12) are the times of vaccine inoculation. Ab, antibody. (B) The mean values of CTL activity at various effector-to-target ratios are shown for four animals in each experimental group. The values of weeks and months correspond to the times of sample collection and of vaccine inoculation, respectively. (C) Mean values of IL-2 production, following in vitro stimulation with native SIVmac251 gp120, by effector cells from four animals in each experimental group. Values of weeks and months correspond to times of sample collection and vaccine inoculation, respectively.
TABLE 2.
Neutralizing antibody titers against the SIVmac251 challenge strain in CEM × 174 cells
| Group | Animal no. | Titers at indicated no. of mo postchallengea | |
|---|---|---|---|
| 8 | 12 | ||
| Slow-progressor vaccinees | 269 | − | − |
| 273 | 27 | − | |
| 274 | − | − | |
| 276 | − | − | |
| Progressor vaccinees | 268 | >80 | − |
| 270 | − | − | |
| 271 | Dead | ||
| 272 | 38 | Dead | |
| 275 | − | Dead | |
| 277 | Dead | ||
| 278 | 10 | >80 | |
| 280 | 31 | 47 | |
| Controls | 279 | − | Dead |
| 281 | − | − | |
| 282 | − | 20 | |
| 283 | − | − | |
| 284 | Dead | ||
| 285 | − | − | |
| 286 | 13 | Dead | |
| 287 | − | Dead |
The CTL results presented in Fig. 1B represent the mean values obtained from four animals in each arm of the immunization protocol. Cytotoxic activity below 5% is insignificant since equivalent CTL activity was observed in control groups A and E (Fig. 1B). In groups A, B, and E no significant CTL activity was detected in the PBMC of the animals after any immunization. CTL activity, however, differed in the animals that received IL-12 (groups C and D). In these groups, CTLs were measurable after the third and the fourth immunizations (group B) (Fig. 1B), further suggesting that IL-12 may have skewed the host response in favor of cell-mediated immunity.
The mean values of helper-T-cell responses were determined by measuring IL-2 production following in vitro stimulation of PBMC with the SIV gp120 envelope. In contrast to what was found for neutralizing antibody and CTL responses, the T-helper response was observed after the first immunization. The third immunization significantly boosted T-cell responses in all three experimental groups. However, T-cell responses were lower for the groups of animals treated with the cytokines (groups C and D) (Fig. 1C) and in all cases were transient. T-cell responses were only marginally increased after the last immunization in all groups except for a single animal in group B, in which there was a large T-cell response after the last immunization (data not shown). T-helper responses were not detected in the animals of the control groups at any time point. Thus, it appeared that IL-12 down-modulated the CD4+-specific antiviral immunological responses elicited by the NYVAC-SIV vaccine and that this effect was evident after the third immunization.
Natural killer cell activity in four animals from each group (A through E) was also measured at the time of viral challenge. No significant differences among the groups of vaccinated and control animals were observed (data not shown).
Viral and clinical parameters following intravenous challenge exposure to SIVmac251.
The eight animals of each group (A through E) were divided into two subgroups of four animals each and exposed to either 10 IU of SIVmac251 (49) by the i.v. route (6 months following the last vaccine inoculation) or to 20 50% MID (MID50) of SIVmac251 i.r. (12) (9 months following the last vaccine inoculation).
All 20 animals (controls and vaccinees) that were challenged by the i.v. route became infected and in most of these (groups A through E), SIVmac251 was consistently isolated from the PBMC (see Table 3). Taking into account the plasma of all animals (8 controls and 12 vaccinees), the median RNA copy number for the vaccinated animals was lower than that for the controls during the acute phase of the virus, defined as the 4-week period after challenge exposure. However, only the difference in virus loads measured at week 4 reached statistical significance (P = 0.003).
TABLE 3.
Frequency of SIVmac251 detection in immunized and naive animals after i.v. challenge (groups A through E)
| Animal | Virus detectiona at indicated no. of mo after challenge | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 4 | 6 | 7 | 8 | 9 | 12 | 14 | 16 | |
| Vaccinees | ||||||||||
| 269 | + | + | + | − | − | + | − | − | − | − |
| 270 | + | + | − | − | − | − | + | + | − | + (dead) |
| 273 | + | − | + | − | − | − | − | − | − | − |
| 274 | + | + | + | + | − | − | − | + | − | − |
| 276 | + | + | + | + | − | − | − | − | − | − |
| 280 | + | + | − | − | + | + | + | ND | − | + |
| 271 | + | + | + | Dead | ||||||
| 277 | + | + | + | Dead | ||||||
| 272 | + | + | + | − | + | Dead | ||||
| 275 | + | + | + | − | + | Dead | ||||
| 268 | + | + | + | + | + | + | + | + | + | + |
| 278 | + | + | + | + | + | + | + | + | + | + |
| Controls | ||||||||||
| 284 | + | + | + | + (dead) | ||||||
| 287 | + | + | + | + | + | + (dead) | ||||
| 282 | + | + | + | + | + | + | + | ND | ND | NDb |
| 279 | + | + | + | ND | + | + | + (dead) | |||
| 283 | + | + | + | + | + | + | + | ND | + | + |
| 286 | + | + | + | + | + | + | ND | Dead | ||
| 285 | + | + | + | − | + | + | + | ND | + | −b |
| 281 | + | + | − | − | + | − | + | ND | − | − |
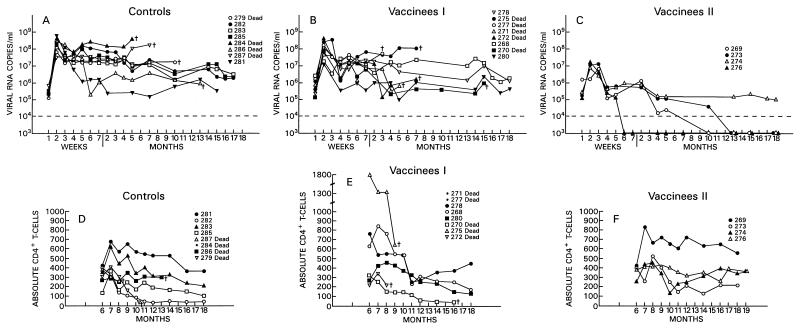
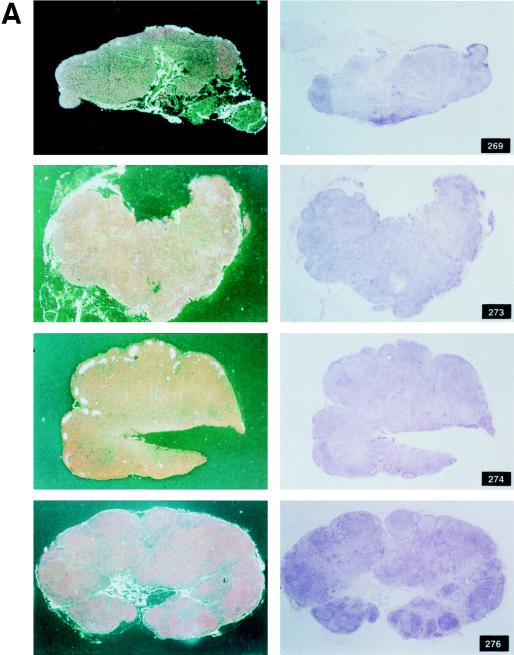
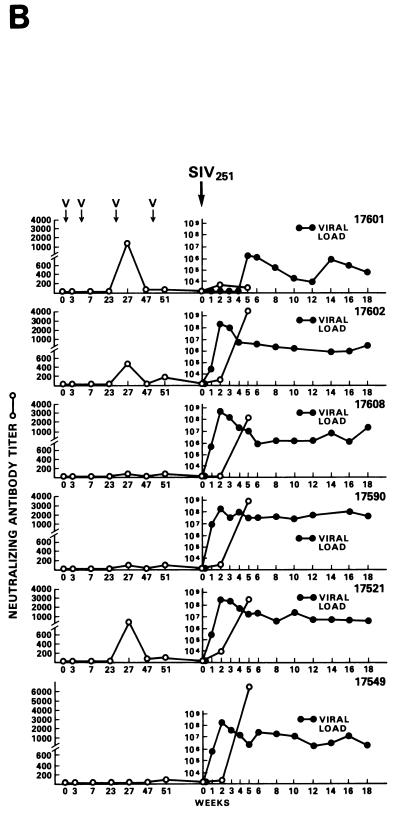
Among the 12 vaccinees, however, a distinct group of 4 animals (Fig. 2C) were identified (animals 269, 273, 274 and 276). In these macaques, viremia never exceeded 107 RNA copies/ml of plasma, and these animals appeared to be able to control viral replication with time, i.e., virus isolation from PBMC became sporadic by 6 to 7 months after viral exposure (Table 3). The overall levels of viremia in the remaining vaccinees (Fig. 2B) and control animals (Fig. 2A) were comparable. Interestingly, the differences in plasma RNA copy number among these three groups were already statistically significant at week 2 (P = 0.0038) and were even more so at week 4 (P = 0.0006) and thereafter. Accordingly, in group C animals, the viral burden in the lymph nodes was lower than it was in control animals or was undetectable (Fig. 3A and B).
FIG. 2.
Plasma virus load measurement and absolute CD4+ T-cell counts for immunized and control macaques following i.v. challenge exposure to SIVmac251. The top panels show the results of virus load measurements for the plasma of control animals (A) and vaccinees (B and C). The bottom panels show absolute CD4+ T-cell counts for controls (D) and vaccinees (E and F).
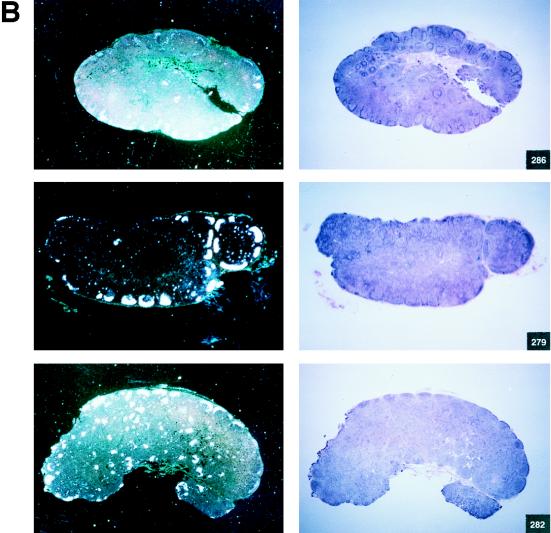
FIG. 3.
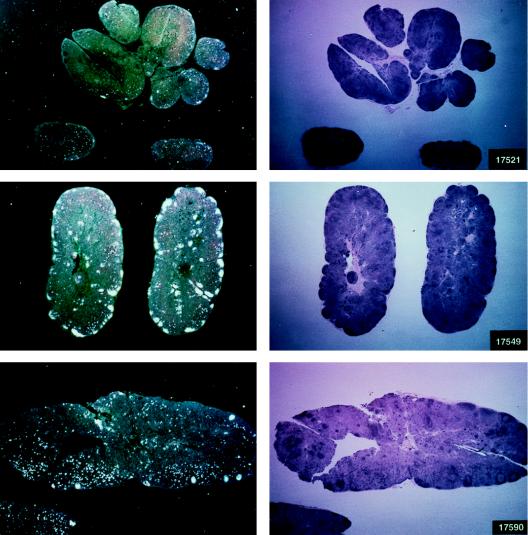
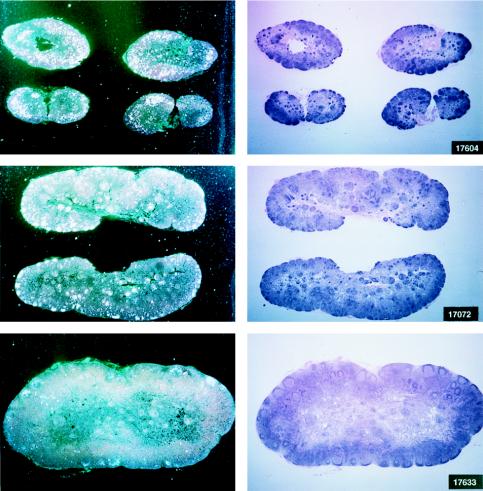
Histological staining and in situ RT-PCR results for lymph nodes from controls and vaccinees. (A) Results obtained from the lymph nodes of slow-progressor animals 269, 273, 274, and 276 at 6 months after viral exposure. (B) Three representative lymph nodes from control animals 279, 282, and 286 collected 6 months after viral exposure.
The frequency of virus isolation and the viral burden in both plasma and lymph nodes in the remaining eight vaccinees, which became infected following the i.v. SIVmac251 challenge (animals 268, 270, 271, 272, 275, 277, 278, and 280), were comparable to those of the control animals (Fig. 2B and Table 3).
All animals have been monitored for 18 months postchallenge, and within this observation period, six of eight control animals have succumbed to AIDS. Of the remaining two, all are experiencing a progressive decrease of CD4+ T cells, though at different rates (Fig. 2D). Among the vaccinees, 5 of 12 have died, and among the survivors, 3 display a progressive decline in CD4+ T cells (animals 268, 278, and 280), whereas animals 269, 273, 274, and 276 appeared to have stable CD4+ counts (Fig. 2E and F).
These results suggest that the level of virus load in the acute phase of infection may correlate with disease progression, as also suggested by others (47). The animals that had virus loads of 107 or fewer RNA copies/ml of plasma at 2 weeks following viral challenge and, more importantly, after week 4 (set point) had levels of viremia not exceeding 106 RNA copies/ml, are clinically asymptomatic, and display stable CD4+ counts (compare Fig. 2D, E, and F). Conversely, most of the controls and the remaining vaccinees which had, at week 2, a virus load between 107 and 109 RNA copies/ml and in which viremia had remained at high levels (∼107 RNA copies/ml or greater) either succumbed to SIVmac251 infection or are progressing to AIDS. However, animal 281, which had a consistently low level of viremia, is experiencing a progressive slow decline in CD4+ T-cell counts.
Suppression of viral replication in vaccinated nonprogressor macaques.
The observation that some of the nonprogressor vaccinated animals were able to control viremia could be attributed to the selection of an attenuated virus rather than truly reflecting the ability of the host to control virus replication over time. To assess these two possibilities, 30 ml of blood from a progressor animal (animal 268) and a nonprogressor animal (animal 276) was transfused into two naive animals each. At the time of blood transfusion, animal 268 had a virus load of 107 viral RNA copies/ml of plasma and animal 276 had no detectable viral RNA. The virus load during the acute phase of infection in the recipient animals (animals 525 and 526), was monitored twice a week within the first 2 weeks following blood transfusion. The macaques that were recipients of blood from animal 268 had identical kinetics of viral replication, as demonstrated by the quantitative measurement of virus load. By day 4, 109 copies of viral RNA per ml were detected in the plasma of both animals. In contrast, a delay of 5 days (7 versus 2 days) in the detection of plasma viral RNA was observed for macaques 527 and 528, recipients of blood from animal 276 (data not shown). The viral load in animals 527 and 528 reached peak levels comparable to those in animals 525 and 526 only at day 13 after blood transfusion. Thus, the overall viral burden and the rate of viral replication appeared to be equivalent in the blood of all four animals, although a delay was observed for the animals that received blood from animal 276. These data suggest that the replicative abilities of the SIV present in the blood of animals 268 and 276 did not substantially differ, and the delay in the spreading of infection in the blood recipients is probably an effect of the different doses of virus in the inocula. Thus, these findings suggest that a host-dependent factor(s), rather than the in vivo selection of a less virulent virus, accounts for the low viremia observed in the nonprogressor vaccinated animals.
Viral and clinical parameters following SIVmac251 mucosal challenge.
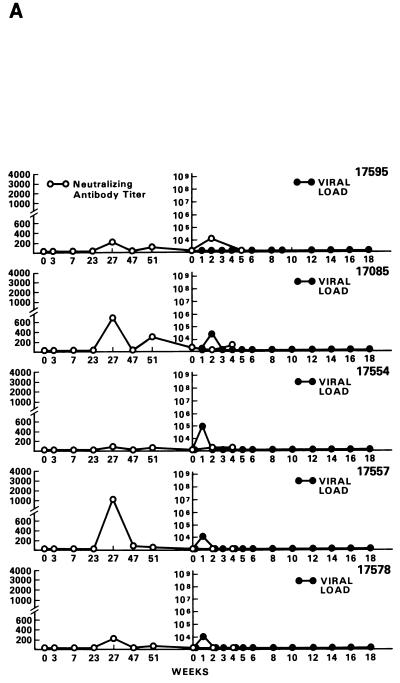
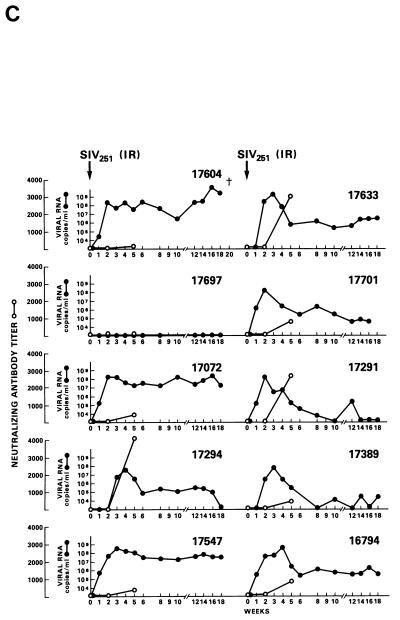
Half of the animals from groups A through E and both animals in group F were exposed i.r. to 20 MID50 of SIVmac251 (24). Virus was isolated from PBMC DNA, and plasma RNA was measured at various intervals following viral exposure. Among the 11 vaccinees (animal 17427 died during the immunization period for unrelated reasons), 5 scored repeatedly negative for virus isolation (animals 17695, 17085, 17554, 17557, and 17578), whereas the other vaccinees and controls scored positive within the 9-month observation time following viral exposure (Table 4). Accordingly, for the five animals that scored negative in the virus isolation assay, viral RNA was detected in the plasma at low levels (between 104 and 105 RNA copies/ml) only at the first week after exposure (Fig. 4A). In the remaining vaccinees (Fig. 4B) and controls (Fig. 4C), high levels (108 to 109 copies of viral RNA per ml) of viral RNA were found during the acute viremic stage and were detected continuously in the plasma of most animals (except animal 17697).
TABLE 4.
Frequency of SIVmac251 detection in cultured PBMC of the i.r.-challenged macaques
| Animal | Virus detectiona at indicated no. of: | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Wk after challenge | Mo after challenge | |||||||||
| 1 | 3 | 5 | 9 | 3 | 4 | 6 | 8 | 10 | 12 | |
| Vaccinees | ||||||||||
| 17554 | − | − | − | − | − | − | − | − | − | − |
| 17557 | − | − | − | − | − | − | − | − | ND | − |
| 17578 | − | − | − | − | − | − | − | − | − | − |
| 17085 | − | − | − | − | − | − | − | − | − | − |
| 17595 | − | − | − | − | − | − | − | − | − | − |
| 17549 | + | + | + | − | + | + | + | + | ND | − |
| 17521 | + | − | − | + | + | + | + | + | − | + |
| 17590 | + | + | + | − | + | + | + | + | Dead | |
| 17601 | − | − | − | − | + | + | + | − | − | + |
| 17602 | + | + | − | + | + | − | + | − | − | − |
| 17608 | + | + | − | + | + | + | + | + | + | + |
| Controls | ||||||||||
| 17604 | + | + | + | + | + | + | Dead | |||
| 17633 | + | + | − | + | + | + | + | + | − | + |
| 17697 | − | − | − | + | − | + | + | + | − | − |
| 17701 | + | + | − | + | + | − | + | + | ND | + |
| 17072 | + | + | + | + | + | + | + | + | ND | + |
| 17291 | + | + | − | + | + | − | − | − | ND | + |
| 17294 | − | + | − | + | + | − | − | − | − | − |
| 17389 | + | + | + | − | + | + | + | + | − | − |
| 16794 | + | − | − | − | + | + | + | + | ND | + |
| 17547 | + | + | + | − | − | + | + | + | + | + |
FIG. 4.
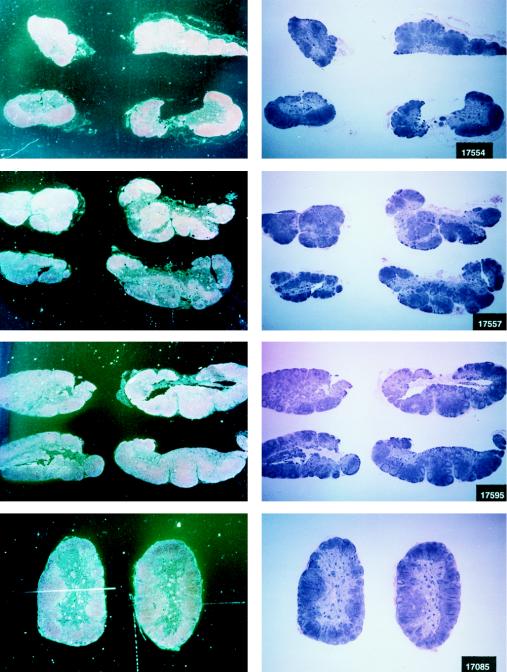
Virus load in control and vaccinated animals following SIVmac251 i.r. challenge. •, viral RNA copies per milliliter of plasma; ○, neutralizing antibody titers against laboratory-adapted SIVK1W. Data are presented for all vaccinated animals (A and B) before and after viral challenge. In the case of control animals (C), neutralizing antibodies were positive only after viral challenge, as expected. On the left side of each figure are results of in situ RT-PCR on viral RNA and histological staining of the lymph nodes of some of the animals in the study. The cross sign indicates the death of the animal.
Statistical analysis of protection data for i.r. exposure was performed for animals grouped as follows: group 1 was represented by the five animals (17085, 17095, 17554, 17557, and 17578) whose PBMC cultures were never positive for virus (in 10 attempts; see Table 4); group 2 included one animal (17697) in the control group that had undetectable plasma virus but scored positive for virus in the PBMC culture a few months after challenge exposure (Table 4); group 3 included all the remaining vaccinees and control animals whose PBMC cultures were positive for plasma viral RNA and virus several times (Table 4). The difference between vaccinated and control animals by the Cochran-Armitage test (for trend) reached statistical significance at the P = 0.035 level.
Neutralizing antibody titers against laboratory-adapted SIVK1W were undetectable at the time of challenge in all the vaccinees and were effectively boosted only in the animals that became persistently infected (Fig. 4B). In contrast, neutralizing antibody titers against the primary SIVmac251 challenge virus, absent at all times in vaccinees before and at the time of viral challenge, were not boosted following viral infection (data not shown). Similarly, several of the control animals developed neutralizing antibody titers, although with different kinetics, against laboratory-adapted SIVK1W.
The protected animals (17595, 17085, 17554, 17557, and 17578) exhibited no or low neutralizing antibody titers (in any of the three assays) at the time of challenge. In contrast to the other vaccinees, which became persistently infected, they exhibited no sustained increase in neutralizing antibody titers following challenge (compare Fig. 4B and C). Similarly, the overall ELISA antibodies against SIV decreased within the 13 months following viral challenge (data not shown). These findings, in conjunction with the observation that plasma viral RNA was undetectable by week 2 after exposure (Fig. 4A), suggest that these animals were able to control viral replication very early on following challenge exposure and presumably cleared the virus. Accordingly, the same animals were negative as regards virus isolation (Table 3) and the detection of viral RNA in the lymph nodes, whereas in the other vaccinees and controls clear evidence of virally infected cells or trapped viral RNA in the cortical region of the lymph nodes was observed (Fig. 4B and C).
Progression to disease in animals infected by the mucosal route appears to be slower than that observed in animals challenged with the same viral strain by the i.v. route (Table 4). However, within the 12-month observation time, 1 control animal (17604) and 1 vaccinee (17590) succumbed to SIVmac251 disease and 5 of the remaining 9 controls and 4 of the remaining 11 vaccinees are experiencing a significant decrease in CD4+ T-cell counts. An assessment of whether vaccination may have influenced disease progression in animals which become infected following mucosal challenge exposure is not warranted at this time. Similarly, an overall assessment of the influence of vaccination on survival of macaque challenge by both the i.v. and mucosal routes will require a longer observation time.
Immune correlates of protection.
Since complete data on all the immunological parameters studied were available from the 20 immunized and i.v.-challenged animals, we compared the immunological responses before, at the time of, and following viral challenge in an attempt to obtain correlates with the clinical outcome of infection. The 12 vaccinees were divided into two groups according to their clinical status (Table 5). Vaccinated animals 269, 273, 274, and 276 were designated slow progressors because of their low virus loads and stable CD4+ T-cell counts (Fig. 2C and F and 3). Vaccinated animals 268, 270, 271, 272, 275, 277, 278, and 280 were designated progressors because of persistent high virus loads and progressive decline of CD4+ T-cell counts. In this group, five animals (270, 271, 272, 275, and 277) have succumbed to disease and the remaining three are experiencing a progressive decline of CD4+ T cells (Fig. 2B and E).
TABLE 5.
Overall clinical and virological status of vaccinated and control animals
| Group | Vaccine(s) | Status of: | |
|---|---|---|---|
| i.v.-challenged group | i.r.-challenged group | ||
| A | NYVAC–IL-12 | 2 dead, 1 progressor, 1 slow progressor | 1 dead, 3 infected |
| B | NYVAC-SIV | 1 dead, 2 progressors, 1 slow progressor | 1 infected, 2 uninfected, 1 NDa |
| C | NYVAC-SIV + NYVAC–IL-12 | 2 dead, 2 slow progressors | 1 dead, 3 infected |
| D | NYVAC-SIV + NYVAC–IL-12 + NYVAC–IL-2 | 2 dead, 2 slow progressors | 1 infected, 3 uninfected |
| E | NYVAC–IL-12 + NYVAC–IL-2 | 3 dead, 1 progressor | 4 infected |
| F | NYVAC | ND | 2 infected |
The magnitudes of neutralizing or antiviral antibody titers, cytotoxic responses, and T-cell responses (IL-2 production) before, at the time of, and following viral challenge for both groups were analyzed to determine whether any of these immune responses would correlate with the ability of the host to control viremia. The antibody results obtained from the sera of animals in the two groups demonstrated no significant difference in the titers of neutralizing antibodies against the viral challenge, as measured in the CEM × 174 assay, or against laboratory-adapted SIVK1W (data not shown) either before or after viral challenge (Table 2). Similarly, ELISA titers of antibodies against total disrupted virions and optical density values of sera containing antibodies against purified p27_gag_ proteins did not correlate with the ability of some animals to control viremia (Table 6), contrary to observations of others (23). Similarly, no significant difference in CTL activity or IL-2 production upon pg120 stimulation was observed for the PBMC of the different animal groups before viral challenge.
TABLE 6.
ELISA antibody titers in the immunized animals challenged by the i.v. route
| Group | Animal no. | Antibody level as indicated by: | |
|---|---|---|---|
| Antibody titers against total disrupted virionsa | ODb | ||
| Slow-progressor vaccinees | 269 | 400 | 0.420 |
| 273 | 400 | 0.411 | |
| 274 | <25 | 0.811 | |
| 276 | 50 | 1.428 | |
| Progressor vaccinees | 268 | <25 | 0.091 |
| 270 | 200 | 0.373 | |
| 271 | 200 | 0.616 | |
| 272 | 200 | 0.174 | |
| 275 | 200 | 0.140 | |
| 277 | 200 | 0.582 | |
| 278 | 3,200 | 1.148 | |
| 280 | 200 | 0.086 | |
| Controls | 279 | −c | 0.090 |
| 281 | − | 0.294 | |
| 282 | − | 0.043 | |
| 283 | − | 0.744 | |
| 284 | − | − | |
| 285 | − | − | |
| 286 | − | 0.322 | |
| 287 | − | 0.618 |
A complete set of data on the animals challenged by the i.r. route was available only for the humoral immune response. Neutralizing antibody titers against the primary challenge virus (SIVmac251) were absent or very low in all vaccinees at the time of challenge and were not elicited (data not shown) following viral infection either in the six vaccinees that became infected or in the five animals which consistently scored negative for viral isolation. Neutralizing antibody titers against laboratory-adapted SIVK1W, also absent or very low in the same animal groups at the time of viral challenge, were elicited only in vaccinees that became infected, not in animals that remained virus negative for all parameters studied (Fig. 4). Thus, these results indicate that neutralizing antibody titers, as measured here by three different assays using both a laboratory-adapted strain (SIVK1W) and primary strain SIVmac251, do not appear to be predictive of the induction of protective immunity against infection by this vaccine approach.
In the group of animals challenged i.v., the induction of CD8+ antiviral activity by the vaccine regimens was observed (26). CD8+ antiviral activity, measured 4 weeks before viral challenge, correlated with plasma SIV RNA levels which developed in the animals by 8 weeks postchallenge. In addition, correlation of postchallenge CD8+ antiviral activity with disease outcome was observed, with high activity levels present in nonprogressor animals, intermediate levels in progressors, and low levels in rapidly progressing animals which died within 8 months of challenge. Thus, vaccination-induced CD8+ antiviral activity may contribute to the control of viral replication in vivo and overall to vaccine protective efficacy.
DISCUSSION
The present study used three different NYVAC recombinant SIV vaccine strategies to test for protection against i.v. and i.r. challenge of macaques with highly pathogenic SIVmac251. We analyzed virologic and clinical parameters after viral challenge and obtained SIV-specific immunological data for neutralizing antibodies, T-helper-cell function, and CTL activation throughout the immunization phase and after challenge.
NYVAC–IL-12 coadministered with NYVAC-SIV appeared to modulate the SIV-specific immune response prior to challenge exposure by dampening humoral immune responses and enhancing the overall CTL activity measurable in the periphery. By using two different routes for viral challenge in animals which received an identical vaccine regimen, we demonstrated that approximately half of the animals exposed by the i.r. route became viremic within the first week after exposure but appeared to clear viral infection. The lack of neutralizing antibody titers against both laboratory-adapted SIVK1W and the primary viral challenge (SIVmac251) in the protected animals at the time of and after viral challenge suggests that alternative immune effector mechanisms were responsible for the apparent viral clearance.
Although all animals became infected following an i.v. challenge exposure, one third of the vaccinees experienced a low virus load in the acute phase of infection, controlled the virus effectively thereafter, and appeared to be slow progressors as judged by stable CD4+ T-cell counts (Table 3 and Fig. 2F). A systematic analysis of CTL activity, neutralizing antibodies against the challenge virus as well as against a laboratory-adapted virus, and T-cell responses before viral challenge did not indicate clear correlates of immunity. As reported elsewhere, however, the vaccination regimen induced a CD8+ T-cell-associated antiviral activity which correlated with low steady-state levels of plasma viremia that developed following the acute phase of infection (30). Furthermore, postchallenge CD8+ T-cell-associated antiviral activity levels appeared to correlate with disease outcome, indicating that this activity may modulate viral replication in vivo and may contribute to protective immunity. Others have also reported an association of postchallenge CD8+ T-cell-associated antiviral activity with protection against infection by the mucosal route (29). Further studies will be necessary to determine the extent to which this activity contributes to vaccine efficacy.
The addition of NYVAC–IL-12, though deemed effective in skewing the immune response toward cell-mediated immunity, did not appear to influence the outcome of viral challenge regardless of the route of viral exposure. The reasons for this result and the lack of any observable synergy with IL-2, as has been observed previously (9), are presently unknown. It is possible that NYVAC-borne expression of these cytokines in vivo was not optimal for providing the qualitative and/or quantitative modulation in immune response necessary to influence vaccine efficacy. Alternatively, the amino acid identity between human and rhesus macaque IL-12 p40 (98.2%) and p35 (94.2%), as well as IL-2 (99.4%) (46), could translate into differential adjuvant properties in vivo across species. In the case of IL-12, these differences have been shown to affect activity in vitro by using the reciprocal human and nonhuman cell sources and to result in the marked immunogenicity of human IL-12 in nonhuman primates (46). However, human IL-12 has been shown to have biological activity in rhesus macaques (24).
Poxvirus-borne cytokine expression has been shown previously to affect immune responses to coexpressed immunogens and to immunogens from whole tumor cells infected with poxvirus-borne cytokines (28, 31, 39, 40). Although adjuvant effects have been observed with soluble IL-12 (26), administration of IL-12 with a vaccinia virus RSVG recombinant was able to reverse TH-2-associated responses with no benefit to the host (26). As such, the results presented in this paper may merely illustrate the complex nature of lentivirus-host interactions and immune regulation in general and not necessarily a deficiency of poxvirus-borne cytokine expression.
Despite the lack of clear immune correlates, it is likely that the differences in infectibility and virus load observed in these animals were due to specific responses elicited by vaccination because five vaccinees resisted the establishment of a chronic viral infection following mucosal challenge. None of the 10 control animals resisted chronic infection except animal 17697, which had undetectable viral RNA in the plasma but which, nevertheless, was sporadically positive for virus in the PBMC. Further, among the animals that became infected after i.v. viral exposure, only the vaccinees experienced low virus load in the acute phase of infection and maintained viremia at a level of 105 viral copies/ml of plasma or lower thereafter. One of the eight control animals appeared to be able to control viremia better (animal 281), but it appears nevertheless to be progressing to AIDS. Finally, transfusion to naive animals of blood from one animal with low virus load demonstrated the establishment of viremia in the recipient animals, suggesting that the low viremia observed in the donor (vaccinated) animal was probably not a consequence of the in vivo selection of a less virulent virus but rather the result of the host’s continuous ability to suppress the virus.
In summary, our results suggest that it is possible to obtain and boost cellular and humoral immune responses against SIV by using repeated doses of a recombinant attenuated poxvirus vector. Further studies will be required to assess the relative effectivenesses of different doses of vaccine in combination with boosting substances such as proteins (25), DNA (7, 32), and other live vector vaccines (36) to substitute for the fourth NYVAC-SIV inoculation, which apparently did not further boost some immune responses in the vaccinated animals.
Of major interest is the finding that the route of exposure seems to have important implications in the evaluation of vaccine efficacy in the SIVmac251-infected macaque model. We believe that the induction of long-lasting protective immunity in 5 of 11 animals following mucosal challenge and the apparent prevention of disease progression in a portion of infected vaccinees following i.v. exposure in this perhaps too-rigorous animal model validate the usefulness of this vaccine approach and warrant further research on how to reach protection in a higher portion of the vaccinated animals. Evaluation of NYVAC recombinant vaccines in combination with other vaccine approaches may help in this endeavor.
ACKNOWLEDGMENTS
We thank Thomas Waldmann for the human anti-IL-2-receptor antibody anti-Tac, Ronald Desrosiers for the generous gift of the SIVmac251 viral challenge strain, Celil Fox for carrying out in situ RT-PCR on tissues, Michel Leno for critical reading of the manuscript, and Kelli Carrington and Sydnye White for editorial assistance. We are also grateful to Ruth Woodworth and Marsha Sowers for excellent animal care.
Part of this work was supported by Pasteur-Merieux-Conaught.
REFERENCES
- 1.Abimiku A G, Franchini G, Tartaglia J, Aldrich K, Myagkikh M, Markham P D, Chong P, Klein M, Kieny M P, Paoletti E, Gallo R C, Robert-Guroff M. HIV-1 recombinant poxvirus vaccine induces cross-protection against HIV-2 challenge in rhesus macaques. Nat Med. 1995;1:321–329. doi: 10.1038/nm0495-321. [DOI] [PubMed] [Google Scholar]
- 2.Abimiku A G, Robert-Guroff M, Benson J, Tartaglia J, Paoletti E, Gallo R C, Markham P D, Franchini G. Long-term survival of SIVmac251-infected macaques previously immunized with NYVAC-SIV vaccines. J Acquired Immune Defic Syndr. 1997;15:S78–S85. [Google Scholar]
- 3.Afonso L C C, Scharton T M, Vieira L Q, Wysocka M, Trinchieri G, Scott P. The adjuvant effect of interleukin-12 in a vaccine against Leishmania major. Science. 1994;263:235. doi: 10.1126/science.7904381. [DOI] [PubMed] [Google Scholar]
- 4.Andersson S, Makitalo B, Thorstensson R, Franchini G, Tartaglia J, Limbach K, Paoletti E, Putkonen P, Biberfeld G. Immunogenicity and protective efficacy of an HIV-2 recombinant canarypox (ALVAC) vaccine candidate in cynomolgus monkeys. J Infect Dis. 1996;174:977–985. doi: 10.1093/infdis/174.5.977. [DOI] [PubMed] [Google Scholar]
- 5.Baba T W, Jeong Y S, Pennick D, Bronson R, Greene M F, Ruprecht R M. Pathogenicity of live, attenuated SIV after mucosal infection of neonatal macaques. Science. 1995;267:1820–1825. doi: 10.1126/science.7892606. [DOI] [PubMed] [Google Scholar]
- 6.Boom R, Sol C J A, Salimans M M M, Jansen C L, Wertheim-Van Dillen P M E, Van Der Noordaa J. Rapid and simple method for purification of nucleic acids. J Clin Microbiol. 1990;28:495–503. doi: 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyer J D, Ugen K E, Wang B, Agadjanyan M, Gilbert L, Bagarazzi M L, Chattergoon M, Frost P, Javadian A, Williams W V, Refaeli Y, Ciccarelli R B, McCallus D, Coney L, Weiner D B. Protection of chimpanzees from high-dose heterologous HIV-1 challenge by DNA vaccination. Nat Med. 1997;3:S26–S32. doi: 10.1038/nm0597-526. [DOI] [PubMed] [Google Scholar]
- 8.Cadoz M, Strady A, Meignier B, Taylor J, Tartaglia J, Paoletti E, Plotkin S. Immunisation with canarypox virus expressing rabies glycoproteins. Lancet. 1992;339:1429–1432. doi: 10.1016/0140-6736(92)92027-d. [DOI] [PubMed] [Google Scholar]
- 9.Chan S H, Perussia B, Gupta J W, Kobayashi M, Posppís̆il M, Young H A, Wolf S F, Young D, Clark S C, Trinchieri G. Induction of interferon γ production by natural killer cell stimulatory factor: characterization of the responder cells and synergy with other inducers. J Exp Med. 1991;173:869–879. doi: 10.1084/jem.173.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark S C, Arya S K, Wong-Staal F, Matsumoto-Kobayashi M, Kay R M, Kaufman R J, Brown E L, Shoemaker C, Copeland T, Oroszlan S, Smith K, Sarngadharan M G, Linder S G, Gallo R C. Human T-cell growth factor: partial amino acid sequence, cDNA cloning, and organization and expression in normal leukemic cells. Proc Natl Acad Sci USA. 1984;81:2543–2547. doi: 10.1073/pnas.81.8.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooney E L. Enhanced immunity to human immunodeficiency virus (HIV) envelope elicited by a compound vaccine regimen consisting of priming with a vaccinia recombinant expressing HIV envelope and boosting with gp 160 protein. Proc Natl Acad Sci USA. 1993;90:1882–1886. doi: 10.1073/pnas.90.5.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cranage M P, Baskerville A, Ashworth L A E, Dennis M, Cook N, Cook R, Sharpe S A, Rose J, Kitchin P, Greenaway P J. Mucosal infection and vaccine studies with macaque SIV. Vaccine Res. 1992;1:311–318. [Google Scholar]
- 13.Daniel M D, Mazzara G P, Simon M A, Sehgal P K, Kodama T, Panicali D L, Desrosiers R C. High-titer immune responses elicited by recombinant vaccinia virus priming and particle boosting are ineffective in preventing virulent SIV infection. AIDS Res Hum Retroviruses. 1994;10:839–851. doi: 10.1089/aid.1994.10.839. [DOI] [PubMed] [Google Scholar]
- 14.Davis L, Lipsky P, Bottomly K. Measurement of human and murine interleukin 2 and interleukin 4. In: Coligan J, et al., editors. Current protocols in immunology. New York, N.Y: Green Publishing and Wiley-Interscience; 1995. pp. 6.3.1–6.3.13. [DOI] [PubMed] [Google Scholar]
- 15.Desrosiers, R. C. 1995. Non-human primate models for AIDS vaccines. AIDS 9(Suppl. A)**:**S137–S141. [PubMed]
- 16.Excler, J.-L., and S. Plotkin. 1997. The prime-boost concept applied to HIV preventive vaccines. AIDS 11(Suppl. A)**:**S127–S137. [PubMed]
- 17.Franchini G, Gurgo C, Guo H G, Gallo R C, Collalti E, Fargnoli K A, Hall L F, Wong-Staal F, Reitz M S., Jr Sequence of simian immunodeficiency virus and its relationship to the human immunodeficiency viruses. Nature. 1987;328:539–542. doi: 10.1038/328539a0. [DOI] [PubMed] [Google Scholar]
- 18.Franchini G, Robert-Guroff M, Tartaglia J, Aggarwal A, Abimiku A, Benson J, Markham P D, Limbach K, Hurteau G, Fullen J, Wills M, Arp J, Dekaban G, Paoletti E, Gallo R C. Highly attenuated HIV type 2 recombinant poxviruses, but not HIV-2 recombinant Salmonella vaccines induce long-lasting protection in rhesus macaques. AIDS Res Hum Retroviruses. 1995;11:909–920. doi: 10.1089/aid.1995.11.909. [DOI] [PubMed] [Google Scholar]
- 19.Gately M, Chizzonite R, Presky D. Measurement of human and mouse interleukin-12. In: Coligan J, et al., editors. Current protocols in immunology. New York, N.Y: Greene Publishing and Wiley-Interscience; 1995. pp. 6.1.6–6.1.15. [DOI] [PubMed] [Google Scholar]
- 20.Goebel S J, Johnson G P, Perkus M E, Davis S W, Winslow J P, Paoletti E. The complete DNA sequence of vaccinia virus. Virology. 1990;179:247–266. doi: 10.1016/0042-6822(90)90294-2. [DOI] [PubMed] [Google Scholar]
- 21.Graham B S, Matthews T J, Bleshe R B, Clements M L, Dolin R, Wright P F, Gorse G J, Schwartz D H, Keefer M C, Bolognesi D P, Corey L, Stablein D M, Esterlitz J R, Hu S-L, Smith G E, Fast P E, Koff N C the NIAID AIDS Vaccine Clinical Trails Network. Augmentation of human immunodeficiency virus type 1 neutralizing antibody by priming with gp160 recombinant vaccinia and boosting with rpg160 in vaccinia-naive adults. J Infect Dis. 1993;167:538–547. doi: 10.1093/infdis/167.3.533. [DOI] [PubMed] [Google Scholar]
- 22.Hirsch M S, Curran J. Human immunodeficiency viruses. In: Fields B N, et al., editors. Fields virology. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 1953–1975. [Google Scholar]
- 23.Hirsch V M, Fuerst T R, Sutter G, Carroll M W, Yang L C, Goldstein S, Piatak M, Jr, Elkins W R, Alvord W G, Montefiori D C, Moss B, Lifson J D. Patterns of viral replication correlate with outcome in simian immunodeficiency virus (SIV)-infected macaques: effect of prior immunization with a trivalent SIV vaccine in modified vaccinia virus Ankara. J Virol. 1996;70:3741–3752. doi: 10.1128/jvi.70.6.3741-3752.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoffman S L, Crutcher J M, Puri S K, Ansari A A, Villinger F, Franke E D, Singh P P, Finkelman F, Gately M K, Dutta G P, Sedegah M. Sterile protection of monkeys against malaria after administration of interleukin-12. Nat Med. 1997;3:80–83. doi: 10.1038/nm0197-80. [DOI] [PubMed] [Google Scholar]
- 25.Hu S-L. Is subunit envelope antigen our best bet for an AIDS vaccine? In: Girard M, Dodet B, editors. Neuvième Colloque des Cent Gardes. Retroviruses of human AIDS and related animal diseases. Paris, France: Marnes-La-Coquette; 1994. pp. 275–281. [Google Scholar]
- 26.Hussel T, Khan U, Openshaw P. IL-12 treatment attenuates T helper cell type 2 and B cell responses but does not improve vaccine-enhanced lung illness. J Immunol. 1997;159:328–334. [PubMed] [Google Scholar]
- 27.Jeannin P, Delnest Y, Seveso M, Life P, Bonnefoy J-Y. IL-12 synergizes with IL-2 and other stimuli in inducing IL-10 production by human T cells. J Immunol. 1996;156:3159–3165. [PubMed] [Google Scholar]
- 28.Kawakita M, Rao G S, Ritchey J K, Ornstein D K, Hudson D K, Tartaglia J, Paoletti E, Humphrey P A, Harmon T J, Ratliff T L. Effect of canarypox virus (ALVAC)-mediated cytokine expression on murine prostate tumor growth. J Natl Cancer Inst. 1997;89:428–436. doi: 10.1093/jnci/89.6.428. [DOI] [PubMed] [Google Scholar]
- 29.Lehner T, Wang Y, Cranage M, Bergmeier L A, Mitchell E, Tao L, Hall G, Denis M, Cook N, Brookes R, Klavinskis L, Jones I, Doyle C, Ward R. Protective mucosal immunity elicited by targeted iliac lymph node immunization with a subunit SIV envelop and core vaccine in macaques. Nat Med. 1996;2:767–775. doi: 10.1038/nm0796-767. [DOI] [PubMed] [Google Scholar]
- 30.Leno, M., L. Carter, L. Romano, P. D. Markham, K. Limbach, J. Tartaglia, E. Paoletti, J. Benson, G. Franchini, and M. Robert-Guroff. Vaccination-induced CD8 antiviral activity in rhesus macaques: correlation with low plasma viremia and slow disease progression following SIV challenge. Submitted for publication.
- 31.Leong K H, Ramsay A J, Boyle D B, Ramshaw I A. Selective induction of immune responses by cytokines coexpressed in recombinant fowlpox virus. J Virol. 1994;68:8125–8130. doi: 10.1128/jvi.68.12.8125-8130.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu S, Arthos S L, Montefiori D C, Yasutomi Y, Manson K, Mustafa F, Johnson E, Santoro J C, Wissink J, Mullins J I, Haynes J R, Letvin N L, Wyand M, Robinson H L. Simian immunodeficiency virus DNA vaccine trial in macaques. J Virol. 1996;70:3978–3991. doi: 10.1128/jvi.70.6.3978-3991.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Montefiori D C, Baba T W, Li A, Bilska M, Ruprecht R M. Neutralizing and infection-enhancing antibody responses do not correlate with the differential pathogenicity of SIVmac251 delta-3 in adult and infant rhesus monkeys. J Immunol. 1996;157:5528–5535. [PubMed] [Google Scholar]
- 34.Montefiori D C, Pantaleo G, Fink L M, Zhou J T, Zhou J Y, Bilska M, Miralles G D, Fauci A S. Neutralizing and infection-enhancing antibody responses to human immunodeficiency virus type 1 in long-term non-progressors. J Infect Dis. 1996;173:60–67. doi: 10.1093/infdis/173.1.60. [DOI] [PubMed] [Google Scholar]
- 35.Myagkikh M, Alipanah S, Markham P D, Tartaglia J, Paoletti E, Gallo R C, Franchini G, Robert-Guroff M. Multiple immunizations with attenuated poxvirus HIV type 2 recombinants and subunit boosts required for protection of rhesus macaques. AIDS Res Hum Retroviruses. 1996;12:985–991. doi: 10.1089/aid.1996.12.985. [DOI] [PubMed] [Google Scholar]
- 36.Perkus M E, Paoletti E. Recombinant virus as vaccination carrier of heterologous antigens. In: Kaufmann S H E, editor. Concepts in vaccine development. Berlin, Germany: Walter de Gruyter; 1996. pp. 379–408. [Google Scholar]
- 37.Perkus M E, Tartaglia J, Paoletti E. Poxvirus-based vaccine candidates for cancer, AIDS, and other infectious diseases. J Leukocyte Biol. 1995;58:1–13. doi: 10.1002/jlb.58.1.1. [DOI] [PubMed] [Google Scholar]
- 38.Pialoux G, Excler J L, Riviere Y, Gonzalez-Canali G, Feuillie V, Coulaud P, Gluckman J C, Matthews T J, Meignier B, Kieny M P, et al. A prime-boost approach to HIV preventive vaccine using a recombinant canarypox virus expressing glycoprotein 160 (MN) followed by a recombinant glycoprotein 160 (MN/LAI) AIDS Res Hum Retroviruses. 1995;11:373–381. doi: 10.1089/aid.1995.11.373. [DOI] [PubMed] [Google Scholar]
- 39.Romano J W, Williams K G, Shurtliff R N, Ginocchio C, Kaplan M. NASBA technology: isothermal RNA amplification in qualitative and quantitative diagnostics. Immunol Invest. 1997;26:15–28. doi: 10.3109/08820139709048912. [DOI] [PubMed] [Google Scholar]
- 40.Ruby J, Ramshaw A, Karupiah G, Ramshaw L. Recombinant virus vectors that coexpress cytokines—a new vaccine strategy. Vaccine Res. 1992;1:347–356. [Google Scholar]
- 41.Schultz, A. M., and S.-L. Hu. 1993. Primate models for HIV vaccines. AIDS 7(Suppl. 1)**:**S161–170. [PubMed]
- 42.Schultz, A. M., and E. J. Stott. 1994. Primate models for AIDS vaccines. AIDS 8(Suppl. 1)**:**S203–S212.
- 43.Seder R A, Kelsall B L, Jankovic D. Differential roles for IL-12 in the maintenance of immune responses in infectious versus autoimmune disease. J Immunol. 1996;157:2745–2748. [PubMed] [Google Scholar]
- 44.Tartaglia J, Perkus M E, Taylor J, Norton E K, Audonnet J-C, Cox W I, Davis S W, Van Der Hoeven J, Meignier B, Riviere M, Languet B, Paoletti E. NYVAC: a highly attenuated strain of vaccinia virus. Virology. 1992;188:217–232. doi: 10.1016/0042-6822(92)90752-b. [DOI] [PubMed] [Google Scholar]
- 45.Taylor J, Edbauer C, Rey-Senelonge A, Bouquet J-F, Norton E, Goebel S, Desmettre P, Paoletti E. Newcastle disease virus fusion protein expressed in a fowlpox virus recombinant confers protection in chickens. J Virol. 1990;64:1441–1450. doi: 10.1128/jvi.64.4.1441-1450.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vilinger F, Brar S S, Mayne A, Chikkala N, Ansari A A. Comparative sequence analysis of cytokine genes from human and nonhuman primates. J Immunol. 1995;155:3946–3954. [PubMed] [Google Scholar]
- 47.Watson A, Ranchalis J, Travis B, McClure J, Sutton W, Johnson P R, Hu S-H, Haigwood N L. Plasma viremia in macaques infected with simian immunodeficiency virus: viral load early in infection predicts survival. J Virol. 1997;71:284–290. doi: 10.1128/jvi.71.1.284-290.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wolf S F, Temple P A, Kobayashi M, Young D, Dicig M, Lowe L, Dzialo R, Fitz L, Ferenz C, Hewick R M, Kelleher K, Herrmann S H, Clark S C, Azzoni L, Chan S H, Trinchieri G, Perussia B. Cloning of cDNA for natural killer cell stimulatory factor, a heterodimeric cytokine with multiple biological effects on T and natural killer cells. J Immunol. 1991;146:3074–3081. [PubMed] [Google Scholar]
- 49.Wyand M S, Manson K H, Garcia-Moll M, Montefiori D, Desrosiers R C. Vaccine protection by a triple deletion mutant of simian immunodeficiency virus. J Virol. 1996;70:3724–3733. doi: 10.1128/jvi.70.6.3724-3733.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wyand M S, Manson K H, Lackner A A, Desrosiers R C. Resistance of neonatal monkeys to live attenuated vaccine strains of simian immunodeficiency virus. Nat Med. 1997;3:32–36. doi: 10.1038/nm0197-32. [DOI] [PubMed] [Google Scholar]