Induction of Antibodies in Guinea Pigs and Rhesus Monkeys against the Human Immunodeficiency Virus Type 1 Envelope: Neutralization of Nonpathogenic and Pathogenic Primary Isolate Simian/Human Immunodeficiency Virus Strains (original) (raw)
Abstract
We have compared the abilities of human immunodeficiency virus type 1 (HIV-1) envelope V3 peptides and recombinant gp120 to induce antibodies that neutralize simian/human immunodeficiency viruses (SHIVs). SHIV-89.6 is a nonpathogenic SHIV that expresses the envelope protein of primary HIV-1 isolate 89.6. SHIV-89.6P, clone KB9, is a pathogenic SHIV variant derived from SHIV-89.6. Infection of rhesus monkeys with these SHIVs rarely induces anti-V3 region antibodies. To determine the availability of the gp120 V3 loop for neutralizing antibody binding on SHIV-89.6 and KB9 virions, we have constructed immunogenic C4-V3 peptides from these SHIVs and induced anti-V3 antibodies in guinea pigs and rhesus monkeys. We found that both SHIV-89.6 and KB9 C4-V3 peptides induced antibodies that neutralized SHIV-89.6 but that only SHIV-KB9 C4-V3 peptide induced antibodies that neutralized SHIV-KB9. Immunoprecipitation assays demonstrated that SHIV-KB9 C4-V3 peptide-induced antibodies had a greater ability to bind SHIV-KB9 envelope proteins than did antibodies raised against SHIV-89.6 C4-V3 peptide. We have used a series of mutant HIV-1 envelope constructs to map the gp120 determinants that affect neutralization by anti-V3 antibodies. The residue change at position 305 of arginine (in SHIV-89.6) to glutamic acid (in SHIV-KB9) played a central role in determining the ability of peptide-induced anti-V3 antiserum to neutralize primary isolate SHIVs. Moreover, residue changes in the SHIV-89.6 V1/V2 loops also played roles in regulating the availability of the V3 neutralizing epitope on SHIV-89.6 and -KB9. Thus, SHIV-89.6 and -KB9 V3 region peptides are capable of inducing neutralizing antibodies against these primary isolate SHIVs, although the pathogenic SHIV-KB9 is less easily neutralized than its nonpathogenic variant SHIV-89.6. In contrast to natural infection with SHIV-89.6, in which few animals make anti-V3 antibodies, C4-V3 peptides frequently induced anti-V3 antibodies that neutralized primary isolate SHIV strains.
A major goal in human immunodeficiency virus type 1 (HIV-1) vaccine development is to design immunogens that will induce anti-HIV-1 antibodies that neutralize HIV-1 primary isolates (2, 5, 14, 23, 26, 30, 37). The gp120 exterior envelope glycoprotein of HIV-1, which contains variable regions (V1 to V5), is a major target for neutralizing antibodies. Whereas antibodies against the third variable (V3) loop of the HIV-1 gp120 envelope glycoproteins consistently neutralize T-cell-line-adapted (TCLA) HIV-1 isolates, they inconsistently neutralize HIV-1 primary isolates (1, 5, 8, 9, 12–14, 19, 30, 35–37). A key question is whether the primary isolate envelope V3 loop is available for anti-V3 region antibody binding on HIV-1 primary isolates (3). If the V3 loop is available for antibody binding to some degree on primary HIV-1 isolates, then perhaps strategies whereby the exposed region(s) may be included as a component of a vaccine candidate designed to induce neutralizing antibodies can be devised.
Primary isolate simian/human immunodeficiency virus (SHIV) strains are genetically engineered viruses comprised of HIV-1 primary isolate envelope and SIVmac239 regulatory and core proteins (22). SHIV-89.6 (32) and its pathogenic variant SHIV-89.6P (31) (and its molecular clone, KB9, hereafter termed SHIV-KB9 [20]) infect rhesus monkeys and are useful for testing HIV-1 envelope-containing immunogens as vaccine candidates in rhesus monkey protection trials (31). SHIV-89.6 and SHIV-KB9 differ by 12 amino acids in their envelope glycoproteins, including one amino acid substitution of glutamic acid (E) (in SHIV-KB9) for arginine (R) (in SHIV-89.6) at position 305 of the V3 region of gp120 (20). Rhesus monkeys infected with SHIV-89.6 produce anti-SHIV neutralizing antibodies with a variety of specificities, most of which are not anti-V3 (11; D. C. Montefiori et al., unpublished data). Although studies with recombinant viruses indicate that V3 sequences can contribute to neutralization epitopes in some SHIV-infected monkeys, these neutralizing antibodies are rarely absorbed by V3 peptides (Montefiori et al., unpublished data). In this study, we have determined if peptides of the C4-V3 design (29) could induce antibodies that neutralized primary isolate SHIVs. Moreover, we have used peptides and mutant SHIV envelope constructs both to probe the specificities of the anti-SHIV V3 antibody responses and to map amino acids that determine anti-V3 antibody reactivity. We found that anti-V3 antibodies against SHIV-89.6 neutralized SHIV-89.6 but did not neutralize SHIV-KB9. However, sera from a subset of animals immunized with SHIV-KB9 V3 peptide neutralized both SHIV variants. Using mutant SHIV-89.6 and SHIV-KB9 envelope constructs, we showed that V3 amino acid 305 as well as sequences in the gp120 V1 and V2 regions contributed to the availability of primary isolate SHIV V3 regions for neutralizing antibody binding.
MATERIALS AND METHODS
Guinea pigs and rhesus monkeys.
Outbred guinea pigs were purchased from Harlan Sprague, Inc., Chicago, Ill., and housed in the Duke University Animal Facility. Animals were studied under AALAC guidelines with an animal use protocol approved by the Duke University Animal Use and Care Committee. Rhesus monkeys were housed in the Oregon Regional Research Primate Center, Portland, and the New England Regional Primate Research Center, Southborough, Mass. These animals were maintained in accordance with the guidelines of the Committee on Animals of Harvard Medical School and the National Institutes of Health guide for the care and use of laboratory animals (28).
HIV-1 synthetic peptides and proteins.
Synthetic peptides were synthesized by SynPep Corporation, Dublin, Calif., and purified by reverse-phase high-pressure liquid chromatography. Peptides used in this study were greater than 95% purified as determined by high-pressure liquid chromatography and mass spectrometry. The SHIV-89.6 V3 peptides (TRPNNNTRRRLSIGPGRAFYARR and NTRRRLSIGPGRAFYARRNIIGDIRQA) were derived from the HIV-1 primary isolate SHIV-89.6 Env gp120 V3 loop, while the SHIV-KB9 V3 peptides (TRPNNNTRERLSIGPGRAFYARR, NTRERLSIGPGRAFYARRNIIGDIRQA, and TRPNNNTRERLSIGPGRAFYARRNIIGDIRQA) were derived from the pathogenic strain SHIV-KB9 and had an arginine-to-glutamic acid substitution at amino acid 305 (20). To enhance peptide immunogenicity, both SHIV-89.6 and SHIV-KB9 V3 peptides were synthesized C terminal to the gp120 C4 region of the T-helper cell determinant (KQIINMWQEVGKAMYA) as described previously (6). For some studies, a control peptide (C4-V3 SHIV-89.6 Scrambled) that had a scrambled amino acid sequence of the SHIV-89.6 V3 loop (RGYFTRRNAPSNTARGRPILRRN), synthesized C terminal to the C4 T-helper-cell determinant, was used. Recombinant HIV-1 SHIV-89.6 Env gp120 monomer was prepared as described previously (33).
Formulation of peptides, dose, route of immunizations, and immunization schedule.
Lyophilized peptides stored at 4°C were reconstituted in H2O, and mixed in an emulsion in CFA or IFA (Sigma Chemical Co., St. Louis, Mo.) at a 1:1 volume ratio of peptide in H2O to CFA (used for the first immunization in guinea pigs) or IFA (used for subsequent immunizations in guinea pigs and for priming and boosting in rhesus monkeys). Two or more guinea pigs were used for each immunogen, and each animal was given either 250 μg of the synthetic peptides per injection or 50 μg of recombinant HIV-1 Env proteins per injection. Guinea pigs were injected with immunogens every 3 weeks (a total of five immunizations), with bleeds 10 days after each injection since the second immunization. Sera were isolated from blood, heat inactivated (56°C, 45 min), and stored at −20°C until use. Rhesus monkeys were given 1 mg of peptide per injection every 6 weeks (a total of three immunizations). Sera were isolated from blood 2 weeks after the third immunization, heat inactivated (56°C, 45 min), and stored at −20°C until use.
Other HIV-1 gp120 antisera.
To characterize the portion of the V3 loop present on the SHIV primary isolate, we used two additional rhesus monkey sera raised against HIV-1 isolate MN (HIV-1-MN) V3, sera 18987 and 17336 (17). Serum 18987 has previously been characterized as neutralizing different TCLA HIV-1 strains and binding to the sequence IGPGRA at the crown of the V3 loop. All neutralizing activity of 18987 serum was absorbed by the DP-2 peptide IGPGRAFIGPGRAFIGPGRAFC (17). As a control, serum 17336 is type specific for HIV-1-MN neutralization and maps to the IHI sequence to the left of the V3 loop crown (17).
HIV neutralization assays.
Neutralization activity of guinea pig sera was measured in the MT-2 cell-killing assay (25) with SHIV-89.6, the pathogenic clone SHIV-KB9, or HIV-1-MN. Viral stocks of both SHIV-89.6 and SHIV-KB9 that were used in neutralization were prepared in human peripheral blood mononuclear cells (PBMC). Neutralization for HIV-1 isolates BAL and JR-FL was measured in mitogen-stimulated human peripheral blood mononuclear cells (PBMC) by using the reduction in viral p24 synthesis as described previously (24).
Antipeptide and anti-gp120 serum antibody assays.
Antibody titers against the immunizing peptide and recombinant HIV-1 Env proteins were determined in standard enzyme-linked immunosorbent assays (ELISAs) (10). The antibody end point binding titers were determined as the reciprocal of the highest dilution of the serum assayed against corresponding peptides or recombinant HIV-1 Env proteins giving an experimental/control optical density ratio of ≥3.0.
Immunoprecipitation, SDS-PAGE, and Western blot analysis.
The ability of guinea pig antisera to recognize HIV-1 Env proteins was determined by radioimmunoprecipitation as described previously (34). Briefly, 35S-radiolabeled soluble gp120 was immunoprecipitated with various amounts of guinea pig sera. The amount of gp120 was normalized with HIV-1-positive patient serum. The immunoprecipitated envelope glycoproteins were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (10% acrylamide gel), and the intensity of each signal was measured by scanning densitometry. SDS-PAGE and Western blot assays were performed as described previously (4).
Envelope determinants of V3 neutralization.
A panel of 13 recombinant SHIV-KB9 or SHIV-89.6 viruses with defined Env glycoproteins were used as described previously (11) to characterize epitopes recognized by neutralizing antibodies (Fig. 1). In SHIV-KB9 mutants, amino acid changes that occurred in SHIV-KB9 during animal passage were reverted, individually or in combination, to the original envelope amino acids in the parent strain of SHIV-89.6. In the SHIV-89.6 mutants, amino acids of the envelope glycoproteins were individually or in combination changed to mimic the mutations that naturally occurred in SHIV-KB9 (20). Specific neutralizing activities of antisera from the SHIV-89.6 C4-V3-immunized guinea pigs and rhesus monkeys were determined by using a single-round Env complementation assay as described previously (11, 18). Briefly, viruses containing chloramphenical acetyltransferase (CAT) in their proviral genomes were pseudotyped with the various recombinant envelope glycoproteins. Viruses were produced in 293T cells, and viral titers were normalized to their reverse transcriptase activity. CEMx174 cells were infected with the recombinant CAT viruses in the presence of preimmune serum or 1:50 dilutions of antisera from immunized guinea pigs and rhesus monkeys. The CAT conversion value from samples with the preimmune serum was used as the baseline value for entry in the cell for each recombinant virus; the CAT conversion values for assays with neutralizing antisera were divided by the CAT conversion values for the corresponding preimmune sera. Thus, the resulting normalized conversion rates range between 0 and 1, where 1 represents the absence of neutralization and 0 represents complete neutralization. All of the neutralization assays were duplicated, and the average values are reported here.
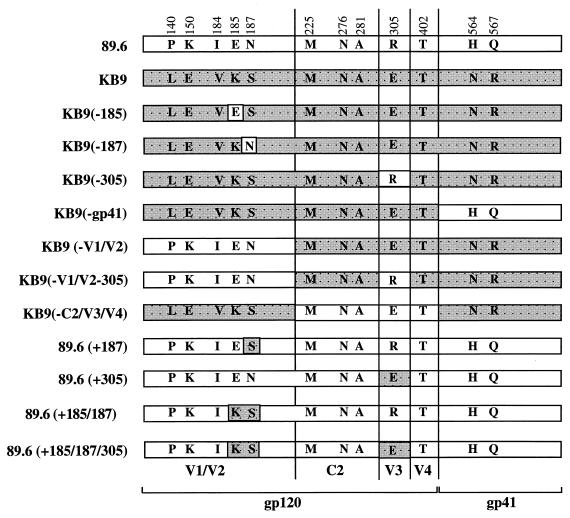
FIG. 1.
Schematic representation of SHIV-89.6 and SHIV-KB9 mutant envelope glycoproteins. Shown are the genetic compositions of 13 different SHIV-89.6 and SHIV-KB9 envelope glycoprotein mutants used in this study. The names of each individual envelope glycoprotein constructs is shown on the left. SHIV-89.6 envelope glycoprotein components are represented by white rectangles, and SHIV-KB9 components are represented by shaded rectangles. The SHIV-KB9 envelope glycoprotein contains 12 amino acid changes in gp120 and gp41 compared to SHIV-89.6 envelope glycoproteins. Amino acid substitutions, represented by single-letter code, are shown and identified by amino acid positions in HIV-1 envelope glycoproteins. KB9(-185), KB9(-187), and KB9(-305) have the same sequences of SHIV-KB9 except that the amino acid at position 185, 187, or 305, respectively, was reverted to wild type. The KB9(-gp41) envelope glycoprotein is identical to the SHIV-KB9 protein except that changes in the gp41 region envelope were reverted back to the wild-type amino acids originally present in SHIV-89.6. The KB9(-V1/V2) mutant contains sequences of SHIV-KB9 except that mutations in the V1/V2 regions have been reverted back to the wild-type sequence of SHIV-89.6. KB9(-V1/V2-305)) is the same as KB9(-V1/V2) except that the amino acid at position 305 was also reverted to the wild-type sequence of SHIV-89.6. KB9(-C2/V3/V4) contains SHIV-KB9-like mutations in the V1/V2 and gp41 regions. 89.6(+187), 89.6(+305), 89.6(+185/187), and 89.6(+185/187/305) contain the wild-type SHIV-89.6 sequence background except for changes that were made at position 185, 187, or 305 or in combination a of two (185 and 187) or three (185, 187, and 305) amino acids to mimic the mutations found in SHIV-KB9.
RESULTS
Ability of anti-SHIV-89.6 V3 sera to neutralize SHIV-89.6.
We have used the C4-V3 peptide design to determine if gp120 V3 peptide can induce neutralizing antibodies against primary isolate SHIVs. A summary of neutralizing activities of antisera from guinea pigs immunized with the SHIV-89.6 C4-V3 peptide is shown in Table 1. All four sera from the SHIV-89.6 C4-V3-immunized guinea pigs neutralized SHIV-89.6, with neutralizing antibody titers ranging from 1:169 to 1:2,501. When these sera were tested against HIV-1-MN, with V3 sequences substantially different from the V3 sequences of SHIV-89.6, two of four sera also cross-neutralized HIV-1-MN, with titers of 1:56 to 1:267 (Table 1). However, none of these sera neutralized the pathogenic SHIV strain, SHIV-KB9, or HIV-1 isolates BAL and JR-FL (Table 1). ELISAs were carried out to determine if anti-SHIV-89.6 V3 sera recognized the SHIV-KB9 C4-V3 sequence. As shown in Fig. 2, anti-SHIV-89.6 V3 sera reacted well to both SHIV-KB9 and SHIV-89.6 V3 peptides.
TABLE 1.
Ability of SHIV-89.6 C4-V3 peptide to induce antibodies that neutralize SHIV-89.6 and HIV-1-MN but not SHIV-KB9
| Guinea pig no. | Neutralization titera with SHIV-89.6 C4-V3 immunogen and SHIV or HIV-1 isolate: | ||||
|---|---|---|---|---|---|
| SHIV-89.6 | SHIV-KB9 | HIV-1-MN | BAL | JR-FL | |
| 33 | 169 | <30 | 56 | <5 | <5 |
| 34 | 2,501 | <30 | 267 | <5 | <5 |
| 51 | 1,617 | <30 | <30 | NDb | ND |
| 52 | 768 | <30 | <30 | ND | ND |
FIG. 2.
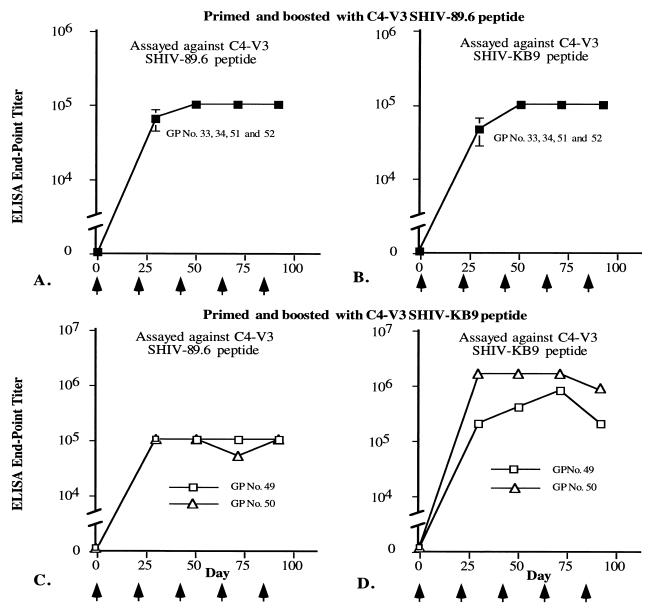
SHIV-89.6 and SHIV-KB9 C4-V3 peptides induced high titers of antibody responses. Serum samples collected from guinea pigs (GPs) 10 days after each injection (as indicated with arrows) with SHIV-89.6 C4-V3 (A and B) or SHIV-KB9 C4-V3 (C and D) were tested against captured SHIV-89.6 C4-V3 (A and C) or SHIV-KB9 C4-V3 (B and D) peptide. The end point titers of guinea pig sera against these peptides, which were determined as described in Materials and Methods, are shown on the y axis.
Ability of SHIV-89.6 and SHIV-KB9 anti-V3 sera to neutralize the pathogenic clone, SHIV-KB9.
When sera from guinea pigs immunized with the SHIV-KB9 C4-V3 peptide were tested for neutralizing antibody responses, three of six anti-SHIV-KB9 sera neutralized SHIV-89.6, with neutralizing titers ranging from 1:175 to 1:1,571, and three of six sera neutralized SHIV-KB9, with titers ranging from 1:45 to 1:150 (Table 2). These sera did not neutralize BAL or JR-FL when tested for their ability to neutralize primary HIV-1 isolates (Table 2). It is important to point out that the neutralization of SHIV-KB9 occurred at up to a log titer less than with SHIV-89.6. Two antisera (no. 49 and 50) that neutralized SHIV-KB9 had high ELISA titers of 1:819,2001 and 1:1,638,400, respectively, to SHIV-KB9 C4-V3 peptide (Fig. 2). Further, guinea pig antisera raised by both SHIV-89.6 and SHIV-KB9 C4-V3 peptides reacted strongly with the recombinant SHIV-89.6 envelope protein gp120 in Western blot assays (Fig. 3).
TABLE 2.
Ability of SHIV-KB9 C4-V3 peptide to induce antibodies that neutralize both SHIV-89.6 and SHIV-KB9
| Guinea pig no. | Neutralization titera with SHIV-KB9 C4-V3 immunogen and SHIV or HIV-1 isolate: | |||
|---|---|---|---|---|
| SHIV-89.6 | SHIV-KB9 | HIV-1-MN | BAL | |
| 49 | <30 | 64 | NDb | ND |
| 50 | 1,571 | 150 | 105 | ND |
| 75 | <30 | <30 | <30 | ND |
| 76 | 175 | 45 | 104 | ND |
| 81 | 176 | <30 | ND | <5 |
| 82 | <30 | <30 | <30 | <5 |
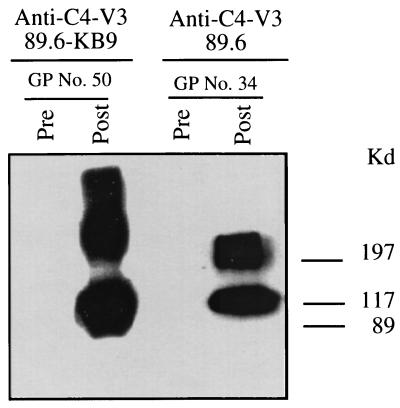
FIG. 3.
SHIV-89.6 and SHIV-KB9 C4-V3 peptides induced antibodies that reacted with the SHIV-89.6 recombinant envelope protein rgp120. SHIV-89.6 rgp120 was separated by SDS-PAGE (7% acrylamide gel) before being transferred to nitrocellulose filters. The immunoblots of SHIV-89.6 rgp120 were incubated with the indicated sera (at a 1:400 dilution) from guinea pigs (GPs) before or after immunization with SHIV-89.6 or SHIV-KB9 C4-V3 peptides, followed by goat anti-guinea pig immunoglobulin G–horseradish peroxidase, and then developed by chemiluminescence. Data are representative of three experiments.
Ability of rgp120 to induce neutralizing antibodies against SHIV-89.6 and SHIV-KB9.
As a control for C4-V3 peptide immunizations, guinea pigs were primed and boosted with the SHIV-89.6 gp120. An additional set of animals was primed once with SHIV-89.6 recombinant gp120 (rgp120) and boosted twice with SHIV-89.6 C4-V3 peptides. Two of two animals immunized with SHIV-89.6 rgp120 developed antibodies that neutralized SHIV-89.6, with neutralizing antibody titers of 1:203 and 1:209, respectively (Table 3), and also weakly neutralized SHIV-KB9 (Table 3). Both animals primed and boosted with SHIV-89.6 rgp120 as well as animals primed with SHIV-89.6 rgp120 and boosted with SHIV-89.6 C4-V3 peptide both neutralized SHIV-89.6 and weakly neutralized SHIV-KB9. Since SHIV-89.6 C4-V3 peptide alone did not induce anti-SHIV-KB9 neutralizing antibodies (Table 1), these data suggested that either additional neutralizing antibody responses against gp120 were induced by rgp120 priming or priming with rgp120 induced V3 antibodies that cross-neutralized SHIV-KB9 better than priming and boosting with SHIV-89.6 C4-V3 peptides alone.
TABLE 3.
Ability of SHIV-89.6 rgp120 to induce antibodies that neutralize both SHIV-89.6 and SHIV-KB9
| Guinea pig no. | Immunogen | Neutralization titera with SHIV isolate: | |
|---|---|---|---|
| SHIV-89.6 | SHIV-KB9 | ||
| 57 | Primed and boosted with SHIV-89.6 rgp120 | 208 | 62 |
| 58 | Primed and boosted with SHIV-89.6 rgp120 | 203 | 49 |
| 59 | Primed once with rgp120 and boosted twice with SHIV-89.6 C4-V3 | 49 | 41 |
| 60 | Primed once with rgp120 and boosted twice with SHIV-89.6 C4-V3 | 176 | 51 |
ELISAs showed that guinea pigs primed and boosted with rgp120 developed high titers of antibody that reacted with the immunizing antigen rgp120 but had only relatively low titers (1:3,200 to 1:102,400) of antibodies against the SHIV-89.6 V3 region (Fig. 4).
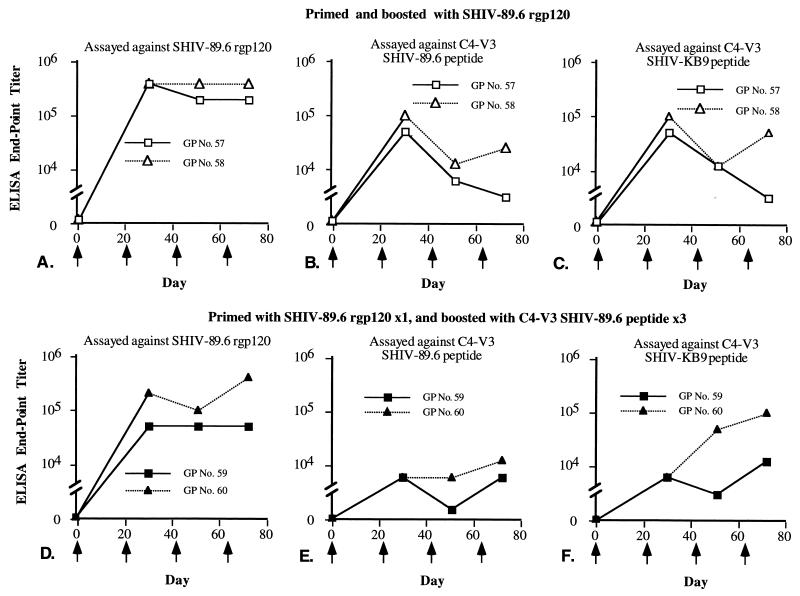
FIG. 4.
SHIV-89.6 rgp120 induced antibodies that reacted with SHIV-89.6 rgp120 and SHIV-89.6 and SHIV-KB9 C4-V3 peptides. Guinea pigs (GPs) were primed and boosted with rgp120 or primed with SHIV-89.6 rgp120 and then boosted three times with SHIV-89.6 C4-V3 peptide. Serum samples collected from guinea pigs 10 days after each immunization (arrows) were tested against captured rgp120 (A and D), SHIV-89.6 C4-V3 peptide (B and E), or SHIV-KB9 C4-V3 peptide (C and F). The end point titers of serum samples are shown on the y axis.
Relative ability of antibodies raised against SHIV-89.6 and SHIV-KB9 C4-V3 peptides to bind to SHIV-89.6 and SHIV-KB9 gp120 monomers.
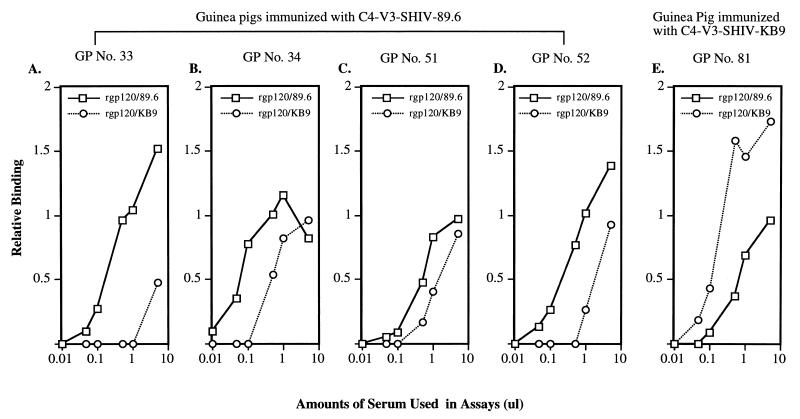
The ability of antibodies raised against SHIV-89.6 and SHIV-KB9 C4-V3 peptides to bind SHIV-89.6 and SHIV-KB9 gp120 monomers was determined by using 35S-labeled SHIV-89.6 and SHIV-KB9 gp120 in immunoprecipitation assays. We found that sera from guinea pigs immunized with either the SHIV-89.6 or SHIV-KB9 C4-V3 peptide bound to SHIV-89.6 and SHIV-KB9 gp120 monomers in solution (Fig. 5). However, sera from guinea pigs immunized with SHIV-89.6 C4-V3 had greater binding to SHIV-89.6 gp120 than to SHIV-KB9 gp120 (Fig. 5A to D). Conversely, sera from SHIV-KB9 C4-V3 peptide-immunized guinea pigs had higher binding to SHIV-KB9 gp120 than to SHIV-89.6 gp120 (Fig. 5E). These results, taken together with neutralization data, demonstrated that mutations in the SHIV-KB9 envelope changed the neutralization sensitivities of SHIV-89.6 and SHIV-89.6P.
FIG. 5.
Ability of antibodies raised against SHIV-89.6 and SHIV-KB9 peptides to bind SHIV-89.6 and SHIV-KB9 gp120 monomers. 35S-radiolabeled soluble gp120 was immunoprecipitated with various amounts of guinea pig (GP) sera. The immunoprecipitated envelope glycoproteins were subjected to SDS-PAGE (10% acrylamide gel), and the intensity of each signal was measured by scanning densitometry. The vertical axis shows relative binding, using arbitrary densitometric values. The x axis shows the amounts of guinea pig sera used in the binding assays.
Mapping of V3 amino acids responsible for neutralization responses of antisera against SHIV-89.6.
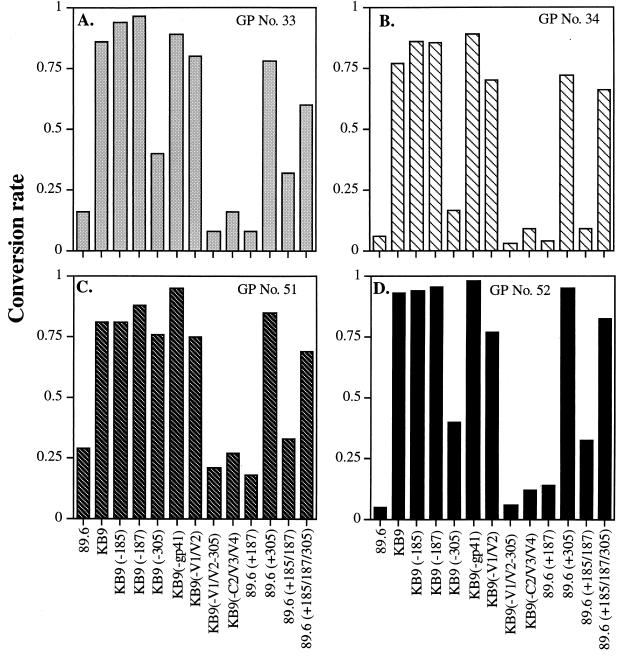
We next tested a panel of SHIV-KB9 and SHIV-89.6 Env mutants (Fig. 1) to characterize the neutralization epitopes recognized by SHIV-89.6 anti-V3 sera. Figure 6A to D shows the results of studies of neutralization of recombinant viruses bearing SHIV-KB9 and SHIV-89.6 Env mutants with four anti-C4-V3 guinea pig sera raised against SHIV-89.6 C4-V3 peptide. Recombinant viruses with SHIV-89.6 envelope glycoproteins were neutralized efficiently by sera from four guinea pigs immunized with SHIV-89.6 C4-V3 peptides, whereas the recombinant viruses with SHIV-KB9 envelope glycoproteins were not neutralized. Viruses containing mutant SHIV-KB9 envelope glycoproteins, in which amino acids were reverted to those of the parental SHIV-89.6 in the V2 region (amino acids 185 and 187) and in gp41, remained resistant to neutralization by SHIV-89.6 anti-V3 sera (Fig. 6). These results indicated that the specific amino acid changes in the V2 region and/or in the gp41 region of SHIV-89.6 did not by themselves play a major role in determining availability of the SHIV-KB9 V3 neutralization epitope.
FIG. 6.
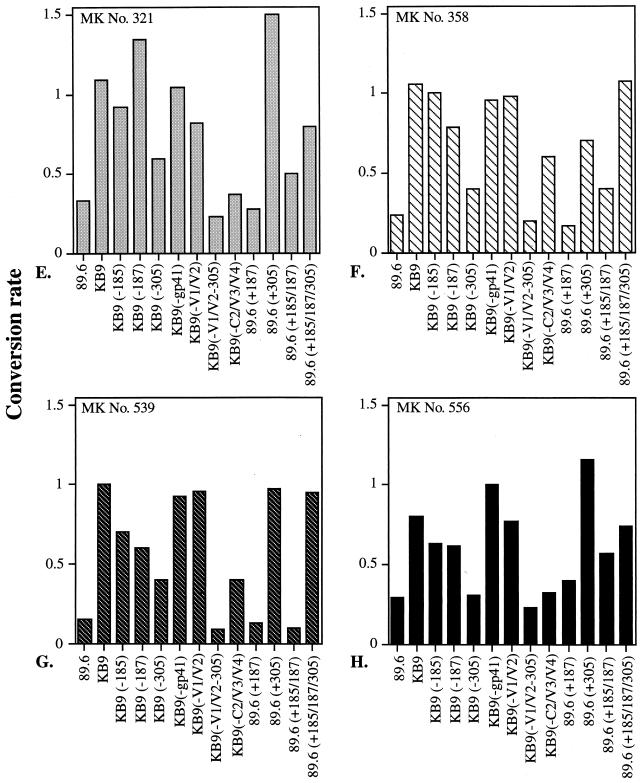
Neutralization of SHIV-89.6 and SHIV-KB9 mutant envelope glycoproteins by anti-C4-V3 SHIV-89.6 sera. Abbreviations for recombinant viruses containing envelope glycoproteins mutants used in this study are illustrated in Fig. 1 and described in detail in the Fig. 1 legend. Recombinant viruses containing envelope glycoprotein mutants as indicated on the x axis were tested for neutralization with serum samples from guinea pigs (GPs) (A to D) and rhesus monkeys (MKs) (E to H) immunized with SHIV-89.6 C4-V3 peptides. Neutralization assays were carried out as described in Materials and Methods. The y axis shows the conversion rates, reflecting the levels of neutralization, which were normalized to the value observed in the presence of preimmune serum. A value of 1 indicates no neutralization, and 0 represents complete neutralization. All of the neutralization assays were duplicated, and the averages are reported here.
In contrast, viruses containing SHIV-KB9 envelope glycoproteins in which glutamic acid (E) at position 305 was reverted back to arginine (R) at position 305 became neutralizable by most of the SHIV-89.6 anti-V3 sera at the same level as viruses containing SHIV-89 envelope glycoproteins (Fig. 6A to D). Viruses containing KB9(-V1/V2) envelope glycoproteins, which contain all mutations in SHIV-KB9, except for the parental SHIV-89.6 sequence in the V1 and V2 regions (Fig. 1), were resistant to neutralization by SHIV-89.6 anti-V3 sera (Fig. 6). The addition of the SHIV-89.6-like amino acid (R) at position 305 rendered the KB9(-V1/V2) envelope glycoproteins completely susceptible to neutralization. These results suggested that mutations at position 305 played a critical role in forming the V3 neutralizing epitope recognized by our SHIV-89.6 C4-V3 peptide antisera. SHIV-KB9 sequences outside the V3 loop, particularly in the V1/V2 regions, influenced the degree of sensitivity to neutralization.
These results were also confirmed by using viruses containing SHIV-89.6 envelope glycoprotein mutants. Viruses containing SHIV-89.6 envelope glycoproteins with mutations at position 185 (V2) or 185 (V2) plus 187 (V2) and viruses containing KB9(-C2/V3/C4) mutant envelope glycoprotein (with the SHIV-89.6 gp120 sequence except for the KB9-like V1/V2 loops and gp41 region) were neutralized to the same degree as viruses with the parental SHIV-89.6 envelope glycoproteins (Fig. 6A to D). Viruses bearing SHIV-89.6 envelope glycoproteins with a SHIV-KB9-like change at position 305 (i.e., R for E), like SHIV-KB9, were resistant to neutralization by SHIV-89.6 anti-V3 sera (Fig. 6).
By comparison of viruses containing envelope glycoproteins from KB9(-V1/V2-305) or from KB9(-305), viruses containing the KB9(-V1/V2-305) envelope glycoproteins were significantly (P < 0.05) more susceptible to SHIV-89.6 anti-V3 sera than were viruses containing KB9(-305) envelope glycoproteins (Fig. 6). These results indicate that mutations in the V1/V2 regions play secondary roles in determining the neutralization sensitivity of viruses with particular V3 neutralization epitopes.
Results similar to those described above were obtained with serum samples from rhesus monkeys. Here, sera from rhesus monkeys immunized with SHIV-89.6 C4-V3 peptide neutralized recombinant viruses containing SHIV-89.6 envelope glycoproteins but not viruses containing SHIV-KB9 envelope glycoproteins (Fig. 6E to H). Also, the ability of those serum samples to neutralize mutant viruses was highly dependent on amino acids changes in V1/V2 and at position 305 in the V3 loop (Fig. 6E to H). These results demonstrate that the primary isolate V3-specific responses generated in guinea pigs can also be achieved in primates.
Availability of the crown of the V3 loop on SHIV-89.6 and SHIV-KB9.
We have previously generated and characterized a rhesus monkey anti-V3 antiserum, 18987, that broadly neutralizes TCLA HIV-1 strains and binds to the sequence IGPGRAF at the crown of the HIV-1-MN V3 loop (17). An additional, control rhesus monkey serum, 17336, that was type specific for HIV-1-MN and binds on the left side of the HIV-1-MN gp120 V3 loop was also made (17). SHIV-89.6 and SHIV-KB9 both have the sequence IGPGRAF at the V3 crown. We found that these sera did not neutralize either SHIV-89.6 or SHIV-KB9 in vitro, while as previously reported, they did neutralize HIV-1-MN (Table 4). ELISA studies demonstrated that 18987 serum bound weakly to SHIV-89.6 and SHIV-KB9 V3 peptides (Table 5). Taken together, these data suggest that the crown of the V3 loop of SHIV-89.6 or SHIV-89.6P was not in a conformation to be recognized by rhesus monkey serum 18987.
TABLE 4.
Ability of sera from rhesus monkeys immunized with HIV-1-MN C4-V3 peptide to neutralize the TCLA HIV-1-MN but not SHIV-89.6 or SHIV-KB9
| Rhesus monkey no. | Neutralization titera with: HIV-1 or SHIV isolate: | ||
|---|---|---|---|
| HIV-1-MN | SHIV-89.6 | SHIV-KB9 | |
| 18987 | 397 | <20 | <20 |
| 17366 | 547 | <20 | <20 |
TABLE 5.
ELISAs of serum samples from rhesus monkeys and guinea pigs against HIV-1-MN, SHIV-89.6, SHIV-KB9, and DP-2 peptides
| Animal and serum sample | C4-V3 immunogen | ELISA titer against the following SHIV or HIV-1 peptidea: | |||
|---|---|---|---|---|---|
| SHIV-89.6 C4-V3 | SHIV-KB9 C4-V3 | DP-2b | HIV-1-MN C4-V3 | ||
| Rhesus monkeys | |||||
| 18987 | HIV-1-MN | 6,400 | 25,600 | 1,600 | 102,400 |
| 17366 | HIV-1-MN | 6,400 | 6,400 | 400 | 102,400 |
| Guinea pigs | |||||
| 33 | SHIV-89.6 | 25,600 | 102,400 | 800 | NDc |
| 34 | SHIV-89.6 | 102,400 | 102,400 | 12,800 | ND |
| 51 | SHIV-89.6 | 204,800 | 409,600 | 25,600 | ND |
| 52 | SHIV-89.6 | 819,200 | 819,200 | 25,600 | ND |
| 49 | SHIV-KB9 | 102,400 | 204,800 | 400 | ND |
| 50 | SHIV-KB9 | 409,600 | 819,200 | 102,400 | ND |
| 75 | SHIV-KB9 | 204,800 | 204,800 | 25,600 | ND |
| 76 | SHIV-KB9 | 819,200 | 409,600 | 102,400 | ND |
| 81 | SHIV-KB9 | 102,400 | 204,800 | 6,400 | ND |
| 82 | SHIV-KB9 | 102,400 | 409,600 | 25,600 | ND |
Next, the six SHIV-KB9 anti-V3 antisera were tested for reactivity to the DP-2 (IGPGRAFIGPGRAFIGPGRAFC) peptide (17). We found that the two sera that had the highest neutralizing titers (Table 2) against SHIV-KB9 also bound well (titer of 1:102,400) to the DP-2 peptide. Taken together, these data suggested that the crown of the SHIV-KB9 V3 loop can be immunogenic in the context of the SHIV-KB9 C4-V3 peptide (albeit inconsistently) and that, again, the IGPGRAF V3 crown of SHIV-89.6 and SHIV-KB9 is in a conformation that is different from that in HIV-1-MN (17) but that the V3 loop and likely the crown are at least in part available for antibody binding on the surfaces of SHIV-89.6 and SHIV-89.6P for neutralization to occur.
DISCUSSION
An important question in HIV-1 vaccine development is to determine an immunogen design that is capable of inducing antibodies that neutralize HIV-1 primary isolates. The fundamental question regarding the V3 loop as a neutralizing antibody target is whether the V3 loop, either alone or in combination with other subunit epitopes, will be a component of an effective HIV-1 vaccine. Recent data suggest that the gp120 V3 loop is near the chemokine coreceptor binding region of gp120 and may in and of itself interact with chemokine receptors (7). The fact that the C4-V3 peptide from SHIV-KB9 can induce antibodies that neutralize SHIV-KB9 suggests that the V3 neutralizing region is available on the surface of SHIV-KB9 for antibody binding. However, the titers of neutralizing antibodies induced by SHIV-KB9 C4-V3 are a log unit lower against SHIV-KB9 than the antibodies induced by SHIV-89.6 V3 peptides against SHIV-89.6 and are consistent with the notion that the V3 region of SHIV-KB9 is less exposed for efficient antibody binding than is that of SHIV-89.6.
Although the C4 region of HIV-1, which is conserved among HIV-1 isolates, may induce mouse neutralizing antibodies (27), the neutralizing activity of sera generated from guinea pigs and rhesus monkeys by C4-V3 peptides of SHIV-89.6 and SHIV-KB9 is V3 specific, since the neutralizing activity can be completely absorbed by V3 peptides (Montefiori et al., unpublished data). Further, we have previously shown that the C4 region is a very poor inducer of antibody in most animal species (38).
Karlsson et al. have demonstrated that in a comparison of SHIV-KB9 to the parental SHIV-89.6, the SHIV-KB9 envelope binds with more avidly to CCR5 and is more difficult to be neutralized by both CD4 binding site and V3 region antibodies (21).
Another critical issue is what parts of the gp120 V3 loop are available for antibody binding on primary isolates. Epitope mapping demonstrated that anti-SHIV-89.6 V3 antibodies required the amino acid R at position 305 to bind. Thus, the left side of the V3 loop is clearly needed for neutralizing antibody binding, although we cannot be certain at this time whether it contains the antibody epitope or affects the structure of the cognate epitope distally. The crown of the V3 loop is also immunogenic in SHIV-KB9 C4-V3 peptide-immunized animals, as those sera that best neutralized SHIV-KB9 also bound best the V3 crown peptide, DP-2. However, since the anti-IGPGRAF serum, 18987, raised against the HIV-1-MN C4-V3 peptide did not neutralize SHIV-89.6 or SHIV-KB9 (both of which contain the V3 crown sequence IGPGRAF) we know that the secondary and higher-order structures of the SHIV-89.6 and -KB9 V3 crowns are different from those of the TCLA strain, HIV-1-MN.
Mutant SHIV viruses containing KB9(-V1/V2-305) envelope glycoproteins were neutralized significantly better (P < 0.05) than viruses containing the KB9(-305) envelope glycoproteins. This was true for sera from immunized guinea pigs and rhesus monkeys and suggested that the changes in the V1/V2 loops participate in creating conformational changes that mask or alter the neutralization epitope in the V3 region. It is also important to recognize that the masking effects on the V3 region by the V1/V2 loops are not strong, as demonstrated by the fact (Fig. 6) that the KB9(-C2/V3/C4) envelope (Fig. 1) was neutralizable by anti-V3 sera, although KB9(-C2/V3/C4) envelope glycoproteins had the same mutations in the V1/V2 and gp41 regions as seen in SHIV-KB9.
It is important to note that rhesus monkeys infected with SHIV-89.6 only occasionally make potent anti-V3 antibodies (11; Montefiori et al., unpublished data), whereas it is possible to directly induce anti-V3 antibodies with C4-V3 peptides. Thus, one advantage of the C4-V3 immunogen design is that C4-V3 peptides induce neutralizing anti-V3 antibodies, whereas natural infection frequently does not.
Finally, our study does not address the issue of HIV-1 variability and the type-specific and restricted nature of anti-V3 neutralizing antibody specificity. If V3 subunits are to be useful for HIV-1 vaccine development, subunits reflecting the sequences of many primary isolate envelopes clearly will be needed (15, 16). Our study does show that when the V3 immunogen sequences match those of the primary isolates, such as in the cases of SHIV-89.6 and SHIV-KB9, one can induce anti-primary isolate neutralizing antibodies. Protection trials with rhesus monkeys to determine if these C4-V3-induced antibodies can protect against SHIV-89.6 and/or SHIV-KB9 infection are under way.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grants AI35351, AI33832, and 31783 from the National Institutes of Health. Duke University and the Dana-Farber Cancer Institute are recipients of Centers for AIDS Research awards from the National Institutes of Health. This work was also supported by the Mathers Charitable Foundation, the Friends 10, Douglas and Judy Krupp, and the late William F. McCarty-Cooper.
We thank Kim R. McClammy for expert secretarial support.
REFERENCES
- 1.Beddows S, Louisirirotchanakul S, Cheingsong-Popov R, Easterbrook P J, Simmonds P, Weber J. Neutralization of primary and T-cell line adapted isolates of human immunodeficiency virus type 1: role of V3-specific antibodies. J Gen Virol. 1998;79:77–82. doi: 10.1099/0022-1317-79-1-77. [DOI] [PubMed] [Google Scholar]
- 2.Belshe R B, Gorse G J, Mulligan M J, Evans T G, Keefer M C, Excler J-L, Duliege A-M, Tartaglia J, McNamara J, Kai-Lin H, Montefiori D, Weinhold K. Rapid induction of HIV-1 immune response by canarypox (ALVAC) HIV-1 and gp120 SF2 recombinant vaccines in uninfected volunteers. AIDS. 1998;12:2407–2415. doi: 10.1097/00002030-199818000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Bou-Habib D C, Roderiquez G, Oravecz T, Berman P W, Lusso P, Norcross M A. Cryptic nature of envelope V3 region epitopes protects primary monocytotropic human immunodeficiency virus type 1 from antibody neutralization. J Virol. 1994;68:6006–6013. doi: 10.1128/jvi.68.9.6006-6013.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bronstein I, Voyta J C, Murphy O J, Bresnick L, Kricka L J. Improved chemiluminescent western blotting procedure. BioTechniques. 1992;12:748–753. [PubMed] [Google Scholar]
- 5.Burton D R, Montefiori D C. The antibody response in HIV-1 infection. AIDS. 1997;11:S87–S98. [PubMed] [Google Scholar]
- 6.Cease K B, Margalit H, Cornette J L, Putney S D, Robey W G, Ouyang C, Streicher H Z, Fischinger P J, Gallo R C, DeLisi C, et al. Helper T-cell antigenic site identification in the acquired immunodeficiency syndrome virus gp120 envelope protein and induction of immunity in mice to the native protein using a 16-residue synthetic peptide. Proc Natl Acad Sci USA. 1987;84:4249–4253. doi: 10.1073/pnas.84.12.4249. . (Erratum, 85:8226, 1988.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cocchi F, DeVico A L, Garzino-Demo A, Cara A, Gallo R C, Lusso P. The V3 domain of the HIV-1 gp120 envelope glycoprotein is critical for chemokine-mediated blockade of infection. Nat Med. 1996;2:1244–1247. doi: 10.1038/nm1196-1244. [DOI] [PubMed] [Google Scholar]
- 8.Conley A J, Conard P, Bondy S, Dolan C A, Hannah J, Leanza W J, Marburg S, Rivetna M, Rusiecki V K, Sugg E E, et al. Immunogenicity of synthetic HIV-1 gp120 V3-loop peptide-conjugate immunogens. Vaccine. 1994;12:445–451. doi: 10.1016/0264-410x(94)90123-6. [DOI] [PubMed] [Google Scholar]
- 9.Conley A J, Gorny M K, Kessler II J A, Boots L J, Ossorio-Castro M, Koenig S, Lineberger D W, Emini E A, Williams C, Zolla-Pazner S. Neutralization of primary human immunodeficiency virus type 1 isolates by the broadly reactive anti-V3 monoclonal antibody, 447-52D. J Virol. 1994;68:6994–7000. doi: 10.1128/jvi.68.11.6994-7000.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engvall E, Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972;109:129–135. [PubMed] [Google Scholar]
- 11.Etemad-Moghadam B, Karlsson G B, Halloran M, Sun Y, Schenten D, Fernandes M, Letvin N L, Sodroski J. Characterization of simian-human immunodeficiency virus envelope glycoprotein epitopes recognized by neutralizing antibodies from infected monkeys. J Virol. 1998;72:8437–8445. doi: 10.1128/jvi.72.10.8437-8445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gorny M K, Mascola J R, Israel Z R, VanCott T C, Williams C, Balfe P, Hioe C, Brodine S, Burda S, Zolla-Pazner S. A human monoclonal antibody specific for the V3 loop of HIV type 1 clade E cross-reacts with other HIV type 1 clades. AIDS Res Hum Retrovir. 1998;14:213–221. doi: 10.1089/aid.1998.14.213. [DOI] [PubMed] [Google Scholar]
- 13.Gorny M K, VanCott T C, Hioe C, Israel Z R, Michael N L, Conley A J, Williams C, Kessler J A, 2nd, Chigurupati P, Burda S, Zolla-Pazner S. Human monoclonal antibodies to the V3 loop of HIV-1 with intra- and interclade cross-reactivity. J Immunol. 1997;159:5114–5122. [PubMed] [Google Scholar]
- 14.Hanson C V. Measuring vaccine-induced HIV neutralization: report of a workshop. AIDS Res Hum Retrovir. 1994;10:645–648. doi: 10.1089/aid.1994.10.645. [DOI] [PubMed] [Google Scholar]
- 15.Haynes B F. HIV vaccines: where we are and where we are going. Lancet. 1996;348:933–937. doi: 10.1016/S0140-6736(96)09339-7. [DOI] [PubMed] [Google Scholar]
- 16.Haynes B F, Putman S B, Weinberg J B. Update on the issues of HIV vaccine development. Ann Med. 1996;28:39–41. doi: 10.3109/07853899608999072. [DOI] [PubMed] [Google Scholar]
- 17.Haynes B F, Torres J V, Langlois A J, Bolognesi D P, Gardner M B, Palker T J, Scearce R M, Jones D M, Moody M A, McDanal C, et al. Induction of HIVMN neutralizing antibodies in primates using a prime-boost regimen of hybrid synthetic gp120 envelope peptides. J Immunol. 1993;151:1646–1653. [PubMed] [Google Scholar]
- 18.Helseth E, Kowalski M, Gabuzda D, Olshevsky U, Haseltine W, Sodroski J. Rapid complementation assays measuring replicative potential of human immunodeficiency virus type 1 envelope glycoprotein mutants. J Virol. 1990;64:2416–2420. doi: 10.1128/jvi.64.5.2416-2420.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Javaherian K, Langlois A J, McDanal C, Ross K L, Eckler L I, Jellis C L, Profy A T, Rusche J R, Bolognesi D P, Putney S D, et al. Principal neutralizing domain of the human immunodeficiency virus type 1 envelope protein. Proc Natl Acad Sci USA. 1989;86:6768–6772. doi: 10.1073/pnas.86.17.6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karlsson G B, Halloran M, Li J, Park I W, Gomila R, Reimann K A, Axthelm M K, Iliff S A, Letvin N L, Sodroski J. Characterization of molecularly cloned simian-human immunodeficiency viruses causing rapid CD4+ lymphocyte depletion in rhesus monkeys. J Virol. 1997;71:4218–4225. doi: 10.1128/jvi.71.6.4218-4225.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karlsson G B, Halloran M, Schenten D, Lee J, Racz P, Tenner-Racz K, Manola J, Gelman R, Etemad-Moghadam B, Desjardins E, Wyatt R, Gerard N P, Marcon L, Margolin D, Fanton J, Axthelm M K, Letvin N L, Sodroski J. The envelope glycoprotein ectodomains determine the efficiency of CD4+ T lymphocyte depletion in simian-human immunodeficiency virus-infected macaques. J Exp Med. 1998;188:1159–1171. doi: 10.1084/jem.188.6.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li J T, Halloran M, Lord C I, Watson A, Ranchalis J, Fung M, Letvin N L, Sodroski J G. Persistent infection of macaques with simian-human immunodeficiency viruses. J Virol. 1995;69:7061–7067. doi: 10.1128/jvi.69.11.7061-7067.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mascola J R, Snyder S W, Weislow O S, Belay S M, Belshe R B, Schwartz D H, Clements M L, Dolin R, Graham B S, Gorse G J, Keefer M C, McElrath M J, Walker M C, Wagner K F, McNeil J G, McCutchan F E, Burke D S. Immunization with envelope subunit vaccine products elicits neutralizing antibodies against laboratory-adapted but not primary isolates of human immunodeficiency virus type 1. The National Institute of Allergy and Infectious Diseases AIDS Vaccine Evaluation Group. J Infect Dis. 1996;173:340–348. doi: 10.1093/infdis/173.2.340. [DOI] [PubMed] [Google Scholar]
- 24.Montefiori D C, Pantaleo G, Fink L M, Zhou J T, Zhou J Y, Bilska M, Miralles G D, Fauci A S. Neutralizing and infection-enhancing antibody responses to human immunodeficiency virus type 1 in long-term nonprogressors. J Infect Dis. 1996;173:60–67. doi: 10.1093/infdis/173.1.60. [DOI] [PubMed] [Google Scholar]
- 25.Montefiori D C, Robinson W E, Jr, Schuffman S S, Mitchell W M. Evaluation of antiviral drugs and neutralizing antibodies to human immunodeficiency virus by a rapid and sensitive microtiter infection assay. J Clin Microbiol. 1988;26:231–235. doi: 10.1128/jcm.26.2.231-235.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moore J P. HIV vaccines. Back to primary school. Nature. 1995;376:115. doi: 10.1038/376115a0. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura G R, Byrn R, Wilkes D M, Fox J A, Hobbs M R, Hastings R, Wessling H C, Norcross M A, Fendly B M, Berman P W. Strain specificity and binding affinity requirements of neutralizing monoclonal antibodies to the C4 domain of gp120 from human immunodeficiency virus type 1. J Virol. 1993;67:6179–6191. doi: 10.1128/jvi.67.10.6179-6191.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Institutes of Health. Guide for the care and use of laboratory animals. U.S. Department of Health and Human Services publication no. 82–23. Bethesda, Md: National Institutes of Health; 1985. [Google Scholar]
- 29.Palker T J, Clark M E, Langlois A J, Matthews T J, Weinhold K J, Randall R R, Bolognesi D P, Haynes B F. Type-specific neutralization of the human immunodeficiency virus with antibodies to env-encoded synthetic peptides. Proc Natl Acad Sci USA. 1988;85:1932–1936. doi: 10.1073/pnas.85.6.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poignard P, Klasse P J, Sattentau Q J. Antibody neutralization of HIV-1. Immunol Today. 1996;17:239–246. doi: 10.1016/0167-5699(96)10007-4. [DOI] [PubMed] [Google Scholar]
- 31.Reimann K A, Li J T, Veazey R, Halloran M, Park I W, Karlsson G B, Sodroski J, Letvin N L. A chimeric simian/human immunodeficiency virus expressing a primary patient human immunodeficiency virus type 1 isolate Env causes an AIDS-like disease after in vivo passage in rhesus monkeys. J Virol. 1996;70:6922–6928. doi: 10.1128/jvi.70.10.6922-6928.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reimann K A, Li J T, Voss G, Lekutis C, Tenner-Racz K, Racz P, Lin W, Montefiori D C, Lee-Parritz D E, Lu Y, Collman R G, Sodroski J, Letvin N L. An env gene derived from a primary human immunodeficiency virus type 1 isolate confers high in vivo replicative capacity to a chimeric simian/human immunodeficiency virus in rhesus monkeys. J Virol. 1996;70:3198–3206. doi: 10.1128/jvi.70.5.3198-3206.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richardson T M, Jr, Stryjewski B L, Broder C C, Hoxie J A, Mascola J R, Earl P L, Doms R W. Humoral response to oligomeric human immunodeficiency virus type 1 envelope protein. J Virol. 1996;70:753–762. doi: 10.1128/jvi.70.2.753-762.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rizzuto C D, Wyatt R, Hernandez-Ramos N, Sun Y, Kwong P D, Hendrickson W A, Sodroski J. A conserved HIV gp120 glycoprotein structure involved in chemokine receptor binding. Science. 1998;280:1949–1953. doi: 10.1126/science.280.5371.1949. [DOI] [PubMed] [Google Scholar]
- 35.Spenlehauer C, Saragosti S, Fleury H J, Kirn A, Aubertin A M, Moog C. Study of the V3 loop as a target epitope for antibodies involved in the neutralization of primary isolates versus T-cell-line-adapted strains of human immunodeficiency virus type 1. J Virol. 1998;72:9855–9864. doi: 10.1128/jvi.72.12.9855-9864.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sullivan N, Sun Y, Li J, Hofmann W, Sodroski J. Replicative function and neutralization sensitivity of envelope glycoproteins from primary and T-cell line-passaged human immunodeficiency virus type 1 isolates. J Virol. 1995;69:4413–4422. doi: 10.1128/jvi.69.7.4413-4422.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vancott T C, Polonis V R, Loomis L D, Michael N L, Nara P L, Birx D L. Differential role of V3-specific antibodies in neutralization assays involving primary and laboratory-adapted isolates of HIV type 1. AIDS Res Hum Retrovir. 1995;11:1379–1391. doi: 10.1089/aid.1995.11.1379. [DOI] [PubMed] [Google Scholar]
- 38.Vu H M, Myers D, de Lorimier R, Matthews T J, Moody M A, Heinly C, Torres J V, Haynes B F, Spicer L. Nuclear magnetic resonance analysis of solution conformations in C4-V3 hybrid peptides derived from human immunodeficiency virus (HIV) type 1 gp120: relation to specificity of peptide-induced anti-HIV neutralizing antibodies. J Virol. 1999;73:746–750. doi: 10.1128/jvi.73.1.746-750.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]