Human Immunodeficiency Virus Type 1 Tat Protein Activates Transcription Factor NF-κB through the Cellular Interferon-Inducible, Double-Stranded RNA-Dependent Protein Kinase, PKR (original) (raw)
Abstract
The transactivator protein of human immunodeficiency virus type 1 (HIV-1) (Tat) is a powerful activator of nuclear factor-κB (NF-κB), acting through degradation of the inhibitor IκB-α (F. Demarchi, F. d’Adda di Fagagna, A. Falaschi, and M. Giacca, J. Virol. 70:4427–4437, 1996). Here, we show that this activity of Tat requires the function of the cellular interferon-inducible protein kinase PKR. Tat-mediated NF-κB activation and transcriptional induction of the HIV-1 long terminal repeat were impaired in murine cells in which the PKR gene was knocked out. Both functions were restored by cotransfection of Tat with the cDNA for PKR. Expression of a dominant-negative mutant of PKR specifically reduced the levels of Tat transactivation in different human cell types. Activation of NF-κB by Tat required integrity of the basic domain of Tat; previous studies have indicated that this domain is necessary for specific Tat-PKR interaction.
Upon infection of susceptible cells and integration into the host genome, transcription of the human immunodeficiency virus type 1 (HIV-1) is dependent on the concerted action of the cellular transcription machinery and of the viral Tat transactivator protein. Tat acts as an extremely powerful activator of viral gene expression. The protein binds to a bulge sequence within TAR, a highly structured RNA element located at the 5′ end of all viral transcripts (8), and acts by a dual mechanism. From one side, it increases the levels of transcriptional elongation, by augmenting the processivity of RNA polymerase II through the interaction with protein complexes possessing protein kinase activity and phosphorylating the carboxyl-terminal domain of the large subunit of the polymerase (13, 21, 36, 43, 46). On the other side, the protein also acts at the level of transcriptional initiation, by increasing the rate at which the RNA polymerase II starts transcription (14). We (33) and others (5, 27) have recently demonstrated that the latter function of Tat is mediated by the specific interaction of Tat with the nuclear factor p300-CREB binding protein and by the recruitment of this acetyltransferase to the viral long terminal repeat (LTR) for chromatin remodeling.
Besides the above-mentioned interactions of cellular proteins with Tat, which require an intact TAR element, experimental evidence indicates that an additional transcriptional function of the protein also occurs in a TAR-independent manner, provided that the enhancer region of the LTR is intact (1, 6, 7, 25, 35). This region contains two tandemly arranged binding sites (κB sites) for the dimeric transcription factors composed of several combinations of members of the Rel/NF-κB family of polypeptides (for a review, see reference 3). The predominant complex binding to the LTR κB sites in activated cells is NF-κB (p50-p65 heterodimer). In unstimulated cells, NF-κB is retained in the cytoplasm through the interaction with inhibitor proteins belonging to the IκB family. Activation of NF-κB occurs through phosphorylation and proteolysis of the IκB inhibitor, with subsequent translocation of the active factor into the nucleus, where it can bind to its cognate binding sites (26). Maximal activation of the HIV LTR requires the concerted action of Tat and of cellular proteins binding to the κB sites (1, 10, 19, 32, 44). Accordingly, Tat-mediated activation of the HIV-1 LTR in Jurkat T cells is strongly inhibited by a degradation-resistant IκB-α mutant (24).
Tat itself is able to activate nuclear translocation of NF-κB (11, 16, 44). We have observed that treatment of T-lymphocytic, monocytic, and epithelial cells with recombinant Tat results in a rapid and transient nuclear translocation of NF-κB which parallels transcriptional activation of the proviral LTR (16).
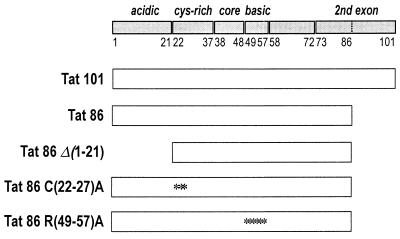
To address the study of the functional domains of the Tat protein involved in the activation of NF-κB, we obtained a series of constructs bearing deletions or point mutations in some of the relevant portions of the protein. A schematic representation of these constructs is presented in Fig. 1. They include the wild-type Tat proteins of 101 amino acids (aa) (present in most primary HIV isolates) and of 86 aa (retaining full activity and present in the prototype laboratory strain HXB2) and mutated derivatives of the latter protein having a deletion of the acidic, N-terminal domain [Tat 86Δ(1-21)], mutations of the cysteines at positions 22, 25, and 27 to alanines [Tat 86 C(22-27)A], and mutations of the arginines in the basic region at positions 49, 52, 53, 55, 56, and 57 to alanines [Tat 86 R(49-57)A]. The construction and purification of these recombinant Tat proteins have been already described (16, 33); all these mutations completely knock out the ability of the protein to _trans_-activate the LTR (not shown).
FIG. 1.
Tat proteins and mutants. The Tat protein of HIV-1 and its functional domains are schematically shown. Tat 101 is the full-length, two-exon Tat of most clinical isolates; Tat 86, lacking 15 amino acids at the C terminus, derives from clone HXB2 and is fully active for LTR transcription activation. The mutant proteins include Tat 86 Δ(1-21), which has a truncation in the first 21 amino acids; Tat 86 C(22-27)A, in which cysteines 22, 25, and 27 were mutated to alanines; and Tat 86 R(49-57)A, in which arginines at positions 49, 52, 53, 55, 56, and 57 were mutated to alanines. Asterisks indicate the positions of the mutated amino acids.
These mutants were tested either as recombinant proteins obtained as glutathione _S_-transferase fusions or after transfection of the corresponding expression vectors. In the former case, recombinant Tat was delivered to the cells by lipofection, as already described (16, 33). Under these conditions, the protein rapidly enters the cells through an endosome-mediated pathway and subsequently escapes from the endosomes and enters the nucleus, where it displays its transactivation capacity.
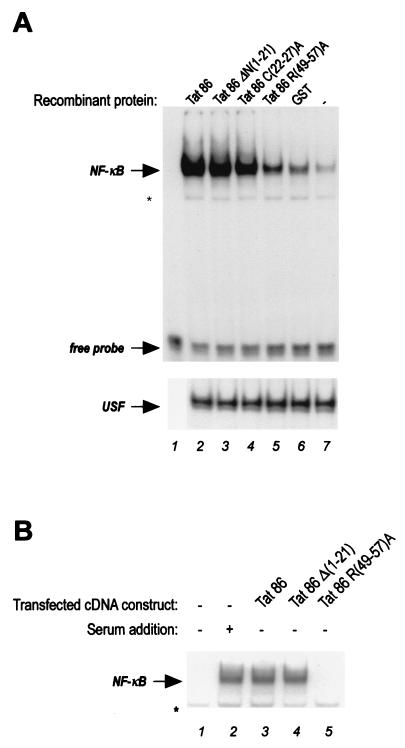
Wild-type Tat and mutant proteins were delivered into HL3T1 cells (containing an integrated LTR-chloramphenicol acetyltransferase [CAT] cassette, the kind gift of B. Felber [20]), and nuclear extracts were prepared according to the microscale preparation protocol described by Li et al. (31) and used in gel retardation assays to monitor the binding activity to a κB site oligonucleotide (17). As shown in Fig. 2A, NF-κB induction appeared considerably reduced when the Tat protein mutated in the basic domain was used, while the other mutations did not have any remarkable effect.
FIG. 2.
The basic domain of Tat is required for NF-κB activation. The figure shows the results of gel retardation analysis of NF-κB complexes present in nuclear extracts of HL3T1 cells either treated with wild-type and mutant Tat proteins (A) or transfected with plasmids expressing the respective cDNAs (B). The arrows indicate the specific NF-κB- or USF-containing complexes, or the free probe, as specified. The asterisks mark unspecific bands. The specificity and identity of the NF-κB-containing complex have been previously verified (16). (A) Seven micrograms of nuclear extracts from control cells (lane 7) or from cells in which 9 μg of GST protein/15-cm plate (lane 6), or the same amount of the Tat 86 protein (lane 1) and mutant derivatives (lanes 3 to 6), had been delivered by lipofection was used in a gel retardation assay with a γ-32P-labeled double-stranded oligonucleotide specific for NF-κB (16). (B) HL3T1 cells were transfected with 1 μg of empty vector/15-cm plate (lanes 1 and 2) or with 1 μg of expression vectors for Tat 86, Tat 86 Δ(1-21) (lane 4), and Tat 86 R(49-57)A (lane 5). Sixteen hours after transfection, serum concentration was lowered to 0.5% for an additional 24 h. Nuclear extracts were prepared and used in a gel retardation assay as described above. Two hours before harvesting, 10% serum was added to control cells (lane 2).
We also examined whether the effects of mutations in the basic region could be observed by transfection of an expression vector for the protein. Transfections were performed by using Lipofectin (Gibco BRL Life Technologies Ltd., Paisley, Scotland) in accordance with the procedure suggested by the manufacturer. To reduce the basal level of NF-κB in cycling HL3T1 cells (shown in Fig. 2, lane 7), 16 h after transfection cells were put in 0.5% serum. Under these conditions, NF-κB induction could be obtained by the expression vectors for Tat 86 and Tat 86Δ(1-21) to levels comparable to those obtained by serum addition (Fig. 1B). On the contrary, transfection of Tat 86 R(49-57)A did not result in the induction of nuclear translocation of NF-κB.
The experiments described above indicate that the basic domain of Tat is essential for NF-κB induction. Interestingly, this domain has been reported to interact with the human RNA-dependent protein kinase PKR both in vitro (12) and in vivo (34). This protein, also referred to as DAI, P1 kinase, and p68 kinase, is a serine/threonine kinase that is induced by interferon and is activated in the presence of double-stranded RNA, polyanions, and some structured single-stranded RNA (reviewed in reference 40). Activation of PKR leads to its autophosphorylation and to the consequent phosphorylation of its cellular targets, which include eukaryotic initiation factor 2 (eIF2) (9, 41) and IκB-α (28), with consequent inhibition of protein synthesis and activation of NF-κB translocation, respectively.
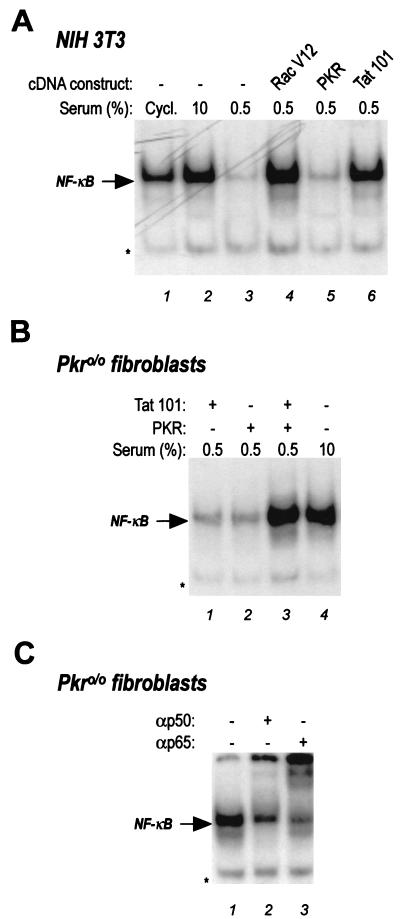
Given these considerations, we addressed the question of whether the molecular pathway initiated by Tat and leading to the activation of NF-κB could be mediated by the activity of PKR. For this purpose, we took advantage of the availability of murine fibroblasts derived from Pkr knockout (Pkr0/0) mice (45), the kind gift of B. Williams. We first checked whether Tat could induce NF-κB in mouse cells. Mouse NIH 3T3 fibroblasts were transfected with expression plasmids coding for Tat 101; for activated Rac, a member of the family of small GTP binding proteins shown to trigger NF-κB nuclear translocation (37) (the kind gift of Alan Hall); and for PKR, donated by B. Williams. Transfected and mock-transfected cells were serum starved for 24 h to reduce the level of constitutive NF-κB present in cycling 3T3 cells, as described previously (37). After serum starvation, rich medium was added to one aliquot of mock-transfected cells to obtain a positive control for NF-κB nuclear translocation. Nuclear extracts were prepared and utilized for band-shift assays with the oligonucleotide probe specific for NF-κB. As shown in Fig. 3A, NF-κB was almost undetectable in serum-starved cells (lane 3), as well as in starved cells previously transfected with the PKR expression vector (lane 4). On the contrary, transfection of both the activated form of Rac and the Tat-expressing plasmids resulted in a remarkable nuclear translocation of the factor (lanes 4 and 6, respectively). Thus, Tat can induce nuclear translocation of NF-κB also in mouse cells, suggesting that the underlying molecular mechanisms are preserved also in this species.
FIG. 3.
Gel retardation analysis of NF-κB complexes induced by Tat in NIH 3T3 and PKR knockout mouse cells. (A) NIH 3T3 cells plated in 15-cm dishes were transiently transfected with 5 μg of the different cDNA constructs as indicated in the figure. The control samples (lanes 1 to 3) were transfected with the empty vector pCDNAIII. After 16 h, cells were serum starved (0.5% serum) for 24 h to lower the endogenous NF-κB levels (37). Gel retardation assays were performed with 7.5 μg of nuclear extract by using an NF-κB-specific oligonucleotide probe. Two hours before cells harvesting, 10% serum was added to control cells in lane 2. Lane 1, endogenous levels of NF-κB in cycling (Cycl.) cells grown in 10% serum. (B) Results of transfection of primary mouse PKR0/0 fibroblasts with plasmids expressing Tat 101 (lane 1), PKR (lane 2), or both (lane 3). Cells were transfected and serum starved as described above. Lane 4, same as lane 2 in panel A. (C) Results of supershift assay to ascertain the identity of the retarded band detected with the κB probe in gel retardation assays with nuclear extract from mouse PKR0/0 fibroblasts. The assay was performed with antibodies against the p50 and p65 subunits of NF-κB as indicated in the figure and nuclear extracts from PKR0/0 fibroblasts cotransfected with PKR and Tat. In all panels, the arrow indicates the specific NF-κB-containing complexes and the asterisks mark the unspecific band.
Contrary to what was observed in NIH 3T3 fibroblasts, in Pkr0/0 mouse cells neither Tat nor PKR alone could trigger NF-κB activation (Fig. 3B, lanes 1 and 2), while cotransfection of the two plasmids resulted in a strong synergistic effect (lane 3), similar to the one obtained by serum addition (lane 4). The activated factor observed by gel retardation assays in these cells was identified as NF-κB containing the p50 and p65 subunits, since p50 antibody supershifted and p65 antibody abolished the complex (Fig. 3C). Supershift assays were performed with NF-κB p50 and p65 polyclonal antibodies (Santa Cruz Biotechnology, Santa Cruz, Calif.) by preincubating the nuclear extracts with 2 μg of the antibody in the reaction buffer for 30 min prior to the gel retardation assays.
The above results clearly indicate that PKR is involved in Tat-induced activation of NF-κB in mouse cells. The next step was to test whether this functional interaction also affects the transcriptional properties of Tat. For this purpose, we studied the role of Tat and PKR in the activation of transcription from a transfected LTR-CAT cassette in mouse cells lacking a functional PKR as well as in control NIH 3T3 cells. Cell extracts were prepared 48 h after transfection, and CAT assays were performed according to standard protocols (39) after normalization for transfection efficiency.
The results are shown in Fig. 4. As expected, the effects of Tat on LTR-driven transcription in mouse cells are not as dramatic as in human cells (2, 4). Therefore, the amount of Tat plasmid that was transfected was 10 times higher (5 μg of DNA/10-cm plate) than the one we generally use for human cells. Under these conditions, Tat considerably enhances the transcription driven by the LTR in NIH 3T3 cells, while PKR does not have any effect (Fig. 4A). In PKR knockout cells (Fig. 4B) the transcriptional effect of Tat is impaired with respect to control cells, but it can be fully restored by cotransfection of a wild-type PKR expression plasmid.
FIG. 4.
PKR is functionally required for Tat transactivation of the LTR promoter in mouse fibroblasts. NIH 3T3 mouse cells and primary mouse fibroblasts (panels A and B, respectively) from PKR knockouts were transiently transfected with a reporter plasmid containing the LTR linked to the CAT gene. Five micrograms of Tat 101 and/or 5 μg of PKR expression constructs was cotransfected, as indicated. The empty vector pCDNAIII was added in order to keep constant the amount of transfected DNA. Forty-eight hours after transfection, cell extracts were prepared and monitored for CAT activity after normalization for transfection efficiency. The bars show the percentage of chloramphenicol acetylation obtained with each extract; the data are the averages of three independent experiments, and the standard deviations are indicated. Standardization for transfection efficiency was also obtained by cotransfection of 0.5 μg of a luciferase expression vector per 60-mm plate.
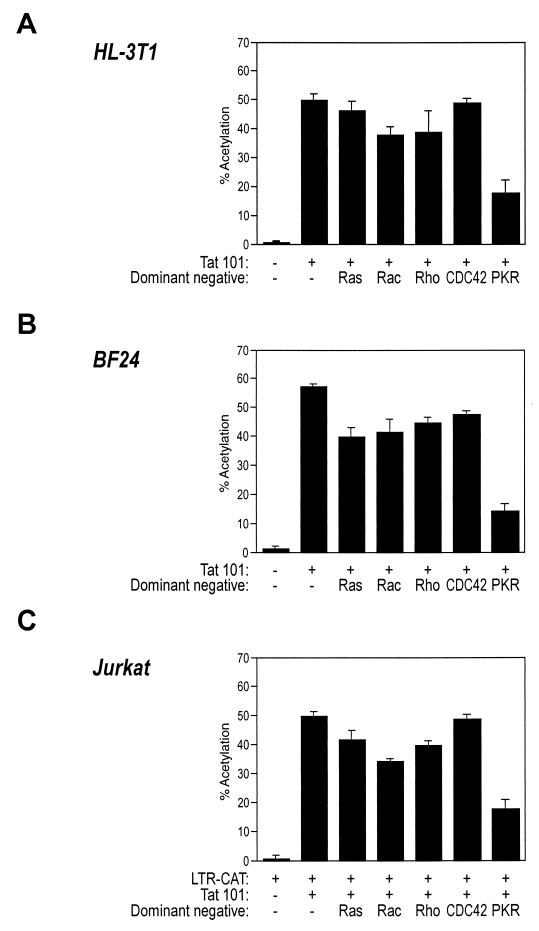
Altogether, the data described above demonstrate that the functions of PKR are required for NF-κB nuclear translocation and LTR transcription activation by Tat in a murine system. Next, it was important to address the question of whether PKR plays a similar role in human cells. For this purpose, we exploited the properties of a series of dominant-negative mutants of different proteins physiologically involved in different pathways of NF-κB activation. These proteins included, besides PKR, a set of small GTP binding proteins of the Rho family (Ras, RhoA, Rac1, and Cdc42), which are known regulators of different protein kinases and act as potent activators of NF-κB in various cell types (37).
For each of these factors (37), as well as for PKR (29), mutants having a _trans_-dominant-negative effect on the wild-type proteins have been described. Expression vectors for dominant-negative PKR, Ras, and Rac were kindly donated by B. Williams, C. J. Marshall, and A. Hall, respectively. Dominant negatives for RhoA and CDC42 were the kind gift of G. Bokoch.
These vectors were cotransfected with Tat in human epithelial HL3T1 and monocytic BF24 cells (both of which contain an integrated LTR-CAT cassette [20, 42]) and in T-lymphocytic Jurkat cells together with an LTR-CAT plasmid. In all these cell lines, the PKR dominant-negative mutant caused a net decrease in Tat-mediated activation of LTR-driven transcription (Fig. 5A, B, and C). On the contrary, dominant negatives of Ras, Rac1, RhoA, and Cdc42 did not have any significant effect. As control, all the dominant-negative mutants were previously checked in cotransfection experiments in Jurkat cells for their ability to prevent the activation of the HIV-1 LTR by their respective wild-type counterparts (not shown).
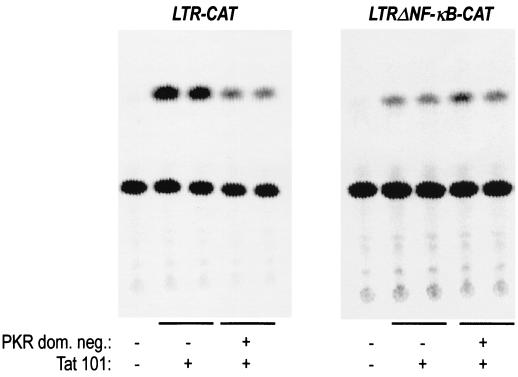
FIG. 5.
Dominant-negative PKR impairs LTR activation induced by Tat. HL3T1, BF24, and Jurkat cells (panels A, B, and C, respectively) were transfected with 2.5 μg of pCDNAIII empty vector (first lanes of each panel) or with 500 ng of Tat 101 expression vector and 2 μg of the dominant-negative mutant cDNAs for Ras (plasmid Ras N17), Rac (Rac N17), RhoA (Rho T19N), CDC42 (Cdc42 T17N), and PKR (PKR-M) as indicated. Jurkat cells were also cotransfected with 1 μg of an LTR-CAT-containing plasmid. Twenty hours after transfection, cell extracts were prepared and used for CAT assays after normalization for transfection efficiency. The results are the mean values from three independent experiments, with error bars indicating standard deviations.
The inhibitory effect of the PKR dominant-negative construct on Tat transactivation was mediated in cis by the enhancer region of the LTR. As shown in Fig. 6, when the vector expressing Tat 101 was cotransfected in HeLa cells together with the PKR dominant-negative construct, transactivation from the wild-type LTR was reduced, while it was unaffected from an LTR promoter bearing the deletion of the NF-κB sites (30).
FIG. 6.
An intact LTR enhancer region is required for downregulation of Tat transactivation by dominant-negative PKR. HeLa cells were transfected with the wild-type LTR-CAT reporter (1 μg; left panel) or plasmid pNFA-CAT (1 μg; right panel). The latter plasmid, described by Leonard et al. (30), was a kind gift of G. Scala and contains a deletion of the NF-κB sites of the LTR. These plasmids were cotransfected with 0.5 μg of pCDNAIII-Tat101 and 1 μg of pPKR-M, as indicated. The empty vector pCDNAIII was added in order to keep constant the amount of transfected DNA in each plate. Experiments were performed in duplicate.
Altogether the experimental data reported above indicate that PKR is involved in Tat transactivation of the LTR both in rodent and in human cells through the activation of NF-κB. Following different experimental strategies, over the last few years, several laboratories have suggested that a complex interplay exists between Tat, TAR, and PKR, although the results obtained have often been controversial. Some reports showed that TAR activates PKR, resulting in trans inhibition of translation (18), while others demonstrated that PKR-TAR interaction had a negative effect on activation of the kinase (22, 23). Also controversial are the findings that the levels and the activity of PKR are decreased (38), increased (15), or unaffected (34) in HIV-1-infected and in Tat-expressing cells. These conflicting data are difficult to reconcile and are likely the results of the different experimental settings in which they were obtained. As far as Tat and PKR are concerned, two independent laboratories have recently shown that a physical interaction exists between the two proteins both in vitro and in vivo and that this interaction requires the integrity of the basic domain of Tat (12, 34). This is the same domain that we found to be essential for the activation of NF-κB in our experiments. However, it should be pointed out that the same laboratories have indicated, by in vitro studies, that Tat behaves as an inhibitor of PKR activation and as a competitive substrate for phosphorylation, in apparent contradiction with the functional positive role of the PKR-Tat interplay that we are suggesting here. In this respect, we believe that the conditions for in vitro functional studies of the kinase are likely to be considerably different from those found within the cells, where the relative concentrations of PKR, TAR, Tat, and accessory factors are not easily quantifiable and might vary during the course of infection. As an alternative explanation, we cannot rule out the possibility that the functional requirement of PKR for Tat-mediated NF-κB activation, which clearly stems from the set of our experiments, is not directly dependent on the PKR-Tat protein-protein interaction but is mediated by an unidentified intermediate pathway.
The results presented in this work suggest that both Tat 101, Tat 86, and Tat 72 (one exon; results not shown) are able to activate NF-κB through the PKR pathway, both in rodent and human cells, and that this pathway requires the integrity of the basic domain of the protein. Thus, this mechanism appears to be clearly different from the one proposed by Westendorp and collaborators (44), which requires the second exon of the protein and involves the repression of the Mn-dependent superoxide dismutase and the subsequent alteration of the redox state of the cell. An alternative pathway proposed to explain NF-κB activation by Tat is the induction of expression of the tumor necrosis factor alpha (TNF-α) gene and the subsequent activation of NF-κB through the TNF-α receptor-mediated signaling mechanism (11). Again, this mechanism is quite distinct from the one suggested by the data presented here, since TNF-α signals responsive promoters in both PKR+/+ and PKR0/0 cells, indicating that this cytokine utilizes a largely non-PKR-dependent transduction pathway (29).
It is conceivable that all the above-mentioned molecular processes of Tat-induced NF-κB activation could operate in different cell types and under different conditions. While it is difficult to rank the relative importance of these mechanisms, we would like to point out that in murine fibroblasts lacking PKR the induction of NF-κB nuclear translocation by Tat is almost completely impaired, thus suggesting that, at least in this cell type, the presence of functional PKR is likely to be crucial for this function.
Acknowledgments
We thank Maria Elena Lopez for excellent assistance in tissue culture and Barbara Boziglav for skillful technical support. We are grateful to Bryan Williams and Patricia Kessler for the kind gift of the PKR knockout cells and PKR constructs, to Gary Bokoch for providing constructs Rho T19N and Cdc42 T17N, to Alan Hall for providing the Rac N17 and Rac V12 constructs, to Christopher J. Marshall for providing the Ras N17 construct, and to Barbara Felber for the BF24 and HL3T1 cell lines. We are grateful to K.-T. Jeang for the initial suggestion to investigate the role of the Tat-PKR interaction in NF-κB induction.
This work was supported by grant 40A.0.50 from the ISS (Istituto Superiore di Sanità), Rome, Italy.
REFERENCES
- 1.Alcamí J, Laín de Lera T, Folgueira L, Pedraza M A, Jacqué J-M, Bachelerie F, Noriega A R, Hay R T, Harrich D, Gaynor R B, Virelizier J-L, Arenzana-Seisdos F. Absolute dependence on κB responsive elements for initiation and Tat-mediated amplification of HIV transcription in blood CD4 T lymphocytes. EMBO J. 1995;14:1552–1560. doi: 10.1002/j.1460-2075.1995.tb07141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alonso A, Cujec T, Peterlin B. Effects of human chromosome 12 on interactions between Tat and TAR of human immunodeficiency virus type 1. J Virol. 1994;66:6505–6513. doi: 10.1128/jvi.68.10.6505-6513.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baeuerle P A, Baltimore D. NF-κB: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 4.Barry P A, Pratt-Lowe E, Unger R E, Luciw P A. Cellular factors regulate transactivation of human immunodeficiency virus type 1. J Virol. 1991;65:1392–1399. doi: 10.1128/jvi.65.3.1392-1399.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benkirane M, Chun R F, Xiao H, Ogryzko V V, Howard B H, Nakatani Y, Jeang K T. Activation of integrated provirus requires histone acetyltransferase. p300 and P/CAF are coactivators for HIV-1 Tat. J Biol Chem. 1998;273:24898–24905. doi: 10.1074/jbc.273.38.24898. [DOI] [PubMed] [Google Scholar]
- 6.Berkhout B, Gatignol A, Rabson A B, Jeang K-T. TAR-independent activation of the HIV-1 LTR: evidence that Tat requires specific regions of the promoter. Cell. 1990;62:757–767. doi: 10.1016/0092-8674(90)90120-4. [DOI] [PubMed] [Google Scholar]
- 7.Berkhout B, Jeang K T. Functional roles for the TATA promoter and enhancers in basal and Tat-induced expression of the human immunodeficiency virus type 1 long terminal repeat. J Virol. 1992;66:139–149. doi: 10.1128/jvi.66.1.139-149.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berkhout B, Silverman R H, Jeang K T. Tat trans-activates the human immunodeficiency virus through a nascent RNA target. Cell. 1989;59:273–282. doi: 10.1016/0092-8674(89)90289-4. [DOI] [PubMed] [Google Scholar]
- 9.Berry M J, Knutson G S, Lasky S R, Munemitsu S M, Samuel C E. Purification and substrate specificities of the double-stranded RNA-dependent protein kinase from untreated and interferon treated mouse fibroblasts. J Biol Chem. 1985;260:240–247. [PubMed] [Google Scholar]
- 10.Biswas D K, Ahlers C M, Dezube B J, Pardee A B. Cooperative inhibition of NF-κB and Tat-induced superactivation of human immunodeficiency virus type 1 long terminal repeat. Proc Natl Acad Sci USA. 1993;90:11044–11048. doi: 10.1073/pnas.90.23.11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biswas D K, Salas T R, Wang F, Ahlers C M, Dezube B J, Pardee A B. A Tat-induced auto-up-regulatory loop superactivation of the human immunodeficiency virus type 1 promoter. J Virol. 1995;69:7437–7444. doi: 10.1128/jvi.69.12.7437-7444.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brand S R, Kobayashi R, Mathews M B. The Tat protein of human immunodeficiency virus type 1 is a substrate and inhibitor of the interferon-induced, virally activated protein kinase, PKR. J Biol Chem. 1997;272:8388–8395. doi: 10.1074/jbc.272.13.8388. [DOI] [PubMed] [Google Scholar]
- 13.Cujec T P, Okamoto H, Fujinaga K, Meyer J, Chamberlin H, Morgan D O, Peterlin B M. The HIV transactivator TAT binds to the CDK-activating kinase and activates the phosphorylation of the carboxy-terminal domain of RNA polymerase II. Genes Dev. 1997;11:2645–2657. doi: 10.1101/gad.11.20.2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cullen B. Does HIV-1 Tat induce a change in viral initiation rights? Cell. 1993;73:417–420. doi: 10.1016/0092-8674(93)90126-b. [DOI] [PubMed] [Google Scholar]
- 15.DeLuca C, Roulston A, Koromilas A, Wainberg M A, Hiscott J. Chronic human immunodeficiency virus type 1 infection of myeloid cells disrupts the autoregulatory control of the NF-κB/Rel pathway via enhanced IκBα degradation. J Virol. 1996;70:5183–5193. doi: 10.1128/jvi.70.8.5183-5193.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Demarchi F, d’Adda di Fagagna F, Falaschi A, Giacca M. Activation of transcription factor NF-κB by the Tat protein of human immunodeficiency virus type 1. J Virol. 1996;70:4427–4437. doi: 10.1128/jvi.70.7.4427-4437.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Demarchi F, D’Agaro P, Falaschi A, Giacca M. In vivo footprinting analysis of constitutive and inducible protein-DNA interactions at the long terminal repeat of human immunodeficiency virus type 1. J Virol. 1993;67:7450–7460. doi: 10.1128/jvi.67.12.7450-7460.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edery I, Petryshyn R, Sonenberg N. Activation of double-stranded RNA-dependent kinase (dsl) by the TAR region of HIV-1 mRNA: a novel translational control mechanism. Cell. 1989;56:303–312. doi: 10.1016/0092-8674(89)90904-5. [DOI] [PubMed] [Google Scholar]
- 19.El Kharroubi A, Piras G, Zensen R, Martin M A. Transcriptional activation of the integrated chromatin-associated human immunodeficiency virus type 1 promoter. Mol Cell Biol. 1998;18:2535–2544. doi: 10.1128/mcb.18.5.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Felber B K, Pavlakis G N. A quantitative bioassay for HIV-1 based on trans-activation. Science. 1988;239:184–186. doi: 10.1126/science.3422113. [DOI] [PubMed] [Google Scholar]
- 21.Gold M O, Yang X, Herrmann C H, Rice A P. PITALRE, the catalytic subunit of TAK, is required for human immunodeficiency virus Tat transactivation in vivo. J Virol. 1998;72:4448–4453. doi: 10.1128/jvi.72.5.4448-4453.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gunnery S, Green S R, Mathews M B. Tat-responsive region RNA of human immunodeficiency virus type 1 stimulates protein synthesis in vivo and in vitro: relationship between structure and function. Proc Natl Acad Sci USA. 1992;89:11557–11561. doi: 10.1073/pnas.89.23.11557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gunnery S, Rice A P, Robertson H D, Mathews M B. Tat-responsive region RNA of human immunodeficiency virus 1 can prevent activation of the double-stranded-RNA-activated protein kinase. Proc Natl Acad Sci USA. 1990;87:8687–8691. doi: 10.1073/pnas.87.22.8687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harhaj E, Blanley J, Millhouse S, Sun S C. Differential effects of IκB molecules on Tat-mediated transactivation of the LTR. Virology. 1996;216:284–287. doi: 10.1006/viro.1996.0062. [DOI] [PubMed] [Google Scholar]
- 25.Harrich D, Garcia J, Mtsuyasu R, Gaynor R. Tar independent activation of the human immunodeficiency virus in phorbol ester stimulated T lymphocytes. EMBO J. 1990;9:4417–4423. doi: 10.1002/j.1460-2075.1990.tb07892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Henkel T, Machleidt T, Alkalay I, Kronke M, Ben-Neriah Y, Baeuerle P A. Rapid proteolysis of IκB-α is necessary for activation of transcription factor NF-κB. Nature. 1993;365:182–185. doi: 10.1038/365182a0. [DOI] [PubMed] [Google Scholar]
- 27.Hottiger M O, Nabel G J. Interaction of human immunodeficiency virus type 1 Tat with the transcriptional coactivators p300 and CREB binding protein. J Virol. 1998;72:8252–8256. doi: 10.1128/jvi.72.10.8252-8256.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar A, Haque J, Lacoste J, Hiscott J, Williams B R G. Double-stranded RNA-dependent protein kinase activates transcription factor NF-κB by phosphorylating IκB. Proc Natl Acad Sci USA. 1994;91:6288–6292. doi: 10.1073/pnas.91.14.6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar A, Yang Y L, Flati V, Der S, Kadereit S, Deb A, Haque J, Reis L, Weissmann C, Williams B R G. Deficient cytokine signaling in mouse embryo fibroblasts with a targeted deletion in the PKR gene: role of IRF-1 and NF-κB. EMBO J. 1997;16:406–416. doi: 10.1093/emboj/16.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leonard J, Parrott C, Buckler-White A J, Turner W, Ross E K, Martin M A, Rabson A B. The NF-κB binding sites in the human immunodeficiency virus type 1 long terminal repeat are not required for virus infectivity. J Virol. 1989;63:4919–4924. doi: 10.1128/jvi.63.11.4919-4924.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Y, Ross J, Scheppler J A, Franza R., Jr An in vitro transcription analysis of early responses of the human immunodeficiency virus type 1 long terminal repeat to different transcription activators. Mol Cell Biol. 1991;11:1883–1893. doi: 10.1128/mcb.11.4.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu J, Perkins N, Schmid R, Nabel G. Specific NF-κB subunits act in concert with Tat to stimulate human immunodeficiency type 1 transcription. J Virol. 1992;66:3883–3887. doi: 10.1128/jvi.66.6.3883-3887.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marzio G, Tyagi M, Gutierrez M I, Giacca M. HIV-1 Tat transactivator recruits p300 and CBP histone acetyl transferases to the viral promoter. Proc Natl Acad Sci USA. 1998;23:13519–13524. doi: 10.1073/pnas.95.23.13519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McMillan N A J, Chun R F, Sideovski D P, Galabru J, Toone W M, Samuel C E, Mak T W, Hovanessian A G, Jeang K T, Williams B R G. HIV-1 Tat directly interacts with the interferon-induced, double-stranded RNA-dependent kinase PKR. Virology. 1995;213:413–424. doi: 10.1006/viro.1995.0014. [DOI] [PubMed] [Google Scholar]
- 35.Nabel G J, Rice S A, Knipe D M, Baltimore D. Alternative mechanisms for activation of human immunodeficiency virus enhancer in T cells. Science. 1988;239:1299–1302. doi: 10.1126/science.2830675. [DOI] [PubMed] [Google Scholar]
- 36.Parada C A, Roeder R G. Enhanced processivity of RNA polymerase II triggered by Tat-induced phosphorylation of its carboxy-terminal domain. Nature. 1996;384:375–378. doi: 10.1038/384375a0. [DOI] [PubMed] [Google Scholar]
- 37.Perona R, Montaner S, Saniger L, Sanchez-Perez I, Bravo R, Lacal J C. Activation of the nuclear factor-κB by Rho, CDC42, and Rac proteins. Genes Dev. 1997;11:463–475. doi: 10.1101/gad.11.4.463. [DOI] [PubMed] [Google Scholar]
- 38.Roy S, Katze M G, Parkin N T, Edery I, Hovanessian A G, Sonenberg N. Control of the interferon-induced 68-kilodalton protein kinase by the HIV-1 tat gene product. Science. 1990;247:1216–1219. doi: 10.1126/science.2180064. [DOI] [PubMed] [Google Scholar]
- 39.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 40.Samuel C E. Antiviral actions of interferon interferon-regulated cellular proteins and their surprisingly selective antiviral activities. Virology. 1991;183:1–11. doi: 10.1016/0042-6822(91)90112-o. [DOI] [PubMed] [Google Scholar]
- 41.Samuel C E. Mechanism of interferon action. Phosphorylation of protein synthesis initiation factor eIF-2 in interferon-treated human cells by a ribosome-associated kinase possessing site specificity similar to hemin-regulated rabbit reticulocyte kinase. Proc Natl Acad Sci USA. 1979;76:600–604. doi: 10.1073/pnas.76.2.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schwartz S, Felber B K, Fenyo E M, Pavlakis G N. Rapidly and slowly replicating human immunodeficiency virus type 1 isolates can be distinguished according to target-cell tropism in T-cell and monocyte cell lines. Proc Natl Acad Sci USA. 1989;86:7200–7203. doi: 10.1073/pnas.86.18.7200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wei P, Garber M E, Fang S-M, Fisher W H, Jones K A. A novel CDK9-associated C-type cyclin interacts directly with HIV-1 Tat and mediates its high-affinity, loop-specific binding to TAR RNA. Cell. 1998;92:451–462. doi: 10.1016/s0092-8674(00)80939-3. [DOI] [PubMed] [Google Scholar]
- 44.Westendorp M O, Shatrov V A, Schulze-Osthoff K, Frank R, Kraft M, Los M, Krammer P H, Droge W, Lehmann V. HIV-1 Tat potentiates TNF-induced NF-κB activation and cytotoxicity by altering the cellular redox state. EMBO J. 1995;14:546–554. doi: 10.1002/j.1460-2075.1995.tb07030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang Y L, Reis F L, Pavlovic J, Aguzzi A, Schafer R, Kumar A, Williams B R G, Aguet M, Weissmann C. Deficient signaling in mice devoid of double-stranded RNA-dependent protein kinase. EMBO J. 1995;14:6095–6106. doi: 10.1002/j.1460-2075.1995.tb00300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu Y, Pe’ery T, Peng J, Ramanathan Y, Marshall N, Marshall T, Amendt B, Mathews M B, Price D H. Transcription elongation factor P-TEFb is required for HIV-1 tat transactivation in vitro. Genes Dev. 1997;11:2622–2632. doi: 10.1101/gad.11.20.2622. [DOI] [PMC free article] [PubMed] [Google Scholar]