Activation of the PAK-Related Kinase by Human Immunodeficiency Virus Type 1 Nef in Primary Human Peripheral Blood Lymphocytes and Macrophages Leads to Phosphorylation of a PIX-p95 Complex (original) (raw)
Abstract
Human immunodeficiency virus type 1 (HIV-1) Nef enhances virus replication in both primary T lymphocytes and monocyte-derived macrophages. This enhancement phenotype has been linked to the ability of Nef to modulate the activity of cellular kinases. We find that despite the reported high-affinity interaction between Nef and the Src kinase Hck in vitro, a Nef-Hck interaction in the context of HIV-1-infected primary macrophages is not detectable. However, Nef binding and activation of the PAK-related kinase and phosphorylation of its substrate could be readily detected in both infected primary T lymphocytes and macrophages. Furthermore, we show that this substrate is a complex composed of the recently characterized PAK interacting partner PIX (PAK-interacting guanine nucleotide exchange factor) and its tightly associated p95 protein. PAK and PIX-p95 appear to be differentially activated and phosphorylated depending on the intracellular environment in which nef is expressed. These results identify the PIX-p95 complex as a novel effector of Nef in primary cells and suggest that the regulation of the PAK signaling pathway may differ in T cells and macrophages.
The human immunodeficiency virus type 1 (HIV-1) nef gene encodes a 25- to 27-kDa myristoylated protein unique to primate lentiviruses that is produced early in the infectious cycle and is estimated to comprise up to 80% of early transcripts (51). Its potential role in HIV-1 pathogenesis was established by the seminal study of Kestler et al., in which Nef was demonstrated to be essential for the maintenance of high viral loads and progression to simian AIDS in adult rhesus macaques (26). The existence of individuals infected with forms of HIV-1 from which nef was deleted who remained asymptomatic for over 15 years further supports the idea of Nef playing a role in disease progression (15, 27, 32). The identification and elucidation of the molecular interactions between Nef and host cell factors will therefore be crucial to understanding how Nef manifests its in vivo phenotype and in the rational design of intervention strategies.
Multiple in vitro functions of Nef have been identified and include the downmodulation of the CD4 (18, 22) and major histocompatibility complex class I (58) molecules. A mechanistic basis for these Nef functions has recently been provided in studies showing a connection between Nef’s ability to down-regulate specific surface molecules and its ability to bind to components of the cellular protein sorting machinery (7, 19, 34, 37, 49, 50). Nef has also been shown to enhance viral infectivity as measured by single-cycle (11, 44) and peripheral blood mononuclear cell (PBMC) infectivity assays (16, 44, 60). The molecular basis for this phenotype is as yet unclear, but some clues have been obtained from genetic studies showing that the interaction of Nef with cellular kinases contributes to the enhanced replication of Nef+ viruses (52, 56, 64). The findings that 10 to 100 molecules of Nef are packaged into the virion (48, 63) and that Nef must be present in the producer cell in order to enhance viral infectivity (1, 45) led to the suggestion that Nef-mediated recruitment of specific cellular factors, such as kinases, results in the modification of the virion so that processes involved in uncoating and/or reverse transcription proceed more efficiently (1, 10, 57).
An interesting property of Nef is its ability to alter T-cell signal transduction pathways (4, 23, 25). This activity of Nef is likely to be mediated through interactions with cellular kinases and signaling proteins. In vitro, Nef has been reported to bind the Src family tyrosine kinases Hck (8, 21, 33, 46), Lyn (52), Lck (3, 12, 20), and Src (31). Interactions with protein kinase C theta and delta (3, 59), the zeta chain of the T-cell receptor (5, 24, 65), mitogen-activated protein kinase (20), and lastly a serine/threonine kinase (36, 47, 54) have also been reported. Of these interactions, only that involving the serine/threonine kinase has been shown to be present in virally infected cells. In vitro kinase assays (IVKAs) performed on Nef immunoprecipitates (IPs) from infected and transfected transformed T-cell lines led to the detection of two serine phosphorylated proteins, p62 and p72 (54). It was subsequently shown that the membrane-targeting domain of Nef, critical proline residues in the SH3 domain, and an arginine residue flanking the SH3 domain were required for Nef binding and autophosphorylation of p62 (39, 53, 64). Elements in the C-terminal end of Nef also appear to be important for Nef association with p62 (38).
Several recent studies have provided evidence that the p62 Nef-associated kinase (NAK) is or is closely related to a member of the (p21-activated kinase PAK) family (36, 47, 55). The PAK family of kinases serve as effectors for the small GTP-binding proteins Rac1 and Cdc42 to activate transcriptional events and induce cytoskeletal rearrangements (35, 41). Like that of PAK, NAK activity can be potentiated by constitutively activated forms of the guanine nucleotide binding proteins Cdc42 and Rac1 (36, 47) and blocked by dominant-negative forms of PAK (36). In addition, a membrane-targeted SH3 domain from the adaptor protein Nck was shown to significantly enhance the ability of NL4-3 R71 Nef to activate PAK (39). Nck, which possesses a classical SH3 binding domain as well as a domain capable of binding to a unique proline-rich interaction motif of PAK, was proposed to serve as a connector between Nef and PAK (39).
Although the Nef-NAK interaction can be readily detected in transfected or virally infected immortalized T-cell lines, it has not been examined in the context of more physiologically relevant cell types such as primary peripheral blood lymphocytes and macrophages. In addition to being targets of HIV-1 infection, macrophages may represent an important viral reservoir in infected patients (29). Yet much less is known about the effects of Nef function or its interaction with cellular factors in these cells. Since macrophages play essential roles in the innate immune responses to viral infection and as antigen-presenting cells, it can be envisioned that any effect of Nef on downmodulation of the major histocompatibility complex class I molecules or on signaling pathways may alter their effector functions. In this regard, the observation that Nef can bind and activate Hck catalytic activity in coexpression systems (8, 46) is of interest, as this Src kinase is primarily expressed in macrophages.
In this study, we assess the function of Nef in primary macrophages and look for a physiological interaction of Nef with the host cell kinases, Hck and NAK. Using an HIV-1-infected human macrophage culture system, we were unable to demonstrate an interaction of Nef with Hck. However, we could readily detect Nef binding and activation of p62 NAK in infected macrophages as well as PBMCs. More importantly, we show that the second phosphoprotein, p72, detected in IVKAs performed on Nef IPs from infected T lymphocytes and macrophages represents the recently identified PAK-interacting guanine nucleotide exchange protein (PIX) and its associated p95 protein (42). This finding provides additional evidence that NAK is PAK. The activation of PAK and the phosphorylation of its downstream effectors by Nef in both primary target cells of HIV-1 suggest that this signal transduction pathway is important for Nef-mediated functions.
MATERIALS AND METHODS
Generation of nef mutant viruses.
The SF162 Δ_nef_ and R25 Δ_nef_ viruses were generated by creating a frameshift mutation at the unique _Xho_I site in nef of SF162 (9) and R25 (30) by fill-in and blunting with Klenow enzyme, followed by religation. R25 is an SF2 env recombinant virus that contains the V1 to V3 regions of SF162 gp120 and is capable of replicating in macrophages (30). Viruses were propagated by cotransfection of linearized plasmids carrying the 3′ and 5′ halves of the genome into 293T cells and coculture with PBMCs as previously described (9). Two clones of each virus were constructed, sequenced, and characterized to minimize the possibility that the observed effects were due to secondary site mutations.
PBMC isolation and replication assays.
PBMCs were isolated from the buffy coats of healthy human donors by Ficoll gradient centrifugation. For the resting cell replication assay, cells were resuspended in RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum (Biowhittaker), 1% glutamine, penicillin, and streptomycin (complete medium). Cells (5 × 106) were infected with 10 ng of virus p24 for 4 h. The cells were washed with Hanks’ buffered saline solution (HBSS) and resuspended in 7 ml of complete medium in T-25 Falcon flasks. At day 4 postinfection (p.i.) the infected cells were stimulated with 3 μg of phytohemagglutinin (PHA; Sigma) and 5 U of recombinant interleukin 2 (IL-2) per ml for 3 days. The infected and stimulated cells were washed with Hanks’ buffered saline solution and resuspended in complete medium containing 5 U of IL-2 per ml. Every 3 to 4 days, 4 ml of medium was removed and saved for p24 quantitation by antigen capture method (Abbott) and 4 ml of fresh medium was added to the cultures. For the stimulated cell assay, cells were resuspended in complete medium containing 5 U of IL-2 and 3 μg of PHA per ml immediately after isolation and stimulated for 3 days. The PBMCs were then washed and resuspended in complete medium with 5 U of IL-2 per ml. Stimulated PBMCs were infected and supernatant was harvested as described above.
Macrophage isolation and virus infection.
Enrichment of PBMCs for the monocyte population was obtained by centrifuging the cells through a 46% Percoll gradient (Pharmacia) (13). The monocytes were resuspended in RPMI 1640 complete medium supplemented with 20% heat-inactivated fetal bovine serum and 5% heat-inactivated human AB serum (Biowhittaker). In some experiments, as specified in the text, monocytes were cultured in macrophage serum-free medium (Gibco BRL). No exogenous cytokines were added to serum plus cultured monocytes or serum-free cultured monocytes. Monocytes were allowed to adhere for 3 days to tissue culture flasks that had been coated with polylysine (Sigma) before washing with HBSS to remove any nonadherent cells. In most experiments, monocyte-derived macrophages (MDM) were infected 10 to 14 days postisolation with equal amounts of p24 of the SF162 and R25 wild-type or nef mutant viruses. Infected macrophages were harvested 7 to 10 days p.i. by incubation of monolayers in phosphate-buffered saline (PBS; Biowhittaker) containing 10 mM EDTA for 3 to 5 min, and then cells were gently scraped and collected in PBS. Cells were washed in PBS, counted, and resuspended in the appropriate lysis buffer. CEMx174 cells (2 × 106; obtained from James Hoxie, University of Pennsylvania) chronically infected with SF2 wild-type, SF2 nef P73A, and SF2 Δ_nef_ were subjected to IVKAs as described below.
IVKA, reimmunoprecipitation, and Western blotting.
Nef was immunoprecipitated from infected or control MDM with rabbit anti-HIV Nef antisera (1:200) (64). PAK was immunoprecipitated with rabbit anti-PAK (N-20; 1:200); Hck was immunoprecipitated with rabbit anti-Hck (N30; 1:200) and Vav was immunoprecipitated with rabbit anti-Vav (H-211; 1:200) antibodies (Santa Cruz Biotechnology). Affinity-purified anti-PIX SH3 antibodies were the generous gift of Edward Manser and Louis Lim (Glaxo-ICMB Group, Crescent, Singapore). Nef and PAK IPs were subjected to IVKAs as previously described (54). Cells subjected to Hck immunoprecipitation were lysed in a solution containing 50 mM Tris-HCl, 50 mM NaCl, 1 mM EDTA, 10 mM MgCl2, and 1% Triton X-100 (8). Hck IPs were then subjected to IVKAs as described previously (8). For reimmunoprecipitation analyses, proteins were eluted from protein G-Sepharose by incubation in 0.5% sodium dodecyl sulfate (SDS) and heating at 70°C for 10 min. Ten volumes of cold KEB (54) were added, and the supernatant was collected. Before the second antibody was added, the eluted sample was precleared of immunoglobulins by incubation with protein G-Sepharose. Labeled immunoprecipitation analyses were run on SDS–10% polyacrylamide gel electrophoresis (SDS–10% PAGE) gels, dried onto Whatman 3MM filter paper, and subjected to phosphorimager analysis. For detection of Nef or Hck protein by Western blot, immunoprecipitation analyses were run on SDS–10% PAGE and blotted onto Hybond C-extra membranes (Amersham). Membranes were blocked with 5% milk–0.2% Tween 20 (Sigma) in PBS for 1 h at room temperature. Blocked membranes were incubated with anti-Nef (1:500) or anti-Hck (1:200) antibody for 1 to 2 h at room temperature. Membranes were washed with 0.2% Tween 20 in PBS. Bands were visualized with secondary antibodies coupled to horseradish peroxidase in conjunction with the ECL Western blotting kit (Amersham). For reprobing, blots were stripped by incubation in a solution containing 100 mM 2-mercaptoethanol, 2% SDS, and 62.5 mM Tris-HCl, pH 7.0, at 50°C for 30 min. They were then washed thoroughly in PBS–0.2% Tween 20 before blocking.
RESULTS
HIV-1 Nef fails to bind and activate Hck in infected human macrophages.
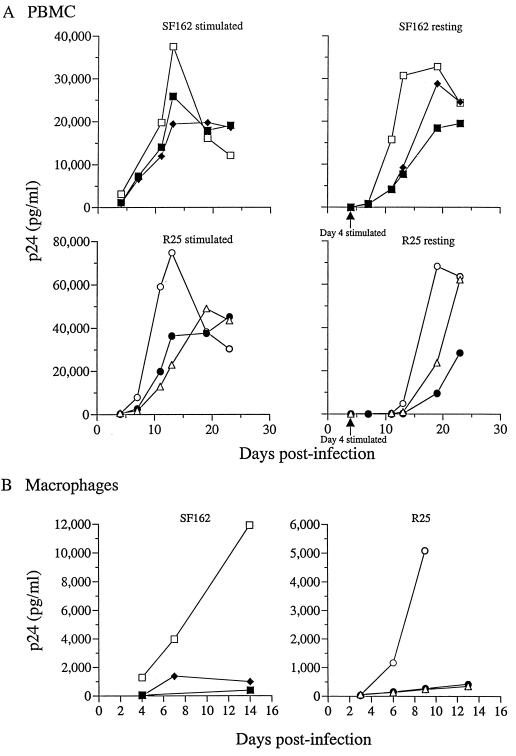
To assess the function of Nef in human macrophages, we generated Δ_nef_ versions of the macrophage-tropic SF162 (9) and R25 (30) viruses. R25 is a recombinant virus that contains regions of the SF162 envelope on the genomic background of the T-cell line-tropic isolate SF2. This construct permitted the study of the well-characterized T-cell line-tropic SF2 nef allele in the context of a macrophage intracellular milieu. Since allelic differences in the dependence on Nef for replication in primary cells have been reported (62), the replication kinetics of the wild-type and Δ_nef_ viruses generated were first examined in PBMCs that were infected immediately after isolation and then stimulated at 4 days p.i. (resting) or in cells that were infected after mitogenic stimulation (stimulated). The two SF162 Δ_nef_ clones replicated with kinetics that were, compared to those of the wild type, slightly delayed in stimulated PBMCs but significantly delayed in resting cells (Fig. 1A). In both cell culture systems, the SF162 Δ_nef_ viruses attained lower viral titers. The kinetics of replication of the R25 wild-type and nef mutant viruses in stimulated PBMCs were comparable to those of the corresponding SF162 viruses. In the resting cell assay, however, wild-type R25 replication was more attenuated than that of wild-type SF162 (Fig. 1A). Only at 7 to 8 days poststimulation was an appreciable amount of p24 detected with R25 wild-type virus. As expected, the delay in replication of the R25 Δ_nef_ viruses was even more pronounced. In MDM, both the SF162 Δ_nef_ and R25 Δ_nef_ viruses showed a highly attenuated replication phenotype (Fig. 1B). These findings are in agreement with previous reports (44, 60) and demonstrate, for these viruses, that Nef is required for efficient growth in the two major target cells of HIV-1.
FIG. 1.
(A) Replication kinetics of SF162 and R25 in stimulated and resting PBMCs. PBMCs were stimulated with IL-2 and PHA before infection (stimulated) or infected and then stimulated 4 days postinfection (resting). The amount of p24 capsid antigen released into the culture supernatant was quantitated every 3 to 4 days over periods of 14 and 25 days, respectively, for infected macrophages and PBMCs. □, SF162 wild type; ⧫, SF162 Δ_nef_ clone 9; ■, SF162 Δ_nef_ clone 10; ○, R25 wild type; ●, R25 Δ_nef_ clone 3; ▵, R25 Δ_nef_ clone 14. (B) Replication kinetics of SF162 and R25 in MDM. MDM were infected on day 10 postisolation with 40 ng of virus p24. □, SF162 wild type; ⧫, SF162 Δ_nef_ clone 9; ■, SF162 Δ_nef_ clone 10; ○, R25 wild type; ●, R25 Δ_nef_ clone 3; ▵, R25 Δ_nef_ clone 14.
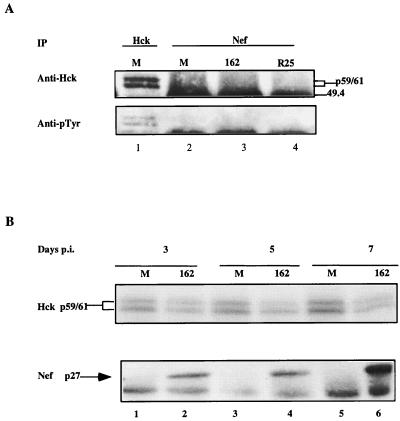
The binding and activation of Hck by Nef has been linked to its ability to enhance viral infectivity (52). We therefore wanted to determine if the Nef-defective phenotype in macrophages correlated with the ability of Nef to bind and activate Hck. Since Hck expression and activation has been reported to increase during macrophage differentiation (66), monocytes were cultured in both the presence and absence of serum. We reasoned that the background level of Hck activation should be reduced in the absence of serum components, thus enabling us to more readily see any effect of Nef on the state of Hck activation. A greatly reduced amount of Hck was present in macrophages grown in the absence of serum compared to those cultured in its presence (data not shown), consistent with the idea that the cells are in different states of differentiation. Coimmunoprecipitation analyses therefore were performed on infected macrophages grown in the presence of serum. When anti-Nef IPs from mock-infected and infected cells were subjected to anti-Hck immunoblotting, Hck was not detected in IPs from SF162- or R25-infected macrophages (Fig. 2A, lanes 3 and 4). The anti-Hck blot was stripped and probed with anti-phosphotyrosine antibodies to detect activated Hck. Although activated Hck protein was readily detected in Hck IPs from uninfected macrophages, no such protein was present in the anti-Nef IPs of infected macrophages (Fig. 2A, lanes 3 and 4). Reimmunoprecipitation of anti-Nef IPs with anti-Hck and anti-Tyr antibodies also failed to demonstrate any interaction of Nef with Hck (data not shown).
FIG. 2.
(A) MDM were grown and infected in medium with serum and harvested as described in Materials and Methods. Approximately 2 × 105 to 5 × 105 cells were used for each immunoprecipitation. Western analysis was performed with an anti-Hck antibody (Anti-Hck) (top row), and the blot was probed with anti-phosphotyrosine antibody (Anti-pTyr) (bottom row). Hck immunoprecipitated from mock-infected cells (lane 1) served as a control for the level of Hck protein present. The two forms of Hck protein are due to alternative translation initiation start sites giving rise to proteins of 59 and 61 kDa. The top edge of a band visible at 49.4 kDa is the immunoglobulin heavy chain. The amount of Nef protein immunoprecipitated was also determined and is shown in Fig. 3, lanes 5 and 6. Anti-Nef IPs from mock-infected cells (lane 2), SF162 wild type-infected cells (lane 3), and R25 wild type-infected cells (lane 4) are shown. (B) IVKAs were performed at 3, 5, and 7 days p.i. on Hck IPs from mock-infected (lanes 1, 3, and 5, respectively) and SF162 wild type-infected (lanes 2, 4, and 6, respectively) macrophages. The reactions were run on SDS–10% PAGE gels and dried on Whatman 3 MM filter paper, and nucleotide incorporation was detected by phosphorimager analysis. The levels of Nef expression were determined by immunoprecipitation and Western analyses.
The failure to detect Hck in Nef IPs could have been due to an interaction that occurs early in viral infection and is transient in nature. To assess this possibility, Nef immunoprecipitation and Western analyses, together with Hck IVKAs, were performed at various time points p.i. Nef protein was expressed at low levels 3 and 5 days p.i. (Fig. 2B, lanes 2 and 4), but expression increased by ∼10-fold at 7 days p.i. (Fig. 2B, lane 6). Despite the increase in Nef protein expression over time, we did not observe a parallel increase in Hck autophosphorylation which would have indicated its activation (Fig. 2B).
HIV-1 Nef activates NAK in infected human macrophages and PBMCs.
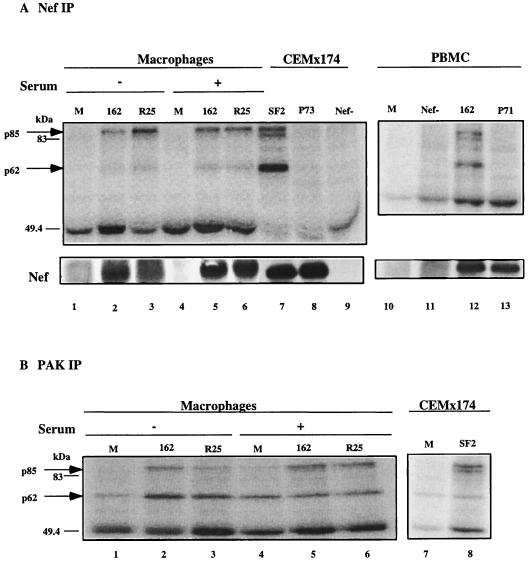
Binding and activation of the PAK-related kinase NAK by Nef was also shown to correlate with the enhancement of viral infectivity (56, 64). Since we could not detect an interaction of Nef with Hck, we looked for the ability of Nef to activate NAK in infected macrophages. The CEMx174 cell line chronically infected with SF2 served as a positive control. We performed IVKAs on Nef IPs from infected CEMx174 cells and from a portion of the same infected macrophages that were used for the Hck experiments whose results are shown in Fig. 2. In contrast to the apparent lack of a Nef-Hck interaction, Nef-mediated activation of NAK was readily detectable in infected human macrophages cultured in the absence or presence of serum. Two phosphorylated proteins were detected, one of 62 kDa and another of ∼85 kDa in cells infected with wild-type virus (Fig. 3A, lanes 2 and 3 and 5 through 7) but not in cells infected with virus lacking Nef (lane 9) or encoding the P73A (lane 8) mutation. This mutation was previously shown to abrogate Nef-mediated activation of NAK and was used as a control for nonspecific interactions (53, 64). As in the Hck experiments, we used the R25 recombinant virus to introduce the T-cell line-tropic SF2 wild-type nef allele into the macrophage intracellular environment. Interestingly, although both p62 and p85 species were present in SF2 Nef IPs from infected macrophages and the immortalized T-cell line CEMx174, the pattern of phosphorylation differed (Fig. 3A, compare lanes 6 and 7). NAK was hyperphosphorylated in the CEMx174 cells, while p85 was more highly phosphorylated in macrophages (Fig. 3A). Furthermore, as shown by the experiment results shown in Fig. 3, p85 appeared predominantly as a single band in the donor macrophages while in CEMx174 cells p85 appeared as a doublet. The single p85 band in infected macrophages corresponds to the slower-migrating band in infected CEMx174 cells. In some macrophage experiments, however, a fainter and faster-migrating band was also visible, giving rise to the doublet pattern seen in CEMx174 cells (data not shown).
FIG. 3.
(A) Nef IPs from macrophages (lanes 1 to 6) infected and cultured in the presence (+) or absence (−) of serum for 10 days were subjected to an IVKA. IVKAs of Nef IPs from the chronically infected T-cell line CEMx174 (lanes 7 to 9) were run side by side with the IPs from macrophages for comparison. Nef IVKAs were performed on infected PBMCs at day 12 p.i. (lanes 10 to 13). Results for mock-infected cells (lanes 1, 4, and 10) and cells infected with SF162 wild type (lanes 2, 5, and 12), SF162 Δ_nef_ (lane 11), SF162 Nef P71A (lane 13), R25 wild type (lanes 3 and 6), SF2 wild type (lane 7), SF2 Nef P73A (lane 8), or SF2 Δ_nef_ (lane 9) are shown. Underneath, the levels of Nef protein present in each IP as detected by Western analysis are shown. (B) PAK IPs from macrophages infected and cultured in the presence (+) or absence (−) of serum were subjected to IVKAs. Results for mock-infected cells (lanes 1 and 4), SF162 wild type-infected cells (lanes 2 and 5) and R25 wild type-infected cells (lanes 3 and 6) are shown. Results of IVKAs of PAK IPs from mock-infected (lane 7) and chronically SF2-infected (lane 8) CEMx174 cells are shown for comparison. The band at 49.4 is the immunoglobulin heavy chain.
To extend our observation of differential phosphorylation of NAK and p85 in primary macrophages and the transformed T-cell line to primary PBMCs, IVKAs on Nef IPs from infected PBMCs were performed. Nef expression together with NAK activation and phosphorylation of p85 was observed in SF162-infected PBMCs but not in PBMCs infected with SF162 Nef P71A that corresponds to the P73A mutation of SF2 Nef (Fig. 3A, lanes 12 and 13). The SF162 Nef P71A virus also failed to activate NAK in infected macrophages (data not shown). Similar to findings with the CEMx174 T-cell line, p85 appeared as a doublet. However, hyperphosphorylation of NAK was less apparent (Fig. 3A, compare lanes 7 and 12). Such variation may be related to the use of a natural infection culture system versus the use of a chronically infected T-cell line. Taken together, these results demonstrate that Nef can activate NAK in both primary macrophages and PBMCs, but the extent of activation might be cell type dependent.
The p85 phosphoprotein is a substrate of PAK.
We next performed IVKAs on PAK IPs obtained from infected or mock-infected macrophages to determine whether p85 is the substrate for PAK. In macrophages grown in the presence or absence of serum, a phosphorylated protein of 62 kDa representing PAK was observed in both mock-infected and infected cells. However, phosphorylation of an ∼85-kDa protein was detected only in infected cells (Fig. 3B, lanes 2, 3, 5, and 6). Phosphorylation of p85 was also observed in IVKAs performed on PAK IPs from CEMx174 cells chronically infected with SF2 wild type as compared to mock-infected cells (Fig. 3B, lanes 7 and 8). Again, the p85 band appears as a doublet in CEMx174 cells. These results demonstrated that p85 is a PAK-associated protein that is phosphorylated in the presence of Nef. In previous studies, the molecular mass of the PAK substrate in SF2- and simian immunodeficiency virus strain mac239-infected CEMx174 cells run on SDS–12% PAGE gels has been assigned a molecular mass of 72 kDa (54, 56). In this study we have assigned a molecular mass of 85 kDa to the PAK substrate. The apparent discrepancy might be related to the fact that different-percentage SDS-PAGE gels were used in these studies. In fact, as shown in Fig. 3, the PAK substrates from T cells and macrophages migrate to the same position on SDS–10% PAGE gels.
PIX complex is the NAK substrate in both HIV-1-infected T lymphocytes and macrophages.
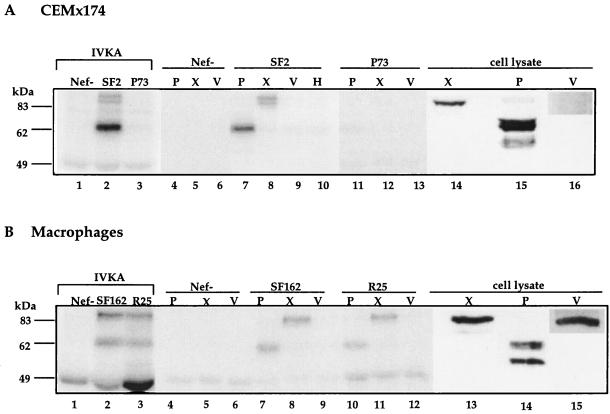
Mounting evidence suggests that NAK is related to the PAK family of kinases (36, 39, 47). The recent description of the PAK-interacting guanine nucleotide exchange factor (GEF), PIX (43), as an 85-kDa interacting partner of PAK prompted us to investigate whether the p85 NAK-associated phosphoprotein is related to PIX. As the 95-kDa proto-oncogene and GEF Vav has also been reported to interact with Nef (17), its presence within the Nef immune complex was also investigated. An IVKA was performed on Nef IPs from CEMx174 cells chronically infected with SF2 wild type, SF2 ΔNef, or SF2 Nef P73A. The phosphorylated protein complexes were eluted from protein G-Sepharose, precleared of released immunoglobulin and then subjected to immunoprecipitation with anti-PAK, anti-PIX, or anti-Vav antibodies. Reimmunoprecipitation with anti-Hck provided a negative control. As expected, no phosphorylated proteins were detected in Nef IPs or from SF2 ΔNef- or SF2 Nef P73A-infected cells (Fig. 4A, lanes 1 and 3, 4 through 6, and 11 through 13). In agreement with previous reports (47, 56), reimmunoprecipitation of SF2 Nef IPs with anti-PAK antibody brought down the expected phosphorylated species of 62 kDa (Fig. 4A, lane 7). The low level of phosphorylated PAK seen in IP after reimmunoprecipitation (lane 7) compared to the level observed in the IVKA (lane 2) is most likely due to the lower reactivity of the anti-rat PAK1 antibody with human PAK1 (47). Reimmunoprecipitation with the anti-Vav antibody did not result in the specific immunoprecipitation of any phosphorylated species as compared to the anti-Hck control (Fig. 4A, lanes 9 and 10). Significantly, the anti-PIX antibody precipitated two phosphorylated species at 85 kDa representing the PIX complex (Fig. 4A, lane 8). Interestingly, Western blot analysis with anti-PIX antibody on uninfected CEMx174 cell lysates run side by side with the IVKA gels detected only a single protein that comigrated with the lower band in the p85 complex (Fig. 4A, lane 13). Furthermore, Vav expression was barely detectable in this cell line (Fig. 4A, lane 16).
FIG. 4.
(A) Reimmunoprecipitation of PAK and PIX from Nef IPs of infected CEMx174 cells. Cell lysates from ∼7 × 106 cells were split into two portions (2.5 × 106 and 4.5 × 106 cells) and subjected to Nef immunoprecipitation and IVKA. One portion was reserved as the IVKA control for SF2 Δ_nef_ (Nef−) (lane 1), SF2 wild type (SF2) (lane 2) and SF2 Nef P73A (P73) (lane 3). The larger portion was subjected to reimmunoprecipitation with anti-PAK (P) (lanes 4, 7, and 11), anti-PIX (X) (lanes 5, 8, and 12), anti-Vav (V) (lanes 6, 9, and 13), or anti-Hck (H) (lane 10) antibodies as described in Materials and Methods. Western analysis with either anti-PAK (P) (lane 14), anti-PIX (X) (lane 15), or anti-Vav (V) (lane 16) antibody was performed on CEMx174 cell lysates. The band at 49.4 kDa is the immunoglobulin heavy chain. (B) Reimmunoprecipitation of PAK and PIX from infected primary macrophages was performed as described above except that ∼1 × 106 cells were used. Results of IVKAs performed as controls for SF162 Δ_nef_-infected (Nef−), SF162 wild-type (SF162), and R25 wild-type (R25) cells are shown in lanes 1, 2, and 3, respectively. Reimmunoprecipitation was performed with anti-PAK (lanes 4, 7, and 10), anti-PIX (lanes 5, 8, and 11), or anti-Vav (lanes 6, 9, and 12) antibodies. Western analysis with anti-PIX, anti-PAK, or anti-Vav antibody was performed on macrophage cell lysates, and results are shown in lanes 13, 14, and 15, respectively.
Nef IVKAs and reimmunoprecipitation analyses were also performed on infected macrophages. As expected, reimmunoprecipitation with anti-PAK antibody brought down the phosphorylated p62 protein (Fig. 4B, lanes 7 and 10) whereas reimmunoprecipitation with anti-Vav antibody was again negative (Fig. 4B, lanes 9 and 12). In Nef IPs from SF162 wild type- and R25 wild type-infected macrophages subjected to reimmunoprecipitation with anti-PIX antibody, a major phosphorylated species of 85 kDa was detected (Fig. 4B, lanes 8 and 11). A minor species just below the major species was also detected. In Western blot analyses of uninfected macrophage cell lysates, the anti-PIX antibody also reacted with a single major species that comigrated with the faint lower band in the p85 complex (Fig. 4B, lane 13). Although the anti-PAK1 antibody used in these studies is reported to be nonreactive with gamma and beta PAK, in both CEMx174 cells and macrophages the antibody recognizes two major species, of which the slower-migrating species comigrates with PAK detected in IVKAs (Fig. 4A and B lane 14). In contrast to what was observed in CEMx174 cells, Vav is abundantly expressed in primary macrophages (Fig. 4B, lane 15).
DISCUSSION
Using a prototypic macrophage-tropic HIV-1 virus and recombinant virus that expressed the nef allele of a T-cell line-tropic isolate, these studies confirmed the importance of Nef function for efficient viral growth in both primary T lymphocytes and MDM. Despite the reported high-affinity interaction of Nef with Src kinase Hck in vitro, we were unable to detect an interaction between these proteins in infected primary macrophages where both are abundantly expressed and in which a Nef phenotype is manifested. Furthermore, activation of Hck as measured by its phosphorylation state (Fig. 2) and ability to phosphorylate an exogenous substrate (enolase; data not shown) was not detected over background level. These findings therefore call into question the physiological relevance of a Nef-Hck interaction. Nevertheless, the possibility that Nef and Hck may interact transiently and/or at a very early step to facilitate viral infectivity, making such an event difficult to capture by the type of experiments performed in this study, cannot be excluded.
Under the same infection conditions, however, Nef interaction with and activation of the PAK-related kinase can be readily detected in primary macrophages as well as primary T lymphocytes (Fig. 3). Importantly, Nef-PAK interaction and activation result in the phosphorylation of a complex containing the PAK-interacting partner PIX (Fig. 4). PIX belongs to a new class of Rho-p21 GEFs that have been shown to bind with high affinity through their N-terminal SH3 domains to a conserved proline-rich sequence of PAK (42). It has been suggested that PIX binding is required for the translocation of PAK to focal complexes. The formation of focal complexes is regulated by the p21 GTP-binding proteins Cdc42 and Rac1 (GTPases) (2, 28, 40, 41, 43) and are important sites for the transduction of signals mediated through the actin cytoskeleton.
The PAK-PIX complex has been shown to contain additional proteins that include the adaptor Nck and a p95 PAK substrate whose identity has recently been revealed (61). Nck and PIX bind to distinct domains of PAK (6, 42), while p95 binds specifically to PIX (61). Several findings suggest that p95 is also present in the Nef-PAK-PIX complex in HIV-1-infected cells. First, similar to Manser et al. (42), who reported that several proteins coimmunoprecipitate with PAK and serve as substrates, we find that in Nef or PAK IPs from infected T lymphocytes, the PAK substrate runs as a doublet (Fig. 3B). Immunoblot analysis demonstrated that PIX is present as a single isoform in these cells and migrates as the faster protein within the substrate (Fig. 4A and B). The slower-migrating band, therefore, is likely to be p95. Second, data from reimmunoprecipitation analyses support the idea that p95 is tightly bound to PIX (Fig. 4). Protein-protein interactions between PAK and PIX could be efficiently disrupted, while under the same conditions, PIX and p95 binding could not be dissociated. Lastly, preliminary studies involving transient cotransfection of Nef, PIX, and p95 expression plasmids into 293T cells support the idea of the formation of a complex by these proteins (data not shown).
The mechanism by which Nef mediates PAK activation remains undefined. Recently, Vav has been reported to bind directly to HIV-1 Nef both in vitro and in vivo, resulting in the activation of Vav GEF activity (17). Since PAK activity is potentiated through binding to the active forms of Rac1 or Cdc42, and Vav bears GEF activity for these GTP-binding proteins, it is conceivable that Nef-mediated activation of Vav may stimulate PAK. However, Vav expression was barely detectable in the T-cell–B-cell hybrid CEMx174 cell line, and in primary macrophages where it is abundantly expressed, Vav was not the phosphorylated protein in the Nef immune complex (Fig. 5). Thus, the activation of PAK observed in infected macrophages and in CEMx174 cells is most likely not due to Vav activity. In this regard, it has been reported that PAK activity can be modulated as a consequence of physical interaction with PIX by mechanisms that require or are independent of exchange factor binding (14). Thus, it will be important to determine whether Rac1 or Cdc42 is recruited within the Nef macromolecular complex and if activation of PAK is mediated through PIX.
In vitro kinase experiments with Nef expressed in 293T (human embryonic kidney cells) (64) and COS cells (simian fibroblasts) (53) led to the conclusion that p85 is present only in the T-cell lineage (53, 54). We have now demonstrated that this PAK substrate, which is composed of PIX and p95, is also present in primary PBMCs and macrophages and that it can coimmunoprecipitate with Nef in infected cells. Nevertheless, Western analyses show that PIX is also present in 293T cells (data not shown) and COS cells (42). Thus, it is surprising that Nef-mediated activation only of PAK and not of PIX was observed in 293T and COS cells. It could be argued that different isoforms of PIX may be expressed in fibroblast and lymphoid or myeloid cells and that the Nef-PAK complex fails to interact with PIX present in 293T or COS cells or induce its phosphorylation. Alternatively, the Nef-PAK complex may require another essential component to recruit PIX that is specifically expressed in T lymphocytes and macrophages and which is absent from 293T and COS cells. Additional studies will be required to address these different possibilities. Since antibody against p95 is currently unavailable, its tissue and cell distribution is as yet unknown. However, Nef+ virions produced in 293T and COS cells still exhibit enhancement of infectivity properties (36, 44, 63), suggesting that PAK activation of another downstream target besides PIX or p95 might be involved in mediating this effect of Nef. As PAK has been shown to participate in multiple signaling pathways, it is not surprising that depending on upstream signals and specific downstream effectors, the binding and activation of this kinase by Nef may lead to pleiotropic effects.
Indeed, our results show that although both PIX and p95 serve as substrates for PAK that is activated as a result of binding to Nef in infected T lymphocytes, p95 appears to be preferentially phosphorylated by PAK in infected macrophages expressing the same nef allele (Fig. 3). These findings of differential phosphorylation of the PAK substrates further support the notion that the regulation of the downstream effector activity of PAK is tightly controlled and is likely to be cell type dependent. Nevertheless, the possibility that another kinase present in the Nef immune complex in infected macrophages specifically phosphorylates p95 cannot be excluded at present.
Nef is synthesized at high levels early in infection and yet is also packaged into the virion at the end of the infectious cycle. Furthermore, Nef exerts its functions in both the producer and target cells at various stages of the viral replication cycle. With the identification of downstream effectors of PAK that are recruited as a result of Nef binding and activation, their role in mediating Nef function can now be addressed. Whether overexpression of transdominant PIX or p95 mutant proteins, or peptides derived from these proteins, affects receptor down-modulation, early postentry events that would include uncoating and/or activation of gene transcription, and virion morphogenesis, can now be assessed. Identification of additional proteins in the Nef-PAK complex should further advance our understanding of Nef function at the molecular level. Although there are major challenges to performing molecular analyses in primary cells, attempts to interfere with the function of the PIX-p95 complex in such cell types will be necessary to understand how the activation and regulation of Nef effectors results in the enhanced ability of HIV-1 virions to infect and spread within the host.
ACKNOWLEDGMENTS
This work was funded by NIH grant RO1 AI38532.
We are grateful to Edward Manser and Louis Lim for the generous gift of anti-PIX antibody. We thank Lisa Chakrabarti and Edward Manser for critical comments on the manuscript, Leo Stamatatos and Lubbertus Mulder for helpful advice on macrophage purification and culture, and Thomas Kawano for technical assistance.
REFERENCES
- 1.Aiken C, Trono D. Nef stimulates human immunodeficiency virus type 1 proviral DNA synthesis. J Virol. 1995;69:5048–5056. doi: 10.1128/jvi.69.8.5048-5056.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagrodia S, Taylor S J, Creasy C L, Chernoff J, Cerione R A. Identification of a mouse p21Cdc42/Rac-activated kinase. J Biol Chem. 1995;270:22731–22737. doi: 10.1074/jbc.270.39.22731. [DOI] [PubMed] [Google Scholar]
- 3.Baur A S, Sass G, Laffert B, Willbold D, Cheng M C, Peterlin B M. The N-terminus of Nef from HIV-1/SIV associates with a protein complex containing Lck and a serine kinase. Immunity. 1997;6:283–291. doi: 10.1016/s1074-7613(00)80331-3. [DOI] [PubMed] [Google Scholar]
- 4.Baur A S, Sawai E T, Dazin P, Fantl W J, Cheng-Mayer C, Peterlin B M. HIV-1 Nef leads to inhibition or activation of T cells depending on its intracellular localization. Immunity. 1994;1:373–384. doi: 10.1016/1074-7613(94)90068-x. [DOI] [PubMed] [Google Scholar]
- 5.Bell I, Ashman C, Maughan J, Hooker E, Cook F, Reinhart T A. Association of simian immunodeficiency virus Nef with the T-cell receptor (TCR) ζ chain leads to TCR down-modulation. J Gen Virol. 1998;79:2717–2727. doi: 10.1099/0022-1317-79-11-2717. [DOI] [PubMed] [Google Scholar]
- 6.Bokoch G M, Wang Y, Bohl B P, Sells M A, Quilliam L A, Knaus U G. Interaction of the Nck adapter protein with p21-activated kinase (PAK1) J Biol Chem. 1996;271:25746–25749. doi: 10.1074/jbc.271.42.25746. [DOI] [PubMed] [Google Scholar]
- 7.Bresnahan P A, Yonemoto W, Ferrell S, Williams-Herman D, Geleziunas R, Greene W C. A dileucine motif in HIV-1 Nef acts as an internalization signal for CD4 downregulation and binds the AP-1 clathrin adaptor. Curr Biol. 1998;8:1235–1238. doi: 10.1016/s0960-9822(07)00517-9. [DOI] [PubMed] [Google Scholar]
- 8.Briggs S D, Sharkey M, Stevenson M, Smithgall T E. SH3-mediated Hck tyrosine kinase activation and fibroblast transformation by the Nef protein of HIV-1. J Biol Chem. 1997;272:17899–17902. doi: 10.1074/jbc.272.29.17899. [DOI] [PubMed] [Google Scholar]
- 9.Cheng-Mayer C, Quiroga M, Tung J W, Dina D, Levy J A. Viral determinants of human immunodeficiency virus type I T-cell and macrophage tropism, cytopathogenicity, and CD4 antigen modulation. J Virol. 1990;64:4390–4398. doi: 10.1128/jvi.64.9.4390-4398.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chowers M Y, Pandori M W, Spina C A, Richman D D, Guatelli J C. The growth advantage conferred by HIV-1 nef is determined at the level of viral DNA formation and is independent of CD4 downregulation. Virology. 1995;212:451–457. doi: 10.1006/viro.1995.1502. [DOI] [PubMed] [Google Scholar]
- 11.Chowers M Y, Spina C A, Kwoh T J, Fitch N J S, Richman D D, Guateli J C. Optimal infectivity in vitro of human immunodeficiency virus type 1 requires an intact nef gene. J Virol. 1994;68:2906–2914. doi: 10.1128/jvi.68.5.2906-2914.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collette Y, Dutartre H, Benziane A, Ramos-Morales F, Benarous R, Harris M, Olive D. Physical and functional interaction of Nef with Lck. J Biol Chem. 1996;271:6333–6341. doi: 10.1074/jbc.271.11.6333. [DOI] [PubMed] [Google Scholar]
- 13.Colotta F, Peri G, Villa A, Mantovani A. Rapid killing of actinomycin D-treated tumor cells by human mononuclear cells. I. Effectors belong to the monocyte-macrophage lineage. J Immunol. 1984;132:936–944. [PubMed] [Google Scholar]
- 14.Daniels R H, Zenke F T, Bokoch G M. α-PIX stimulates p21-activated kinase activity through exchange factor-dependent and -independent mechanisms. J Biol Chem. 1999;274:6047–6050. doi: 10.1074/jbc.274.10.6047. [DOI] [PubMed] [Google Scholar]
- 15.Deacon N J, Tsykin A, Solomon A, Smith K, Ludford-Menting M, Hooker K J, McPhee D A, Greenway A, Ellet L A, Chatfeld C, Lawson V A, Crowe S, Maerz A, Sonza S, Learment J, Sullivan J S, Cunningham A, Dwyer D, Dowton D, Mills J. Genomic structure of an attenuated quasi species from a blood transfusion donor and recipients. Science. 1995;270:988–991. doi: 10.1126/science.270.5238.988. [DOI] [PubMed] [Google Scholar]
- 16.de Ronde A, Klaver B, Keulen W, Smit L, Goudsmit J. Natural HIV-1 Nef accelerates virus replication in primary human lymphocytes. Virology. 1992;188:391–395. doi: 10.1016/0042-6822(92)90772-h. [DOI] [PubMed] [Google Scholar]
- 17.Fackler O, Luo W, Geyer M, Alberts A S, Peterlin B M. Presented at the Retrovirus Conference. N.Y: Cold Spring Harbor; 1999. [Google Scholar]
- 18.Garcia J V, Miller A D. Serine phosphorylation-independent downregulation of cell-surface CD4 by nef. Nature. 1991;350:508–511. doi: 10.1038/350508a0. [DOI] [PubMed] [Google Scholar]
- 19.Greenberg M E, Bronson S, Lock M, Neumann M, Pavlakis G N, Skowronski J. Co-localization of HIV-1 Nef with the AP-2 adaptor protein complex correlates with Nef-induced CD4 down-regulation. EMBO J. 1997;16:6964–6976. doi: 10.1093/emboj/16.23.6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greenway A L, Azad A, McPhee D A. Human immunodeficiency virus type 1 Nef protein inhibits activation pathways in peripheral blood mononuclear cells and T-cell lines. J Virol. 1995;69:1842–1850. doi: 10.1128/jvi.69.3.1842-1850.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grzesiek S, Bax A, Clore G M, Gronenborn A M, Hu J-S, Kaufman J, Palmer I, Stahl S, Wingfield P T. The solution structure of HIV-1 Nef reveals an unexpected fold and permits delineation of the binding surface for the SH3 domain of Hck tyrosine protein kinase. Nat Struct Biol. 1996;3:340–345. doi: 10.1038/nsb0496-340. [DOI] [PubMed] [Google Scholar]
- 22.Guy B, Kieny M P, Riviere Y, Le Peuch C, Dott K, Girard M, Montagnier L, Lecocq J P. HIV F/3′ orf encodes a phosphorylated GTP-binding protein resembling an oncogene product. Nature. 1987;330:266–269. doi: 10.1038/330266a0. [DOI] [PubMed] [Google Scholar]
- 23.Hanna Z, Kay D G, Rebai N, Guimond A, Jothy S, Jolicoeur P. Nef harbors a major determinant of pathogenicity for an AIDS-like disease induced by HIV-1 in transgenic mice. Cell. 1998;95:163–175. doi: 10.1016/s0092-8674(00)81748-1. [DOI] [PubMed] [Google Scholar]
- 24.Howe A Y, Jung J U, Desrosiers R C. Zeta chain of the T-cell receptor interacts with Nef of simian immunodeficiency virus and human immunodeficiency virus type 2. J Virol. 1998;72:9827–9834. doi: 10.1128/jvi.72.12.9827-9834.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iafrate J A, Bronson S, Skowronski J. Separable functions of Nef disrupt two aspects of T cell receptor machinery: CD4 expression and CD3 signalling. EMBO J. 1997;16:673–684. doi: 10.1093/emboj/16.4.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kestler H W I, Ringler D J, Mori K, Panicali D L, Sehgal P K, Daniel M D, Desrosiers R C. Importance of the nef gene for maintenance of high virus loads and for the development of AIDS. Cell. 1991;65:651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- 27.Kirchoff F, Geenough T C, Brettler D B, Sullivan J L, Desrosiers R C. Absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N Engl J Med. 1995;332:228–232. doi: 10.1056/NEJM199501263320405. [DOI] [PubMed] [Google Scholar]
- 28.Knaus U G, Morris S, Dong H, Chernoff J, Bokoch G M. Regulation of human leukocyte p21-activated kinases through G protein-coupled receptors. Science. 1995;269:221–223. doi: 10.1126/science.7618083. [DOI] [PubMed] [Google Scholar]
- 29.Koenig S, Gendelman J M, Orenstein J M, Dal Canto M, Pezeshkpour G H, Yungbluth M, Janotta F, Akasamit A, Martin M A, Fauci A S. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science. 1986;233:1089–1093. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- 30.Koito A, Harrowe G, Levy J A, Cheng-Mayer C. Functional role of the V1/V2 region of human immunodeficiency virus type 1 envelope glycoprotein gp120 in infection of primary macrophages and soluble CD4 neutralization. J Virol. 1994;68:2253–2259. doi: 10.1128/jvi.68.4.2253-2259.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lang S M, Iafrate A J, Stahl-Henning C, Kuhn E M, Nisslein T, Kaup F-J, Haupt M, Hunsmann G, Skowronski J, Kirchhoff F. Association of simian immunodeficiency virus Nef with cellular serine/threonine kinases is dispensable for the development of AIDS in rhesus macaques. Nat Med. 1997;3:860–865. doi: 10.1038/nm0897-860. [DOI] [PubMed] [Google Scholar]
- 32.Learmont J C, Geczy A F, Mills J, Ashton L J, Raynes-Greenow C H, Garsia R, Dyer W, McIntyre L, Oelrichs R B, Rhodes D I, Deacon N J, Sullivan J S. Immunologic and virologic status after 14 to 18 years of infection with an attenuated strain of HIV-1. N Engl J Med. 1999;340:1715–1722. doi: 10.1056/NEJM199906033402203. [DOI] [PubMed] [Google Scholar]
- 33.Lee C-H, Leung B, Lemmon M A, Zheng J, Cowburn D, Kuriyan J, Saksela K. A single amino acid in the SH3 domain of Hck determines its high affinity and specificity in binding to HIV-1 Nef protein. EMBO J. 1995;14:5006–5015. doi: 10.1002/j.1460-2075.1995.tb00183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le Gall S, Erdtmann L, Benichou S, Berlioz-Torrent C, Liu L, Benarous R, Heard J-M, Schwartz O. Nef interacts with the μ subunit of clathrin adaptor complexes and reveals a cryptic sorting signal in MHC I molecules. Immunity. 1998;8:483–495. doi: 10.1016/s1074-7613(00)80553-1. [DOI] [PubMed] [Google Scholar]
- 35.Lim L, Manser E, Leung T, Hall C. Regulation of phosphorylation pathways by p21 GTPases. Eur J Biochem. 1996;242:171–185. doi: 10.1111/j.1432-1033.1996.0171r.x. [DOI] [PubMed] [Google Scholar]
- 36.Lu X, Wu X, Plemenitas A, Yu H, Sawai E T, Abo A, Peterlin B M. CDC42 and Rac1 are implicated in the activation of the Nef-associated kinase and replication of HIV-1. Curr Biol. 1996;6:1677–1684. doi: 10.1016/s0960-9822(02)70792-6. [DOI] [PubMed] [Google Scholar]
- 37.Lu X, Yu H, Liu S-H, Brodsky F M, Peterlin B M. Interactions between HIV-1 Nef and vacuolar ATPase facilitate the internalization of CD4. Immunity. 1998;8:647–656. doi: 10.1016/s1074-7613(00)80569-5. [DOI] [PubMed] [Google Scholar]
- 38.Luo T, Livingston R A, Garcia J V. Infectivity enhancement by human immunodeficiency virus type 1 Nef is independent of its association with a cellular serine/threonine kinase. J Virol. 1997;71:9524–9530. doi: 10.1128/jvi.71.12.9524-9530.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manninen A, Hiipakkam M, Vihinen M, Lu W, Mayer B J, Saksela K. SH3-domain binding function of HIV-1 Nef is required for association with PAK-related kinase. Virology. 1998;250:273–282. doi: 10.1006/viro.1998.9381. [DOI] [PubMed] [Google Scholar]
- 40.Manser E, Chong C, Zhao Z, Leung T, Michael G, Hall C, Lim L. Molecular cloning of a new member of the p21Cdc42/Rac-activated kinase (PAK) family. J Biol Chem. 1995;270:25070–25078. doi: 10.1074/jbc.270.42.25070. [DOI] [PubMed] [Google Scholar]
- 41.Manser E, Leung T, Salihuddin H, Zhao Z, Lim L. A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature. 1994;367:40–46. doi: 10.1038/367040a0. [DOI] [PubMed] [Google Scholar]
- 42.Manser E, Loo T-H, Koh C-G, Zhao Z-S, Chen X-Q, Tan L, Tan I, Leung T, Lim L. PAK kinases are directly coupled to the PIX family of nucleotide exchange factors. Mol Cell. 1998;1:183–192. doi: 10.1016/s1097-2765(00)80019-2. [DOI] [PubMed] [Google Scholar]
- 43.Martin G A, Bollag G, McCormick F, Abo A. A novel serine kinase activated by rac1/CDC42Hs-dependent autophosphorylation is related to PAK65 and STE20. EMBO J. 1995;14:1970–1978. doi: 10.1002/j.1460-2075.1995.tb07189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller M D, Warmerdam M T, Gaston I, Greene W C, Feinberg M B. The human immunodeficiency virus-1 nef gene product: a positive factor for viral infection and replication in primary lymphocytes and macrophages. J Exp Med. 1994;179:101–113. doi: 10.1084/jem.179.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller M D, Warmerdam M T, Page K A, Feinberg M B, Greene W C. Expression of human immunodeficiency virus type I (HIV-1) nef gene during HIV-1 production increases progeny particle infectivity independently of gp120 or viral entry. J Virol. 1995;69:579–584. doi: 10.1128/jvi.69.1.579-584.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moarefi I, LaFevre-Bernt M, Sicheri F, Huse M, Lee C-H, Kuriyan J, Miller W T. Activation of the Src-family tyrosine kinase Hck by SH3 domain displacement. Nature. 1997;385:650–653. doi: 10.1038/385650a0. [DOI] [PubMed] [Google Scholar]
- 47.Nunn M F, Marsh J W. Human immunodeficiency virus type 1 Nef associates with a member of the p21-activated kinase family. J Virol. 1996;70:6157–6161. doi: 10.1128/jvi.70.9.6157-6161.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pandori M W, Fitch N J S, Craig H M, Richman D D, Spina C A, Guatelli J C. Producer-cell modification of human immunodeficiency virus type 1: Nef is a virion protein. J Virol. 1996;70:4283–4290. doi: 10.1128/jvi.70.7.4283-4290.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Piguet V, Chen Y-L, Mangasarian A, Foti M, Carpentier J-L, Trono D. Mechanism of Nef-induced CD4 endocytosis: Nef connects CD4 with the μ chain of adaptor complexes. EMBO J. 1998;17:2472–2481. doi: 10.1093/emboj/17.9.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Piguet V, Gu F, Foti M, Demaurex N, Gruenberg J, Carpentier J-L, Trono D. Nef-induced CD4 degradation: a diacidic-based motif in Nef functions as a lysosomal targeting signal through the binding of β-COP in endosomes. Cell. 1999;97:63–73. doi: 10.1016/s0092-8674(00)80715-1. [DOI] [PubMed] [Google Scholar]
- 51.Robert-Guroff M, Popovic M, Gartner S, Markham P, Gallo R C, Reitz M S. Structure and expression of tat-, rev-, and nef-specific transcripts of human immunodeficiency virus type 1 in infected lymphocytes and macrophages. J Virol. 1990;64:3391. doi: 10.1128/jvi.64.7.3391-3398.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saksela K, Cheng G, Baltimore D. Proline-rich (PxxP) motifs in HIV-1 Nef bind to SH3 domains of a subset of Src kinases and are required for the enhanced growth of Nef+ viruses but not for down-regulation of CD4. EMBO J. 1995;14:484–491. doi: 10.1002/j.1460-2075.1995.tb07024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sawai E, Baur A S, Peterlin B M, Levy J A, Cheng-Mayer C. A conserved domain and membrane targeting of Nef from HIV and SIV are required for association with a cellular serine kinase activity. J Biol Chem. 1995;270:15307–15314. doi: 10.1074/jbc.270.25.15307. [DOI] [PubMed] [Google Scholar]
- 54.Sawai E T, Baur A, Struble H, Peterlin B M, Levy J A, Cheng-Mayer C. Human immunodeficiency virus type 1 Nef associates with a cellular serine kinase in T lymphocytes. Proc Natl Acad Sci USA. 1994;91:1539–1543. doi: 10.1073/pnas.91.4.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sawai E T, Cheng-Mayer I C, Luciw P A. Nef and the Nef-associated kinase. Res Virol. 1997;148:47–52. doi: 10.1016/s0923-2516(97)81913-9. [DOI] [PubMed] [Google Scholar]
- 56.Sawai E T, Khan I H, Montbriand P, Cheng-Mayer C, Luciw P A. Activation of PAK by HIV and SIV Nef: importance for AIDS in rhesus macaques. Curr Biol. 1996;6:1519–1527. doi: 10.1016/s0960-9822(96)00757-9. [DOI] [PubMed] [Google Scholar]
- 57.Schwartz O, Marechal V, Danos O, Heard J-M. Human immunodeficiency virus type 1 Nef increases the efficiency of reverse transcription in the infected cell. J Virol. 1995;69:4053–4059. doi: 10.1128/jvi.69.7.4053-4059.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schwartz O, Marechal V, Le Gall S, Lemonnier F, Heard J M. Endocytosis of MHC-1 molecules is induced by HIV-1 Nef. Nat Med. 1996;2:338–342. doi: 10.1038/nm0396-338. [DOI] [PubMed] [Google Scholar]
- 59.Smith B L, Krushelnycky B W, Mochly R D, Berg P. The HIV Nef protein associates with protein kinase C theta. J Biol Chem. 1996;271:16753–16757. doi: 10.1074/jbc.271.28.16753. [DOI] [PubMed] [Google Scholar]
- 60.Spina C A, Kwoh T J, Chowers M Y, Guatelli J C, Richman D D. The importance of nef in the induction of human immunodeficiency virus type 1 replication from primary quiescent CD4 lymphocytes. J Exp Med. 1994;179:115–123. doi: 10.1084/jem.179.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Turner C E, Brown M C, Perrotta J A, Riedy M C, Nikolopoulos S N, McDonald A R, Bagrodia S, Thomas S, Leventhal P S. Paxillin LD4 motif binds PAK and PIX through a novel 95-kD ankyrin repeat, ARF-GAP protein: a role in cytoskeletal remodeling. J Cell Biol. 1999;145:851–863. doi: 10.1083/jcb.145.4.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Twerllinger E, Langhoff E, Gabuzda D, Zazopoulos E, Haseltine W. Allelic variation in the effects of the nef gene on replication of human immunodeficiency virus type I. Proc Natl Acad Sci USA. 1991;88:10971–10975. doi: 10.1073/pnas.88.23.10971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Welker R, Kottler H, Kalbitzer H R, Krausslich H-G. Human immunodeficiency virus type 1 Nef protein is incorporated into virus particles and specifically cleaved by the viral proteinase. Virology. 1996;219:228–236. doi: 10.1006/viro.1996.0240. [DOI] [PubMed] [Google Scholar]
- 64.Wiskerchen M, Cheng-Mayer C. HIV-1 Nef association with cellular serine kinase correlates with enhanced virion infectivity and efficient proviral DNA synthesis. Virology. 1996;224:292–301. doi: 10.1006/viro.1996.0531. [DOI] [PubMed] [Google Scholar]
- 65.Xu X-N, Laffert B, Screaton G R, Kraft M, Wolf D, Kolanus W, Mongkolsapay J, McMichael A J, Baur A S. Induction of fas ligand expression by HIV involves the interaction of Nef with the T cell receptor ζ chain. J Exp Med. 1999;189:1489–1496. doi: 10.1084/jem.189.9.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ziegler S F, Wilson C B, Perlmutter R M. Augmented expression of a myeloid-specific protein tyrosine kinase gene (hck) after macrophage activation. J Exp Med. 1988;168:1801–1810. doi: 10.1084/jem.168.5.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]