Virion Incorporation of Human Immunodeficiency Virus Type 1 Nef Is Mediated by a Bipartite Membrane-Targeting Signal: Analysis of Its Role in Enhancement of Viral Infectivity (original) (raw)
Abstract
The nef gene of primate immunodeficiency viruses is essential for high-titer virus replication and AIDS pathogenesis in vivo. In tissue culture, Nef is not required for human immunodeficiency virus (HIV) infection but enhances viral infectivity. We and others have shown that Nef is incorporated into HIV-1 particles and cleaved by the viral proteinase. To determine the signal for Nef incorporation and to analyze whether virion-associated Nef is responsible for enhancement of infectivity, we generated a panel of nef mutants and analyzed them for virion incorporation of Nef and for their relative infectivities. We report that N-terminal truncations of Nef abolished its incorporation into HIV particles. Incorporation was reconstituted by targeting the respective proteins to the plasma membrane by using a heterologous signal. Mutational analysis revealed that both myristoylation and an N-terminal cluster of basic amino acids were required for virion incorporation and for plasma membrane targeting of Nef. Grafting the N-terminal anchor domain of Nef onto the green fluorescent protein led to membrane targeting and virion incorporation of the resulting fusion protein. These results indicate that Nef incorporation into HIV-1 particles is mediated by plasma membrane targeting via an N-terminal bipartite signal which is reminiscent of a Src homology region 4. Virion incorporation of Nef correlated with enhanced infectivity of the respective viruses in a single-round replication assay. However, the phenotypes of HIV mutants with reduced Nef incorporation only partly correlated with their ability to replicate in primary lymphocytes, indicating that additional or different mechanisms may be involved in this system.
In addition to the prototypic retroviral gag, pol, and env genes, lentiviruses harbor a number of accessory genes, including nef, which is unique to primate immunodeficiency viruses (27). A functional Nef protein appears to be essential for high-titer virus replication and AIDS pathogenesis in vivo (6, 17, 30, 32). In contrast, Nef is dispensable for virus replication in tissue culture, and only moderate effects have been observed for _nef_-defective viruses in selected culture systems. Nef is a 27-kDa myristoylated protein, which is observed predominantly in the cytoplasm of infected cells, associated with cellular membranes and the cytoskeleton (19, 20, 42; reviewed in reference 27). Structural analysis revealed that Nef is a two-domain protein (21): the C-terminal core domain (amino acids 58 to 206) showed structural similarities to DNA binding proteins containing a winged helix-turn-helix motif (25, 35), while the N-terminal part showed no defined structure and was proposed to function as a membrane anchor (21, 22). Most conserved regions in primary isolates of Nef map to the core domain (27). Interestingly, Nef is cleaved in vitro by the viral proteinase (PR) between tryptophan 57 and leucine 58, separating the putative anchor domain from the core domain (22).
Analysis of Nef function in vitro has been largely hampered by the fact that Nef is not essential for virus growth in tissue culture. In a variety of experimental studies, three effects have been consistantly observed upon expression of Nef in cultured cells: (i) downregulation of cell surface CD4, (ii) modulation of cellular activation and signal transduction pathways, and (iii) enhancement of viral infectivity. It appears likely that some or all of these effects also influence human immunodeficiency virus (HIV) replication in vivo, although their relative contributions toward AIDS pathogenesis have not been defined to date. In tissue culture, expression of HIV Nef led to the accelerated internalization of CD4 and major histocompatibility complex class I molecules from the cell surface (3, 26, 50; reviewed in reference 27). These effects require localization of Nef at the plasma membrane (3, 26, 29), where it affects vesicular sorting pathways by altering the function of adaptor complexes (3, 12, 24, 37). In addition, Nef has been reported to modulate cellular signal transduction pathways, most probably by associating with cellular protein kinases (11, 43, 46, 47). Both cellular activation and inhibition of activation have been reported (10, 27). Opposite effects may be induced depending on the intracellular localization of Nef, with activation of the target cell being dependent on localization of Nef at the plasma membrane (10). A _nef_-dependent phenotype is observed when nonprestimulated primary human lymphocytes are infected at a low multiplicity of infection (40, 51). Similar Nef-dependent enhancement of infectivity was observed in single-cycle replication assays, in which _nef_-defective viruses exhibited a moderate and strain-dependent decrease in infectious titer (36, 40). Enhancement of infectivity is dependent on myristoylation of Nef as well, but it can be genetically separated from the effect of Nef expression on CD4 downregulation (2, 23).
All three phenotypes are strictly dependent on the subcellular localization of Nef and its transient association with the plasma membrane. Membrane association is dependent on N-terminal myristoylation, but myristoylation is unlikely to be its sole determinant. Interestingly, Nef-mediated enhancement of infectivity can be complemented by Nef provided in trans in the virus-producing cell but not in the target cell (2). This result indicated that Nef influences the composition of the virion, either by modifying a structural component or by being incorporated into the particle itself. We and others have recently shown that approximately 10 molecules of Nef are incorporated into HIV-1 particles, where they are cleaved by the viral PR (18, 44, 53). This observation suggested that virion-associated Nef may be responsible for some of the Nef-dependent phenotypes. Here, we report that incorporation of Nef into HIV particles is mediated by a bipartite membrane localization signal. Plasma membrane targeting and virion incorporation of Nef correlated with enhancement of infectivity in single-cycle infectivity assays, but additional or alternative effects may play a role in unstimulated primary lymphocytes.
MATERIALS AND METHODS
Expression plasmids.
All proviral plasmids were derived from pNL4-3 (1). Plasmids containing deletions from (with the numbers specifying the first and last codon deleted in nef) 8 to 17, 25 to 35, 41 to 49, 57 to 66 and 73 to 82 (see Fig. 1) were a gift from G. Aldrovandi and J. Zack (7). All the other plasmids were generated by PCR and standard cloning techniques (9), and mutations and PCR-amplified regions were verified by sequence analysis. Oligonucleotide sequences are available upon request.
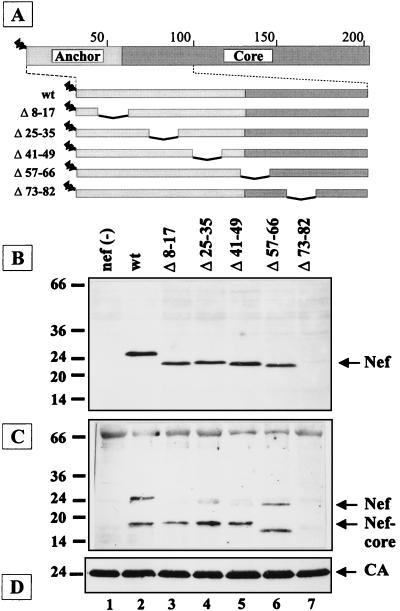
FIG. 1.
Effect of internal deletions on virion incorporation of HIV-1 Nef proteins. (A) Schematic representation of the HIV-1 Nef polypeptide and a panel of nef mutants containing internal deletions. The anchor domain is indicated by light shading, and the core domain is indicated by dark shading. Amino acid numbers are given above the box. N-terminal myristoylation is depicted as a zigzag line. (B to D) Western blot analysis of cell lysates (B) and particle preparations (C and D) derived from C8166 cells infected with HIV-1 NL4-3 (wt) or the nef mutants indicated above each lane. The blots in panels B and C were reacted with antiserum against Nef, and the blot in panel D was reacted with antiserum against CA. The additional band migrating at approximately 33 kDa in panel C probably corresponds to HIV integrase. Molecular mass standards (in kilodaltons) are indicated on the left; the positions of full-length Nef, the cleaved core fragment, and CA are indicated on the right.
The _nef_-deficient proviral plasmid pNL4-3/nef(−) was generated by deleting codons 1 to 74 of nef. To ensure equal gene expression of N-terminally altered Nef proteins, a consensus sequence for optimized eukaryotic translation initiation was engineered between the termination codon of env and the initiation codon of nef, also creating a novel _Nco_I restriction site (CTATAATCGATAGCACCATGGGT; termination and start codons underlined). Comparison of Nef expression in human T-cell lines infected with the parental virus or virus derived from this proviral clone (pNL4-3/nef-wt) showed no detectable difference (data not shown). The codon for Gly2 of nef was replaced by an Ala codon to generate a myristoylation-defective variant (pNL4-3/nef-Δmyr). The N-terminally truncated Nef proteins Nef-33 and Nef-45 contain Gly and Leu residues in positions 2 and 3 followed by Val33 or Ser45 of Nef, respectively (see Fig. 2). The newly generated N termini of these proteins do not correspond to consensus signals for myristoylation (45). Proviral plasmids pNL4-3/src-nef-33 and pNL4-3/src-nef-45 encode chimeric Nef proteins containing the first 10 codons of pp60 c-src (MGSSKSKPKD) (45), followed by Ser and Leu codons and _nef_-derived sequences from Val33 and Ser45, respectively (Fig. 2). pNL4-3/nefΔ7–22 contains a deletion of codons 7 to 22 of nef and a substitution of Val33 by Ala (see Fig. 4). The gene for the jellyfish Aquaefora victoriae green fluorescent protein (GFP) (Clontech) (28) was inserted in the position of codons 1 to 72 of nef to generate plasmid pNL4-3/GFP (see Fig. 3). In the case of pNL4-3/Anchor-GFP, the GFP gene was fused in frame behind the anchor domain of nef (codons 1 to 56; see Fig. 3). Expression plasmids encoding the Nef protein of HIV-1 BH10 (pSG5.Nef) and a myristoylation-defective variant thereof (pSG5.BH10 G2S) have been described previously (15). Plasmid pSG5.BH10Nef Δ7–22 is a derivative of pSG5.Nef with a deletion of the indicated codons of nef.
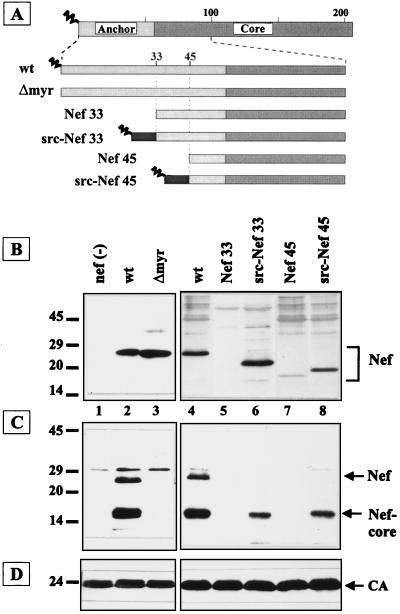
FIG. 2.
Analysis of virion incorporation of chimeric Nef proteins, with a heterologous N terminus. (A) Schematic representation of Nef proteins containing a Gly-to-Ala substitution at position 2 (Δmyr) or N-terminal truncations to residue 33 and 45, respectively (Nef 33 and Nef 45). Black boxes with a zigzag line indicate fusion of the myristoylated membrane-targeting signal of pp60_src_ to the truncated Nef proteins (src-Nef 33 and src-Nef 45). (B to D) Western blot analysis of cell lysates (B) or particle preparations (C and D) derived from C8166 cells (B) or MT-4 cells (C and D) infected with HIV-1 NL4-3 (wt) or the nef mutants indicated above the lanes. Blots were reacted with antisera against Nef (B and C) or CA (D). Immunoreactive proteins were revealed by ECL, except for the right part of panel B and the left part of panel D, where color detection was used. Molecular mass standards (in kilodaltons) and the positions of relevant proteins are indicated.
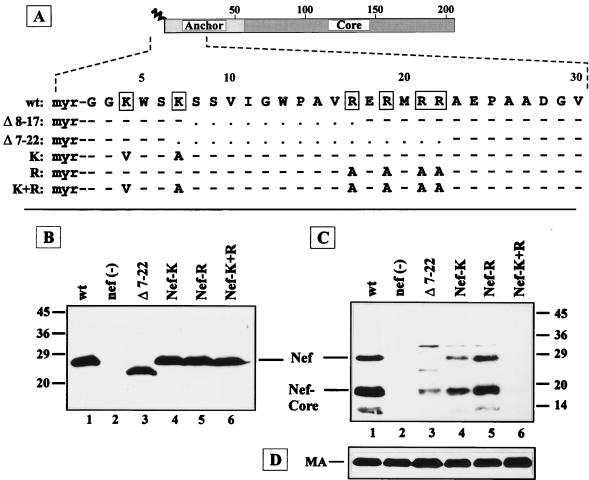
FIG. 4.
Analysis of the role of basic amino acids for incorporation of Nef into the virion. (A) The N-terminal 30 codons of NL4-3 Nef (wt) and of deletion and substitution mutants are depicted in single-letter code. Basic amino acids in the wild-type sequence are boxed. Dashes indicate that the wild-type sequence was preserved, and dots indicate gaps introduced by the deletions. Analysis of Nef/Δ8–17 is shown in Fig. 1. (B to D) Western blot analysis of cell lysates (B) or particle preparations (C and D) derived from C8166 cells infected with HIV-1 NL4-3 or the nef mutants indicated above the lanes. The blots were reacted with antisera against Nef (B and C) or against MA (D). Molecular mass standards (in kilodaltons) and the positions of relevant proteins are indicated.
FIG. 3.
(A) Fluorescence micrographs of MT-4 cells infected with viruses expressing GFP or an Anchor-GFP fusion protein. (B) Western blot analysis of particle preparations derived from infected MT-4 cells. The blots were reacted with antisera against GFP (upper panel) or CA (lower panel), and immunoreactive proteins were revealed by ECL.
Cells, transfections, and infections.
COS-7 cells, HeLa cells, and HeLa-CD4-LTR-βGal cells (31) were maintained by using standard procedures (9, 39). HeLa cell lines expressing Nef from tetracycline-inducible promoters (15, 16) were grown in the presence of 1 μg of tetracycline per ml. COS-7 cells were transfected by lipofection, and HeLa cells were transfected by the modified calcium phosphate method (9). For production of virus stocks, culture media were harvested 48 h after transfection, filtered (0.45-μm-pore-size filters), analyzed for capsid (CA) antigen by enzyme-linked immunosorbent assay (33), and stored at −80°C. HIV-1-permissive MT-4 and C8166 cells were infected either with culture medium from transfected cells normalized for CA antigen or by cocultivation with infected cells and harvested 2 to 5 days following infection.
Single-round infectivity assays were performed by infecting HeLa-CD4-LTR-βGal cells with virus supernatant (derived from parallel transfections and normalized for CA antigen) containing 20 μg of DEAE-dextran per ml. Infected cells were fixed and stained with X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) 2 days postinfection. The number of blue foci was counted under the light microscope. Human peripheral blood mononuclear cells (PBMC) from healthy donors were infected overnight with 2 ml of virus supernatant derived from parallel transfections (diluted to 0.1 to 10 ng of CA antigen per ml). Following infection, the cells were washed once and resuspended in the appropriate culture medium. PBMC were either infected immediately after isolation or following activation with 3 μg of phytohemagglutinin (PHA) per ml and 20 U of recombinant human interleukin 2 (IL-2) per ml for 2 days. After overnight infection, prestimulated PBMC were cultivated in culture medium supplemented with 20 U of IL-2 per ml. Cells that had not been prestimulated were incubated in culture medium for 2 to 3 days following infection and were subsequently stimulated for 3 days by addition of PHA and IL-2 as described above. Following activation, the cells were cultivated for the time indicated in medium supplemented with 20 U of IL-2 per ml. Aliquots of culture medium were removed for antigen enzyme-linked immunosorbent assay every 2 to 4 days and were replaced by fresh medium.
Analysis of viral proteins in infected cells and virus particles.
For biochemical analysis of virus particles, filtered medium was centrifuged through a cushion of 20% (wt/vol) sucrose in phosphate-buffered saline (PBS) at 120,000 × g for 90 min at 4°C. Western blot analysis of cell or particle extracts was performed as described previously with polyclonal antisera against Nef (15, 53), CA (39), and integrase (IN) (33). Rabbit antiserum was raised against purified histidine-tagged GFP (28); antiserum against matrix (MA) protein was obtained through the National Institutes of Health AIDS Research and Reference Reagent Program. Immunoreactive proteins were revealed by using color detection (9) or enhanced chemiluminescence (ECL; Amersham). For fluorescence microscopy, cells infected with HIV variants expressing GFP or GFP fusion proteins were washed with PBS, fixed with 4% formaldehyde and 0.1% glutaraldehyde in PBS for 15 min, and mounted in elvanol. Microscopy was performed on a Zeiss Axiophot with a charge-coupled device camera (Photometrics), using the IP-LAB software package.
Radioactive labeling, immunoprecipitation, and subcellular fractionation.
COS-7 cells were metabolically labeled 24 h posttransfection with either 100 μCi of Tran 35S-label (ICN-Flow; >1,000 Ci/mmol) per ml for 4 h or 200 μCi of [3H]myristic acid (Amersham; 40 to 60 Ci/mmol) per ml for 16 h as described previously (15). Following immunoprecipitation with a rat monoclonal antibody against Nef and separation on sodium dodecyl sulfate-polyacrylamide gels, labeled Nef proteins were detected by autoradiography. For subcellular fractionation, stably transfected HeLa cell lines (16) were plated in the absence of tetracycline for 24 h. The cells were harvested, swollen in hypotonic buffer [10 mM piperazine-N,_N_′-bis(2-ethanesulfonic acid) (PIPES)-NaOH (pH 7.2), 0.5 mM MgCl2], homogenized with a Dounce homogenizer, and adjusted to 120 mM KCl and 30 mM NaCl. Postnuclear supernatants were centrifuged at 100,000 × g for 30 min at 4°C, and the resulting supernatant was termed cytosol (C). The pellet was washed with the same buffer containing 10% glycerol, resuspended in buffer containing 1% Triton X-100, and centrifuged at 50,000 × g for 10 min at 4°C. The resulting supernatant, termed the Triton-soluble membrane fraction (P), and the Triton-insoluble pellet (PP) were resuspended in sodium dodecyl sulfate sample buffer and analyzed by Western blotting.
RESULTS
The anchor domain of Nef is required for virion incorporation but can be functionally replaced by a heterologous membrane-targeting signal.
To determine the role of the anchor domain of Nef in virion incorporation, we analyzed a panel of mutants containing internal deletions dispersed through the N-terminal segment of Nef (Fig. 1A). Except for the Δ73–82 variant, which lacks part of the N-terminal PXXP motifs (46), all the Nef proteins were expressed at levels comparable to wild-type Nef in infected cells (Fig. 1B). Substitution of four Pro residues in these PXXP motifs also greatly reduced the steady-state levels of Nef (data not shown), indicating that they are important for protein stability. Analysis of virion preparations revealed that altered Nef proteins (except for Δ73–82) were incorporated into virus particles at similar levels to those observed for wild-type Nef and cleaved by the viral PR (Fig. 1C). Interestingly, Nef/Δ57–66, which lacks the PR cleavage site, was also processed in the virus particle but yielded a smaller core fragment (lane 6).
We subsequently analyzed the effect of myristoylation and of N-terminal truncations of Nef on virion incorporation. In infected T cells, Nef and a nonmyristoylated variant (Δmyr) were present at similar levels (Fig. 2B, lanes 2 and 3). However, only trace amounts of nonmyristoylated Nef were detected in virion preparations, while full-length Nef and the cleaved core fragment were readily observed in wild-type virus (Fig. 2C, lanes 2 and 3). Deletion of the first 33 or 45 codons of nef (Fig. 2A) yielded low levels of truncated nonmyristoylated Nef proteins in infected cells and no detectable Nef in virus particles (Fig. 2B and C, lanes 5 and 7). This phenotype is not due to lack of myristoylation, since nonmyristoylated Nef was stably expressed in infected cells. Therefore, it appears likely that the N terminus of Nef is important for protein stability. Since myristoylation is important for virion incorporation, we analyzed whether a heterologous plasma membrane-targeting signal (containing a myristoylation consensus sequence) could functionally replace the N-terminal part of the putative anchor domain of Nef. To this end, the first 33 or 45 codons of nef were replaced by the first 10 codons of pp60_src_ (45) (Fig. 2A). These chimeric Src-Nef proteins were expressed at similar levels to wild-type Nef in infected cells (Fig. 2B, lanes 6 and 8), were incorporated into virus particles, and were cleaved by the viral PR (Fig. 2C, lanes 6 and 8).
The anchor domain of Nef can direct plasma membrane targeting and virion incorporation of a heterologous protein.
To analyze whether the anchor domain of Nef can direct a heterologous protein to the plasma membrane of HIV-infected cells and facilitate its incorporation into virus particles, we constructed proviral clones expressing GFP (pNL4-3/GFP) or GFP fused to the N-terminal anchor domain of Nef (pNL4-3/Anchor-GFP) in the position of nef (Fig. 3). In infected MT-4 cells, GFP fluorescence was readily detectable in both instances but with a different subcellular pattern. The chimeric Anchor-GFP localized predominantly to the cell periphery, while GFP was distributed throughout the cell (Fig. 3A). Peripheral localization of Anchor-GFP is likely to reflect association with the plasma membrane but may also correspond to cytoplasmic protein in part, since lymphocytes contain only a narrow cytoplasmic area. Analysis of virion preparations derived from infected cells revealed that Anchor-GFP but not GFP was incorporated into HIV-1 particles (Fig. 3B), indicating that the Nef anchor domain is sufficient to direct a heterologous protein into virus particles. Similar results were observed for infected C8166 and H9 cells and particle preparations derived thereof and for a chimeric GFP containing the Src membrane-targeting signal instead of the Nef anchor domain (data not shown).
A bipartite signal mediates membrane targeting and virion incorporation of Nef.
The functional replacement of the Nef anchor by the Src membrane-targeting signal suggested that incorporation of Nef into the virion may depend primarily on its localization at the plasma membrane. Similar to Src (45), HIV-1 Nef is myristoylated and contains two clusters of basic residues within the N-terminal region of the protein (Fig. 4A), suggesting that its membrane-targeting signal may be similar. Accordingly, an internal deletion in the N-terminal region removing five of the six basic residues (Δ7–22; Fig. 4A) almost completely abolished virion incorporation of the corresponding Nef protein (Fig. 4C, lane 3), while a smaller deletion (Δ8–17) removing only a single positively charged residue had no effect on virion incorporation (Fig. 1C, lane 3). Mutations in the N-terminal region may also affect myristoylation of Nef proteins and indirectly prevent membrane binding of the respective protein. Therefore, we analyzed myristoylation of wild-type Nef, a G2S variant, and Nef/Δ7–22 by metabolic labeling with [35S]methionine or [3H]myristic acid and subsequent immunoprecipitation. Wild-type Nef and Nef/Δ7–22, but not the G2S variant, were myristoylated equally well (Fig. 5A, lanes 4 to 6).
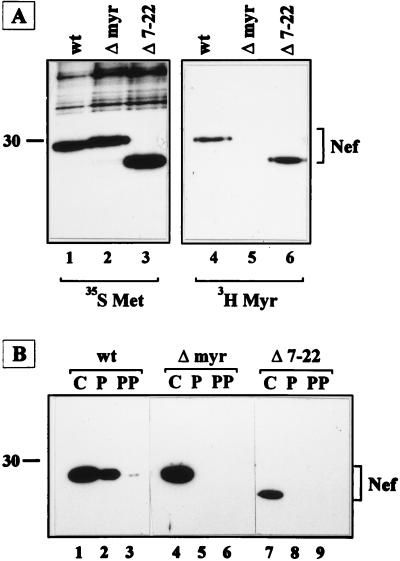
FIG. 5.
Analysis of myristoylation and membrane association of mutant Nef proteins. (A) Immunoprecipitation of Nef proteins from COS-7 cells transiently transfected with pSG5.BH10 Nef (lanes 1 and 4), pSG5.BH10 G2S (Δ-myr; lanes 2 and 5), or pSG5.BH10 Δ7–22 (lanes 3 and 6) after metabolic labeling with [35S]methionine (lanes 1 to 3) or [3H]myristic acid (lanes 4 to 6). (B) HeLa cell lines expressing wt Nef, G2S-Nef (Δ-myr), or Δ7–22 Nef were fractionated into cytosolic (C), Triton-soluble membrane (P), and Triton-insoluble cytoskeletal (PP) fractions as described in Materials and Methods. Molecular mass standards (in kilodaltons) and the positions of Nef proteins are indicated.
To analyze the effect of N-terminal alterations on intracellular localization of Nef, we performed subcellular fractionation experiments (Fig. 5B). HeLa cell lines expressing wild-type, Δmyr, and Δ7–22 Nef proteins under control of a tetracycline-regulated promoter (16) were fractionated into cytosolic, membrane, and cytoskeletal fractions by ultracentrifugation and detergent extraction. Following Western blot analysis of these fractions, wild-type Nef was observed predominantly in the cytoplasmic and membrane fractions (Fig. 5B; lanes 1 and 2), while nonmyristoylated Nef and Nef/Δ7–22 were detected only in the cytosolic fraction but not in the membrane fraction (lanes 4 to 9). These results demonstrated that myristoylation of Nef was not sufficient for membrane binding and virion incorporation, since Nef/Δ7–22 was myristoylated but did not associate with membranes and was not incorporated into HIV particles.
To investigate whether the basic amino acids in the N-terminal region of Nef contribute to virion incorporation, nef variants with substitutions of either lysine codons 4 and 7 (Nef-K) or arginine codons 17, 19, 21, and 22 (Nef-R) or a combination thereof (Nef-K+R) (Fig. 4A) were constructed. In infected cells, the steady-state levels of these altered proteins were similar to that of wild-type Nef, except for that of Nef/Δ7–22, which was slightly reduced (Fig. 4B, lane 3). Virion incorporation of Nef proteins with substitutions or deletion of N-terminal basic residues, on the other hand, was significantly reduced or undetectable in all cases. No virion-associated Nef was observed for the mutant lacking all basic residues (Fig. 4C, lane 6), while the corresponding Nef protein was present at a level equal to wild-type Nef in infected cells (Fig. 4B, lane 6). Replacing both Lys residues in the first 7 amino acids significantly reduced Nef incorporation as well (Fig. 4C, lane 4), while replacing the downstream Arg residues had a less dramatic effect on virion incorporation (Fig. 4C, lane 5). It is unlikely that these alterations affect Nef myristoylation, since all mutant proteins still conform to consensus signals for N-terminal myristoylation and since labeling experiments showed that substitution of residues 7 and 8 did not adversely affect myristoylation of Nef (data not shown). These results suggest that positively charged residues in the N-terminal anchor domain function additively and are essential for membrane targeting and virion incorporation of Nef.
Effect of N-terminal alterations in Nef on viral infectivity.
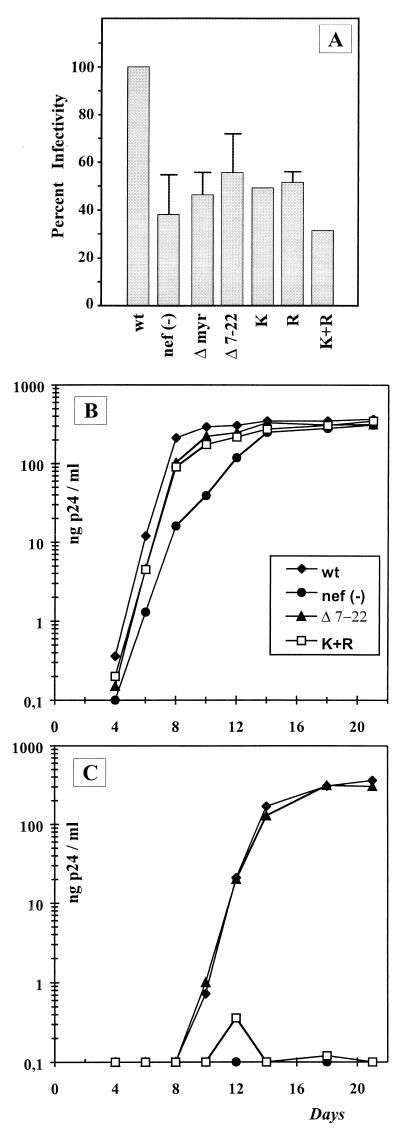
To assess the relevance of Nef incorporation for viral infectivity, we performed single-round replication assays on HeLa-CD4-LTR-βGal cells (31, 40). The infectivity of wild-type HIV-1 NL4-3 was consistently two- to threefold higher than the infectivity of a _nef_-deficient variant in eight independent experiments, each performed in quadruplicate (Fig. 6A). This result is in good agreement with published data for this HIV-1 strain (36). A nonmyristoylated Nef protein (Δmyr) which was not incorporated into virus particles also yielded a Nef-minus phenotype in this assay (Fig. 6A). Viruses containing Nef proteins with internal deletions in the N-terminal region of Nef that had no effect on virion incorporation (Δ8–17, Δ25–35, and Δ41–49) were similar to wild-type virus (data not shown). In contrast, HIV-1 NL4-3 (Δ7–22) expressing a Nef protein with reduced membrane binding and virion incorporation properties showed a Nef-minus phenotype (Fig. 6A). Similar results were obtained for the three viruses containing nef genes with substitutions of basic codons (Nef-K, Nef-R, and Nef-K+R [Fig. 6A]). Taken together, these results indicate that the infectivity phenotype of mutant viruses in single round replication assays correlated with incorporation of the respective Nef proteins into the virion.
FIG. 6.
Analysis of _nef_-mediated enhancement of viral infectivity. (A) Single-round replication assay. HeLa-CD4-LTR-βGal cells were infected with HIV-1 NL4-3 (wt) or derivatives as indicated underneath the bars. Viral infectivity (as a percentage of the wild-type value) was calculated by dividing the mean number of blue foci for each mutant by the mean number of blue foci observed for the wild type. Numbers were calculated from eight independent experiments, each performed in quadruplicate. Error bars indicate one standard deviation. No error bars are given for wild-type NL4-3, because it was defined as 100% and for the mutants K and K+R, for which only two experiments were performed. (B and C) Infection of primary human lymphocytes that were either stimulated prior to infection (B) or infected without prestimulation (C). Cells were infected with HIV-1 NL4-3 (wt) or the Nef mutants nef(−), Δ7–22, and K+R, as indicated in panel B. Viruses were diluted to 0.5 ng of CA antigen per ml prior to infection. The graphs show the increase in the p24 concentration in the culture medium over time after infection on a semilogarithmic scale. Mutant viruses corresponding to Nef/Δmyr, Nef-K, and Nef-R were analyzed in the same experiment but omitted from the graph for reasons of clarity.
The Nef phenotype can also be revealed by infection of primary human lymphocytes that have or have not been stimulated prior to infection (40, 51). No significant difference was observed for wild-type HIV-1 NL4-3 and nef mutants on the infection of PHA- and IL-2-activated PBMC (Fig. 6B). In contrast, infection of nonprestimulated PBMC yielded a productive infection for wild-type HIV-1 NL4-3 but not for the Nef-defective variant [nef(−); Fig. 6C]. The nef mutants showed discordent behavior in this assay. Unexpectedly, HIV-1 NL4-3 (Δ7–22) replicated efficiently in nonprestimulated PBMC (Fig. 6C) but exhibited a Nef-minus phenotype in a single-round replication assay (Fig. 6A). Furthermore, viruses containing substitutions of some basic residues in the anchor domain (NL4-3/Nef-K and NL4-3/Nef-R) also replicated like wild-type HIV in nonprestimulated lymphocytes (data not shown) but exhibited a Nef-defective phenotype in single-round replication assays (Fig. 6A). Removal of all basic charges, on the other hand (NL4-3/Nef-K+R), caused a Nef-minus phenotype in both assay systems (Fig. 6B and C). All experiments were performed in parallel with virus from parallel transfections, and similar results were obtained with PBMC from different donors and for different virus stocks (data not shown).
DISCUSSION
In this study, we have defined the signal for virion incorporation of HIV-1 Nef as a bipartite membrane-targeting signal which consists of covalently attached myristic acid and a cluster of positive charges in the N-terminal region of the protein. This signal is reminiscent of a Src homology region 4 (SH-4), which mediates plasma membrane targeting of protein tyrosine kinases of the Src family (45). It also resembles the membrane-targeting signal in the N-terminal MA domain of retroviral Gag polyproteins, which is essential for virus budding (reviewed in reference 34). Membrane binding of Nef and of N-terminally altered Nef proteins correlated with their incorporation into virus particles and was independent of any other viral protein. Furthermore, the N-terminal anchor domain was sufficient for plasma membrane localization and virion incorporation of a chimeric protein (Anchor-GFP). Recently, the N-terminal 10 amino acids of Nef have been shown to target a chimeric GFP to the plasma membrane of transfected T cells as well (24). Most probably, Nef and possibly other plasma membrane-associated proteins are incorporated into HIV-1 particles by their presence at the assembly site, provided that they are not actively excluded. Accordingly, HIV-1 Nef is also incorporated into murine leukemia virus particles when expressed to high levels in murine leukemia virus-infected cells (13).
N-terminal deletions abolished the membrane targeting of Nef and reduced its stability in infected cells. Both effects were reversed when the plasma membrane-targeting signal of the Src protein was fused to the N terminus of truncated Nef proteins, indicating that heterologous targeting signals can functionally replace the anchor domain. In agreement with previous studies, we observed that myristoylation of Nef was required for virion incorporation (13, 41). However, myristoylation was not sufficient, and two distinct clusters of basic residues, apparently functioning in an additive manner, were also needed. Studies with peptides have shown that the binding energy of a myristoyl group in a membrane bilayer is not sufficient to stably anchor peptides in biological membranes (38, 45). Positively charged side chains of Lys and Arg residues can form electrostatic interactions with negatively charged phospholipid head groups to provide additional binding energy for stable association (38, 45). The two clusters of basic residues in Nef were not equivalent, since alteration of the N-terminal Lys residues more severely affected membrane targeting and incorporation into the virion. Similarly, positive charges within the first 10 residues of Src family kinases are essential for membrane binding whereas downstream basic residues influence membrane binding to a lesser degree (45).
Alterations in the N-terminal region of Nef that did not significantly affect Nef incorporation into virus particles led to an infectivity phenotype indistinguishable from that of wild-type HIV-1 in single-round replication assays. Substitution of positively charged residues in either one or both basic clusters, on the other hand, reduced the relative infectivity of the respective virus to that of a _nef_-deficient HIV-1. These Nef proteins were incorporated into virus particles at reduced levels but were clearly not absent, at least in some cases, suggesting that a threshold concentration of Nef is required for enhancement of infectivity. Alternatively, substitutions of basic residues may cause additional defects besides affecting membrane transport and virion incorporation (11). Surprisingly, upon single-round infection of HeLa cells, the infectivity phenotype did not always correlate with the ability of the respective virus to replicate in nonprestimulated primary lymphocytes. It is generally assumed that viral phenotypes in single-cycle replication assays correspond to growth in primary lymphocytes and reflect the same functions (23, 40, 44, 49). Clearly, myristoylation and positive charges in the N-terminal region of Nef are needed for Nef-mediated enhancement of infectivity in both systems. However, several mutant viruses (Δ7–22, Nef-K, and Nef-R) replicated to wild-type levels in nonprestimulated lymphocytes while exhibiting a Nef-defective phenotype upon single-round infection of HeLa cells. This discrepancy indicates that different or additional effects may be important for Nef function in different target cells. Recently, Nef has been shown to stimulate autocrine IL-2 secretion in infected herpesvirus saimiri-transformed T-cells, leading to cell division and enhanced virus replication (8). Similarly, Nef may provide activating stimuli to infected primary lymphocytes, eventually leading to enhanced virus replication (40, 51). The residual membrane binding capacity of some of the altered Nef proteins may suffice for this function but not for virion incorporation.
How may virion-associated HIV-1 Nef increase viral infectivity? The enhancing effect of Nef becomes evident after virus entry into the target cell (2, 49), most probably following uncoating (5), and eventually leads to increased proviral DNA synthesis (2, 49). Since only a few molecules of Nef are incorporated into HIV-1 particles, Nef may exert its function indirectly through interaction with cellular proteins. Recently, several protein kinases were shown to interact with Nef (11, 43, 46, 47; reviewed in reference 27) and some of these kinases were implicated in phosphorylation of viral Gag proteins (52). It is conceivable that Nef recruits cellular proteins to the site of virus assembly or into virus particles, thereby modulating the function of other viral proteins. Alternatively, Nef or the core domain of Nef may be a component of the viral ribonucleoprotein complex exerting a function in genome replication or transport. In this case, cleavage of Nef by the viral PR could play a regulatory role (22), which would explain the complementation of Nef in the producer cell but not in the target cell (2). Nef cleavage site mutants are generally impaired in infectivity assays, and alternative cleavage sites can be used instead (Nef/Δ57–66) (data not shown) (14, 41). However, there is no clear correlation between infectivity phenotype and relative Nef cleavage for several cleavage site mutants (14, 41), and simian immunodeficiency virus Nef is not proteolytically cleaved, although it is incorporated into virus particles and can functionally substitute for HIV-1 Nef-mediated enhancement of infectivity (14).
Plasma membrane targeting is critical not only for virion incorporation of Nef and for enhancement of viral infectivity but also for most if not all of its other functions. Consistent with this hypothesis, myristoylation of Nef is essential for CD4 downregulation (3, 26, 29), while mutation of single clusters of basic residues in the N terminus of Nef impairs but does not completely abolish this process (4, 29). Moreover, Nef has been shown to differentially modulate the state of cellular activation depending on its intracellular localization in various cell types (10; reviewed in reference 27). This interference is likely to be mediated at least in part by interaction of Nef with and activation or inhibition of cellular protein kinases. Myristoylation and membrane localization of Nef appear to be important for this modulation (11, 15, 48), further underlining the importance of membrane targeting for Nef function.
ACKNOWLEDGMENTS
We are indebted to G. Aldrovandi, J. Zack, and K. Saksela for providing Nef mutants and for helpful discussions. We are grateful to R. Tsien for providing GFP expression vectors, H. J. Stellbrink for providing patient sera, C. Löliger for providing buffy coats, and M. Schreiber for performing oligonucleotide synthesis. We thank D. Mann and A. Baur for discussions, K. Coates for subcellular fractionation, B. Henkel and M. Dittmar for help with tissue culture, and B. Müller for critical reading of the manuscript.
This work was supported in part by a grant from the German Ministry for Education and Research to H.-G.K.
REFERENCES
- 1.Adachi A, Gendelman H E, Koenig S, Folks T, Willey R, Rabson A, Martin M A. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol. 1986;59:284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aiken C, Trono D. Nef stimulates human immunodeficiency virus type 1 proviral DNA synthesis. J Virol. 1995;69:5048–5056. doi: 10.1128/jvi.69.8.5048-5056.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aiken C, Konner J, Landau N, Lenburg M, Trono D. Nef induces CD4 endocytosis: requirement for a critical dileucine motif in the membrane-proximal CD4 cytoplasmic domain. Cell. 1994;76:853–864. doi: 10.1016/0092-8674(94)90360-3. [DOI] [PubMed] [Google Scholar]
- 4.Aiken C, Krause L, Chen Y L, Trono D. Mutational analysis of HIV 1 Nef: identification of two mutants that are temperature-sensitive for CD4 downregulation. Virology. 1996;217:293–300. [Google Scholar]
- 5.Aiken C. Pseudotyping human immunodeficiency virus type 1 (HIV-1) by the glycoprotein of vesicular stomatitis virus targets HIV-1 entry to an endocytic pathway and suppresses both the requirement for Nef and the sensitivity to cyclosporin A. J Virol. 1997;71:5871–5877. doi: 10.1128/jvi.71.8.5871-5877.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aldrovandi G M, Zack J. Replication and pathogenicity of human immunodeficiency virus type 1 accessory gene mutants in SCID-hu mice. J Virol. 1996;70:1505–1511. doi: 10.1128/jvi.70.3.1505-1511.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aldrovandi G M, Gao L Y, Bristol G, Zack J. Regions of human immunodeficiency virus type 1 required for function in vivo. J Virol. 1998;72:7032–7039. doi: 10.1128/jvi.72.9.7032-7039.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alexander L, Du Z, Rosenzweig M, Jung J U, Desrosiers R C. A role for natural simian immunodeficiency virus and human immunodeficiency virus type 1 nef alleles in lymphocyte activation. J Virol. 1997;71:6094–6099. doi: 10.1128/jvi.71.8.6094-6099.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ausubel F, Brent R, Kingston R E, Moore D D, Smith J A, Seidman J G, Struhl K. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1987. [Google Scholar]
- 10.Baur A, Sawai E, Dazin P, Fantl W, Cheng-Mayer C, Peterlin M B. HIV-1 Nef leads to inhibition or activation of T cells depending on its intracellular localization. Immunity. 1994;1:373–384. doi: 10.1016/1074-7613(94)90068-x. [DOI] [PubMed] [Google Scholar]
- 11.Baur A S, Sass G, Laffert B, Willbold D, Cheng-Mayer C, Peterlin B M. The N-terminus of Nef from HIV-1/SIV associates with a protein complex containing Lck and a serine kinase. Immunity. 1997;6:283–291. doi: 10.1016/s1074-7613(00)80331-3. [DOI] [PubMed] [Google Scholar]
- 12.Benichou S, Bomsel M, Bodeus M, Durand H, Doute M, Letourneur F, Camonis J, Benarous R. Physical interaction of the HIV-1 Nef protein with β-Cop, a component of non-clathrin-coated vesicles essential for membrane traffic. J Biol Chem. 1994;269:30073–30076. [PubMed] [Google Scholar]
- 13.Bukovsky A A, Dorfman T, Weimann A, Göttlinger H G. Nef association with human immunodeficiency virus type 1 virions and cleavage by the viral protease. J Virol. 1997;71:1013–1018. doi: 10.1128/jvi.71.2.1013-1018.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Y-L, Trono D, Camaur D. The proteolytic cleavage of human immunodeficiency virus type 1 Nef does not correlate with its ability to stimulate virion infectivity. J Virol. 1998;72:3178–3184. doi: 10.1128/jvi.72.4.3178-3184.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coates K, Cooke S J, Mann D A, Harris M P G. Protein kinase C-mediated phosphorylation of HIV-I Nef in human cell lines. J Biol Chem. 1997;272:12289–12294. doi: 10.1074/jbc.272.19.12289. [DOI] [PubMed] [Google Scholar]
- 16.Cooke S J, Coates K, Barton C H, Biggs T E, Barrett S J, Cochrane A, Oliver K, McKeating J A, Harris M P, Mann D A. Regulated expression vectors demonstrate cell-type-specific sensitivity to human immunodeficiency virus type 1 Nef-induced cytostasis. J Gen Virol. 1997;78:381–392. doi: 10.1099/0022-1317-78-2-381. [DOI] [PubMed] [Google Scholar]
- 17.Deacon N J, Tsykin A, Solomon A, Smith K, Ludford A, Menting M, Hooker D J, McPhee D A, Greenway A L, Ellett A, Chatfield C. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science. 1995;270:988–991. doi: 10.1126/science.270.5238.988. [DOI] [PubMed] [Google Scholar]
- 18.Fackler O T, Kremmer E, Mueller-Lantzsch N. Evidence for the association of Nef protein with HIV-2 virions. Virus Res. 1996;46:105–110. doi: 10.1016/s0168-1702(96)01389-5. [DOI] [PubMed] [Google Scholar]
- 19.Fackler O T, Kienzle N, Kremmer E, Boese A, Schramm B, Klimkait T, Kucherer C, Mueller-Lantzsch N. Association of human immunodeficiency virus Nef protein with actin is myristoylation dependent and influences its subcellular localization. Eur J Biochem. 1997;247:843–851. doi: 10.1111/j.1432-1033.1997.00843.x. [DOI] [PubMed] [Google Scholar]
- 20.Franchini G, Robert-Guroff M, Ghrayeb J, Chang N, Wong-Staal F. Cytoplasmic localization of the HTLV III 3′ orf in cultured T cells. Virology. 1986;155:593–599. doi: 10.1016/0042-6822(86)90219-9. [DOI] [PubMed] [Google Scholar]
- 21.Freund J, Kellner R, Houthaeve T, Kalbitzer H R. Stability and proteolytic domains of Nef protein from human immunodeficiency virus (HIV) type 1. Eur J Biochem. 1994;221:811–819. doi: 10.1111/j.1432-1033.1994.tb18795.x. [DOI] [PubMed] [Google Scholar]
- 22.Freund J, Kellner R, Konvalinka J, Wolber V, Krausslich H G, Kalbitzer H R. A possible regulation of negative factor (Nef) activity of human immunodeficiency virus type 1 by the viral protease. Eur J Biochem. 1994;223:589–593. doi: 10.1111/j.1432-1033.1994.tb19029.x. [DOI] [PubMed] [Google Scholar]
- 23.Goldsmith M A, Warmerdam M T, Atchison R E, Miller M D, Greene W C. Dissociation of the CD4 downregulation and viral infectivity enhancement functions of human immunodeficiency virus type 1 Nef. J Virol. 1995;69:4112–4121. doi: 10.1128/jvi.69.7.4112-4121.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greenberg M E, Bronson S, Lock M, Neumann M, Pavlakis G N, Skowronski J. Co-localization of HIV-1 Nef with the AP-2 adaptor protein complex correlates with Nef-induced CD4 down-regulation. EMBO J. 1997;16:6964–6976. doi: 10.1093/emboj/16.23.6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grzesiek S, Bax A, Clore G M, Gronenborn A M, Hu J S, Kaufman J, Palmer I, Stahl S J, Wingfield P T. The solution structure of HIV-1 Nef reveals an unexpected fold and permits delineation of the binding surface for the SH3 domain of Hck tyrosine protein kinase. Nat Struct Biol. 1996;3:340–345. doi: 10.1038/nsb0496-340. [DOI] [PubMed] [Google Scholar]
- 26.Guy B, Riviere Y, Dott K, Regnault A, Kieny M P. Mutational analysis of the HIV nef protein. Virology. 1990;176:413–425. doi: 10.1016/0042-6822(90)90011-f. [DOI] [PubMed] [Google Scholar]
- 27.Harris M. From negative factor to a critical role in virus pathogenesis: the changing fortunes of Nef. J Gen Virol. 1996;77:2379–2392. doi: 10.1099/0022-1317-77-10-2379. [DOI] [PubMed] [Google Scholar]
- 28.Heim R, Tsien R. Engineering green fluorescent protein for improved brightness, longer wavelengths, and fluorescence resonance energy transfer. Curr Biol. 1996;6:178–182. doi: 10.1016/s0960-9822(02)00450-5. [DOI] [PubMed] [Google Scholar]
- 29.Iafrate A J, Bronson S, Skowronski J. Separable functions of Nef disrupt two aspects of T cell receptor machinery: CD4 expression and CD3 signaling. EMBO J. 1997;16:673–684. doi: 10.1093/emboj/16.4.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kestler H, Ringler D, Mori K, Panicali D, Sehgal P, Daniel M, Desrosiers R. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell. 1991;65:651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- 31.Kimptom J, Emerman M. Detection of replication-competent and pseudotyped HIV with a sensitive cell line on the basis of activation of an integrated β-galactosidase gene. J Virol. 1992;66:2232–2239. doi: 10.1128/jvi.66.4.2232-2239.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kirchhoff F, Greenough T, Brettler D, Sullivan J, Desrosiers R. Brief report: absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N Engl J Med. 1995;332:228–323. doi: 10.1056/NEJM199501263320405. [DOI] [PubMed] [Google Scholar]
- 33.Konvalinka J, Litterst M A, Welker R, Kottler H, Rippmann F, Heuser A M, Kräusslich H G. An active-site mutation in the human immunodeficiency virus type 1 proteinase (PR) causes reduced PR activity and loss of PR-mediated cytotoxicity without apparent effect on virus maturation and infectivity. J Virol. 1995;69:7180–7186. doi: 10.1128/jvi.69.11.7180-7186.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kräusslich H G, Welker R. Intracellular transport of retroviral capsid components. Curr Top Microbiol Immunol. 1996;214:25–64. doi: 10.1007/978-3-642-80145-7_2. [DOI] [PubMed] [Google Scholar]
- 35.Lee C H, Saksela K, Mirza U A, Chait B T, Kuriyan J. Crystal structure of the conserved core of HIV-1 Nef complexed with a Src family SH3 domain. Cell. 1996;85:931–942. doi: 10.1016/s0092-8674(00)81276-3. [DOI] [PubMed] [Google Scholar]
- 36.Luo T, Garcia J V. Association of Nef with a cellular serine/threonine kinase and its enhancement of infectivity are isolate dependent. J Virol. 1996;70:6493–6496. doi: 10.1128/jvi.70.9.6493-6496.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mangasarian A, Foti M, Aiken C, Chin D, Carpentier J L, Trono D. The HIV-1 Nef protein acts as a connector with sorting pathways in the Golgi and at the plasma membrane. Immunity. 1997;6:67–77. doi: 10.1016/s1074-7613(00)80243-5. [DOI] [PubMed] [Google Scholar]
- 38.McLaughlin S, Aderem A. The myristoyl-electrostatic swich: a modulator of reversible protein-membrane interactions. Trends Biochem Sci. 1995;20:272–276. doi: 10.1016/s0968-0004(00)89042-8. [DOI] [PubMed] [Google Scholar]
- 39.Mergener K, Fäcke M, Welker R, Brinkmann V, Gelderblom H, Kräusslich H G. Analysis of HIV-1 particle formation using transient expression of subviral constructs in mammalian cells. Virology. 1992;186:25–39. doi: 10.1016/0042-6822(92)90058-w. [DOI] [PubMed] [Google Scholar]
- 40.Miller M, Warmerdam M, Gaston I, Greene W, Feinberg M. The human immunodeficiency virus-1 nef gene product: a positive factor for viral infection and replication in primary lymphocytes and macrophages. J Exp Med. 1994;179:101–113. doi: 10.1084/jem.179.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller M D, Warmerdam M T, Ferrell S S, Benitez R, Greene W C. Intravirion generation of the C-terminal core domain of HIV-1 Nef by the HIV-1 protease is insufficient to enhance viral infectivity. Virology. 1997;234:215–225. doi: 10.1006/viro.1997.8641. [DOI] [PubMed] [Google Scholar]
- 42.Niederman T M, Hastings W R, Ratner L. Myristoylation-enhanced binding of the HIV-1 Nef protein to T cell skeletal matrix. Virology. 1993;197:420–425. doi: 10.1006/viro.1993.1605. [DOI] [PubMed] [Google Scholar]
- 43.Nunn M F, Marsh J W. Human immunodeficiency virus type 1 Nef associates with a member of the p21-activated kinase family. J Virol. 1996;70:6157–6161. doi: 10.1128/jvi.70.9.6157-6161.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pandori M W, Fitch N J, Craig H M, Richman D D, Spina C A, Guatelli J C. Producer-cell modification of human immunodeficiency virus type 1: Nef is a virion protein. J Virol. 1996;70:4283–4290. doi: 10.1128/jvi.70.7.4283-4290.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Resh M D. Myristylation and palmitylation of Src-family members: the fats of the matter. Cell. 1994;76:411–413. doi: 10.1016/0092-8674(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 46.Saksela K, Cheng G, Baltimore D. Proline-rich (PxxP) motifs in HIV-1 Nef bind to SH3 domains of a subset of src kinases and are required for the enhanced growth of Nef(+) viruses but not for downregulation of CD4. EMBO J. 1995;14:484–491. doi: 10.1002/j.1460-2075.1995.tb07024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sawai E, Baur A, Struble H, Peterlin B M, Levy J, Cheng-Mayer C. Human immunodeficiency virus type 1 Nef associates with a cellular serine kinase in T lymphocytes. Proc Natl Acad Sci USA. 1994;91:1539–1543. doi: 10.1073/pnas.91.4.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sawai E T, Baur A S, Peterlin B M, Levy J A, Cheng-Mayer C. A conserved domain and membrane targeting of Nef from HIV and SIV are required for association with a cellular serine kinase activity. J Biol Chem. 1995;270:15307–15314. doi: 10.1074/jbc.270.25.15307. [DOI] [PubMed] [Google Scholar]
- 49.Schwartz O, Marechal V, Danos O, Heard J. Human immunodeficiency virus type 1 Nef increases the efficiency of reverse transcription in the infected cell. J Virol. 1995;69:4053–4059. doi: 10.1128/jvi.69.7.4053-4059.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schwartz O, Marechal V, Le-Gall S, Lemonnier F, Heard J M. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat Med. 1996;2:338–342. doi: 10.1038/nm0396-338. [DOI] [PubMed] [Google Scholar]
- 51.Spina C, Kwoh T, Chowers M, Guatelli J, Richman D. The importance of nef in the induction of human immunodeficiency virus type 1 replication from primary quiescent CD4 lymphocytes. J Exp Med. 1994;179:115–123. doi: 10.1084/jem.179.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Swingler S, Gallay P, Camaur D, Song J, Abo A, Trono D. The Nef protein of human immunodeficiency virus type 1 enhances serine phosphorylation of the viral matrix. J Virol. 1997;71:4372–4377. doi: 10.1128/jvi.71.6.4372-4377.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Welker R, Kottler H, Kalbitzer H R, Kräusslich H G. Human immunodeficiency virus type 1 Nef protein is incorporated into virus particles and specifically cleaved by the viral proteinase. Virology. 1996;219:228–236. doi: 10.1006/viro.1996.0240. [DOI] [PubMed] [Google Scholar]