Cytotoxic T-Lymphocyte Epitope Immunodominance in the Control of Choroid Plexus Tumors in Simian Virus 40 Large T Antigen Transgenic Mice (original) (raw)
Abstract
The simian virus 40 (SV40) large tumor antigen (Tag) is a virus-encoded oncoprotein which is the target of a strong cytotoxic T-lymphocyte (CTL) response. Three immunodominant H-2b-restricted epitopes, designated epitopes I, II/III, and IV, have been defined. We investigated whether induction of CTLs directed against these Tag epitopes might control Tag-induced tumors in SV11+ (H-2b) mice. SV11+ mice develop spontaneous tumors of the choroid plexus due to expression of SV40 Tag as a transgene. We demonstrate that SV11+ mice are functionally tolerant to the immunodominant Tag CTL epitopes. CTLs specific for the H-2Kb-restricted Tag epitope IV were induced in SV11+ mice following adoptive transfer with unprimed C57BL/6 spleen cells and immunization with recombinant vaccinia viruses expressing either full-length Tag or the H-2Kb-restricted epitope IV as a minigene. In addition, irradiation of SV11+ mice prior to adoptive transfer with unprimed C57BL/6 spleen cells led to the priming of epitope IV-specific CTLs by the endogenous Tag. Induction of epitope IV-specific CTLs in SV11+ mice by either approach correlated with increased life span and control of the choroid plexus tumor progression, indicating that CTLs specific for the immunodominant Tag epitope IV control the progressive growth of spontaneous tumors induced by this DNA virus oncogene in transgenic mice.
Simian virus 40 (SV40) large tumor antigen (Tag) is a virus-encoded oncoprotein which can transform a variety of cell types in vitro and induces tumors in mice when expressed as a transgene (7, 19, 30, 43, 53). In addition to possessing transforming properties, Tag serves as the target of a vigorous class I major histocompatibility complex (MHC)-restricted cytotoxic T-lymphocyte (CTL) response (65) which is involved in immunity to SV40 Tag-induced tumors (16, 27, 54, 66). The Tag-specific CTL response in C57BL/6 (B6) mice is characterized by a hierarchical response against multiple epitopes (9, 48, 62, 63). CTLs specific for the immunodominant H-2Db-restricted epitopes I (residues 206 to 215) (40, 63) and II/III (residues 223 to 231) (15) and the H-2Kb-restricted epitope IV (residues 404 to 411) (49, 63) are induced following immunization with Tag-transformed cells (48), SV40, or Tag expressed by a recombinant vaccinia virus (rVV) (20). A third H-2Db-restricted Tag epitope, epitope V (residues 489 to 497), is immunorecessive (15, 62). Previous studies (48) using limiting-dilution analysis have established that epitope IV is clearly the most dominant H-2b-restricted epitope.
Mice of the line SV11 express the SV40 Tag as a transgene in the B6 background under the influence of the viral enhancer-promoter which drives expression of the protein in the choroid plexus (53). As a result, SV11+ mice develop papillomas of the choroid plexus which grow progressively until death at a mean age of 104 days (67). Tag protein in the choroid plexus can be detected as early as 14 days after birth, but the first stages of neoplasia are not observed until the mice reach an age of 36 to 41 days. Tumors grow rapidly in mice around the age of 80 days, with a corresponding increase in Tag expression. The consistent time frame of tumor progression in these mice makes the SV11+ tumor system an attractive model for analyzing immunotherapeutic approaches in the control of oncogene-induced tumors in vivo.
We have used SV11+ mice to determine if the immunodominant H-2b-restricted Tag CTL epitopes can induce control of spontaneously arising choroid plexus tumors in vivo. As SV11+ mice are tolerant of the immunodominant Tag CTL epitopes I, II/III, and IV, they were given adoptive transfers with unprimed B6 spleen cells and immunized with rVVs expressing either full-length Tag or individual Tag epitope minigenes. This resulted in the generation of a strong CTL response against epitope IV and led to the control of choroid plexus tumor progression. In addition, we demonstrate that irradiation of SV11+ mice prior to adoptive transfer with unprimed B6 spleen cells led to the induction of epitope IV-specific CTLs against endogenous Tag and a highly significant increase in the life span of SV11+ mice due to decreased tumor progression.
MATERIALS AND METHODS
Mice.
C57BL/6 (H-2b) mice were purchased from the Jackson Laboratory (Bar Harbor, Maine) and maintained in conventional housing at the animal facility of the M. S. Hershey Medical Center. Mice were routinely used between the ages of 6 and 12 weeks. SV11 mice were generated by Palmiter et al. (53), and the kinetics of tumor progression have been described previously (67). SV11+ (H-2b) mice, expressing the SV40 Tag transgene under the control of the viral enhancer-promoter, were bred on the B6 background for over 30 generations in the animal facility at Princeton University (Princeton, N.J.). An SV11+ founder male was obtained from Terry Van Dyke (University of North Carolina, Chapel Hill) via Edward Roy (University of Illinois, Urbana-Champaign), and the line has been maintained in the animal facility of the M. S. Hershey Medical center by backcrossing Tag transgene-positive males with B6 females.
PCR analysis of tail-derived DNA.
Offspring of SV11+ mice were routinely screened for the presence of the Tag transgene at 20 to 25 days of age by PCR amplification of Tag-specific sequences from tail-derived genomic DNA by using the antisense STEV 313 (5′-AGGCATTCGACCACTGCTCCCATTCA-3′) and sense STEV 314 (5′GACTTTGGAGGCTTCTGGGATGCA-3′) primers which flank the SV40 Tag intron sequence and amplify a 154-bp product from the SV11 transgene due to a 268-bp deletion within the Tag intron (14). Amplification was carried out in a Perkin-Elmer model 9600 Gene Amp thermal cycler with Taq polymerase (PGC Scientific) for 30 cycles of amplification.
Cell lines and media.
B6/WT-19 is an SV40-transformed B6 mouse embryo fibroblast line which expresses wild-type Tag (48). B6/350gB cells were derived by transformation of B6 mouse embryo fibroblasts with an SV40 Tag construct containing the H-2Kb-restricted herpes simplex virus (HSV) glycoprotein B residues 498 to 505 (gB498-505) CTL epitope inserted at position 350 of Tag (21). B6/K-1,4,5 was derived from a Tag-transformed B6 kidney cell line by sequential immunoselection with Tag-specific CTL clones and lacks the Tag epitopes I, II/III, IV, and V (64). All Tag-transformed cell lines were maintained in Dulbecco’s modified Eagle medium (DMEM) supplemented with 100 U of penicillin per ml, 100 μg of streptomycin per ml, 100 μg of kanamycin per ml, 2 mM l-glutamine, 10 mM HEPES buffer, 0.075% (wt/vol) NaHCO3, and 5 to 10% fetal bovine serum (FBS). RMA (H-2b) cells (41) were maintained in suspension by using RPMI-1640 medium supplemented with 10% FBS, 100 U of penicillin per ml, 100 μg of streptomycin per ml, 2 mM l-glutamine, and 50 μM 2-mercaptoethanol.
Synthetic peptides.
All peptides used were synthesized at the Macromolecular Core Facility of the M. S. Hershey Medical Center by 9-fluroenylmethoxycarbonyl chemistry on an automated peptide synthesizer (9050 MilliGen PepSynthesizer). Peptides used in these experiments correspond to SV40 Tag epitopes I (SAINNYAQKL), II/III (CKGVNKEYL), and IV (VVYDFLKC), as well as the optimized H-2Db-binding peptide DbN5 (SMIKNLEYM) (23, 48) and two H-2Kb-restricted HSV-derived peptides, gB498-505 (SSIEFARL) (6) and ribonucleotide reductase (RR1) 822-829 (QTFDFGRL) (59).
Viruses.
rVVs used in this study have been described previously and include rVV-941T (20), which encodes full-length SV40 Tag, and a series of rVVs encoding Tag epitope minigenes preceded by the adenovirus E3/19K endoplasmic reticulum insertion sequence (ES) and designated rVV-ES I (Tag sequence 206 to 215), rVV-ES II/III (Tag sequence 223 to 231) (20), and rVV-ES IV (Tag sequence 404 to 411) (5). An rVV encoding the H-2Kb-restricted CTL epitope gB498-505 derived from HSV preceded by ES is designated rVV-ES-gB498-505 (5). The wild-type vaccinia virus strain WR (VV-WR [ATCC VR-119]), from which all the rVVs were derived, was used in these experiments.
Irradiation, adoptive transfer, and immunization.
Spleen cells used for adoptive transfer were obtained from the appropriate sex of normal B6 or SV11+ mice, as indicated. SV11+ mice were reconstituted by intravenous (i.v.) injection of 5 × 107 erythrocyte-depleted naive B6 spleen cells suspended in 0.2 ml of Hanks balanced salt solution into the tail vein at 40 to 45 days of age. Some mice received 450 rads of gamma irradiation from a 60Co source GammaCell 220 (Nordion International) 1 day prior to reconstitution with B6 spleen cells. After 2 days, reconstituted and control unmanipulated mice were immunized i.v. with 107 PFU of the indicated rVV in 0.2 ml of phosphate-buffered saline (PBS) containing 0.1% bovine serum albumin (BSA).
Maintenance of CTL clones.
SV40 Tag-specific CTL clones used in this study were maintained by in vitro passage as described previously (40) and include clones K-11, K-19 (9), and Y-4 (63), which recognize Tag epitopes I (residues 206 to 215), II/III (residues 223 to 231), and IV (residues 404 to 411), respectively.
In vitro stimulation of bulk CTL.
Spleens were harvested at the times indicated (3 to 8 weeks after immunization) and single-cell suspensions of erythrocyte-depleted spleen cells were restimulated in vitro with gamma-irradiated B6/WT-19 cells as described previously (48). Briefly, 107 spleen cells from immunized animals were mixed with 5 × 105 gamma-irradiated (10,000 rads) B6/WT-19 cells in 4 ml of complete RPMI-1640 medium supplemented with 10% FBS per well of a 12-well tissue culture plate. To verify infection, 107 spleen cells from each vaccinia virus-infected mouse were restimulated in vitro with 5 × 105 irradiated (60,000 rads) VV-WR-infected B6 spleen cells in 4 ml of complete RPMI-1640 medium supplemented with 10% FBS per well of a 12-well plate.
Cytotoxicity assays.
Assays for CTL lysis were performed on day 6 after in vitro restimulation as previously described (20). Briefly, RMA target cells were labeled with 100 μCi of sodium 51chromate (51Cr) per 106 cells for 1 h at 37°C. Cells were washed once and resuspended in complete RPMI-1640 medium containing a 1 μM concentration of the appropriate peptide, and the resulting suspensions were rocked for 2 h at 37°C. Excess peptide was removed with three washes, and cells were resuspended in an appropriate volume of complete RPMI-1640 medium. Tag-transformed cell lines were treated with gamma interferon (40 U/ml; Pharmingen, San Diego, Calif.) for 48 h prior to labeling with 250 μCi of 51Cr per T-25 flask for 3 to 4 h at 37°C. Cells were harvested by trypsinization and washed three times prior to final resuspension in complete RPMI-1640 medium. For vaccinia virus-infected target cells, monolayers of B6/K-1,4,5 cells were labeled with 250 μCi of 51Cr overnight at 37°C in T-25 flasks. Labeled cells were trypsinized, washed once with PBS–0.1% BSA and resuspended in PBS–0.1% BSA at 5 × 106 cells per ml. Cells were either mock infected or infected with VV-WR at a multiplicity of infection of 10 for 1 h at 37°C with occasional agitation. Cells were then diluted with 5 ml of complete DMEM containing 5% FBS, rocked at 37°C for 3 to 4 h, and washed three times prior to final suspension in complete RPMI-1640 medium. All target cells were added in 100-μl aliquots to 96-well V-bottom microtiter plates to yield 0.5 × 104 to 1 × 104 cells per well. Dilutions of effector cells were added to target cells in 100-μl aliquots to give the indicated effector-to-target cell ratios and were incubated at 37°C in 5% CO2 for 5 h. Cells were then pelleted by centrifugation at 180 × g for 6 min; 100 μl of supernatant was harvested, and the radioactivity was counted in a Packard Cobra model 5005 gamma radiation counter. Percent specific lysis was calculated as follows: % specific lysis = [(experimental − spontaneous)/(maximum − spontaneous)] × 100, where experimental is the counts per minute released from target cells incubated with CTL effectors, spontaneous is the counts per minute released from target cells incubated with medium alone, and maximum is the counts per minute obtained from target cells added to wells containing 100 μl of 5% sodium dodecyl sulfate. All data points represent the means of triplicate samples.
Histology and immunohistochemistry.
For routine histology, freshly harvested mouse brains were snap frozen in isopentane and stored at −80°C. Ten-micrometer-thick sections were cut with a microtome at −20°C, and frozen sections were fixed with ethanol-acetone prior to staining with hematoxylin and eosin (H&E). For immunohistochemistry, mice were perfused with 4% paraformaldehyde, and their brains were fixed with 4% paraformaldehyde for a further 24 h prior to embedding in paraffin blocks. Seven-micrometer-thick sections were cut on a microtome and collected onto positively charged slides. Parallel sections were stained with H&E or by immunohistochemistry for Tag. For immunohistochemistry, sections were deparaffinized in xylene and rehydrated in ethanol. After two washes in PBS, slides were boiled in PBS twice for 5 min using a microwave oven (low setting). Cooled slides were incubated with 10% normal goat serum in PBS containing 0.1% Tween 20 for 30 min, washed, and incubated with primary anti-Tag antibody for 1 h at room temperature. Primary antibody consisted of an equal mixture of monoclonal antibody Pab419 (32) and Pab901 (10) culture supernatants which bind to epitopes in the amino- and carboxyl-terminal portions of SV40 Tag, respectively. Sections were then incubated for 1 h with goat anti-mouse immunoglobulin G (1:200; Sigma) followed by incubation for 1 h with mouse peroxidase antiperoxidase (1:200; Sigma). Sections were stained with diaminobenzidine substrate (DAB substrate kit for peroxidase; Vector) for 2 to 10 min, as needed.
Life span analysis and derivation of choroid plexus tumor cell lines.
SV11+ mice in life span analysis studies were monitored for the development of cranial enlargement, indicative of end-stage choroid plexus tumors, and were sacrificed when they became moribund or distressed. The presence of choroid plexus tumors in the brains of end-stage mice was confirmed by necropsy. In some cases, tumors were harvested from end-stage mice and cell lines were derived by digestion of minced tumors in a solution of EDTA containing 1% trypsin for 20 min at 37°C. Following neutralization with DMEM, cultures were established in closed-cap T-75 tissue culture flasks in DMEM containing 10% FBS. Fresh culture medium was added every 3 to 4 days until flasks contained confluent monolayers of transformed cells which were then passaged sequentially. All established lines were 100% positive for nuclear SV40 Tag as determined by immunofluorescent staining.
Statistical analysis.
Survival curves were constructed by the Kaplan-Meier method with DeltaGraph software (Deltapoint, Inc., Monterey, Calif.), and statistical analysis was performed by a single-factor analysis of variance and validated with Fisher’s protected least-significant-difference test found on SUPERANOVA software (Abacus Concepts, Inc., Berkeley, Calif.). P values of <0.05 were considered significant.
RESULTS
Progression of choroid plexus tumors in SV11+ mice.
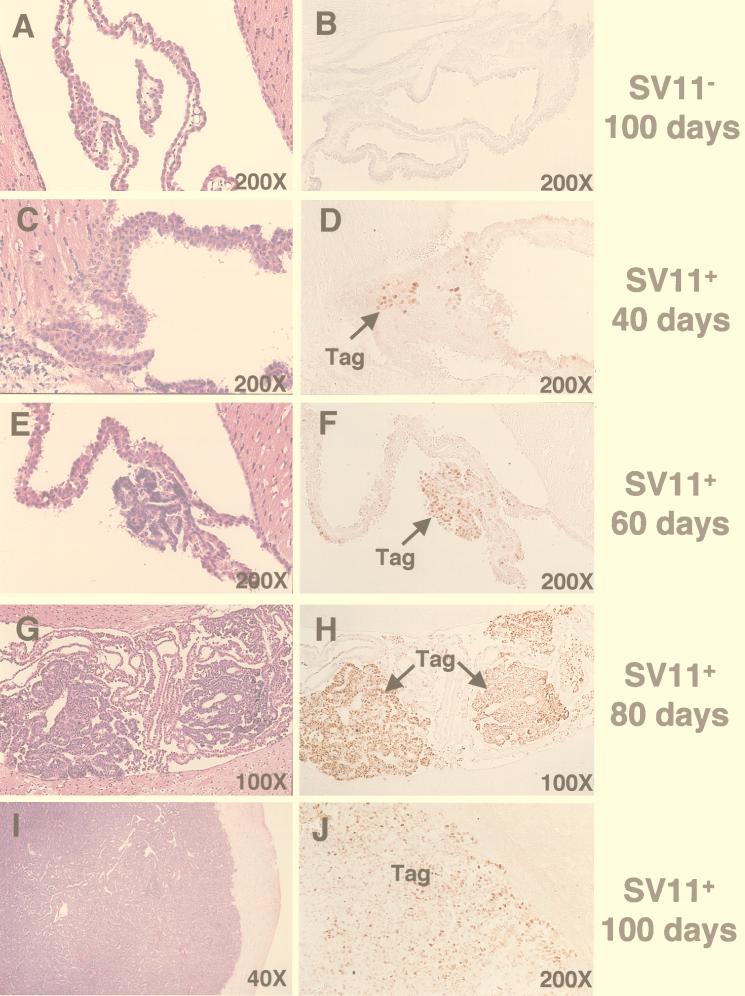
The progression of tumors and kinetics of Tag expression in the choroid plexus of SV11+ mice has been described previously (67). In order to follow choroid plexus tumor progression for SV11+ mice given immunotherapy in the current study, we examined a series of sections derived from the brains of unmanipulated SV11+ mice of increasing age. Sections were stained in parallel with H&E or by immunohistochemistry for Tag (Fig. 1C through J). The normal architecture of the choroid plexus within the ventricles of the brain is shown for a 100-day-old SV11− littermate in Fig. 1A. Tag-specific staining of the choroid plexus was not detected in these mice (Fig. 1B). In contrast, small clusters of Tag+ cells were detected in the choroid plexus tissue of SV11+ mice by 40 days of age, although no tumors were apparent (Fig. 1C and D). Microscopic grade I tumors were observed in 60-day-old mice (Fig. 1E and F), and these tumors progressed to macroscopic size by day 80 (Fig. 1G and H). All tumors stained positively for Tag, and isolated Tag+ nuclei outside of the tumor bed also were observed in the choroid plexus tissue. By 100 days of age, most unmanipulated SV11+ mice had massive grade IV tumors which distorted the ventricles and invaded the surrounding tissue (Fig. 1I and Table 1, group A). These large tumors retained Tag expression (Fig. 1J).
FIG. 1.
Progression of choroid plexus tumors in unmanipulated SV11+ mice. Serial sections from paraffin-embedded brains of 100-day-old SV11− mice (A and B) and SV11+ mice which were 40 (C and D), 60 (E and F), 80 (G and H), and 100 (I and J) days of age were stained with H&E (A, C, E, G, and I) or anti-Tag monoclonal antibody plus peroxidase antiperoxidase (B, D, F, H, and J) to detect Tag+ cells (brown). Arrows indicate the location of Tag+ cells. Magnifications are indicated.
TABLE 1.
Summary of tumor progression in reconstituted and immunized SV11+ mice at 100 days of agea
| Group | No. of mice/group | Adoptive transfer | rVV | % of mice with indicated tumor gradeb: | |||
|---|---|---|---|---|---|---|---|
| I | II | III | IV | ||||
| A | 8 | No | None | 13 | 87 | ||
| B | 6 | Yes | None | 100 | |||
| C | 15 | Yes | ES-gB | 7 | 20 | 73 | |
| D | 3 | No | 941T | 100 | |||
| E | 16 | Yes | 941T | 19 | 12 | 38 | 31 |
| F | 3 | No | ES-IV | 100 | |||
| G | 17 | Yes | ES-IV | 30 | 35 | 35 |
SV11+ mice are tolerant to SV40 Tag immunodominant CTL epitopes.
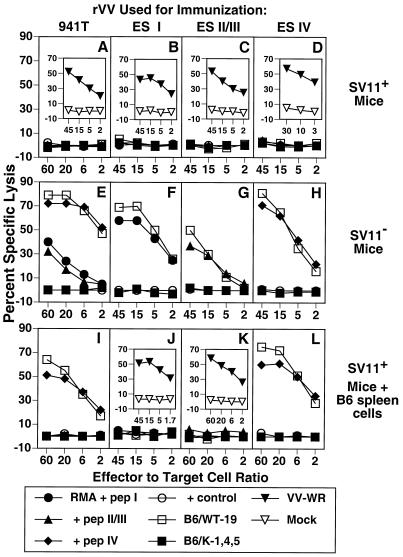
Since SV11+ mice express the SV40 Tag transgene early in life (53, 67), it is likely that they develop tolerance against the Tag self protein as has been demonstrated for other SV40 transgenic mice (16). To investigate this possibility, we determined the ability of SV11+ mice to develop Tag-specific CTLs following Tag immunization. SV11+ mice and their SV11− littermates were immunized i.v. with 107 PFU of an rVV expressing full-length Tag, rVV-941T, at approximately 50 days of age. After 3 weeks, mice were sacrificed and spleen cells were restimulated in vitro for 6 days with the syngeneic Tag-transformed cell line B6/WT-19. Responder cells were tested for their ability to lyse syngeneic RMA target cells pulsed with synthetic peptides corresponding to Tag CTL epitopes I, II/III, and IV as well as Tag-transformed cells. SV11− mice immunized with rVV-941T developed CTLs directed against the three immunodominant Tag epitopes I, II/III, and IV (Fig. 2E). Immunization of B6 mice with rVV-941T reproducibility elicited a more vigorous CTL response against epitope IV than against epitopes I and II/III. These CTLs also lysed the Tag-transformed cell line B6/WT-19, which expresses wild-type SV40 Tag, but failed to lyse B6/K-1,4,5 cells which contain a mutant form of Tag devoid of epitopes I, II/III, IV, and the immunorecessive epitope V (39, 49, 64). In contrast, immunization of SV11+ mice with rVV-941T failed to elicit CTLs against any of the three immunodominant Tag epitopes (Fig. 2A). Cells from these cultures also failed to lyse B6/WT-19 or B6/K-1,4,5 target cells, demonstrating that CTLs directed against cryptic Tag epitopes were not induced in SV11+ mice following immunization with full-length Tag. Identical results were obtained by immunization with syngeneic Tag-transformed cell lines (data not shown). To ensure that SV11+ mice were infected with rVV-941T, a portion of the spleen cells from the same mice were restimulated in vitro with B6 spleen cells which had been infected with VV-WR. Responder cells lysed VV-WR-infected B6/K-1,4,5 cells but not mock-infected cells (Fig. 2A, inset), demonstrating that the SV11+ mice were efficiently infected with rVV-941T and capable of mounting a strong CTL response against an unrelated antigen.
FIG. 2.
SV11+ mice were tolerant to Tag CTL epitopes. SV11+ (A to D), SV11− (E to H), and SV11+ mice reconstituted with 5 × 107 naive B6 spleen cells (I to L) were immunized i.v. with 107 PFU of rVV-941T (A, E, and I), rVV-ES I (B, F, and J), rVV-ES II/III (C, G, and K), or rVV-ES IV (D, H, and L). After 3 weeks, spleen cells were restimulated in vitro with gamma-irradiated B6/WT-19 cells as described in Materials and Methods. Responder cells were tested on day 6 for their ability to lyse 51Cr-labeled RMA cells pulsed with 1 μM concentrations of the indicated peptides or the Tag-transformed cell lines B6/WT-19 (full-length Tag) and B6/K-1,4,5 (CTL epitope loss Tag) in a 5-h assay. The control peptide for all panels was DbN5, except that HSV gB498-505 was used in panels D and H and HSV RR1 822-829 was used in panels I and L. Insets show response of spleen cells from the same mice against B6/K-1,4,5 target cells infected with VV-WR or mock infected following 6 days of in vitro restimulation with VV-WR-infected B6 spleen cells as described in Materials and Methods.
To more thoroughly investigate the extent of Tag epitope tolerance in SV11+ mice, SV11+ and SV11− littermates were immunized with rVVs encoding the immunodominant Tag epitopes I, II/III, or IV as minigenes preceded by the adenovirus E3/19K ES, designated rVV-ES I, rVV-ES II/III (20), and rVV-ES IV (5). This strategy bypasses the need for proteolytic processing of the epitope from full-length protein in the cytosol as well as transport into the endoplasmic reticulum through the transporter associated with antigen processing (3), has been shown to enhance the number of class I MHC-peptide complexes (2), and increased the immunogenicity of an immunorecessive Tag CTL epitope (20). Three weeks following immunization with these rVVs, spleen cells were restimulated in vitro with B6/WT-19 cells, and the CTL response was determined on day 6 after restimulation. As expected, SV11− littermates developed Tag epitope-specific CTLs in accordance with the rVV used for immunization (Fig. 2F through H). In contrast, SV11+ mice failed to develop Tag epitope-specific CTLs after immunization with these minigene constructs (Fig. 2B through D). All SV11+ mice immunized with rVVs developed strong vaccinia virus-specific CTL responses (Fig. 2B through D, inset). These results demonstrate that SV11+ mice are tolerant to the immunodominant Tag CTL epitopes but are capable of responding to unrelated CTL epitopes. Previous studies have shown that the expression of a specific antigen in the thymus can lead to clonal deletion of CTL precursors specific for that antigen (35, 36), resulting in central tolerance. We have demonstrated expression of Tag RNA transcripts in the thymus of SV11+ mice by reverse transcription-PCR (data not shown). Thus, tolerance to Tag CTL epitopes in SV11+ mice is consistent with a mechanism for deletion of reactive CTLs during T-cell development.
Reconstituted SV11+ mice develop Tag epitope IV-specific CTLs following immunization with full-length Tag or a minigene encoding epitope IV.
In order to provide SV11+ mice with a population of CTL precursors which could respond to the immunodominant Tag epitopes, they were given adoptive transfers with unprimed B6 spleen cells prior to immunization with rVVs expressing full-length Tag or Tag epitope minigenes. Adoptive transfers were given to the mice at 40 to 45 days of age, when choroid plexus tumors are still microscopic (Fig. 1C and D). After 2 days, these reconstituted SV11+ mice were immunized with rVV-941T. Three weeks later, mice were sacrificed and spleen cells were restimulated in vitro with B6/WT-19 cells or VV-WR-infected B6 spleen cells. SV11+ mice reconstituted with naive B6 spleen cells developed a strong epitope IV-specific CTL response following immunization with rVV-941T (Fig. 2I). In contrast, CTL responses directed against Tag epitopes I and II/III were not detected in these same mice. This may be due to the lower frequency of CTL precursors specific for Tag epitopes I and II/III than for epitope IV detected in B6 mice or the possibility that CTLs specific for epitopes I and II/III become tolerant after exposure to Tag in SV11+ mice (see Discussion). Importantly, CTLs from these reconstituted SV11+ mice immunized with rVV-941T also recognized epitope IV processed from full-length Tag by B6/WT-19 cells. All mice immunized with rVV-941T developed strong vaccinia virus-specific CTL responses (data not shown). SV11+ mice which received only adoptive transfers with normal B6 spleen cells did not develop Tag-specific CTLs (data not shown), indicating that the endogenous Tag was not involved in CTL priming.
We also determined if immunization with Tag epitope minigenes could induce CTLs in SV11+ mice following adoptive transfers with naive B6 spleen cells. Reconstituted SV11+ mice immunized with rVV-ES I and rVV-ES II/III failed to develop detectable levels of CTLs specific for these epitopes after restimulation in vitro with B6/WT-19 cells (Fig. 2J and K, respectively), as was observed following immunization of reconstituted SV11+ mice with full-length Tag (Fig. 2I). Mice immunized with these minigene constructs developed strong vaccinia virus-specific CTL responses (Fig. 2J and K, insets), demonstrating that CTLs directed against vaccinia virus epitopes were effectively induced. In contrast, reconstituted SV11+ mice immunized with rVV-ES IV developed a strong CTL response directed against epitope IV (Fig. 2L). The rVV-ES IV-induced CTLs also recognized epitope IV processed from endogenous Tag as shown by lysis of B6/WT-19 cells. These results demonstrate that CTLs specific for the immunodominant H-2Kb-restricted epitope IV were induced in SV11+ mice following adoptive transfer with unprimed B6 spleen cells and immunization with rVVs which encode either full-length Tag or epitope IV as a minigene.
Reconstituted SV11+ mice immunized with rVV-941T or rVV-ES IV have increased life spans.
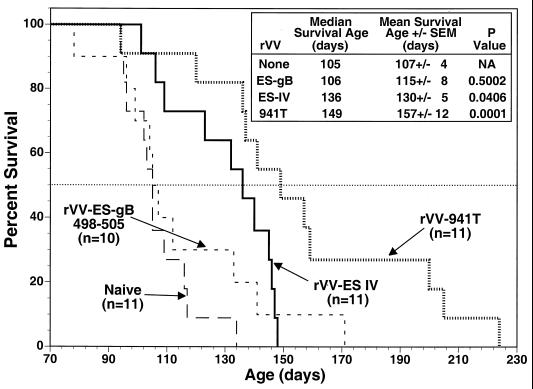
Preliminary experiments in our laboratories indicated that adoptive transfer of spleen cells from Tag-immunized B6 mice resulted in increased life spans for SV11+ mice. Thus, we determined whether induction of epitope IV-specific CTLs in SV11+ mice by active immunization correlated with an increased life span. Groups of SV11+ mice were reconstituted with B6 spleen cells at 45 days of age and immunized 2 days later with rVV-ES IV, rVV-941T, or rVV-ES-gB498-505, which encodes an unrelated H-2Kb-restricted CTL epitope from HSV (5). Resulting life spans were determined and compared with those of unmanipulated SV11+ mice. The mean survival age for unmanipulated SV11+ mice was 107 days, with a median survival age of 105 days (Fig. 3), which is in agreement with previous observations (67). Only one mouse from this group survived beyond 120 days of age. Reconstituted SV11+ mice immunized with rVV-ES-gB498-505 had a mean survival age of 115 days and a median survival age of 106 days, which is not significantly different from those for the unmanipulated SV11+ mice. This group included three mice which survived beyond 120 days, suggesting that a limited response against choroid plexus tumors might have occurred in these individuals. In contrast, reconstituted SV11+ mice immunized with rVV-ES IV had a mean survival age of 130 days and a median survival age of 136 days, which is significantly longer than those in the unmanipulated control group. Only three mice within this group died prior to 120 days of age, indicating an overall advantage in the control of tumor progression for reconstituted mice immunized with rVV-ES IV. Reconstituted SV11+ mice immunized with rVV-941T also demonstrated increased survival compared to control SV11+ mice, having a mean survival age of 149 days with a median survival age of 157 days. Only one mouse from this group died prior to 120 days of age. Three mice from the rVV-941T-immunized group greatly exceeded the life spans of other mice within the group, surviving for over 200 days. These results suggest that the induction of Tag epitope IV-specific CTLs in reconstituted SV11+ mice by active immunization could delay choroid plexus tumor progression.
FIG. 3.
The life span of reconstituted SV11+ mice is prolonged by immunization with rVV-ES IV or rVV-941T. SV11+ mice remained unmanipulated (long-dashed line) or were reconstituted at 45 days of age with 5 × 107 naive B6 spleen cells and immunized 2 days later with rVV-ES IV (solid line), rVV-941T (dotted line), or rVV-ES-gB498-505 (short-dashed line). Survival was monitored and plotted as percentage of surviving animals versus age. The horizontal dotted line indicates 50% survival. The inset shows survival times and P values as determined for mean survival ages compared to unimmunized animals, with a P value of <0.05 considered significant. The numbers of animals per group are indicated in the figure.
Reconstituted SV11+ mice immunized with rVV-941T or rVV-ES IV maintain epitope IV-specific memory CTLs and have reduced tumor progression at 100 days of age.
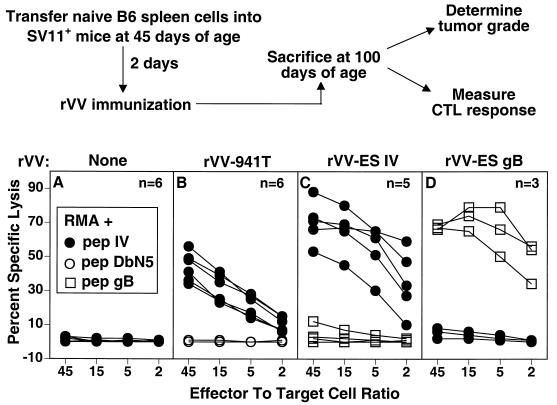
Since unmanipulated SV11+ mice are tolerant to epitope IV, we determined whether epitope IV-specific memory CTLs persisted after prolonged exposure to the endogenous Tag expressed by SV11+ mice. Groups of SV11+ mice at 45 days of age were reconstituted with unprimed B6 spleen cells. After 2 days, groups of reconstituted SV11+ and unmanipulated SV11+ and SV11− mice were immunized with rVV-941T, rVV-ES IV, or rVV-ES-gB498-505 (Fig. 4). Some mice remained unimmunized to determine the effect of reconstitution alone on tumor development. Mice from each group were sacrificed at 100 days of age, a time when the majority of unmanipulated SV11+ mice had large, end-stage tumors and became moribund.
FIG. 4.
Reconstituted SV11+ mice develop epitope IV-specific memory CTLs following active immunization. The scheme for determining the effect of epitope IV-specific CTL response on choroid plexus tumor development in SV11+ mice is shown in the upper portion of the figure. SV11+ mice were reconstituted with 5 × 107 naive B6 spleen cells i.v. at 45 days of age followed 2 days later by no immunization or immunization with rVV-941T, rVV-ES IV, or rVV-ES-gB498-505. At 100 days of age, mice were sacrificed and spleen cells were restimulated in vitro with gamma-irradiated B6/WT-19 (A to C) or B6/350gB (D) cells. After 6 days, responder cells were tested for lysis of RMA target cells which had been pulsed with a 1 μM concentration of the indicated peptide in a 5-h 51Cr release assay. Each curve within a given panel represents the response of an individual mouse to RMA cells pulsed with the indicated target or control peptide. The number of mice in each group is indicated.
Memory CTL responses were determined for individual mice following in vitro restimulation, and representative data are shown in Fig. 4. SV11+ mice reconstituted with B6 spleen cells, but which remained unimmunized, failed to develop Tag epitope IV-specific CTLs (Fig. 4A), demonstrating that the B6 donor spleen cells were not primed by the endogenous Tag expressed in SV11+ mice. In contrast, reconstituted SV11+ mice immunized either with rVV-941T or rVV-ES IV developed epitope IV-specific CTL memory responses (Fig. 4B and C). Spleen cells from reconstituted SV11+ mice immunized with rVV-ES-gB498-505 were restimulated in vitro with B6/350gB cells which express full-length SV40 Tag containing gB498-505 inserted at Tag position 350 (21) in order to expand gB498-505 specific CTLs. The data in Fig. 4D demonstrate that immunization with rVV-ES-gB498-505 induced CTLs specific for gB498-505 but not Tag epitope IV. Thus, the induction of CTLs against an unrelated epitope does not lead to priming of CTLs against endogenous Tag in reconstituted SV11+ mice. These results demonstrate that epitope IV-specific memory CTLs were readily detectable in 100-day-old SV11+ mice following adoptive transfer with naive B6 spleen cells and immunization at 45 days of age with either rVV-941T or rVV-ES IV.
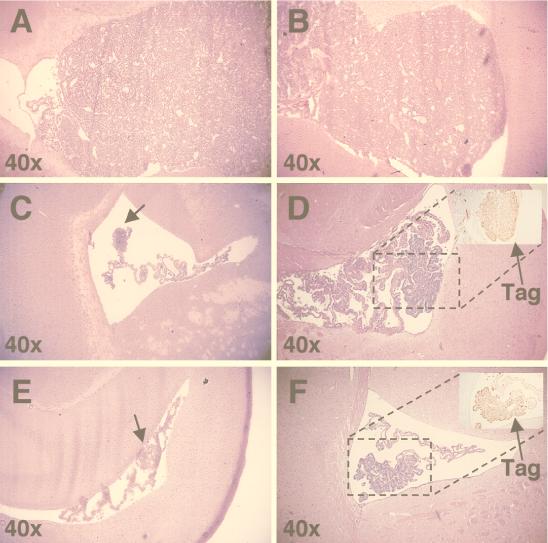
To determine if the increased life span of epitope IV immune SV11+ mice was due to control of choroid plexus tumor progression, brains from corresponding 100-day-old mice were fixed, sectioned, stained with H&E, and examined for the presence of choroid plexus tumors. Tumors were classified as described by Van Dyke et al. (67) and based on sections containing the highest grade of tumor found for each animal. SV11+ mice reconstituted with unprimed B6 spleen cells, but which remained unimmunized, developed grade IV tumors by 100 days of age (Fig. 5A and Table 1, group B), indicating that reconstitution alone had no effect on choroid plexus tumor progression. The majority, 73%, of SV11+ mice given adoptive transfers with naive B6 spleen cells and immunized with rVV-ES-gB498-505 also progressed to grade IV choroid plexus tumors by 100 days of age (Fig. 5B and Table 1, group C), which demonstrates that the induction of an unrelated CTL response did not effectively lead to the control of tumor development. In contrast, the majority of reconstituted SV11+ mice immunized with rVV-941T (Fig. 5C and D and Table 1, group E) or rVV-ES IV (Fig. 5E and F and Table 1, group G) had reduced tumor burdens in the choroid plexus. Only 31 and 35%, respectively, of these animals contained tumors which progressed to grade IV by 100 days of age. Tumors were found in all animals, however, indicating that control of tumor progression was incomplete. Tumors from epitope IV immune mice retained expression of Tag in the nucleus, indicating that tumor outgrowth was not due to development of Tag loss variants (Fig. 5D and F, insets). Immunization with rVV-941T appeared to enhance control of tumor progression compared to immunization with rVV-ES IV, as several mice were identified among rVV-941T-immunized mice which contained only the lowest grade of tumor, grade I, by 100 days of age (Table 1, group E). All of the SV11+ mice immunized with rVV-941T or rVV-ES IV without prior adoptive transfer with naive B6 spleen cells developed grade IV choroid plexus tumors by 100 days of age (Table 1, groups D and F, respectively). Thus, immunization of reconstituted SV11+ mice with rVV-ES IV or rVV-941T leads to a reduced choroid plexus tumor burden by 100 days of age. These results, along with those demonstrating an increased life span for SV11+ mice (Fig. 3), indicate that the induction of epitope IV-specific CTLs in SV11+ mice leads to the control of choroid plexus tumor progression.
FIG. 5.
Reconstituted SV11+ mice immunized with rVV-ES IV and rVV-941T have reduced choroid plexus tumor burdens by 100 days of age. Brains were harvested from SV11+ and SV11− mice at 100 days of age, sectioned, and stained with H&E as described in Materials and Methods. All sections are shown at ×40 magnification unless indicated otherwise. (A) Grade IV tumor from an SV11+ mouse which received naive B6 spleen cells at 45 days of age but remained unimmunized. (B) Grade IV tumor from an SV11+ mouse which received naive B6 spleen cells at 45 days of age followed by immunization with rVV-ES-gB498-505. (C and D) Grade I and II choroid plexus tumors, respectively, from two different SV11+ mice reconstituted with naive B6 spleen cells at 45 days of age and immunized with rVV-941T. (E and F) Grade II choroid plexus tumors from two representative SV11+ mice reconstituted with naive B6 spleen cells at 45 days of age and immunized with rVV-ES IV. Insets in panels D and F show immunohistochemical staining for Tag of parallel paraffin-embedded sections. The dashed boxes indicate the relative location of Tag staining with the corresponding H&E-stained sections. The magnification of the insets is ×100. Arrows indicate the location of small tumors.
Naive B6 spleen cells are primed by endogenous Tag in irradiated and reconstituted SV11+ mice.
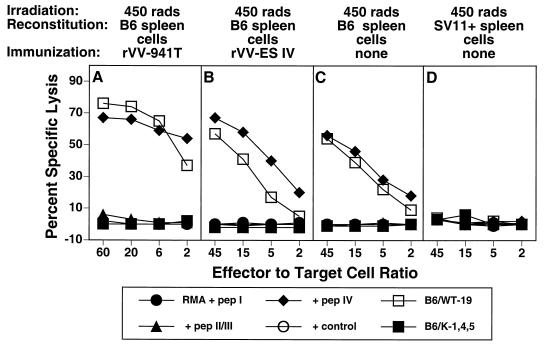
Irradiation of the tumor-bearing host has been shown to synergize with adoptive transfer in the induction of tumor immunity (8, 50, 58). Thus, we determined the effect of gamma irradiation of SV11+ mice on the induction of epitope IV-specific CTLs and tumor immunity. SV11+ mice received 450 rads of radiation 16 to 24 h prior to reconstitution with naive B6 spleen cells, followed by immunization with rVV-941T or rVV-ES IV or no immunization. CTL responses were determined following in vitro restimulation with B6/WT-19 cells. As demonstrated with nonirradiated SV11+ mice (Fig. 2I and L), irradiated SV11+ mice reconstituted with naive B6 spleen cells and immunized with either rVV-941T or rVV-ES IV developed epitope IV-specific CTLs (Fig. 6A and B). CTLs specific for epitopes I and II/III were not detected. Interestingly, irradiated mice which received only adoptive transfer also developed epitope IV-specific CTLs following in vitro restimulation, indicating that naive B6 spleen cells were primed by the endogenous Tag (Fig. 6C). B6 donor spleen cells were required for this priming since irradiated SV11+ mice reconstituted with SV11+ spleen cells failed to develop epitope IV-specific CTLs under the same conditions (Fig. 6D). Irradiation of B6 mice followed by reconstitution with unprimed B6 spleen cells failed to elicit epitope IV-specific CTLs (data not shown) and demonstrates that the endogenous Tag expressed by SV11+ mice is required to prime B6 donor spleen cells. Thus, in contrast to our findings using nonirradiated SV11+ mice (Fig. 4A), irradiation of SV11+ mice prior to adoptive transfer with naive B6 spleen cells allowed priming of epitope IV-specific CTLs of the donor against the endogenous Tag expressed by SV11+ mice.
FIG. 6.
Epitope-specific CTLs are primed by endogenous Tag in irradiated SV11+ mice. SV11+ mice were irradiated at 450 rads at 40 days of age and then reconstituted with 5 × 107 B6 (A to C) or SV11+ (D) spleen cells. Some mice remained unimmunized (C and D) or were immunized 2 days later with rVV-941T (A) or rVV-ES-IV (B) After 3 to 5 weeks, spleen cells were restimulated in vitro with B6/WT-19 cells and tested on day 6 in a 51Cr release assay against RMA target cells pulsed with 1 μM concentrations of the indicated peptides or the Tag-transformed cell lines B6/WT-19 (full-length Tag) and B6/K-1,4,5 (CTL epitope loss Tag). Data represent the response of individual mice from representative groups of three to five mice.
Reconstitution of irradiated SV11+ mice with naive B6 spleen cells leads to control of choroid plexus tumor progression.
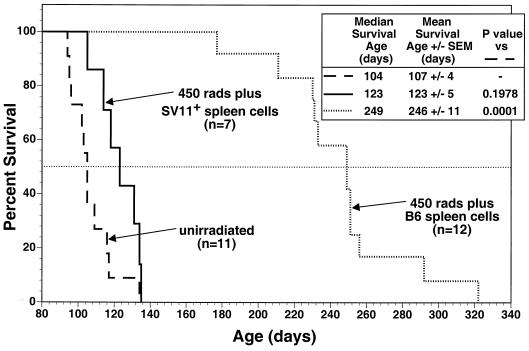
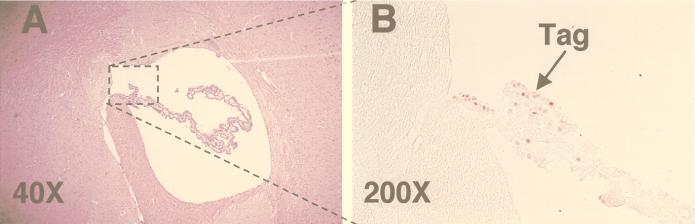
To determine if epitope IV-specific CTLs primed by endogenous Tag in irradiated SV11+ mice might also mediate control of choroid plexus tumor progression, life spans were determined for SV11+ mice which were irradiated and reconstituted with naive B6 spleen cells at 40 to 45 days of age. These mice had substantially increased life spans compared to unmanipulated SV11+ mice, with mean survival ages of 246 and 107 days, respectively (Fig. 7). In contast, SV11+ mice which were irradiated and reconstituted with SV11+ spleen cells failed to live significantly longer than unmanipulated SV11+ mice (Fig. 7 and inset). Histological analysis of brains from 100-day-old SV11+ mice which were irradiated and reconstituted with naive B6 spleen cells revealed that of six mice examined, one had a grade II tumor, three had grade I tumors, and two had no detectable tumors. Normal choroid plexus architecture was observed for the tumor-free SV11+ mice (Fig. 8A), and choroid plexus cells expressing nuclear Tag were identified (Fig. 8B) which appeared similar to those observed in 40-day-old unmanipulated SV11+ mice prior to tumor formation (Fig. 1D). Similar results were obtained with 150-day-old SV11+ mice which had received irradiation and reconstitution with B6 spleen cells at 45 days of age (data not shown). Thus, the induction of epitope IV-specific CTLs primed against endogenous Tag correlates with a highly significant reduction of choroid plexus tumor progression in irradiated SV11+ mice.
FIG. 7.
Irradiated SV11+ mice given adoptive transfers with naive B6 spleen cells have significantly increased life spans. SV11+ mice were irradiated at 450 rads at 40 days of age and reconstituted the following day with 5 × 107 naive B6 (dotted line) or SV11+ (solid line) spleen cells. Some SV11+ mice remained unmanipulated (dashed line). Survival was monitored and plotted as percentage of surviving animals versus age. The inset shows survival times and statistics for each group. P values were determined for mean survival ages compared to unmanipulated SV11+ mice, with a value of <0.01 considered significant. The number of animals per group are indicated in the figure. The dashed horizontal line indicates 50% survival.
FIG. 8.
Irradiated SV11+ mice given adoptive transfers with naive B6 spleen cells have reduced tumor burdens by 100 days of age. SV11+ mice were irradiated at 450 rads at 40 days of age and reconstituted the following day with 5 × 107 naive B6 spleen cells. Brains were harvested at 100 days of age, fixed, paraffin embedded, sectioned, and stained with H&E (A) or by immunohistochemistry for Tag (B). The arrow indicates the location of Tag-positive cells (brown), and the box in panel A indicates the relative location of the tissue stained in panel B. Magnifications are indicated.
Choroid plexus tumor outgrowth is not due to loss of Tag epitope IV or MHC class I expression.
Although we have shown that induction of Tag epitope IV-specific CTLs in SV11+ mice correlates with the control of choroid plexus tumor progression, even mice with prolonged life spans eventually die from choroid plexus tumors (Fig. 3 and 7). Previous investigations have indicated that tumor cells can escape from CTL-mediated lysis by downregulation of class I MHC molecules (25) or by mutation of the relevant CTL epitopes (39, 42, 74). To determine if such a mechanism might explain the outgrowth of choroid plexus tumors in epitope IV-immune SV11+ mice, cell lines were derived from the choroid plexus tumors of end-stage mice and characterized for their susceptibility to lysis by CTL clones specific for Tag epitopes I, II/III, and IV. Cell lines were derived from SV11+ mice which lived beyond the expected life span of 105 days for unmanipulated mice (survival age range of 123 to 322 days). While some variation was observed, all tumor cell lines were lysed efficiently by CTL clones specific for each Tag epitope, including the epitope IV-specific CTL clone Y-4 (Table 2). In addition, all cell lines tested expressed comparable levels of surface H-2Kb and H-2Db molecules as well as nuclear Tag (data not shown). These results indicate that the outgrowth of choroid plexus tumors in epitope IV-immune SV11+ mice is not due to the loss of the immunodominant Tag epitope IV or downregulation of class I MHC surface expression.
TABLE 2.
Lysis of choroid plexus tumor-derived cell lines by Tag-specific CTL clones
| Cell linea | Mouse treatment | % Lysis of target cells by CTL cloneb: | ||||
|---|---|---|---|---|---|---|
| 450 radsc | Source of spleen cells | Immunization | K-11 | K-19 | Y-4 | |
| B6/WT-19−d | 69 | 76 | 73 | |||
| B6/K-1,4,5−e | 0 | 19 | 2 | |||
| SV11-440A | − | B6 | rVV-ESIV | 35 | 66 | 52 |
| SV11-449 | − | B6 | rVV-ESIV | 42 | 67 | 61 |
| SV11-640 | − | B6 | rVV-941T | 68 | 71 | 67 |
| SV11-656 | − | B6 | rVV-941T | 43 | 56 | 39 |
| SV11-553 | + | B6 | None | 70 | 86 | 65 |
| SV11-557 | + | B6 | None | 54 | 69 | 25 |
| SV11-942 | + | SV11+ | None | 55 | 71 | 41 |
| SV11-982 | + | SV11+ | None | 62 | 77 | 58 |
DISCUSSION
An important goal in tumor immunology is the induction of effective tumor-specific immunity in the host which develops spontaneous tumors. While multiple studies have shown that the induction of epitope-specific CTL can lead to protection against tumor challenge or the eradication of preestablished tumor transplants in mice (4, 13, 17, 18, 45, 46), only a limited number of studies have examined whether the control of naturally arising tumors can be mediated by tumor antigen-specific CTLs (57, 61,76). The capacity of the host to respond immunologically in this situation must be considered, since the expression of the tumor antigen as a self antigen may lead to the induction of tolerance or anergy (35, 36, 56, 72). The use of transgenic mice which spontaneously develop tumors should provide insight into the role of tumor-specific CTLs in a tolerance-inducing environment. Mice which express the SV40 Tag as a transgene are particularly well suited for these studies, since the same protein which induced tumors in a particular tissue also serves as the target for an antitumor immune response.
We determined the ability of active immunization against the Tag H-2b-restricted immunodominant CTL epitopes to control the progression of spontaneous choroid plexus tumors in SV11+ mice. We demonstrate that SV11+ mice are functionally tolerant to the SV40 Tag epitopes I, II/III, and IV. This finding is consistent with previous investigations using Tag transgenic mice in which the failure to detect Tag-specific CTL precursors also was associated with thymic expression of the transgene (16, 26, 34), possibly due to clonal deletion of Tag-reactive CTL precursors. Since Tag is also expressed in the periphery of SV11+ mice, an extrathymic mechanism of tolerance induction cannot be excluded (56, 72). The major finding of this study is that the induction of CTLs specific for the immunodominant epitope IV in SV11+ mice correlates with control of choroid plexus tumor progression. This was accomplished by two approaches. In the first approach SV11+ mice were given adoptive transfers with unprimed B6 spleen cells and then immunized with rVVs expressing either full-length Tag or epitope IV as a minigene. In the second approach, exposure of SV11+ mice to gamma radiation followed by adoptive transfer with unprimed B6 spleen cells led to the induction of epitope IV-specific CTLs primed against endogenous Tag as well as increased tumor immunity. Thus, epitope IV-specific CTLs may play an important role in the control of choroid plexus tumor progression. Eventual tumor outgrowth did not involve loss of epitope IV-specific memory CTLs or mutation of the relevant CTL epitope by the tumor.
The role of H-2b-restricted, Tag-specific CTLs in the control of endogenous tumor progression was previously addressed with RIP1-Tag4 transgenic mice (76) that develop insulinomas. In contrast to the tolerance observed in SV11+ mice, RIP1-Tag4 mice developed Tag-specific CTLs when immunized with SV40. Immunization resulted in significantly prolonged life spans for RIP1-Tag4 mice if given prior to the onset of Tag expression at 4 to 5 weeks of age. All immunized RIP1-Tag4 mice eventually succumbed to the tumor burden, similar to our findings. In contrast to our results, however, Ye et al. (76) found that tumors derived from immunized mice had greatly reduced levels of class I MHC surface expression,which might have contributed to tumor escape from CTL surveillance. The role of individual H-2b-restricted Tag epitopes in control of tumor progression was not addressed in this study. Speiser et al. (61) used a related transgenic mouse line RIP(GP × Tag2), which expresses SV40 Tag and the glycoprotein (GP) of lymphocytic choriomeningitis virus (LCMV) under the control of the rat insulin promoter. These mice express Tag and glycoprotein in the β cells of the pancreas an develop insulinomas. While the RIP(GP × Tag2) transgenic mice are tolerant to SV40 Tag CTL epitopes (34), they do not develop immunological tolerance to glycoprotein CTL epitopes. The authors (61) demonstrate that the induction of glycoprotein specific CTL by immunization with LCMV leads to a reduced tumor burden but that this effect is short-lived and the tumors continue to progress after a delay. Tumor outgrowth was not due to loss of glycoprotein-specific memory CTLs, and our results support their conclusion that tumor outgrowth is not due to CTL exhaustion. The role of H-2Db-restricted Tag epitope II/III-specific CTLs in the control of Tag-induced liver tumors was recently addressed in Tag transgenic mice which display partial tolerance to the Tag H-2b epitopes (57). These authors found that adoptive transfer with a CTL line induced by peptide immunization and specific for Tag epitope II/III led to a slight reduction in liver mass. We failed, however, to detect Tag epitope II/III-specific CTLs in SV11+ mice reconstituted with naive B6 spleen cells and immunized with rVVs expressing full-length Tag or epitope II/III as a minigene.
We observed that while adoptive transfer of naive B6 spleen cells to unmanipulated SV11+ mice had no effect on tumor development, irradiation of SV11+ mice prior to adoptive transfer with naive B6 spleen cells resulted in priming of epitope IV-specific CTLs by endogenous Tag and efficient control of tumor progression. Thus, endogenous Tag failed to prime Tag-specific CTLs in nonirradiated SV11+ mice, and the B6 donor spleen cells remained immunologically ignorant of endogenous Tag unless activated by specific immunization. Similar observations regarding immunologic ignorance were made previously with transgenic mice which express LCMV glycoprotein or nucleoprotein in the pancreatic β cells (51, 52, 68). These mice are not tolerant to glycoprotein or nucleoprotein CTL epitopes but remain immunologically ignorant of antigen expressed in the periphery unless activated by immunization with LCMV. Further experiments with LCMV transgenic mice (31, 60) and mice which express a nontransforming Tag fragment as a transgene in the pancreatic β cells (60) have indicated that immunological ignorance can be overcome if costimulatory molecules are coexpressed on the same tissue as the antigen and a high frequency of CTL precursors are present. Tag fragment transgenic mice, however, only developed autoimmunity following adoptive transfer of activated Tag-specific CTLs. Additional experiments also indicate an important role for local cytokine production in overcoming immunological ignorance to peripheral antigens (28, 29, 33, 70), most likely by recruitment of professional antigen-presenting cells as well as other immune effectors to the target tissue. Together, these findings suggest that the induction of an inflammatory response is important for the activation of resting CTLs against antigen expressed by peripheral tissues. Based on these results, we suggest that donor B6 spleen cells remain immunologically ignorant of endogenous Tag after adoptive transfer into SV11+ mice unless an immune response is initiated by specific immunization or host irradiation prior to adoptive transfer.
Previous investigations have shown that irradiation of the tumor-bearing host synergizes with the adoptive transfer of tumor-activated lymphocytes to produce an effective response against established tumors (8, 24, 50, 58). Various mechanisms have been proposed to explain this synergistic effect. (i) An increased number of lymphocytes may seed into irradiated compared to nonirradiated recipients due to destruction of host lymphocytes, which creates “space” in lymphoid organs. Using our adoptive transfer protocol, preliminary experiments indicate that an increased number of donor spleen cells from Thy1.1+ mice can seed into the spleens of irradiated versus unirradiated Thy1.2+ mice. (ii) Radiation-sensitive immunoregulation, which normally maintains tolerance to self tumor antigens, may be lost. (iii) Radiation may exert direct effects on the tumor that results in killing or increased susceptibility to the immune response. A recent study by Ganss and Hanahan (24) indicates that irradiation induces changes in the tumor microenvironment, resulting in increased tumor infiltration by lymphocytes. (iv) A combination of these factors might contribute to the increased immunity to tumors. The increased life span of irradiated SV11+ mice reconstituted with naive B6 spleen cells was not due to radiation-induced regression of tumors alone, since reconstitution of irradiated SV11+ mice with SV11+ spleen cells did not significantly control tumor progression or extend their life span. At this point, we cannot determine which of these mechanisms might lead to enhanced tumor immunity in this Tag transgenic system, although the priming of naive B6 spleen cells against Tag epitope IV in vivo should involve processing of endogenous Tag by professional antigen-presenting cells. Thus, it is tempting to speculate that irradiation of the host leads to enhanced antigen processing and presentation of Tag and may serve as a danger signal to initiate an immune response against Tag (44).
Irradiated SV11+ mice reconstituted with B6 spleen cells also had increased life spans (median survival age of 249 days [Fig. 7]) compared to nonirradiated recipients which were immunized with rVV-941T or rVV-ES IV (median survival ages of 136 and 149 days, respectively [Fig. 3]). This result indicates that the induction of epitope IV-specific CTL against endogenous Tag in SV11+ mice leads to more-effective control of tumor growth than immunization with rVVs expressing epitopes IV. Preliminary experiments indicate this might be due to seeding increased numbers of lymphocytes into irradiated recipients. The recent advent of MHC class I-peptide tetramer technology (1, 47) should allow accurate estimates to be made of the number of Tag epitope-specific CTLs induced following adoptive transfer of naive B6 spleen cells into irradiated and nonirradiated SV11+ mice. Subsequent immunization of irradiated SV11+ mice with rVV-941T or rVV-ES IV shortly after reconstitution with B6 spleen cells did not further prolong the life span of these mice (data not shown), suggesting that a threshold of antitumor activity was reached by irradiation and adoptive transfer alone. We have yet to determine if boosting the epitope IV response in immune SV11+ mice at time points distant from the initial effector phase might lead to enhanced tumor immunity.
While epitope IV-specific CTLs were successfully reconstituted in SV11+ mice following immunization with rVV-941T (Fig. 1I), we failed to detect CTLs specific for epitopes I or II/III in these mice, even if recipients were irradiated prior to reconstitution and immunization. In addition epitope I- and II/III-specific CTLs were not detected following immunization of reconstituted SV11+ mice with rVV-ES I or rVV-ES II/III. A possible explanation for these results is that too few CTL precursors specific for these epitopes graft into the recipient mouse to mount a detectable response following immunization. Previous studies have shown that the frequency of B6 responder CTLs directed against epitope IV is 5- to 10-fold higher than the frequency of responder CTLs directed against epitopes I and II/III following immunization with Tag-transformed cells (48). Alternatively, epitope I- and II/III-specific CTL precursors may be rendered tolerant after transfer into SV11+ mice by mechanisms which induce CTL anergy or lead to clonal deletion.
Since all of the SV11+ mice eventually died from choroid plexus tumor burden despite control of progression early in life, it is likely that tumors escape immune control after an initial effector phase. Tumors may escape activated CTLs through a variety of mechanisms, including downregulation of class I MHC surface molecules (25, 27, 54) or antigen processing machinery (55), the loss of CTL epitopes (39, 42, 74), and resistance to tumor necrosis factor- or Fas-mediated killing (12). The ability of tumor cells to neutralize responding CTLs via Fas-mediated killing (71) or secretion of immunosuppressive cytokines such as transforming growth factor β or interleukin 10 (12) may also lead to tumor escape. Knowles and colleagues (75, 76) have shown previously that insulinomas derived from RIP1-Tag4 SV40 Tag transgenic mice, as well as in vitro established cell lines from these tumors, have greatly reduced MHC class I cell surface expression which might contribute to tumor escape from SV40 Tag-specific CTLs. In addition, previous studies have indicated that Tag in transformed cell lines undergoes spontaneous mutations which can result in the expansion of CTL epitope loss variants following coculture with Tag epitope specific-CTL clones (39, 64). Immunohistochemical analysis of tumors from epitope IV immune SV11+ mice revealed that these tumors retain expression of nuclear Tag. In addition, our analysis of tumor cell lines derived from the choroid plexus tumors of epitope IV-immune SV11+ mice indicates that tumor escape is not due to the downregulation of class I MHC molecule surface expression or a mutation in the coding region of the relevant CTL epitopes, since all choroid plexus tumor-derived cell lines analyzed expressed comparable levels of cell surface class I MHC molecules and were efficiently lysed by Tag-specific CTL clones.
One alternative explanation for eventual choroid plexus tumor outgrowth in epitope IV-immune SV11+ mice is that Tag-specific CTLs return to a resting state after the initial immune response has subsided. This might be explained by extended exposure to Tag epitopes presented by the tumor cells in the absence of costimulation or downregulation due to engagement of CTLA-4 molecules (11, 22, 37). Blockade of CTLA-4 molecules by injection of monoclonal antibody specific for CTLA-4 has been shown to enhance tumor immunity, possibly through prevention of T-cell inhibitory signals (38). This seems a likely explanation, since tumor-bearing SV11+ mice still had strong epitope IV-specific memory CTL responses at 100 days of age, indicating that epitope IV-specific CTLs were not rendered tolerant. If such a mechanism is in operation, additional immunization may be beneficial in order to reactivate memory CTLs against the target epitope as was suggested by Speiser and coworkers (61). Finally, tumors may simply outgrow the effective immune response due to the inability of the immune response to penetrate the surrounding stroma of the growing tumor (73). Since multiple tumor foci can arise within the choroid plexus of SV11+ mice and tumors of different grades can be observed simultaneously (67), new tumors may arise in epitope IV immune mice after the initial immune response has been downregulated. Thus, these new tumors might not activate the memory CTL to respond in the absence of an inflammatory response (22).
Our results support the idea that the induction of CTLs against an immunodominant tumor-derived epitope can lead to the control of spontaneous tumors. Thus, the tumorigenic events and immunological consequences that occur in SV11+ transgenic mice provide an excellent model to investigate the induction of effective immunity against spontaneously arising tumors.
ACKNOWLEDGMENTS
We thank Melanie Epler and Andrew Gaydos for excellent technical assistance and Sebastian Joyce for critically reading the manuscript.
This work was supported by research grant CA 25000 from the National Cancer Institute, National Institutes of Health. Todd Schell is supported by the Concern Foundation for Cancer Research/Cancer Research Institute Fellowship.
REFERENCES
- 1.Altman J D, Moss P A H, Goulder P J R, Barouch D H, McHeyzer-Williams M G, Bell J I, McMichael A J, Davis M M. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 2.Antón L C, Yewdell J W, Bennink J R. MCH class I-associated peptides produced from endogenous gene products with vastly different efficiencies. J Immunol. 1997;158:2535–2542. [PubMed] [Google Scholar]
- 3.Bacik I, Cox J H, Anderson R, Yewdell J W, Bennink J R. TAP (transporter associated with antigen processing)-independent presentation of endogenously synthesized peptides is enhanced by endoplasmic reticulum insertion sequences located at the amino- but not carboxyl-terminus of the peptide. J Immunol. 1994;152:381–387. [PubMed] [Google Scholar]
- 4.Blachere N E, Li Z, Chandawarkar R Y, Suto R, Jaikarai N S, Basu S, Udono H, Srivastava P K. Heat shock protein-peptide complexes, reconstituted in vitro, elicit peptide-specific cytotoxic T lymphocyte response and tumor immunity. J Exp Med. 1997;186:1315–1322. doi: 10.1084/jem.186.8.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blaney J E, Jr, Nobusawa E, Brehm M A, Bonneau R H, Mylin L M, Fu T-M, Kawaoka Y, Tevethia S S. Immunization with a single major histocompatibility complex class I-restricted cytotoxic T-lymphocyte recognition epitope of herpes simplex virus type 2 confers protective immunity. J Virol. 1998;72:9567–9574. doi: 10.1128/jvi.72.12.9567-9574.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonneau R H, Salvucci L H, Johnson D C, Tevethia S S. Epitope specificity of H-2Kb-restricted, HSV-1-, and HSV-2-cross-reactive cytotoxic T lymphocyte clones. Virology. 1993;195:62–70. doi: 10.1006/viro.1993.1346. [DOI] [PubMed] [Google Scholar]
- 7.Brinster R L, Chen H Y, Messing A, van Dyek T, Levine A J, Palmiter R D. Transgenic mice harboring SV40 T-antigen genes develop characteristic brain tumors. Cell. 1984;37:367–379. doi: 10.1016/0092-8674(84)90367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cameron R B, Spiess P J, Rosenberg S A. Synergistic antitumor activity of tumor-infiltrating lymphocytes, interleukin-2, and local tumor irradiation. Studies on the mechanism of action. J Exp Med. 1990;171:249–263. doi: 10.1084/jem.171.1.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell A E, Foley F L, Tevethia S S. Demonstration of multiple antigenic sites of the SV40 translation rejection antigen by using cytotoxic lymphocyte clones. J Immunol. 1983;130:490–492. [PubMed] [Google Scholar]
- 10.Cavender J F, Conn A, Epler M, Lacko H, Tevethia M J. Simian virus 40 large T antigen contains two independent activities that cooperate with a ras oncogene to transform rat embryo fibroblasts. J Virol. 1995;69:923–934. doi: 10.1128/jvi.69.2.923-934.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen L. Immunological ignorance of silent antigens as an explanation of tumor evasion. Immunol Today. 1998;19:27–30. doi: 10.1016/s0167-5699(97)01180-8. [DOI] [PubMed] [Google Scholar]
- 12.Chouaib S, Asselin-Paturel C, Mami-Chouaib F, Caignard A, Blay J Y. The host-tumor immune conflict: from immunosuppression to resistance and destruction. Immunol Today. 1997;18:493–497. doi: 10.1016/s0167-5699(97)01115-8. [DOI] [PubMed] [Google Scholar]
- 13.Ciernik I F, Berzofsky J A, Carbone D P. Induction of cytotoxic T lymphocytes and antitumor immunity with DNA vaccines expressing single T cell epitopes. J Immunol. 1996;156:2369–2375. [PubMed] [Google Scholar]
- 14.Colby W W, Shenk T. Fragments of the simian virus 40 transforming gene facilitate transformation of rat embryo cells. Proc Natl Acad Sci USA. 1982;79:5189–5193. doi: 10.1073/pnas.79.17.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deckhut A M, Lippolis J D, Tevethia S S. Comparative analysis of core amino acid residues of H-2Db-restricted cytotoxic T-lymphocyte recognition epitopes in simian virus 40 T antigen. J Virol. 1992;66:440–447. doi: 10.1128/jvi.66.1.440-447.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faas S J, Pan S, Pinkert C A, Brinster R L, Knowles B B. Simian virus 40 (SV40)-transgenic mice that develop tumors are specifically tolerant to SV40 T antigen. J Exp Med. 1987;165:417–427. doi: 10.1084/jem.165.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feltkamp M C W, Smits H L, Vierboom M P M, Minnaar R P, de Jongh B M, Drijfhout J W, ter Schegget J, Melief C J M, Kast W M. Vaccination with cytotoxic T lymphocyte epitope-containing peptide protects against a tumour induced by human papillomavirus type 16-transformed cells. Eur J Immunol. 1993;23:2242–2249. doi: 10.1002/eji.1830230929. [DOI] [PubMed] [Google Scholar]
- 18.Feltkamp M C W, Vreugdenhil GR, Vierboom M P M, Ras E, van der Burg S H, ter Schegget J, Melief C J M, Kast W M. Cytotoxic T lymphocytes raised against a subdominant epitope offered as a synthetic peptide eradicate human papillomavirus type 16-induced tumors. Eur J Immunol. 1995;25:2638–2642. doi: 10.1002/eji.1830250935. [DOI] [PubMed] [Google Scholar]
- 19.Fox N, Crooke R, Hwang L-HS, Schibler U, Knowles B B, Solter D. Metastatic hibernomas in transgenic mice expressing an α-amylase-SV40 T antigen hybrid gene. Science. 1989;244:460–463. doi: 10.1126/science.2785714. [DOI] [PubMed] [Google Scholar]
- 20.Fu T-M, Mylin L M, Schell T D, Bacik I, Russ G, Yewdell J W, Bennink J R, Tevethia S S. An endoplasmic reticulum-targeting signal sequence enhances the immunogenicity of an immunorecessive simian virus 40 large T antigen cytotoxic T-lymphocyte epitope. J Virol. 1998;72:1469–1481. doi: 10.1128/jvi.72.2.1469-1481.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fu T-M, Bonneau R H, Tevethia M J, Tevethia S S. Simian virus 40 T antigen as a carrier for the expression of cytotoxic T-lymphocyte recognition epitopes. J Virol. 1993;67:6866–6871. doi: 10.1128/jvi.67.11.6866-6871.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fuchs E J, Matzinger P. Is cancer dangerous to the immune system? Semin Immunol. 1996;8:271–280. doi: 10.1006/smim.1996.0035. [DOI] [PubMed] [Google Scholar]
- 23.Gairin J E, Oldstone M B A. Design of high-affinity major histocompatibility complex-specific antagonist peptides that inhibit cytotoxic T-lymphocyte activity: implications for control of viral disease. J Virol. 1992;66:6755–6762. doi: 10.1128/jvi.66.11.6755-6762.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ganss R, Hanahan D. Tumor microenvironment can restrict the effectiveness of activated antitumor lymphocytes. Cancer Res. 1998;58:4673–4681. [PubMed] [Google Scholar]
- 25.Garrido F, Ruiz-Cabello F, Cabrera T, Pérez-Villar J J, López-Botet M, Duggan-Keen M, Stern P L. Implications for immunosurveillance of altered HLA class I phenotypes in human tumours. Immunol Today. 1997;18:89–95. doi: 10.1016/s0167-5699(96)10075-x. [DOI] [PubMed] [Google Scholar]
- 26.Geiger T, Soldevila G, Flavell R A. T cells are responsive to the simian virus 40 large tumor antigen transgenically expressed in pancreatis islets. J Immunol. 1993;151:7030–7036. [PubMed] [Google Scholar]
- 27.Gooding L R. Characterization of a progressive tumor from C3H fibroblasts transformed in vitro with SV40 virus. Immunoresistance in vivo correlates with phenotypic loss of H-2Kk. J Immunol. 1982;129:1306–1312. [PubMed] [Google Scholar]
- 28.Guerder S, Eynon E E, Flavell R A. Autoimmunity without diabetes in transgenic mice expressing β cell-specific CD86, but not CD80: parameters that trigger progression to diabetes. J Immunol. 1998;161:2128–2140. [PubMed] [Google Scholar]
- 29.Guerder S, Picarella D E, Linsley P S, Flavell R A. Costimulator B7-1 confers antigen-presenting-cell function to parenchymal tissue and in conjunction with tumor necrosis factor α leads to autoimmunity in transgenic mice. Proc Natl Acad Sci USA. 1994;91:5138–5142. doi: 10.1073/pnas.91.11.5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hanahan D. Heritable formation of pancreatic β-cell tumors in transgenic mice expressing recombinant insulins/simian virus 40 oncogenes. Nature. 1985;315:115–122. doi: 10.1038/315115a0. [DOI] [PubMed] [Google Scholar]
- 31.Harlan D M, Hengartner H, Huang M L, Kang Y H, Abe R, Moreadith R W, Pircher H, Gray G S, Ohashi P S, Freeman G J, et al. Mice expressing both B7-1 and viral glycoprotein on pancreatic beta cells along with glycoprotein-specific transgenic T cells develop diabetes due to a breakdown of T-lymphocyte unresponsiveness. Proc Natl Acad Sci USA. 1994;91:3137–3141. doi: 10.1073/pnas.91.8.3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harlow E, Crawford L V, Pim D C, Williamson N M. Monoclonal antibodies specific for simian virus 40 tumor antigens. J Virol. 1981;39:861–869. doi: 10.1128/jvi.39.3.861-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heath W R, Allison J, Hoffmann M W, Schönrich G, Hämmerling G, Arnold B, Miller J F A P. Autoimmune diabetes as a consequence of locally produced interleukin-2. Nature. 1992;359:547–549. doi: 10.1038/359547a0. [DOI] [PubMed] [Google Scholar]
- 34.Jolicoeur C, Hanahan D, Smith K M. T cell tolerance towards a transgenic β-cell antigen and transcription of endogenous pancreatic genes in the thymus. Proc Natl Acad Sci USA. 1994;91:6707–6711. doi: 10.1073/pnas.91.14.6707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kappler J W, Roehm N, Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987;49:273–280. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- 36.Kisielow P, Blüthmann H, Staerz U D, Steinmetz M, von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 1988;333:742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- 37.Krummel M F, Allison J P. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leach D R, Krummel M F, Allison J P. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 39.Lill N L, Tevethia M J, Hendrickson W G, Tevethia S S. Cytotoxic T lymphocytes (CTL) against a transforming gene product slect for transformed cells with point mutations within sequences encoding CTL recognition epitopes. J Exp Med. 1992;176:449–457. doi: 10.1084/jem.176.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lippolis J D, Mylin L M, Simmons D T, Tevethia S S. Functional analysis of amino acid residues encompassing and surrounding two neighboring H-2Db-restricted cytotoxic T lymphocyte epitopes in simian virus 40 tumor antigen. J Virol. 1995;69:3134–3146. doi: 10.1128/jvi.69.5.3134-3146.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ljunggren H-G, Kärre K. Host resistance directed selectively against H-2-deficient lymphoma variants. J Exp Med. 1985;162:1745–1759. doi: 10.1084/jem.162.6.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lurquin C, Van Pel A, Mariamé B, De Plaen E, Szikora J-P, Janssens C, Reddehase M J, Lejeune J, Boon T. Structure of the gene of tum− transplantation antigen P91A: the mutated exon encodes a peptide recognized with Ld by cytolytic T cells. Cell. 1989;58:293–303. doi: 10.1016/0092-8674(89)90844-1. [DOI] [PubMed] [Google Scholar]
- 43.Manfredi J J, Prives C. The transforming activity of simian virus 40 large tumor antigen. Biochim Biophys Acta. 1994;1198:65–83. doi: 10.1016/0304-419x(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 44.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 45.Mayordomo J I, Zorina T, Storkus W J, Zitvogel L, Celluzzi C, Falo L D, Melief C J, Ildstad S T, Kast W M, DeLeo A B, Lotze M T. Bone marrow-derived dendritic cells pulsed with synthetic peptides elicit protective and therapeutic antitumor immunity. Nat Med. 1995;1:1297–1302. doi: 10.1038/nm1295-1297. [DOI] [PubMed] [Google Scholar]
- 46.Minev B R, McFarland B J, Spiess P J, Rosenberg S A, Restifo N P. Insertion signal sequence fused to minimal peptides elicits specific CD8+ T cell responses and prolongs survival of thymoma-bearing mice. Cancer Res. 1994;54:4155–4161. [PMC free article] [PubMed] [Google Scholar]
- 47.Murali-Krishna K, Altman J D, Suresh M, Sourdive D J, Zajac A J, Miller J D, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 48.Mylin L M, Bonneau R H, Lippolis J D, Tevethia S S. Hierarchy among multiple H-2b-restricted cytotoxic T-lymphocyte epitopes within simian virus 40 T antigen. J Virol. 1995;69:6665–6677. doi: 10.1128/jvi.69.11.6665-6677.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mylin L M, Deckhut A M, Bonneau R H, Kierstead T D, Tevethia M J, Simmons D T, Tevethia S S. Cytotoxic T lymphocyte escape variants, induced mutations, and synthetic peptides define a dominant H-2Kb-restricted determinant in simian virus 40 tumor antigen. Virology. 1995;208:159–172. doi: 10.1006/viro.1995.1139. [DOI] [PubMed] [Google Scholar]
- 50.North R J. Gamma-irradiation facilitates the expression of adoptive immunity against established tumors by eliminating suppressor T cells. Cancer Immunol Immunother. 1984;16:175–181. doi: 10.1007/BF00205425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ohashi P S, Oehen S, Buerki K, Pircher H, Ohashi C T, Odermatt B, Malissen B, Zinkernagel R M, Hengartner H. Ablation of “tolerance” and induction of diabetes by virus infection in viral antigen transgenic mice. Cell. 1991;65:305–317. doi: 10.1016/0092-8674(91)90164-t. [DOI] [PubMed] [Google Scholar]
- 52.Oldstone M B A, Nerenberg M, Southern P, Price J, Lewicki H. Virus infection triggers insulin-dependent diabetes mellitus in a transgenic model: role of anti-self (virus) immune response. Cell. 1991;65:319–331. doi: 10.1016/0092-8674(91)90165-u. [DOI] [PubMed] [Google Scholar]
- 53.Palmiter R D, Chen H Y, Messing A, Brinster R L. SV40 enhancer and large-T antigen are instrumental in development of choroid plexus tumors in transgenic mice. Nature. 1985;316:457–460. doi: 10.1038/316457a0. [DOI] [PubMed] [Google Scholar]
- 54.Pan S, Abramczuk J, Knowles B B. Immune control of SV40-induced tumors in mice. Int J Cancer. 1987;39:722–728. doi: 10.1002/ijc.2910390612. [DOI] [PubMed] [Google Scholar]
- 55.Restifo N P, Esquivel F, Asher A L, Stotter H, Barth R J, Bennink J R, Mulé J J, Yewdell J W, Rosenberg S A. Defective presentation of endogenous antigens by a murine sarcoma. Implications for the failure of an anti-tumor immune response. J Immunol. 1991;147:1453–1459. [PMC free article] [PubMed] [Google Scholar]
- 56.Rocha B, von Boehmer H. Peripheral selection of the T cell repertoire. Science. 1991;251:1225–1228. doi: 10.1126/science.1900951. [DOI] [PubMed] [Google Scholar]
- 57.Romieu R, Baratin M, Kayibanda M, Lacabanne V, Ziol M, Guillet J-G, Viguier M. Passive but not active CD8+ T cell-based immunotherapy interferes with liver tumor progression in a transgenic mouse model. J Immunol. 1998;161:5133–5137. [PubMed] [Google Scholar]
- 58.Rosenberg S A, Spiess P, Lafreniere R. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science. 1986;233:1318–1321. doi: 10.1126/science.3489291. [DOI] [PubMed] [Google Scholar]
- 59.Salvucci L A, Bonneau R H, Tevethia S S. Polymorphism within the herpes simplex virus (HSV) ribonucleotide reductase large subunit (ICP6) confers type specificity for recognition by HSV type 1-specific cytotoxic T lymphocytes. J Virol. 1995;69:1122–1131. doi: 10.1128/jvi.69.2.1122-1131.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Soldevila G, Geiger T, Flavell R A. Breaking immunologic ignorance to an antigenic peptide of simian virus 40 large T antigen. J Immunol. 1995;155:5590–5600. [PubMed] [Google Scholar]
- 61.Speiser D E, Miranda R, Zakarian A, Bachmann M F, McKall-Faienza K, Odermatt B, Hanahan D, Zinkernagel R M, Ohashi P S. Self antigens expressed by solid tumors do not efficiently stimulate naive or activated T cells: implications for immunotherapy. J Exp Med. 1997;186:645–653. doi: 10.1084/jem.186.5.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tanaka Y, Anderson R W, Maloy W L, Tevethia S S. Localization of an immunorecessive epitope on SV40 T antigen by H-2Db-restricted cytotoxic T-lymphocyte clones and a synthetic peptide. Virology. 1989;171:205–213. doi: 10.1016/0042-6822(89)90527-8. [DOI] [PubMed] [Google Scholar]
- 63.Tanaka Y, Tevethia S S. In vitro selection of SV40 T antigen epitope loss variants by site-specific cytotoxic T lymphocyte clones. J Immunol. 1988;140:4348–4354. [PubMed] [Google Scholar]
- 64.Tanaka Y, Tevethia S S. Loss of immunorecessive cytotoxic T lymphocyte determinant V on SV40 T antigen following cocultivation with site-specific cytotoxic T lymphocyte clone Y-5. Intervirology. 199;31:197–202. doi: 10.1159/000150154. [DOI] [PubMed] [Google Scholar]
- 65.Tevethia S S. Recognition of simian virus 40 T antigen by cytotoxic T lymphocytes. Mol Biol Med. 1990;7:83–96. [PubMed] [Google Scholar]
- 66.Tevethia S S, Blasecki J W, Waneck G, Goldstein A L. Requirement of thymus-derived θ-positive lymphocytes for rejection of DNA virus (SV40) tumors in mice. J Immunol. 1974;113:1417–1423. [PubMed] [Google Scholar]
- 67.Van Dyke T A, Finlay C, Miller D, Marks J, Lozano G, Levine A J. Relationship between simian virus 40 large tumor antigen expression and tumor formation in transgenic mice. J Virol. 1987;61:2029–2032. doi: 10.1128/jvi.61.6.2029-2032.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.von Herrath M G, Dockter J, Oldstone M B A. How virus induces a rapid or slow onset insulin-dependent diabetes mellitus in a transgenic model. Immunity. 1994;1:231–242. doi: 10.1016/1074-7613(94)90101-5. [DOI] [PubMed] [Google Scholar]
- 69.von Herrath M G, Guerder S, Lewicki H, Flavell R A, Oldstone M B A. Coexpression of B7-1 and viral (“self”) transgenes in pancreatic β cells can break peripheral ignorance and lead to spontaneous autoimmune diabetes. Immunity. 1995;3:727–738. doi: 10.1016/1074-7613(95)90062-4. [DOI] [PubMed] [Google Scholar]
- 70.von Herrath M G, Oldstone M B A. Interferon-γ is essential for destruction of β cells and development of insulin-dependent diabetes mellitus. J Exp Med. 1997;185:531–539. doi: 10.1084/jem.185.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Walker P R, Saas P, Dietrich P Y. Role of Fas ligand (CD95L) in immune escape: the tumor cell strikes back. J Immunol. 1997;158:4521–4524. [PubMed] [Google Scholar]
- 72.Webb S, Morris C, Sprent J. Extrathymic tolerance of mature T cells: clonal elimination as a consequence of immunity. Cell. 1990;63:1249–1256. doi: 10.1016/0092-8674(90)90420-j. [DOI] [PubMed] [Google Scholar]
- 73.Wick M, Dubey P, Koeppen H, Siegel C T, Fields P E, Chen L, Bluestone J A, Schreiber H. Antigenic cancer cells grow progressively in immune hosts without evidence for T cell exhaustion or systemic anergy. J Exp Med. 1997;186:229–238. doi: 10.1084/jem.186.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wortzel R D, Urban J L, Schreiber H. Malignant growth in the normal host after variant selection in vitro with cytolytic T-cell lines. Proc Natl Acad Sci USA. 1984;81:2186–2190. doi: 10.1073/pnas.81.7.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ye X, Kralli A, Ge R, Ricciardi R P, Knowles B B. Down-regulation of MHC class I antigen in insulinoma cells controlled by the R1 element of the H-2 enhancer. Oncogene. 1994;9:1195–1204. [PubMed] [Google Scholar]
- 76.Ye X, McCarrick J, Jewett L, Knowles B B. Timely immunization subverts the development of peripheral nonresponsiveness and suppresses tumor development in simian virus 40 tumor antigen-transgenic mice. Proc Natl Acad Sci USA. 1994;91:3916–3920. doi: 10.1073/pnas.91.9.3916. [DOI] [PMC free article] [PubMed] [Google Scholar]