Lentivirus Nef Specifically Activates Pak2 (original) (raw)
Abstract
Nef proteins from human immunodeficiency virus type 1 (HIV-1) and simian immunodeficiency virus (SIV) have been found to associate with an active cellular serine/threonine kinase designated Nef-associated kinase (Nak). The exact identity of Nak remains controversial, with two recent studies indicating that Nak may be either Pak1 or Pak2. In this study, we investigated the hypothesis that such discrepancies arise from the use of different Nef alleles or different cell types by individual investigators. We first confirm that Pak2 but not Pak1 is cleaved by caspase 3 in vitro and then demonstrate that Nak is caspase 3 sensitive, regardless of Nef allele or cell type used. We tested nef alleles from three lentiviruses (HIV-1 SF2, HIV-1 NL4-3, and SIVmac239) and used multiple cell lines of myeloid, lymphoid, and nonhematopoietic origin to evaluate the identity of Nak. We demonstrate that ectopically expressed Pak2 can substitute for Nak, while ectopically expressed Pak1 cannot. We then show that Nef specifically mediates the robust activation of ectopically expressed Pak2, directly demonstrating that Nef regulates Pak2 activity and does not merely associate with activated Pak2. We report that most of the active Pak2 is found bound to Nef, although a fraction is not. In contrast, only a small amount of Nef is found associated with Pak2. We conclude that Nak is Pak2 and that Nef specifically mediates Pak2 activation in a low-abundance complex. These results will facilitate both the elucidation of the role of Nef in pathogenesis and the development of specific inhibitors of this highly conserved function of Nef.
The nef genes of human immunodeficiency virus (HIV) and simian immunodeficiency virus (SIV) are major determinants of the in vivo pathogenicity of these lentiviruses (8). Nef plays a crucial role in the maintenance of high virus load and subsequent development of AIDS in adult macaques infected with SIV (17) or HIV/SIV chimeric viruses (2, 21, 32). Consistent with an essential role for Nef in HIV pathogenesis, several long-term nonprogressors have been documented to be infected with _nef_-defective viruses (9, 20, 41, 53). The significance of Nef in viral pathogenesis has also been highlighted in studies using a SCID-hu mouse model of HIV infection (1, 16). Finally, transgenic mice expressing Nef have been shown to manifest a variety of hematological and immunological abnormalities (15, 25). These in vivo findings together suggest an important role for Nef in HIV and SIV replication and the development of AIDS.
The HIV and SIV nef genes encode a 27- to 34-kDa myristoylated phosphoprotein (29). In vitro studies have suggested a number of mechanisms by which Nef may enhance viral replication and pathogenesis in vivo. Nef downregulates cell surface levels of CD4 (3, 14, 34), the primary HIV and SIV receptor, suggesting possible roles for Nef in preventing superinfection and promoting efficient viral budding (4, 24, 39). Nef may also aid in immune evasion by mediating the downregulation of major histocompatibility complex class I surface expression (7, 46). Nef, moreover, enhances viral particle infectivity (6, 35, 45, 49) and is packaged into viral cores (23). Nef-mediated cytokine and chemokine production in T cells and macrophages, respectively, has also been suggested to promote viral replication and spread (50, 52). As the sequence diversity between nef isolates is second only to that of the env gene and different Nef isolates possess distinct functions (30), Nef may enhance viral replication in vivo by multiple mechanisms that may vary with cell type or allele expressed.
Nef tightly associates with a 62-kDa active protein kinase referred to as the Nef-associated kinase (Nak) (30, 42). We have shown that Nak association is isolate dependent and that Nak is expressed in a wide variety of cell types (30). The exact identity of Nak has remained elusive, with several lines of evidence suggesting that Nak belongs to the p21-activated kinase (Pak) family (27, 36, 43, 44). Two recent reports have identified Nak as either Pak2 (37) or Pak1 (11). Renkema et al. used Nef from HIV type 1 (HIV-1) NL4-3 (NefNL4-3) transiently expressed in 293T cells to identify Nak as Pak2 (37), while Fackler et al. expressed Nef from HIV-1 SF2 (NefSF2) in Jurkat cells to identify Nak as Pak1 (11). The latter group suggests that Nak may actually represent both of these Pak family members, with the specific interaction depending on the particular nef allele studied or the cell type used (11). The role of Nef in mediating Nak activation has also remained contentious. While some argue that Nef mediates Nak activation (27, 44), others suggest that Nef preferentially binds to already active Nak but does not mediate Nak activation (38).
It is possible that subtle differences in experimental systems have led different investigators to regard two distinct activities as Nak. Pak1 (65 kDa) and Pak2 (62 kDa) are highly homologous Pak family members with common regulatory mechanisms (22). In the inactive state, the regulatory regions of Paks interact with their catalytic domains and inhibit catalytic activity. During activation by GTP-bound Rac or CDC42, autoinhibition is relieved and the kinase achieves an open state in which the regulatory and catalytic domains no longer interact. This allows for autophosphorylation of a specific threonine residue in the catalytic domain and activates the kinase. In vitro, active Paks autophosphorylate on serine residues in the N-terminal regulatory region (22).
In this study we used three nef alleles and a variety of cell types to investigate the identity of Nak. We also addressed whether or not Nef mediates the activation of Nak. We conclude that HIV and SIV Nefs associate with Pak2 in hematopoietic and nonhematopoietic cell lines. We also show that ectopically expressed Pak2, but not Pak1, efficiently substitutes for Nak and provide direct evidence that Nef mediates the potent activation of Pak2 and does not activate Pak1. Last, we demonstrate that Nef-activated Pak2 is found mostly in a low-abundance Nef-Pak2 complex, although a clearly detectable fraction of Pak2 is free of Nef.
MATERIALS AND METHODS
Cell lines and culture conditions.
Human HuT-78, CEM, and Jurkat T cells as well as U937 and THP-1 human monocytic cells were transduced to express only the neomycin phosphotransferase gene (neo) or NefSF2 and neo as described previously (3, 28). Cells were cultured in RPMI 1640 medium supplemented with either 10% heat-inactivated (HuT-78) or non-heat-inactivated (CEM, Jurkat, THP-1, and U937) fetal bovine serum (HyClone, Logan, Utah), 50 IU of penicillin, 50 μg of streptomycin per ml, 2 mM l-glutamine, and 1 mM sodium pyruvate. Transduced cells were selected by the addition of G418 (1.5 mg/ml) to the culture medium. 293T cells were cultured in Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum, 50 IU of penicillin, 50 μg of streptomycin per ml, and 2 mM l-glutamine. Cell lines were maintained at 37°C in a humidified incubator with 5% CO2.
Plasmid expression constructs and transfections.
Plasmid DNA constructs of Pak1 and Pak2 hemagglutinin epitope (HA) tagged at the amino terminus (48) were used to subclone both Paks into pcDNAI/AMP (Invitrogen). Myc-tagged CDC42G12V cloned into pCMV6 was kindly provided by M. Cobb (13). Alleles encoding NefNL4-3 and SIVmac239 Nef (SNef) were also cloned into pcDNAI/AMP. Plasmids were cotransfected into 293T cells by the calcium phosphate method as previously described (10). Cells were harvested for analysis by Western blot and in vitro kinase assays 36 to 48 h after transfection.
Production and purification of recombinant caspase 3.
Bacteria expressing six-histidine-tagged caspase 3 were generously provided by X. Wang (26). Bacterial cultures were grown at 37°C to an optical density (_A_600) of 0.6. Isopropyl-1-thio-β-d-galactopyranoside was then added to a final concentration of 2 mM. After a 2-h induction, bacteria were pelleted and lysed by sonication in buffer A (20 mM HEPES-KOH [pH 7.4], 10 mM KCl, 1.0 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1 mM dithiothreitol, 0.1 mM phenylmethylsulfonyl fluoride). After centrifugation, supernatants were loaded on to a 2-ml nickel-Sepharose column (Qiagen) equilibrated with buffer A. The column was washed once with 10 ml of buffer A and once with 10 ml of buffer A containing 1 M NaCl and then rinsed with 10 ml of buffer A. Caspase 3 was then eluted (1-ml fractions) with buffer A containing 250 mM imidazole. Relative activity of fractions was determined using a colorimetric substrate (caspase 3 substrate I; Calbiochem).
Western blot analysis.
With the one exception indicated below, HIV and SIV Nef expression was determined with sheep polyclonal anti-HIV or anti-SIV Nef serum (1:4,000 or 1:2,000 dilution, respectively), followed by horseradish peroxidase (HRP)-conjugated anti-sheep immunoglobulin G (IgG; 1:20,000; Chemicon International). Anti-HA tag (Boehringer) and anti-Myc tag (Invitrogen) mouse monoclonal antibodies followed by HRP-conjugated anti-mouse IgG (1:10,000; Zymed) were used to detect Pak1 or Pak2 and CDC42G12v expression, respectively. Nef Western blot analyses following immunoprecipitation with sheep polyclonal antibodies were performed using EH1 mouse monoclonal anti-Nef antibody (1:2,500; kindly provided by J. Hoxie) followed by HRP-conjugated anti-mouse IgG as indicated above. HRP conjugates were visualized by enhanced chemiluminescence (Amersham).
In vitro kinase assay and caspase 3 treatment.
The assay for the cellular kinase activity associated with Nef was performed essentially as described by Sawai et al. (42), modified as previously described (31) by the addition of a 1 M MgCl2 wash prior to the kinase assay. Protein content of lysates was determined by the Bio-Rad protein assay. Immunoprecipitations were performed with anti-Nef (5 μl of sheep polyclonal antibody per 300 μg of lysate) or anti-HA (1.6 μg of mouse monoclonal antibody per 300 μg lysate) antibody. All lanes represent immunoprecipitates from 250 to 300 μg of cell lysate unless otherwise indicated. For caspase 3 treatment experiments, 900-μg aliquots of Nef-containing lysates were immunoprecipitated. Following the kinase assay, reactions were stopped with the addition EDTA to a final concentration of 33 mM. The kinase reaction mixtures were then placed on ice, and the protein A beads were washed twice with ice-cold caspase 3 buffer (50 mM HEPES [pH 7.5], 100 mM sodium chloride, 0.1% Triton X-100, 5 mM dithiothreitol, 20 mM sodium fluoride, 2 mM sodium vanadate, 20 mM β-glycerophosphate). During the last wash, the samples were divided into three aliquots. The immunoprecipitates were then mock treated, treated with caspase 3, or treated with caspase 3 plus the caspase 3 inhibitor ZVAD (40 μM) and incubated for 30 min at 37°C. Reactions were stopped by the addition of 1.5× Laemmli protein loading buffer, and the proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Dried gels were then exposed to a phosphorimager screen (Packard) or to film.
Immunodepletion experiments.
Nef/Pak2-transfected cell lysates (250 μg) were first immunoprecipitated with sheep anti-Nef or mouse anti-HA antibody as described above. Protein A beads were then pelleted, and supernatants were removed. After a second immunoprecipitation with the same antibody to ensure complete depletion of the immunoprecipitated protein, a third immunoprecipitation using the complementary antibody was carried out. All immunoprecipitations were carried out for 4 to 12 h at 4°C and kept on ice until used for the kinase assay. During the last wash step before the kinase assay, one-fifth of the protein A was removed and pelleted separately for direct elution into Laemmli protein loading buffer and subsequent Western blot analysis.
RESULTS
Caspase 3-catalyzed cleavage serves as a diagnostic test to differentiate between Pak1 and Pak2.
Despite the extensive similarity of Pak family members, only Pak2 has a consensus caspase 3 cleavage site. Pak2 is cleaved by caspase 3 into an N-terminal 27-kDa fragment that contains most of the kinase regulatory domain and a constitutively active C-terminal 34-kDa fragment containing the catalytic domain (Fig. 1A) (40, 51). Autophosphorylation of serine residues in the N-terminal regulatory region following activation increases the apparent molecular mass of the 27-kDa fragment generated during caspase 3 cleavage to approximately 32 kDa (51). We confirmed that in our experimental system caspase 3 specifically cleaves Pak2 by performing in vitro kinase assays and caspase 3 treatments as described in Materials and Methods on anti-HA immunoprecipitates from 293T cells coexpressing either HA-Pak1 or HA-Pak2 and constitutively active CDC42G12V (data not shown).
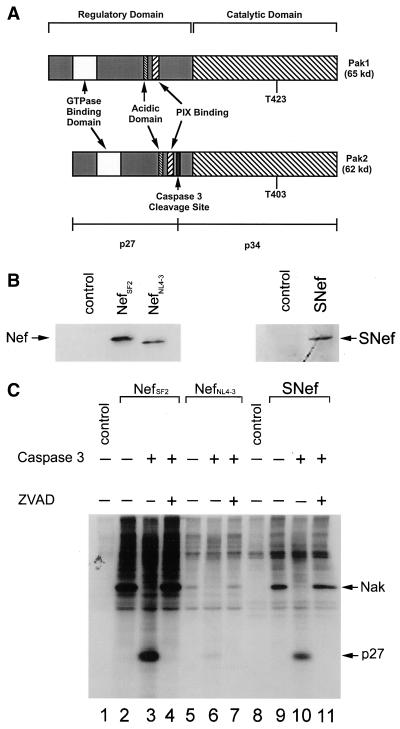
FIG. 1.
Caspase 3-mediated cleavage of Nak is nef allele independent. (A) Diagrammatic representation of human Pak1 and Pak2. The two highly homologous kinases contain an N-terminal regulatory region and a C-terminal catalytic domain. Threonine phosphorylation of either residue 423 in Pak1 or residue 403 in Pak2 activates the kinases and primes them for autophosphorylation in vitro. Particularly germane to this study is the caspase 3 cleavage site found in Pak2 but not Pak1. Cleavage at this site generates a 27-kDa N-terminal fragment and a 34-kDa C-terminal fragment. Serine phosphorylation of the 27-kDa N-terminal fragment increases its apparent molecular mass to 32 kDa (51). Also indicated are the p21 GTPase binding domain, the acidic domain, and the PIX binding domain. (B) Western blot analysis of HIV-1 and SIV Nef expression in lysates of transfected 293T cells. (C) In vitro kinase assay and caspase 3 treatments of the same lysates. Note that all three Nefs associate with a caspase 3-sensitive Nak. Positions of Nak and its 32-kDa cleavage product (p27) are indicated on the right. Lanes 1 and 8, samples from 293T cells transfected with control (empty) expression plasmid; lanes 2 to 4, 5 to 7, and 9 to 11, samples from 293T cells transfected with plasmids expressing NefSF2, NefNL4-3, and SNef, respectively. Samples in lanes 3, 6, and 10 were treated with caspase 3 following the kinase assay; samples in lanes 4, 7, and 11 were treated with caspase 3 in the presence of the inhibitor ZVAD.
The identity of Nak as Pak2 is nef allele independent.
Fackler et al. hypothesized that different nef alleles could bind preferentially to Pak1 or Pak2 (11). To test this hypothesis, we performed transient transfections of 293T cells with the HIV NL4-3 and SF2 nef alleles, as well as with the nef allele of SIVmac239, which is functional in vivo (17). As shown in Fig. 1B, all three Nef proteins were expressed in 293T cells as determined by Western blot analysis. In vitro protein kinase assays were then performed on Nef immunoprecipitates from extracts of cells expressing the different nef alleles. All three Nef proteins associated with Nak (Fig. 1C). Consistent with our previous results, NefNL4-3 associated with less Nak activity than NefSF2 or SNef (30). To distinguish whether the kinase autophosphorylation activity present in the immunoprecipitates corresponded to either Pak1 or Pak2, we investigated its sensitivity to caspase 3. In all three cases, the band corresponding to Nak was found to be susceptible to cleavage by caspase 3 under conditions that failed to cleave Pak1 (Fig. 1C). The specificity of the caspase 3 digestion of Nak was further confirmed by addition of ZVAD. Addition of this inhibitor completely blocked the cleavage of Nak associated with NefSF2, NefNL4-3, and SNef (Fig. 1C). In agreement with Renkema et al. (37), these results demonstrate that in 293T cells, HIV-1 and SIV Nefs associate with a caspase 3-sensitive kinase, suggesting that in all three cases Nak is Pak2, not Pak1.
The identity of NefSF2-associated Nak is cell type independent.
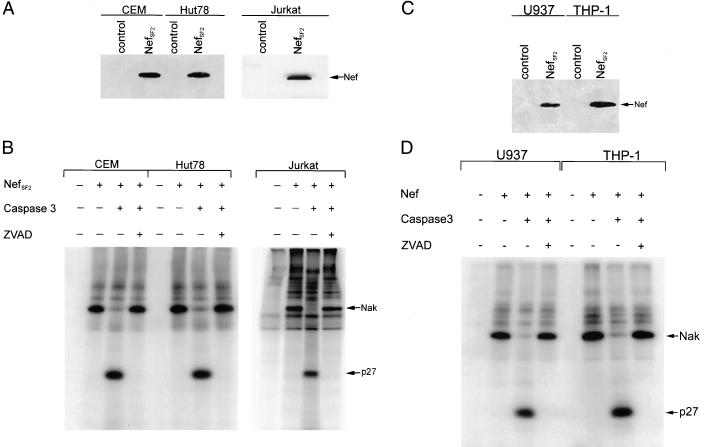
We also investigated the hypothesis that Nef binds Pak1 in a cell-type-dependent manner (11). To address this question, we stably transduced three T-cell lines (CEM, HuT-78, and Jurkat) and two monocytic cell lines (U937 and THP-1) with a retrovirus vector expressing NefSF2, the isolate used to identify Nak as Pak1. Expression of NefSF2 in these cell lines was analyzed by Western blotting (Fig. 2A and C). In vitro kinase assays confirmed that Nef associates with Nak in both T cells (Fig. 2B) and monocytic cells (Fig. 2D). To determine whether the active kinase bound to Nef was Pak1 or Pak2, we tested its sensitivity to caspase 3-mediated proteolysis. Our results show that Nak activity is susceptible to cleavage by caspase 3 in both cell types and that cleavage of Nak by caspase 3 is specifically inhibited by ZVAD. Thus, in three T-cell and two monocytic cell lines, the NefSF2-associated Nak activity is Pak2.
FIG. 2.
Nef binds Pak2 in human T cells and monocytic cells. (A) Western blot analysis for NefSF2 expression in the human T-cell lines CEM, HuT-78, and Jurkat. (B) In vitro kinase assay followed by caspase 3 cleavage of anti-Nef immunoprecipitates from cell lysates obtained from each cell line. In all three cases the Nak immunoprecipitated was sensitive to caspase 3 treatment, and this cleavage was inhibited by ZVAD. (C) Western blot analysis for NefSF2 expression in two human monocytic cell lines, U937 and THP-1. (D) In vitro kinase assay followed by caspase 3 treatment of Nef immunoprecipitates from cell lysates obtained from each cell line. Nak was found to be caspase 3 sensitive in both monocytic cell lines.
Ectopically expressed Pak2, but not Pak1, efficiently substitutes for Nak.
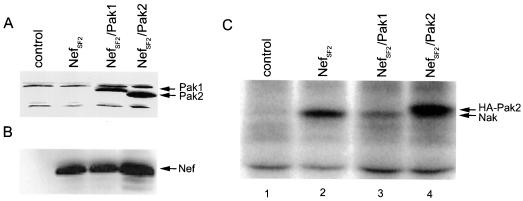
To demonstrate directly that Nak is Pak1 or Pak2, 293T cells were cotransfected with expression constructs for NefSF2 and either HA-Pak1 or HA-Pak2. Expression of Nef, HA-Pak1, and HA-Pak2 was confirmed by Western blot analysis (Fig. 3A and B). Cell extracts were immunoprecipitated with anti-Nef antibodies, and in vitro kinase assays were performed. Immunoprecipitates from extracts of cells expressing NefSF2 alone showed typical Nak activity (Fig. 3C, lane 2). Due to the presence of the HA tag, which slightly increases the size of the ectopically expressed Pak1 and Pak2, a shift in mobility would be expected if ectopic Pak1 or Pak2 could efficiently substitute for Nak. Coexpression of tagged Pak1 with NefSF2 did not produce a shift in the apparent mobility of Nak (Fig. 3C, lane 3). In contrast, in vitro kinase assays of extracts from cells coexpressing Pak2 and NefSF2 consistently showed high levels of phosphorylation of a protein that migrated with a mobility slightly higher than that of endogenous Nak and that corresponded to the mobility of activated HA-tagged Pak2 (Fig. 3C, lane 4). These results indicate that only ectopically expressed Pak2 can efficiently substitute for endogenous Nak.
FIG. 3.
Exogenous Pak2 substitutes for endogenous Nak. (A) Western blot analysis demonstrating expression of both Pak1 and Pak2 (arrows at right) in 293T cells transfected with NefSF2 and either Pak1 or Pak2, as indicated; (B) Western blot analysis demonstrating Nef expression in the transfected cells; (C) in vitro kinase assay of anti-Nef immunoprecipitates from the transfected cells. Positions of the phosphorylated HA-tagged Pak2 and endogenous Nak are indicated on the right in panel C.
NefSF2 activates Pak2 but not Pak1.
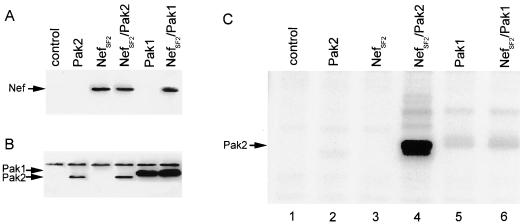
To determine whether Nef mediates the activation of Pak2 or simply binds to an already active pool of Pak2, we transfected cells with Pak expression constructs and either a control plasmid or a NefSF2 expression plasmid. Expression of Paks and NefSF2 was confirmed by Western blot analysis (Fig. 4A and B). Because we did not include an active form of p21 in the cotransfection, we were able to directly assess the effect of Nef on Pak activation by performing in vitro kinase assays on anti-HA immunoprecipitates. As expected, in the absence of Nef or CDC42V12G, no active Pak2 was detected (Fig. 4, lane 2). Also as expected, anti-HA immunoprecipitates of cells transfected with Nef alone did not show kinase activity (Fig. 4, lane 3). However, in the HA-tagged immunoprecipitates, the presence of Nef clearly caused robust (>90-fold) activation of HA-tagged Pak2 (Fig. 4C, lane 4). In contrast, the presence of Nef consistently had no significant effect on Pak1 activation (Fig. 4C, lanes 5 and 6). These results demonstrate the activation of Pak2 mediated by Nef, using an approach that eliminates the bias inherent to assaying only Pak2 activity bound to Nef. Moreover, the lack of activation of Pak1 by Nef further confirms that Nak is Pak2.
FIG. 4.
Specific activation of Pak2 by Nef. (A and B) Western blot analyses of Nef and Pak1/Pak2 expression, respectively, in transfected 293T cells; (C) in vitro kinase assays of anti-HA immunoprecipitates from the transfected cells. Note that Pak2 activity was clearly increased in the presence of NefSF2 (compare lanes 2 and 4), whereas Nef had no significant effect on basal Pak1 activity (lanes 5 and 6).
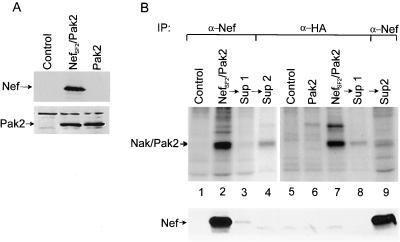
Nef-activated Pak2 is found mostly in a Nef-Pak2 complex, but a fraction of active Pak2 is not bound to Nef.
We sought to investigate if all of the Nef-activated Pak2 is found associated with Nef. 293T cells were transfected with control plasmids or cotransfected with NefSF2 and Pak2 expression plasmids, and expression was confirmed by Western blot analysis (Fig. 5A). Successive immunodepletions followed by in vitro kinase assays were then performed to determine if anti-Nef antibodies could deplete all of the active HA-Pak2 (Fig. 5B, top). In addition, during the last wash of the protein A beads before the in vitro kinase assay, one-fifth of the beads were removed. The presence of Nef in these samples was then assessed by Western blot analysis (Fig. 5B, bottom). In vitro kinase assays performed on anti-Nef immunoprecipitates from cotransfected cells produced not only the expected activity corresponding mostly to HA-Pak2 but also some endogenous Pak2 (Fig. 5B, lane 2, top). A second immunoprecipitation of the supernatant with anti-Nef antibodies confirmed the near-complete depletion of Nef-bound active Pak2 as well as Nef (Fig. 5B, lane 3, top and bottom). After anti-HA immunoprecipitation of the supernatant from the second immunodepletion with anti-Nef antibodies, a small amount of active HA-Pak2 remained, despite the depletion of the Nef bound active Pak2 (Fig. 5B, lane 4, top). These results indicate that active Pak2 is found both free of and bound to Nef, although the majority of the activity associates with Nef. Anti-HA immunoprecipitation of lysates from cells coexpressing Nef and Pak2 effectively depletes the HA-Pak2 activity (Fig. 5B, lanes 7 and 8). The anti-Nef immunoprecipitate of the resulting supernatant contains a residual amount of mostly endogenous Pak2 activity (Fig. 5B, lane 9), clearly showing that anti-HA immunoprecipitation depletes the majority of the Nef-associated Pak2 activity found in lysates of cotransfected cells. In contrast, all of the detectable Nef remained in the supernatant (Fig. 5B, lanes 7 to 9, bottom). Thus, relative to total Nef expression, the Nef-Pak2 complex is of extremely low abundance even when Pak2 is overexpressed, indicating the complex contains other limiting factors necessary for Pak2 association.
FIG. 5.
Nef-activated Pak2 is found mostly in a low-abundance Nef-Pak2 complex, but a fraction of active Pak2 is free. (A) Western blot analysis to confirm Nef (top) and Pak2 (bottom) expression in transfected 293T cells. (B) In vitro kinase assays (top) and Nef Western blot analysis (bottom) of immunoprecipitates (IP) from Nef/Pak2 expression plasmid (or control) transfections, using antibodies to Nef and HA, as indicated. Samples in lanes 2 to 4 represent successive immunoprecipitations of the same lysate, as do samples in lanes 7 to 9. In lanes 2 and 3 (top), immunoprecipitation with anti-Nef depletes all of the Nef-associated Pak2 and most, but not all, of the total active Pak2 (lane 4); also, depletion of HA-tagged Pak2 (lanes 7 and 8, top) depletes the majority of Nak but does not deplete endogenous Pak2 (lane 9). Interestingly, HA-Pak2 immunodepletion did not remove an appreciable amount of the total Nef in the lysates (lanes 7 to 9, bottom), indicating that only a small amount of Nef interacts with Pak2 even in the presence of overexpressed Pak2. Sup, supernatant.
DISCUSSION
In this report we address two controversial issues regarding Nak, the active cellular kinase associated with HIV and SIV Nef. Both Pak1 and Pak2 have been previously identified as Nak (11, 27, 36, 37), and the ability of Nef to activate Nak has remained unclear (27, 38, 44). Here, we provide evidence that Nak is Pak2 and that Nef mediates robust activation of Pak2 but not Pak1. The high degree of structural similarity among the Pak family members is likely the cause of discrepancies regarding the identity of Nak. Commercial antibodies used to identify Nak as Pak1 have indeed been demonstrated to be cross-reactive with Pak2 (37). Peptide inhibitors of Pak1 have also been used to identify Nak as Pak1 (11). While it has been argued, based on sequence comparison, that these peptides specifically inhibit Pak1, no experimental evidence to support this claim has been offered. Given the extensive homology of the Pak1 and Pak2 catalytic domains, it is likely that these peptide inhibitors effectively inhibit the kinase activity of both proteins. In light of the extensive homology between Pak1 and Pak2, moreover, it may well be that under certain in vitro conditions, both proteins associate with Nef. However, our results show that Nef does not mediate Pak1 activation in a cellular context, even when Pak1 is overexpressed.
An important difference between our results and those of Renkema et al. (37), who also identify Nak as Pak2, is that we did not require the coexpression of a constitutively active form of a p21 GTP binding protein such as Rac1 or CDC42 to observe robust Nak activity. The most likely explanation for this difference is allelic variation between NefNL4-3, used by Renkema et al., and the NefSF2 used for most of our experiments. We confirmed our previous observation that in the presence of similar amounts of Nef, NefSF2 produces significantly greater Nak activity than NefNL4-3 (30). Activation of endogenous Pak2 by CDC42G12V could have certainly facilitated the detection of active Pak2 in their experiments, but there is also the possibility that the activity observed was distinct from that found in the absence of activated p21. The work presented here, however, precludes that possibility. The absence of a constitutively active p21 in our experiments, furthermore, allowed us to directly demonstrate that Nef mediates Pak2 activation.
This work as well as other reports suggest a possible mechanism of Nef-mediated Pak2 activation. As Pak2 does not contain an SH3 domain, reports that the Nef SH3 binding domain is critical for Nak activity suggest that at least one other SH3 domain-containing protein might play an essential role in mediating Pak2 activation by Nef (19, 33). This hypothesis is consistent with our observation that the Nef-Pak2 complex is of low abundance even when Pak2 is overexpressed, suggesting that at least one other limiting cellular factor is important for Nef-mediated Pak activation. One study has indicated that the SH3 domain-containing protein may be Vav (12), while others propose that it is PIX (5). Both of these candidates have guanine nucleotide exchange activity toward p21 GTP binding proteins, which in the GTP-bound state could bind to the Pak2 GTPase binding domain and serve as the final effectors in Nef-mediated Pak2 activation. Thus, Nef may coordinate the formation of a complex comprising an SH3 domain-containing guanine nucleotide exchange factor, a p21, and Pak2. As we show here that a small fraction of active Pak2 is not bound to Nef, activated Pak2 may then leave the complex before inactivation occurs. To our knowledge, there is no report of an endogenous activator of full-length Pak2 that does not also activate Pak1. Thus, Nef must either specifically recruit Pak2 to the activation complex or exploit an undescribed endogenous activation factor that acts on only Pak2. Identification of remaining factors in the Nef-Pak2 complex as well as mutational analysis of Pak2 regulatory domains will further elucidate the molecular mechanism of Pak2 activation by Nef.
In vivo studies have clearly demonstrated the importance of Nef in virus replication and pathogenesis. The role of Pak2 in mediating Nef function, however, is not yet understood. Disease progression in macaques has correlated with reversion of Nef to a Nak activity-producing phenotype (18). A number of functions of Pak2 have been described, including the regulation of cellular motility and morphology, apoptosis, and mitogen-activated protein kinase signaling cascades (22, 47). Pak2 could potentially play an additional role in mediating Nef function. Elucidation of the actual role of Nef-mediated Pak2 activation will be facilitated by studies using specific inhibitors of Pak2 or cells with Pak2 null backgrounds. In this study, we focused on conclusively identifying the functionally defined Nak protein and investigating whether Nef mediates Nak activation. Based on our results, we propose that the 62-kDa active protein kinase that is bound to Nef be designated Pak2, rather than Nak or Pak1/2, and conclude that Nef specifically activates Pak2. This information will aid in future studies on the role of Nef-mediated activation of Pak2 in vivo and in the design of drugs that target this function of Nef.
ACKNOWLEDGMENTS
We thank X. Wang and Lili Li for the caspase 3-expressing bacteria and Deepak Nijhawan for help with the colorimetric caspase 3 activity assays. We also thank Melanie Cobb for the pCMV(Myc)CDC42G12V construct and R. Desrosiers for the SIVmac239 nef. The Hut78 and U937 cell lines and the NL4-3 nef allele were obtained from the AIDS Research and Reference Reagent Program. Monoclonal anti-Nef antibodies were a generous gift from J. Hoxie. We thank Richard Koup and Daniel Foster for continued support of this work.
This work was supported by National Institutes of Health grants AI-33331 and GM-60805 (J.V.G.) and GM54168 (J.C.) and by American Cancer Society grant CB-189 (J.C.). V.K.A. was supported in part by training grant CA-09082.
REFERENCES
- 1.Aldrovandi G M, Gao L, Bristol G, Zack J A. Regions of human immunodeficiency virus type 1 nef required for function in vivo. J Virol. 1998;72:7032–7039. doi: 10.1128/jvi.72.9.7032-7039.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander L, Du Z, Howe A Y, Czajak S, Desrosiers R C. Induction of AIDS in rhesus monkeys by a recombinant simian immunodeficiency virus expressing nef of human immunodeficiency virus type 1. J Virol. 1999;73:5814–5825. doi: 10.1128/jvi.73.7.5814-5825.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson S, Shugars D C, Swanstrom R, Garcia J V. Nef from primary isolates of human immunodeficiency virus type 1 suppresses surface CD4 expression in human and mouse T cells. J Virol. 1993;67:4923–4931. doi: 10.1128/jvi.67.8.4923-4931.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benson R E, Sanfridson A, Ottinger J S, Doyle C, Cullen B R. Downregulation of cell-surface CD4 expression by simian immunodeficiency virus Nef prevents viral super infection. J Exp Med. 1993;177:1561–1566. doi: 10.1084/jem.177.6.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown A, Wang X, Sawai E, Cheng-Mayer C. Activation of the PAK-related kinase by human immunodeficiency virus type 1 Nef in primary human peripheral blood lymphocytes and macrophages leads to phosphorylation of a PIX-p95 complex. J Virol. 1999;73:9899–9907. doi: 10.1128/jvi.73.12.9899-9907.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chowers M Y, Spina C A, Kwoh T J, Fitch N J, Richman D D, Guatelli J C. Optimal infectivity in vitro of human immunodeficiency virus type 1 requires an intact nef gene. J Virol. 1994;68:2906–2914. doi: 10.1128/jvi.68.5.2906-2914.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collins K L, Chen B K, Kalams S A, Walker B D, Baltimore D. HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature. 1998;391:397–401. doi: 10.1038/34929. [DOI] [PubMed] [Google Scholar]
- 8.Cullen B R. HIV-1 auxiliary proteins: making connections in a dying cell. Cell. 1998;93:685–692. doi: 10.1016/s0092-8674(00)81431-2. [DOI] [PubMed] [Google Scholar]
- 9.Deacon N J, Tsykin A, Solomon A, Smith K, Ludford-Menting M, Hooker D J, McPhee D A, Greenway A L, Ellett A, Chatfield C. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science. 1995;270:988–991. doi: 10.1126/science.270.5238.988. [DOI] [PubMed] [Google Scholar]
- 10.Evans J T, Kelly P F, O'Neill E, Garcia J V. Human cord blood CD34+CD38− cell transduction via lentivirus-based gene transfer vectors. Hum Gene Ther. 1999;10:1479–1489. doi: 10.1089/10430349950017815. [DOI] [PubMed] [Google Scholar]
- 11.Fackler O T, Lu X, Frost J A, Geyer M, Jiang B, Luo W, Abo A, Alberts A S, Peterlin B M. p21-activated kinase 1 plays a critical role in cellular activation by Nef. Mol Cell Biol. 2000;20:2619–2627. doi: 10.1128/mcb.20.7.2619-2627.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fackler O T, Luo W, Geyer M, Alberts A S, Peterlin B M. Activation of Vav by Nef induces cytoskeletal rearrangements and downstream effector functions. Mol Cell. 1999;3:729–739. doi: 10.1016/s1097-2765(01)80005-8. [DOI] [PubMed] [Google Scholar]
- 13.Frost J A, Xu S, Hutchison M R, Marcus S, Cobb M H. Actions of Rho family small G proteins and p21-activated protein kinases on mitogen-activated protein kinase family members. Mol Cell Biol. 1996;16:3707–3713. doi: 10.1128/mcb.16.7.3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia J V, Miller A D. Downregulation of cell surface CD4 by nef. Res Virol. 1992;143:52–55. doi: 10.1016/s0923-2516(06)80080-4. [DOI] [PubMed] [Google Scholar]
- 15.Hanna Z, Kay D G, Rebai N, Guimond A, Jothy S, Jolicoeur P. Nef harbors a major determinant of pathogenicity for an AIDS-like disease induced by HIV-1 in transgenic mice. Cell. 1998;95:163–175. doi: 10.1016/s0092-8674(00)81748-1. [DOI] [PubMed] [Google Scholar]
- 16.Jamieson B D, Aldrovandi G M, Planelles V, Jowett J B, Gao L, Bloch L M, Chen I S, Zack J A. Requirement of human immunodeficiency virus type 1 nef for in vivo replication and pathogenicity. J Virol. 1994;68:3478–3485. doi: 10.1128/jvi.68.6.3478-3485.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kestler H W, III, Ringler D J, Mori K, Panicali D L, Sehgal P K, Daniel M D, Desrosiers R C. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell. 1991;65:651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- 18.Khan I H, Sawai E T, Antonio E, Weber C J, Mandell C P, Montbriand P, Luciw P A. Role of the SH3-ligand domain of simian immunodeficiency virus Nef in interaction with Nef-associated kinase and simian AIDS in rhesus macaques. J Virol. 1998;72:5820–5830. doi: 10.1128/jvi.72.7.5820-5830.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khan I H, Sawai E T, Antonio E, Weber C J, Mandell C P, Montbriand P, Luciw P A. Role of the SH3-ligand domain of simian immunodeficiency virus Nef in interaction with Nef-associated kinase and simian AIDS in rhesus macaques. J Virol. 1998;72:5820–5830. doi: 10.1128/jvi.72.7.5820-5830.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirchhoff F, Greenough T C, Brettler D B, Sullivan J L, Desrosiers R C. Brief report: absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N Engl J Med. 1995;332:228–232. doi: 10.1056/NEJM199501263320405. [DOI] [PubMed] [Google Scholar]
- 21.Kirchhoff F, Munch J, Carl S, Stolte N, Matz-Rensing K, Fuchs D, Haaft P T, Heeney J L, Swigut T, Skowronski J, Stahl-Hennig C. The human immunodeficiency virus type 1 nef gene can to a large extent replace simian immunodeficiency virus nef in vivo. J Virol. 1999;73:8371–8383. doi: 10.1128/jvi.73.10.8371-8383.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knaus U G, Bokoch G M. The p21Rac/Cdc42-activated kinases (PAKs) Int J Biochem Cell Biol. 1998;30:857–862. doi: 10.1016/s1357-2725(98)00059-4. [DOI] [PubMed] [Google Scholar]
- 23.Kotov A, Zhou J, Flicker P, Aiken C. Association of Nef with the human immunodeficiency virus type 1 core. J Virol. 1999;73:8824–8830. doi: 10.1128/jvi.73.10.8824-8830.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lama J, Mangasarian A, Trono D. Cell-surface expression of CD4 reduces HIV-1 infectivity by blocking Env incorporation in a Nef- and Vpu-inhibitable manner. Curr Biol. 1999;9:622–631. doi: 10.1016/s0960-9822(99)80284-x. [DOI] [PubMed] [Google Scholar]
- 25.Lindemann D, Wilhelm R, Renard P, Althage A, Zinkernagel R, Mous J. Severe immunodeficiency associated with a human immunodeficiency virus 1 NEF/3′-long terminal repeat transgene. J Exp Med. 1994;179:797–807. doi: 10.1084/jem.179.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu X, Kim C N, Pohl J, Wang X. Purification and characterization of an interleukin-1beta-converting enzyme family protease that activates cysteine protease P32 (CPP32) J Biol Chem. 1996;271:13371–13376. [PubMed] [Google Scholar]
- 27.Lu X, Wu X, Plemenitas A, Yu H, Sawai E T, Abo A, Peterlin B M. CDC42 and Rac1 are implicated in the activation of the Nef-associated kinase and replication of HIV-1. Curr Biol. 1996;6:1677–1684. doi: 10.1016/s0960-9822(02)70792-6. [DOI] [PubMed] [Google Scholar]
- 28.Luo T, Douglas J L, Livingston R L, Garcia J V. Infectivity enhancement by HIV-1 Nef is dependent on the pathway of virus entry: implications for HIV-based gene transfer systems. Virology. 1998;241:224–233. doi: 10.1006/viro.1997.8966. [DOI] [PubMed] [Google Scholar]
- 29.Luo T, Downing J R, Garcia J V. Induction of phosphorylation of human immunodeficiency virus type 1 Nef and enhancement of CD4 downregulation by phorbol myristate acetate. J Virol. 1997;71:2535–2539. doi: 10.1128/jvi.71.3.2535-2539.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luo T, Garcia J V. The association of Nef with a cellular serine/threonine kinase and its enhancement of infectivity are viral isolate dependent. J Virol. 1996;70:6493–6496. doi: 10.1128/jvi.70.9.6493-6496.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luo T, Livingston R A, Garcia J V. Infectivity enhancement by human immunodeficiency virus type 1 Nef is independent of its association with a cellular serine/threonine kinase. J Virol. 1997;71:9524–9530. doi: 10.1128/jvi.71.12.9524-9530.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mandell C P, Reyes R A, Cho K, Sawai E T, Fang A L, Schmidt K A, Luciw P A. SIV/HIV Nef recombinant virus (SHIVnef) produces simian AIDS in rhesus macaques. Virology. 1999;265:235–251. doi: 10.1006/viro.1999.0051. [DOI] [PubMed] [Google Scholar]
- 33.Manninen A, Hiipakka M, Vihinen M, Lu W, Mayer B J, Saksela K. SH3-domain binding function of HIV-1 Nef is required for association with a PAK-related kinase. Virology. 1998;250:273–282. doi: 10.1006/viro.1998.9381. [DOI] [PubMed] [Google Scholar]
- 34.Mariani R, Skowronski J. CD4 down-regulation by nef alleles isolated from human immunodeficiency virus type 1-infected individuals. Proc Natl Acad Sci USA. 1993;90:5549–5553. doi: 10.1073/pnas.90.12.5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller M D, Warmerdam M T, Gaston I, Greene W C, Feinberg M B. The human immunodeficiency virus-1 nef gene product: a positive factor for viral infection and replication in primary lymphocytes and macrophages. J Exp Med. 1994;179:101–113. doi: 10.1084/jem.179.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nunn M F, Marsh J W. Human immunodeficiency virus type 1 Nef associates with a member of the p21-activated kinase family. J Virol. 1996;70:6157–6161. doi: 10.1128/jvi.70.9.6157-6161.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Renkema G H, Manninen A, Mann D A, Harris M, Saksela K. Identification of the Nef-associated kinase as p21-activated kinase 2. Curr Biol. 1999;9:1407–1410. doi: 10.1016/s0960-9822(00)80086-x. [DOI] [PubMed] [Google Scholar]
- 38.Renkema H G, Saksela K. Interactions of HIV-1 NEF with cellular signal transducing proteins. Front Biosci. 2000;5:D268–D283. doi: 10.2741/renkema. [DOI] [PubMed] [Google Scholar]
- 39.Ross T M, Oran A E, Cullen B R. Inhibition of HIV-1 progeny virion release by cell-surface CD4 is relieved by expression of the viral Nef protein. Curr Biol. 1999;9:613–621. doi: 10.1016/s0960-9822(99)80283-8. [DOI] [PubMed] [Google Scholar]
- 40.Rudel T, Bokoch G M. Membrane and morphological changes in apoptotic cells regulated by caspase-mediated activation of PAK2. Science. 1997;276:1571–1574. doi: 10.1126/science.276.5318.1571. [DOI] [PubMed] [Google Scholar]
- 41.Salvi R, Garbuglia A R, Di Caro A, Pulciani S, Montella F, Benedetto A. Grossly defective nef gene sequences in a human immunodeficiency virus type 1-seropositive long-term nonprogressor. J Virol. 1998;72:3646–3657. doi: 10.1128/jvi.72.5.3646-3657.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sawai E T, Baur A, Struble H, Peterlin B M, Levy J A, Cheng-Mayer C. Human immunodeficiency virus type 1 Nef associates with a cellular serine kinase in T lymphocytes. Proc Natl Acad Sci USA. 1994;91:1539–1543. doi: 10.1073/pnas.91.4.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sawai E T, Cheng-Mayer C, Luciw P A. Nef and the Nef-associated kinase. Res Virol. 1997;148:47–52. doi: 10.1016/s0923-2516(97)81913-9. [DOI] [PubMed] [Google Scholar]
- 44.Sawai E T, Khan I H, Montbriand P M, Peterlin B M, Cheng-Mayer C, Luciw P A. Activation of PAK by HIV and SIV Nef: importance for AIDS in rhesus macaques. Curr Biol. 1996;6:1519–1527. doi: 10.1016/s0960-9822(96)00757-9. [DOI] [PubMed] [Google Scholar]
- 45.Schwartz O, Marechal V, Danos O, Heard J M. Human immunodeficiency virus type 1 Nef increases the efficiency of reverse transcription in the infected cell. J Virol. 1995;69:4053–4059. doi: 10.1128/jvi.69.7.4053-4059.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwartz O, Marechal V, Le Gall S, Lemonnier F, Heard J M. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat Med. 1996;2:338–342. doi: 10.1038/nm0396-338. [DOI] [PubMed] [Google Scholar]
- 47.Sells M A, Chernoff J. Emerging from the Pak: the p21-activated protein kinase family. Trends Cell Biol. 2000;7:162–167. doi: 10.1016/S0962-8924(97)01003-9. [DOI] [PubMed] [Google Scholar]
- 48.Sells M A, Knaus U G, Bagrodia S, Ambrose D M, Bokoch G M, Chernoff J. Human p21-activated kinase (Pak1) regulates actin organization in mammalian cells. Curr Biol. 1997;7:202–210. doi: 10.1016/s0960-9822(97)70091-5. [DOI] [PubMed] [Google Scholar]
- 49.Spina C A, Kwoh T J, Chowers M Y, Guatelli J C, Richman D D. The importance of nef in the induction of human immunodeficiency virus type 1 replication from primary quiescent CD4 lymphocytes. J Exp Med. 1994;179:115–123. doi: 10.1084/jem.179.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Swingler S, Mann A, Jacque J, Brichacek B, Sasseville V G, Williams K, Lackner A A, Janoff E N, Wang R, Fisher D, Stevenson M. HIV-1 Nef mediates lymphocyte chemotaxis and activation by infected macrophages. Nat Med. 1999;5:997–103. doi: 10.1038/12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Walter B N, Huang Z, Jakobi R, Tuazon P T, Alnemri E S, Litwack G, Traugh J A. Cleavage and activation of p21-activated protein kinase gamma-PAK by CPP32 (caspase 3). Effects of autophosphorylation on activity. J Biol Chem. 1998;273:28733–28739. doi: 10.1074/jbc.273.44.28733. [DOI] [PubMed] [Google Scholar]
- 52.Wang J K, Kiyokawa E, Verdin E, Trono D. The Nef protein of HIV-1 associates with rafts and primes T cells for activation. Proc Natl Acad Sci USA. 2000;97:394–399. doi: 10.1073/pnas.97.1.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zaunders J J, Geczy A F, Dyer W B, McIntyre L B, Cooley M A, Ashton L J, Raynes-Greenow C H, Learmont J, Cooper D A, Sullivan J S. Effect of long-term infection with nef-defective attenuated HIV type 1 on CD4+ and CD8+ T lymphocytes: increased CD45RO+CD4+ T lymphocytes and limited activation of CD8+ T lymphocytes. AIDS Res Hum Retroviruses. 1999;15:1519–1527. doi: 10.1089/088922299309801. [DOI] [PubMed] [Google Scholar]