PLAC-24 Is a Cytoplasmic Dynein-Binding Protein That Is Recruited to Sites of Cell-Cell Contact (original) (raw)
Abstract
We screened for polypeptides that interact specifically with dynein and identified a novel 24-kDa protein (PLAC-24) that binds directly to dynein intermediate chain (DIC). PLAC-24 is not a dynactin subunit, and the binding of PLAC-24 to the dynein intermediate chain is independent of the association between dynein and dynactin. Immunocytochemistry using PLAC-24–specific polyclonal antibodies revealed a punctate perinuclear distribution of the polypeptide in fibroblasts and isolated epithelial cells. However, as epithelial cells in culture make contact with adjacent cells, PLAC-24 is specifically recruited to the cortex at sites of contact, where the protein colocalizes with components of the adherens junction. Disruption of the cellular cytoskeleton with latrunculin or nocodazole indicates that the localization of PLAC-24 to the cortex is dependent on intact actin filaments but not on microtubules. Overexpression of β-catenin also leads to a loss of PLAC-24 from sites of cell-cell contact. On the basis of these data and the recent observation that cytoplasmic dynein is also localized to sites of cell-cell contact in epithelial cells, we propose that PLAC-24 is part of a multiprotein complex localized to sites of intercellular contact that may function to tether microtubule plus ends to the actin-rich cellular cortex.
INTRODUCTION
The microtubule motor cytoplasmic dynein provides motive force for critical cellular functions in both interphase and dividing cells. In interphase, dynein moves vesicular cargo from the cell periphery toward the cell center. For example, the retrograde transport of organelles along the axon and the trafficking of vesicles from endoplasmic reticulum to Golgi are dynein-dependent processes (reviewed inKarki and Holzbaur, 1999). In mitosis, dynein is required for the assembly of the bipolar spindle and is also involved in mediating the attachment of microtubules to kinetochores. Furthermore, dynein is required for the rotation of spindles during polarized cell division (Karki and Holzbaur, 1999).
The ability of a single motor to interact specifically with such diverse cargo as a vesicle and a kinetochore is not well understood. One focus of investigation has been dynactin. Dynactin is a multisubunit complex that is a required activator for many of the functions of dynein (reviewed in Holleran et al., 1998). Although dynactin may function to increase the efficiency of the dynein motor by enhancing processivity (Waterman-Storer et al., 1995; King and Schroer, 2000), dynactin has also been shown to link dynein both to vesicles (Steffen et al., 1997;Waterman-Storer et al., 1997) and to the kinetochore (Starr et al., 1998).
Mutational analyses in yeast and in Drosophila have indicated that disruption of either dynein or dynactin function gives similar phenotypes. However, it is not clear whether dynactin is a required activator for all dynein functions. Several studies have suggested that dynactin is not always necessary to link dynein to its cargo. For example, pericentrin has been shown to bind directly to the light intermediate chain of cytoplasmic dynein (Purohit et al., 1999), and the transport of rhodopsin-bearing vesicles along microtubules in photoreceptor cells has been shown to be mediated by the direct binding of rhodopsin to the dynein light chain Tctex-1 (Tai_et al._, 1999). Similarly, the 8-kDa dynein light chain has been found to bind to a 3′-untranslated sequence of mRNA encoding parathyroid hormone (Epstein et al., 2000) and to the protein Swallow, which binds in turn to bicoid RNA (Schnorrer et al., 2000), raising the possibility that dynein actively transports specific mRNAs in the cytoplasm.
Studies on the kinesin superfamily of microtubule motors have established a paradigm in which a common motor domain is fused to a wide variety of head or tail domains that specify cargo linkage. For dynein, it appears that functional diversity is obtained through the interaction of distinct dynein subunits with different cellular proteins. Therefore, we hypothesized that the identification and characterization of additional dynein-binding proteins may provide further insight into the specific cellular cargos that are transported by dynein and the mechanisms that govern the targeting and regulation of dynein within the cell. We sought to identify dynein-binding proteins by means of a dynein IC (DIC) affinity column. The intermediate chain of cytoplasmic dynein is located at the base of the motor, at the presumed site of cargo attachment. Using this approach, we have previously identified casein kinase II as a dynein-binding kinase capable of phosphorylating both recombinant and native cytoplasmic dynein in vitro (Karki et al., 1997).
In this study, we screened for proteins from rat brain cytosol that interact with the cytoplasmic dynein intermediate chain and isolated a novel 24-kDa polypeptide. This polypeptide, which we now refer to as PLAC-24 (a 24-kDa _P_rotein that _L_ocalizes_A_t Cell-cell _C_ontacts), binds directly to dynein. PLAC-24 is not a dynactin subunit, and the binding of PLAC-24 to dynein is independent of the association between dynein and dynactin. Immunocytochemistry using anti-PLAC-24 antibodies demonstrates that this protein has a punctate perinuclear distribution in fibroblasts and in isolated epithelial cells. However, when epithelial cells in culture make contact with adjacent cells, PLAC-24 becomes localized to the developing adherens junction. Localization of PLAC-24 to adherens junctions is dependent on an intact actin cytoskeleton but not on the microtubule cytoskeleton. Overexpression of β-catenin leads to the loss of PLAC-24 from sites of cell-cell contact. On the basis of these observations and the recent localization of dynein to cellular adherens junctions, we propose that PLAC-24 and dynein may be components of a multiprotein complex localizing to intercellular contact sites. This complex may act to tether microtubule plus ends to the actin-rich cortex and therefore may provide a link between the cellular microtubule and actin cytoskeletons.
MATERIALS AND METHODS
Cloning and Molecular Characterization of PLAC-24 cDNA
Rat brain cytosol was fractionated on a recombinant DIC affinity column as previously described (Karki et al., 1997). Proteins that bound to the matrix were eluted with 0.5 M NaCl and resolved by SDS-PAGE. A prominent band of 24 kDa was excised and subjected to in situ proteolysis and microsequencing. A 14-mer peptide sequence was obtained: ENAYDLEANLAVLK and was used to search the NCBI database in a BLAST search (Altschul et al., 1990). A human expressed sequence tag (EST) (Adams et al., 1993) prepared from parathyroid mRNA source (clone 320842) was identified by homology, and the corresponding cDNA was obtained from Incyte Genomics (Palo Alto, CA). The EST clone was completely sequenced from both ends and was found to contain the sequenced peptide. The cDNA insert was labeled with the Prime-It II Random Primer Kit (Stratagene, La Jolla, CA) and used to probe a human multiple tissue Northern blot (Clontech, Palo Alto, CA).
Antibodies and Western Blotting
The coding region of PLAC-24 was subcloned into the pET15b expression vector (Novagen, Madison, WI) and expressed in E. coli as a fusion protein with an amino-terminal histidine tag. Recombinant protein was purified on a Ni2+affinity column and used as an antigen to immunize both rabbits and rats. The resulting antisera, rabbit polyclonal antibody UP1076 and rat polyclonal antibody UP-R47, were affinity-purified on a column of recombinant PLAC-24 bound to activated CH-Sepharose 4B beads (Amersham Pharmacia Biotech, Piscataway, NJ). An additional antibody, UP1447, was generated to the peptide sequence CRYNPENLATLERYVETQAKEC, which corresponds to residues 20–39 of the predicted PLAC-24 sequence, flanked by N-terminal and C-terminal cysteine residues, and was affinity-purified against full-length recombinant PLAC-24. Affinity-purified polyclonal antibodies to p150Glued, Arp1, and the DIC have been described previously (Holleran et al., 1996; Tokito et al., 1996; Ligon et al., 2001). A rabbit polyclonal antibody, UP1097, was generated to full-length recombinant human dynamitin and was affinity-purified before use as previously described (Tokito_et al._, 1996). Antibody to dynein heavy chain was provided by R. Vallee (University of Massachusetts Medical School, Worcester, MA), antibody to Tctex-1 was provided by S. King (University of Connecticut Health Center, Farmington, CT), and antibody to rp3 was provided by C. H. Sung (Cornell University Medical College, New York, NY). Antibodies to DIC (monoclonal from Chemicon, Temecula, CA, and Sigma-Aldrich, St. Louis, MO), β-catenin (monoclonal from BD Biosciences Pharmigen, San Diego, CA), E-cadherin (monoclonal from Zymed, South San Francisco, CA), tubulin (monoclonals from Sigma-Aldrich, St. Louis, MO and Serotec, Raleigh, NC), actin (monoclonal from Chemicon), and myosin II (monoclonal from Chemicon) were purchased commercially. Alexa-350–, Alexa-488–, Alexa-594–, FITC-, and Texas Red-conjugated secondary antibodies were purchased from Molecular Probes (Eugene, OR) or Jackson Immunoresearch (West Grove, PA).
To examine the tissue distribution of PLAC-24, protein extracts from a range of human tissues were purchased from Clontech and resolved by SDS-PAGE using a 12% gel, then transferred to Immobilon-P (Millipore, Bedford, MA) and probed with affinity-purified anti-PLAC-24 antibody. Approximately 100 μg of total protein was loaded per gel lane.
Sucrose Gradient Fractionation, Gel Filtration, and Immunoprecipitations
Cytosol was prepared from either rat brain or PtK2 cells, as noted, by homogenization in an equal volume of PHEM buffer (50 mM Na-PIPES, 50 mM Na-HEPES, 1 mM EDTA, 2 mM MgCl2, pH 6.9) supplemented with the protease inhibitors phenylmethylsulfonyl fluoride, leupeptin, TAME, and pepstatin-A as previously described (Karki et al., 1997). Dithiothreitol at 1 mM was included for experiments involving sucrose gradient fractionation, but not for immunoprecipitation experiments. The cytosolic fraction was clarified by centrifugation at 100,000 × g for 1 h. A 500-μl aliquot of cytosol was resolved on a 5–25% linear sucrose gradient (in PHEM with dithiothreitol) by ultracentrifugation, and the resulting fractions were analyzed by SDS-PAGE and Western blotting using antibodies to p150Glued, DIC, and PLAC-24. Gradients were calibrated using the standards glutamate dehydrogenase (26.6 S), thyroglobulin (19.4 S), catalase (11.3 S), aldolase (7.3 S), tubulin (6S), and BSA (4.5 S). Immunoprecipitations were performed as described previously (Tokito et al., 1996) from brain or PtK2 cytosol using an affinity-purified rabbit polyclonal anti-p150Glued antibody, an anti-DIC monoclonal antibody (Chemicon), or an affinity-purified antibody to PLAC-24. The resulting immunoprecipitates were resolved by SDS-PAGE, transferred to Immobilon-P, and probed with antibodies to PLAC-24, DIC, or the p150Glued, Arp1, and dynamitin, subunits of dynactin as noted.
Affinity Chromatography
Affinity matrices were prepared by cross-linking recombinant PLAC-24, DIC, or BSA, as noted, to activated CH-Sepharose 4B (Amersham Pharmacia Biotech) beads at 2 mg/ml ligand. Rat brain cytosol and ATP extract of rat brain microtubules were prepared as previously described (Karki et al., 1997). Either cytosol or purified proteins were loaded onto affinity columns as noted, and the columns were washed extensively with buffer to remove loosely bound proteins. Specifically retained proteins were eluted with either 0.5 or 1 M NaCl as noted, and the eluates were resolved by SDS-PAGE, followed where noted by Western blotting. For blocking experiments, identical DIC affinity columns were constructed in parallel and were either untreated or blocked with excess recombinant PLAC-24. Equal volumes of35S-labeled p150Glued or PLAC-24 were loaded onto the columns, and the columns were washed in the continued absence (control) or presence of excess recombinant PLAC-24. Specifically bound proteins were eluted with 1 M NaCl and analyzed by SDS-PAGE followed by fluorography.
Yeast Two-Hybrid Interaction Screen
The Lex A yeast two-hybrid system was used to screen for proteins interacting with PLAC-24. Because full-length PLAC-24 was found to autoactivate the reporter genes, a construct lacking the amino-terminal 68 residues, cloned into plasmid pJK202, was used to screen a human brain cDNA library from Invitrogen. Positive clones were identified by replica-plating the cotransformants onto induction media, requiring the activation of the LEU2 reporter gene for growth, and allowing the activation of the lacZ reporter gene. Screening 2 × 106 library clones led to 317 putative positives, of which 84 were sequenced.
Cell Culture, Transient Transfections, and Immunocytochemistry
Rat2 or PtK2 cells were seeded onto 18 × 18-mm glass coverslips and grown to 75% confluence. The cells were then rapidly fixed in −20°C 100% methanol with 1 mM EGTA for 10 min and processed for immunocytochemistry as described (Karki et al., 1998). To investigate the effects of latrunculin and nocodazole on the cortical distribution of PLAC-24, semiconfluent PtK2 cells were treated with nocodazole (5 μg/ml) or latrunculin B (0.5 μg/ml) for 30 min at 37°C. Cells were then immediately fixed in cold methanol and processed for immunocytochemistry. A GFP-PLAC-24 construct was generated by subcloning the coding region of PLAC-24 into a GFP expression vector (Clontech) and transfected into cultured PtK2 cells with the transfection reagent Fugene (Roche, Indianapolis, IN). The effects of β-catenin overexpression on the cellular localization of PLAC-24 was investigated with a β-catenin construct generously provided by Drs. A. Barth and W. J. Nelson of the University of California at San Francisco, as previously described (Ligon et al., 2001).
RESULTS
Molecular Characterization of PLAC-24
To identify novel dynein-binding proteins expressed in rat brain, we constructed an affinity column with recombinant DIC covalently linked to a Sepharose matrix (Karki and Holzbaur, 1995; Karki et al., 1997). We loaded rat brain cytosol onto the affinity matrix, washed extensively, and then eluted bound protein with 0.5 M NaCl. The resulting eluate was analyzed by SDS-PAGE and Western blotting. As expected, dynactin subunits p150Glued, p62, dynamitin, Arp1, capping protein subunits α and β, and p22 were present in the column eluate, as well as casein kinase II, as previously described (Karki et al., 1997). Additional polypeptides were also observed in this fraction, including a prominent band of 24 kDa evident on Coomassie-stained gels of the DIC column eluate. We excised the 24-kDa polypeptide from the gel and performed in situ proteolysis and microsequencing, which resulted in a 14-mer sequence: ENAYDLEANLAVLK (peptide sequence is underlined in Figure1A).
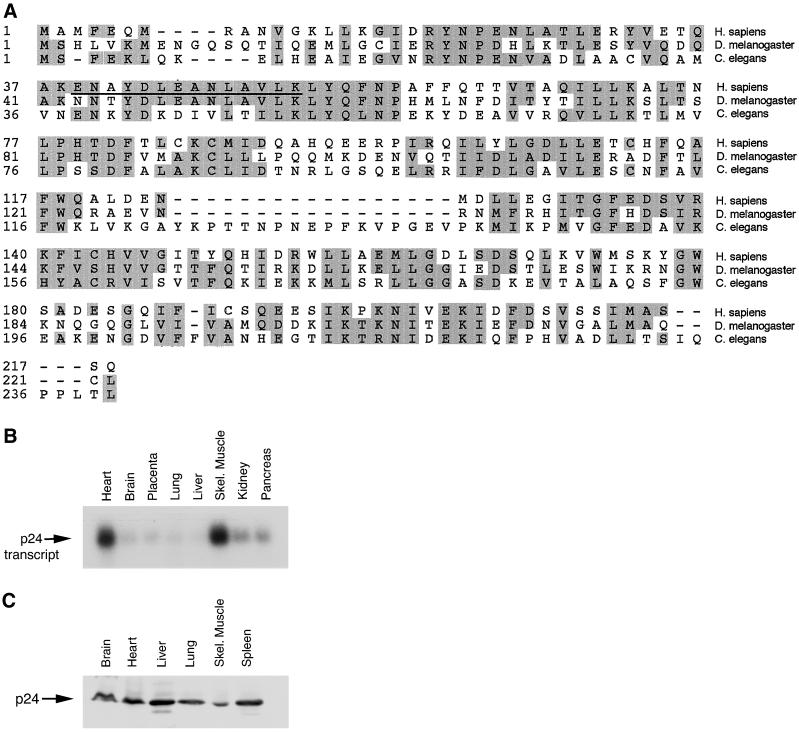
Figure 1.
PLAC-24 is a conserved protein in higher eukaryotes. (A) Comparison of the predicted protein sequences for PLAC-24 from human D. melanogaster, and C. elegans. The human sequence is from this work and accession numbers BAA76626 and AAD40193, the _D. melanogaster_sequence is from AAF46753 (Adams, 2000), and the C. elegans is from T24957. Areas of sequence identity or conservative substitution are shaded. The peptide sequence obtained from microsequencing of rat PLAC-24 protein is underlined. Note the extensive similarity throughout the lengths of the sequences of the homologues. (B) Northern blot analysis of human tissues reveals that an ∼1-kb mRNA transcript encoding PLAC-24 is expressed at a low level in all tissues examined. Significantly higher expression levels were observed in heart and skeletal (Skel.) muscle than in other tissues. (C) Western blot analysis of human tissues using an affinity-purified anti-PLAC-24 polyclonal antibody demonstrates that the PLAC-24 polypeptide is expressed in all tissues examined.
Database searches with this sequence identified a human EST that encoded the peptide sequence. Further characterization of this EST revealed that it encodes the complete sequence of a 24-kDa polypeptide (Figure 1A). Subsequently, the sequence for this polypeptide was entered into the database as a protein whose expression is enriched in muscle (accession numbers BAA76626 and AAD40193). Translation of the open reading frame predicts a protein of ∼25 kDa, consistent with the observed migration of the protein through SDS-PAGE. Analysis of the PLAC-24 sequence revealed no identifiable motifs or domains. However, database searches revealed predicted homologues in Drosophila melanogaster and Caenorhabditis elegans (Figure 1A). Comparisons of these sequences reveal domains of significant homology that may indicate conserved binding motifs.
We used the cDNA encoding PLAC-24 to probe a multiple-tissue Northern blot and found that the polypeptide is encoded by an ∼1-kb transcript. This transcript appears to be expressed ubiquitously at a relatively low level, consistent with our biochemical isolation of PLAC-24 from brain cytosol. Significantly higher levels of PLAC-24 mRNA were detected in human heart and skeletal muscle (Figure 1B). Antibodies were raised to recombinant PLAC-24, and the resulting affinity-purified antibodies were used to probe Western blots of human tissue extracts. As shown in Figure 1C, we observed PLAC-24 expression in all tissues examined. The observed differences between mRNA and protein expression levels may reflect tissue-specific differences in message and/or protein stability.
PLAC-24 Is Not an Integral Subunit of Dynactin or Cytoplasmic Dynein
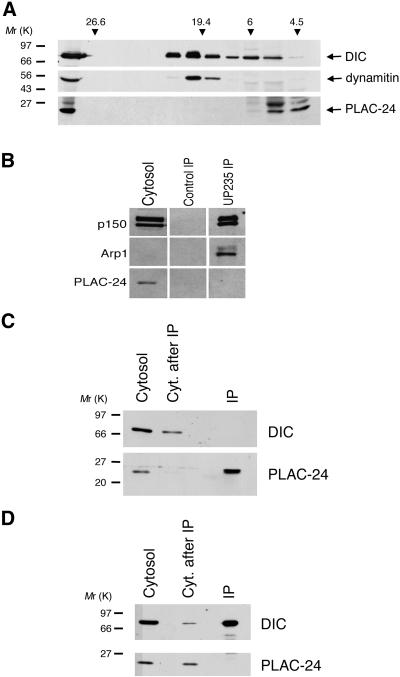
PLAC-24 was identified initially on the basis of its retention on a dynein affinity column. Dynactin also binds to the DIC affinity column (Karki and Holzbaur, 1995) and is coeluted from the affinity column along with PLAC-24. Because several polypeptides in the 22- to 25-kDa range copurify with dynactin, we tested to see whether PLAC-24 is a dynactin subunit. Dynactin sediments with a characteristically large S value, so rat brain cytosol was fractionated on 5–25% linear density sucrose gradients calibrated with known standards. Western blots of the resulting fractions were probed with antibodies to the dynactin subunit dynamitin (p50), to DIC, and to PLAC-24. Dynein and dynactin both sedimented at ∼19–20 S. Cosedimentation of PLAC-24 with dynein and dynactin was not observed. Instead, a single peak of PLAC-24 immunoreactivity was observed at 4.5 S (Figure2A).
Figure 2.
PLAC-24 is not a subunit of dynactin or cytoplasmic dynein. (A) Rat brain cytosol was fractionated over a 12-ml 5–25% sucrose gradient. Fractions (1 ml) along the gradient were analyzed by SDS-PAGE and Western blot using antibodies to dynein (DIC), dynactin (dynamitin), and PLAC-24. The first lane shown is cytosol, and subsequent lanes are gradient fractions. Gradients were calibrated using the standards glutamate dehydrogenase (26.6 S), thyroglobulin (19.4 S), tubulin (6S), and BSA (4.5 S). (B) Dynactin was immunoprecipitated from rat brain cytosol using a polyclonal anti-p150Glued antibody and probed with antibodies to the p150Glued and Arp1 subunits of dynactin and to PLAC-24. PLAC-24 is absent from the dynactin immunoprecipitate. Arp1, although present in the cytosol, can be clearly detected only in more concentrated fractions with this antibody. (C) PLAC-24 was immunoprecipitated from PtK2 cell cytosol using an affinity-purified polyclonal antibody. The resulting fractions: cytosol, cytosol after immunoprecipitation (Cyt. after IP), and immunoprecipitate (IP) were analyzed by SDS-PAGE and Western blot using antibodies to DIC and PLAC-24. DIC was not observed to coprecipitate with PLAC-24. (D) Cytoplasmic dynein was immunoprecipitated from PtK2 cell cytosol using an mAb to DIC. The resulting fractions: cytosol, cytosol after immunoprecipitation (Cyt. after IP), and immunoprecipitate (IP) were analyzed by SDS-PAGE and Western blot using antibodies to DIC and PLAC-24. PLAC-24 was not observed to coimmunoprecipitate with dynein from cellular cytosol.
To address this question further, we also tested whether the PLAC-24 polypeptide was coimmunoprecipitated with dynactin from rat brain cytosol. Previous studies have demonstrated that the p150Glued, p62, dynamitin, Arp1, and p22 subunits of dynactin coimmunoprecipitate under these conditions (Holleran_et al._, 1996; Karki et al., 1998; Karki et al., 2000). Dynactin was immunoprecipitated with an affinity-purified polyclonal antibody to p150Glued. The resulting immunoprecipitate was resolved by SDS-PAGE and analyzed by Western blot using antibodies to the p150Glued and Arp1 subunits of dynactin and an affinity-purified antibody to PLAC-24. Both p150Glued and Arp1 were present in the immunoprecipitate, whereas PLAC-24 was absent (Figure 2B). Together, the sucrose gradient fractionation and immunoprecipitation data indicate that PLAC-24 is not an integral subunit of dynactin.
We also examined the interaction between PLAC-24 and cytoplasmic dynein by immunoprecipitation assays. PtK2 cell cytosol was immunoprecipitated with either an affinity-purified antibody to PLAC-24 or a monoclonal antibody to DIC. As shown in Figure 2C, no dynein was observed to coimmunoprecipitate with PLAC-24. In the converse experiment, no PLAC-24 was observed in the dynein immunoprecipitate (Figure 2D). Therefore, although PLAC-24 can bind to dynein, PLAC-24 is not a dynein subunit, and the affinity of the interaction is not sufficiently high to result in the coimmunoprecipitation of these proteins.
PLAC-24 Binds Directly to Dynein
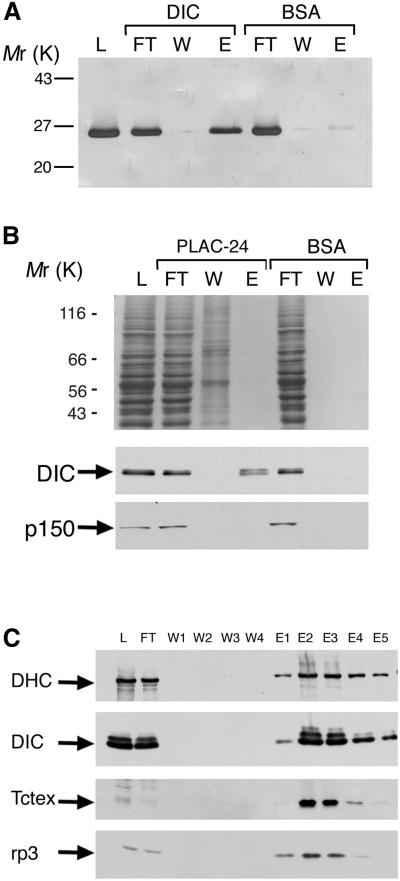
Although PLAC-24 was isolated because of its retention on a DIC affinity column, this interaction could be direct or indirect and mediated by other proteins. We tested for a direct interaction by examining the binding of recombinant PLAC-24 to a DIC affinity column. As shown in Figure 3A, purified recombinant PLAC-24 bound to the DIC column but was not significantly retained on the control BSA column.
Figure 3.
PLAC-24 binds directly to the intermediate chain of cytoplasmic dynein. (A) PLAC-24 binds to recombinant DIC. Purified recombinant PLAC-24 bound to a DIC affinity column but not to a control BSA column. Shown are the load (L), flowthrough (FT), wash (W), and eluate (E) fractions resolved by SDS-PAGE and stained for total protein with Coomassie blue. (B) Dynein from rat brain cytosol binds to a PLAC-24 affinity column. Shown are the load (L), flowthrough (FT), wash (W), and eluate (E) fractions resolved by SDS-PAGE and stained for total protein with Coomassie Blue (top) and corresponding Western blots probed with antibodies to dynein (DIC) and dynactin (p150Glued). Although dynein is specifically bound to the PLAC-24 matrix, dynactin is not retained by the PLAC-24 affinity column. Neither dynein nor dynactin was retained by the BSA control column. (C) Rat brain cytosol was loaded onto a PLAC-24 affinity column. After extensive buffer washes, bound proteins were eluted with 1 M NaCl. Load (L), flowthrough (FT), wash (W1, W2, W3, W4), and eluate fractions (E1, E2, E3, E4, E5) were analyzed by SDS-PAGE and Western blot using antibodies to dynein heavy chain (DHC), DIC, and to the Tctex-1 and rp3 dynein light chains.
We also performed the inverse experiment, testing for the binding of dynein and dynactin to a PLAC-24 affinity matrix. As shown in Figure2B, dynein from cytosol was retained on a PLAC-24 affinity matrix and not on a parallel control column with BSA bound. In contrast, dynactin did not bind to PLAC-24, as judged by immunoblotting the column eluate with anti-p150Glued antibody (Figure 3B). Together, these data indicate that PLAC-24 binds directly to DIC and that dynactin does not bind to PLAC-24.
To determine whether PLAC-24 binds to intact dynein or only to the intermediate chain, we fractionated rat brain cytosol on a PLAC-24 affinity column. After extensive washes, the bound proteins were eluted with high salt and analyzed by SDS-PAGE and Western blot. Antibodies to dynein heavy chain, and the Tctex-1 and rp3 light chains of dynein revealed the cofractionation of these dynein subunits on the PLAC-24 column (Figure 3C), suggesting that PLAC-24 is capable of binding to intact dynein.
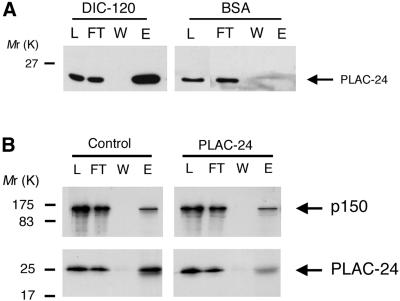
Further binding studies were performed using the yeast two-hybrid assay to screen for PLAC-24 interacting proteins. One of the clones isolated in this screen partially encoded the human 2C isoform of the the intermediate chain of cytoplasmic dynein. This observation confirms the binding data above and indicates that residues 28–178 of DIC are sufficient to bind to PLAC-24. Further mapping of the binding domain using affinity chromatography assays indicates that a fragment of DIC encoding residues 1–120 was sufficient to retain PLAC-24 (Figure4A). Together, these data localize the PLAC-24 binding site to residues 28–120 of DIC. This is in close proximity to the binding site for p150Glued, which has been mapped to residues 1–123 of DIC (Karki and Holzbaur, 1995; Vaughan and Vallee, 1995). Therefore, it is possible that PLAC-24 and dynactin compete for the same binding site on the DIC. To test this possibility, the binding of 35S-labeled p150Glued and that of35S-labeled PLAC-24 to dynein IC were each examined in the absence and in the presence of excess unlabeled recombinant PLAC-24 (Figure 4B). Comparisons of the eluate fractions from these columns indicates that the binding of35S-labeled PLAC-24 to DIC was inhibited by excess PLAC-24 to a much greater extent than was observed for the binding of p150Glued to DIC. This observation suggests that although both PLAC-24 and p150Gluedbind to the amino-terminal domain of DIC, the binding of these proteins to dynein may not be mutually exclusive.
Figure 4.
PLAC-24 interacts with the amino-terminal domain of the DIC. (A) PLAC-24 from cytosol bound to a recombinant fragment corresponding to residues 1–120 of DIC, covalently cross-linked to Sepharose beads. Load (L), flowthrough (FT), wash (W), and eluate (E) fractions were analyzed by SDS-PAGE and Western blotting, using an affinity-purified antibody to PLAC-24. No significant binding of PLAC-24 from cytosol to a control column was observed. (B) To test whether the binding of PLAC-24 to the DIC blocks the interaction of dynactin with dynein, the binding of 35S-labeled p150Glued and 35S-labeled PLAC-24 to DIC affinity columns was examined in the presence (PLAC-24) and absence (Control) of excess unlabeled recombinant PLAC-24. The binding of35S-labeled PLAC-24 to DIC was inhibited by excess unlabeled PLAC-24 to a much greater extent than was observed for the binding of 35S-labeled p150Glued to DIC, suggesting that PLAC-24 and p150Glued do not compete for binding to dynein. Load (L), flowthrough (FT), wash (W), and eluate (E) fractions were analyzed by SDS-PAGE and fluorography.
PLAC-24 Localizes to the Cortex at Sites of Cell-Cell Contact
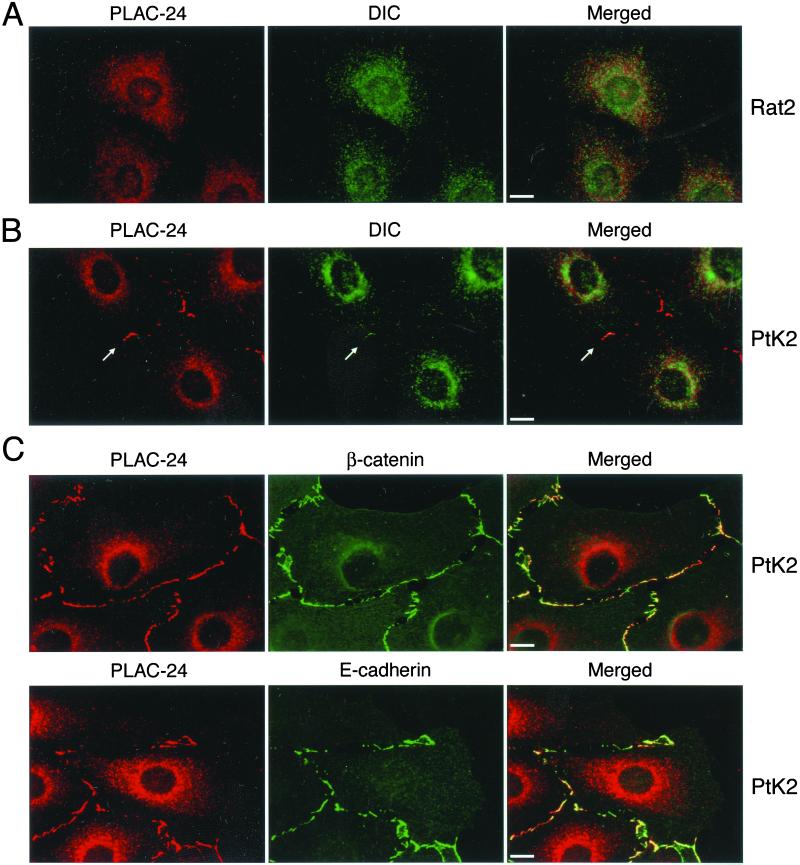
We used antibodies raised either to recombinant PLAC-24 or to an immunogenic peptide from the PLAC-24 sequence to localize the protein in fibroblast (Rat2) and epithelial (PtK2) cell lines. In both Rat2 (Figure 5A) and PtK2 (Figure 5B) cells, the cytoplasmic pool of PLAC-24 is concentrated in the perinuclear region, where its distribution closely resembles that of dynein, although the two proteins are not completely colocalized.
Figure 5.
PLAC-24 is recruited to the cortex in epithelial cells. (A) Semiconfluent Rat2 fibroblasts were double-labeled with antibodies to PLAC-24 (red) and DIC (green). Both PLAC-24 and dynein show a partially overlapping punctate distribution throughout the cytoplasm that is enriched in the perinuclear region of the cell. No localization of PLAC-24 to the cortex was observed in these cells. (B) PtK2 epithelial cells were double-labeled with an affinity-purified polyclonal anti-PLAC-24 antibody (red) and monoclonal antibodies to the DIC (green). PLAC-24 was observed to localize to the cortex at sites of cell-cell contact, where it partially colocalizes with DIC. (C) PLAC-24 (red) colocalizes with the adherens junction proteins β-catenin (green, top row) and E-cadherin (green, bottom row) at sites of cell-cell contact in PtK2 cells.
In semiconfluent monolayers of PtK2 cells, we also noted the distinct localization of PLAC-24 to the cell cortex at sites of cell-cell contact (Figure 5, B and C). This peripheral staining was not observed in Rat2 cells, suggesting that the localization may be specific to epithelial cells. Double-label immunocytochemistry with antibodies to PLAC-24 and β-catenin or E-cadherin show a striking colocalization of PLAC-24 with components of the adherens junction (Figure 5C). This colocalization is extensive, but the overlap may not be complete.
Recent work from our laboratory has demonstrated that cytoplasmic dynein also localizes to the cell cortex, with the most prominent staining observed at adherens junctions (Ligon et al., 2001). Although this cortical dynein is best resolved by polyclonal antibodies to the dynein heavy, intermediate, and light chains, some cortical localization was observed with a monoclonal DIC antibody, allowing us to compare the distributions of PLAC-24 and dynein directly (Figure 5B, arrow). Partial colocalization of the two proteins was observed at cortical sites of cell-cell contact, although the PLAC-24 labeling is more extensive in these regions.
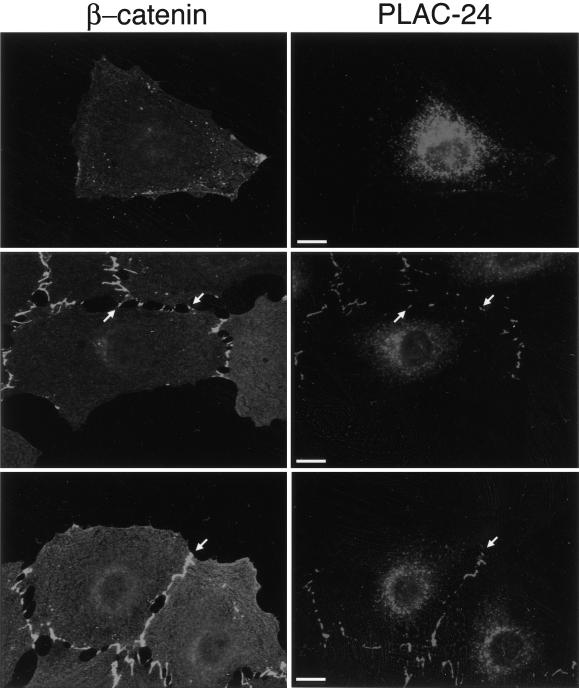
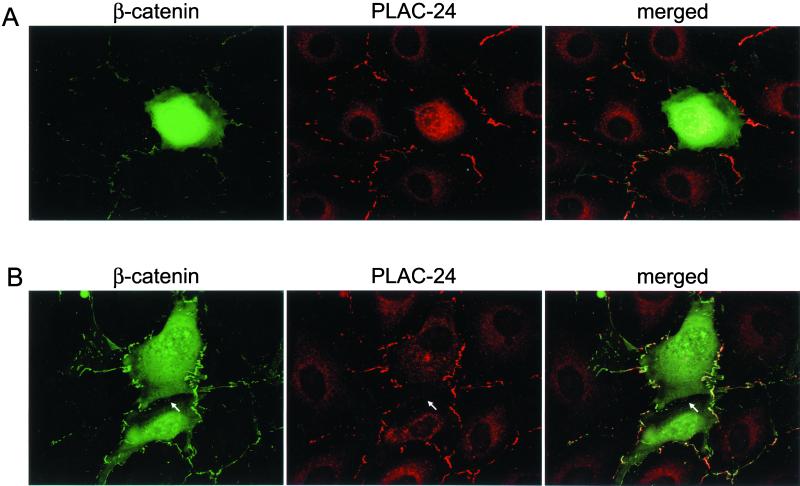
Vasioukhin et al. (2000) have characterized the formation of adhesion zippers in the development of adhesion junctions between adjacent epithelial cells. These structures were found to stain with antibodies to E-cadherin, α-actinin, vinculin, zyxin, VASP, and Mena. We examined the localization of PLAC-24 to developing adhesion junctions in PtK2 cells to see whether the recruitment of PLAC-24 is an early or late step in the formation of these contacts. Neither β-catenin nor PLAC-24 shows significant localization to the periphery in isolated PtK2 cells (Figure 6, top row). As cells begin to make contact, however, β-catenin is recruited to these sites (Figure 6, bottom row). In parallel, we observed the recruitment of PLAC-24 to sites of initial contact between cells, where it colocalizes with β-catenin (Figure 6, bottom row, arrows). Within the limits of resolution of this experiment, the time courses of recruitment of β-catenin and PLAC-24 to developing cell-cell contact sites were indistinguishable.
Figure 6.
PLAC-24 and β-catenin are recruited to developing adhesion zippers as cell-cell contacts are formed. PtK2 cells were plated and fixed at low density to allow the observation of initial stages of cell-cell contact formation and then double-labeled with antibodies to β-catenin (green) and PLAC-24 (red). In isolated cells, neither protein shows significant localization to the cortex (top row). As cells make initial contacts, structures referred to as adhesion zippers were observed to stain with antibodies to both PLAC-24 and β-catenin (arrows, middle row). As the cell-cell contacts mature, PLAC-24 and β-catenin colocalize extensively along the length of the contacts (arrows, bottom row).
PLAC-24 Localization to Intercellular Contacts Is Dependent on an Intact Actin Cytoskeleton
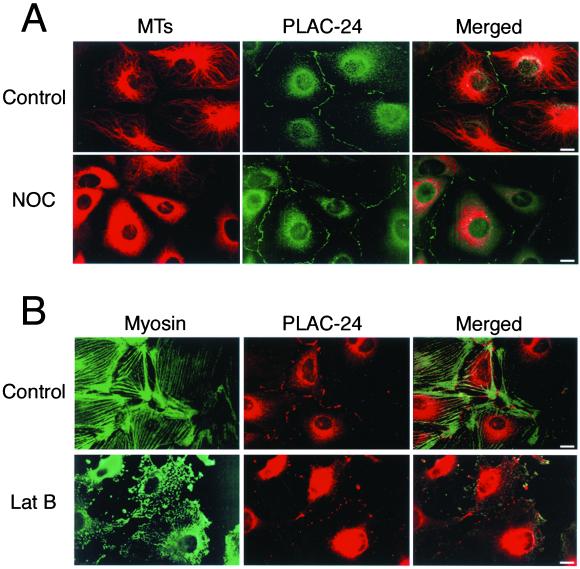
Because PLAC-24 is associated with a microtubule motor, we tested to see whether the localization of PLAC-24 to the cortex is dependent on an intact microtubule cytoskeleton. Semiconfluent monolayers of PtK2 cells were treated with nocodazole to disrupt cytoplasmic microtubules and then processed for double-label immunocytochemistry using antibodies to tubulin and PLAC-24. No significant perturbation in the distribution of either cytosolic or peripheral PLAC-24 was observed in nocodazole-treated cells compared with untreated control cells (Figure7A), suggesting that the localization of PLAC-24 to the cell periphery at sites of cell-cell contact is not dependent on an intact microtubule cytoskeleton.
Figure 7.
The localization of PLAC-24 to cortical sites of cell-cell contact is dependent on an intact actin cytoskeleton. Semiconfluent PtK2 cells were treated with either 5 μg/ml nocodazole (A, NOC) or 0.5 μg/ml latrunculin (B, Lat B) for 30 min at 37°C followed by immediate fixation in cold methanol to disrupt the microtubule or actin cytoskeletons, respectively. Double-labeling of nocodazole-treated cells with antibodies to tubulin and to PLAC-24 revealed that the localization of PLAC-24 to the cortex was not affected by the disruption of the microtubule cytoskeleton (A). In contrast, double-labeling of cells with antibodies to myosin II, to visualize the actin cytoskeleton, and to PLAC-24 revealed that the localization of PLAC-24 to the cortex was significantly disrupted by the loss of an intact actin cytoskeleton in response to latrunculin treatment (B).
To investigate whether PLAC-24 localization to cell-cell contacts is dependent on an intact actin cytoskeleton, we treated PtK2 cells with latrunculin B before fixation. As shown in Figure 7B, we noted a significant disruption of the actin cytoskeleton after latrunculin B treatment. Under these conditions, PLAC-24 distribution at the cell cortex is clearly affected. Although PLAC-24 continues to localize to the periphery, it is distributed in discrete spots, rather than as a continuous line along cell-cell contact sites. As adjacent cells round up and lose their contacts with each other, PLAC-24 staining is lost from the cell periphery. These data support the initial observations that PLAC-24 is localized to the cell periphery only at sites of cell-cell contact and that the distribution of PLAC-24 to these sites is dependent either directly or indirectly on an intact actin cytoskeleton.
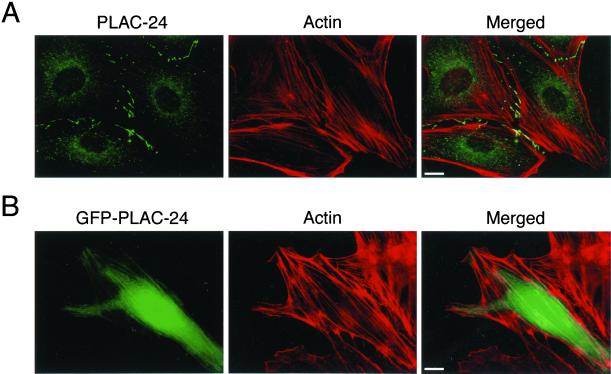
To further examine the association of PLAC-24 with the actin cytoskeleton, double-label immunocytochemistry was performed with antibodies to actin and PLAC-24. Although some localization of PLAC-24 to the ends of stress fibers was observed, no significant localization of PLAC-24 along the lengths of actin filaments was seen (Figure8A). In contrast, overexpression of a GFP-tagged PLAC-24 construct led to the more extensive decoration of stress fibers (Figure 8B). Together, these data suggest that PLAC-24 is associated with the actin cytoskeleton but suggest that the localization of endogenous PLAC-24 is specifically regulated in the cell.
Figure 8.
PLAC-24 is concentrated at the ends of stress fibers. (A) In PtK2 cells double-labeled with antibodies to PLAC-24 (green) and to actin (red), PLAC-24 is clearly concentrated at the ends of stress fibers. No significant decoration along actin filaments was observed. (B) In contrast, in transfected cells overexpressing a GFP-PLAC-24 construct, decoration along actin filaments was observed.
PLAC-24 Localization to Intercellular Contacts Is Disrupted by the Overexpression of β-Catenin
We recently demonstrated a direct binding interaction between dynein and β-catenin (Ligon et al., 2001). Overexpression of β-catenin in transfected cells was found to disrupt both the cytosolic and cortical pools of dynein. Furthermore, in cells overexpressing β-catenin, we noted that microtubules no longer projected directly to sites of cell-cell contact but instead curved away from the cell cortex. Therefore, we examined the effects of β-catenin overexpression on the cellular localization of PLAC-24 using transient transfection assays. We found that elevated levels of β-catenin led to the disruption of the cytosolic distribution of PLAC-24 (Figure 9A). We also noted loss of PLAC-24 from the cortex. This loss of PLAC-24 from adherens junctions is most readily observed when we examine the junctions between pairs of transfected cells (Figure 9B). Because PLAC-24 appears to be more readily lost from adherens junctions with β-catenin overexpression than was previously observed for dynein (Ligon et al., 2001), PLAC-24 is not likely to be required for the localization of dynein to sites of cell-cell contact but instead may participate in mediating a network of interactions.
Figure 9.
Overexpression of β-catenin disrupts the intracellular localization of PLAC-24. (A) PtK2 cells were transiently transfected with β-catenin, then fixed and stained with antibodies to β-catenin (green) and to PLAC-24 (red). High levels of β-catenin led to the disruption of the cytosolic pool of PLAC-24. (B) Analysis of regions of contact between adjacent cells expressing high levels of β-catenin indicates that the localization of PLAC-24 to cell-cell contacts is also disrupted (arrows). This loss of PLAC-24 from adherens junctions is most readily observed when we examine the junctions between pairs of transfected cells.
DISCUSSION
Coordination between the actin and microtubule cytoskeletons is essential for many cellular functions, including cell division, the development and maintenance of cell polarity, and cell migration. Mechanisms that enable cytoskeletal cross-talk are now being identified. This cross-talk may involve proteins that can directly cross-link cytoskeletal networks, such as BPAGn1/BPAGn2 and MACF/kakapo (reviewed in Fuchs and Yang, 1999; Klymkowsky, 1999), or it may involve the formation of macromolecular complexes. Other examples of cross-talk involve the coordination between actin-based and microtubule-based transport, such as the switching of organelles from movement along microtubules to movement along the actin filaments (Kuznetsov et al., 1992; Rodionov et al., 1998; reviewed in Goode_et al._, 2000).
Interactions between microtubules and the actin-rich cortex are important in processes such as nuclear migration and spindle rotation, and dynein and dynactin have been proposed to mediate these contacts (reviewed in Karki and Holzbaur, 1999). Interactions between microtubules and the cortex have also been proposed to be involved in cell adhesion. The capture and stabilization of microtubule plus ends at early focal adhesions has been observed in locomoting fibroblasts (Kaverina et al., 1998), and the presence of adherens junctions appears to enhance microtubule stability in newt lung epithelial cells (Waterman-Storer et al., 2000). A similar association between cell-cell contact and microtubule dynamics was also observed by Chausovsky et al. (2000). In addition,Waterman-Storer et al. (2000) observed that nocodazole treatment of primary epithelial cells led to the disruption of cell-cell contacts. Together, these observations support the hypothesis of cross-talk between adherens junctions and microtubules and also suggest that this cross-talk involves a dialog: microtubule behavior is affected by adherens junctions, but the stability of adherens junctions is dependent on intact microtubules.
The mechanism or mechanisms that mediate this cross-talk are now being explored. Chausovsky et al. (2000) proposed that cadherins are involved in a signaling mechanism that results in the stabilization of microtubule ends. We have recently observed microtubules projecting toward dynein patches localized at the cortex at developing sites of cell-cell contact and have hypothesized that dynein may be acting to tether and thus stabilize microtubules projecting toward these sites (Ligon et al., 2001).
The specific targeting of dynein to sites of cell-cell contact at the cortex that we have observed suggests that there are specific binding partners at these sites that specifically mediate the association of dynein either with the plasma membrane or, more likely, with the actin-rich cortical cytoskeleton. In this study, we have isolated and characterized a novel 24-kDa protein (PLAC-24) because of its binding affinity for dynein. We have determined that PLAC-24 binds directly to the dynein IC and that this interaction is independent of the dynein/dynactin interaction. Using antibodies to PLAC-24, we have demonstrated that this protein is specifically recruited to sites of cell-cell contact in epithelial cell monolayers and that this occurs at an early step in the development of these junctions. Therefore, PLAC-24 is a good candidate for a protein that targets dynein to cell-cell contact sites. Alternatively, the dynein-β-catenin interaction we have recently observed (Ligon et al., 2001) may be sufficient to target dynein to these sites. In this case, PLAC-24 may serve either to increase the affinity of the association or to transduce signals from dynein to the actin cytoskeleton. Although we have not observed the direct association of endogenous PLAC-24 with actin filaments, immunocytochemistry suggests that PLAC-24 may be associated with the ends of actin stress fibers in the cell (Figure6A). It is also interesting to note that the localization of PLAC-24 to sites of intercellular contact was not dependent on microtubules but was dependent on an intact actin cytoskeleton. This observation is consistent with the possibility that PLAC-24 associates with the actin-rich contact sites by its association, either direct or indirect, with actin.
Comparisons of predicted sequences of PLAC-24 homologues from humans,Drosophila, and C. elegans indicates that there is significant conservation of sequence along the length of the polypeptide. Although this analysis did not reveal any well-defined motifs or domains, it is likely that these highly conserved sequences may represent sites of interaction with other cellular proteins. We have attempted to map the dynein-binding domain within PLAC-24 using either the yeast two-hybrid assay or affinity chromatography (data not shown). Data from both approaches suggest that there are multiple binding determinants throughout the linear polypeptide sequence of PLAC-24 that are essential for binding to DIC. It is possible that these sites are brought together when PLAC-24 is correctly folded in vitro; alternatively, this polypeptide may dimerize in vivo.
It is possible that PLAC-24 may be recruited to cell-cell junctions as part of a multisubunit complex, which also includes dynein. Results from a two-hybrid screen for PLAC-24 interactors suggest that PLAC-24 may associate with other cellular proteins. A second clone isolated as a potential PLAC-24 interactor in our yeast two-hybrid screen corresponds to residues 4108–4242 of MACF2 (Sun_et al._, 2001). MACF2 is a member of the plectin family, with sequence similarity to MACF (Sun et al., 2001), which has been shown to interact with both the microtubule and actin cytoskeletons (Karakesisoglou et al., 2000; Leung et al., 1999). The Drosophila homolog of MACF is kakapo (also known as short stop), which has been shown to be involved in both axonal outgrowth of neurons and muscle attachment (Gregory and Brown, 1998; Prokop et al., 1998; Strumpf and Volk, 1998; Lee_et al._, 2000). Kakapo was found to be prominently concentrated at the apical and basal surfaces of muscle attachment cells at the ends of microtubule bundles (Gregory and Brown, 1998). The localization of PLAC-24 to intercellular contact sites and the interaction detected between PLAC-24 and MACF2 in the yeast two-hybrid screen suggests that PLAC-24, along with MACF or MACF2, may be part of a cortical attachment complex. These data further support the hypothesis that PLAC-24 may be part of the machinery that captures and stabilizes microtubules projecting toward sites of cell-cell contact.
In summary, we have cloned and characterized a novel 24-kDa polypeptide that binds to cytoplasmic dynein and is recruited to the actin-rich cortex at sites of intercellular contact in epithelial cells. PLAC-24 sequences from humans, Drosophila, and _C. elegans_show significant homology, suggesting that this polypeptide is evolutionarily conserved across species because of a conserved and possibly essential function. Given the localization of PLAC-24 at intercellular attachment sites and its binding interaction with the microtubule motor dynein, we propose that PLAC-24 may be involved in the capture and localization of microtubule plus ends at sites of cell-cell contact. Further studies will be needed to dissect whether PLAC-24 is merely a structural component at adherens junctions or actively participates in modulating actin and/or microtubule dynamics.
ACKNOWLEDGMENTS
We gratefully acknowledge the contributions of Jennifer Schumacher for the PLAC-24–MACF2 binding studies and Dr. Ron Liem of Columbia University for providing MACF2 subclones. This work was supported National Institutes of Health grant GM-48661 and American Heart Association grant 0150418N to E.L.F.H. E.L.F.H. is an Established Investigator of the American Heart Association. S.K. was supported by a postdoctoral fellowship from the American Heart Association, Southeastern Pennsylvania Affiliate, and L.A.L. was supported by a National Institutes of Health postdoctoral fellowship. J.D.S. was partially supported by a Merck Summer Research Fellowship.
Footnotes
REFERENCES
- Adams MD, et al. The genome sequence of Drosophila melanogaster. Science. 2000;287:2185–2195. doi: 10.1126/science.287.5461.2185. [DOI] [PubMed] [Google Scholar]
- Adams MD, Bento Soares M, Kerlavage AR, Fields C, Ventor JC. Rapid cDNA sequencing (expressed sequence tags) from a directionally cloned human infant brain cDNA library. Nat Genet. 1993;4:373–380. doi: 10.1038/ng0893-373. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Meyers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Chausovsky A, Bershadsky AD, Borisy GG. Cadherin-mediated regulation of microtubule dynamics. Nat Cell Biol. 2000;2:797–804. doi: 10.1038/35041037. [DOI] [PubMed] [Google Scholar]
- Epstein E, Sela-Brown A, Ringel I, Kilav R, King SM, Benashki SE, Yisraeli JK, Silver J, Naveh-Many T. Dynein light chain binding to a 3′-untranslated sequence mediates parathyroid hormone mRNA association with microtubules. JClin Invest. 2000;105:505–512. doi: 10.1172/JCI8557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E, Yang Y. Crossroads on cytoskeletal highways. Cell. 1999;98:547–550. doi: 10.1016/s0092-8674(00)80041-0. [DOI] [PubMed] [Google Scholar]
- Goode BL, Drubin DG, Barnes G. Functional cooperation between the microtubule and actin cytoskeletons. Curr Opin Cell Biol. 2000;12:63–71. doi: 10.1016/s0955-0674(99)00058-7. [DOI] [PubMed] [Google Scholar]
- Gregory, S.L., and Brown, N.H. (1998). Kakapo, a gene required for adhesion between and within cell layers in Drosophila, encodes a large cytoskeletal linker protein related to plectin and dystrophin. 143, 1271–1282. [DOI] [PMC free article] [PubMed]
- Holleran EA, Karki S, Holzbaur ELF. The role of dynactin complex in intracellular motility. Int Rev Cytol. 1998;182:69–109. doi: 10.1016/s0074-7696(08)62168-3. [DOI] [PubMed] [Google Scholar]
- Holleran EA, Tokito MK, Karki S, Holzbaur ELF. Centractin (ARP1) associates with spectrin revealing potential mechanism to link dynactin to intracellular organelles. J Cell Biol. 1996;135:1815–1829. doi: 10.1083/jcb.135.6.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakesisoglou I, Yang Y, Fuchs E. An epidermal plakin that integrates actin and microtubule networks at cellular junctions. J Cell Biol. 2000;149:195–208. doi: 10.1083/jcb.149.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki S, Holzbaur ELF. Affinity chromatography demonstrates a direct binding between cytoplasmic dynein and the dynactin complex. J Biol Chem. 1995;270:28806–28811. doi: 10.1074/jbc.270.48.28806. [DOI] [PubMed] [Google Scholar]
- Karki S, Holzbaur ELF. Cytoplasmic dynein and dynactin in cell division and intracellular transport. Curr Opin Cell Biol. 1999;11:45–53. doi: 10.1016/s0955-0674(99)80006-4. [DOI] [PubMed] [Google Scholar]
- Karki S, LaMonte B, Holzbaur ELF. Characterization of the p22 subunit of dynactin reveals the localization of cytoplasmic dynein and dynactin to the midbody of dividing cells. J Cell Biol. 1998;142:1023–1034. doi: 10.1083/jcb.142.4.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki S, Tokito MK, Holzbaur ELF. Casein kinase II binds to and phosphorylates cytoplasmic dynein. J Biol Chem. 1997;272:5887–5891. doi: 10.1074/jbc.272.9.5887. [DOI] [PubMed] [Google Scholar]
- Karki S, Tokito MK, Holzbaur ELF. A dynactin subunit with a highly conserved cysteine-rich motif interacts directly with Arp1. J Biol Chem. 2000;275:4834–4839. doi: 10.1074/jbc.275.7.4834. [DOI] [PubMed] [Google Scholar]
- Kaverina I, Rottner K, Small JV. Targeting, capture, and stabilization of microtubules at early focal adhesions. J Cell Biol. 1998;142:181–190. doi: 10.1083/jcb.142.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King SJ, Schroer TA. Dynactin increases the processivity of the cytoplasmic dynein motor. Nat Cell Biol. 2000;2:20–24. doi: 10.1038/71338. [DOI] [PubMed] [Google Scholar]
- Klymkowsky MW. Weaving a tangled web: the interconnected cytoskeleton. Nat Cell Biol. 1999;1:E1121–E1123. doi: 10.1038/12950. [DOI] [PubMed] [Google Scholar]
- Kuznetsov SA, Langford GM, Weiss DG. Actin-dependent organelle movement in squid axoplasm. Nature. 1992;256:722–725. doi: 10.1038/356722a0. [DOI] [PubMed] [Google Scholar]
- Lee S, Harris KL, Whittington PM, Kolodziej PA. Short stop is allelic to kakapo, and encodes rod-like cytoskeletal-associated proteins required for axon extension. J Neurosci. 2000;20:1096–1108. doi: 10.1523/JNEUROSCI.20-03-01096.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung CL, Sun D, Zheng M, Knowles DR, Liem RKH. Microtubule actin cross-linking factor (MACF): a hybrid of dystonin and dystrophin that can interact with the actin and microtubule cytoskeletons. J Cell Biol. 1999;147:1275–1285. doi: 10.1083/jcb.147.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligon LA, Karki S, Tokito M, Holzbaur ELF. Dynein binds to β-catenin and tethers microtubules at adherens junctions. Nat Cell Biol. 2001;3:913–917. doi: 10.1038/ncb1001-913. [DOI] [PubMed] [Google Scholar]
- Prokop A, Uhler J, Roote J, Bate M. The kakapo mutation affects terminal arborization and central dendritic sprouting of Drosophila motorneurons. J Cell Biol. 1998;143:1283–1294. doi: 10.1083/jcb.143.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purohit A, Tynan SH, Vallee RB, Doxsey SJ. Direct interaction of pericentrin with cytoplasmic dynein light intermediate chain contributes to mitotic spindle organization. J Cell Biol. 1999;147:481–492. doi: 10.1083/jcb.147.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodionov VI, Hope AJ, Svitkina TM, Borisy GG. Functional coordination of microtubule-based and actin-based motility in melanophores. Curr Biol. 1998;8:161–163. doi: 10.1016/s0960-9822(98)70064-8. [DOI] [PubMed] [Google Scholar]
- Schnorrer F, Bohmann K, Nusslein-Volhard C. The molecular motor dynein is involved in targeting swallow and bicoid RNA to the anterior pole of Drosophila oocytes. Nat Cell Biol. 2000;2:185–190. doi: 10.1038/35008601. [DOI] [PubMed] [Google Scholar]
- Starr DA, Williams BC, Hays TS, Goldberg ML. ZW10 helps recruit dynactin and dynein to the kinetochore. J Cell Biol. 1998;142:763–774. doi: 10.1083/jcb.142.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen W, Karki S, Vaughan KT, Vallee RB, Holzbaur ELF, Weiss DG, Kuznetsov SA. The involvement of the intermediate chain of cytoplasmic dynein in binding the motor complex to membranous organelles of Xenopusoocytes. Mol Biol Cell. 1997;8:2077–2088. doi: 10.1091/mbc.8.10.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strumpf D, Volk T. Kakapo, a novel cytoskeletal-associated protein is essential for the restricted localization of the neuregulin-like factor, vein, at the muscle-tendon junction site. J Cell Biol. 1998;143:1259–1270. doi: 10.1083/jcb.143.5.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Leung CL, Liem RKH. Characterization of the microtubule binding domain of microtubule actin crosslinking factor (MACF): identification of a novel group of microtubule associated proteins. J Cell Sci. 2001;114:161–172. doi: 10.1242/jcs.114.1.161. [DOI] [PubMed] [Google Scholar]
- Tai AW, Chuang J-Z, Bode C, Wolfrum U, Sung C-H. Rhodopsin's carboxy-terminal cytoplasmic tail acts as a membrane receptor for cytoplasmic dynein by binding to the dynein light chain Tctex-1. Cell. 1999;97:877–887. doi: 10.1016/s0092-8674(00)80800-4. [DOI] [PubMed] [Google Scholar]
- Tokito MK, Howland DS, Lee VM-Y, Holzbaur ELF. Functionally distinct isoforms of dynactin are expressed in human neurons. Mol Biol Cell. 1996;7:1167–1180. doi: 10.1091/mbc.7.8.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasioukhin V, Bauer C, Yin M, Fuchs E. Directed actin polymerization is the driving force for epithelial cell-cell adhesion. Cell. 2000;100:209–219. doi: 10.1016/s0092-8674(00)81559-7. [DOI] [PubMed] [Google Scholar]
- Vaughan KT, Vallee RB. Cytoplasmic dynein binds dynactin through a direct interaction between the intermediate chains and p150Glued. J Cell Biol. 1995;131:1507–1516. doi: 10.1083/jcb.131.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman-Storer CM, Karki S, Holzbaur ELF. The p150Gluedcomponent of the dynactin complex binds to both microtubules and the actin-related protein centractin (Arp-1) Proc Natl Acad Sci USA. 1995;92:1634–1638. doi: 10.1073/pnas.92.5.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman-Storer CM, Kuznetsov SA, Karki S, Tabb JS, Weiss DG, Langford GM, Holzbaur ELF. The interaction between cytoplasmic dynein and dynactin is required for fast axonal transport. Proc Natl Acad Sci USA. 1997;94:12180–12185. doi: 10.1073/pnas.94.22.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman-Storer CM, Salmon WC, Salmon ED. Feedback interactions between cell-cell adherens junctions and cytoskeletal dynamics in newt lung epithelial cells. Mol Biol Cell. 2000;11:2471–2483. doi: 10.1091/mbc.11.7.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]