Productive Replication of Human Adenoviruses in Mouse Epidermal Cells (original) (raw)
Abstract
In contrast to most cells of mouse origin, cell lines derived from mouse epidermis are permissive for replication of human adenovirus type 5. The extent of epidermal cell differentiation correlated with the level of E1A expression and virus replication. Mouse epidermal cells may provide useful models for cancer therapy using replication-competent human adenoviruses.
Human adenoviruses have great potential for human gene therapy as replication-defective delivery vehicles or as replication-competent viruses for the treatment of cancer (6, 8, 13, 20, 23). These approaches would benefit from the availability of suitable mouse models to test the efficacy of different therapeutic strategies. At present, there is no immunocompetent mouse model to test replicating adenoviruses (2, 11) as previous work suggests that both the infectivity and productive replication of adenoviruses in rodent cells are poor (4, 9). In these model systems, although some evidence for limited replication has been obtained (12, 17, 18), a productive infection leading to an efficient viral burst has not been seen. This apparent block has been attributed to species-specific properties of mouse cells leading to repression of early viral protein expression (7) or defects at other points of the viral life cycle (5, 16).
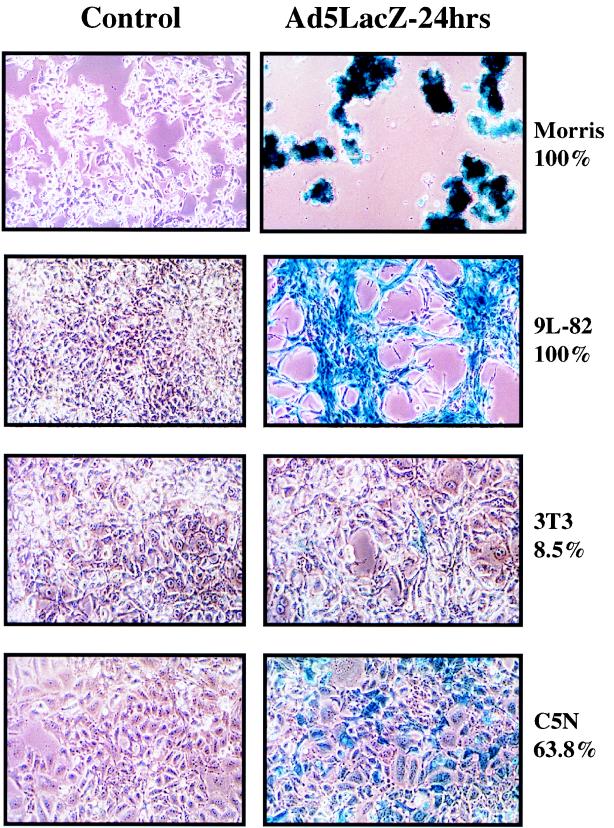
We have investigated replication of adenovirus type 2 (Ad2) in a series of mouse epidermal cell lines (3, 19, 22), together with cells from a variety of mouse tissues (Table 1). A nonreplicating E1-deleted adenovirus with a lacZ reporter construct (CMVlacZ virus) (14) was used to determine infectivity. The percentages of β-galactosidase (β-Gal)-positive cells at multiplicities of infection (MOIs) of 10 and 100 PFU/cell for several different rodent cell lines are shown in Table 1. Infectivity was very high with specific tissue types, notably mouse epidermal cells (Fig. 1), mouse kidney adenocarcinoma TCMK1 cells, rat glioblastoma 9L-82 cells, rat thyroid carcinoma VH1 VRS2 cells, and rat Morris hepatoma cells. Infectivity was very low in mouse Lewis lung carcinoma cells, rat colon carcinoma K12/TrB cells, and 3T3 fibroblasts (21) (Fig. 1). Surprisingly, infectivity was higher for many of the rodent cell types than for the human ovarian cell line A2780Cp.
TABLE 1.
Infectivity, relative E1A expression, CPE, and immunofluorescence for hexon protein of wild-type adenovirus-infected rodent cell linesa
| Cell line | Species | Tissue | Infectivity (% of β-Gal-positive cells at 24 h [mean ± SE]) at indicated MOI | Relative E1A expression (%) | CPEb | IFb | |
|---|---|---|---|---|---|---|---|
| 10 PFU/cell | 100 PFU/cell | ||||||
| A2780Cp70 | Human | Ovarian adenocarcinoma | 14.8 ± 0.9 | 40.4 ± 1.6 | ND | + | + |
| B16F1 | Mouse | Melanoma | 0.7 ± 0 | 4.8 ± 0.8 | 30.8 | − | − |
| Lewis | Mouse | Lung carcinoma | 0.5 ± 0.2 | 1.5 ± 0.2 | 12.3 | − | − |
| 3T3 | Mouse | Fibroblasts | 1 ± 0 | 8.5 ± 1.3 | ND | − | − |
| K12/TrB | Rat | Colon carcinoma | 3.5 ± 0.6 | 8.3 ± 1.1 | 48.9 | + | + |
| TCMK1 | Mouse | Kidney adenocarcinoma | 19.5 ± 0.8 | 36.6 ± 1.6 | 6.3 | − | − |
| Morris | Rat | Liver hepatoma | 59.6 ± 4.1 | 100 ± 0 | 100 | − | − |
| 9L-82 | Rat | Glioblastoma | 36.7 ± 2.4 | 100 ± 0 | 19.2 | − | − |
| VH1 VRS2 | Rat | Thyroid carcinoma | 31.3 ± 3.7 | 46.4 ± 3.2 | 85.2 | + | + |
| A5 | Mouse | Epidermal spindle cell carcinoma | 22 ± 1.8 | 63.5 ± 1.9 | 11.3 | + | + |
| B9 | Mouse | Epidermal squamous cell carcinoma | 26.7 ± 1.9 | 68 ± 3 | 36.2 | + | + |
| SN161 | Mouse | Epidermal spindle-squamous cell carcinoma | 16.3 ± 1.3 | 57.5 ± 4.7 | 20.8 | + | + |
| CarB | Mouse | Epidermal spindle cell carcinoma | 23.8 ± 2 | 49.8 ± 4.3 | 14.8 | + | + |
| P6 | Mouse | Epidermal papilloma | 16.9 ± 2.2 | 44.5 ± 2.8 | 56.8 | + | + |
FIG. 1.
Infectivity of rodent cell lines by Ad5. For details, see the legend to Table 1. The percentages shown correspond to the proportion of blue cells in the culture after infection with the CMVlacZ virus. (Left panels) Control uninfected cells; (right panels) virus-infected cells.
Cytopathic effect (CPE) assays using wild-type Ad2 were carried out at an MOI of 10 PFU/cell. Table 1 summarizes the results showing that a clear CPE was found in all of the mouse epidermal cells and to a lesser extent in the rat colon carcinoma K12/TrB and thyroid carcinoma VH1 VRS2 cell lines. All cell lines which showed positive CPEs were positive by immunofluorescence for hexon protein, when IMAGEN adenovirus reagent containing a fluorescein isothiocyanate-labeled mouse monoclonal antibody to adenovirus hexon protein was used. The cell lines 9L-82 and TCMK1, which were highly infectable with the CMVlacZ virus, showed no CPE or positive immunofluorescence, indicating the presence of a specific barrier to replication or late protein expression in these cells.
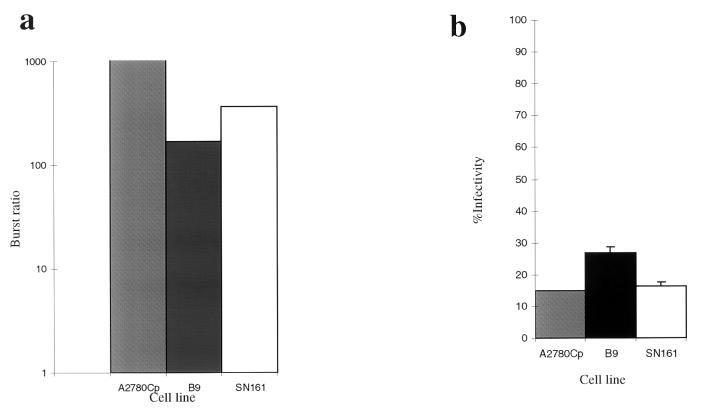
To determine if a productive infection could be produced in mouse cells, burst assays were done with wild-type Ad2 (10). The burst ratio was expressed as the concentration of virus at 72 h relative to the concentration of virus at 4 h postinfection. Lysates were prepared by three cycles of freezing and thawing. Titers of serial dilutions were determined on HEK293 cells. Figure 2a shows the results of burst assays for cell lines B9 and SN161 in comparison to those for the human ovarian cell line A2780Cp. The burst ratio for A2780Cp is 50 times greater than that in B9, and 25 times greater than that in SN161. Figure 2b shows that the infectivities of each cell line are similar at 10 PFU/cell, as determined by using the LacZ adenovirus. These results show that several mouse epidermal cell lines can produce a productive viral yield but generally at reduced efficiency (25- to 50-fold less) in comparison to that produced by human A2780cp cells.
FIG. 2.
Burst assay and infectivity assay of mouse epidermal cell lines in comparison to the human ovarian cell line A2780Cp. (a) Simultaneous burst assays were determined for the human A2780 cells (gray column), squamous B9 cells (black column), and SN161 cells (white column). Burst ratio is shown on a log scale on the left. (b) Infectivity was determined by using the CMVlacZ adenovirus at an MOI of 10 PFU/cell for each of the cell lines.
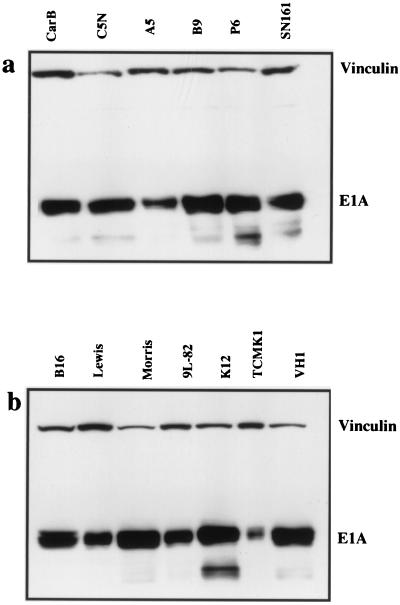
Previous results with rodent fibroblasts have suggested that the expression of adenovirus E1A is repressed following viral uptake by the presence of a _trans_-acting transcriptional repressor protein (7). To determine whether this mechanism is operative in a wide range of rodent cell types, protein lysates were made from cells 24 h after infection with Ad2 at an MOI of 100 PFU/cell. Equivalent amounts of protein were run on Western blots with the mouse monoclonal antibody M73 (Oncogene Science; dilution 1/1,000), which detects the E1A proteins. A vinculin mouse monoclonal antibody (VIN-11-1; Sigma) was used at a concentration of 1/1,000.
Figure 3 shows that all rodent cells were able to express the early gene E1A proteins after infection. E1A expression was expressed relative to vinculin expression for each cell line by densitometry (Table 1) and then expressed as a percentage relative to the cell line with maximum E1A expression (Morris liver hepatoma cell line). There was no obvious correlation between E1A expression and infectivity or ability to support virus replication. Overall, the levels of E1A expression did not differ markedly between cells that showed no evidence for virus replication and those that showed either a weak CPE and low-level hexon staining (the colon cell line K12/TrB and the thyroid cell line VH1 VRS2) or a strong CPE and substantial hexon expression (the epidermal cell lines). This indicated that there was a block to late-phase protein expression in most tissue types with the exception of mouse epidermal cells.
FIG. 3.
Western blots of E1A expression of Ad2-infected rodent cells. (a) A number of epidermal cell lines were infected with Ad2 at an MOI of 10 PFU/cell and harvested 24 h later. Western blotting was carried out with the anti-E1A monoclonal antibody M73. Vinculin was used as a control for protein loading. (b) A range of cell lines from various mouse tissues (see Table 1) was infected and adenovirus E1A levels were assessed as described above.
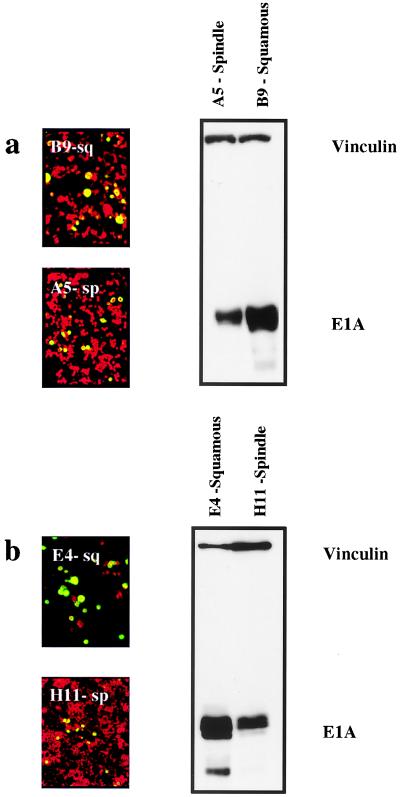
Although these experiments did not reveal any correlation between infectivity, E1A expression, and replication across a panel of different cell types, within the series of epidermal cell lines we found a relatively good correlation with the degree of cell differentiation. We compared E1A expression levels in two independent pairs of well-differentiated squamous cell lines (B9 and E4) with their undifferentiated spindle counterparts (A5 and H11, respectively). In each of these pairs of cells, the spindle variant was derived from the same primary tumor as the squamous cell (3, 19, 22; J. Liddell and A. Balmain, unpublished data). Figure 4 shows that E1A expression correlated with the degree of cellular differentiation, with poorly differentiated H11 and A5 cells showing reduced expression. The more-differentiated cells also were more permissive for replication, as shown by the relatively increased staining with anti-hexon protein antibodies. Preferential replication of Ad2 in more-differentiated cell populations has also been reported for human keratinocytes (1, 15). Our results on squamous and spindle carcinoma cells would also indicate that replication is better in the more-differentiated cell phenotype, although the mechanistic basis for these observations is unclear. Previous work by others (7) has shown that fibroblasts produce a _trans_-acting transcriptional repressor which suppresses the activity of the E1A promoter. Indeed, we have shown that the expression level of this nuclear factor, φAP3, a zinc finger-containing DNA-binding protein related to the GLI-Kruppel protein (7), is approximately 20-fold higher in the spindle phenotype (data not shown), and this may account for the lower E1A levels in the spindle cells.
FIG. 4.
Hexon protein expression and E1A expression in squamous-spindle paired cell lines. Two matched pairs of squamous and spindle cells (panel a, B9 [squamous] and A5 [spindle]; panel b, E4 [squamous] and H11 [spindle]) were stained for hexon protein expression 24 h after infection with Ad2 at an MOI of 10 PFU/cell. Green staining indicates positive hexon protein expression, and red staining shows counterstaining with evans blue. The level of hexon protein staining was consistently higher in the squamous cells than in their spindle counterparts. Levels of E1A were assessed by Western blotting with anti-E1A antibodies and antivinculin antibodies as shown on the right side of the figure. The relative expression levels of E1A are higher in both squamous cells in comparison with those of the corresponding spindle cells.
We have shown that a series of restriction points that control the ability of human adenoviruses to replicate in rodent cells exist, including infectivity, expression of the early gene product E1A, and subsequent initiation of viral replication and late protein synthesis. Most or all of these restrictions are surprisingly absent in mouse epidermal cells, leading us to suggest that epidermal tumor models may be useful for testing replication-competent adenoviral therapy for cancer (2, 11).
Acknowledgments
We thank Wilma Steegenga and Hans Bos for the CMVlacZ virus, Byron Hann for critical reading of the manuscript, and numerous colleagues in the adenovirus group at Onyx Pharmaceuticals (Richmond, Calif.) for interesting discussions.
This work was supported by funding from the Cancer Research Campaign (United Kingdom) and from Onyx Pharmaceuticals.
REFERENCES
- 1.Aneskievich B J, Lee J I, Taichman L B. Analysis of adenovirus early and late gene expression in cultured epidermal keratinocytes. Differentiation. 1990;94:183–186. doi: 10.1111/1523-1747.ep12874480. [DOI] [PubMed] [Google Scholar]
- 2.Bischoff J R, Kirn D H, Williams A, Heise C, Horn S, Muna M, Ng L, Nye J A, Sampson-Johannes A, Fattaey A, McCormick F. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274:373–376. doi: 10.1126/science.274.5286.373. [DOI] [PubMed] [Google Scholar]
- 3.Burns P A, Kemp C J, Gannon J V, Lane D P, Bremner R, Balmain A. Loss of heterozygosity and mutational alterations of the p53 gene in skin tumors of interspecific hybrid mice. Oncogene. 1991;6:2363–2369. [PubMed] [Google Scholar]
- 4.Duncan S J, Gordon F C, Gregory D W, McPhie J L, Postlethwaite R, White R, Willcox H N. Infection of mouse liver by human adenovirus type 5. J Gen Virol. 1978;40:45–61. doi: 10.1099/0022-1317-40-1-45. [DOI] [PubMed] [Google Scholar]
- 5.Eggerding F A, Pierce W C. Molecular biology of adenovirus type 2 semipermissive infections. I. Viral growth and expression of viral replicative functions during restricted adenovirus infection. Virology. 1986;148:97–113. doi: 10.1016/0042-6822(86)90406-x. [DOI] [PubMed] [Google Scholar]
- 6.Esandi M C, van Someren G D, Vincent A J, van Bekkum D W, Valerio D, Bout A, Noteboom J L. Gene therapy of experimental malignant mesothelioma using adenovirus vectors encoding the HSVtk gene. Gene Ther. 1997;4:280–287. doi: 10.1038/sj.gt.3300385. [DOI] [PubMed] [Google Scholar]
- 7.Fognani C, Della V G, Babiss L E. Repression of adenovirus E1A enhancer activity by a novel zinc finger-containing DNA-binding protein related to the GLI-Kruppel protein. EMBO J. 1993;12:4985–4992. doi: 10.1002/j.1460-2075.1993.tb06192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ginsberg H S. The ups and downs of adenovirus vectors. Bull N Y Acad Med. 1996;73:53–58. [PMC free article] [PubMed] [Google Scholar]
- 9.Ginsberg H S, Moldawer L L, Sehgal P B, Redington M, Kilian P L, Chanock R M, Prince G A. A mouse model for investigating the molecular pathogenesis of adenovirus pneumonia. Proc Natl Acad Sci USA. 1991;88:1651–1655. doi: 10.1073/pnas.88.5.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graham F L, Prevec L. Methods in molecular biology. Vol. 7. Clifton, N.J: Humana Press; 1991. Manipulation of adenovirus vectors; pp. 109–128. [DOI] [PubMed] [Google Scholar]
- 11.Heise C, Sampson-Johannes A, Williams A, McCormick F, Von Hoff D D, Kirn D H. ONYX-015, an E1B gene-attenuated adenovirus, causes tumor-specific cytolysis and antitumoral efficacy that can be augmented by standard chemotherapeutic agents. Nat Med. 1997;3:639–645. doi: 10.1038/nm0697-639. [DOI] [PubMed] [Google Scholar]
- 12.Kelly F, Boccara M. Susceptibility of teratocarcinoma cells to adenovirus type 2. Nature. 1976;262:409–411. doi: 10.1038/262409a0. [DOI] [PubMed] [Google Scholar]
- 13.Ko S C, Gotoh A, Thalmann G N, Zhau H E, Johnston D A, Zhang W W, Kao C, Chung L W. Molecular therapy with recombinant p53 adenovirus in an androgen-independent, metastatic human prostate cancer model. Hum Gene Ther. 1996;7:1683–1691. doi: 10.1089/hum.1996.7.14-1683. [DOI] [PubMed] [Google Scholar]
- 14.Kolls J, Peppel K, Silva M, Beutler B. Prolonged and effective blockade of tumor necrosis factor activity through adenovirus-mediated gene transfer. Proc Natl Acad Sci USA. 1994;91:215–219. doi: 10.1073/pnas.91.1.215. . (Erratum, 91:1979, 1994.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laporta R F, Taichman L B. Adenovirus type-2 infection of human keratinocytes: viral expression dependent upon the state of cellular maturation. Virology. 1981;110:137–146. doi: 10.1016/0042-6822(81)90015-5. [DOI] [PubMed] [Google Scholar]
- 16.Lucher L A. Abortive adenovirus infection and host range determinants. In: Doerfler W, Bohm P, editors. The molecular repertoire of adenoviruses I. Berlin, Germany: Springer Verlag; 1995. pp. 119–152. [DOI] [PubMed] [Google Scholar]
- 17.Oualikene W, Gonin P, Eloit M. Short and long term dissemination of deletion mutants of adenovirus in permissive (cotton rat) and non-permissive (mouse) species. J Gen Virol. 1994;75:2765–2768. doi: 10.1099/0022-1317-75-10-2765. [DOI] [PubMed] [Google Scholar]
- 18.Pacini D L, Dubovi E J, Clyde W A J. A new animal model for human respiratory tract disease due to adenovirus. J Infect Dis. 1984;150:92–97. doi: 10.1093/infdis/150.1.92. [DOI] [PubMed] [Google Scholar]
- 19.Portella G, Cumming S A, Liddell J, Cui W, Ireland H, Akhurst R J, Balmain A. Transforming growth factor beta is essential for spindle cell conversion of mouse skin carcinoma in vivo: implications for tumor invasion. Cell Growth Differ. 1998;9:393–404. [PubMed] [Google Scholar]
- 20.Quillien V, Heresbach L B, Dufour T, Denais A, Guegan Y, Ferry N, Bloin V. Gene therapy of a model of glioblastoma in rats using adenovirus vector encoding the HSVtk gene. Bull Cancer. 1997;84:1047–1052. [PubMed] [Google Scholar]
- 21.Silverstein G, Strohl W A. Restricted replication of adenovirus type 2 in mouse Balb/3T3 cells. Arch Virol. 1986;87:241–264. doi: 10.1007/BF01315303. [DOI] [PubMed] [Google Scholar]
- 22.Stoler A B, Stenback F, Balmain A. The conversion of mouse skin squamous cell carcinomas to spindle cell carcinomas is a recessive event. J Cell Biol. 1993;122:1103–1117. doi: 10.1083/jcb.122.5.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson J M. Adenoviruses as gene-delivery vehicles. N Engl J Med. 1996;334:1185–1187. doi: 10.1056/NEJM199605023341809. [DOI] [PubMed] [Google Scholar]