Functional Interaction between Pleiotropic Transactivator pUL69 of Human Cytomegalovirus and the Human Homolog of Yeast Chromatin Regulatory Protein SPT6 (original) (raw)
Abstract
The phosphoprotein pUL69 of human cytomegalovirus (HCMV), which is a herpesvirus of considerable medical importance in immunosuppressed patients and newborns, has previously been identified as an early-late viral protein that can stimulate several viral and cellular promoters and thus exerts a rather broad activation pattern. To gain insight into the mechanism of this transactivation process, we looked for cellular factors interacting with pUL69 in a yeast two-hybrid screen. Using a B-lymphocyte cDNA library fused to the GAL4 activation domain, we identified 34 clones, 11 of which comprised one distinct gene. Interaction with this gene turned out to be very strong, producing β-galactosidase levels 100-fold greater than the background as measured in an ONPG (_o_-nitrophenyl-β-d-galactopyranoside) assay. Sequencing identified this gene as the human homolog of the yeast factor SPT6, which is thought to be involved in the regulation of chromatin structure. A direct interaction of pUL69 and the carboxy terminus of hSPT6 could be demonstrated using in vitro pull-down experiments. After having generated a specific antiserum that is able to detect the endogenous hSPT6 protein, we were able to observe an in vivo interaction of both proteins by coimmunoprecipitation analysis. The interaction domain within pUL69 was mapped to a central domain of this viral protein that is conserved within the homologous proteins of other herpesviruses such as the ICP27 protein of herpes simplex virus. Internal deletions within this central domain, as well as a single amino acid exchange at position C495, resulted in a loss of interaction. This correlated with a loss of the transactivation potential of the respective mutants, suggesting that the hSPT6 interaction of pUL69 is essential for stimulating gene expression. Furthermore, we demonstrate that the carboxy terminus of hSPT6 also binds to histon H3 and that this interaction can be antagonized by pUL69. This allows the deduction of a model by which pUL69 acts as an antirepressor by competing for binding of histones to hSPT6, thereby antagonizing the chromatin remodeling function of this cellular protein.
Human cytomegalovirus (HCMV), a member of the beta-subgroup of herpesviruses, is characterized by its narrow host range and prolonged replicative cycle in cell culture as well as in the infected human host. Generally, HCMV demonstrates low pathogenicity when infecting healthy individuals. However, it is of considerable clinical importance in immunocompromised patients such as transplant recipients or patients suffering from AIDS as well as in prenatally infected newborns (2). Like other herpesviruses, the lytic cycle gene expression of HCMV occurs in a sequential fashion. Initially after infection, the immediate-early (IE) gene products are the first to be synthesized, followed by the early and late genes (17, 36, 62, 63).
In addition to the IE gene products of HCMV, for which important functions in gene regulation are well documented, it has been reported that the viral protein pUL69 also acts as a regulatory polypeptide (64). Due to differential phosphorylation, three isoforms of this protein of 105, 110, and 116 kDa can be detected in lysates of HCMV-infected fibroblast cells (66). Expression from the UL69 gene locus occurs with an overall early-late kinetics during the viral replicative cycle; however, since the 110-kDa isoform of pUL69 is incorporated into the tegument of viral particles, this protein may also exert effects during the IE phase of gene expression (64, 66). Consistent with this, transactivation of the major IE enhancer-promoter by pUL69 could be observed, and this transactivation was synergistically enhanced in the presence of an additional tegument protein encoded by the open reading frame UL82, the so-called upper-matrix protein pp71 (65). However, transactivation was not confined to the major IE enhancer-promoter but could also be detected with a rather broad spectrum of promoters, such as the long terminal repeats (LTRs) of human immunodeficiency virus type 1 (HIV-1) and Rous sarcoma virus; the cellular promoters driving expression of thymidine kinase, beta-actin, or phosphoglycerol pyruvate kinase; or several early promoters of HCMV (64). In addition, results of transient replication assays indicated that pUL69 may also be able to stimulate lytic replication from the orilyt of HCMV, suggesting an even broader effect of this protein that is not confined to the stimulation of gene expression (56). This is further supported by the recent observation that pUL69 induces cells to arrest in the G1 phase of the cell cycle after transient expression of this protein (33).
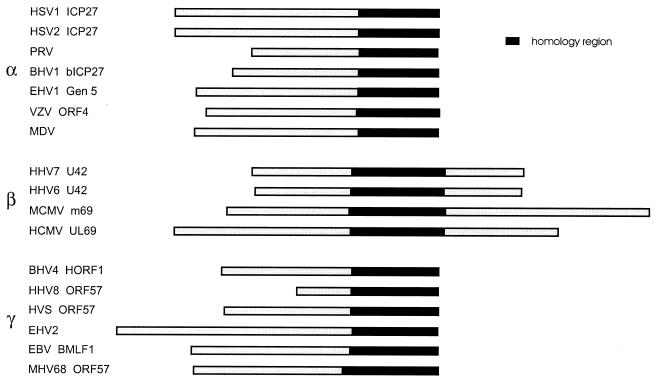
Our studies on pUL69 were initiated since this protein is a member of a family of homologous proteins that are conserved within all subclasses of the herpesviruses. These include ICP27 and ORF4 of the alphaherpesviruses herpes simplex virus (HSV) and varicella-zoster virus (29, 53), the IE genes BMLF1 and IE-52k of the gammaherpesviruses Epstein-Barr virus and herpesvirus saimiri (12, 42), and the UL69 and m69 genes of the betaherpesviruses HCMV and murine CMV (13, 48) (see Fig. 1). The amino acid identities among the encoded proteins range from 17 to 36%; however, the C-terminal part of ICP27, which is known to be of functional importance, shows a higher conservation of approximately 40% amino acid identity with several positionally conserved amino acid residues (55). This highly conserved amino acid sequence corresponds to a central domain within the betaherpesvirus proteins UL69 and M69, since the betaherpesvirus polypeptides differ from their homologous proteins by a unique C-terminal extension that is not contained within the alpha- or gammaherpesvirus members of this protein family (Fig. 1). Whereas the respective proteins encoded by the alpha- and gammaherpesviruses appear to regulate gene expression mainly via a posttranscriptional mechanism (45), various lines of evidence suggest important functional differences between pUL69 and ICP27. For instance, whereas ICP27 has been reported to repress gene expression when an intron is contained either 5′ or 3′ to the target gene-coding sequences (55), UL69 exerted no negative regulation depending on the presence of introns (64). Furthermore, a redistribution of the cellular splicing snRNPs from a widespread diffuse speckled pattern to a highly punctate organization is induced by ICP27, which colocalizes with the redistributed snRNPs (44, 54). This could also not be observed with pUL69 (M. Winkler and T. Stamminger, unpublished data).
FIG. 1.
Alignment of proteins encoded by alpha-, beta-, and gammaherpesviruses that show homology to the ICP27 of HSV-1. The region of highest homology is indicated by a black bar (homology region). The betaherpesvirus members of the protein family are characterized by a C-terminal extension that is not present within the alpha- and gammaherpesvirus proteins. The alignment shown was obtained using the program DIALIGN 2 (39). The following sequences were used for the alignment: HSV-1 (HSV1) ICP27 (38), HSV-2 (HSV2) ICP27 (37), pseudorabies virus (PRV) (9), bovine herpesvirus 1 (BHV1) bICP27 (20), equine herpesvirus 1 (EHV1) gene 5 (59), varicella-zoster virus (VZV) ORF4 (16), Marek's disease virus (MDV) (50), human herpesvirus 7 (HHV-7) U42 (41), human herpesvirus 6 (HHV6) U42 (23), murine CMV (MCMV) m69 (48), HCMV UL69 (13), bovine herpesvirus 4 (BHV4) HORF1 (60), human herpesvirus 8 (HHV8) ORF57 (52), herpesvirus saimiri (HVS) ORF57 (1), equine herpesvirus 2 (EHV2) (58), Epstein-Barr virus (EBV) BMLF1 (6), and murine herpesvirus 68 (MHV68) ORF57 (34).
In an attempt to further elucidate the mechanism that is used by pUL69 to stimulate gene expression, a yeast two-hybrid screen was performed in order to detect cellular cofactors of this viral protein. Here, we report the identification of hSPT6, the human homolog of the yeast chromatin regulatory protein ySPT6, as an interaction partner of pUL69. Our results suggest that at least part of the activation by pUL69 may be mediated by antagonizing a potential repressing function of the hSPT6 protein.
MATERIALS AND METHODS
Oligonucleotides.
Oligonucleotides were obtained from Eurogentec (Seraing, Belgium), MWG Biotech (Ebersberg, Germany), or ARK (Darmstadt, Germany). The sequences of oligonucleotides used in this study are listed in Table 1.
TABLE 1.
Oligonucleotides used for sequencing, PCR, and mutagenesis
| Oligo- nucleotide | Sequence |
|---|---|
| BAMLI-A | 5′-GATCCGGACCATGGGGGCCCTACTAGT-3′ |
| BAMLI-B | 5′-GATCACTAGTAGGGCCCCCATGGTCCG-3′ |
| UL69-CD1 | 5′-CGAATTCTAGATATCTTGCGGTACCGGTGGTGG-3′ |
| UL69-CD3 | 5′-GCGAATTCTAGA TATCCGCGGTCGTCTTGTAGAC-3′ |
| UL69-CD4 | 5′-GCGAATTCTAGATATCGCCCTCAGAGTAGTGCTG-3′ |
| UL69-CD5 | 5′-GCGAATTCTAGATATCCGTTGCTGGGTCCGTCCT-3′ |
| UL69-CD6 | 5′-GCGAATTCTAGATATCGATCAGGTCCAGGACAGC-3′ |
| UL69-CD7 | 5′-GCGAATTCTAGATATCGTAGCGAGACGGCAACGC-3′ |
| UL69-CD8 | 5′-GCGAATTCTAGATATCCCAGGCGCTCATTTGACC-3′ |
| UL69-Konst-3′ | 5′-GCGGATCCTAGATATCGGTACCCATGGCGTTGTT-3′ |
| UL69-Konst-5′ | 5′-TATTAGGATCCCCATGGAGCTGCACTCACGC-3′ |
| UL69-var-3′ | 5′-GCGATATCTAGGATCCTTAGTCATCCATATCATC-3′ |
| UL69-var-574-5′ | 5′-GAATTCATGGATATCACGCTGACAGCCTACGAT-3′ |
| UL69-var-520-5′ | 5′-GAATTCAT GGATATCCTCACCGTCTTTGCCGGT-3′ |
| UL69C-495a | 5′-CCTGCAGTGCCACGAGGCTCAGAACGAGATGTGC-3′ |
| UL69C-495b | 5′-GCACATCTCGTTCTGAGCCTCGTGGCACTGCAGG-3′ |
| UL69L-502a | 5′-ACCGAGATGTGCGAAGCGCGCATCCAACGCGC-3′ |
| UL69L-502b | 5′-GCGCGTTGGATGCGCGCTTCGCACATCTCGTT-3′ |
| UL69-5′ | 5′-TATTAGGATCCCCATGGAGCTGCACTCAC-3′ |
| UL69-744-3′ | 5′-GCGGATCCTAGATATCTTAGTCATCCA TATCATC-3′ |
| KIA162-3 | 5′-GACAGATATCCACTTCACGCCACTTTATG-3′ |
| KIA162-5 | 5′-AGGCGATATCAATGTCTGATTTTGTGGAA-3′ |
Plasmid constructions and in vitro mutagenesis.
The bait plasmid pHM300 for the yeast two-hybrid screen was generated by cloning of the _Bam_HI/_Eco_RV fragment of pHM162 containing the UL69 reading frame (64) into pGBT9 digested with _Pst_I, blunted by treatment with T4 DNA polymerase, and finally cut with _Bam_HI. The carboxy-terminal UL69 deletion mutants for the yeast interaction analysis were constructed by PCR amplification using the oligonucleotides UL69-CD1 and UL69-CD3 to UL69-CD8 and pHM160 (64) as a template. The fragments were inserted into the pBluescript KS II vector (Stratagene, San Diego, Calif.) using the _Eco_RI and _Bam_HI restriction sites (pHM748 and pHM750 to pHM755). From these plasmids fragments were isolated using the enzymes _Bam_HI and _Xho_I and then inserted into the yeast vector pGBT9 (Clontech, Palo Alto, Calif.) via the _Sal_I and _Bam_HI restriction sites, resulting in plasmids pHM791 and pHM793 to pHM798, respectively. The amino-terminal UL69 deletion mutants were created using the double-stranded nested deletion kit (Pharmacia, Freiburg, Germany) as described by the manufacturer. For this, the procaryotic expression vector pHM164 (64) was first modified by inserting a linker oligonucleotide (BAMLI-A–BAMLI-B) into a singular _Bam_HI site. The resulting plasmid, pHM466, was linearized with _Spe_I, and the DNA ends were made resistant for exonuclease III digestion by a fill-in reaction with thionucleotides. After digestion with _Sal_I that cleaves at the amino terminus of UL69, the unidirectional exonuclease digestion was started. Several aliquots of the reaction were stopped after different time points to generate a nested deletion series with a spacing of approximately 150 bp. In the resulting plasmids pHM507, pHM515, pHM516, pHM517, and pHM521, the UL69 reading frame starts at amino acids 92, 269, 315, 380, and 478, respectively. For eukaryotic expression as FLAG-tagged proteins, the UL69 fragments were isolated from plasmids pHM507, pHM515, pHM516, pHM517, and pHM521 and inserted into the pCATCH-NLS vector (22) using the _Bam_HI and _Eco_RV restriction sites. Subsequently, the resulting plasmids pHM743 to pHM747 were cleaved with _Bam_HI and _Xho_I. The isolated fragments were inserted into pGBT9 using the _Sal_I and _Bam_HI restriction sites. The internal UL69 deletion mutants UL69-D478-572 (pHM937) and UL69-D478-527 (pHM938) were constructed by PCR amplification of an N-terminal UL69 fragment (nucleotides [nt] 1 to 1431) using the oligonucleotides UL69-Konst-5′ and UL69-Konst-3′ and two different C-terminal UL69 fragments (nt 1719 to 2232 and nt 1584 to 2232) using the oligonucleotides UL69-var-574-5′, UL69-var-520-5′, and UL69-var-3′. The N-terminal fragment was cloned into pcDNA3 (Invitrogen Corp., San Diego, Calif.) using the _Bam_HI restriction site. Thereafter, the C-terminal UL69 fragments were inserted into the resulting vector pHM936 via the _Eco_RV restriction site. For expression in yeast cells, the internal deletion mutants pHM937 and pHM938 were cleaved with _Bam_HI, followed by insertion into the pGBT9 vector. Site-directed mutagenesis was performed using the QuikChange Site-Directed Mutagenesis Kit according to the manufacturer's protocol (Stratagene, Heidelberg, Germany). The pUL69 single-amino-acid mutants (pHM940 and pHM939) were constructed by PCR using plasmid pHM160 and oligonucleotides UL69C-495a and UL69C-495b (resulting in mutant UL69-C495 carrying a C-A substitution at amino acid position 495 of pUL69) or oligonucleotides UL69L-502a and UL69L-502b (resulting in mutant UL69-L502 with a L-A substitution at amino acid position 502 in pUL69). For expression in yeast, both mutants were amplified by PCR using the oligonucleotides UL69-5′ and UL69-744-3′ and then cloned into the _Bam_HI-digested pGBT9 vector (pHM967 and pHM979). The hSPT6 reading frame was amplified by PCR using primers KIA162-5 and KIA162-3 and template KIAA0162 (40) and, after digestion with _Eco_RV, was cloned into pBluescript KS II, resulting in plasmid pHM632. To construct a FLAG-tagged hSPT6 for expression in eukaryotic cells, the hSPT6 reading frame was isolated from pHM632 as an _Eco_RV fragment and cloned into _Eco_RV-digested pSUPERCATCH (22) to give plasmid pHM635. For prokaryotic expression, the C terminus of hSPT6 was isolated as _Sma_I/_Xho_I fragment from Y69-1 and cloned into _Sma_I/_Sal_I-cut pQE30 (Qiagen, Hilden, Germany), resulting in plasmid pHM504. To create hSPT6 deletion mutants, pHM632 was cleaved with _Eco_RV and _Dra_I (nt 1 to 1919), _Dra_I and _Hpa_I (nt 1920 to 3458), or _Hpa_I and _Stu_I (nt 3459 to 5178). The fragments were inserted into the pGEX-4T1 vector (Pharmacia Biotech, Freiburg, Germany) cut with _Sma_I (pHM725 to pHM727). Additionally, a C-terminal fragment of hSPT6 was isolated from yeast vector Y69-145 (as isolated in the yeast two-hybrid screen) by _Xho_I digestion and then cloned into the _Sal_I-digested vector pGEX-4T1 vector to give plasmid pHM608. All plasmid constructs were confirmed by nucleotide sequence analysis. Plasmids pHM124 (alternatively termed pBSIE1, encoding IE1-p72), used as a control in pull-down assays, and pHM134, used as a control in coimmunoprecipitation analysis, were described previously (21). Luciferase reporter plasmids pHM287 (containing the IE1/2 enhancer-promoter [including the so-called modulator region]), HIVluc, and RSVluc were also as described previously (64).
Yeast two-hybrid screening.
Yeast two-hybrid screening was performed using GAL4 fusion proteins and Saccharomyces cerevisiae Y153 as described previously (28). Yeast strain Y153 containing the bait plasmid pHM300 was transformed with a cDNA library derived from human B lymphocytes fused to the GAL4 activation domain in the pACT vector (19). The primary transformants (0.9 × 106) were selected for growth on histidine dropout plates containing 30 mM 3-aminotriazole. His+ colonies were subsequently analyzed for β-galactosidase activity by filter-lift experiments (11). The interaction was then quantified by _o_-nitrophenyl-β-d-galactopyranoside (ONPG) assays as described earlier (24). Interactor plasmids from clones positive in both tests were rescued by transformation of competent KC8 bacteria with total yeast DNA (26). For mapping of interaction domains using the yeast two-hybrid system, the respective UL69 deletion mutants within yeast vector pGBT9 were transformed together with the interactor plasmids into yeast strain Y153 and tested as described above.
GST fusion proteins and pull-down assays.
Purification of glutathione _S_-transferase (GST) fusion proteins was performed as described previously (31). For pull-down assays, 5 to 30 μl of the GST fusion proteins on beads were preincubated for 10 min in 200 μl of ELB buffer (125 mM NaCl; 50 mM HEPES, pH 7.0; 0.1% Nonidet P-40 [NP-40]; 1 mM phenylmethylsulfonyl fluoride [PMSF]; 0.5 mM dithiothreitol [DTT]; 0.5 mM EDTA) containing bovine serum albumin (final concentration, 1 mg/ml). To avoid DNA-dependent protein associations, ethidium-bromide was added to a final concentration of 50 μg/ml (30). After addition of 1 to 6 μl of in vitro-translated test protein which had been generated by using the TNT system (Promega, Heidelberg, Germany), the beads loaded with GST fusion proteins were gently mixed for 2 h at 4°C. For competition assays, 10 to 30 μl of GST-hSPT6 fusion protein was incubated with a constant amount of in vitro-translated histone H3 (1 μl) (27) and increasing amounts (1, 4, 8, and 16 μl) of in vitro-translated pUL69 or IE1-p72. The beads were then washed five times in 1 ml of ELB buffer, pelleted, and boiled in 2× sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer, and bound proteins were resolved using SDS-polyacrylamide gels. The gels were fixed, incubated in Amplify (Amersham Life Science) for 15 min, and dried before autoradiography and quantification of signals using a phosphorimager.
Cell culture, transfection, and reporter assays.
Human foreskin fibroblasts (HFFs) and U373MG and COS-7 cells were cultured as described previously (64). Infection of HFF cells with HCMV (AD169) was performed exactly as described previously (47). The day before transfection, COS-7 cells were plated in 100-mm-diameter plastic dishes at 106 cells per dish. DNA transfection was performed by the calcium phosphate coprecipitation method using BES as described earlier (5). Cells were harvested 48 h after transfection and used for Western blotting or immunoprecipitation.
For luciferase assays, U373MG cells were plated onto six-well dishes at 2.8 × 105 cells per well the day before transfection. Plasmid transfection was performed by the DEAE-dextran method as described previously (4). Routinely, 1 μg of luciferase target and 2.3 μg of the cotransfected transactivator plasmid were used. The total amount of transfected DNA was kept constant by using the cloning vector pCB6 in order to replace the missing transactivator plasmid. At 48 h after transfection, cells were harvested and luciferase assays were performed using a lysis buffer containing 50 mM Tris-H3PO4 (pH 7.8), 0.1732 g of trans N,N,_N_′-1,2-diaminocyclohexane, 2% Triton X-100, 4 mM DTT, and 20% glycerol. Luciferase activity in the supernatant was determined using a luminometer (Bertholt, Freiburg, Germany). Each transfection was performed in triplicate and was repeated at least three times.
Antibodies.
The polyclonal antisera against pUL69 of HCMV (64) and hSPT6 were generated by immunizing rabbits with the respective procaryotically expressed proteins. For procaryotic expression of hSPT6, plasmid pHM504, containing the C terminus of hSPT6 (amino acids 1509 to 1726) fused to an amino-terminal His tag, was transformed into Escherichia coli M15/pREP4. Procaryotic expression, purification, and preparation for immunization were performed as described previously (32, 64). Immunization of rabbits and bleeding was done by Eurogentec (Seraing, Begium). The monoclonal antibody 69-66 (directed against pUL69) was obtained from B. Britt (Birmingham, Ala.). The monoclonal antibodies p63-72 (directed against IE1-p72) and SMX (directed against IE2) were as described elsewhere (3, 46). Monoclonal antibody anti-FLAG M2, which is directed against the synthetic FLAG octapeptide N-Asp-Tyr-Lys-Asp-Asp-Asp-Asp-Lys-C, was purchased from INTEGRA Bioscience (Fernwald, Germany). Anti-mouse and anti-rabbit horseradish peroxidase-conjugated secondary antibodies were obtained from Dianova (Hamburg, Germany).
Western blotting and immunoprecipitation analysis.
For Western blot analysis, transfected or infected cells were lysed in SDS-Laemmli buffer and boiled at 94°C for 10 min. Samples were electrophoresed by SDS-PAGE on 8 to 12.5% polyacrylamide gels, and the proteins were transferred onto nitrocellulose membranes (Schleicher & Schuell, Dassel, Germany). Western blotting and chemiluminescence detection were performed according to the manufacturer's protocol (ECL Western Detection Kit; Amersham Pharmacia Biotech Europe, Freiburg, Germany). Coimmunoprecipitation analysis for detection of noncovalent protein interactions was performed as described elsewhere (8). Briefly, transfected or infected cells were lysed in 1 ml of NP-40 lysis buffer (50 mM Tris-HCl, pH 8.0; 150 mM NaCl; 5 mM EDTA; 0.5% NP-40; 1 mM PMSF; 2 μg of aprotinin per ml) and incubated for 20 min at 4°C. After centrifugation, the supernatant was incubated with the appropriate antibody for 2 h at 4°C and, thereafter, a 50% protein A-Sepharose suspension was added and incubation continued for another 2 h at 4°C. The Sepharose beads were collected and washed three times in phosphate-buffered saline–0.5% NP-40. Antigen-antibody complexes were recovered by boiling in SDS sample buffer and analyzed by Western blotting.
RESULTS
Identification of hSPT6 as cellular interaction partner of the HCMV pUL69 transactivator protein by yeast two-hybrid experiments.
In order to identify novel cellular interaction partners of the pUL69 protein of HCMV, a yeast two-hybrid screen was carried out. For this, the coding sequence of UL69 was cloned into the yeast vector pGBT9, resulting in an in-frame fusion of the UL69 sequence to the GAL4 DNA-binding domain. After transformation of S. cerevisiae Y153, the presence of the GAL4-UL69 expression plasmid pHM300 was stably maintained by selection in liquid dropout culture medium lacking tryptophan, and the expression of the respective fusion protein was confirmed by Western blot analysis (data not shown). In order to determine whether the bait protein was able to activate transcription in yeast by itself, β-galactosidase expression of the yeast strain Y153/pHM300 that was transformed with the GAL4 activation domain plasmid pGAD424 was tested by filter lift experiments. No β-galactosidase expression could be detected with this combination, indicating that GAL4-UL69 alone does not activate expression of the reporter genes in yeast (Fig. 2C, row 12).
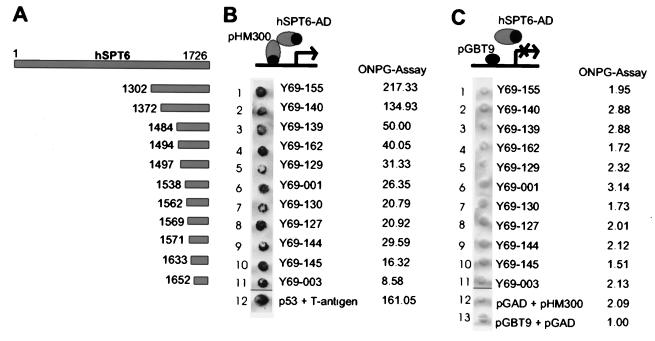
FIG. 2.
Specific interaction between HCMV pUL69 and hSPT6 in yeast cells. Yeast cells were transformed with two separate vectors, one of which encoded either pUL69 fused to the GAL4 DNA-binding domain (pHM300) or the DNA-binding domain alone (pGBT9). The second plasmid encoded either the GAL4 activation domain alone (pGAD) or carboxy-terminal fragments of hSPT6 (as isolated in the yeast two-hybrid screen) as fusion with the GAL4 activation domain, respectively. Yeast colonies were selected for the presence of both plasmids with dropout media lacking tryptophane and leucine and subsequently analyzed for the expression of β-galactosidase by filter lift assays. The association of murine p53 (encoded by plasmid pVA3 [Clontech]) and SV40 large T antigen (plasmid pTD1 [Clontech]) served as a positive control (lane 12); as a negative control, the activation domain vector pGAD424 (pGAD) was either transformed with plasmid pHM300 (encoding pUL69 in fusion with the GAL4 DNA-binding domain) or the GAL4 DNA-binding domain vector pGBT (lanes 12 and 13, respectively). (A) Schematic diagram illustrating the hSPT6 fragments isolated in the screen that are contained within the respective GAL4 activation domain fusion vectors (hSPT6 fusion plasmids termed Y69-155, Y69-140, Y69-139, Y69-162, Y69-129, Y69-001, Y69-130, Y69-127, Y69-144, Y69-145, and Y69-003). (B) Qualitative and quantitative analysis of the respective interaction between pUL69 and the various hSPT6 fragments as determined in filter lift experiments (left part of panel B) and by liquid β-galactosidase assays (results of ONPG assays in Miller units, right part of panel B). (C) Qualitative and quantitative analysis of the respective interaction between the DNA-binding domain vector pGBT9 and the various hSPT6 fragments as determined in filter lift experiments (left part) and by liquid β-galactosidase assays (results of ONPG assays in Miller units, right part of panel C).
The yeast two-hybrid screen was performed by transformation of the yeast strain Y153 containing plasmid pHM300 with a cDNA library derived from B lymphocytes in the vector pACT (19). Plasmids encoding putative interactors of pUL69 were isolated from double-positive clones and retransformed into yeast strain Y153/pHM300 in order to confirm the interaction. Positive clones after this retransformation were characterized by automated sequencing and a search for homologies in the NCBI databases. We report here the identification of human SPT6 (hSPT6) as a specific interaction partner of the pUL69 protein. For this interaction partner, 11 independent clones representing the C terminus of the hSPT6 protein were found in the yeast two-hybrid screen, indicating a sufficient complexity of the cDNA library and the specificity of the interaction with pUL69 (Fig. 2A). In cotransformation experiments of the individual interactor clones and the empty pGBT9 vector, it was excluded that the hSPT6 fusions with the GAL4 activation domain were able to activate the reporter genes in yeast in the absence of a bait protein (Fig. 2C). Additionally, liquid β-galactosidase assays (ONPG [_o_-nitrophenyl-β-d-galactopyranoside] assays) were performed in order to quantify the strength of interaction of individual hSPT6 clones with pUL69 (Fig. 2B). Interestingly, the interaction between pUL69 and the longest fragment of hSPT6 that was selected in the yeast two-hybrid screen turned out to be even stronger than the interaction between p53 and the simian virus 40 (SV40) T antigen, which served as a positive control, indicating a very strong binding of these two proteins. hSPT6 has previously been identified due to its amino acid identity of 34% to the yeast protein ySPT6 (14, 40). Although no data on the function of hSPT6 are available as yet, the yeast SPT6 has been reported to function as a global repressor of gene expression. Thus, an interaction of pUL69 with this protein could potentially antagonize its repressing function and thus explain the broad transactivation pattern observed with pUL69.
GST pull-down analysis reveals a direct interaction between pUL69 and the carboxy terminus of hSPT6.
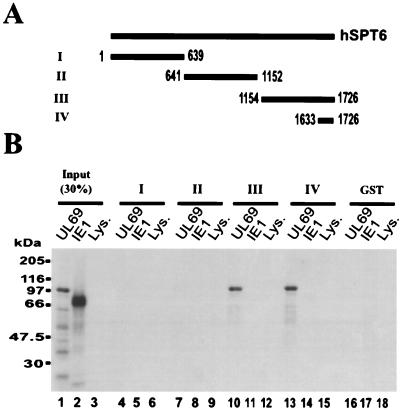
Having identified the hSPT6 protein as a potential interaction partner of HCMV pUL69 in the yeast two-hybrid screen, we wanted to confirm the interaction between these two proteins by an independent experimental approach. For this purpose, the coding sequence of hSPT6 was cloned in three nonoverlapping fragments downstream of GST into the procaryotic expression vector pGEX-4T1 (Fig. 3A). Additionally, one fragment isolated in the yeast two-hybrid screen was used as a GST fusion protein. After purification of the respective GST fusion proteins, GST pull-down assays were performed. The viral proteins pUL69 and IE1-p72 (IE1) were in vitro translated in reticulocyte lysates in the presence of [35S]methionine (Fig. 3, lanes 1 and 2). The radiolabeled proteins were then incubated with the bacterially expressed GST-hSPT6 fusions. As a further control for nonspecific binding, the GST fusion proteins were incubated with reticulocyte lysate that had not been programmed for the production of a specific protein (Fig. 3, lanes 6, 9, 12, and 15). As shown in Fig. 3, lanes 10 and 13, pUL69 was able to interact strongly with GST fusions representing the carboxy terminus of hSPT6. No interaction was observed with the amino-terminal or the central portion of hSPT6. In addition, none of the GST-hSPT6 proteins bound to IE1 (Fig. 3, lanes 5, 8, 11, and 14), and a GST protein alone was not able to interact with pUL69 (Fig. 3, lane 16), arguing against a nonspecific interaction of pUL69 with hSPT6. In summary, this experiment shows that pUL69 can interact directly with the carboxy terminus of hSPT6 in an in vitro binding assay.
FIG. 3.
The carboxy terminus of hSPT6 physically interacts with pUL69 in a GST pull-down assay. (A) Schematic diagram illustrating the fragments of hSPT6 that were expressed as GST fusion proteins (I to IV). (B) In vitro-translated 35S-labeled UL69 (lane 1) and IE1-p72 (lane 2) proteins and unprogrammed reticulocyte lysate (lane 3) were used for pull-down assays. Lanes 1 to 3 show the SDS-PAGE results of input proteins (30% of the amount used in the pull-down assay); lanes 4 to 18 show proteins that were recovered after GST pull-down analysis. Lanes: 4, 7, 10, 13, and 16, in vitro-translated pUL69 was incubated with the GST fusion proteins; 5, 8, 11, 14, and 17, in vitro-translated IE1-p72 was incubated with the GST fusion proteins; 6, 9, 12, 15, and 18; unprogrammed reticulocyte lysate was incubated with the GST fusion proteins. The following GST fusions were used for pull-down analysis: lanes 4 to 6, GST-hSPT6 fusion I (amino acids 1 to 639); lanes 7 to 9, GST-hSPT6 fusion II (amino acids 641 to 1152); lanes 10 to 12, GST-hSPT6 fusion III (amino acids 1154 to 1726); lanes 13 to 15, GST-hSPT6 fusion IV (amino acids 1633 to 1726); lanes 16 to 18, GST protein alone. The sizes of the molecular mass markers are indicated on the left of the figure.
Coimmunoprecipitation of pUL69 and hSPT6 confirms the in vivo interaction of both proteins after transfection and HCMV infection of cells.
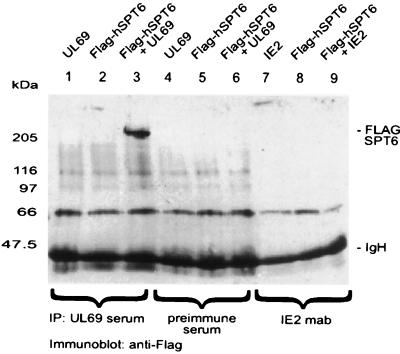
Although the results of the yeast two-hybrid screen and the in vitro interaction experiments strongly suggested a direct contact between pUL69 and hSPT6, we sought to confirm this within the context of a mammalian cell. In order to be able to detect hSPT6 in mammalian cell extracts, a eucaryotic vector was constructed (pHM635) that expresses hSPT6 in fusion with the FLAG epitope. Then we performed cotransfection experiments in COS-7 cells using expression vectors for pUL69 and FLAG-tagged hSPT6, followed by immunoprecipitation with a pUL69-specific antiserum. FLAG-tagged hSPT6 was detected by Western blot analyses of the precipitates using the anti-FLAG monoclonal antibody (Fig. 4). After coexpression of pUL69 together with hSPT6, a strong signal of approximately 220 kDa, corresponding to the FLAG-tagged hSPT6, could be observed in reactions with the UL69-specific antiserum (Fig. 4, lane 3), indicating an interaction of both proteins. This reaction was specific, since no signals were present when each of the proteins was expressed alone or when the preimmune serum was used for precipitation (Fig. 4, lanes 1, 2, and 4 to 6). In addition, after cotransfection of hSPT6 together with the IE2 transactivator of HCMV and coimmunoprecipitation with an IE2-specific monoclonal antibody, no FLAG-tagged hSPT6 was detectable (Fig. 4, lanes 7 to 9), thus further confirming the specificity of the interaction.
FIG. 4.
Analysis of the interaction between pUL69 and hSPT6 by coimmunoprecipitation from transfected cells. COS-7 cells were transfected with expression vectors encoding pUL69, FLAG-hSPT6, or IE2-p86 as indicated and lysed in NP-40 lysis buffer. Immunoprecipitations were performed using the UL69 antiserum (lanes 1 to 3) or the UL69 preimmune serum (lanes 4 to 6) or monoclonal antibody SMX directed against IE2-p86 (lanes 7 to 9). Precipitates were washed three times and separated by SDS–8% PAGE. Thereafter, coprecipitated interactor proteins were detected by Western blot analysis using the anti-FLAG monoclonal antibody. Lanes: 1 and 4, transfection with the pUL69 expression vector alone; 2, 5, and 8, transfection with the FLAG-hSPT6 expression vector alone; 3 and 6, transfection with a combination of vectors encoding pUL69 and FLAG-hSPT6; 7, transfection with the IE2-p86 expression vector pHM134; 9, transfection with a combination of vectors encoding IE2-p86 and FLAG-hSPT6. The sizes of the molecular mass markers are indicated on the left; the position of FLAG-tagged hSPT6 (FLAG-SPT6) and the immunoglobulin heavy chain (IgH) are shown on the right.
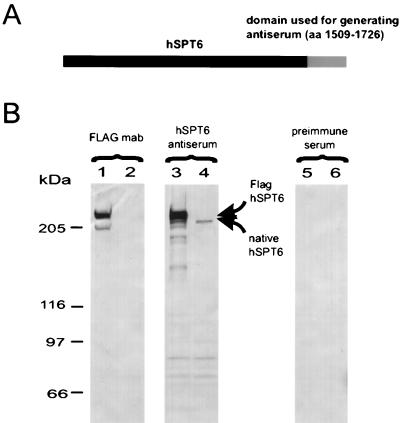
Since we also wanted to detect the interaction between pUL69 and the endogenous hSPT6 in HCMV-infected fibroblast cells, a specific antiserum against hSPT6 was generated and tested for its reactivity in Western blot analysis (Fig. 5). As shown in Fig. 5B, lane 3, the antiserum against hSPT6 was able to recognize the FLAG-tagged hSPT6 as expressed in COS-7 cells. An additional band migrating slightly faster than FLAG-tagged hSPT6 was visible with lysates from mock-transfected cells (Fig. 5, lane 4). Since no signals could be detected with the preimmune serum (Fig. 5, lanes 5 and 6), the reactivity detected with nontransfected cells represents the endogenously expressed SPT6 protein of COS-7 cells.
FIG. 5.
Eucaryotic expression analysis of hSPT6 with a specific anti-hSPT6 antiserum. (A) Schematic diagram indicating the domain of hSPT6 that was used for procaryotic expression and immunization of rabbits in order to generate a specific antiserum. (B) Western blot analysis of extracts derived from COS-7 cells with either the anti-FLAG monoclonal antibody (lanes 1 and 2), the anti-hSPT6 antiserum (lanes 3 and 4), or the preimmune serum (lanes 5 and 6). Lanes: 1, 3, and 5, extracts from COS-7 cells that were transfected with expression vector pHM635 encoding FLAG-tagged hSPT6; 2, 4, and 6, extracts from COS-7 cells that were transfected with the empty expression vector pSuperCatch. The positions of FLAG-tagged and endogenous SPT6 are indicated by arrows. The molecular mass markers are shown on the left of panel B.
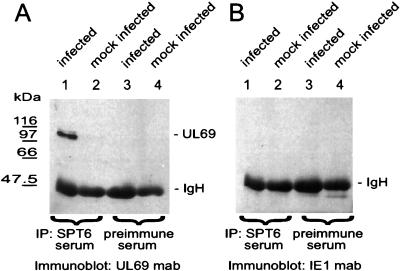
This antiserum was then used for coimmunoprecipitation analysis of the interaction between pUL69 and hSPT6 in HCMV-infected primary human fibroblast cells. Cell lysates from either mock-infected cells or cells infected for 72 h with HCMV were incubated with the anti-hSPT6 serum. After immunoprecipitation, protein complexes were resolved using SDS-PAGE, and pUL69 was detected by Western blot analyses with monoclonal antibody 69-66 directed against pUL69. As shown in Fig. 6A, lanes 1 and 2, a signal of approximately 110 kDa, corresponding to pUL69, could be detected with lysates from HCMV-infected fibroblasts but not with lysates from mock-infected cells. No pUL69 was detectable either when the preimmune serum was used for precipitation or when an IE1-specific antibody was used for Western blot analysis (Fig. 6A, lanes 3 and 4, and Fig. 6B). On the basis of these results, we conclude that pUL69 is able to interact with endogenous hSPT6 in mammalian cells.
FIG. 6.
Analysis of the interaction between endogenous hSPT6 and pUL69 by coimmunoprecipitation with lysates from HCMV-infected primary HFFs. HFFs were infected with either HCMV strain AD169 or were mock infected. At 72 h postinfection lysates were prepared in NP-40 lysis buffer. Immunoprecipitations were performed using the hSPT6 antiserum (lanes 1 and 2) or the hSPT6 preimmune serum (lanes 3 and 4). Precipitates were washed three times and separated by SDS–10% PAGE. Thereafter, coprecipitated interactor proteins were detected by Western blot analysis using monoclonal antibody 69-66 against pUL69 (A) or monoclonal antibody p63-27 against IE1-p72 (B). Lanes 1 and 3, lysates from HCMV-infected cells were used; lanes 2 and 4, lysates from mock-infected cells were used. The sizes of the molecular mass markers are indicated on the left; the positions of pUL69 (UL69) and the immunoglobulin heavy chain (IgH) are shown on the right.
The hSPT6 interaction domain of pUL69 is located within a central domain that is conserved within the homologous proteins of other herpesviruses.
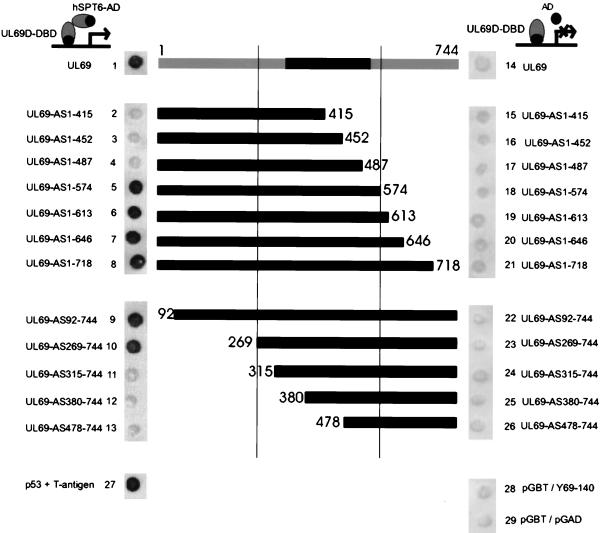
After having confirmed the in vivo interaction of pUL69 with hSPT6, we were interested in defining the domain within pUL69 that is required for binding. In particular, we wanted to elucidate whether the unique carboxy terminus of pUL69 is involved in the hSPT6 interaction, thus potentially explaining the functional differences between pUL69 and the homologous protein of HSV type 1 (HSV-1), the ICP27. For this, a set of C-terminal deletion mutants of pUL69 in fusion with the GAL4 DNA-binding domain was constructed within the yeast vector pGBT9. After transformation of yeast strain Y153/Y69-140 expressing hSPT6 (amino acids 1372 to 1726) fused to the GAL4 activation domain with the resulting UL69 deletion clones, β-galactosidase expression was analyzed by filter lift assays. As shown in Fig. 7, lanes 4 and 5, a UL69 deletion clone comprising amino acids 1 to 574 was still able to bind to hSPT6, while a further deletion of 87 amino acids from the carboxy terminus (deletion mutant UL69-AS1-487) resulted in a complete loss of interaction. Thus, the unique carboxy terminus of pUL69 is not required for binding to hSPT6. To delineate the amino-terminal sequences involved in binding, an analogous series of N-terminally deleted UL69 expression vectors was generated. A test for interaction in the yeast two-hybrid system revealed that the first 268 amino acids of pUL69 are not necessary for binding, since deletion mutant UL69-AS269-744 gave a strong reaction in the filter lift assay (see Fig. 7, lane 10). However, mutant UL69-AS315-744 was no longer able to interact (Fig. 7, lane 11). By cotransformation of the UL69 mutants with the empty activation domain vector pGAD424, it was excluded that the pUL69 fusions with the GAL4 DNA-binding domain were able to activate the reporter genes in yeast in the absence of hSPT6 (Fig. 7, lanes 14 to 26). Thus, the hSPT6 interaction domain within pUL69 maps to the central region between amino acids 269 and 574, which corresponds to the domain that is most highly conserved between the homologous proteins of other herpesviruses (see Fig. 1).
FIG. 7.
Mapping of the hSPT6 interaction domain within pUL69 using the yeast two-hybrid system. A series of N- and C-terminally deleted versions of pUL69 in fusion with the GAL4 DNA-binding domain was constructed within the yeast vector pGBT9. The domain of pUL69 contained within each vector is illustrated in the central part of the figure. Yeast cells were then transformed with two separate vectors, one of which encoded the pUL69 deletion mutant fused to the GAL4 DNA-binding domain. The second plasmid (pGAD) encoded either the GAL4 activation domain alone (lanes 14 to 26) or a carboxy-terminal fragment of hSPT6 (plasmid Y69-140, as isolated in the yeast two-hybrid screen [see Fig. 2]) in fusion with the GAL4 activation domain (lanes 1 to 13), respectively. Yeast colonies were selected for the presence of both plasmids with dropout media lacking tryptophane and leucine and subsequently analyzed for the expression of β-galactosidase by filter lift assays. The association of murine p53 (encoded by plasmid pVA3 [Clontech]) and SV40 large T antigen (plasmid pTD1 [Clontech]) served as a positive control (lane 27); as a negative control, the DNA-binding domain vector pGBT9 (pGBT) was either transformed with plasmid pY69-140 (encoding the hSPT6 C-terminal fragment in fusion with the GAL4 activation domain) or the GAL4 activation domain vector pGAD424 (pGAD) (lanes 28 and 29, respectively).
Correlation between hSPT6 interaction and stimulation of gene expression by pUL69 suggests a functional relevance of hSPT6 binding for pUL69-mediated transactivation.
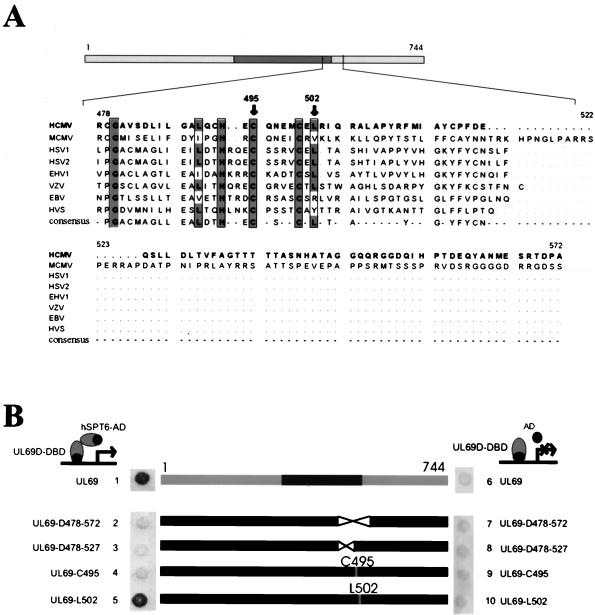
Next, we were interested in determining whether the interaction of pUL69 with hSPT6 plays a role for the trans-acting function of this viral protein. For this, we intended to construct mutants of pUL69 that were no longer able to interact with hSPT6. Since we observed a loss of interaction after deletion of the C-terminal amino acids between positions 487 and 574 (see Fig. 7, lanes 4 and 5), an internal deletion mutant lacking these amino acids was constructed within the context of the yeast DNA-binding domain vector pGBT9 (plasmid pUL69-D478-574). A test for interaction using the yeast two-hybrid system showed that this internal deletion abrogated the binding of pUL69 to hSPT6 (Fig. 8B, lane 2). A closer inspection of the amino acid sequence between positions 478 and 572 revealed that it represented the junction between the ICP27 homology domain and the unique domain that is only found in the CMV-encoded proteins (Fig. 8A). As shown in Fig. 8B, lane 3, an internal deletion of the conserved amino acids between positions 478 and 527 also resulted in a loss of interaction with hSPT6, suggesting that this amino acid sequence contains residues with a critical role for binding. In order to further investigate this amino acid sequence, we performed a PCR mutagenesis by which we substituted either cysteine 495 (UL69-C495) or leucine 502 (UL69-L502) by alanine. Both amino acids corresponded to well-conserved positions within the ICP27 homology domain (Fig. 8A). Interestingly, mutant UL69-L502 still showed an interaction with hSPT6, whereas mutant UL69-C495 was no longer able to bind (Fig. 8, lanes 4 and 5).
FIG. 8.
Interaction of internal deletion mutants and single amino acid mutants of pUL69 with hSPT6 in yeast. (A) Amino acid sequence of the pUL69 domain between amino acid residues 477 and 573 and alignment with the homologous sequences encoded by various alpha-, beta-, and gammaherpesviruses. Conserved amino acid residues are indicated by boxes. Arrows show the localization of amino acid residues that were mutagenized. (B) Interaction between pUL69 internal deletion mutants or single amino acid mutants and hSPT6 in yeast cells. The central part of panel B illustrates the mutations that were introduced into the pUL69 coding sequence. Yeast cells were transformed with two separate vectors, one of which encoded the pUL69 mutant fused to the GAL4 DNA-binding domain. The second plasmid (pGAD) encoded either the GAL4 activation domain alone (lanes 6 to 10) or a carboxy-terminal fragment of hSPT6 (plasmid Y69-140, as isolated in the yeast two-hybrid screen; see Fig. 2) in fusion with the GAL4 activation domain (lanes 1 to 5), respectively. Yeast colonies were selected for the presence of both plasmids with dropout media lacking tryptophane and leucine and subsequently analyzed for the expression of β-galactosidase by filter lift assays.
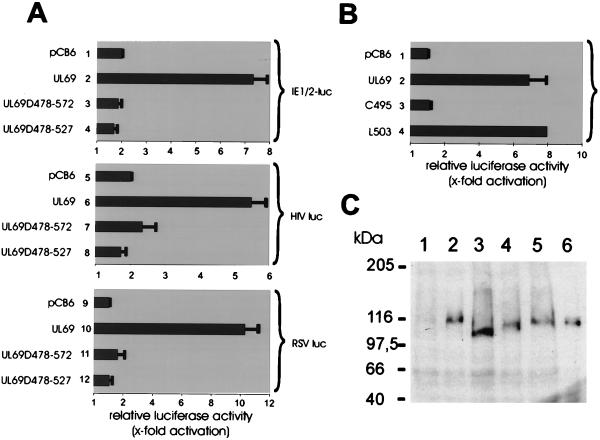
Both the internal deletion mutants and the single amino acid mutants were then tested for their capability to transactivate. For this, transient-expression assays were performed with U373MG cells using various promoters fused to the luciferase gene as a reporter. As reported previously, cotransfection of the UL69 expression vector pHM160 with the luciferase reporter plasmids IE1/2-luc (IE1/2 enhancer-promoter upstream of luciferase), HIVluc (HIV-1 LTR upstream of luciferase) or RSVluc (Rous sarcoma virus LTR upstream of luciferase) resulted in an approximately 5- to 10-fold stimulation of promoter activities (Fig. 9A, bars 2, 6, and 10, respectively) (64). In contrast, no significant stimulation was observed when the internal deletion mutants UL69-D478-572 and UL69-D478-527 were used for cotransfection (Fig. 9A, bars 3, 4, 7, 8, 11, and 12). Moreover, the single amino acid mutant UL69-C495 was no longer able to transactivate, whereas mutant UL69-L502, which was able to bind to hSPT6, stimulated the IE1/2 enhancer-promoter as efficiently as did the wild-type UL69 (Fig. 9B). As determined by Western blot analysis, all mutant UL69 proteins were expressed at comparable levels after transient expression. Thus, these results suggest a functional relevance of the hSPT6 interaction for pUL69-mediated transactivation, since we were able to observe a correlation between binding to hSPT6 and the capability to stimulate gene expression.
FIG. 9.
Luciferase analysis after cotransfection of U373 MG cells with various luciferase reporter constructs and genuine or mutagenized pUL69 expression plasmids. (A and B) Schematic diagrams of activation values obtained in cotransfection experiments. The relative luciferase activity is expressed as the fold activation relative to the activity of the respective luciferase construct in the absence of a UL69 expression vector. Results are from at least three independent experiments; standard deviations are indicated by error bars. (A) Luciferase assay after cotransfection of internal deletion mutants of pUL69 with luciferase plasmids containing either the IE1/2 enhancer-promoter plasmid pHM287 (IE1/2-luc, bars 1 to 4) or the HIV-1 LTR (HIV luc, bars 5 to 8) or the RSV LTR (RSV luc, bars 9 to 12). Lanes: 1, 5, and 9, cotransfection with the empty expression vector pCB6; 2, 6, and 10, cotransfection with plasmid pHM160 expressing wild-type pUL69; 3, 7, and 11, cotransfection with the internal UL69 deletion mutant UL69-D478-572; 4, 8, and 12, cotransfection with the internal UL69 deletion mutant UL69-D478-527. (B) Luciferase assay after cotransfection of single amino acid mutants of UL69 with luciferase plasmid IE1/2-luc (pHM287) containing the IE1/2 enhancer-promoter. Lanes: 1, cotransfection with the empty expression vector pCB6; 2, cotransfection with plasmid pHM160 (wild-type pUL69); 3, cotransfection with UL69 mutant UL69-C495; 4, cotransfection with UL69 mutant UL69-L502. (C) Western blot analysis of 293 cell extracts after transfection of various UL69 expression plasmids using the UL69-specific monoclonal antibody 69-66. Lanes: 1, transfection was performed with expression vector pCB6; 2, transfection was performed with vector pHM160; 3, transfection was performed with the vector encoding the internal deletion mutant UL69-D478-572; 4, transfection was performed with the vector encoding mutant UL69-D478-527; 5, transfection was performed with the vector encoding UL69 single-amino-acid mutant UL69-C495; 6, transfection was performed with the vector encoding UL69 single-amino-acid mutant UL69-L502.
The carboxy terminus of hSPT6 interacts with histones.
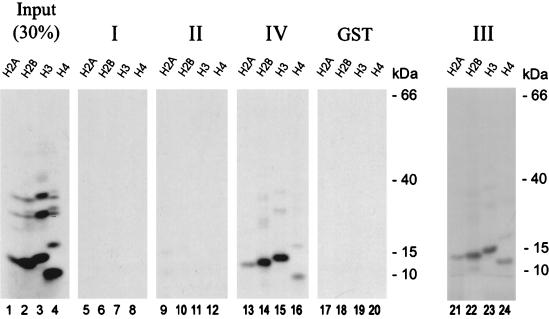
Since it was published previously that the yeast SPT6 protein is able to interact with histones, we asked the question whether this is also true for the human homolog hSPT6 (10). In order to investigate this, we performed GST pull-down assays using in vitro-translated histones H2A, H2B, H3, and H4. The radiolabeled histones were incubated together with the GST-hSPT6 fusions that had been used to confirm the binding of pUL69 to the carboxy terminus of hSPT6 (Fig. 3A). As shown in Fig. 10, lanes 13 to 16 and lanes 21 to 24, binding of histones was observed with the GST fusions comprising the carboxy terminus. As few as amino acids 1633 to 1726 of hSPT6 were sufficient for the binding of histones. As also described for the yeast SPT6, all histones were able to interact, but histones H3 and H2B bound preferentially (10). No significant interaction was observed with GST-hSPT6 fusions comprising amino-terminal and central sequences of hSPT6 (Fig. 10, lanes 5 to 12) or GST alone (Fig. 10, lanes 17 to 20). Thus, the interaction domain of both histones and pUL69 maps to C-terminal sequences within hSPT6.
FIG. 10.
The carboxy terminus of hSPT6 interacts with histones in a GST pull-down assay. Fragments I to IV of hSPT6 in fusion with GST (see Fig. 3) were incubated with in vitro-translated histones H2A, H2B, H3, and H4, respectively. After an extensive washing, the bound proteins were resolved by SDS-PAGE, and an autoradiograph of the gels is shown. The sizes of the molecular mass markers are indicated on the left of the figure. Lanes: 1 to 4, in vitro-translated 35S-labeled histone H2A (lane 1), histone H2B (lane 2), histone H3 (lane 3), and histone H4 (lane 4) (30% of the amount used in the pull-down assay). Lanes 5 to 24 show proteins that were recovered after GST pull-down analysis. The following GST fusions were used for pull-down analysis: lanes 5 to 8, GST-hSPT6 fusion I (amino acids 1 to 639); lanes 9 to 12, GST-hSPT6 fusion II (amino acids 641 to 1152); lanes 13 to 16, GST-hSPT6 fusion IV (amino acids 1633 to 1726); lanes 17 to 20, GST protein alone; lanes 21 to 24, GST-hSPT6 fusion III (amino acids 1152 to 1726). The in vitro-translated histones were added to the reactions as follows: lanes 5, 9, 13, 17, and 21, histone H2A; lanes 6, 10, 14, 18, and 22, histone H2B; lanes 7, 11, 15, 19, and 23, histone H3; lanes 8, 12, 16, 20, and 24, histone H4.
The pUL69 protein antagonizes the binding of histones to hSPT6.
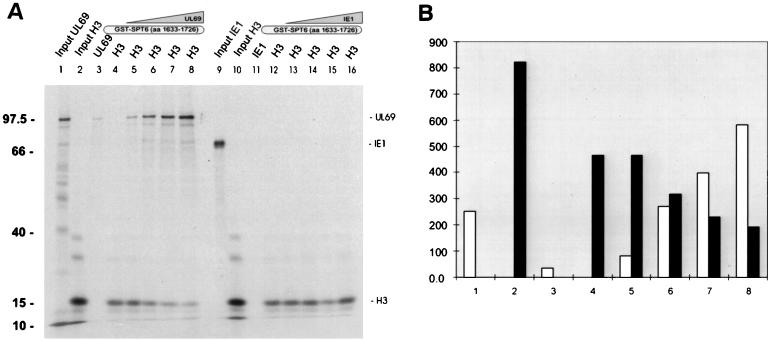
The detection of a histone-binding domain within the same region of hSPT6 that is also used by pUL69 suggested the possibility of a cooperative binding of both proteins resulting in an enhancement of histone binding in the presence of the viral transactivator. Alternatively, pUL69 might be able to compete with histone H3 for binding to hSPT6. In order to distinguish between these two possibilities, an additional GST pull-down experiment was performed. The GST-hSPT6 fusion comprising amino acids 1633 to 1726 (fragment IV; see Fig. 3A) was incubated with a constant amount of histon H3 in the presence of increasing amounts of in vitro-translated pUL69. After an extensive washing, separation of bound proteins by SDS-PAGE, autoradiography, and quantification of bound proteins using a phosphorimager, we could observe that the addition of pUL69 to the reaction resulted in a decrease in the amount of histone H3 that was bound to hSPT6 (Fig. 11, lanes 4 to 8). This effect was not observed when histone H3 was incubated with GST-hSPT6 in the presence of increasing amounts of the IE1-p72 transactivator protein, arguing against a nonspecific influence of pUL69 on the interaction between histone H3 and hSPT6 (Fig. 11, lanes 12 to 16). In summary, this experiment demonstrates that pUL69 can antagonize the binding of histone H3 to hSPT6.
FIG. 11.
The viral transactivator pUL69 and histone H3 compete for binding to hSPT6 in a pull-down assay. (A) Lanes: 1, 2, 9, and 10, SDS-PAGE of the in vitro-translated input proteins; lanes 3 to 8 and lanes 11 to 16, SDS-PAGE analysis of proteins after incubation with a GST-SPT6 fusion protein (amino acids 1633 to 1726). In lanes 3 to 4 and lanes 11 to 12, the in vitro-translated proteins (lane 3, pUL69; lanes 4 and 12, histone H3; lane 11, IE1) were incubated with the GST-hSPT6 fusion protein. Lanes: 5 to 8, a constant amount of histone H3 was used, and increasing amounts of pUL69 were added to the binding reaction; 13 to 16, a constant amount of histone H3 was used, and increasing amounts of IE1 were added to the binding reaction. Molecular mass markers are shown on the left and refer to proteins of 97.4, 40, 15, and 10 kDa, as indicated on the left of the figure. (B) Quantification of pUL69 and histone H3 protein levels as contained within lanes 1 to 8 of the pull-down assay shown in panel A. The graph shows the relative amounts of radioactive pUL69 (open bars) or histone H3 (black bars) (measured in relative photostimulated luminescence per square millimeter) of lanes 1 to 8 of the pull-down assay, as quantitated by using a phosphorimager.
DISCUSSION
The pUL69 protein of HCMV acts as a pleiotropic regulator of various target promoters in transient-expression assays (7, 64). Moreover, this protein may also be involved in the control of lytic DNA replication as suggested by results of transient replication assays and by the localization of pUL69 within viral replication centers at late times after infection (56, 64). The pUL69 protein is the homolog of the IE protein ICP27 of HSV-1 (13). The relationship between these two proteins is based on an amino acid identity of ca. 24% and on their location at identical positions within a gene block that is conserved between the various subclasses of herpesviruses. Since ICP27 has been shown to regulate gene expression on the posttranscriptional level by various mechanisms, including enhancement of 3′ RNA processing, inhibition of splicing, and nucleocytoplasmic shuttling (for a review, see reference 45), initial experiments tried to confirm an analogous mechanism for pUL69 (64). However, these studies detected important differences between the two viral regulators. For instance, no repression was observed with intron-containing genes, demonstrating that pUL69 does not inhibit splicing (64). In addition, we did not observe a colocalization of pUL69 with splicing speckles recognized by monoclonal antibody SC35 (M. Winkler and T. Stamminger, unpublished data) as reported for ICP27 (44, 54). Finally, pUL69 was not able to complement an ICP27 null mutant of HSV-1, further supporting the assumption of a nonanalogous mode of action (64).
In order to unravel the mechanism that is used by pUL69 to activate gene expression and to stimulate lytic DNA replication, we decided to search for cellular interaction partners of this viral protein. Using the yeast two-hybrid screen, we were able to isolate several copies of a polypeptide termed either hSPT6 or SUPT6H as a specific interaction partner of pUL69 in yeast (14, 40). This protein is the human homolog of the yeast regulatory protein SPT6, a large acidic nuclear polypeptide that is essential for growth in S. cerevisiae and has been implicated as a regulator of chromatin structure (10, 15). The human SPT6 is a predicted protein of 1,726 amino acids, which is highly conserved (99% identity) between mouse and human and shows 34% amino acid identity with the yeast SPT6 (14). Additional homologues have been isolated from Caenorhabditis elegans (emb-5) and Drosophila melanogaster (43). Computer analysis showed that all proteins share an acidic amino terminus which comprises a weakly conserved NAP domain, implicated in nucleosome remodeling (51). Additionally, a weakly conserved carboxy-terminal SH2 domain is contained in the C terminus of all homologs (35, 40). The central part contains a potential S1 RNA-binding domain and a helix-hairpin-helix motif implicated in non-sequence-specific DNA binding (18). Besides the yeast SPT6, very little more is known about these reading frames. The mRNAs of both the human and mouse SPT6 are ubiquitously expressed (14, 40). Consistent with this, after having generated a specific antiserum against hSPT6, we were able to detect the respective protein both in monkey COS-7 cells and in primary human foreskin fibroblasts, thus supporting the ubiquitous expression of the encoded protein. Furthermore, it was possible to coimmunoprecipitate hSPT6 and pUL69 from lysates of HCMV-infected HFFs, thus demonstrating an interaction under natural conditions. This also strongly suggests that the hSPT6 interaction is relevant for the function of pUL69 during viral replication.
Since pUL69 differs in both functional and structural aspects from ICP27, we initially hypothesized that binding of hSPT6 to the nonconserved carboxy-terminal sequence within pUL69 may explain some unique properties of this viral regulator. Surprisingly, however, our mapping studies revealed that the hSPT6 binding domain within pUL69 is located in the central region that shows the highest sequence identity to ICP27. Moreover, we observed a loss of interaction after internal deletion of a homologous sequence and after mutation of a cysteine residue of pUL69 that is highly conserved within all of the homologous proteins of other herpesviruses. This may suggest that the binding of hSPT6 is not confined to pUL69 but may also occur with other members of the ICP27 homology family. Further studies will be necessary to clarify this.
The functional relevance of the hSPT6 interaction for pUL69-mediated transactivation was investigated by the construction of mutants that showed a loss of interaction and the consecutive test of these mutants for transactivation. This revealed a correlation between binding to hSPT6 and the capacity of pUL69 mutants to stimulate promoter activities, indicating that hSPT6 is important for pUL69-mediated transactivation. Whether this is also true for other functions of this pleiotropic regulatory protein, such as the stimulation of lytic DNA replication or effects on cell cycle regulation, remains to be determined.
Up to now, little was known about the functions of hSPT6. Its homolog in yeasts, the ySPT6, was initially identified due to the observation that mutations in ySPT6 are able to overcome transcriptional defects in strains lacking the Snf-Swi protein complex, suggesting that the ySPT6 protein is required for the control of chromatin structure in yeasts (57). This was further supported by experiments demonstrating that a ySPT6 mutation causes changes in chromatin structure in vivo (10). In addition, ySPT6, along with ySPT4 and ySPT5, is important for mediating the repressive effect of histones on gene expression, as shown with a LexA-H2B fusion protein that was expressed in the context of yeast strains with defects in ySPT4, ySPT5, or ySPT6 (49, 67). This finding is in accordance with the demonstration of a direct interaction between ySPT6 and histones, primarily histone H3, and the ability of ySPT6 to assemble nucleosomes in vitro (10). We could confirm an interaction with all histones for the human SPT6; however, here the histones H2B and H3 showed the highest affinity in pull-down assays. Furthermore, we demonstrate that pUL69 competes with histone H3 for binding to the carboxy terminus of hSPT6 in an in vitro binding assay. Since the histone interaction was proposed to be critical for the nucleosome assembly function of ySPT6, pUL69 may be able to inhibit such a function of the human homolog. A potential antagonization of the assembly of nucleosomes which is thought to be repressive for transcription might both explain the broad transactivation capacity of pUL69 and the influence of this protein on lytic viral DNA replication. It will therefore be interesting to investigate whether pUL69 is able to inhibit the association of hSPT6 with histones in vivo and whether the chromatin structure of both viral and cellular genes is modified in the presence of pUL69.
Alternatively, genetic data recently suggested that ySPT6 may be involved in the regulation of transcriptional elongation (25). Although it was initially thought that ySPT6 forms a complex with two other proteins, ySPT4 and ySPT5, biochemical studies revealed that ySPT4 and ySPT5 are tightly associated in a complex that does not contain ySPT6 (25). The human homologs of ySPT4 and ySPT5, termed Supt4h and Supt5h, have recently been identified as constituents of the transcription elongation factor DSIF (DRB-sensitivity-inducing factor), that causes pausing of RNA polymerase II in conjunction with the transcription inhibitor DRB (61). Consistent with this, spt4 and spt5 mutants in yeasts have phenotypes indicating an elongation defect, and this was also observed for spt6 mutants, suggesting at least a functional interaction between these three proteins (25). Thus, the modulation of a potential role of hSPT6 in transcriptional elongation might also be an explanation for pleiotropic transactivation by pUL69.
In summary, we have identified the hSPT6 protein as a strong interaction partner of the HCMV regulatory protein pUL69. The targeting of a chromatin regulatory protein acting as a repressor of gene expression may explain both the broad transactivation mediated by this viral protein as well as its effects on lytic DNA replication.
ACKNOWLEDGMENTS
The first two authors contributed equally to this work.
We thank Donatella de Gaspero-Hoops for excellent technical assistance, Nobuo Nomura for plasmid KIAA0162 encoding hSPT6, B. Britt for his kind gift of monoclonal antibody 69-66, and B. Fleckenstein for continuous support.
This work was supported by the Deutsche Forschungsgemeinschaft (grant Sta 357/3-1 and SFB 473) and the Bundesministerium für Forschung und Technologie (IZKF Erlangen).
REFERENCES
- 1.Albrecht J C, Nicholas J, Biller D, Cameron K R, Biesinger B, Newman C, Wittmann S, Craxton M A, Coleman H, Fleckenstein B. Primary structure of the herpesvirus saimiri genome. J Virol. 1992;66:5047–5058. doi: 10.1128/jvi.66.8.5047-5058.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alford C A, Britt W J. Cytomegalovirus. In: Fields B N, Knipe D M, editors. Virology. New York, N.Y: Raven Press, Ltd.; 1990. pp. 1981–2010. [Google Scholar]
- 3.Andreoni M, Faircloth M, Vugler L, Britt W J. A rapid microneutralization assay for the measurement of neutralizing antibody reactive with human cytomegalovirus. J Virol Methods. 1989;23:157–167. doi: 10.1016/0166-0934(89)90129-8. [DOI] [PubMed] [Google Scholar]
- 4.Arlt H, Lang D, Gebert S, Stamminger T. Identification of binding sites for the 86-kilodalton IE2 protein of human cytomegalovirus within an IE2-responsive viral early promoter. J Virol. 1994;68:4117–4125. doi: 10.1128/jvi.68.7.4117-4125.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ausubel F, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1989. [Google Scholar]
- 6.Baer R, Bankier A T, Biggin M D, Deininger P L, Farrell P J, Gibson T J, Hatfull G, Hudson G S, Satchwell S C, Seguin C, Tufnell P S, Barrell B G. DNA sequence and expression of the B96-8 Epstein-Barr virus genome. Nature. 1984;348:344–346. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- 7.Baldick C J, Jr, Marchini A, Patterson C E, Shenk T. Human cytomegalovirus tegument protein pp71 (ppUL82) enhances the infectivity of viral DNA and accelerates the infectious cycle. J Virol. 1997;71:4400–4408. doi: 10.1128/jvi.71.6.4400-4408.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bannister A J, Kouzarides T. The CBP co-activator is a histone acetyltransferase. Nature. 1996;384:641–643. doi: 10.1038/384641a0. [DOI] [PubMed] [Google Scholar]
- 9.Baumeister J, Klupp B G, Mettenleiter T C. Pseudorabies virus and equine herpesvirus 1 share a nonessential gene which is absent in other herpesviruses and located adjacent to a highly conserved gene cluster. J Virol. 1995;69:5560–5567. doi: 10.1128/jvi.69.9.5560-5567.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bortvin A, Winston F. Evidence that Spt6p controls chromatin structure by a direct interaction with histones. Science. 1996;272:1473–1476. doi: 10.1126/science.272.5267.1473. [DOI] [PubMed] [Google Scholar]
- 11.Breeden L, Nasmyth K. Regulation of the yeast HO gene. Cold Spring Harb Symp Quant Biol. 1985;50:643–650. doi: 10.1101/sqb.1985.050.01.078. [DOI] [PubMed] [Google Scholar]
- 12.Buisson M, Manet E, Trescol-Biemont M C, Gruffat H, Durand B, Sergeant A. The Epstein-Barr virus (EBV) early protein EB2 is a posttranscriptional activator expressed under the control of EBV transcription factors EB1 and R. J Virol. 1989;63:5276–5284. doi: 10.1128/jvi.63.12.5276-5284.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chee M S, Bankier A T, Beck S, Bohni R, Brown C M, Cerny R, Horsnell T, Hutchison C A, Kouzarides T, Martignetti J A, et al. Analysis of the protein-coding content of the sequence of human cytomegalovirus strain AD169. Curr Top Microbiol Immunol. 1990;154:125–169. doi: 10.1007/978-3-642-74980-3_6. [DOI] [PubMed] [Google Scholar]
- 14.Chiang P W, Wang S, Smithivas P, Song W J, Ramamoorthy S, Hillman J, Puett S, Van Keuren M L, Crombez E, Kumar A, Glover T W, Miller D E, Tsai C H, Blackburn C C, Chen X N, Sun Z, Cheng J F, Korenberg J R, Kurnit D M. Identification and analysis of the human and murine putative chromatin structure regulator SUPT6H and Supt6h. Genomics. 1996;34:328–333. doi: 10.1006/geno.1996.0294. [DOI] [PubMed] [Google Scholar]
- 15.Clark-Adams C D, Winston F. The SPT6 gene is essential for growth and is required for delta-mediated transcription in Saccharomyces cerevisiae. Mol Cell Biol. 1987;7:679–686. doi: 10.1128/mcb.7.2.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davison A J, Scott J E. The complete DNA sequence of varicella-zoster virus. J Gen Virol. 1986;67:1759–1816. doi: 10.1099/0022-1317-67-9-1759. [DOI] [PubMed] [Google Scholar]
- 17.DeMarchi J M. Human cytomegalovirus DNA: restriction enzyme cleavage maps and map locations for immediate-early, early, and late RNAs. Virology. 1981;114:23–38. doi: 10.1016/0042-6822(81)90249-x. [DOI] [PubMed] [Google Scholar]
- 18.Doherty A J, Serpell L C, Ponting C P. The helix-hairpin-helix DNA-binding motif: a structural basis for non-sequence-specific recognition of DNA. Nucleic Acids Res. 1996;24:2488–2497. doi: 10.1093/nar/24.13.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Durfee T, Becherer K, Chen P L, Yeh S H, Yang Y, Kilburn A E, Lee W H, Elledge S J. The retinoblastoma protein associates with the protein phosphatase type 1 catalytic subunit. Genes Dev. 1993;7:555–569. doi: 10.1101/gad.7.4.555. [DOI] [PubMed] [Google Scholar]
- 20.Fraefel C, Wirth U V, Vogt B, Schwyzer M. Immediate-early transcription over covalently joined genome ends of bovine herpesvirus 1: the circ gene. J Virol. 1993;67:1328–1333. doi: 10.1128/jvi.67.3.1328-1333.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gebert S, Schmolke S, Sorg G, Floss S, Plachter B, Stamminger T. The UL84 protein of human cytomegalovirus acts as a transdominant inhibitor of immediate-early-mediated transactivation that is able to prevent viral replication. J Virol. 1997;71:7048–7060. doi: 10.1128/jvi.71.9.7048-7060.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Georgiev O, Bourquin J P, Gstaiger M, Knoepfel L, Schaffner W, Hovens C. Two versatile eukaryotic vectors permitting epitope tagging, radiolabelling and nuclear localisation of expressed proteins. Gene. 1996;168:165–167. doi: 10.1016/0378-1119(95)00764-4. [DOI] [PubMed] [Google Scholar]
- 23.Gompels U A, Nicholas J, Lawrence G, Jones M, Thomson B J, Martin M E, Efstathiou S, Craxton M, Macaulay H A. The DNA sequence of human herpesvirus-6: structure, coding content, and genome evolution. Virology. 1995;209:29–51. doi: 10.1006/viro.1995.1228. [DOI] [PubMed] [Google Scholar]
- 24.Guarente L. Yeast promoters and lacZ fusions designed to study expression of cloned genes in yeast. Methods Enzymol. 1983;101:181–191. doi: 10.1016/0076-6879(83)01013-7. [DOI] [PubMed] [Google Scholar]
- 25.Hartzog G A, Wada T, Handa H, Winston F. Evidence that Spt4, Spt5, and Spt6 control transcription elongation by RNA polymerase II in Saccharomyces cerevisiae. Genes Dev. 1998;12:357–369. doi: 10.1101/gad.12.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoffman C S, Winston F. A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of Escherichia coli. Gene. 1987;57:267–272. doi: 10.1016/0378-1119(87)90131-4. [DOI] [PubMed] [Google Scholar]
- 27.Hoffmann A, Chiang C M, Oelgeschlager T, Xie X, Burley S K, Nakatani Y, Roeder R G. A histone octamer-like structure within TFIID. Nature. 1996;380:356–359. doi: 10.1038/380356a0. [DOI] [PubMed] [Google Scholar]
- 28.Hofmann H, Floss S, Stamminger T. Covalent modification of the transactivator protein IE2-p86 of human cytomegalovirus by conjugation to the ubiquitin-homologous proteins SUMO-1 and hSMT3b. J Virol. 2000;74:2510–2524. doi: 10.1128/jvi.74.6.2510-2524.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inchauspe G, Ostrove J M. Differential regulation by varicella-zoster virus (VZV) and herpes simplex virus type-1 trans-activating genes. Virology. 1989;173:710–714. doi: 10.1016/0042-6822(89)90584-9. [DOI] [PubMed] [Google Scholar]
- 30.Lai J S, Herr W. Ethidium bromide provides a simple tool for identifying genuine DNA-independent protein associations. Proc Natl Acad Sci USA. 1992;89:6958–6962. doi: 10.1073/pnas.89.15.6958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lang D, Gebert S, Arlt H, Stamminger T. Functional interaction between the human cytomegalovirus 86-kilodalton IE2 protein and the cellular transcription factor CREB. J Virol. 1995;69:6030–6037. doi: 10.1128/jvi.69.10.6030-6037.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lang D, Stamminger T. The 86-kilodalton IE-2 protein of human cytomegalovirus is a sequence-specific DNA-binding protein that interacts directly with the negative autoregulatory response element located near the cap site of the IE1/2 enhancer-promoter. J Virol. 1993;67:323–331. doi: 10.1128/jvi.67.1.323-331.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu M, Shenk T. Human cytomegalovirus UL69 protein induces cells to accumulate in G1 phase of the cell cycle. J Virol. 1999;73:676–683. doi: 10.1128/jvi.73.1.676-683.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mackett M, Stewart J P, Pepper S de V, Chee M, Efstathiou S, Nash A A, Arrand J R. Genetic content and preliminary transcriptional analysis of a representative region of murine gammaherpesvirus 68. J Gen Virol. 1997;78:1425–1433. doi: 10.1099/0022-1317-78-6-1425. [DOI] [PubMed] [Google Scholar]
- 35.Maclennan A J, Shaw G. A yeast SH2 domain. Trends Biochem Sci. 1993;18:464–465. doi: 10.1016/0968-0004(93)90006-9. [DOI] [PubMed] [Google Scholar]
- 36.McDonough S H, Spector D H. Transcription in human fibroblasts permissively infected by human cytomegalovirus strain AD169. Virology. 1983;125:31–46. doi: 10.1016/0042-6822(83)90061-2. [DOI] [PubMed] [Google Scholar]
- 37.McGeoch D J, Cunningham C, McIntyre G, Dolan A. Comparative sequence analysis of the long repeat regions and adjoining parts of the long unique regions in the genomes of herpes simplex viruses types 1 and 2. J Gen Virol. 1991;72:3057–3075. doi: 10.1099/0022-1317-72-12-3057. [DOI] [PubMed] [Google Scholar]
- 38.McGeoch D J, Dalrymple M A, Davison A J, Dolan A, Frame M C, McNab D, Perry L J, Scott J E, Taylor P. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol. 1988;69:1531–1574. doi: 10.1099/0022-1317-69-7-1531. [DOI] [PubMed] [Google Scholar]
- 39.Morgenstern B. DIALIGN 2: improvement of the segment-to-segment approach to multiple sequence alignment. Bioinformatics. 1999;15:211–218. doi: 10.1093/bioinformatics/15.3.211. [DOI] [PubMed] [Google Scholar]
- 40.Nagase T, Seki N, Ishikawa K, Tanaka A, Nomura N. Prediction of the coding sequences of unidentified human genes. V. The coding sequences of 40 new genes (KIAA0161-KIAA0200) deduced by analysis of cDNA clones from human cell line KG-1. DNA Res. 1996;3:17–24. doi: 10.1093/dnares/3.1.17. [DOI] [PubMed] [Google Scholar]
- 41.Nicholas J. Determination and analysis of the complete nucleotide sequence of human herpesvirus. J Virol. 1996;70:5975–5989. doi: 10.1128/jvi.70.9.5975-5989.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nicholas J, Cameron K R, Coleman H, Newman C, Honess R W. Analysis of nucleotide sequence of the rightmost 43 kbp of herpesvirus saimiri (HVS) L-DNA: general conservation of genetic organization between HVS and Epstein-Barr virus. Virology. 1992;188:296–310. doi: 10.1016/0042-6822(92)90759-i. [DOI] [PubMed] [Google Scholar]
- 43.Nishiwaki K, Sano T, Miwa J. emb-5, a gene required for the correct timing of gut precursor cell division during gastrulation in Caenorhabditis elegans, encodes a protein similar to the yeast nuclear protein SPT6. Mol Gen Genet. 1993;239:313–322. doi: 10.1007/BF00276929. [DOI] [PubMed] [Google Scholar]
- 44.Phelan A, Carmo-Fonseca M, McLaughlan J, Lamond A I, Clements J B. A herpes simplex virus type 1 immediate-early gene product, IE63, regulates small nuclear ribonucleoprotein distribution. Proc Natl Acad Sci USA. 1993;90:9056–9060. doi: 10.1073/pnas.90.19.9056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Phelan A, Clements J B. Posttranscriptional regulation in herpes simplex virus. Semin Virol. 1998;8:309–318. [Google Scholar]
- 46.Plachter B, Britt W, Vornhagen R, Stamminger T, Jahn G. Analysis of proteins encoded by IE regions 1 and 2 of human cytomegalovirus using monoclonal antibodies generated against recombinant antigens. Virology. 1993;193:642–652. doi: 10.1006/viro.1993.1172. [DOI] [PubMed] [Google Scholar]
- 47.Puchtler E, Stamminger T. An inducible promoter mediates abundant expression from the immediate-early 2 gene region of human cytomegalovirus at late times after infection. J Virol. 1991;65:6301–6306. doi: 10.1128/jvi.65.11.6301-6306.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rawlinson W D, Farrell H E, Barrell B G. Analysis of the complete DNA sequence of murine cytomegalovirus. J Virol. 1996;70:8833–8849. doi: 10.1128/jvi.70.12.8833-8849.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Recht J, Dunn B, Raff A, Osley M A. Functional analysis of histones H2A and H2B in transcriptional repression in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:2545–2553. doi: 10.1128/mcb.16.6.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ren D, Lee L F, Coussens P M. Identification and characterization of Marek's disease virus genes homologous to ICP27 and glycoprotein K of herpes simplex virus-1. Virology. 1994;204:242–250. doi: 10.1006/viro.1994.1528. [DOI] [PubMed] [Google Scholar]
- 51.Rodriguez P, Munroe D, Prawitt D, Chu L L, Bric E, Kim J, Reid L H, Davies C, Nakagama H, Loebbert R, Winterpacht A, Petruzzi M J, Higgins M J, Nowak N, Evans G, Shows T, Weissman B E, Zabel B, Housman D E, Pelletier J. Functional characterization of human nucleosome assembly protein-2 (NAP1L4) suggests a role as a histone chaperone. Genomics. 1997;44:253–265. doi: 10.1006/geno.1997.4868. [DOI] [PubMed] [Google Scholar]
- 52.Russo J J, Bohenzky R A, Chien M C, Chen J, Yan M, Maddalena D, Parry J P, Peruzzi D, Edelman I S, Chang Y, Moore P S. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8) Proc Natl Acad Sci USA. 1996;93:14862–14867. doi: 10.1073/pnas.93.25.14862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sacks W R, Greene C C, Aschman D P, Schaffer P A. Herpes simplex virus type 1 ICP27 is an essential regulatory protein. J Virol. 1985;55:796–805. doi: 10.1128/jvi.55.3.796-805.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sandri-Goldin R M, Hibbard M K, Hardwicke M A. The C-terminal repressor region of herpes simplex virus type 1 ICP27 is required for the redistribution of small nuclear ribonucleoprotein particles and splicing factor SC35; however, these alterations are not sufficient to inhibit host cell splicing. J Virol. 1995;69:6063–6076. doi: 10.1128/jvi.69.10.6063-6076.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sandri-Goldin R M, Mendoza G E. A herpesvirus regulatory protein appears to act post-transcriptionally by affecting mRNA processing. Genes Dev. 1992;6:848–863. doi: 10.1101/gad.6.5.848. [DOI] [PubMed] [Google Scholar]
- 56.Sarisky R T, Hayward G S. Evidence that the UL84 gene product of human cytomegalovirus is essential for promoting oriLyt-dependent DNA replication and formation of replication compartments in cotransfection assays. J Virol. 1996;70:7398–7413. doi: 10.1128/jvi.70.11.7398-7413.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Swanson M S, Winston F. SPT4, SPT5 and SPT6 interactions: effects on transcription and viability in Saccharomyces cerevisiae. Genetics. 1992;132:325–336. doi: 10.1093/genetics/132.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Telford E A, Watson M S, Aird H C, Perry J, Davison A J. The DNA sequence of equine herpesvirus 2. J Mol Biol. 1995;249:520–528. doi: 10.1006/jmbi.1995.0314. [DOI] [PubMed] [Google Scholar]
- 59.Telford E A, Watson M S, McBride K, Davison A J. The DNA sequence of equine herpesvirus-1. Virology. 1992;189:304–316. doi: 10.1016/0042-6822(92)90706-u. [DOI] [PubMed] [Google Scholar]
- 60.van Santen V L, Chang L Y. Cloning and mapping of EcoRI, HindIII, and PstI fragments of bovine herpesvirus 4 (DN-599) genome. Intervirology. 1992;34:44–52. doi: 10.1159/000150262. [DOI] [PubMed] [Google Scholar]
- 61.Wada T, Takagi T, Yamaguchi Y, Ferdous A, Imai T, Hirose S, Sugimoto S, Yano K, Hartzog G A, Winston F, Buratowski S, Handa H. DSIF, a novel transcription elongation factor that regulates RNA polymerase II processivity, is composed of human Spt4 and Spt5 homologs. Genes Dev. 1998;12:343–356. doi: 10.1101/gad.12.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wathen M W, Stinski M F. Temporal patterns of human cytomegalovirus transcription: mapping the viral RNAs synthesized at immediate early, early, and late times after infection. J Virol. 1982;41:462–477. doi: 10.1128/jvi.41.2.462-477.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wathen M W, Thomsen D R, Stinski M F. Temporal regulation of human cytomegalovirus transcription at immediate early and early times after infection. J Virol. 1981;38:446–459. doi: 10.1128/jvi.38.2.446-459.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Winkler M, Rice S A, Stamminger T. UL69 of human cytomegalovirus, an open reading frame with homology to ICP27 of herpes simplex virus, encodes a transactivator of gene expression. J Virol. 1994;68:3943–3954. doi: 10.1128/jvi.68.6.3943-3954.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Winkler M, Schmolke S, Plachter B, Stamminger T. UL69 of HCMV, a homolog of the HSV ICP27, is contained within the tegument of virions and activates the major IE enhancer of HCMV in synergy with the tegument protein pp71. Scand J Infect Dis Suppl. 1995;99:8–9. [Google Scholar]
- 66.Winkler M, Stamminger T. A specific subform of the human cytomegalovirus transactivator protein pUL69 is contained within the tegument of virus particles. J Virol. 1996;70:8984–8987. doi: 10.1128/jvi.70.12.8984-8987.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wolffe A P, Pruss D. Hanging on to histones: chromatin. Curr Biol. 1996;6:234–237. doi: 10.1016/s0960-9822(02)00465-7. [DOI] [PubMed] [Google Scholar]