Involvement of clathrin and AP-2 in the trafficking of MHC class II molecules to antigen-processing compartments (original) (raw)
Abstract
Major histocompatibility complex class II (MHC-II) molecules are composed of two polymorphic chains, α and β, which assemble with an invariant chain, Ii, in the endoplasmic reticulum. The assembled MHC-II complexes are transported to the Golgi complex and then to late endosomes/lysosomes, where Ii is degraded and αβ dimers bind peptides derived from exogenous antigens. Targeting of MHC-II molecules to these compartments is mediated by two dileucine-based signals in the cytoplasmic domain of Ii. These signals bind in vitro to two adaptor protein (AP) complexes, AP-1 and AP-2, which are components of clathrin coats involved in vesicle formation and cargo sorting. The physiological roles of these proteins in MHC-II molecule trafficking, however, remain to be addressed. Here, we report the use of RNA interference to examine the involvement of clathrin and four AP complexes (AP-1, AP-2, AP-3, and AP-4) in MHC-II molecule trafficking in vivo. We found that depletion of clathrin or AP-2 caused >10-fold increases in Ii expression on the cell surface and a concomitant decrease in Ii localization to endosomal/lysosomal vesicles. In addition, depletion of clathrin or AP-2 delayed the degradation of Ii and reduced the surface expression of peptide-loaded αβ dimers. In contrast, depletion of AP-1, AP-3, or AP-4 had little or no effect. These findings demonstrate that clathrin and AP-2 participate in MHC-II molecule trafficking in vivo. Because AP-2 is only associated with the plasma membrane, these results also indicate that a significant pool of MHC-II molecules traffic to the endosomal–lysosomal system by means of the cell surface.
Keywords: invariant chain, adaptor proteins, endosomes, antigen presentation
A key event in the mounting of immune responses to extracellular pathogens is the interaction of specific T cell antigen receptors present on the surface of CD4+ T lymphocytes with antigenic peptides bound to major histocompatibility complex class II (MHC-II) molecules on the surface of antigen-presenting cells (1). The production of antigenic peptides and their loading onto MHC-II molecules occur within compartments of the endosomal–lysosomal system (2–4). Antigens enter these compartments by endocytosis from the extracellular medium and are subsequently degraded by lysosomal hydrolases. Some of the resulting peptides are captured by MHC-II molecules arriving from the biosynthetic pathway. This pathway begins with the synthesis of MHC-II chains in the endoplasmic reticulum (ER). MHC-II molecules are composed of two variable polypeptides, α and β, and an invariant polypeptide known as the invariant chain (Ii), which assemble into nonameric (αβIi)3 complexes soon after insertion into the ER membrane (5). These complexes exit the ER, pass through the Golgi cisternae, and reach the trans-Golgi network (TGN), from whence they are delivered to the endosomal–lysosomal system. Whereas the α and β chains are resistant to proteolysis, Ii undergoes proteolytic degradation to various fragments. One of these fragments, the ≈3-kDa class II-associated Ii-derived peptide (CLIP), remains bound to the peptide-binding groove on the surface of the αβ dimers. CLIP is eventually exchanged by antigenic peptides in a process that is facilitated by two MHC-like accessory molecules, HLA-DM and HLA-DO, which reside in the endosomal–lysosomal system (6). The peptide-loaded MHC-II molecules are eventually transported to the cell surface for presentation to T lymphocytes (7, 8).
Although the overall process outlined in the previous paragraph enjoys consensus among most investigators in the field, detailed aspects of this process remain matters of debate. Chief among these questions is the precise route followed by the bulk of newly synthesized (αβIi)3 complexes as they traffic from the TGN to the endosomal–lysosomal system. Whereas some studies have argued for the prevalence of a direct, intracellular transport from the TGN to either early or late endosomes and then to lysosomes (9, 10), other studies have favored an indirect pathway involving transport from the TGN to the plasma membrane, followed by endocytic delivery to early endosomes, late endosomes, and finally lysosomes (11–13). An important first step in understanding the pathway used to deliver (αβIi)3 complexes to the endosomal–lysosomal system is the identification of the molecular machinery involved. It is well known that the main signals that mediate the targeting of MHC-II molecules to the endocytic pathway are two dileucine-based motifs present in the cytosolic tail of Ii (14–16). These signals bind in vitro to two adaptor protein (AP) complexes named AP-1 and AP-2 (17, 18), which are components of clathrin coats associated with the TGN/endosomes and the plasma membrane, respectively (19). So, in principle, these interactions could be responsible for the sorting of (αβIi)3 complexes. However, the actual involvement of AP-1, AP-2, and clathrin for MHC-II trafficking in cells in vivo remains to be addressed experimentally.
We have taken advantage of the ability to ablate expression of specific AP complexes and clathrin by using the technique of RNA interference. Herein, we report that depletion of AP-2 or clathrin causes increased expression of Ii and decreased expression of peptide-loaded αβ dimers at the cell surface. Moreover, depletion of AP-2 or clathrin impairs Ii degradation. Our study thus demonstrates that AP-2 and clathrin are critical for MHC-II trafficking and supports the notion that a substantial population of (αβIi)3 complexes traffic to antigen-processing compartments by means of the plasma membrane.
Materials and Methods
Cell Culture. HeLa-CIITA cells were a gift from Philippe Pierre (Centre d'Immunologie de Marseille-Luminy, Marseille, France). Mel JuSo cells were gifts from Jacques Neefjes (Netherlands Cancer Institute, Amsterdam) and Markus Thali (University of Vermont, Burlington). All cells were grown in DMEM with 10% FCS, 100 units/ml penicillin, and 100 μg/ml streptomycin.
RNA-Mediated Interference. RNA interference of the clathrin heavy chain (CHC) and the μ-subunits of the AP complexes was performed by using small interfering RNA (siRNA) duplexes (Qiagen, Valencia, CA) to the following human target sequences: GGCAUCAAGUAUCGGAAGA for μ1A, GUGGAUGCCUUUCGGGUCA for μ2, GGAGAACAGUUCUUGCGGC for μ3A, GUCUCGUUUCACAGCUCUG for μ4, and UCCAAUUCGAAGACCAAUU for CHC. Cells were transfected twice at 72-h intervals with the siRNAs by using Oligofectamine (Invitrogen) according to the manufacturer's protocol. The cells were analyzed 48–72 h after the second round of transfection.
Antibodies. Antibodies were as follows: Ii, Pin-1 (ascites) (20) and M-B741 (Research Diagnostics, Flanders, NJ); MHC-II αβ, L243 (ascites) (21), DA6.147 (ascites) (22), and TDR31.1 (Research Diagnostics); transferrin receptor (TfR), DF 1513 (Sigma); MHC-I, W6/32 (ascites) (American Type Culture Collection); CHC, X22 (American Type Culture Collection) and clone 23 (BD Biosciences); μ1, rabbit RY/1 (a gift from L. Traub, University of Pittsburgh, Pittsburgh); μ2, rabbit anti-μ2 (23); μ3A, rabbit anti-μ3A (24); β4, rabbit anti-β4 (25); donkey Alexa Fluor 594-conjugated anti-mouse and anti-rabbit IgG (Molecular Probes); PE-conjugated anti-mouse IgG (Jackson ImmunoResearch); and horseradish peroxidase-conjugated anti-mouse and anti-rabbit IgG (Amersham Pharmacia Biosciences).
Immunoblotting. Cells were lysed by using 1% (vol/vol) Triton X-100 in 150 mM NaCl/50 mM Tris·HCl, pH 7.5 at 4°C. The cells were centrifuged at 13,000 rpm for 5 min to remove insoluble material, and Laemmli sample buffer was added to the supernatant. The samples were separated by using SDS/PAGE. The gels were then transferred to poly(vinylidene difluoride) membranes. Membranes were blocked in 5% milk/0.5% (wt/vol) Tween 20 in PBS for 1 h. Primary antibodies were added in the same milk mixture for 1 h. The membranes were washed four times with PBS-T [PBS plus 0.05% (vol/vol) Tween 20]. The secondary antibody was incubated for 1 h. The membranes were then washed, and proteins were detected by using ECL Plus (Amersham Pharmacia Biosciences).
Immunofluorescence Microscopy. For surface staining, cells were grown overnight on glass coverslips. They were subsequently incubated on ice for 1 h in the presence of primary antibody, washed for 5 min in ice-cold PBS, and incubated for an additional 1 h on ice in the presence of an appropriate secondary antibody in PBS. Cells were then washed in ice-cold PBS for 5 min, fixed with 4% (wt/vol) formaldehyde in PBS for 10 min, and mounted onto glass slides by using Fluoromount (Southern Biotechnology Associates). Permeabilized cells were fixed for 10 min at room temperature with 4% (wt/vol) formaldehyde in PBS. Successive incubations with primary and secondary antibodies [diluted in PBS and 0.1% (wt/vol) saponin] were carried out for 1 h at room temperature. The cells were washed with PBS after each incubation. The coverslips were mounted onto glass slides.
FACS Analysis. Cells were first washed by using PBS and subsequently harvested by adding 5 mM EDTA in PBS and incubating for 5 min at 37°C. The cells were then washed twice with PBS containing 3% wt/vol BSA. Primary antibody was added, and the cells incubated on ice for 1 h. The cells were washed three times with PBS/BSA, and phycoerythrin-conjugated secondary antibody was added for 1 h. Finally, the cells were washed and analyzed by using a three-color FACSCalibur flow cytometer equipped with cellquest pro software (Becton Dickinson).
Pulse–Chase Analysis. Cells were washed by using medium lacking methionine and cysteine and then starved in the same medium for 30 min at 37°C. The cells were then labeled by using medium containing 1.0 mCi of [35S]methionine-cysteine (1 Ci = 37 GBq) for 30 min at 37°C. Upon labeling, the cells were rapidly washed with complete medium and then incubated at 37°C for different times. In some experiments, the cells were preincubated for 4 h in 1 mM leupeptin, which was also contained in the pulse and chase media. Immunoprecipitations were performed as described in ref. 24.
Surface Biotinylation. Cells were first treated with siRNA as described previously. At 48 h after the second round of siRNA treatment, the cells were washed three times with ice-cold PBS, and the surface proteins were biotinylated by using EZ-link Sulfo-NHS-LC-LC-Biotin (0.3 mg/ml, Pierce) in PBS at 4°C for 30 min. The reaction was quenched by washing the cells with Tris-buffered saline three times, and the cells were harvested in 1% (vol/vol) Triton X-100/150 mM NaCl/50 mM Tris·HCl, pH 7.5. Immunoprecipitations were performed first with the Pin-1 antibody bound to protein A-Sepharose. The beads were then washed and resuspended in Laemmli sample buffer to elute the isolated proteins from the beads. The Laemmli sample buffer was then diluted out by using 0.2% (vol/vol) Triton X-100/150 mM NaCl/50 mM Tris·HCl, pH 7.5 to 1 ml and reimmunoprecipitated with the DA6.147 antibody. The samples were then separated by using SDS/PAGE and transferred to nitrocellulose, and biotinylated protein was detected by using streptavidin-conjugated horseradish peroxidase (Amersham Pharmacia Biosciences).
Cathepsin Detection. Cathepsin D activity was measured by using fluorescent peptide substrates obtained from Peptides International, following the protocol outlined in ref. 26. The cleaved substrate was detected by using a PerkinElmer Victor3 plate reader at 460 nm. The mock sample was set to 1, and the AP-2- and clathrin-depleted samples were displayed as a ratio relative to mock. Cathepsin K was detected by using the Cathepsin K Detection Assay kit from Sigma according to the manufacturer's protocol for adherent cells. The cells were visualized by using a TCS confocal microscope equipped with krypton, argon, and helium-neon lasers (Leica, Deerfield, IL). Images were acquired with the use of a 63× Plan-Apochromat objective (numerical aperture = 1.4) and the appropriate filter combination.
Results
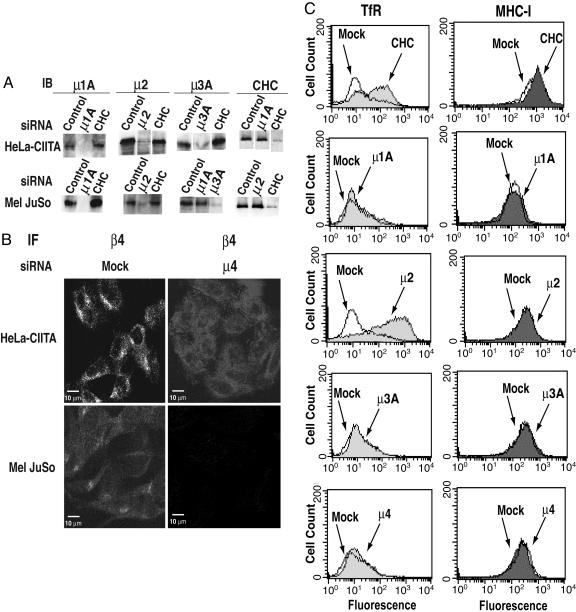
Elimination of the Expression of Clathrin or AP Complexes by RNA Interference. The expression of clathrin, AP-1, AP-2, AP-3, or AP-4 was ablated by treatment of cultured human cells with siRNA directed to the CHC or the μ1A, μ2, μ3A, or μ4 subunits of the respective complexes (μ1A and μ3A are the ubiquitously expressed subunit isoforms). Two MHC-II-expressing cell lines were used in these experiments: HeLa-CIITA and Mel JuSo. HeLa-CIITA is a derivative of the HeLa epithelial cell line that was induced to express MHC-II molecules endogenously by transfection with the transcriptional activator CIITA (27, 28), whereas Mel JuSo is a melanoma cell line that expresses MHC-II molecules naturally (29). Most experiments reported here were done by using both cells lines, with similar results. The levels of CHC, μ1A, μ2, or μ3A in these cells could be greatly reduced (>90%) by treatment with the corresponding siRNAs, as determined by immunoblot analysis (Fig. 1_A_). The depletion of each subunit was specific; only the protein targeted by siRNA was affected (Fig. 1_A_). The effect of siRNA treatment on μ4 levels could not be tested by immunoblotting, because there are no antibodies suitable for this technique. However, immunofluorescence microscopy of β4, another subunit of AP-4, revealed a loss of staining for this protein in the μ4-siRNA-treated cells (Fig. 1_B_). This observation is in line with previous reports that depletion of one subunit of an AP complex leads to degradation of other subunits or failure of the remaining subunits to associate with their target membranes (25, 30–32).
Fig. 1.
Efficient knockdown of CHC and AP-μ subunits in Mel JuSo and HeLa-CIITA cells. (A) HeLa-CIITA and Mel JuSo cells were mock-treated or treated with siRNA oligonucleotides for μ1A, μ2, μ3A, and CHC. Immunoblot (IB) analysis was performed by using antibodies to the proteins indicated. (B) Immunofluorescence (IF) microscopy of mock-treated and μ4-siRNA-treated HeLa-CIITA and Mel JuSo cells with rabbit antibody to β4. (C) FACS analysis of TfR and MHC-I surface expression in HeLa-CIITA cells that were either mock-treated (open curves) or treated with siRNAs directed to the indicated proteins (shaded curves). Primary antibodies to TfR (DF 1513) and MHC-I (W6/32) were used, followed by incubation with phycoerythrin-conjugated anti-mouse IgG.
In addition to testing for the absence of the target proteins in cells treated with siRNA, it was important to show that depletion of a particular AP had the expected effect on trafficking of known cargo proteins. To test this, HeLa-CIITA cells were depleted of clathrin or AP-2, and the surface levels of both the TfR and major histocompatibility complex class I (MHC-I) molecules were determined by FACS analysis (Fig. 1_C_). As expected, the surface levels of the TfR increased up to ≈15-fold in clathrin- or AP-2-depleted cells (Fig. 1_C_), consistent with the requirement of these proteins for rapid internalization of the TfR from the plasma membrane (32, 33, 34). In contrast, the levels of MHC-I, which is slowly internalized in a clathrin-independent fashion (35, 36), were virtually unchanged in the clathrin- or AP-2-deficient cells (Fig. 1_C_). Depletion of AP-1, AP-3, or AP-4 had no discernible effect on the surface levels of the TfR (Fig. 1_C_), which was also in agreement with previous observations made in mutant cells or in cells treated with antisense RNA (25, 30, 37). These experiments thus verified the fidelity of the siRNA approach used in our study.
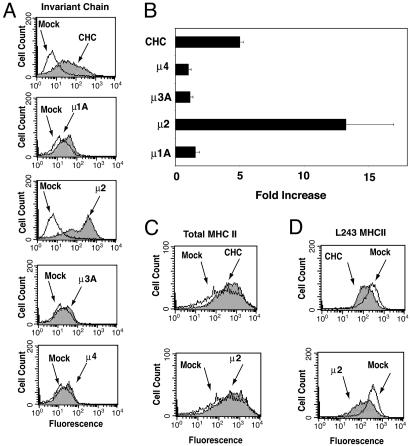
Increased Expression of Ii and Decreased Expression of Peptide-Loaded αβ Dimers on the Surface of Clathrin- or AP-2-Depleted Cells. Having established the siRNA approach to study the function of clathrin and the AP complexes, we examined their role in MHC-II trafficking. HeLa-CIITA cells were depleted of clathrin or each of the four AP complexes. FACS analyses showed that depletion of clathrin or AP-2 in HeLa-CIITA cells caused ≈4-fold or ≈13-fold increases, respectively, of the surface levels of Ii, whereas depletion of AP-1, AP-3, or AP-4 caused little or no increase (Fig. 2 A and B). The levels of total MHC-II αβ chains detected with the TDR31.1 antibody were unchanged or only slightly elevated upon depletion of clathrin or AP-2 (Fig. 2_C_). However, staining with the L243 antibody, which recognizes αβ dimers loaded with antigenic peptides, showed decreased staining in the clathrin- or AP-2-depleted cells (Fig. 2_D_). Similar results were obtained with Mel JuSo cells (data not shown). Thus, depletion of clathrin or AP-2 resulted in increased Ii and decreased peptide-loaded MHC-II at the cell surface, changes that are suggestive of decreased delivery of (αβIi)3 complexes to antigen-processing compartments.
Fig. 2.
FACS analysis of Ii and MHC II in HeLa-CIITA cells depleted of CHC and AP-μ subunits. (A) FACS analysis of Ii surface expression in HeLa-CIITA cells that were either mock-treated (open curves) or treated with siRNAs to the indicated proteins (shaded curves). Staining was with primary antibody to Ii (M-B741), followed by phycoerythrin-conjugated anti-mouse IgG. (B) Results (mean ± SD) from five separate experiments were quantified and graphed as a fold increase relative to the mock experiment. (C and D) HeLa-CIITA cells were either mock-treated (open curves) or treated with siRNAs to μ2orCHC (shaded curves). Cells were stained with either the TDR31.1 antibody (to measure total MHC-II molecule surface expression) (C) or the L243 antibody (to measure peptide-loaded MHC-II molecules) (D) and analyzed by FACS.
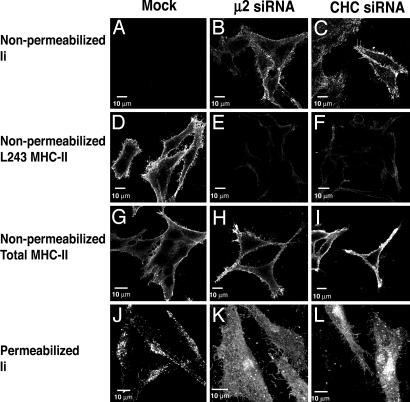
Alterations in the Cellular Distribution of Ii and Levels of Peptide-Loaded αβ Dimers in Cells Depleted of Clathrin or AP-2. To assess the impact of the depletion of clathrin or AP-2 on the overall cellular distribution of Ii and peptide-loaded αβ dimers, we performed immunofluorescence microscopy of nonpermeabilized and permeabilized HeLa-CIITA cells. In nonpermeabilized, mock-treated cells, there was a clear lack of surface staining using antibodies against Ii (Fig. 3_A_). In contrast, nonpermeabilized cells depleted of clathrin or AP-2 displayed strong surface staining of Ii (Fig. 3 B and C). These observations were in agreement with those made by FACS analysis (Fig. 2). In permeabilized, mock-treated cells, Ii was mainly localized to cytoplasmic vesicles (Fig. 3_J_) previously shown to correspond to late endosomes or lysosomes (35, 38). Clathrin- or AP-2-depleted, permeabilized cells, however, exhibited staining for Ii at the plasma membrane and comparatively reduced Ii staining of cytoplasmic vesicles (Fig. 3 K and L). These data indicated that the increased levels of Ii at the cell surface represented a redistribution of Ii to the plasma membrane, to the detriment of Ii localization to cytoplasmic vesicles. Also in agreement with the FACS analyses (Fig. 2), depletion of clathrin or AP-2 caused decreased expression of peptide-loaded αβ (Fig. 3 D_–_F), but not total αβ (Fig. 3 G_–_I), on the surface of nonpermeabilized cells. This finding was consistent with a failure of MHC-II molecules to acquire antigenic peptides in cells depleted of clathrin or AP-2.
Fig. 3.
Immunofluorescence microscopy of nonpermeabilized (A_–_I) and permeabilized (J_–_L) Mel JuSo cells that were either mock-treated or treated with siRNAs to μ2 or CHC. Cells were stained with antibodies to Ii (M-B741 or Pin-1), total MHC-II (TDR31.1), and peptide-loaded MHC-II (L243).
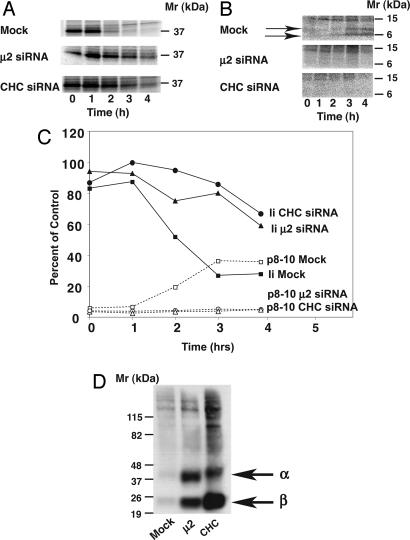
Decreased Ii Degradation in Clathrin- or AP-2-Deficient Cells. If the increase in surface expression of Ii in clathrin- or AP-2-depleted cells represents the missorting of a sizeable population of the protein, then there should be a decrease in Ii degradation in these cells. To analyze this possibility, we performed pulse–chase analyses in Mel JuSo cells. In mock-treated cells, newly synthesized Ii-containing complexes isolated with an antibody to Ii disappeared with a _t_1/2 of ≈2 h (Fig. 4 A and C). Depletion of clathrin or AP-2 resulted in a dramatic decrease in the rate of disappearance of Ii-containing complexes (Fig. 4 A and C); quantification of the results showed that 60% of the newly synthesized complexes could still be detected after 4 h of chase.
Fig. 4.
Effects of CHC and μ2 depletion on Ii degradation and surface expression of Ii-associated α and β chains. (A) Mock-, CHC-, or μ2-depleted Mel JuSo cells were pulse-labeled with [35S]methionine-cysteine for 20 min and chased for the times indicated. Immunoprecipitations were then performed by using the Pin-1 antibody to Ii and analyzed by SDS/PAGE. (B) Mock-, CHC-, or μ2-depleted Mel JuSo cells were incubated, pulse-labeled, and chased for the indicated times in the presence of 1 mM leupeptin. Pin-1 immunoprecipitations were again performed, and the samples were resolved by SDS/PAGE. The p8–10 Ii degradation intermediates are indicated. The positions of molecular mass markers (in kDa) are indicated. (C) The results from A and B were quantified by using imagequant software (Molecular Dynamics) as a percentage of protein at time = 0. (D) HeLa-CIITA cells were either mock-treated or treated with siRNAs to μ2 or CHC as before. The cells were then surface-biotinylated, lysed, and immunoprecipitated with the Pin-1 antibody to Ii. The redissolved immunoprecipitates were then reimmunoprecipitated with the DA6.147 antibody to MHC-II. Next, the samples were analyzed by SDS/PAGE, and biotinylated proteins were detected by using strepdavidin coupled to horseradish peroxidase. The positions of molecular mass markers and the α and β chains are indicated.
The degradation of Ii is catalyzed by lysosomal hydrolases as the (αβIi)3 complexes reach endosomal–lysosomal antigen-processing compartments. Accumulation of Ii degradation intermediates can be achieved by incubation of cells with the protease inhibitor leupeptin. In leupeptin-incubated, mock-treated cells, lower molecular weight species, most likely corresponding to the p8 and p10 fragments (39) of Ii, appeared at ≈3–4 h (Fig. 4 B and C). In contrast, in leupeptin-incubated cells depleted of clathrin or AP-2, there was no accumulation of lower molecular weight species (Fig. 4 B and C), consistent with decreased proteolytic processing of Ii.
Surface Ii Is Associated with αβ Chains. To determine whether the Ii that accumulated on the surface of clathrin- or AP-2-depleted cells was complexed with αβ chains, we labeled the cell surface proteins with biotin. Ii was isolated by immunoprecipitation, and the immunoprecipitates were redisolved in denaturing buffer. αβ chains were reimmunoprecipitated with specific antibodies and then analyzed for biotin labeling. As shown in Fig. 4_D_, in mock-treated cells, there was little to no biotinylated αβ associated with Ii at the cell surface. In contrast, in AP-2- or clathrin-depleted cells, there were large amounts of biotinylated αβ associated with Ii. Thus, the Ii that accumulates at the surface of clathrin- or AP-2-depleted cells is associated with αβ chains.
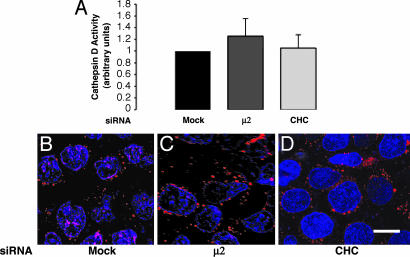
Lysosomal Enzymes Are Properly Localized and Functional in Clathrin or AP-2-Depleted Cells. One possible cause for the decreases in antigenic peptide acquisition and Ii degradation in clathrin- or AP-2-depleted cells was that the lysosomal proteases responsible for these processes were either nonfunctional or mislocalized. Two different assays, one biochemical and the other microscopic, were used to investigate this possibility. The biochemical assay consisted of measuring the activity of the lysosomal protease, cathepsin D, in lysates of mock-, clathrin-, and AP-2-depleted cells by using a specific fluorogenic substrate. We did not observe statistically significant differences among lysates from these cells (Fig. 5_A_). Similar results were obtained for two other lysosomal hydrolases, β-hexosaminidase and β-glucuronidase (data not shown). In the morphologic assay, we examined the activity and localization of another lysosomal protease, cathepsin K, by incubating mock-, clathrin-, or AP-2-depleted live HeLa-CIITA cells with a specific fluorogenic substrate. We observed that cathepsin K activity was contained within vesicles (i.e., endosomes and lysosomes) that were similar in intensity and appearance in the three cell samples (Fig. 5 B_–_D). Therefore, depletion of clathrin or AP-2 does not lead to decreased levels of or mislocalization of cathepsins D and K. The decreased acquisition of antigenic peptides and stabilization of Ii observed in clathrin- or AP-2-depleted cells are therefore not likely caused by missorting of lysosomal proteases but to the inability of (αβIi)3 complexes to access the degradative compartments.
Fig. 5.
HeLa-CIITA cells were either mock-treated or treated with siRNAs directed to CHC or μ2. (A) Cathepsin D activity was measured biochemically in cell lysates by using a fluorogenic substrate specific for this enzyme. Values represent the mean enzymatic activity (in arbitrary units) ± SD from three separate experiments done in triplicate. (B_–_D) Cathepsin K activity was visualized by fluorescence microscopy of live cells incubated with a specific fluorogenic substrate. The cells were also labeled with DAPI to stain the nuclei.
Discussion
The results of our experiments show that clathrin and AP-2 are required for efficient transport of MHC-II (αβIi)3 complexes to the endosomal–lysosomal system and for the expression of peptide-loaded αβ dimers on the cell surface of the nonhematopoietic HeLa-CIITA and Mel JuSo cell lines. In the absence of clathrin or AP-2, Ii accumulates at the cell surface in complex with αβ chains and fails to undergo proteolytic degradation. In addition, we observed that the surface expression of αβ chains recognized by the L243 monoclonal antibody is reduced. This antibody is known to recognize peptide-loaded αβ dimers, as well as some αβ haplotypes bound to Ii (21). The fact that L243 immunoreactivity decreases while total αβ immunoreactivity is unchanged and Ii immunoreactivity increases on the surface of clathrin- or AP-2-depleted cells is most consistent with L243 recognizing mainly peptide-loaded αβ dimers in the cells used in our study. The unchanged levels of total αβ immunoreactivity on the surface of the clathrin- or AP-2-depleted cells can be explained by the replacement of peptide-loaded αβ dimers by an equivalent amount of (αβIi)3 complexes. Depletion of clathrin or AP-2 does not cause significant changes in the contents and/or localization of cathepsins D and K, which participate in both the production of antigenic peptides and degradation of Ii. Thus, our results indicate that the effects of clathrin or AP-2 on MHC-II molecules are not due to defects in the degradative machinery but to impaired delivery of (αβIi)3 complexes to the degradative compartments.
Clathrin is the main constituent of protein coats associated with the TGN, the plasma membrane, and endosomes and could, in principle, play roles in the sorting of MHC-II molecules at any of these compartments. AP-2, however, is only associated with plasma membrane clathrin-coated pits and derived clathrin-coated vesicles. Therefore, clathrin must function in conjunction with AP-2 in the sorting of MHC-II molecules at the plasma membrane, indicating that a substantial population of (αβIi)3 complexes traffic by means of the plasma membrane en route to the endosomal–lysosomal system. The role of clathrin and AP complexes in MHC-II molecule trafficking was unclear before our work. Liu et al. (40) showed that a dominant-negative clathrin “hub” construct led to accumulation of (αβIi)3 complexes at the cell surface, but only when it was concurrently overexpressed with (αβIi)3 complexes in HeLa cells. In addition, Wang et al. (13) demonstrated that overexpression of a dominant-negative form of the clathrin-associated protein dynamin also resulted in cell surface accumulation of (αβIi)3 complexes. Both of these approaches, however, could have inhibited other pathways in addition to endocytosis. A study by Glickman et al. (41) on the role of clathrin and AP-1 in trafficking of (αβIi)3 complexes found that (αβIi)3 complexes did not colocalize with clathrin and AP-1 in buds and vesicles in the TGN of I-cell disease B lymphoblasts. Our results show consistency with all of these observations by demonstrating that clathrin is indeed involved in MHC-II trafficking, but in association with the endocytic AP-2 adaptor.
Our results also show that AP-1, AP-3, and AP-4 are largely dispensable for MHC-II trafficking. The results on AP-3 agree with previous observations that B cells from AP-3-deficient humans and mice exhibit normal trafficking of MHC-II molecules (42, 43). These results are also in line with the report that the dileucine-based signals of Ii do not interact with AP-3 (17). The role of the non-clathrin-associated AP-4 in MHC-II trafficking had not been addressed before. This complex, however, has been implicated in targeting to the basolateral plasma membrane of polarized epithelial cells (37), so it is not surprising that its depletion has no major impact in MHC-II trafficking.
Our observations provide additional evidence for previous proposals that many of the newly synthesized (αβIi)3 complexes appear at the cell surface en route to the endosomal–lysosomal system (11–13). To understand why the cell would use such an indirect route for the (αβIi)3 complexes, it is important to recall that the antigenic peptides destined to be loaded on the αβ chains derive from antigens that are endocytosed from the extracellular milieu. It may therefore be advantageous for the (αβIi)3 complexes and antigens to undergo degradation in the same compartments, such that the αβ dimers and antigenic peptides can come together as soon as they are formed. Indeed, it has been shown that peptide loading can occur not only in late endosomes and lysosomes, but also in early endosomes (4). Having the MHC-II complexes follow a path similar to that of the peptides may ensure that a wide array of antigenic peptides is represented in the MHC-II molecule display.
Author contributions: P.J.M. and J.S.B. designed research; P.J.M. and J.A.M. performed research; P.J.M., J.A.M., and J.S.B. analyzed data; and P.J.M. and J.S.B. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: MHC-II, MHC class II; MHC-I, MHC class I; AP, adaptor protein; TfR, transferrin receptor; TGN, trans-Golgi network; Ii, invariant chain; CHC, clathrin heavy chain; siRNA, small interfering RNA.
Note Added in Proof. An article by Dugast et al. (44) showing similar findings is in press.
References
- 1.Holling, T. M., Schooten, E. & Van Den Elsen, P. J. (2004) Hum. Immunol. 65**,** 282–290. [DOI] [PubMed] [Google Scholar]
- 2.Peters, P. J., Neefjes, J. J., Oorschot, V., Ploegh, H. L. & Geuze, H. J. (1991) Nature 349**,** 669–676. [DOI] [PubMed] [Google Scholar]
- 3.Amigorena, S., Webster, P., Drake, J., Newcomb, J., Cresswell, P. & Mellman, I. (1995) J. Exp. Med. 181**,** 1729–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castellino, F. & Germain, R. N. (1995) Immunity 2**,** 73–88. [DOI] [PubMed] [Google Scholar]
- 5.Cresswell, P. (1994) Annu. Rev. Immunol. 12**,** 259–293. [DOI] [PubMed] [Google Scholar]
- 6.Brocke, P., Garbi, N., Momburg, F. & Hammerling, G. J. (2002) Curr. Opin. Immunol. 14**,** 22–29. [DOI] [PubMed] [Google Scholar]
- 7.Wubbolts, R., Fernandez-Borja, M., Oomen, L., Verwoerd, D., Janssen, H., Calafat, J., Tulp, A., Dusseljee, S. & Neefjes, J. (1996) J. Cell Biol. 135**,** 611–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turley, S. J., Inaba, K., Garrett, W. S., Ebersold, M., Unternaehrer, J., Steinman, R. M. & Mellman, I. (2000) Science 288**,** 522–527. [DOI] [PubMed] [Google Scholar]
- 9.Benaroch, P., Yilla, M., Raposo, G., Ito, K., Miwa, K., Geuze, H. J. & Ploegh, H. L. (1995) EMBO J. 14**,** 37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davidson, H. W. (1999) J. Biol. Chem. 274**,** 27315–27322. [DOI] [PubMed] [Google Scholar]
- 11.Roche, P. A., Teletski, C. L., Stang, E., Bakke, O. & Long, E. O. (1993) Proc. Natl. Acad. Sci. USA 90**,** 8581–8585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hansen, H. J., Ong, G. L., Diril, H., Valdez, A., Roche, P. A., Griffiths, G. L., Goldenberg, D. M. & Mattes, M. J. (1996) Biochem. J. 320**,** 293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang, K., Peterson, P. A. & Karlsson, L. (1997) J. Biol. Chem. 272**,** 17055–17060. [DOI] [PubMed] [Google Scholar]
- 14.Bakke, O. & Dobberstein, B. (1990) Cell 63**,** 707–716. [DOI] [PubMed] [Google Scholar]
- 15.Pieters, J., Bakke, O. & Dobberstein, B. (1993) J. Cell Sci. 106**,** 831–846. [DOI] [PubMed] [Google Scholar]
- 16.Pond, L., Kuhn, L. A., Teyton, L., Schutze, M. P., Tainer, J. A., Jackson, M. R. & Peterson, P. A. (1995) J. Biol. Chem. 270**,** 19989–19997. [DOI] [PubMed] [Google Scholar]
- 17.Hofmann, M. W., Honing, S., Rodionov, D., Dobberstein, B., von Figura, K. & Bakke, O. (1999) J. Biol. Chem. 274**,** 36153–36158. [DOI] [PubMed] [Google Scholar]
- 18.Kongsvik, T. L., Honing, S., Bakke, O. & Rodionov, D. G. (2002) J. Biol. Chem. 277**,** 16484–16488. [DOI] [PubMed] [Google Scholar]
- 19.Kirchhausen, T. (1999) Annu. Rev. Cell Dev. Biol. 15**,** 705–732. [DOI] [PubMed] [Google Scholar]
- 20.Denzin, L. K. & Cresswell, P. (1995) Cell 82**,** 155–165. [DOI] [PubMed] [Google Scholar]
- 21.Shackelford, D., Lampson, L. & Strominger, J. (1981) J. Immunol. 127**,** 1403–1410. [PubMed] [Google Scholar]
- 22.Guy, K., Van Heyningen, V., Cohen, B. B., Deane, D. L. & Steel, C. M. (1982) Eur. J. Immunol. 12**,** 942–948. [DOI] [PubMed] [Google Scholar]
- 23.Aguilar, R. C., Ohno, H., Roche, K. C. & Bonifacino, J. S. (1997) J. Biol. Chem. 272**,** 2760–2766. [DOI] [PubMed] [Google Scholar]
- 24.Dell'Angelica, E. C., Ohno, H., Ooi, C. E., Rabinovich, E., Roche, K. W. & Bonifacino, J. S. (1997) EMBO J. 15**,** 917–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dell'Angelica, E. C., Shotelersuk, V., Aguilar, R. C., Gahl, W. A. & Bonifacino, J. S. (1999) Mol. Cell 3**,** 11–21. [DOI] [PubMed] [Google Scholar]
- 26.Yasuda, Y., Kageyama, T., Akamine, A., Shibata, M., Kominami, E., Uchiyama, Y. & Yamamoto, K. (1999) J. Biochem. (Tokyo) 125**,** 1137–1143. [DOI] [PubMed] [Google Scholar]
- 27.Steimle, V., Otten, L. A., Zufferey, M. & Mach, B. (1993) Cell 75**,** 135–146. [PubMed] [Google Scholar]
- 28.Chang, C. H. & Flavell, R. A. (1995) J. Exp. Med. 181**,** 765–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pieters, J., Horstmann, H., Bakke, O., Griffiths, G. & Lipp, J. (1991) J. Cell Biol. 115**,** 1213–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meyer, C., Zizioli, D., Lausmann, S., Eskelinen, E. L., Hamann, J., Saftig, P., von Figura, K. & Schu, P. (2000) EMBO J. 19**,** 2193–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peden, A. A., Rudge, R. E., Lui, W. W. & Robinson, M. S. (2002) J. Cell Biol. 156**,** 327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Motley, A., Bright, N. A., Seaman, M. N. & Robinson, M. S. (2003) J. Cell Biol. 162**,** 909–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hinrichsen, L., Harborth, J., Andrees, L., Weber, K. & Ungewickell, E. J. (2003) J. Biol. Chem. 278**,** 45160–45170. [DOI] [PubMed] [Google Scholar]
- 34.Huang, F., Khvorova, A., Marshall, W. & Sorkin, A. (2004) J. Biol. Chem. 279**,** 16657–16661. [DOI] [PubMed] [Google Scholar]
- 35.Neefjes, J. J., Stollorz, V., Peters, P. J., Geuze, H. J. & Ploegh, H. L. (1990) Cell 61**,** 171–183. [DOI] [PubMed] [Google Scholar]
- 36.Naslavsky, N., Weigert, R. & Donaldson, J. G. (2003) Mol. Biol. Cell 14**,** 417–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simmen, T., Honing, S., Icking, A., Tikkanen, R. & Hunziker, W. (2002) Nat. Cell Biol. 4**,** 154–159. [DOI] [PubMed] [Google Scholar]
- 38.Peters, P. J., Raposo, G., Neefjes, J. J., Oorschot, V., Leijendekker, R. L., Geuze, H. J. & Ploegh, H. L. (1995) J. Exp. Med. 182**,** 325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bania, J., Gatti, E., Lelouard, H., David, A., Cappello, F., Weber, E., Camosseto, V. & Pierre, P. (2003) Proc. Natl. Acad. Sci. USA 100**,** 6664–6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu, S. H., Marks, M. S. & Brodsky, F. M. (1998) J. Cell Biol. 140**,** 1023–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Glickman, J. N., Morton, P. A., Slot, J. W., Kornfeld, S., Geuze, H. J. (1996) J. Cell Biol. 132**,** 769–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Caplan, S., Dell'Angelica, E. C., Gahl, W. A. & Bonifacino, J. S. (2000) Immunol. Lett. 72**,** 113–117. [DOI] [PubMed] [Google Scholar]
- 43.Sevilla, L. M., Richter, S. S. & Miller, J. (2001) Cell Immunol. 210**,** 143–153. [DOI] [PubMed] [Google Scholar]
- 44.Dugast, M., Toussaint, H., Dousset, C. & Benaroch, P. (2005) J. Biol. Chem., in press. [DOI] [PubMed]