Vaccination with a Recombinant Vesicular Stomatitis Virus Expressing an Influenza Virus Hemagglutinin Provides Complete Protection from Influenza Virus Challenge (original) (raw)
Abstract
Since the development of a system for generating vesicular stomatitis virus (VSV) from plasmid DNAs, our laboratory has reported the expression of several different glycoproteins from recombinant VSVs. In one of these studies, high-level expression of an influenza virus hemagglutinin (HA) from a recombinant VSV-HA and efficient incorporation of the HA protein into the virions was reported (E. Kretzschmar, L. Buonocore, M. J. Schnell, and J. K. Rose, J. Virol. 71:5982–5989, 1997). We report here that VSV-HA is an effective intranasal vaccine vector that raises high levels of neutralizing antibody to influenza virus and completely protects mice from bronchial pneumonia caused by challenge with a lethal dose of influenza A virus. Additionally, these recombinant VSVs are less pathogenic than wild-type VSV (serotype Indiana). This vector-associated pathogenicity was subsequently eliminated through introduction of specific attenuating deletions. These live attenuated recombinant VSVs have great potential as vaccine vectors.
Vesicular stomatitis virus (VSV) is a nonsegmented, negative-strand RNA virus and the prototypic member of the Rhabdoviridae family. The 11-kb VSV genome encodes five structural proteins: nucleocapsid (N), phosphoprotein (P), matrix (M), glycoprotein (G), and an RNA-dependent RNA polymerase (L). Expression levels of the proteins are attenuated in a stepwise fashion following the order of transcription from the 3′ proximal end to the 5′ proximal end of the viral genome (17).
VSV is a natural pathogen of livestock and is probably transmitted by an arthropod vector (10). Although VSV can cause significant disease in livestock, including vesicular lesions around the mouth, hoofs, and teats and loss of milk production, the disease is rarely fatal (7). VSV infections of humans have been observed in rural areas where disease is enzootic and in individuals exposed to VSV in laboratory environments (13). VSV infections are usually asymptomatic in humans. However, febrile illness including chills, myalgia, and nausea has been associated with some symptomatic cases of VSV in humans (8, 10, 11, 13). VSV infects many different species in addition to humans and livestock. A mouse model for VSV infection exists, in which susceptible mice suffer hind limb paralysis and fatal encephalitis within 12 days of infection. The rate and degree of pathogenesis and the lethality of VSV in mice are dependent on several factors, including the strain of virus, the route and titer of inoculation, and the age and sex of the mice (1, 9).
In 1995 Lawson et al. first reported a system for generating recombinant VSVs from plasmid DNAs (18), and similar results were subsequently reported by others (22). Since then our laboratory has developed VSV into an efficient expression vector and reported the recovery of several recombinant VSVs expressing foreign proteins (12, 15, 22, 23). With the advent of this system for generating recombinant VSVs, we have begun to explore the potential of VSV as a recombinant vaccine vector.
Certain characteristics of VSV suggest that recombinant VSVs expressing foreign viral glycoproteins would be very good vaccine candidates. VSV grows to very high titers in many cell lines in vitro (>109 PFU/ml) and elicits strong humoral and cellular immune responses in vivo (7, 24, 25). VSV naturally infects at mucosal surfaces, and mucosal immunization has been shown to elicit both mucosal and systemic immunity (16, 19, 20). Additionally, the low percentage of VSV seropositivity in the general population (3) and the lack of serious pathogenicity in humans are some of the possible advantages of using recombinant VSV vaccines in humans.
Vaccines based on live VSV recombinants would also have advantages over other live recombinant vaccine vectors. Compared to the large, complex genomes of viruses from the Poxviridae family, which encode hundreds of proteins (including immunoevasive and immunosuppressive proteins), the VSV genome is relatively simple, more fully understood, and easier to manipulate. Compared to the segmented genomes of viruses in the Orthomyxoviridae family, the single-stranded genome of VSV does not undergo reassortment and therefore cannot reassort with wild-type viruses in vivo. Cytoplasmic replication, the lack of genetic recombination in VSV, and the ability to accommodate large inserts and multiple genes into its genome are additional attributes of VSV as a recombinant vaccine vector.
A VSV expressing the influenza A virus hemagglutinin (HA) protein (VSV-HA) was reported recently (15). Because antibodies capable of neutralizing influenza virus are directed to the HA protein and because antibodies to HA are sufficient to protect animals from disease (5, 17), we chose to examine the potential of the recombinant VSV-HA as a vaccine against influenza A/WSN/33 (H1N1) virus (a mouse-adapted strain). The expression of influenza virus HA proteins in recombinant vaccinia virus and in recombinant Venezuelan equine encephalitis virus (members of the Poxviridae and Togaviridae families, respectively) has successfully demonstrated the use of a mouse model system for evaluation of vectored influenza virus vaccines (2, 6). Although modifications to the VSV vector may be required before it is a viable influenza virus vaccine candidate, we employ a mouse model system and demonstrate the ability of VSV-HA administered intranasally to elicit humoral immune responses to the expressed influenza virus HA protein and to confer protection from lethal influenza virus challenge. Additionally, we report here on the reduced pathogenicity associated with the vaccine vector (recombinant wild-type VSV compared to wild-type VSV Indiana) and on the further attenuation of vectors containing cytoplasmic tail truncations of the VSV surface glycoprotein (21).
MATERIALS AND METHODS
Viruses and inoculum.
Recombinant wild-type VSV (VSVrwt), VSV-CT1, VSV-CT9, and recombinant VSV expressing influenza A/WSN virus HA protein (VSV-HA) were grown on BHK cells in Dulbecco modified Eagle medium (DMEM) (with l-glutamine, sodium pyruvate, and high glucose and bicarbonate concentrations [3.7 g/liter]; product no. 56-499; GRH Biosciences, Lenexa, Kans.) containing 5% fetal bovine serum (FBS) and penicillin-streptomycin (PS; 100 U/ml). Viral titers were determined from thawed stocks by standard plaque assays. Plaque assays were done in duplicate on BHK cells by using a 1% methylcellulose–1× DMEM (with 5% FBS and PS) overlay. Recombinant VSVs were thawed and diluted with DMEM (serum free) to appropriate titers immediately prior to inoculation. Influenza A/WSN/33 (H1N1) virus (5.3 × 107 PFU/ml), used for challenges, was grown in MDBK cells as described by Castrucci et al. (4). The influenza A/WSN virus was thawed immediately prior to challenge and administered undiluted in a 50-μl total volume. Influenza A/WSN virus used for neutralization assays was grown on MDBK cells in DMEM complemented with 10% FBS and PS, and titers were determined by standard plaque assay on MDBK cells with 1% agarose–1× DMEM (serum free)–trypsin-tolylsulfonyl phenylalanyl chloromethyl ketone (3 μg/ml) overlays. Viruses, DMEM (serum free), or phosphate-buffered saline (PBS) were administered intranasally as described below.
Inoculation of mice.
Five- to six-week-old, female, BALB/c mice from Charles River Laboratories were housed in filter-isolette cages upon arrival. Mice were inoculated no earlier than 4 days after arrival. Prior to inoculation (day 0) mice were lightly anesthetized with Metofane (methoxyflurane; Mallinckrodt Veterinary, Inc., Mundelein, Ill.) and marked by ear punch. Inoculum (25 μl) was administered intranasally with a 200-μl pipette to each anesthetized mouse, and mice were weighed in a plastic beaker on a Sartorius balance (model 1409) to ±0.02 g. Booster doses were administered in an identical fashion with viruses of equal kind, titer, and volume on day 21 unless indicated otherwise. Challenge doses were also administered as described but in a total volume of 50 μl per mouse (day 35 or day 21). Mice were weighed unanesthetized on a daily basis.
Neutralization assays and 50% plaque reduction.
Blood samples from mice inoculated with the same virus preparation (or with DMEM) were pooled and allowed to clot at room temperature. Clots were removed, and samples were centrifuged in a TOMY MTX-150 centrifuge (TMA-11 fixed-angle rotor) at 4°C for 15 min at 5,500 rpm. Clarified sera were transferred to sterile Eppendorf tubes and heat inactivated at 56°C for 30 to 60 min. Heat-inactivated sera were diluted with PBS in serial twofold dilutions in a 96-well plate. Generally, 50 μl of serum was mixed with 50 μl of PBS, and 50 μl was then transferred for serial dilutions. An equal volume (50 μl) of influenza A/WSN virus (∼1.4 × 103 PFU/ml) was then added to the remaining 50 μl of diluted sera and mixed. The 96-well plates containing virus and sera were incubated at 37°C for 30 to 45 min. Virus-serum solutions were transferred from 96-well plates to confluent monolayers of MDBK cells in 6-well plates. Each well of the 96-well plate was washed with 150 μl of DMEM and then transferred to the corresponding well in the 6-well plate. Six-well plates were rocked at room temperature for 30 to 60 min, media were aspirated, and 3 ml of overlay was added to each well. Plates were incubated at 37°C with 5% CO2 for 2 days. In each assay, sera were diluted and analyzed in duplicate and each assay was repeated at least once. Neutralization titers are those dilutions which correspond to at least a 50% plaque reduction compared to the control. Neutralization titers are reported as averages from all assays.
Neutralization assays were also conducted to obtain titers of serum antibody to the vector virus. Assays were performed as previously described but sera were incubated in the presence of VSVrwt, and neutralization titers were determined by total inhibition of viral cytopathic effect (CPE) on BHK cells.
Necropsy and tissue preparation.
Mice were asphyxiated with CO2. Lungs were inflated with 10% neutral buffered formalin via the trachea with a 21-gauge needle. After overnight fixation in neutral buffered formalin, tissues were embedded in paraffin, sectioned, and stained with hemoxylin and eosin. Photomicroscopy was done with an Olympus microscope with Techpan film at an original magnification of ×400.
RESULTS
Preliminary experiments demonstrated the ability of VSV- HA to elicit an antibody response to HA, including neutralizing antibodies to influenza A/WSN virus (and to VSVrwt [vector virus]), and to confer protection from a lethal influenza A/WSN virus challenge (data not shown). In these preliminary studies mice were inoculated via an intraperitoneal route with live or UV-inactivated VSV-HA or VSVrwt, given booster doses intraperitoneally 3 weeks after initial inoculation, and challenged intranasally with 50 μl of influenza A/WSN virus 2 weeks after boosting. Mice inoculated with live VSV-HA had neutralizing antibody titers of >1:2,000 to influenza A/WSN virus and were protected from lethal challenge. Mice receiving UV-inactivated VSV-HA (in the presence of adjuvant) were also protected from lethal challenge. Control mice receiving live or UV-inactivated VSVrwt did not produce neutralizing titers of antibody to influenza A/WSN virus (although they did produce neutralizing titers of antibody to VSV) and were not protected from influenza A/WSN virus challenge.
These preliminary data suggested that VSV-HA might be a useful tool in examining recombinant VSVs as potential recombinant vector vaccines. Although inoculation of mice through an intraperitoneal route and the use of adjuvant with the UV-inactivated virus are procedures often used in studying the efficacy of potential immunogens, it is not an ideal method for vaccine delivery. These drawbacks, along with the following three observations, contributed to the strategy employed in the present study: (i) VSV naturally infects at mucosal surfaces; (ii) influenza virus is naturally transmitted through the respiratory route; and (iii) a mouse model system exists for intranasal inoculations of mice with both viruses. We therefore examined the efficacy of VSV-HA in protecting mice from a lethal influenza A/WSN virus challenge when both inoculations and challenge were delivered intranasally.
Determination of a mouse 50% lethal dose (LD50) for the live recombinant vector VSVrwt.
When wild-type VSV (serotype Indiana) is administered to young mice (5 to 6 weeks old) via an intranasal route, the mice often develop hind-limb paralysis and die from a lethal encephalitis within 7 to 12 days after inoculation, with titers as low as 104 PFU/mouse proving fatal (our data and reference 9). Therefore, before examining the efficacy of a recombinant VSV vaccine, we first addressed the pathogenicity of the vaccine vector.
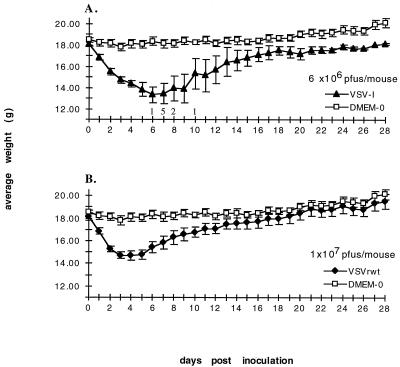
To determine whether the recombinant VSVs derived from plasmid DNAs were pathogenic in mice, wild-type VSV Indiana (VSVI) or VSVrwt (recombinant VSV) were plaque purified. Four plaques of each virus were picked, viral stocks were grown, and titers were determined. Six-week-old female BALB/c mice were inoculated intranasally with VSVI or VSVrwt in a total volume of 25 μl. Mice were weighed and observed daily for signs of pathogenesis as indicated by weight loss, paralysis, or death. A graphic representation of the average daily weights of mice inoculated with VSVI, VSVrwt, or medium only is presented in Fig. 1, in which numbers above the x axis indicate the number of mice dying that day.
FIG. 1.
Effects of viral inoculation on average weight and survival. (A) Twelve 6-week-old female BALB/c mice were divided into four groups of three mice each. Four plaque-purified viral stocks of VSVI were administered intranasally (one plaque-purified stock/group of three mice) at 6 × 106 PFU/mouse. Control mice (DMEM-0) received 25 μl of medium only. Mice were inoculated on day 0 and then weighed daily. Datum points represent the average daily weights. Numbers above the x axis represent the number of mice found dead that day. Error bars, ±0.5 × standard deviation. (B) Same as panel A except mice were inoculated with one of four plaque-purified stocks of VSVrwt at 107 PFU/mouse.
Mice inoculated with VSVI experienced much greater pathogenesis than did mice inoculated with VSVrwt as indicated by initial weight loss, time to weight loss recovery (if any), onset of hind-limb paralysis, and lethality. Of the mice receiving VSVI, 75% suffered weakness and paralysis within 4 to 7 days and subsequently died within 10 days. The 25% of VSVI-inoculated mice that survived all received virus from the same plaque-purified stock, perhaps indicating a slight decrease in pathogenesis for that particular stock. Two of these three surviving mice showed signs of increased pathogenesis compared to VSVrwt-inoculated mice. These two mice lost 20.7 and 35.4% of initial body weight and began regaining weight at 9 and 10 days postinoculation, respectively. The other mouse lost 15.4% of its initial body weight and began regaining weight at 5 days postinoculation (similar to mice receiving VSVrwt). No paralysis or death was observed in mice receiving VSVrwt. The average percent loss of initial body weight in mice receiving VSVrwt was 20.7% ± 3.1%, and most mice began regaining initial weight loss within 4 to 6 days of inoculation. As a control for average daily weights, four mice were inoculated with DMEM only.
From our own data and from lethality data of VSVI reported by Forger et al. (9), a mouse LD50 of approximately 106 PFU/mouse was determined for intranasal inoculations of BALB/c mice (5 to 6 weeks old). The lethality of VSVI varies slightly, depending on the virus preparation and the total volume of the virus inoculum. No LD50 has been reached for mice inoculated with VSVrwt under the same conditions even at titers that are 10-fold higher (107 PFU/mouse) than the LD50 for VSVI. The attenuation of VSVrwt is apparent although further attenuation (one in which mice lack weight loss associated with pathogenesis) would be ideal. The attenuation of VSVrwt compared to VSVI, however, indicates that VSVrwt-based vectors may potentially be used as a recombinant vaccine vectors.
Immunogenicity and vaccine efficacy of recombinant VSV-HA.
Having determined that intranasal inoculation with VSVrwt vector at titers of ≤107 PFU/mouse was not lethal, we examined the immunogenicity of recombinant VSV-HA. VSV-HA was administered at three titers (104, 105, and 106 PFU/mouse). Each titer was examined for its ability to raise neutralizing antibodies to influenza A/WSN virus and was further evaluated for its efficacy in protecting mice from lethal influenza A/WSN virus challenge. Mice were similarly inoculated with VSVrwt at matching titers or with DMEM as control groups.
Six-week-old female BALB/c mice were lightly anesthetized with Metofane and inoculated intranasally with live VSV-HA or VSVrwt in a total volume of 25 μl. Mice were observed daily for signs of paralysis and/or death, and none occurred. However, mice did appear somewhat less active and less well groomed during the first few days immediately following the initial inoculations. At day 18 postinoculation two mice per group were bled. Sera from these bleeds were pooled for each group and heat inactivated. Mice receiving VSV-HA virus produced antibodies to both VSV and influenza A/WSN virus as determined by indirect immunofluorescence assays (IFA) to VSV-infected BHK cells and to influenza A/WSN virus-infected MDBK cells, respectively. Mice inoculated with VSVrwt produced antibodies to VSV only. Sera were also assayed for their ability to neutralize influenza A/WSN virus by using a 50% plaque reduction assays on MDBK cells (Table 1). Sera from mice inoculated with VSV-HA contained neutralizing antibodies to influenza A/WSN virus at titers of 1:512, indicating that intranasal inoculation with recombinant VSV-HA raised a systemic immune response to the heterologous influenza A/WSN virus when administered through a mucosal route. Sera collected from mice prior to inoculation showed no antibodies to either VSV or influenza A/WSN by IFA or by neutralization assays.
TABLE 1.
Antibody response and percent survival
| Initial inoculum and boost (PFU/mouse) | Antibodya | NTb to influenza A/WSN virus at: | % Survivalc after challenge | |
|---|---|---|---|---|
| Initial bleed | Second bleed | |||
| VSVrwt (106) | −WSN-HA, +VSV | <1:8 | <1:8 | 0 |
| VSVrwt (105) | −WSN-HA, +VSV | ND | ND | 0 |
| VSVrwt (104) | −WSN-HA, +VSV | ND | ND | 0 |
| VSV-HA (106) | +WSN-HA, +VSV | ≥1:512 | ≥1:1,024 | 100 |
| VSV-HA (105) | +WSN-HA, +VSV | ≥1:512 | ≥1:1,024 | 100 |
| VSV-HA (104) | +WSN-HA, +VSV | ≥1:512 | ≥1:1,024 | 100 |
Mice were given booster doses 21 days after initial inoculation and observed for an additional 14 days. As before, paralysis and/or death were not observed in any mice, and all mice appeared healthy. At day 32, mice were bled and sera were tested for antibodies by IFA and neutralization assays. The results (Table 1) were similar to those obtained at 18 days. At day 35, mice were challenged intranasally with a lethal dose of influenza A/WSN virus (10 LD100 in 50 μl). Mice were observed for an additional 14 days after influenza A/WSN virus challenge. Within 5 days of influenza A/WSN virus challenge, all mice inoculated with VSVrwt had died. No mice inoculated with VSV-HA died or showed any signs of sickness. These data show that recombinant VSVs delivered intranasally are not only efficacious in raising systemic immunity but also completely protect mice from morbidity and death associated with a lethal challenge when the recombinant VSV expresses a foreign antigen corresponding to the challenge virus. These data also show that VSV-HA titers as low as 104 PFU/mouse are sufficient to protect mice from a lethal influenza A/WSN virus challenge.
Pathogenicity, immunogenicity, and vaccine efficacy of recombinant VSV-HA.
Several observations led to further experiments examining the potential of recombinant VSVs as vaccine vectors. Taken together, the ability of low titers of VSV-HA to protect from lethal influenza A/WSN virus challenge and the reduced pathogenicity of the VSV vector compared to VSVI suggested that we should examine the pathogenicity of the recombinant VSV-HA and VSVrwt at lower titers. Therefore, pathogenicity (as indicated by weight loss), immunogenicity (as indicated by antibody production), and vaccine efficacy (as indicated by an ability to protect mice from lethal influenza A/WSN virus challenge) were examined in mice inoculated with VSV-HA or VSVrwt at titers of 5 × 104 PFU/mouse.
Mice were inoculated intranasally with VSV-HA, VSVrwt, or DMEM only on day 0 and weighed daily. At 18 days after initial inoculation, two mice per group were bled and sera were pooled and heat inactivated. These pooled sera were assayed for neutralizing titers to influenza A/WSN virus by 50% plaque reduction assays on MDBK cells. Mice inoculated with VSV-HA had neutralizing serum titers of 1:640 of antibody to influenza A/WSN virus (Table 2). Mice were boosted with recombinant VSVs (or DMEM) on day 21, and sera were subsequently collected, pooled, and assayed for neutralizing titers of antibody to influenza A/WSN virus on day 32. Mice inoculated with VSV-HA had neutralizing serum titers of 1:2,560 of antibody to influenza A/WSN virus. No neutralizing antibodies to influenza A/WSN virus were detected in mice prior to inoculation or in mice inoculated with VSVrwt or DMEM (Table 2). Mice inoculated with recombinant VSVs were challenged with a lethal dose of influenza A/WSN virus (50 μl) on day 35. Mice inoculated and boosted with DMEM were boosted with 50 μl of DMEM on day 35 and maintained as a weight control group.
TABLE 2.
Neutralization titers and percent survival of mice challenged with influenza A/WSN virus
| Inoculuma | No. of inoculations | NTb to influenza A/WSN virus at: | % Survivalc after challenge | |
|---|---|---|---|---|
| Initial bleed | Second bleed | |||
| VSVrwt | 2 | <1:8 | <1:8 | 60d |
| VSV-HA | 2 | 1:640 | 1:2,560 | 100 |
| VSV-HA | 1 | 1:640 | NBe | 100 |
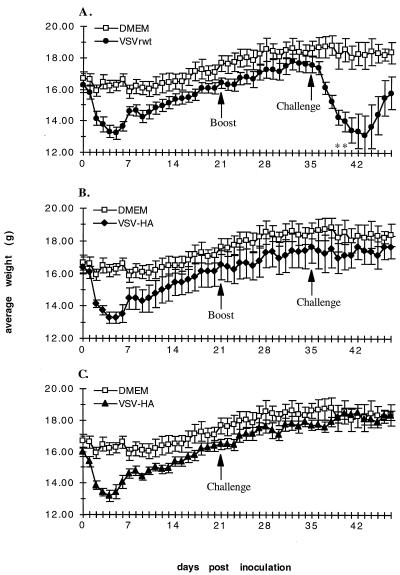
Mice receiving initial inoculations of recombinant VSVs showed signs of pathogenesis as indicated by weight loss. This is demonstrated in Fig. 2A and B by the dips in daily weight averages (days 0 to 5). This initial weight loss was similar in both VSVrwt- and VSV-HA-inoculated mice. The percentages of initial body weight loss were 19.3 ± 4.5 and 18.1 ± 3.4 in mice inoculated with VSVrwt and VSV-HA, respectively. In contrast, the percentage of initial body weight loss in DMEM-inoculated mice was only 4.4 ± 2.0. After the boosting treatment, no significant weight loss occurred in any mice (days 21 to 23), indicating immunity to the vector. On day 35 mice were challenged with a lethal dose of influenza A/WSN virus. VSV-HA-inoculated mice were completely protected from lethal challenge as indicated by the absence of significant weight loss (Fig. 2B) and 100% survival (Table 2). In contrast, control mice inoculated with VSVrwt were not protected from challenge with influenza A/WSN virus. Two of these five control mice died within 5 days of influenza A/WSN virus challenge (Table 2). Although three mice did survive the challenge in this particular experiment, these mice were extremely ill, as indicated by extensive weight loss (26.1 ± 9.4%), as well as by ruffled coats and inactivity, and did not begin recovering weight until day 44 (9 days after challenge).
FIG. 2.
Effects of vaccination and challenge on average weight and survival. (A) Mice were inoculated intranasally with VSVrwt at 5 × 104 PFU/mouse on day 0. Mice were boosted with an equal amount of inoculum on day 21. Mice were challenged with a lethal dose of influenza A/WSN virus on day 35. Asterisks indicate one mouse found dead that day in influenza A/WSN virus-challenged mice. (B) Same as panel A except mice were inoculated and boosted with VSV-HA at 5 × 104 PFU/mouse. (C) Mice were inoculated with VSV-HA at 5 × 104 PFU/mouse on day 0. Mice were challenged with a lethal dose of influenza A/WSN virus on day 21. Error bars, ±0.5 × standard deviation.
In an experiment parallel to those described above, we examined the pathogenicity, immunogenicity, and efficacy of VSV-HA in mice receiving a single intranasal inoculation on day 0 and challenged with a lethal dose of influenza A/WSN virus on day 21. These mice showed an initial weight loss of 17.6 ± 2.3% from the vector inoculation (Fig. 2C). Sera were collected, pooled, and assayed for neutralizing titers of antibody to influenza A/WSN virus at day 18 (Table 2). Mice were then challenged with a lethal dose of influenza A/WSN virus on day 21. These results showed that, even after a single inoculation, VSV-HA-immunized mice were completely protected from lethal influenza A/WSN virus challenge. Complete protection was indicated by negligible weight loss (Fig. 2C, days 21 to 23), the presence of neutralizing titers of antibody to influenza A/WSN virus, and 100% survival (Table 2).
VSV-HA infection protects against influenza virus-induced bronchopneumonia.
To further assess the immune reaction against VSVrwt and VSV-HA, we inoculated two mice each as described above with 5 × 104 PFU/mouse of VSVrwt, VSV-HA, or DMEM alone and examined the spleens of the mice 7 days after initial infection for reactive germinal centers. The spleens from mice infected with VSVrwt and VSV-HA showed active germinal centers, indicating an active immune response, whereas spleens from mice inoculated with DMEM showed no active germinal centers. Lung sections taken from the same mice showed lymphocytic infiltrates in the bronchi of VSVrwt and VSV-HA recipients, indicating possible viral infection; lung sections from DMEM-inoculated mice showed no infiltrates. No signs of pneumonia (i.e., the alveolar spaces were clear) were found in any lung sections (data not shown).
The survival analysis presented above indicated that inoculation of mice with VSV-HA led to complete resistance against influenza A/WSN virus challenge, including protection from influenza A/WSN virus-induced pneumonia. To assess this directly, we examined the lungs of VSV-HA-inoculated mice challenged with influenza A/WSN virus. Two mice each were inoculated with PBS, VSV-HA, or VSVrwt on day 0, boosted with the same agent on day 21, and then challenged with influenza A/WSN virus on day 35. At 3 days after challenge, mice were sacrificed and the lungs were examined for histopathology. Lung sections from mice inoculated with VSVrwt (Fig. 3D) or PBS (Fig. 3B) and challenged with influenza A/WSN virus showed evidence of acute viral bronchopneumonia, as manifested by cytopathic changes in the bronchial epithelium and cellular debris in the bronchial lumens and alveolar spaces. Additional signs of infection and reaction included a marked peribronchial lymphocytic infiltrate and, in the pulmonary vessels, a marked thickening of both the vessel wall and its endothelial lining (Fig. 3B and D). In contrast, lung sections of mice inoculated with VSV-HA and challenged with influenza A/WSN virus showed an intact bronchial epithelium, no thickening of the vessels, and clear alveolar spaces (Fig. 3C). Lungs from mice inoculated with DMEM, which were boosted and challenged with DMEM alone, also showed no signs of pathology (Fig. 3A).
FIG. 3.
Histopathology in lungs in mice 3 days after WSN virus challenge. Mice were inoculated and boosted with DMEM (A), PBS (B), VSV-HA (C), or VSVrwt (D) and then challenged with either influenza A/WSN virus (B, C, and D) or DMEM (A). Abbreviations: BE, bronchial epithelium; V, small arterial vessel; A, alveolar space. Original magnification, ×400.
Pathogenicity and immunogenicity of recombinant VSV-CT1 and VSV-CT9.
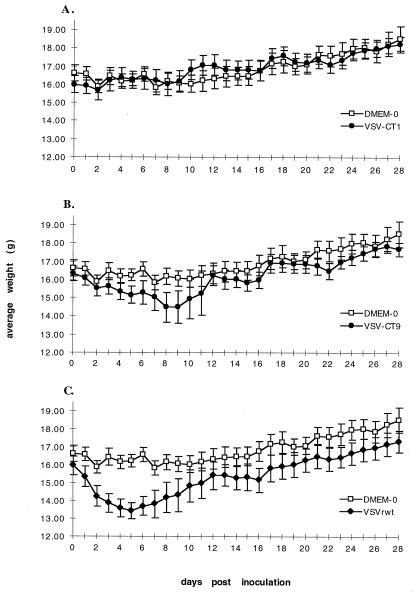
These experiments demonstrated the immunogenicity and complete efficacy of a recombinant VSV vaccine expressing influenza A/WSN/33 HA protein in protecting mice from a lethal influenza A/WSN virus challenge. However, the pathogenicity associated with the immunizing vector remained a concern. Although this initial pathogenicity is limited (mice began regaining initial weight loss within 6 days of immunization), further attenuation of the VSV vaccine vector was addressed. To this end, we examined recombinant VSVs which have previously indicated attenuation in vitro (reduced plaque size and reduced viral titers) compared to VSVrwt. Specifically, two recombinant VSV constructs, VSV-CT1 and VSV-CT9 (21), which have deletions truncating the glycoprotein cytoplasmic tails from 29 amino acids to 1 and 9 amino acids, respectively, were examined for pathogenicity and immunogenicity in the mouse model system.
Six-week-old female BALB/c mice were lightly anesthetized with Metofane and inoculated intranasally with VSVrwt, VSV-CT1, VSV-CT9, or DMEM in a total volume of 25 μl. Mice were observed and weighed daily (Fig. 4). At 14 days after inoculation two mice per group were bled, and sera were pooled within each group. Again, at 28 days after inoculation two mice per group were bled, and sera were pooled within each group. These pooled sera were heat inactivated and assayed for neutralizing titers of antibody to VSVrwt by complete inhibition of the CPE in BHK cells (Table 3). Neutralization titers are reported as ranges obtained in multiple assays.
FIG. 4.
Effects of inoculation with attenuated viral vectors on average daily weights. (A) Six-week-old female BALB/c mice were inoculated intranasally with VSV-CT1 at 105 PFU/mouse in a 25-μl total volume. Control mice (DMEM-0) received 25 μl of medium only. Mice were inoculated on day 0 and weighed daily thereafter. Mice were bled on days 14 and 28. Datum points represent the average daily weights of five mice per group. Error bars, ±0.5 × standard deviation. (B) Same as panel A except mice were inoculated with VSV-CT9 (105 PFU/mouse). (C) Same as panel A except mice were inoculated with VSVrwt (105 PFU/mouse).
TABLE 3.
Neutralizing antibody titers to VSVrwt
| Virus inoculum | Titer in serum causing complete inhibition of CPE at: | |
|---|---|---|
| Day 14a | Day 28b | |
| VSVrwt | 1:320–1:1,280 | 1:640–1:2,560 |
| VSV-CT1 | 1:320–1:640 | 1:320–1:1,280 |
| VSV-CT9 | 1:160–1:640 | 1:640–1:1,280 |
| Pre-bleedc | <1:8 |
Mice inoculated with VSVrwt (105 PFU/mouse) showed signs of initial pathogenesis as indicated by weight loss as previously observed. However, in stark contrast, mice inoculated with VSV-CT1 (105 PFU/mouse) experienced negligible weight loss, a finding comparable to that observed in DMEM-inoculated control mice (Fig. 4A). Furthermore, mice inoculated with VSV-CT1 or DMEM remained active and well groomed in the days following inoculation. Mice receiving VSVrwt had reduced activities and were poorly groomed on days 1 to 5, corresponding to the days of weight loss (Fig. 4C).
Mice inoculated with VSV-CT9 (105 PFU/mouse) showed delayed and reduced pathogenesis compared to VSVrwt-inoculated mice. The amount of weight loss and the rate of weight loss in VSV-CT9-inoculated mice were not as great as those seen in VSVrwt-inoculated mice but were still measurably significant compared to the DMEM-inoculated mice (Fig. 4B). Although pathogenesis was eliminated for VSV-CT1-inoculated mice and reduced for VSV-CT9-inoculated mice, sera collected from both groups at 14 and 28 days after inoculation contained neutralizing antibodies to VSVrwt. These neutralizing antibody titers were similar to those obtained in VSVrwt-inoculated mice (Table 3). Sera from DMEM-inoculated mice and sera collected from mice 3 days prior to inoculation did not contain neutralizing titers of antibodies to VSVrwt at dilutions of 1:8 (the lowest dilution assayed).
DISCUSSION
Intranasal inoculation of mice with recombinant VSVs expressing influenza A/WSN virus HA protected mice from a homologous, lethal influenza A/WSN virus challenge. The protection was complete in preventing pathogenesis as measured by a lack of weight loss and by the normal, healthy appearance of inoculated mice. Protection correlated with the presence of neutralizing titers of serum antibodies to influenza A/WSN virus and with the absence of virally induced pathology in the lungs. The recombinant VSVs (VSVrwt and VSV-HA) were attenuated in pathogenesis in the mouse model, as indicated by the inability of high titers of virus to cause the paralysis and fatal encephalitis that are seen with the wild-type VSV Indiana. Taken together, these data show that recombinant VSVs hold potential for live attenuated vaccines. Additionally, the recombinant VSV-CT9 and VSV-CT1 vectors were further attenuated from VSVrwt, as indicated by reduced and negligible weight losses in mice, respectively, but they were still able to elicit humoral immune responses. These data suggest that VSV vector vaccines can be developed which express foreign viral glycoproteins and which are nonpathogenic, immunogenic, and efficacious. An additional benefit is that these VSV vector vaccines can be administered through a mucosal route, producing systemic immunity and protecting from mucosal challenges.
The basis for the attenuation of VSVrwt and VSV-HA has not been determined, but it may be due to the recombinant nature of all the VSVs generated in our recovery assays. These viruses are derived from an infectious clone that is a hybrid of two VSV subtypes. In the full-length VSV antigenomic vector constructs, the L gene (encoding the viral RNA-dependent RNA polymerase) and the N-terminal 49 amino acids of the N gene are derived from the Mudd-Summers subtype of VSV (serogroup Indiana). This differs from the other genes and noncoding sequences, which are derived from the San Juan subtype of VSV (serogroup Indiana). The recovered wild-type VSV is not attenuated in tissue culture (18), but it has a definite attenuation of pathogenesis in a mouse model system.
When considering the potential of a live recombinant virus as a vaccine vector, the ability to attenuate the vector itself is obviously favorable as long as immunogenicity is retained. The current study shows that the recombinant VSV-CT1 and VSV-CT9 vectors are further attenuated in pathogenesis but still elicit a strong humoral response after a single intranasal inoculation. Preliminary data indicate that fully attenuated vectors expressing HA can also protect from influenza virus challenge.
We have observed high neutralizing titers of antibody (1:8,192) to the VSV used as a vector in these immunization studies. These neutralizing antibodies specific to VSV are directed solely to the VSV-G (14) and might limit the ability to use VSV recombinants for multiple vaccine applications. Influenza viruses undergo both antigenic drift and antigenic shift, and therefore, influenza virus vaccines incorporate the different HA proteins which are present in the environment and are anticipated to be the predominant subtypes in a particular influenza season. If VSV vectors were to be employed in an influenza virus vaccine system, which requires readministration of influenza vaccines on an annual basis, it would likely be necessary to eliminate the neutralizing response to the vector. Although this potential impediment exists, we believe the present study shows the potential of VSV vector vaccines for foreign viruses and other foreign antigens.
We are currently constructing viruses which lack the entire VSV G gene (VSVΔGs) and which express influenza A/WSN virus HA in an attempt to create a highly attenuated virus. Such a virus should be limited in budding efficiency and release from infected cells due to the absence of VSV-G (23) and to the absence of influenza virus neuraminidase that would enhance virus release from cells. In addition, neutralizing antibodies should be raised only to the expressed HA protein and not to the VSVΔG vector. By changing the antigenicity of the expressed attachment protein, the same vector delivery system (VSVΔG) could most likely be used multiple times in a single individual and would provide a powerful recombinant vaccine vector.
We did not examine the ability of VSV-HA to induce cytotoxic T-cell responses. However, recombinant VSVs, like wild-type VSV, would be expected to elicit a strong cell-mediated response. In preliminary experiments with VSV recombinants expressing human immunodeficiency virus (HIV) envelope, a strong cytotoxic T lymphocyte response to the HIV envelope protein has been observed (23a). Therefore, recombinant VSV vaccines would likely be useful in inducing humoral and/or cell-mediated immunity. The role of cell-mediated immunity would also need to be addressed in a multiple-use vector such as the proposed VSVΔG.
Live attenuated recombinant VSVs hold potential as vaccine vectors, with applications in immunizing humans against various viral diseases, as well as in the immunization of livestock to VSV itself. We are excited by this potential of using recombinant VSVs as vaccine vectors and anticipate a broad range of applications since protein expression within recombinant VSVs need not be limited to foreign viral proteins.
ACKNOWLEDGMENTS
We thank the other members of the Rose, Stern, and Perkins laboratories for their critiques and support of this work. We thank Jo Ann Falato for assistance. We also thank Roxanne Swinsick, Bill Nazzaro, and other members of the Yale Animal Resource Center, BCMM, as well as Deborah Caruso and Edward “Z” Zelazny for their care and assistance with all of our mice.
This work was supported by NIH grant AI30374. A. Roberts is supported by NIH postdoctoral training grant CA09159. J. Forman is supported by a fellowship from Howard Hughes Medical Institute.
REFERENCES
- 1.Barna M, Komatsu T, Bi Z, Reiss C S. Sex differences in susceptibility to viral infection of the central nervous system. J Neuroimmunol. 1996;67:31–39. doi: 10.1016/0165-5728(96)00022-7. [DOI] [PubMed] [Google Scholar]
- 2.Bender B S, Rowe C A, Taylor S F, Wyatt L S, Moss B, Small P A., Jr Oral immunization with a replication-deficient recombinant virus protects mice against influenza. J Virol. 1996;70:6418–6424. doi: 10.1128/jvi.70.9.6418-6424.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brody J A, Fischer G F, Peralta P H. Vesicular stomatitis virus in Panama. Human serologic patterns in a cattle raising area. Am J Epidemiol. 1967;86:158–161. doi: 10.1093/oxfordjournals.aje.a120721. [DOI] [PubMed] [Google Scholar]
- 4.Castrucci M R, Bilsel P, Kawaoka Y. Attenuation of influenza A virus by insertion of a foreign epitope into the neuraminidase. J Virol. 1992;66:4647–4653. doi: 10.1128/jvi.66.8.4647-4653.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Couch R B. Advances in influenza virus vaccine research. Ann N Y Acad Sci. 1993;685:803–812. doi: 10.1111/j.1749-6632.1993.tb35946.x. [DOI] [PubMed] [Google Scholar]
- 6.Davis N L, Brown K W, Johnston R E. A viral vaccine vector that expresses foreign genes in lymph nodes. J Virol. 1996;70:3781–3787. doi: 10.1128/jvi.70.6.3781-3787.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dietzschold B, Rupprecht C E, Fu Z F, Koprowski H. Rhabdoviruses. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Vol. 1. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 1137–1159. [Google Scholar]
- 8.Fellowes O N, Dimopoullos G T, Callis J J. Isolation of vesicular stomatitis virus from an infected laboratory worker. Am J Vet Res. 1955;16:623–626. [PubMed] [Google Scholar]
- 9.Forger J M, III, Bronson R T, Huang A S, Reiss C S. Murine infection by vesicular stomatitis virus: initial characterization of the H-2d system. J Virol. 1991;65:4950–4958. doi: 10.1128/jvi.65.9.4950-4958.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanson R P, Brandly C A. Epizootiology of vesicular stomatitis. Am J Public Health. 1957;47:205–209. doi: 10.2105/ajph.47.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanson R P, Rasmussen A F, Jr, Brandly C A, Brown J W. Human infection with the virus of vesicular stomatitis. J Clin Lab Med. 1950;36:754–758. [PubMed] [Google Scholar]
- 12.Johnson J E, Schnell M J, Buonocore L, Rose J K. Specific targeting to CD4+ cells of recombinant vesicular stomatitis viruses encoding human immunodeficiency virus envelope proteins. J Virol. 1997;71:5060–5068. doi: 10.1128/jvi.71.7.5060-5068.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson K M, Vogel J E, Peralta P H. Clinical and serological response to laboratory-acquired human infection by Indiana type vesicular stomatitis virus (VSV) Am J Trop Med Hyg. 1966;15:244–246. doi: 10.4269/ajtmh.1966.15.244. [DOI] [PubMed] [Google Scholar]
- 14.Kelley J M, Emerson S U, Wagner R R. The glycoprotein of vesicular stomatitis virus is the antigen that gives rise to and reacts with neutralizing antibody. J Virol. 1972;10:1231–1235. doi: 10.1128/jvi.10.6.1231-1235.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kretzschmar E, Buonocore L, Schnell M J, Rose J K. High-efficiency incorporation of functional influenza virus glycoproteins into recombinant vesicular stomatitis viruses. J Virol. 1997;71:5982–5989. doi: 10.1128/jvi.71.8.5982-5989.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuno-Sakai H, Kimura M, Ohta K, Shimojima R, Oh Y, Fukumi H. Developments in mucosal influenza virus vaccines. Vaccine. 1994;12:1303–1310. doi: 10.1016/s0264-410x(94)80056-6. [DOI] [PubMed] [Google Scholar]
- 17.Lamb R A, Krug R M. Orthomyxoviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Vol. 1. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 1353–1395. [Google Scholar]
- 18.Lawson N D, Stillman E A, Whitt M A, Rose J K. Recombinant vesicular stomatitis viruses from DNA. Proc Natl Acad Sci USA. 1995;92:4477–4481. doi: 10.1073/pnas.92.10.4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moldoveanu Z, Clements M L, Prince S J, Murphy B R, Mestecky J. Human immune responses to influenza virus vaccines administered by systemic or mucosal routes. Vaccine. 1995;13:1006–1012. doi: 10.1016/0264-410x(95)00016-t. [DOI] [PubMed] [Google Scholar]
- 20.Moldoveanu Z, Novak M, Huang W Q, Gilley R M, Staas J K, Schafer D, Compans R W, Mestecky J. Oral immunization with influenza virus in biodegradable microspheres. J Infect Dis. 1993;167:84–90. doi: 10.1093/infdis/167.1.84. [DOI] [PubMed] [Google Scholar]
- 21.Schnell M J, Buonocore L, Boritz E, Ghosh H P, Chernish R, Rose J K. Requirement for a non-specific glycoprotein cytoplasmic domain sequence to drive efficient budding of vesicular stomatitis virus. EMBO J. 1998;17:1289–1296. doi: 10.1093/emboj/17.5.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schnell M J, Buonocore L, Kretzschmar E, Johnson E, Rose J K. Foreign glycoproteins expressed from recombinant vesicular stomatitis viruses are incorporated efficiently into virus particles. Proc Natl Acad Sci USA. 1996;93:11359–11365. doi: 10.1073/pnas.93.21.11359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schnell M J, Johnson J E, Buonocore L, Rose J K. Construction of a novel virus that targets HIV-1-infected cells and controls HIV-1 infection. Cell. 1997;90:849–857. doi: 10.1016/s0092-8674(00)80350-5. [DOI] [PubMed] [Google Scholar]
- 23a.Tobery, T., E. Johnson, J. K. Rose, and R. Siciliano. Unpublished results.
- 24.Wagner R R, Rose J K. Rhabdoviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Vol. 1. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 1121–1135. [Google Scholar]
- 25.Zinkernagel R M, Adler B, Holland J J. Cell-mediated immunity to vesicular stomatitis virus infections in mice. Exp Cell Biol. 1978;46:53–70. doi: 10.1159/000162882. [DOI] [PubMed] [Google Scholar]