Human Papillomavirus Type 11 Recombinant L1 Capsomeres Induce Virus-Neutralizing Antibodies (original) (raw)
Abstract
The human papillomavirus type 11 (HPV-11) L1 major capsid protein can be trypsinized to generate recombinant capsomeres that retain HPV genotype-restricted capsid antigenicity (M. Li, T. P. Cripe, P. A. Estes, M. K. Lyon, R. C. Rose, and R. L. Garcea, J. Virol. 71:2988–2995, 1997). In the present study, HPV-11 virion-neutralizing monoclonal antibodies H11.F1 and H11.H3, previously characterized as recognizing two distinct HPV-11 capsid-neutralizing antigenic domains (S. W. Ludmerer, D. Benincasa, and G. E. Mark III, J. Virol. 70:4791–4794, 1996), were each found to be highly immunoreactive with trypsin-generated capsomeres in an enzyme-linked immunosorbent assay (ELISA). Capsomeres were used to generate high-titer polyclonal immune sera that demonstrated HPV genotype-restricted reactivity by ELISA. The capsomere antisera were then tested in an in vitro infectivity assay and found to neutralize HPV-11 virions. In this assay, HPV-11 capsomere polyclonal antisera exhibited neutralization titers (10−5 to 10−6) comparable to those obtained with a virion-neutralizing antiserum raised previously against intact HPV-11 VLPs (R. C. Rose, R. C. Reichman, and W. Bonnez, J. Gen. Virol. 75:2075–2079, 1994). These results indicate that highly immunogenic, genotype-restricted HPV capsid-neutralizing antigenic domains are contained entirely within capsomeres. Thus, capsomeres may be viable vaccine candidates for the prevention of HPV disease.
Papillomaviruses cause hyperproliferative cutaneous and/or mucosal epithelial lesions in higher vertebrates, including humans (31). More than 70 genotypically distinct human papillomaviruses (HPVs) have been identified (12) and can be categorized on the basis of observed differences in virus phenotype (i.e., preferred tissue tropisms and/or disease associations). For example, most HPVs preferentially infect cutaneous skin and usually cause only benign disease (e.g., plantar or common warts), while other types more often infect oral or anogenital mucosal epithelium. Mucosal epitheliotropic HPVs have been associated with a variety of lesions, including benign anogenital warts, premalignant intraepithelial neoplasias, and invasive cancers, particularly of the uterine cervix (4, 23, 35). These observations have focused attention on vaccine efforts to prevent HPV infection.
HPV was first propagated outside the natural host in host-derived epithelial xenografts implanted in immunodeficient mice (19). This advance resulted in the ability to produce sufficient quantities of virions to allow the study of important viral determinants of host immune responses (3, 10). However, because it was initially possible to propagate virions of only one HPV genotype in that system (i.e., HPV type 11 [HPV-11]), several groups sought to reproduce the antigenic properties of intact virions by producing empty capsids, or virus-like particles (VLPs), through recombinant expression of the major capsid protein L1 (14, 16, 29). VLPs possess important antigenic features of native HPV virions (7, 18, 29, 30). Such antigenicity depends upon maintaining native virion structure, and VLPs have been shown to be structurally identical to virions at a 35-Å resolution (13). VLP vaccinations have been shown to stimulate immune responses which protect animal hosts from diseases caused by papillomaviruses (6, 17, 34). Thus, VLPs are promising vaccine candidates for preventing HPV infection in humans (15, 33).
Papillomavirus virions have a T=7 icosahedral capsid comprised of 72 pentamers (i.e., capsomeres) of the major capsid protein L1 (2). As with the VP1 capsid protein of the related polyomaviruses (21), several noncontiguous domains of the papillomavirus L1 major capsid protein are likely exposed on the surface of the virion, and determine the conformationally dependent capsid-neutralizing antigenic domains of the virion. Recently, the HPV-11 L1 protein was purified after expression in Escherichia coli (20). This recombinant L1 protein was shown to be capable of self-assembly into capsids in vitro and was also found to be specifically sensitive to trypsin cleavage at R415 near the L1 carboxyl terminus (20). The resulting digestion product is a truncated L1 protein, which appears by electron microscopy as a pentameric capsomere. Unlike capsomeres generated from HPV-11 L1 VLPs upon exposure to high concentrations of reducing agent (25), capsomeres produced by trypsin digestion are unable to reassemble into capsids (20). We previously demonstrated that trypsin-generated HPV-11 capsomeres exhibit an antigenicity comparable to that of intact HPV-11 VLPs when examined by ELISA with polyclonal antisera generated against HPV-11 virions and recombinant HPV-11, -16, and -18 VLPs (20). These results suggested that capsomeres share strong antigenic similarities with native HPV-11 virions and intact VLPs, including genotype specificity. In the present study, we further evaluated capsomere immunogenicity. Our results indicate that HPV capsid-neutralizing antigenic domains are contained entirely within capsomeres and that capsomeres induce the synthesis of virus-neutralizing antibodies.
Preparation of HPV-11 L1 capsomeres.
Methods for the purification of the recombinant HPV-11 L1 protein after expression in Escherichia coli, as well as conditions for complete trypsin digestion of L1 to capsomeres, have been previously described (20). After trypsinization, the L1 protein was purified further with DEAE-cellulose, followed by phosphocellulose chromatography in buffer A (10 mM Tris-HCl [pH 7.2], 5% glycerol, 2 mM EDTA, 15 mM 2-mercaptoethanol, 100 mM NaCl). In this buffer, the trypsinized L1 was found in the flowthrough fractions from both columns (trace amounts of residual undigested L1 eluted at salt concentrations of between 0.5 and 1 M NaCl). Complete digestion of the L1 protein eluting from the phosphocellulose column in buffer A was confirmed by immunoblotting, and the absence of any assembly beyond capsomeres was confirmed by electron microscopy. By these criteria, the purified L1 protein used for immunization was in capsomere form only and was incapable of further assembly (20).
HPV-11 virion-neutralizing MAbs are immunoreactive with HPV-11 L1 capsomeres.
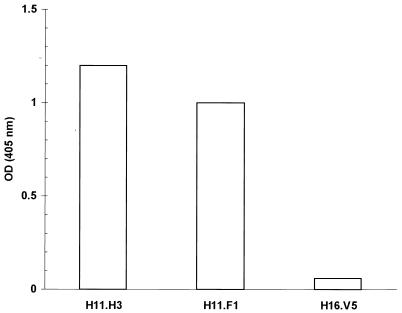
Ascites containing HPV-11 virion-neutralizing monoclonal antibodies (MAbs) H11.F1 and H11.H3 (11) (obtained from Neil Christensen) was diluted 1:128,000 and then evaluated in an enzyme-linked immunosorbent assay (ELISA) against trypsin-generated HPV-11 capsomeres. MAbs were tested in duplicate wells containing 80 ng of purified capsomeres each. As a control, MAb H16.V5 (8) (obtained from Chemicon International, Inc., Temecula, Calif.) was used at a dilution of 1:250. Previously, MAbs H11.F1 and H11.H3 have been used to identify two conformationally dependent neutralization sites on HPV-11 virions (11, 24). The first site, recognized by MAb H11.F1, is centered at residues 131 and 132 on the HPV-11 L1 protein, and the second distinct, although possibly overlapping, site is defined by MAb H11.H3 (24). We tested these MAbs in an ELISA and found that both reacted strongly and specifically with trypsin-generated HPV-11 capsomeres, whereas a third MAb, raised against HPV-16 VLPs (H16.V5) (8), did not react (Fig. 1). These results confirmed our previous observation of strong H11.F1 reactivity with capsomeres generated via thiol reduction of VLPs (25) and indicated that HPV-11 capsomeres contain both of the previously characterized HPV-11 capsid-neutralizing domains.
FIG. 1.
HPV-11-neutralizing antibodies bind HPV-11 capsomeres. H11.F1 and H11.H3 ascites were diluted 1:128,000 and then tested in an ELISA against trypsin-generated HPV-11 capsomeres. As a control, ascites containing an HPV-16 capsid-specific MAb (H16.V5) (8) was assayed at a dilution of 1:250. OD, optical density.
Capsomeres induce polyclonal antibodies that demonstrate HPV genotype specificity.
Trypsin-generated HPV-11 capsomeres were used to immunize each of two rabbits as previously described (30), and the resulting antisera were tested in an ELISA against the same antigen used for immunization and against intact VLPs of HPV-11, -16, and -18 (Fig. 2). The capsomere antibodies reacted strongly with both the homologous capsomere immunogen and with intact HPV-11 VLPs, but not with HPV-16 VLPs. Thus, the capsomere antisera were generally HPV type specific. However, both of the HPV-11 capsomere antisera cross-reacted slightly at low dilutions with HPV-18 VLPs (Fig. 2). A similar pattern of antigenic cross-reactivity was observed when these antisera were tested against HPV-11, -16, and -18 recombinant L1 proteins in a Western blot immunoassay (data not shown). These findings suggest that one or more conserved linear epitopes of the HPV-11 L1 protein may become accessible to the immune system when capsomeres are presented in an unassembled state.
FIG. 2.
HPV-11 capsomeres are highly immunogenic and display HPV genotype-restricted antigenicity. Threefold serial dilutions of rabbit capsomere polyclonal antisera R6-311 (solid symbols) and R6-312 (open symbols) were tested in an ELISA in duplicate against HPV-11 capsomeres (triangles), HPV-11 VLPs (squares), HPV-16 VLPs (circles), and HPV-18 VLPs (diamonds).
Capsomeres elicit virus-neutralizing antibodies.
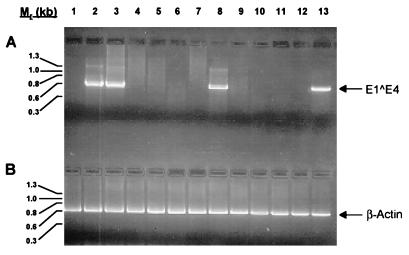
Capsomere polyclonal antisera were also evaluated for virus-neutralizing activity in an in vitro infectivity assay (32). The reverse transcriptase-PCR (RT-PCR) assay was carried out with an HPV-11Hershey virus stock (19) (obtained from John Kreider) and HaCaT cells, an immortalized human keratinocyte cell line (5) (obtained from Norbert Fusenig), as described previously (32) with modifications (25). Samples were analyzed by RT-PCR for the presence of HPV-11 E1̂E4 spliced mRNA, and, as a control, for the presence of spliced cellular β-actin mRNA. Previous work conducted with an in vivo infectivity model has shown that HPV-11 virions and recombinant HPV-11 VLPs induce antibodies that neutralize infectious HPV-11 virions (3, 9, 10, 30). In the in vitro assay used in the present study (32), infection is defined as generation of PCR-detectable early viral transcripts in cells incubated with virus. Neutralizing titer is defined as the highest dilution of serum which, when preincubated with virus, prevents the detection of viral transcripts. Titration of the virus stock used in these experiments on multiple occasions resulted in the consistent detection of the HPV-11 E1̂E4 spliced mRNA with dilutions from 1:102 (the lowest dilution tested) to 1:105. The virus-specific RNA product was not amplified from cells exposed to virus stock diluted 1:106. Therefore, the neutralization assay was conducted with a 1:104 dilution of virus stock. This allowed for consistent assay results while conserving the virus, which is in limited supply. As shown in Fig. 3, HPV-11 capsomere antibodies efficiently neutralized HPV-11 virions (lanes 4 to 8), whereas preimmune serum (lane 2) and HPV-16 VLP postimmune serum (lane 3) demonstrated no neutralizing activity. Anticapsomere neutralization titers were comparable to that of an antiserum raised against intact HPV-11 VLPs (i.e., 10−5 to 10−6) (compare lanes 4 to 8 with lanes 9 to 13), which was produced by the same immunization protocol (30). Thus, capsomeres and VLPs differ structurally but are immunologically equivalent.
FIG. 3.
HPV-11 capsomeres induce virus-neutralizing antibodies. (A) HPV-11 virions were incubated with antiserum before addition to HaCaT cells. Six days postinfection, cells were harvested, and the presence of an E1̂E4 spliced message, diagnostic of HPV-11 infection (3), was determined by RT-PCR. PCR products were separated on 2% agarose gels, stained with ethidium bromide, and examined under UV light for the presence of the ∼0.6-kb E1̂E4 band. Lane 1, HaCaT cells alone, no virus, no antibodies (negative control); lane 2, HPV-11 virions plus preimmune serum from an animal subsequently immunized with HPV-11 capsomeres (10−3 dilution); lane 3, HPV-11 virions plus HPV-16 L1 VLP postimmune serum (10−3 dilution); lanes 4 to 8, HPV-11 virions plus HPV-11 capsomere antiserum (dilutions 10−3 to 10−7, respectively); lanes 9 to 13, HPV-11 virions plus HPV-11 L1 VLP antiserum (dilutions 10−3 to 10−7, respectively). A second rabbit anti-HPV-11 capsomere antiserum tested produced comparable results (i.e., a neutralization titer of 10−5) (data not shown). (B) PCR amplification of β-actin was performed on all cDNA samples as an internal control. The expected size of the β-actin band is ∼0.6 kb. The lanes are the same as in panel A. The HPV-11Hershey virus stock was titrated on HaCat cells on multiple occasions prior to being used in the present study. The titer of the inoculum (i.e., the last dilution which resulted in the detection of spliced E1̂E4 mRNA by RT-PCR) consistently was between 10−5 and 10−6. In the present study, the inoculum was used at a dilution of 10−4.
Results from several studies indicate that the papillomavirus L1 capsid protein is a major neutralization antigen (6, 9, 17, 22, 30, 34). For this reason, significant effort has been directed towards producing recombinant L1 preparations that maintain the antigenic characteristics of native HPV virions. The present results indicate that a smaller unit of the virus capsid, the capsomere, is sufficient to reproduce important antigenic features of the native virion. Capsomere polyclonal antibody recognition patterns and virion neutralization titers paralleled those found with antibodies raised against native virions or recombinant VLPs. Because the capsomeres used to generate these antibodies lacked 86 amino acids from the L1 carboxyl-terminal arm, which mediates contacts between pentamers (20), these results cannot be attributed to either partial or complete reassembly of capsomeres into capsids. Thus, at least two HPV capsid-neutralizing antigenic domains, defined previously by MAbs (24), are contained entirely within pentameric L1 capsomeres, and interpentamer associations are not required for the induction of virus-neutralizing antibodies.
Unexpectedly, ELISA results also indicated that HPV-11 capsomere antibodies cross-react at low dilutions with HPV-18, but not with HPV-16 VLPs. This cross-reactivity may be due to the exposure in isolated capsomeres of a conserved linear epitope of L1, because a similar pattern of cross-reactivity was also seen in a Western blot immunoassay (data not shown). Since previously reported results indicate a minimal level of antigenic cross-reactivity between VLPs of HPV-11 and HPV-18 (references 27 and 28 and unpublished observations), the present results suggest that a linear epitope of HPV-11 L1 conserved with HPV-18 may become more accessible to the immune system when capsomeres are presented in isolation. Unmasking of antigenically cross-reactive L1 epitopes may therefore occur when capsomeres are used as antigens for serologic investigations.
Since the first reports describing isolation of HPV VLPs, it has been speculated that these particles might form the basis for an efficacious vaccine to prevent HPV infection. The observation that capsomeres display immunogenic, virus-neutralizing epitopes found on intact VLPs suggests that capsomeres might also be viable vaccine candidates. Capsomeres are homogeneous in size (20) and may prove to be more stable and less costly to produce than VLPs. Thus, they may be an attractive alternative to the use of VLPs as an immunogen for preventing HPV infection. Although it was not the purpose of the present study to make a dose-response comparison between VLPs and capsomeres, it will be important to assess the relative amplitude of the response against each of these antigens to determine the relative efficacy of capsomeres as vaccine antigens. Studies with different adjuvants and species, as well as various protein concentrations, will be required. The growing consensus that effective HPV vaccines may reduce the incidence of uterine cervical carcinoma (1, 26) underscores the importance of evaluating capsomere efficacy for immunoprophylaxis against genital HPV disease.
Acknowledgments
We thank Neil Christensen for the gift of H11.F1 and H11.H3 MAbs.
This work was supported in part by grant CA37667 from the National Cancer Institute (R.L.G.).
REFERENCES
- 1.Anonymous. National Institutes of Health Consensus Development Conference Statement: cervical cancer, April 1–3, 1996. National Institutes of Health Consensus Development Panel. J Natl Cancer Inst Monogr. 1996;21:vii–xix. [PubMed] [Google Scholar]
- 2.Baker T S, Newcomb W W, Olson N H, Cowsert L M, Olson C, Brown J C. Structures of bovine and human papillomaviruses: analysis by cryoelectron microscopy and three-dimensional image reconstruction. Biophys J. 1991;60:1445–1456. doi: 10.1016/S0006-3495(91)82181-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonnez W, Rose R C, Reichman R C. Antibody-mediated neutralization of human papillomavirus type 11 (HPV-11) infection in the nude mouse: detection of HPV-11 mRNAs. J Infect Dis. 1992;165:376–380. doi: 10.1093/infdis/165.2.376. [DOI] [PubMed] [Google Scholar]
- 4.Bosch F X, Manos M M, Munoz N, Sherman M, Jansen A M, Peto J, Schiffman M H, Moreno V, Kurman R, Shah K V. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International Biological Study on Cervical Cancer (IBSCC) Study Group. J Natl Cancer Inst. 1995;87:796–802. doi: 10.1093/jnci/87.11.796. [DOI] [PubMed] [Google Scholar]
- 5.Boukamp P, Petrussevska R T, Breitkreutz D, Hornung J, Markham A, Fusenig N E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988;106:761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breitburd F, Kirnbauer R, Hubbert N L, Nonnenmacher B, Trin-Dinh-Desmarquet C, Orth G, Schiller J T, Lowy D R. Immunization with viruslike particles from cottontail rabbit papillomavirus (CRPV) can protect against experimental CRPV infection. J Virol. 1995;69:3959–3963. doi: 10.1128/jvi.69.6.3959-3963.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carter J J, Hagensee M, Taflin M C, Lee S K, Koutsky L A, Galloway D A. HPV-1 capsids expressed in vitro detect human serum antibodies associated with foot warts. Virology. 1993;195:456–462. doi: 10.1006/viro.1993.1396. [DOI] [PubMed] [Google Scholar]
- 8.Christensen N D, Dillner J, Eklund C, Carter J J, Wipf G C, Reed C A, Cladel N M, Galloway D A. Surface conformational and linear epitopes on HPV-16 and HPV-18 L1 virus-like particles as defined by monoclonal antibodies. Virology. 1996;223:174–184. doi: 10.1006/viro.1996.0466. [DOI] [PubMed] [Google Scholar]
- 9.Christensen N D, Hopfl R, DiAngelo S L, Cladel N M, Patrick S D, Welsh P A, Budgeon L R, Reed C A, Kreider J W. Assembled baculovirus-expressed human papillomavirus type 11 L1 capsid protein virus-like particles are recognized by neutralizing monoclonal antibodies and induce high titres of neutralizing antibodies. J Gen Virol. 1994;75:2271–2276. doi: 10.1099/0022-1317-75-9-2271. [DOI] [PubMed] [Google Scholar]
- 10.Christensen N D, Kreider J W. Antibody-mediated neutralization in vivo of infectious papillomaviruses. J Virol. 1990;64:3151–3156. doi: 10.1128/jvi.64.7.3151-3156.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christensen N D, Kreider J W, Cladel N M, Patrick S D, Welsh P A. Monoclonal antibody-mediated neutralization of infectious human papillomavirus type 11. J Virol. 1990;64:5678–5681. doi: 10.1128/jvi.64.11.5678-5681.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Villiers E-M. Human pathogenic papillomavirus types: an update. Curr Top Microbiol Immunol. 1994;186:1–12. doi: 10.1007/978-3-642-78487-3_1. [DOI] [PubMed] [Google Scholar]
- 13.Hagensee M E, Olson N H, Baker T S, Galloway D A. Three-dimensional structure of vaccinia virus-produced human papillomavirus type 1 capsids. J Virol. 1994;68:4503–4505. doi: 10.1128/jvi.68.7.4503-4505.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hagensee M E, Yaegashi N, Galloway D A. Self-assembly of human papillomavirus type 1 capsids by expression of the L1 protein alone or by coexpression of the L1 and L2 capsid proteins. J Virol. 1993;67:315–322. doi: 10.1128/jvi.67.1.315-322.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hines J F, Ghim S, Schlegel R, Jenson A B. Prospects for a vaccine against human papillomavirus. Obstet Gynecol. 1995;86:860–866. doi: 10.1016/0029-7844(95)00248-p. [DOI] [PubMed] [Google Scholar]
- 16.Kirnbauer R, Booy F, Cheng N, Lowy D R, Schiller J T. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc Natl Acad Sci USA. 1992;89:12180–12184. doi: 10.1073/pnas.89.24.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirnbauer R, Chandrachud L M, O’Neil B W, Wagner E R, Grindlay G J, Armstrong A, McGarvie G M, Schiller J T, Lowy D R, Campo M S. Virus-like particles of bovine papillomavirus type 4 in prophylactic and therapeutic immunization. Virology. 1996;219:37–44. doi: 10.1006/viro.1996.0220. [DOI] [PubMed] [Google Scholar]
- 18.Kirnbauer R, Hubbert N L, Wheeler C M, Becker T M, Lowy D R, Schiller J T. A virus-like particle enzyme-linked immunosorbent assay detects serum antibodies in a majority of women infected with human papillomavirus type 16. J Natl Cancer Inst. 1994;86:494–499. doi: 10.1093/jnci/86.7.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kreider J W, Howett M K, Leure-Dupree A E, Zaino R J, Weber J A. Laboratory production in vivo of infectious human papillomavirus type 11. J Virol. 1987;61:590–593. doi: 10.1128/jvi.61.2.590-593.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li M, Cripe T P, Estes P A, Lyon M K, Rose R C, Garcea R L. Expression of the human papillomavirus type 11 L1 capsid protein in Escherichia coli: characterization of protein domains involved in DNA binding and capsid assembly. J Virol. 1997;71:2988–2995. doi: 10.1128/jvi.71.4.2988-2995.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liddington R C, Yan Y, Moulai J, Sahli R, Benjamin T L, Harrison S C. Structure of simian virus 40 at 3.8-A resolution. Nature. 1991;354:278–284. doi: 10.1038/354278a0. [DOI] [PubMed] [Google Scholar]
- 22.Lin Y-L, Borenstein L A, Ahmed R, Wettstein F O. Cottontail rabbit papillomavirus L1 protein-based vaccines: protection is achieved only with a full-length, nondenatured product. J Virol. 1993;67:4154–4162. doi: 10.1128/jvi.67.7.4154-4162.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lorincz A T, Reid R, Jenson A B, Greenberg M D, Lancaster W, Kurman R J. Human papillomavirus infection of the cervix: relative risk associations of 15 common anogenital types. Obstet Gynecol. 1992;79:328–337. doi: 10.1097/00006250-199203000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Ludmerer S W, Benincasa D, Mark G E., III Two amino acid residues confer type specificity to a neutralizing, conformationally dependent epitope on human papillomavirus type 11. J Virol. 1996;70:4791–4794. doi: 10.1128/jvi.70.7.4791-4794.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCarthy M P, White W I, Palmer-Hill F, Koenig S, Suzich J A. Quantitative disassembly and reassembly of human papillomavirus type 11 viruslike particles in vitro. J Virol. 1998;72:32–41. doi: 10.1128/jvi.72.1.32-41.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Munoz N, Bosch F X. The causal link between HPV and cervical cancer and its implications for prevention of cervical cancer. Bull Pan Am Hlth Org. 1996;30:362–377. [PubMed] [Google Scholar]
- 27.Roden R B S, Hubbert N L, Kirnbauer R, Christensen N D, Lowy D R, Schiller J T. Assessment of the serological relatedness of genital human papillomaviruses by hemagglutination inhibition. J Virol. 1996;70:3298–3301. doi: 10.1128/jvi.70.5.3298-3301.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rose R C, Bonnez W, Da Rin C, McCance D J, Reichman R C. Serological differentiation of human papillomavirus types 11, 16 and 18 using recombinant virus-like particles. J Gen Virol. 1994;75:2445–2449. doi: 10.1099/0022-1317-75-9-2445. [DOI] [PubMed] [Google Scholar]
- 29.Rose R C, Bonnez W, Reichman R C, Garcea R L. Expression of human papillomavirus type 11 L1 protein in insect cells: in vivo and in vitro assembly of viruslike particles. J Virol. 1993;67:1936–1944. doi: 10.1128/jvi.67.4.1936-1944.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rose R C, Reichman R C, Bonnez W. Human papillomavirus (HPV) type 11 recombinant virus-like particles induce the formation of neutralizing antibodies and detect HPV-specific antibodies in human sera. J Gen Virol. 1994;75:2075–2079. doi: 10.1099/0022-1317-75-8-2075. [DOI] [PubMed] [Google Scholar]
- 31.Shah K V, Howley P M. Papillomaviruses. In: Fields B N, Knipe D M, Howley P M, editors. Virology. 3rd ed. Vol. 2. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2077–2110. [Google Scholar]
- 32.Smith L H, Foster C, Hitchcock M E, Leiserowitz G S, Hall K, Isseroff R, Christensen N D, Kreider J W. Titration of HPV-11 infectivity and antibody neutralization can be measured in vitro. J Investig Dermatol. 1995;105:438–444. doi: 10.1111/1523-1747.ep12321173. [DOI] [PubMed] [Google Scholar]
- 33.Steller M A, Schiller J T. Human papillomavirus immunology and vaccine prospects. J Natl Cancer Inst Monogr. 1996;21:145–148. [PubMed] [Google Scholar]
- 34.Suzich J A, Ghim S-J, Palmer-Hill F J, White W I, Tamura J K, Bell J A, Newsome J A, Jenson A B, Schlegel R. Systemic immunization with papillomavirus L1 protein completely prevents the development of viral mucosal papillomas. Proc Natl Acad Sci USA. 1995;92:11553–11557. doi: 10.1073/pnas.92.25.11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.zur Hausen H. Molecular pathogenesis of cancer of the cervix and its causation by specific human papillomavirus types. Curr Top Microbiol Immunol. 1994;186:131–156. doi: 10.1007/978-3-642-78487-3_8. [DOI] [PubMed] [Google Scholar]