Adenovirus Internalization and Infection Require Dynamin (original) (raw)
Abstract
The cell receptors that facilitate adenovirus internalization into cells have been identified; however, the infectious pathway of virus entry has not been established. Adenovirus entry and infection were examined in HeLa cells lacking or overexpressing mutant dynamin, a protein that specifically regulates clathrin-mediated endocytosis. Expression of mutant dynamin significantly reduced adenovirus internalization and gene delivery, indicating a functional requirement for this molecule. These findings are consistent with virus entry via the clathrin-coated pit pathway.
Entry of human adenoviruses (Ads) into cells is a complex process that involves interactions of several viral capsid proteins with different cell receptors. A recently identified 46-kDa cell membrane protein mediates Ad attachment to cells via the fiber protein (2, 21). A second interaction of the virus penton base protein with αv integrins promotes virus internalization (1, 25). The expression of αv integrins on host cells has also been shown to determine the efficiency with which Ad can deliver foreign genes (6, 11, 12). While the receptors involved in Ad attachment and internalization have been identified, relatively little information exists on the precise entry pathway that leads to infection. Previous electron microscopic studies (3, 16), as well as biochemical analyses (5, 8, 17, 23), have suggested that Ad particles are internalized into cells via the coated-pit pathway. However, the majority of virus particles entering host cells appear to be in uncoated vesicles (16); thus, the infectious pathway of Ad entry has not been determined.
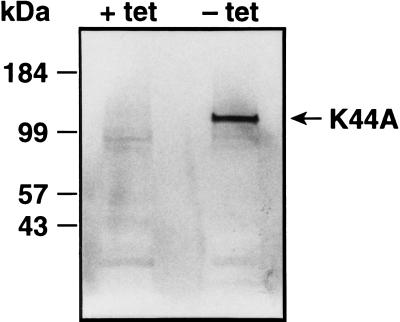
The recent identification of host cell proteins that regulate clathrin-mediated endocytosis has provided an opportunity to more precisely define the pathway of Ad entry. Dynamin is a 100-kDa cytosolic GTPase which selectively regulates clathrin-mediated endocytosis. Dynamin associates with clathrin-coated membrane invaginations and has been proposed to mediate the constriction of coated pits and the budding of coated vesicles from the plasma membrane (10, 20). A dominant-negative mutant form of dynamin containing a point mutation in the GTP binding site (lys44 to ala44, K44A) blocks clathrin-mediated endocytosis of transferrin and epidermal growth factor but does not significantly alter nonclathrin internalization pathways (4). We used tTA-HeLa cells stably transfected with the K44A dominant-negative dynamin (24) under the control of the tetracycline-inducible promoter (7) to determine whether Ad entry and infection are mediated by the clathrin-coated pit pathway. The K44A mutant protein contains an influenza virus hemagglutinin epitope tag that allows its detection in cells by immunoblotting. A 100-kDa protein, consistent with the expected size of dynamin, was expressed in cells cultured in medium lacking tetracycline (lane − tet in Fig. 1) but not in uninduced cells (lane + tet in Fig. 1). These results demonstrate the relatively tight control of mutant dynamin expression using the tetracycline-regulated promoter.
FIG. 1.
Detection of mutant dynamin expression by immunoblotting. tTA-HeLa cells stably transfected with the K44A dynamin mutant were cultured in the presence (+ tet) or absence (− tet) of tetracycline for 48 h and then solubilized in sodium dodecyl sulfate sample buffer. Lysates prepared from 105 induced or uninduced cells were separated on a sodium dodecyl sulfate–7%-polyacrylamide gel under reducing conditions. Following transfer of the proteins to a nitrocellulose filter (Immobilon P; Amersham), the filter was probed with an antihemagglutinin epitope tag monoclonal antibody (12CA5) and then incubated with a goat anti-mouse immunoglobulin antibody conjugated to alkaline phosphatase. The blot was then developed by addition of a chromogenic substrate (Nitro Blue Tetrazolium).
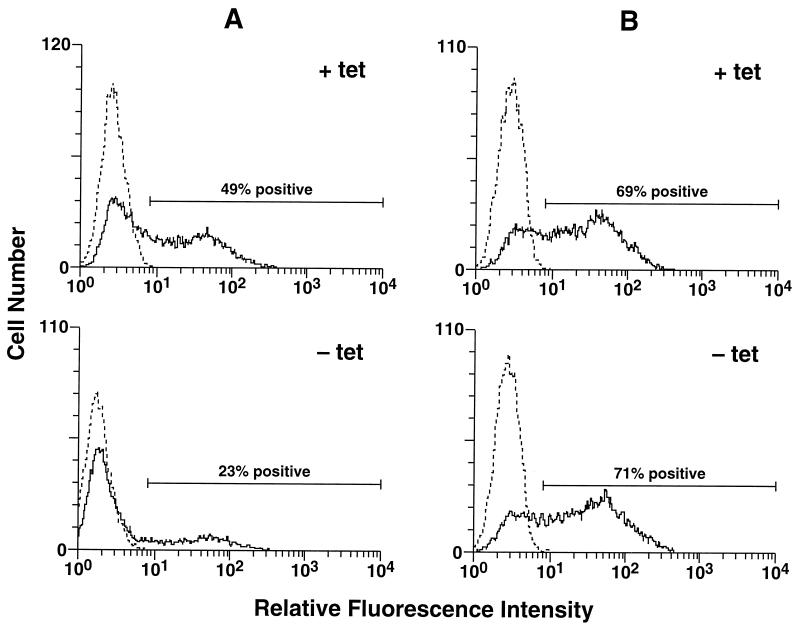
Ad infection of HeLa cells expressing the K44A mutant dynamin was measured by using a recombinant Ad vector, Ad.RSV.GFP (12), encoding green fluorescent protein (GFP). Uninduced tTA-HeLa cells or cells which had been induced by removal of tetracycline for 48 h were infected with Ad.RSV.GFP at a virus particle-to-cell ratio of 300. The number of cells expressing the GFP reporter gene was then quantitated 48 h postinfection by flow cytometry. A significant decrease in Ad-delivered GFP was observed in HeLa cells without tetracycline compared to cells with tetracycline (Fig. 2A). Ad-mediated gene delivery to HeLa cells with tetracycline was very similar to delivery to HeLa cells overexpressing wild-type dynamin under the control of the tetracycline-regulated promoter (data not shown). Tetracycline-treated HeLa cells infected with Ad.RSV.GFP also showed levels of GFP expression similar to those of cells infected with Ad.RSV.GFP and then subsequently induced by removal of tetracycline (Fig. 2B), indicating that the mutant dynamin protein did not inhibit expression of the Ad-delivered reporter gene.
FIG. 2.
Flow cytometric analysis of Ad-mediated gene delivery to control or mutant-dynamin-expressing HeLa cells. (A) tTA-HeLa cells were cultured in the presence (+ tet) or absence (− tet) of tetracycline for 48 h and then infected by incubation with Ad.RSV.GFP at a virus particle/cell ratio of 300 for 1 h at 37°C. The cells were then washed and recultured for 48 h in the presence or absence of tetracycline prior to flow cytometric analysis. Control cells (dotted lines) were incubated in medium without virus. (B) tTA-HeLa cells were cultured in the presence of tetracycline and then infected with Ad.RSV.GFP. The cells were then divided into two equal samples; one was cultured for 48 h in the presence of tetracycline (+ tet), and the other was cultured in the absence (− tet) of tetracycline. Both were then analyzed by flow cytometry.
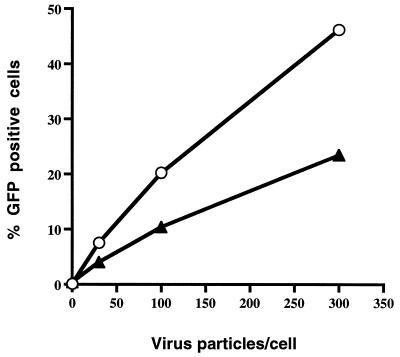
In further studies, we examined mutant dynamin-expressing and control (tetracycline-treated) cells for Ad-mediated gene delivery at different virus particle-to-cell ratios. HeLa cells expressing mutant dynamin showed significantly lower levels of Ad-mediated gene delivery than did tetracycline-treated cells at each virus particle-to-cell ratio (Fig. 3). Inhibition of Ad-mediated gene delivery was also observed when cells were incubated in the absence of tetracycline prior to infection and then cultured in the presence of tetracycline following infection (data not shown; see Fig. 5B).
FIG. 3.
Dose-dependent delivery of GFP to HeLa cells by use of recombinant Ad. tTA-HeLa cells were incubated in the presence (circles) or absence (triangles) of tetracycline for 48 h and then infected with Ad.RSV.GFP at various particle/cell ratios. GFP expression was analyzed 48 h later by flow cytometry. The data are representative of at least three experiments.
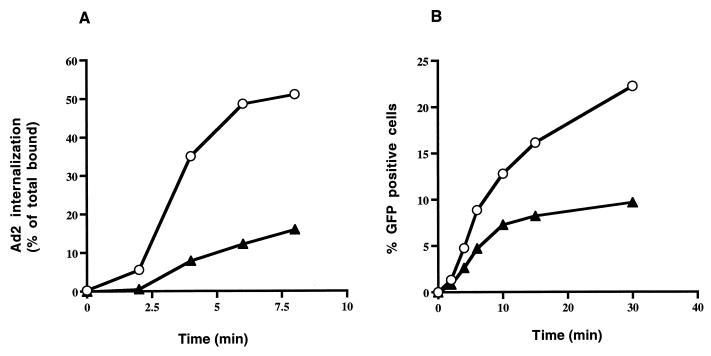
FIG. 5.
Ad internalization and gene delivery into HeLa cells expressing or lacking mutant dynamin. (A) Internalization of 125I-labeled Ad was measured in HeLa cells grown in the presence (circles) or absence (triangles) of tetracycline as previously described (25). Following warming of the cells to 37°C for various lengths of time, uninternalized virus particles were removed by incubating the cells in trypsin-EDTA and then washing them with HEPES-buffered saline. The data are the mean ± the standard deviation of triplicate samples. (B) In parallel studies, Ad-mediated gene delivery was examined in cells grown in the presence (circles) or absence (triangles) of tetracycline. Cells were incubated at 4°C with Ad.RSV.GFP at a particle/cell ratio of 300 and then warmed to 37°C for various lengths of time. After removal of uninternalized virus particles with trypsin-EDTA, the cells were cultured in the presence of tetracycline for 48 h prior to flow cytometric analysis. The data are representative of two experiments. Ad2, Ad type 2.
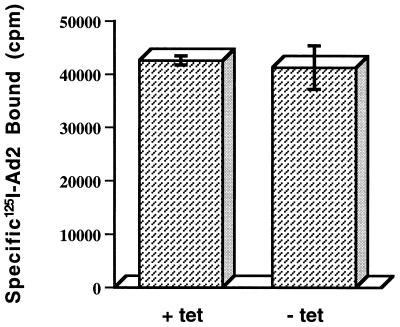
Studies were next undertaken to determine the stage at which expression of mutant dynamin inhibited Ad-mediated gene delivery. We first examined whether expression of the K44A mutant dynamin was capable of interfering with Ad attachment to cells. Tetracycline-free HeLa cells expressing mutant dynamin supported levels of radiolabeled Ad binding similar to those supported by tetracycline-treated HeLa cells (Fig. 4). Therefore, inhibition of Ad-mediated gene delivery was not due to inhibition of virus attachment. Further studies were performed to examine whether Ad internalization into cells was affected by expression of mutant dynamin. As shown in Fig. 5A, Ad was rapidly internalized in HeLa cells lacking mutant dynamin within 5 to 10 min after warming to 37°C. In contrast, only a low level of viral entry occurred in HeLa cells expressing the K44A mutant dynamin. In parallel studies, we examined Ad-mediated gene delivery in cells expressing or lacking mutant dynamin. Cells were warmed to 37°C for various lengths of time to allow virus uptake, and then virus particles remaining on the plasma membrane (uninternalized) were removed by trypsin digestion (11). Following culturing of cells in the presence of tetracycline for 48 h, reporter gene expression was measured by flow cytometry. Cells expressing mutant dynamin showed a significant decrease in Ad-mediated gene delivery over time compared to uninduced cells lacking mutant dynamin (Fig. 5B). These findings indicate that efficient Ad entry and infection are both regulated by dynamin, an essential component of the clathrin-coated pit endocytic pathway.
FIG. 4.
Ad binding to HeLa cells expressing or lacking mutant dynamin. Cells were incubated for 48 h in the presence (+ tet) or absence (− tet) of tetracycline and then assayed for binding of 125I-labeled Ad type 2 (Ad2) particles as previously described (11). Nonspecific virus binding, which was subtracted from the total, was determined by incubating cells with a 200-fold excess of unlabeled virus particles. The data are the mean ± the standard deviation of triplicate samples.
Ad entry into cells has been shown to be promoted by interaction of the virus penton base protein with integrins αvβ3 and αvβ5 (1, 25). αv integrin clustering by Ad particles could facilitate localization of virus particles to coated pits that are destined for internalization. Although direct evidence for this is lacking, the cytoplasmic tails of the β3 and β5 subunits of αv integrins contain the NPXY motif (18, 19), which has been shown to be necessary for the localization of certain receptors to coated pits (15). Disruption of the NPXY sequence in the cytoplasmic tail of β1 integrins has also been reported to inhibit clathrin-mediated bacterial uptake into cells (22). Further studies are necessary to determine if specific internalization sequences in αv integrins mediate Ad uptake into clathrin-coated pits.
While mutant dynamin expression significantly inhibited virus uptake and gene delivery, it did not completely abolish these activities. The present findings are consistent with previous reports that induction of mutant dynamin expression does not completely block internalization of transferrin and epidermal growth factor. These ligands have been well documented to enter cells via the clathrin-coated pit pathway (4). The kinetically slower and less efficient endocytic processes which are not affected by mutant dynamin expression may represent clathrin-independent entry mechanisms such as fluid-phase pinocytosis (13). In certain cell types, pinocytic uptake of solutes has been reported to account for as much as 50% of the total volume of endocytosis (14). Interestingly, Ad-mediated gene delivery is not inhibited by treatment of cells with amiloride or hexamethylamiloride (data not shown), which are potent inhibitors of macropinocytosis (9), suggesting that viral entry probably does not involve this type of endocytic pathway. While further studies are necessary to fully characterize the molecular events involved in Ad internalization, these studies provide direct evidence for the role of dynamin in Ad entry and infection.
Acknowledgments
We thank Erguang Li for helpful discussions and Sandra Schmid for providing tTa-HeLa cells expressing the K44A mutant dynamin.
This work was supported by National Institutes of Health grants HL54352 and EY11431.
Footnotes
†
Publication 11132-IMM from The Scripps Research Institute.
REFERENCES
- 1.Bai M, Harfe B, Freimuth P. Mutations that alter an Arg-Gly-Asp (RGD) sequence in the adenovirus type 2 penton base protein abolish its cell-rounding activity and delay virus reproduction in flat cells. J Virol. 1993;67:5198–5205. doi: 10.1128/jvi.67.9.5198-5205.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergelson J M, Cunningham J A, Droguett G, Kurt-Jones E A, Krithivas A, Hong J S, Horwitz M S, Crowell R L, Finberg R W. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 3.Chardonnet Y, Dales S. Early events in the interaction of adenoviruses with HeLa cells. I. Penetration of type 5 and intracellular release of the DNA genome. Virology. 1970;40:462–477. doi: 10.1016/0042-6822(70)90189-3. [DOI] [PubMed] [Google Scholar]
- 4.Damke H, Baba T, Warnock D E, Schmid S L. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J Cell Biol. 1994;127:915–934. doi: 10.1083/jcb.127.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fitzgerald D J P, Padmanabhan R, Pastan I, Willingham M C. Adenovirus-induced release of epidermal growth factor and pseudomonas toxin into the cytosol of KB cells during receptor-mediated endocytosis. Cell. 1983;32:607–617. doi: 10.1016/0092-8674(83)90480-4. [DOI] [PubMed] [Google Scholar]
- 6.Goldman M J, Wilson J M. Expression of αvβ5 integrin is necessary for efficient adenovirus-mediated gene transfer in the human airway. J Virol. 1995;69:5951–5958. doi: 10.1128/jvi.69.10.5951-5958.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greber U F, Willetts M, Webster P, Helenius A. Stepwise dismantling of adenovirus 2 during entry into cells. Cell. 1993;75:477–486. doi: 10.1016/0092-8674(93)90382-z. [DOI] [PubMed] [Google Scholar]
- 9.Hewlett L J, Prescott A R, Watts C. The coated pit and macropinocytic pathways serve distinct endosome populations. J Cell Biol. 1994;124:689–703. doi: 10.1083/jcb.124.5.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hinshaw J E, Schmid S L. Dynamin self-assembles into rings suggesting a mechanism for coated vesicle budding. Nature. 1995;374:190–192. doi: 10.1038/374190a0. [DOI] [PubMed] [Google Scholar]
- 11.Huang S, Endo R I, Nemerow G R. Upregulation of integrins αvβ3 and αvβ5 on human monocytes and T lymphocytes facilitates adenovirus-mediated gene delivery. J Virol. 1995;69:2257–2263. doi: 10.1128/jvi.69.4.2257-2263.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang S, Stupack D G, Mathias P, Wang Y, Nemerow G. Growth arrest of Epstein-Barr virus immortalized B lymphocytes by adenovirus-delivered ribozymes. Proc Natl Acad Sci USA. 1997;94:8156–8161. doi: 10.1073/pnas.94.15.8156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lamaze C, Schmid S L. The emergence of clathrin-independent pinocytic pathways. Curr Biol. 1995;7:573–580. doi: 10.1016/0955-0674(95)80015-8. [DOI] [PubMed] [Google Scholar]
- 14.McKinley D N, Wiley H S. Reassessment of fluid-phase endocytosis and diacytosis in monolayer cultures of human fibroblasts. J Cell Physiol. 1988;136:389–397. doi: 10.1002/jcp.1041360302. [DOI] [PubMed] [Google Scholar]
- 15.Mukherjee S, Ghosh R N, Maxfield F R. Endocytosis. Physiol Rev. 1997;77:759–803. doi: 10.1152/physrev.1997.77.3.759. [DOI] [PubMed] [Google Scholar]
- 16.Patterson S, Russell W C. Ultrastructural and immunofluorescence studies of early events in adenovirus-HeLa cell interactions. J Gen Virol. 1983;64:1091–1099. doi: 10.1099/0022-1317-64-5-1091. [DOI] [PubMed] [Google Scholar]
- 17.Perez L, Carrasco L. Involvement of the vacuolar H+-ATPase in animal virus entry. J Gen Virol. 1994;75:2595–2606. doi: 10.1099/0022-1317-75-10-2595. [DOI] [PubMed] [Google Scholar]
- 18.Ramaswamy H, Hemler M E. Cloning, primary structure and properties of a novel human integrin β subunit. EMBO J. 1990;9:1561–1568. doi: 10.1002/j.1460-2075.1990.tb08275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suzuki S, Argraves W S, Pytela R, Arai H, Krusius T, Pierschbacher M D, Ruoslahti E. cDNA and amino acid sequences of the cell adhesion protein receptor recognizing vitronectin reveal a transmembrane domain and homologies with other adhesion protein receptors. Proc Natl Acad Sci USA. 1986;83:8614–8618. doi: 10.1073/pnas.83.22.8614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takei K, McPherson P S, Schmid S L, Camilli P D. Tubular membrane invaginations coated by dynamin rings are induced by GTP-gammaS in nerve terminals. Nature. 1995;374:186–190. doi: 10.1038/374186a0. [DOI] [PubMed] [Google Scholar]
- 21.Tomko R P, Xu R, Philipson L. HCAR and MAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc Natl Acad Sci USA. 1997;94:3352–3356. doi: 10.1073/pnas.94.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Nhieu G T, Krukonis E S, Reszka A A, Horwitz A F. Mutations in the cytoplasmic domain of the integrin β1 chain indicate a role for endocytosis factors in bacterial internalization. J Biol Chem. 1996;271:7665–7672. doi: 10.1074/jbc.271.13.7665. [DOI] [PubMed] [Google Scholar]
- 23.Varga M J, Weibull C, Everitt E. Infectious entry pathway of adenovirus type 2. J Virol. 1991;65:6061–6070. doi: 10.1128/jvi.65.11.6061-6070.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vieira A V, Lamaze C, Schmid S L. Control of EGF receptor signaling by clathrin-mediated endocytosis. Science. 1996;274:2086–2089. doi: 10.1126/science.274.5295.2086. [DOI] [PubMed] [Google Scholar]
- 25.Wickham T J, Mathias P, Cheresh D A, Nemerow G R. Integrins αvβ3 and αvβ5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]