Latent Membrane Protein 2A-Mediated Effects on the Phosphatidylinositol 3-Kinase/Akt Pathway (original) (raw)
Abstract
Epstein-Barr virus (EBV) latent membrane protein 2A (LMP2A) is expressed on the membranes of B lymphocytes and blocks B-cell receptor (BCR) signaling in EBV-transformed B lymphocytes in vitro. The phosphotyrosine motifs at positions 74 or 85 and 112 within the LMP2A amino-terminal domain are essential for the LMP2A-mediated block of B-cell signal transduction. In vivo studies indicate that LMP2A allows B-cell survival in the absence of normal BCR signals. A possible role for Akt in the LMP2A-mediated B-cell survival was investigated. The protein kinase Akt is a crucial regulator of cell survival and is activated within B lymphocytes upon BCR cross-linking. LMP2A expression resulted in the constitutive phosphorylation of Akt, and this LMP2A effect is dependent on phosphatidylinositol 3-kinase activity. In addition, recruitment of Syk and Lyn protein tyrosine kinases (PTKs) to tyrosines 74 or 85 and 112, respectively, are critical for LMP2A-mediated Akt phosphorylation. However, the ability of LMP2A to mediate a survival phenotype downstream of Akt could not be detected in EBV-negative Akata cells. This would indicate that LMP2A is not responsible for EBV-dependent Burkitt's lymphoma cell survival.
Epstein-Barr virus (EBV), a gammaherpesvirus, causes infectious mononucleosis in normal adolescents and persists in the B lymphocytes of a majority of adults. EBV is potentially oncogenic, being etiologically linked to a variety of hematopoietic diseases, such as African Burkitt's lymphoma (BL), Hodgkin's disease, adult T-cell leukemia, and lymphoproliferative diseases in immunocompromised individuals (58). In addition, EBV is associated with two epithelial pathologies: nasopharyngeal carcinoma and oral hairy leukoplakia (for reviews, see references 39 and 58).
Primary human B lymphocytes infected in vitro with EBV become immortalized, establishing lymphoblastoid cell lines (LCLs). These EBV-transformed LCLs contain the EBV episome and express a restricted set of latently encoded viral proteins: six nuclear proteins (EBNA-1, EBNA-2, EBNA-3A, EBNA-3B, EBNA-3C, and EBNA-LP), three integral membrane proteins (latent membrane protein 1 [LMP1], LMP2A, and LMP2B), and two small RNAs (EBERs) (for reviews, see references 34 and 41). However, of the proteins expressed during latency, messages of EBNA-1, EBERs, BARTs, and LMP2A have been detected in peripheral B lymphocytes from individuals harboring latent infections (12, 13, 17, 55, 68). In addition, the LMP2A transcript is consistently detected in NPC and other EBV-related malignancies (for reviews, see references 39, 41, and 58). Thus, LMP2A may have significant roles in vivo for viral persistence and EBV-related diseases.
LMP2A forms aggregates, which localize to the plasma membrane of latently infected B cells. LMP2A contains a 119-amino-acid cytoplasmic amino terminus, 12 hydrophobic transmembrane domains, and a 27-amino-acid cytoplasmic carboxyl terminus (43, 61). The amino-terminal domain includes eight tyrosine residues, two of which form an immunoreceptor tyrosine-based motif (10, 57). In addition, the amino-terminal domain has been shown to be tyrosine phosphorylated and necessary for LMP2A association with Src family protein tyrosine kinases (PTKs) and Syk PTK (8, 42). Phosphorylated tyrosines provide potential binding sites for cellular proteins containing Src homology 2 domains. Src homology 2 domains are noncatalytic domains that are conserved among cytoplasmic signaling molecules and bind to phosphotyrosine motifs (for a review, see reference 45). Studies have demonstrated the requirement of LMP2A phosphotyrosine motifs at positions 74 or 85 and 112 to bind Syk and Lyn, respectively (25, 26).
In addition to PTKs associating with LMP2A, LMP2A has a dramatic effect on B-cell signaling. In primary B lymphocytes, ligation of the B-cell receptor (BCR) initially induces the up-regulation of protein tyrosine phosphorylation by recruiting and activating Src family PTKs. This event is followed by the recruitment of other tyrosine and serine/threonine kinases and phosphatases, the hydrolysis of phospholipids, activation of protein kinase C, mobilization of calcium, and activation of nuclear transcription factors that are specific for transcribing the BCR signal-specific genes required for mediating clonal expansion, differentiation, and apoptosis (for a review, see reference 18). In in vitro studies, the expression of LMP2A blocks BCR-mediated signal transduction (47). In vivo, LMP2A expression prevents BCR-negative B lymphocytes from undergoing apoptosis and enables these BCR-negative cells to persist within peripheral lymphoid organs (9). This suggests that LMP2A can provide both a developmental signal and a survival signal to normal B cells.
B-cell survival can be promoted by activation of the serine-threonine kinase Akt. Akt was identified as a proto-oncogene and is the cellular homologue of a protein encoded in the genome of the retrovirus AKT8 (6). Furthermore, the deregulation of Akt activity has been linked to tumorigenesis in humans (5, 64, 65). Upon BCR activation, there is a rapid activation of the nonreceptor PTK, phosphatidylinositol 3-kinase (PI3-K) (27). Activation of PI3-K results in the production of phosphatidylinositol 3,4,5-triphosphate [PtdIns(3,4,5)P3] and phosphatidylinositol 3,4-biphosphate [PtdIns(3,4)P2], which recruit Akt to the plasma membrane by binding the pleckstrin homology domain in Akt. Membrane-anchored Akt is subsequently phosphorylated on Thr308 and Ser473 by PtdIns(3,4,5)P3-dependent kinase-1 and integrin-linked kinase/PtdIns(3,4,5)P3-dependent kinase-2, respectively (for reviews, see references 14 and 21). Phosphorylation of both residues is required for maximal activation of Akt.
Akt is a multifunctional mediator of PI3-K involved in regulating several cellular functions by interacting with a variety of downstream substrates. Through various cytokines, growth factors, and chemokines, Akt can deliver antiapoptotic signals through various mechanisms. Interleukin-2-induced activation of Akt results in promotion of T-cell survival by upregulating the expression of Bcl-2 and c-MYC (1). Akt activation has also been found to block apoptosis by inhibiting caspase activity in a variety of cell types (11, 32, 50). Moreover, studies have demonstrated the ability of Akt to induce phosphorylation of the BAD protein, preventing its association with Bcl-2 and resulting in cell survival (16, 20, 49, 53). More specifically, Akt, which can be regulated by PTKs Syk, Btk, and Lyn (15, 38), can inactivate glycogen synthase kinase 3 (GSK-3) following BCR activation (28), which may promote cell survival and activate nuclear gene transcription (4, 52).
Since previous studies indicated that LMP2A provides a B-cell survival signal, we investigated the regulation of Akt by LMP2A. We demonstrate that the expression of LMP2A leads to the constitutive phosphorylation of Akt. Furthermore, we show that LMP2A mediates the constitutive phosphorylation of Akt via PI3-K and requires the recruitment of Syk and Lyn to mediate this effect. However, the ability of LMP2A to mediate an overall cell survival effect via Akt could not be detected in tissue culture or upon transfer of LMP2A-expressing cells to SCID mice.
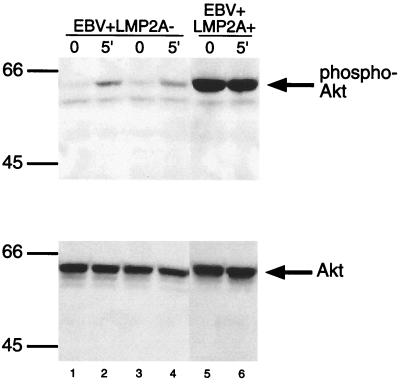
Constitutive phosphorylation of Akt in EBV+ LMP2A+ LCLs.
Previous studies had indicated that LMP2A mediates a survival signal in peripheral B cells (9). To investigate a possible role of Akt in this LMP2A-mediated effect, the phosphorylation state of Akt in cleared lysates from BCR-stimulated and unstimulated EBV+ LMP2A+ and EBV+ LMP2A− LCLs was determined using the phospho-Akt Pathway kit from Cell Signaling Technology (Fig. 1, top panel). EBV+ LMP2A− LCLs demonstrate an increase in Akt phosphorylation upon BCR activation (Fig. 1, lanes 1 to 4), as expected. Whereas in EBV+ LMP2A+ LCLs, there was a dramatic phosphorylation of Akt in the absence of BCR activation, and the amount of Akt phosphorylation was unchanged upon BCR stimulation (Fig. 1, lanes 5 and 6). Therefore, LMP2A results in the constitutive phosphorylation of Akt. Equal loading of Akt in each lane was verified by immunoblotting with anti-Akt antibodies (Fig. 1, lower panel). Whole-cell lysates from untreated and platelet-derived growth factor treated NIH 3T3 cells were used to confirm the unphosphorylated and phosphorylated forms of Akt (60-kDa protein) (data not shown).
FIG. 1.
Akt is constitutively phosphorylated in EBV+ LMP2A+ LCLs. EBV+ LMP2A− LCLs demonstrate an increase in Akt phosphorylation upon activation of the BCR, as expected (lanes 1 through 4, top panel). EBV+ LMP2A+ LCLs indicate that Akt phosphorylation is present without BCR activation and does not change upon activation of the BCR (lanes 5 and 6, top panel). The amounts of protein loaded and Akt expressed for each LCL were similar (bottom panel). EBV+ LMP2A+ cells and EBV+ LMP2A− cells (107) were left untreated or treated with anti-sIg for 5 min, lysed with 1.0% Triton X, and separated by sodium dodecyl sulfate–9% polyacrylamide gel electrophoresis (SDS–9% PAGE). Immobilon membranes were immunoblotted with phospho-specific Akt (top panel) or Akt (bottom panel) polyclonal antibodies, incubated with rabbit HRP-conjugated immunoglobulin G (IgG) antibody, and detected by enhanced chemiluminescence (ECL). Protein standards are indicated at the left in kilodaltons.
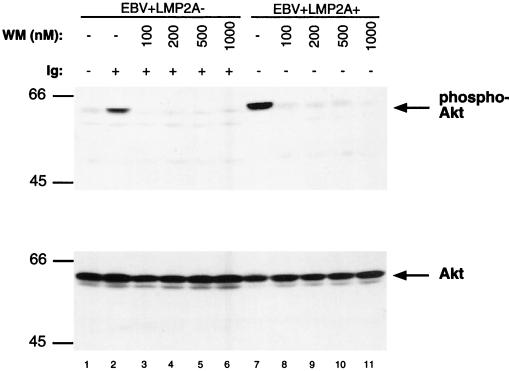
Wortmannin inhibits constitutive and induced phosphorylation of Akt in EBV+ LMP2A+ LCLs.
In B lymphocytes, induction of Akt phosphorylation is mediated through the PI3-K pathway upon activation of the BCR (28, 38). Additionally, LMP2A has been shown to induce the constitutive phosphorylation of the p85 subunit of PI3-K (46). To determine if LMP2A-mediated Akt phosphorylation is PI3-K dependent, Akt phosphorylation was analyzed in EBV+ LMP2A− LCLs and EBV+ LMP2A+ LCLs following treatment with wortmannin. Wortmannin is a microbial secondary metabolite found in a variety of fungal species and is a potent and selective inhibitor of PI3-K (54). Upon treatment of the EBV+ LMP2A+ and EBV+ LMP2A− LCLs with increasing concentrations of wortmannin (100, 200, 500, and 1,000 nM) for 5 min at 37°C, Akt phosphorylation was lost (Fig. 2, lanes 3 to 6, and 8 to 11, upper panel). The EBV+ LMP2A− LCL was used as a positive control to illustrate the inhibition of PI3-K activity by wortmannin upon activation of the BCR (Fig. 2, lanes 1 to 6, upper panel). Thus, the constitutive phosphorylation of Akt by LMP2A is mediated through the PI3-K pathway. This data would indicate that PI3-K is constitutively active in LMP2A-expressing cells. Treatment of LCLs with dimethyl sulfoxide alone did not have any effect on the level of Akt phosphorylation or cell survival (data not shown).
FIG. 2.
The effect of Wortmannin on Akt phosphorylation in LCLs. The EBV+ LMP2A+ LCL shows Akt phosphorylation without BCR stimulation (lane 7, top panel); however, treatment of the cells with increasing doses of wortmannin demonstrates a loss of Akt phosphorylation (lanes 8 through 11, top panel). The EBV+ LMP2A− LCL shows that Akt phosphorylation is increased upon BCR stimulation (lanes 1 and 2, top panel), and Akt phosphorylation was blocked upon treatment of the cells with increasing doses of wortmannin (lanes 3 through 6, top panel). Stimulation of the BCR following wortmannin treatment of the EBV+ LMP2A+ cells did not reverse the loss of Akt phosphorylation. Equivalent amounts of Akt were loaded for each lane (bottom panel). EBV+ LMP2A− LCLs and EBV+ LMP2A+ LCLs (107) were not stimulated (−), BCR stimulated (+), and/or treated with 100, 200, 500, and 1,000 nM concentrations of wortmannin (WN). The cells were lysed, separated by SDS-PAGE, immunoblotted with phospho-specific Akt (top panel) or Akt (bottom panel) antibodies, and detected by ECL. Protein standards are indicated at the left in kilodaltons.
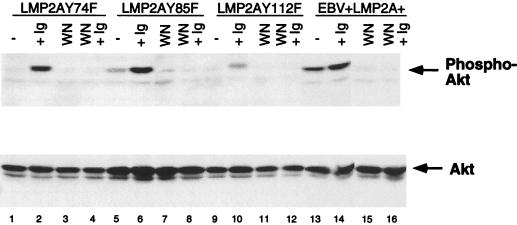
Constitutive phosphorylation of Akt is abolished in EBV+ LMP2AY74F LCLs and EBV+ LMP2AY112F LCLs.
Previous studies have shown that LMP2A function is dependent upon tyrosines at positions 74 or 85 and 112. Specifically, tyrosines 74 or 85 and 112 are required for LMP2A to bind Syk and Lyn, respectively, allowing LMP2A to regulate their activity (25, 26). Furthermore, Syk and Lyn PTKs have been found to affect Akt activity in BCR-activated B lymphocytes (38). Therefore, Akt phosphorylation in EBV+ LMP2AY74F LCLs, EBV+ LMP2AY85F LCLs, and EBV+ LMP2AY112F LCLs was analyzed (Fig. 3). EBV+ LMP2AY74F LCLs and EBV+ LMP2AY112F LCLs showed a loss of constitutive Akt phosphorylation (Fig. 3, lanes 1 and 9, upper panel), resulting in the restoration of BCR regulation of Akt phosphorylation. The loss of tyrosine 85 resulted in only a partial loss of constitutive Akt phosphorylation (Fig. 3, lane 5, upper panel). Upon activation of the BCR in these tyrosine point mutant LCLs, Akt phosphorylation levels increased as shown previously in EBV+ LMP2A− LCLs (Fig. 3, lanes 2, 6, and 10, upper panel). Interestingly, the EBV+ LMP2AY112F LCL showed a reduction of Akt phosphorylation upon BCR stimulation, which is consistently observed in LCLs containing this mutation. The significance of this finding is currently being investigated.
FIG. 3.
Constitutive phosphorylation of Akt is lost in LMP2A point mutant LCLs. The EBV+ LMP2A+ LCL shows constitutive phosphorylation of Akt (lanes 13 and 14, top panel), which is irreversibly blocked upon treatment of the cells with wortmannin, as expected (lanes 15 and 16, top panel). EBV+ LMP2AY74F LCLs, EBV+ LMP2AY85F LCLs, and EBV+ LMP2AY112F LCLs demonstrate a loss of constitutively phosphorylated Akt (lanes 1, 2, 5, 6, 9, and 10, top panel), and treating the cells with wortmannin still results in blocking of Akt phosphorylation (lanes 3, 4, 7, 8, 11, and 12, top panel). The bottom panel shows that relatively similar amounts of Akt are present within each lane. EBV+ LMP2A+ LCLs, EBV+ LMP2AY74F LCLs, EBV+ LMP2AY85F LCLs, and EBV+ LMP2AY112F LCLs (2 × 107) were not stimulated (−), BCR stimulated (+Ig), treated with wortmannin (WN), or treated with wortmannin and then BCR stimulated for 5 min (WN+Ig). The cells were lysed, divided equally, and separated by SDS–8% PAGE. Membranes were immunoblotted with phospho-specific Akt (top panel) or Akt (bottom panel) polyclonal antibodies and detected by ECL.
A small amount of Akt phosphorylation was shown for the unstimulated EBV+ LMP2AY85F LCL (Fig. 3, lane 5, upper panel). Previous studies have shown that binding of Syk to LMP2A is defective when either tyrosine 74 or tyrosine 85 is mutated. In the case of the tyrosine 85 mutant, Syk may weakly interact with tyrosine 74 of LMP2A and become partially activated, whereas any Syk interaction with LMP2A is completely deficient in the tyrosine 74 mutant. This may be an explanation for the weak level of Akt phosphorylation apparent in the unstimulated EBV+ LMP2AY85F LCL (Fig. 3, lane 5, upper panel). In addition, treatment of these cells with wortmannin alone or with wortmannin followed by BCR stimulation showed no Akt phosphorylation, demonstrating the ability of wortmannin to irreversibly block Akt phosphorylation.
Akt phosphorylation was also analyzed for EBV+ LMP2AY23F LCLs, EBV+ LMP2AY31F LCLs, EBV+ LMP2AY60F LCLs, EBV+ LMP2AY64F LCLs, and EBV+ LMP2AY101F LCLs, and all of these mutants showed a phenotype similar to that of EBV+ LMP2A+ LCLs (data not shown). Thus, tyrosines 74 or 85 and 112 play important roles in the constitutive phosphorylation of Akt by LMP2A.
Expression of LMP2A in EBV− Akata cells.
LCLs express LMP1, which has been reported to mimic CD40 receptor-mediated signaling and to upregulate Bcl-2 expression, resulting in cell survival (22, 29, 31). Furthermore, previous studies have not been able to show any difference in cell survival between EBV+ LMP2A+ LCLs and EBV+ LMP2A− LCLs (44, 63). This may be because the LMP1 signal is dominant, and any LMP2A-mediated survival signal would be concealed as a result. Thus, to investigate the mechanism by which LMP2A may mediate the survival of B lymphocytes via the Akt pathway, EBV− Akata BL cells expressing LMP2A were derived as previously described (30). The Akata cell line contains a characteristic t(8;14) translocation that results in deregulation of the proto-oncogene c-MYC (66). Although the parental Akata BL cells are EBV+, upon serial passage in culture, a small percentage of cells spontaneously lose the EBV genome (62). Comparative studies have indicated that whereas EBV+ Akata cells are modestly resistant to apoptotic stimuli and are tumorigenic, the EBV− cells have lost these characteristics (36, 60). Since LMP2A is expressed in the absence of LMP1 within the primary B-cell reservoir of latent EBV in vivo (17, 55, 68), EBV− Akata cells may provide an appropriate cell system with which to assess the effect of LMP2A within B cells in the absence of LMP1.
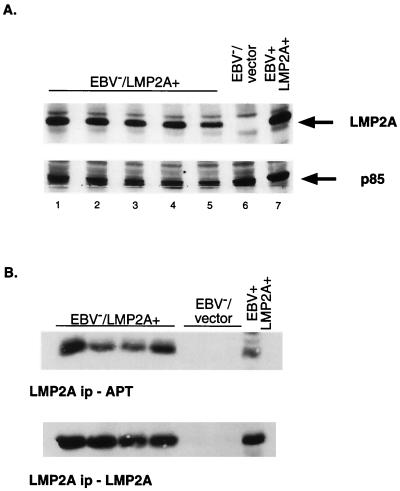
EBV− Akata cells stably expressing LMP2A or containing vector alone were generated, and LMP2A expression was verified by a Western blot (24) (Fig. 4A). LMP2A expression is evident in the EBV+ LMP2A+ LCL positive control (Fig. 4A, lane 7, upper panel), whereas no LMP2A was detected in the vector control EBV− Akata cells, as expected (Fig. 4A, lane 6, upper panel). All of the EBV−/LMP2A+ Akata cells expressed LMP2A at or near the levels of an EBV+ LMP2A+ LCL (Fig. 4A, lanes 1 through 5, upper panel). Previous studies have shown that LMP2A function is dependent upon the protein being phosphorylated (26, 46). Thus, the phosphorylation state of LMP2A in the EBV−/LMP2A+ Akata cells was determined by equally dividing LMP2A immunoprecipitations and immunoblotting with an HRP-linked antiphosphotyrosine antibody or an LMP2A antibody (Fig. 4B) as previously described (25). LMP2A was constitutively phosphorylated in all representative EBV−/LMP2A+ Akata cells, as shown in the control EBV+ LMP2A+ LCL lane. The amount of LMP2A immunoprecipitated from each cell line was approximately the same (Fig. 4B, lower panel).
FIG. 4.
LMP2A expression and phosphorylation in retrovirally infected EBV− Akata cells. (A) The EBV+ LMP2A+ LCL (lane 7) demonstrates the wild-type LMP2A size (54 kDa). EBV−/LMP2A+ Akata cells (lanes 1 through 5) show the same size band and at relatively the same level of expression. The EBV−/vector lane, as expected, does not contain a LMP2A band. Equal amounts of protein were loaded for each lane, as demonstrated by the detection of the PI3-K subunit p85. EBV−/LMP2A+ Akata cells, EBV−/vector cells, and EBV+ LMP2A+ LCLs (5 × 105) were lysed, separated by SDS–7.5% PAGE, and transferred to Immobilon. The membrane was divided in half and immunoblotted with LMP2A antibody, followed by incubation with a secondary horseradish peroxidase (HRP)-linked anti-rat IgG antibody (top panel) or with a monoclonal PI3-K antibody followed by the secondary antibody, HRP-linked anti-mouse IgG (bottom panel). (B) LMP2A shows constitutive phosphorylation in the positive control EBV+ LMP2A+ LCLs lane (top panel). In the EBV−/LMP2A+ lanes, LMP2A demonstrates the same constitutive phosphorylation as the positive control. Phosphorylated LMP2A was not detected in the EBV−/vector lane. Similar amounts of LMP2A were immunoprecipitated (ip) from each cell line (bottom panel). No LMP2A band was present in the EBV−/vector lane included as a negative control. EBV−/LMP2A+ Akata cells, EBV−/vector Akata cells, and EBV+ LMP2A+ LCLs (2 × 107) were equilibrated in serum-free media and lysed with 1% Triton X-100 lysis buffer, and the insoluble lysates were cleared by centrifugation. Cleared lysates were incubated with LMP2A monoclonal antibodies, and protein G-Sepharose beads were used to capture immune complexes. Immunoprecipitated proteins were equally divided, separated by SDS-PAGE, and immunoblotted with HRP-linked anti-phosphotyrosine antibody (top panel) or biotinylated-LMP2A antibody followed by a NeutrAvidin-HRP-conjugated antibody (bottom panel). Proteins were detected by ECL.
EBV−/LMP2A+ Akata cells constitutively phosphorylate Akt.
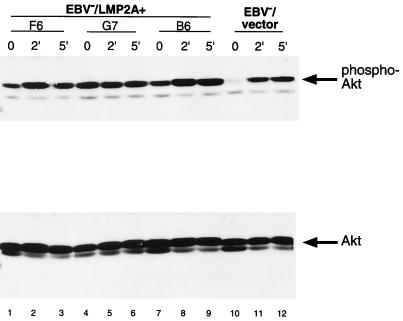
To verify that LMP2A mediates the constitutive phosphorylation of Akt in Akata cells, cell lysates were generated from EBV−/LMP2A+ and EBV−/vector control Akata cells following BCR activation and subjected to immunoblotting with phospho-specific Akt antibodies. EBV−/LMP2A+ Akata cells demonstrated a constitutive phosphorylation of Akt (Fig. 5, lanes 1, 4, and 7, upper panel) comparable to that seen in LCLs (Fig. 1). In addition, the level of Akt phosphorylation did not change upon stimulation of the BCR for 2 or 5 min (Fig. 5). This data also confirmed that it is LMP2A that regulates the phosphorylation of Akt in the absence of BCR activation in EBV-infected B lymphocytes.
FIG. 5.
Expression of LMP2A in EBV− Akata cells induces the constitutive phosphorylation of Akt. EBV−/vector Akata cells show an increase in Akt phosphorylation upon activation of the BCR for 2 or 5 min (lanes 10, 11, and 12, upper panel), whereas EBV−/LMP2A+ Akata cells demonstrate no change in Akt phosphorylation upon BCR stimulation for 0, 2, and 5 min (lanes 1 through 9, upper panel). The lower panel shows equal loading of protein and expression of Akt. Akata cells (5 × 106) expressing LMP2A or containing vector only were lysed, separated by SDS–8% PAGE, and immunoblotted with antibodies to phospho-specific Akt (top panel) or Akt (bottom panel), followed by incubation with a rabbit HRP-conjugated IgG antibody. Proteins were detected by ECL.
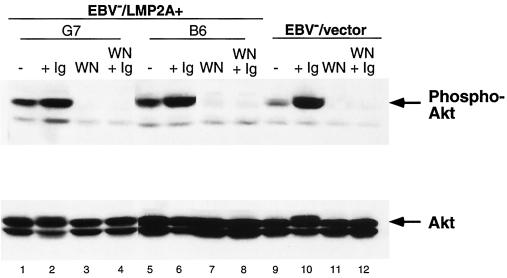
To verify that the PI3-K pathway was responsible for the LMP2A-mediated constitutive phosphorylation of Akt, EBV−/LMP2A+ and EBV−/vector Akata cells were treated with wortmannin or left untreated, and immunoblotting was performed using phospho-specific Akt antibodies. Treatment of cells with wortmannin blocked the constitutive phosphorylation of Akt in EBV−/LMP2A+ Akata cells (Fig. 6, lanes 3 and 7, upper panel). Activation of the BCR following wortmannin treatment did not restore Akt phosphorylation in those cells, confirming the irreversible effect of wortmannin in blocking PI3-K activity. The EBV−/vector Akata cell was used as a control (Fig. 6, lanes 9 to 12, upper panel). Thus, LMP2A appears to be functionally equivalent with respect to Akt phosphorylation when expressed in either an LCL or a BL background.
FIG. 6.
Wortmannin blocks the constitutive phosphorylation of Akt in LMP2A-expressing EBV− Akata cells. EBV−/vector Akata cells show an increase in Akt phosphorylation upon BCR activation (lanes 9 and 10, upper panel). Akt phosphorylation was blocked when the cells were treated with wortmannin (lane 11, upper panel). EBV−/LMP2A+ Akata cells show a loss of constitutive phosphorylation upon treatment with wortmannin (lanes 3 and 7, upper panel). Activation of the BCR following wortmannin treatment did not reverse the loss of Akt phosphorylation (lanes 4, 8, and 12, upper panel). Amounts of Akt loaded and expressed within each lane were similar (lower panel). EBV−/LMP2A+ and EBV−/vector Akata cells (2 × 107) were not treated or were treated with surface immunoglobulin antibodies, wortmannin (WN), or both for 5 min, lysed, and separated by SDS–8% PAGE (8%). Membranes were immunoblotted with anti-phospho-specific Akt (upper panel) or anti-Akt (lower panel) antibodies, incubated with HRP-conjugated rabbit IgG antibody, and detected by ECL.
LMP2A does not promote tumorigenicity or enhance survival in Akata BL cells.
In light of our observation that LMP2A expression induced phosphorylation of Akt in EBV− Akata BL cells, we next addressed whether this effect of LMP2A contributes to the enhanced survival and tumorigenic potential of EBV+ Akata cells (36, 60). Although these EBV-dependent properties have been attributed to the EBER RNAs (35), we have found that relative to EBV infection, the EBERs only partially restore tumorigenic potential to EBV− Akata cells and do so independently of an effect on cell survival (59). Since LMP2A expression has been detected in both EBV+ Akata cells (36) and a majority of BL biopsies analyzed in one study (67), it seemed likely that LMP2A, through its activation of Akt, may contribute to the tumorigenic potential of Akata BL cells by promoting cell survival. To test this, the tumorigenic potential of three EBV− Akata lines expressing LMP2A was evaluated in SCID mice as previously described (59). As shown in Table 1, expression of LMP2A in EBV− Akata cells did not increase their tumorigenic potential. Although two of the LMP2A-expressing lines induced tumors in one of five mice injected, these tumors arose very late in the assay, at 19 and 20 weeks postinjection, in contrast to the 5-week latency period for generation of tumors derived from EBV+ Akata cells. Furthermore, neither of these tumors expressed detectable LMP2A. Additionally, we observed no ability of LMP2A expression to either up-regulate Bcl-2 expression or down-regulate c-MYC levels (data not shown), as previously reported for EBV infection (36, 60). Thus, LMP2A is not responsible for these previously noted effects of EBV infection in Akata cells.
TABLE 1.
LMP2A does not enhance the tumorigenic potential of EBV-negative Akata BL cells
| Cell line | No. of mice that developed tumors/no. studied | Tumor latency period (wk) |
|---|---|---|
| EBV+ | 4/4 | 5 |
| EBV− | 0/4 | NAa |
| EBV−/LMP2A+.1 | 0/5 | NA |
| EBV−/LMP2A+.2 | 1/5b | 19 |
| EBV−/LMP2A+.3 | 1/5b | 20 |
| EBV−/Vector.1 | 0/10 | NA |
| EBV−/Vector.2 | 0/5 | NA |
LMP2A does not enhance cell survival in vitro.
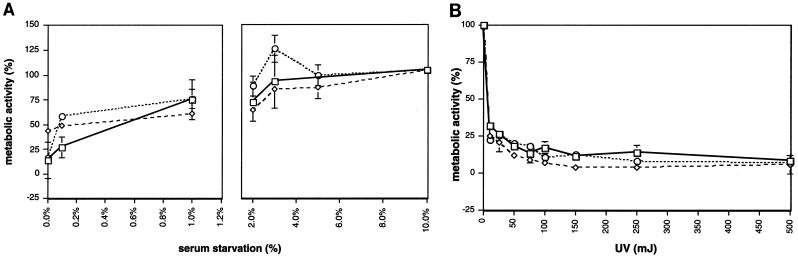
To determine whether LMP2A promotes a cell survival signal via the Akt pathway, EBV−/LMP2A+ and EBV−/vector Akata cells were subjected to apoptotic stimuli (serum deprivation or UV irradiation) and monitored as previously described (36). Previous studies have shown that the EBV− Akata cell line is susceptible to the induction of apoptosis by serum deprivation and UV treatment (36, 60). As expected, the EBV−/vector Akata cell (B7) showed a substantial decrease in the percentage of metabolic activity from 100 to less than 10 when cells were exposed to decreasing serum concentrations (10 to 0%) (Fig. 7A). The B6 and G7 EBV−/LMP2A+ Akata cell lines demonstrated a similar decrease in metabolic activity. UV treatment of EBV−/LMP2A+ Akata cells G7 and F6 also showed no difference in the percentage of metabolic activity compared to the EBV−/vector Akata clone, B7. Thus, despite the fact that LMP2A induced constitutive phosphorylation of Akt, we found no evidence that this translates to increased cell survival within BL cells.
FIG. 7.
The expression of LMP2A does not enhance cell survival. The data presented here are typical results from three independent experiments. EBV− Akata cells (2 × 107) expressing LMP2A (B6, G7, F6) or containing vector alone (B7) were grown in a range of serum concentrations (10, 5, 3, 2, 1, 0.1, and 0%) for 16 h (A) or UV treated (0 to 500 mJ) (B) to induce apoptosis. The cells were plated in triplicate for each apoptotic stimulus, and cellular proliferation was measured using the Promega Cell Titer Assay kit. The average metabolic activity (percent) and error bars (standard error of the percent mean) are shown. Symbols: □, B6 (LMP2A+ (A) or G7 (LMP2A+) (B); ◊, G7 (LMP2A+) (A) or F6 (LMP2A+) (B); ○, B7 (LMP2A−) (A and B).
LMP2A blocks normal BCR signal transduction by recruiting and modulating the activity of the Src family PTKs and the Syk PTK (8, 46–48). In addition, the 85-kDa subunit (p85) of PI3-K is constitutively phosphorylated when LMP2A is expressed in EBV-transformed LCLs grown in culture (46). In an EBV− B-lymphoma cell line, the phosphorylation of p85 results in phosphorylation and activation of the kinase subunit of PI3-K, p110 (37). These observations strongly suggest that LMP2A expression may result in the constitutive activation of PI3-K. In vivo studies have previously shown that LMP2A provides a survival signal to normal B cells (9). Since PI3-K is an important regulator of Akt, whose activation is important for cell survival, we investigated whether LMP2A may activate the PI3-K–Akt signaling pathway.
This analysis demonstrated that LMP2A mediates the constitutive phosphorylation of Akt both in EBV-transformed LCLs and LMP2A-expressing EBV− BL cells. BCR activation of EBV+ LMP2A+ LCLs and EBV−/LMP2A+ Akata cells did not significantly alter the level of Akt phosphorylation. Use of the PI3-K inhibitor wortmannin indicated that the observed Akt phosphorylation was mediated through the PI3-K pathway. In addition, tyrosines 74 or 85 and 112, which are necessary for LMP2A function in blocking BCR signaling, were critical for LMP2A to mediate the constitutive phosphorylation of Akt. However, we were unable to demonstrate any growth advantage for B cells expressing LMP2A compared to control cells.
The serine-threonine kinase Akt has emerged as a major target for PI3-K (14) and is stimulated in response to BCR activation (3, 38). Both Syk and Btk have been proven necessary for maximal Akt activation (15, 38), whereas Lyn may serve as a negative regulator of Akt activity. Phosphorylation of Akt on threonine308 and serine473 is essential for its activation. This is mediated by PDK-1 and PDK-2, respectively (2, 19). Besides PI3-K, several PTKs are required for modulating Akt function. Both Syk and Lyn have been found to play important roles in LMP2A function. LMP2A functional studies have shown that the immunoreceptor tyrosine-based motif-like tyrosines 74 and 85 in the amino-terminal domain of LMP2A are necessary for recruiting Syk PTK, resulting in a constitutively phosphorylated and activated kinase (25) which may result in the activation of Akt. In addition, Syk has also been found to associate with PI3-K through the adapter protein c-cbl (51). It is possible that LMP2A uses its association with Syk to constitutively activate the PI3-K–Akt pathway; however, how LMP2A exerts its effects on PI3-K is currently not known. Previous studies have also shown that LMP2A phosphorylation and the LMP2A-mediated block on BCR signaling are dependent on the binding of the Src family kinase Lyn to the tyrosine 112 in the amino-terminal domain of LMP2A (26). Moreover, by binding Lyn, LMP2A is able to down-modulate Lyn kinase activity and direct the ubiquitin-dependent degradation of Lyn through its association with WW domain-containing Nedd4-like ubiquitin-protein ligases (30).
Previous studies have demonstrated the role of Akt in preventing apoptosis (23, 32, 33). Akt can mediate this function by inhibiting BAD, a death-promoting member of the Bcl-2 family (49), by preventing proteolytic activation of caspase 9 (11), and by inhibiting the induction of proapoptotic gene expression through its phosphorylation of members of the Forkhead family of transcription factors (7). It is somewhat surprising, therefore, that LMP2A-induced phosphorylation of Akt in EBV− Akata BL cells had no detectable effect on cell survival. Previous work from our laboratory and others has indicated that Akata cells that have lost the EBV genome are highly sensitive, relative to their EBV+ counterparts, to apoptotic stimuli, such as serum deprivation, cycloheximide, and hypoxia (36, 60). This has been attributed to the ability of EBV to modestly up-regulate Bcl-2 expression in these cells and to induce a repression of c-MYC levels under growth-limiting conditions. Presumably this ability to inhibit apoptosis contributes to the EBV-dependent tumorigenic potential of Akata cells (36, 60). Indeed, we have shown recently that enforced expression of Bcl-2 in EBV− Akata cells does promote BL cell survival and as a likely result enhances tumorigenic potential (Ruf et al., unpublished observation). Thus, the failure of LMP2A to enhance the survival of EBV− Akata cells is not due to an inherent inability of these cells to respond to antiapoptotic factors. The failure of LMP2A to enhance survival of EBV− Akata cells in the face of proapoptotic stimuli or to increase their tumorigenic potential suggests that if LMP2A-induced phosphorylation of Akt does provide a survival signal, this signal alone is not sufficient to protect Akata BL cells from apoptosis. This is consistent with the earlier observation that BCR-mediated activation of Akt is not sufficient to promote cell survival in the EBV− BL cell line RAMOS (28). By contrast, recent work has indicated that LMP2A is able to promote cell survival in epithelial cells (61a). However, transgenic mice in which LMP2A is expressed in the basal layer of the epithelia do not demonstrate any cell survival or tumorigenic phenotype (40). Thus, any survival signal induced by LMP2A in BL cells is unlikely to be sufficient to block apoptosis induced as a consequence of the deregulation of c-MYC expression.
Another major downstream target of Akt is GSK-3, a constitutively active serine-threonine kinase whose activity is inhibited by Akt. Studies have primarily focused on insulin receptor-mediated signaling activation of Akt resulting in a reduction in GSK-3 activity (69). One of the major cellular processes regulated by GSK-3 is the subcellular localization of the NF-ATc transcription factor. GSK-3 phosphorylates NF-ATc, resulting in its rapid export from the nucleus (4). Recently, it has been demonstrated that activation of the BCR results in the activation of the PI3-K–Akt–GSK-3 pathway (28). Induction of the BCR also causes an increase in intracellular Ca2+ concentrations, leading to the activation of the Ca2+-dependent phosphatase calcineurin, which dephosphorylates NF-ATc (56). LMP2A blocks calcium mobilization upon BCR activation (48). Thus, LMP2A may use the PI3-K–Akt pathway to block the phosphorylation of NF-ATc by GSK-3, resulting in transcriptional activation. This would allow LMP2A to control B-cell gene transcription directly while preventing the activation of Ca2+-dependent signaling pathways.
In summary, we have provided evidence that LMP2A promotes the constitutive phosphorylation of Akt via the PI3-K pathway. In addition, the activation of the PI3-K–Akt pathway by LMP2A does not promote survival of BL cells grown in culture or models of BL tumorigenesis in vivo. Since the PI3-K–Akt–GSK-3 pathway can regulate a variety of cellular processes, in addition to cell survival, such as cell cycle progression and transcription, LMP2A may be using this signal to regulate different aspects of B-cell signal transduction. Future studies which delineate the mechanism by which LMP2A is able to take advantage of normal B cell processes should help define the strategy EBV uses to persist in the human host.
Acknowledgments
We thank the members of the Longnecker and Spear laboratories for providing invaluable advice. A special thanks goes to Luz Longan for producing the LMP2A retrovirus.
R.S. was supported by a minority research supplement from the National Institutes of Health. I.K.R. was supported by PHS grant T32-AI07372. J.S. is supported by USPHS grant CA73544 and Cancer Center Support (CORE) grant CA21765 from the National Cancer Institute and by the American Lebanese-Syrian Associated Charities (ALSAC). R.L. is a scholar of the Leukemia and Lymphoma Society of America. R.L. is supported by Public Health Service grants CA62234 and CA73507 from the National Cancer Institute and DE13127 from the National Institute of Dental and Craniofacial Research.
REFERENCES
- 1.Ahmed N N, Grimes H L, Bellacosa A, Chan T O, Tsichlis P N. Transduction of interleukin-2 antiapoptotic and proliferative signals via Akt protein kinase. Proc Natl Acad Sci USA. 1997;94:3627–3632. doi: 10.1073/pnas.94.8.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alessi D R, James S R, Downes C P, Holmes A B, Gaffney P R, Reese C B, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase B alpha. Curr Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 3.Astoul E, Watton S, Cantrell D. The dynamics of protein kinase B regulation during B cell antigen receptor engagement. J Cell Biol. 1999;145:1511–1520. doi: 10.1083/jcb.145.7.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beals C R, Sheridan C M, Turck C W, Gardner P, Crabtree G R. Nuclear export of NF-ATc enhanced by glycogen synthase kinase-3. Science. 1997;275:1930–1934. doi: 10.1126/science.275.5308.1930. [DOI] [PubMed] [Google Scholar]
- 5.Bellacosa A, de Feo D, Godwin A K, Bell D W, Cheng J Q, Altomare D A, Wan M, Dubeau L, Scambia G, Masciullo V. Molecular alterations of the AKT2 oncogene in ovarian and breast carcinomas. Int J Cancer. 1995;64:280–285. doi: 10.1002/ijc.2910640412. [DOI] [PubMed] [Google Scholar]
- 6.Bellacosa A, Testa J R, Staal S P, Tsichlis P N. A retroviral oncogene, akt, encoding a serine-threonine kinase containing an SH2-like region. Science. 1991;254:274–277. doi: 10.1126/science.254.5029.274. [DOI] [PubMed] [Google Scholar]
- 7.Brunet A, Bonni A, Zigmond M J, Lin M Z, Juo P, Hu L S, Anderson M J, Arden K C, Blenis J, Greenberg M E. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 8.Burkhardt A L, Bolen J B, Kieff E, Longnecker R. An Epstein-Barr virus transformation-associated membrane protein interacts with src family tyrosine kinases. J Virol. 1992;66:5161–5167. doi: 10.1128/jvi.66.8.5161-5167.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caldwell R G, Wilson J B, Anderson S J, Longnecker R. Epstein-Barr virus LMP2A drives B cell development and survival in the absence of normal B cell receptor signals. Immunity. 1998;9:405–411. doi: 10.1016/s1074-7613(00)80623-8. [DOI] [PubMed] [Google Scholar]
- 10.Cambier J C. New nomenclature for the Reth motif (or ARH1/TAM/ARAM/YXXL) Immunol Today. 1995;16:110. doi: 10.1016/0167-5699(95)80105-7. [DOI] [PubMed] [Google Scholar]
- 11.Cardone M H, Roy N, Stennicke H R, Salvesen G S, Franke T F, Stanbridge E, Frisch S, Reed J C. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 12.Chen F, Zou J Z, di Renzo L, Winberg G, Hu L F, Klein E, Klein G, Ernberg I. A subpopulation of normal B cells latently infected with Epstein-Barr virus resembles Burkitt lymphoma cells in expressing EBNA-1 but not EBNA-2 or LMP1. J Virol. 1995;69:3752–3758. doi: 10.1128/jvi.69.6.3752-3758.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen H, Smith P, Ambinder R F, Hayward S D. Expression of Epstein-Barr virus BamHI-A rightward transcripts in latently infected B cells from peripheral blood. Blood. 1999;93:3026–3032. [PubMed] [Google Scholar]
- 14.Coffer P J, Jin J, Woodgett J R. Protein kinase B (c-Akt): a multifunctional mediator of phosphatidylinositol 3-kinase activation. Biochem J. 1998;335:1–13. doi: 10.1042/bj3350001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Craxton A, Jiang A, Kurosaki T, Clark E A. Syk and Bruton's tyrosine kinase are required for B cell antigen receptor-mediated activation of the kinase Akt. J Biol Chem. 1999;274:30644–30650. doi: 10.1074/jbc.274.43.30644. [DOI] [PubMed] [Google Scholar]
- 16.Datta S R, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg M E. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 17.Decker L L, Klaman L D, Thorley-Lawson D A. Detection of the latent form of Epstein-Barr virus DNA in the peripheral blood of healthy individuals. J Virol. 1996;70:3286–3289. doi: 10.1128/jvi.70.5.3286-3289.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeFranco A L. The complexity of signaling pathways activated by the BCR. Curr Opin Immunol. 1997;9:296–308. doi: 10.1016/s0952-7915(97)80074-x. [DOI] [PubMed] [Google Scholar]
- 19.Delcommenne M, Tan C, Gray V, Rue L, Woodgett J, Dedhar S. Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/AKT by the integrin-linked kinase. Proc Natl Acad Sci USA. 1998;95:11211–11216. doi: 10.1073/pnas.95.19.11211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science. 1997;278:687–689. doi: 10.1126/science.278.5338.687. [DOI] [PubMed] [Google Scholar]
- 21.Downward J. Mechanisms and consequences of activation of protein kinase B/Akt. Curr Opin Cell Biol. 1998;10:262–267. doi: 10.1016/s0955-0674(98)80149-x. [DOI] [PubMed] [Google Scholar]
- 22.Eliopoulos A G, Dawson C W, Mosialos G, Floettmann J E, Rowe M, Armitage R J, Dawson J, Zapata J M, Kerr D J, Wakelam M J, Reed J C, Kieff E, Young L S. CD40-induced growth inhibition in epithelial cells is mimicked by Epstein-Barr virus-encoded LMP1: involvement of TRAF3 as a common mediator. Oncogene. 1996;13:2243–2254. [PubMed] [Google Scholar]
- 23.Eves E M, Xiong W, Bellacosa A, Kennedy S G, Tsichlis P N, Rosner M R, Hay N. Akt, a target of phosphatidylinositol 3-kinase, inhibits apoptosis in a differentiating neuronal cell line. Mol Cell Biol. 1998;18:2143–2152. doi: 10.1128/mcb.18.4.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fruehling S, Lee S, Herrold R, Frech B, Laux G, Kremmer E, Grässer F A, Longnecker R. Identification of latent membrane protein 2A (LMP2A) domains essential for the LMP2A dominant-negative effect on B-lymphocyte surface immunoglobulin signal transduction. J Virol. 1996;70:6216–6226. doi: 10.1128/jvi.70.9.6216-6226.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fruehling S, Longnecker R. The immunoreceptor tyrosine-based activation motif of Epstein-Barr virus LMP2A is essential for blocking BCR-mediated signal transduction. Virology. 1997;235:241–251. doi: 10.1006/viro.1997.8690. [DOI] [PubMed] [Google Scholar]
- 26.Fruehling S, Swart R, Dolwick K M, Kremmer E, Longnecker R. Tyrosine 112 of latent membrane protein 2A is essential for protein tyrosine kinase loading and regulation of Epstein-Barr virus latency. J Virol. 1998;72:7796–7806. doi: 10.1128/jvi.72.10.7796-7806.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gold M R, Chan V W, Turck C W, DeFranco A L. Membrane Ig cross-linking regulates phosphatidylinositol 3-kinase in B lymphocytes. J Immunol. 1992;148:2012–2022. [PubMed] [Google Scholar]
- 28.Gold M R, Scheid M P, Santos L, Dang-Lawson M, Roth R A, Matsuuchi L, Duronio V, Krebs D L. The B cell antigen receptor activates the Akt (protein kinase B)/glycogen synthase kinase-3 signaling pathway via phosphatidylinositol 3-kinase. J Immunol. 1999;163:1894–1905. [PubMed] [Google Scholar]
- 29.Henderson S, Rowe M, Gregory C, Croom-Carter D, Wang F, Longnecker R, Kieff E, Rickinson A. Induction of bcl-2 expression in Epstein-Barr virus latent membrane protein 1 protects infected B cells from programmed cell death. Cell. 1991;65:1107–1115. doi: 10.1016/0092-8674(91)90007-l. [DOI] [PubMed] [Google Scholar]
- 30.Ikeda M, Ikeda A, Longan L C, Longnecker R. The Epstein-Barr virus (EBV) latent membrane protein 2A (LMP2A) PY motif recruits WW domain-containing ubiquitin-protein ligases. Virology. 2000;268:178–191. doi: 10.1006/viro.1999.0166. [DOI] [PubMed] [Google Scholar]
- 31.Izumi K M, Kieff E D. The Epstein-Barr virus oncogene product latent membrane protein 1 engages the tumor necrosis factor receptor-associated death domain protein to mediate B lymphocyte growth transformation and activate NF-κB. Proc Natl Acad Sci USA. 1997;94:12592–12597. doi: 10.1073/pnas.94.23.12592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelley T W, Graham M M, Doseff A I, Pomerantz R W, Lau S M, Ostrowski M C, Franke T F, Marsh C B. Macrophage colony-stimulating factor promotes cell survival through Akt/protein kinase B. J Biol Chem. 1999;274:26393–26398. doi: 10.1074/jbc.274.37.26393. [DOI] [PubMed] [Google Scholar]
- 33.Kennedy S G, Kandel E S, Cross T K, Hay N. Akt/protein kinase B inhibits cell death by preventing the release of cytochrome c from mitochondria. Mol Cell Biol. 1999;19:5800–5810. doi: 10.1128/mcb.19.8.5800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kieff E. Epstein-Barr virus and its replication. In: Fields B N, Knipe D M, Howley P M, et al., editors. Fundamental virology. New York, N.Y: Raven Press; 1996. pp. 1109–1162. [Google Scholar]
- 35.Komano J, Maruo S, Kurozumi K, Oda T, Takada K. Oncogenic role of Epstein-Barr virus-encoded RNAs in Burkitt's lymphoma cell line Akata. J Virol. 1999;73:9827–9831. doi: 10.1128/jvi.73.12.9827-9831.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Komano J, Sugiura M, Takada K. Epstein-Barr virus contributes to the malignant phenotype and to apoptosis resistance in Burkitt's lymphoma cell line Akata. J Virol. 1998;72:9150–9156. doi: 10.1128/jvi.72.11.9150-9156.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuwahara K, Kawai T, Mitsuyoshi S, Matsuo Y, Kikuchi H, Imajoh-Ohmi S, Hashimoto E, Inui S, Cooper M D, Sakaguchi N. Cross-linking of B cell antigen receptor-related structure of pre-B cell lines induces tyrosine phosphorylation of p85 and p110 subunits and activation of phosphatidylinositol 3-kinase. Int Immunol. 1996;8:1273–1285. doi: 10.1093/intimm/8.8.1273. [DOI] [PubMed] [Google Scholar]
- 38.Li H L, Davis W W, Whiteman E L, Birnbaum M J, Pure E. The tyrosine kinases Syk and Lyn exert opposing effects on the activation of protein kinase Akt/PKB in B lymphocytes. Proc Natl Acad Sci USA. 1999;96:6890–6895. doi: 10.1073/pnas.96.12.6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liebowitz D. Epstein-Barr pathogenesis. In: McCance D, editor. Human tumor viruses. Washington, D.C.: American Society for Microbiology; 1998. pp. 173–198. [Google Scholar]
- 40.Longan L, Longnecker R. Epstein-Barr virus latent membrane protein 2A has no growth-altering effects when expressed in differentiating epithelia. J Gen Virol. 2000;81:2245–2252. doi: 10.1099/0022-1317-81-9-2245. [DOI] [PubMed] [Google Scholar]
- 41.Longnecker R. Molecular biology of Epstein-Barr virus. In: McCance D, editor. Human tumor viruses. Washington, D.C.: American Society for Microbiology; 1998. pp. 133–171. [Google Scholar]
- 42.Longnecker R, Druker B, Roberts T M, Kieff E. An Epstein-Barr virus protein associated with cell growth transformation interacts with a tyrosine kinase. J Virol. 1991;65:3681–3692. doi: 10.1128/jvi.65.7.3681-3692.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Longnecker R, Kieff E. A second Epstein-Barr virus membrane protein (LMP2) is expressed in latent infection and colocalizes with LMP1. J Virol. 1990;64:2319–2326. doi: 10.1128/jvi.64.5.2319-2326.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Longnecker R, Miller C L, Tomkinson B, Miao X Q, Kieff E. Deletion of DNA encoding the first five transmembrane domains of Epstein-Barr virus latent membrane proteins 2A and 2B. J Virol. 1993;67:5068–5074. doi: 10.1128/jvi.67.8.5068-5074.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mayer B J, Gupta R. Functions of SH2 and SH3 domains. Curr Top Microbiol Immunol. 1998;228:1–22. doi: 10.1007/978-3-642-80481-6_1. [DOI] [PubMed] [Google Scholar]
- 46.Miller C L, Burkhardt A L, Lee J H, Stealey B, Longnecker R, Bolen J B, Kieff E. Integral membrane protein 2 of Epstein-Barr virus regulates reactivation from latency through dominant negative effects on protein-tyrosine kinases. Immunity. 1995;2:155–166. doi: 10.1016/s1074-7613(95)80040-9. [DOI] [PubMed] [Google Scholar]
- 47.Miller C L, Lee J H, Kieff E, Longnecker R. An integral membrane protein (LMP2) blocks reactivation of Epstein-Barr virus from latency following surface immunoglobulin crosslinking. Proc Natl Acad Sci USA. 1994;91:772–776. doi: 10.1073/pnas.91.2.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miller C L, Longnecker R, Kieff E. Epstein-Barr virus latent membrane protein 2A blocks calcium mobilization in B lymphocytes. J Virol. 1993;67:3087–3094. doi: 10.1128/jvi.67.6.3087-3094.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mok C L, Gil-Gomez G, Williams O, Coles M, Taga S, Tolaini M, Norton T, Kioussis D, Brady H J. Bad can act as a key regulator of T cell apoptosis and T cell development. J Exp Med. 1999;189:575–586. doi: 10.1084/jem.189.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Owada K, Sanjo N, Kobayashi T, Mizusawa H, Muramatsu H, Muramatsu T, Michikawa M. Midkine inhibits caspase-dependent apoptosis via the activation of mitogen-activated protein kinase and phosphatidylinositol 3-kinase in cultured neurons. J Neurochem. 1999;73:2084–2092. [PubMed] [Google Scholar]
- 51.Panchamoorthy G, Fukazawa T, Miyake S, Soltoff S, Reedquist K, Druker B, Shoelson S, Cantley L, Band H. p120cbl is a major substrate of tyrosine phosphorylation upon B cell antigen receptor stimulation and interacts in vivo with Fyn and Syk tyrosine kinases, Grb2 and Shc adaptors, and the p85 subunit of phosphatidylinositol 3-kinase. J Biol Chem. 1996;271:3187–3194. doi: 10.1074/jbc.271.6.3187. [DOI] [PubMed] [Google Scholar]
- 52.Pap M, Cooper G M. Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-kinase/Akt cell survival pathway. J Biol Chem. 1998;273:19929–19932. doi: 10.1074/jbc.273.32.19929. [DOI] [PubMed] [Google Scholar]
- 53.Pastorino J G, Tafani M, Farber J L. Tumor necrosis factor induces phosphorylation and translocation of BAD through a phosphatidylinositide-3-OH kinase-dependent pathway. J Biol Chem. 1999;274:19411–19416. doi: 10.1074/jbc.274.27.19411. [DOI] [PubMed] [Google Scholar]
- 54.Powis G, Bonjouklian R, Berggren M M, Gallegos A, Abraham R, Ashendel C, Zalkow L, Matter W F, Dodge J, Grindey G. Wortmannin, a potent and selective inhibitor of phosphatidylinositol-3-kinase. Cancer Res. 1994;54:2419–2423. [PubMed] [Google Scholar]
- 55.Qu L, Rowe D. Epstein-Barr virus latent gene expression in uncultured peripheral blood lymphocytes. J Virol. 1992;66:3715–3724. doi: 10.1128/jvi.66.6.3715-3724.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rao A, Luo C, Hogan P G. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- 57.Reth M. Antigen receptor tail clue. Nature. 1989;338:383–384. [PubMed] [Google Scholar]
- 58.Rickinson A, Kieff E. Epstein-Barr virus. In: Fields B N, Howley P M, et al., editors. Virology. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2397–2446. [Google Scholar]
- 59.Ruf I K, Rhyne P W, Yang C, Cleveland J L, Sample J T. Epstein-Barr virus small RNAs potentiate tumorigenicity of Burkitt lymphoma cells independently of an effect on apoptosis. J Virol. 2000;74:10223–10228. doi: 10.1128/jvi.74.21.10223-10228.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ruf I K, Rhyne P W, Yang H, Borza C M, Hutt-Fletcher L M, Cleveland J L, Sample J T. Epstein-Barr virus regulates c-MYC, apoptosis, and tumorigenicity in Burkitt lymphoma. Mol Cell Biol. 1999;19:1651–1660. doi: 10.1128/mcb.19.3.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sample J, Liebowitz D, Kieff E. Two related Epstein-Barr virus membrane proteins are encoded by separate genes. J Virol. 1989;63:933–937. doi: 10.1128/jvi.63.2.933-937.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61a.Scholle F, Bendt K M, Raab-Traub N. Epstein-Barr virus LMP2A transforms epithelial cells, inhibits cell differentiation, and activates Akt. J Virol. 2000;74:10681–10689. doi: 10.1128/jvi.74.22.10681-10689.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shimizu N, Tanabe-Tochikura A, Kuroiwa Y, Takada K. Isolation of Epstein-Barr virus (EBV)-negative cell clones from the EBV-positive Burkitt's lymphoma (BL) line Akata: malignant phenotypes of BL cells are dependent on EBV. J Virol. 1994;68:6069–6073. doi: 10.1128/jvi.68.9.6069-6073.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Speck P, Kline K A, Cheresh P, Longnecker R. Epstein-Barr virus lacking latent membrane protein 2 immortalizes B cells with efficiency indistinguishable from that of wild-type virus. J Gen Virol. 1999;80:2193–2203. doi: 10.1099/0022-1317-80-8-2193. [DOI] [PubMed] [Google Scholar]
- 64.Staal S P. Molecular cloning of the akt oncogene and its human homologues AKT1 and AKT2: amplification of AKT1 in a primary human gastric adenocarcinoma. Proc Natl Acad Sci USA. 1987;84:5034–5037. doi: 10.1073/pnas.84.14.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Staal S P, Hartley J W. Thymic lymphoma induction by the AKT8 murine retrovirus. J Exp Med. 1988;167:1259–1264. doi: 10.1084/jem.167.3.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Takada K, Horinouchi K, Ono Y, Aya T, Osato T, Takahashi M, Hayasaka S. An Epstein-Barr virus-producer line Akata: establishment of the cell line and analysis of viral DNA. Virus Genes. 1991;5:147–156. doi: 10.1007/BF00571929. [DOI] [PubMed] [Google Scholar]
- 67.Tao Q, Robertson K D, Manns A, Hildesheim A, Ambinder R F. Epstein-Barr virus (EBV) in endemic Burkitt's lymphoma: molecular analysis of primary tumor tissue. Blood. 1998;91:1373–1381. . (Erratum, 91:3091.) [PubMed] [Google Scholar]
- 68.Tierney R J, Steven N, Young L S, Rickinson A B. Epstein-Barr virus latency in blood mononuclear cells: analysis of viral gene transcription during primary infection and in the carrier state. J Virol. 1994;68:7374–7385. doi: 10.1128/jvi.68.11.7374-7385.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van Weeren P C, de Bruyn K M, de Vries-Smits A M, van Lint J, Burgering B M. Essential role for protein kinase B (PKB) in insulin-induced glycogen synthase kinase 3 inactivation. Characterization of dominant-negative mutant of PKB. J Biol Chem. 1998;273:13150–13156. doi: 10.1074/jbc.273.21.13150. [DOI] [PubMed] [Google Scholar]