Interaction of HSP90 to N-WASP leads to activation and protection from proteasome-dependent degradation (original) (raw)
Abstract
Neural Wiskott–Aldrich syndrome protein (N-WASP) regulates reorganization of the actin cytoskeleton through activation of the Arp2/3 complex. Here, we show that heat shock protein 90 (HSP90) regulates N-WASP-induced actin polymerization in cooperation with phosphorylation of N-WASP. HSP90 binds directly to N-WASP, but binding alone does not affect the rate of N-WASP/Arp2/3 complex-induced in vitro actin polymerization. An Src family tyrosine kinase, v-Src, phosphorylates and activates N-WASP. HSP90 increases the phosphorylation of N-WASP by v-Src, leading to enhanced N-WASP-dependent actin polymerization. In addition, HSP90 protects phosphorylated and activated N-WASP from proteasome-dependent degradation, resulting in amplification of N-WASP-dependent actin polymerization. Association between HSP90 and N-WASP is increased in proportion to activation of N-WASP by phosphorylation. HSP90 is colocalized and associated with active N-WASP at podosomes in 3Y1/v-Src cells and at growing neurites in PC12 cells, whose actin structures are clearly inhibited by blocking the binding of HSP90 to N-WASP. These findings suggest that HSP90 induces efficient activation of N-WASP downstream of phosphorylation signal by Src family kinases and is critical for N-WASP-dependent podosome formation and neurite extension.
Keywords: HSP90, neurite, N-WASP, phosphorylation, podosome
Introduction
Wiskott–Aldrich syndrome protein (WASP) family members such as WASP, N-WASP, and WAVEs 1, 2, and 3 play essential roles in actin polymerization through activation of the actin-related protein 2/3 (Arp2/3) complex (Miki et al, 1998; Machesky et al, 1999; Rohatgi et al, 1999). In particular, N-WASP-induced cortical actin polymerization is involved in the formation of filopodia (Miki et al, 1998; Svitkina et al, 2003).
We have shown that N-WASP is critically involved in adhesive podosome formation in _v_-_src_-transformed 3Y1 cells (3Y1/v-Src cells). Overexpression of dominant-negative mutants of N-WASP that are incapable of activating the Arp2/3 complex suppressed podosome formation in these cells (Mizutani et al, 2002), indicating that N-WASP-induced actin polymerization is essential for podosome formation in 3Y1/v-Src cells. However, the regulation of N-WASP downstream of v-Src is not clear.
N-WASP exists in an inactive and closed conformation formed by intramolecular interactions until upstream signals are activated. In response to extracellular stimuli, the VCA region of N-WASP, the region essential for activation of the Arp2/3 complex, is exposed, and actin nucleation is initiated. Direct binding of Cdc42, PIP2 or proteins containing SH3 domain to N-WASP releases the autoinhibitory conformation of N-WASP (Carlier et al, 2000; Rohatgi et al, 2000, 2001). Src family tyrosine kinases such as Hck and Fyn have been found to phosphorylate and activate WASP and N-WASP. The respective phospho-mimicking mutants induce neurite extension in PC12 cells and filopodia formation in primary macrophages (Cory et al, 2002; Suetsugu et al, 2002; Torres and Rosen, 2003). However, phosphorylation of N-WASP alone was not sufficient for full activation of N-WASP for actin polymerization, at least in vitro (Suetsugu et al, 2002), which suggests the possibility that other activation mechanisms in conjunction with phosphorylation are necessary for full activation of WASP family proteins. Phosphorylation of N-WASP by Fyn induces cytosolic localization of nuclear N-WASP, which is also required for expression of HSP90 (Suetsugu and Takenawa, 2003). HSP90 is a molecular chaperone protein that is known to regulate the catalytic activity, conformational maturation, and intracellular transport of a variety of proteins and thus contributes to cell growth and survival (Mayer and Bukau, 1999; Neckers, 2002). The functional relation between phosphorylated N-WASP and HSP90 is unclear.
We found that HSP90 binds to N-WASP and that this binding is dependent on the activation of N-WASP by phosphorylation. HSP90 stimulates the phosphorylation of N-WASP by Src family tyrosine kinases, leading to N-WASP-induced actin polymerization. Importantly, HSP90 attenuates proteasome-dependent degradation of active N-WASP, which amplifies activation of N-WASP and induces podosome formation and neurite extension.
Results
HSP90 associates with N-WASP
To investigate the potential interaction between HSP90 and N-WASP, we first performed coimmunoprecipitation experiments of the two endogenous proteins. As we know that endogenous N-WASP exists exclusively in podosomes in 3Y1 rat fibroblasts transformed with v-src (3Y1/v-Src) (Mizutani et al, 2002), lysates of 3Y1/v-Src cells were immunoprecipitated with antibody against N-WASP. As shown in Figure 1A, N-WASP was coimmunoprecipitated with HSP90. Immunoprecipitation with anti-HSP90 antibody coimmunoprecipitated N-WASP, indicating a specific association of endogenous HSP90 and N-WASP in 3Y1/v-Src cells. Interestingly, HSP90 bound little to N-WASP in parental 3Y1 cells, suggesting that the association between HSP90 and N-WASP occurs predominantly downstream of v-Src.
Figure 1.
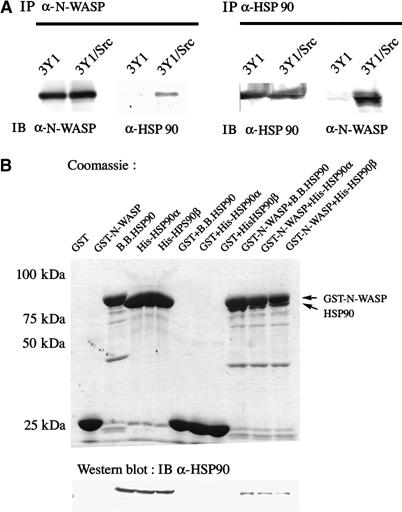
HSP90 associates with N-WASP. (A) Specific association between HSP90 and N-WASP in 3Y1/v-Src cells. N-WASP and HSP90 were immunoprecipitated from 3Y1 or 3Y1/v-Src cells with anti-N-WASP and anti-HSP90 antibodies. Precipitates were analyzed by Western blotting with anti-N-WASP and anti-HSP90 antibodies. (B) Direct binding of HSP90 and N-WASP in vitro. GST-fusion full-length protein of N-WASP was immobilized on glutathione-agarose beads and incubated with bovine brain HSP90, His-fusion human HSP90α or HSP90β expressed in Sf9 cells. Bound proteins were detected by Coomassie Blue staining and Western blotting with anti-HSP90 antibody.
To investigate a direct association between the two proteins, we next performed in vitro pull-down assays with purified N-WASP and HSP90 proteins. Bovine HSP90, human HSP90α, or HSP90β recombinantly expressed in Sf9 cells was incubated with a full-length GST fusion protein of N-WASP. As shown in Figure 1B, all HSP90 proteins bound directly to N-WASP.
Identification of domains responsible for HSP90 and N-WASP interaction
N-WASP is composed of several well-characterized domains. We determined which domains of N-WASP are responsible for binding to HSP90 (Figure 2A and B). Pull-down assays with various GST fusion proteins of N-WASP were first performed. As described above, HSP90 bound directly to full-length N-WASP. An active mutant of N-WASP (Δa) containing only three acidic amino acids at the C-terminus showed somewhat stronger association than did full-length N-WASP. Mutant N-WASP lacking the N-terminal sequence from the IQ motif to the basic region (ΔIQ-Basic) showed little binding to HSP90. In contrast, fragments of N-WASP containing the IQ motif and basic region bound to HSP90. The VCA fragment of N-WASP also showed a little binding. Similar results were obtained in experiments with His-fusion proteins of N-WASP. Deletion of the IQ-Basic region of N-WASP resulted in almost complete loss of binding to HSP90. These results suggest that HSP90 binds predominantly to the region of N-WASP between the IQ motif and the basic region. To better identify the region of N-WASP necessary for HSP90 binding, we carried out competition assays between HSP90 and GTPγS-loaded Cdc42 or PIP2. Addition of up to 500 nM Cdc42 did not affect binding of HSP90 to N-WASP. PIP2 affects binding of HSP90 to N-WASP in a concentration-dependent manner. PIP2 did not compete with HSP90 for N-WASP binding at a low concentration such as 1 μM. At high concentrations over 50 μM, PIP2 inhibited binding of HSP90 to N-WASP, suggesting that HSP90 binds to the basic region of N-WASP (Figure 2C).
Figure 2.
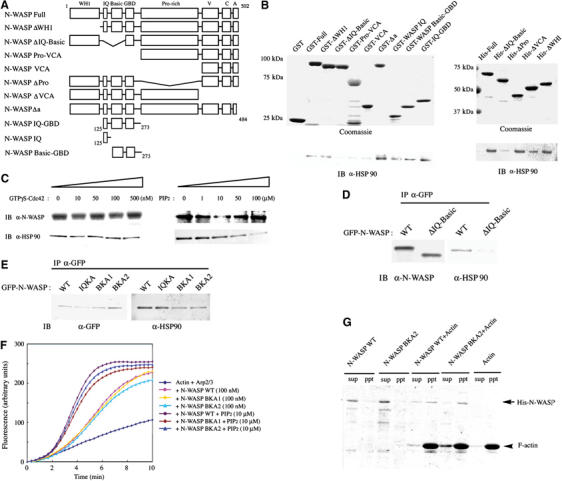
Identification of the binding domain of N-WASP for HSP90. (A) Domain structures of N-WASP used in the in vitro pull-down assays. (B) GST- or His-fusion proteins of N-WASP were immobilized on beads and incubated with bovine HSP90. (C) Competition of HSP90 and PIP2 for binding to N-WASP. A final concentration of 100 nM GST-N-WASP was incubated with various concentrations of GTPγS-Cdc42 or PIP2 and then mixed with 1 μM HSP90. (D) In vivo interaction of HSP90 with the IQ-Basic region of N-WASP. GFP-tagged N-WASP WT and the ΔIQ-Basic mutant were transfected into 3Y1/v-Src cells. The resulting cell lysates were immunoprecipitated with anti-GFP antibody. (E) IQKA (K125A, K126A, R137A, and R138A), BKA1 (K183A and K187A), and BKA2 (K183A, K187A, K191A, R194A, and K197A) N-WASP mutants were produced by site-directed mutagenesis. Each GFP-tagged mutant was expressed in 3Y1/v-Src cells. (F) His-fusion proteins of N-WASP WT, BKA1, or BKA2 expressed in Sf9 cells was subjected to Arp2/3 complex-induced actin polymerization assays in the absence or presence of 10 μM PIP2. (G) N-WASP proteins (1.5 μM) were mixed with F-actin (4 μM). The association with F-actin was assayed by cosedimentation with F-actin after ultracentrifugation at 70 000 g.
To determine whether this region is responsible for in vivo association of N-WASP to HSP90, GFP-tagged wild-type (WT) N-WASP or the N-WASP ΔIQ-Basic mutant lacking the IQ motif and basic region was expressed in 3Y1/v-Src cells (Figure 2D). Levels of ectopically expressed N-WASP were three to five times greater than that of endogenous N-WASP (data not shown). Examination of the immunoprecipitates with anti-GFP antibody revealed that HSP90 associates with full-length N-WASP but not with the N-WASP ΔIQ-Basic mutant. We confirmed that the N-terminal IQ motif and basic region of N-WASP are predominantly involved in in vivo binding between N-WASP and HSP90.
We then examined regions of HSP90 required for direct interaction with N-WASP. GST-fusion proteins of HSP90β were prepared as indicated in Figure 3A and used for in vitro pull-down assays. Fragments of HSP90 containing the N-terminal (HSP90 N) or middle regions, specifically the latter part of the middle region (HSP90 M1 and M3), bound strongly to N-WASP, suggesting two major N-WASP-binding sites in HSP90 (Figure 3B). We also transiently transfected Myc-tagged expression plasmids of HSP90 in 3Y1/v-Src cells and detected interactions with N-WASP (Figure 3C). Full-length HSP90 clearly associated with endogenous N-WASP in these cells. Unexpectedly, the HSP90 N mutant containing only the N-terminal region of HSP90 showed a very weak interaction. A ΔN mutant of HSP90 lacking the N-terminal region of HSP90 still bound to N-WASP. An HSP90 ΔM3 mutant lacking the latter part of the middle region did not co-precipitate N-WASP, indicating that the M3 middle region of HSP90 is predominantly responsible for the in vivo interaction of HSP90 with N-WASP. The difference between the in vitro and in vivo interaction regions for N-WASP may provide important information on HSP90 to bind target proteins.
Figure 3.
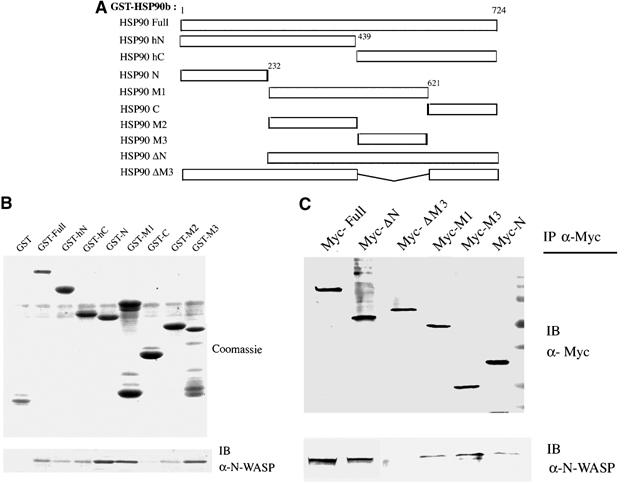
Identification of the HSP90β domain responsible for binding to N-WASP. (A) Structural domains of human HSP90β used in these experiments. (B) GST-fusion proteins of HSP90β were immobilized on beads and incubated with native N-WASP from Sf9 cells. (C) Myc-tagged HSP90 plasmids were expressed in 3Y1/v-Src cells.
HSP90 is essential for N-WASP-mediated podosome formation in 3Y1/v-Src cells
To evaluate the functional significance of the interaction between HSP90 and N-WASP, we examined whether HSP90 is involved in N-WASP-dependent podosome formation in 3Y1/v-Src cells. We first determined intracellular localization of endogenous HSP90 in these cells. HSP90 was localized throughout the cell, including the cytoplasm and the plasma membrane, and was also concentrated in dot-like clusters of podosomes (Figure 4A and Supplementary Figure 1A). Double staining with anti-HSP90 and anti-N-WASP antibodies showed that these proteins were colocalized in podosomes, suggesting that HSP90 functions in N-WASP-induced podosome formation.
Figure 4.
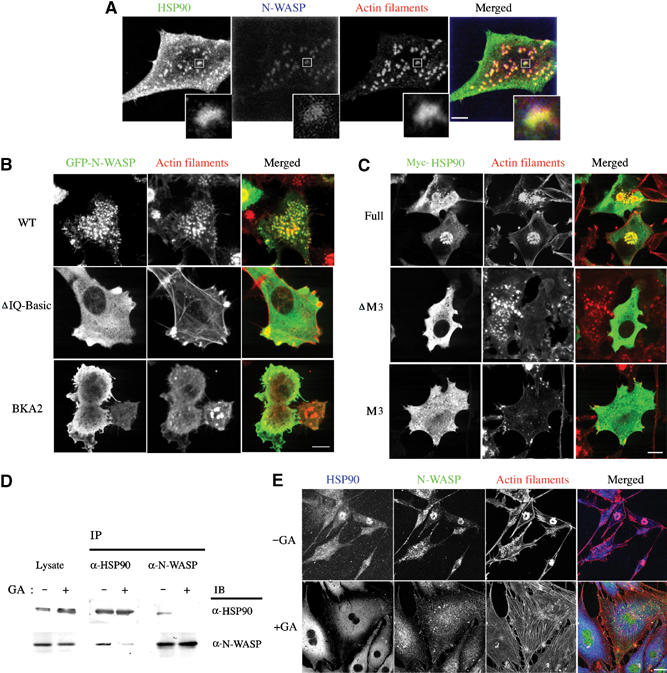
HSP90 is required for podosome formation in 3Y1/v-Src cells. (A) Intracellular localization of HSP90 and N-WASP in 3Y1/v-Src cells. 3Y1/v-Src cells were stained with anti-HSP90 antibody, anti-N-WASP antibody, and rhodamine–phalloidin. Scale bar, 10 μm. Images of HSP90 and N-WASP in podosomes are also shown in enlarged squares. (B) Inhibitory effect of ΔIQ-Basic and BKA2 mutants of N-WASP on podosome formation. Scale bar, 20 μm. (C) 3Y1/v-Src cells were transfected with expression plasmids for Myc-tagged HSP90β WT, ΔM3, or M3. Scale bar, 20 μm. (D) 3Y1/v-Src cells were cultured with or without GA (100 nM) for 12 h. HSP90 or N-WASP immunoprecipitated with anti-HSP90 antibody or anti-N-WASP antibody were immunoblotted with the indicated antibody. (E) Effect of 100 nM GA treatment on podosome formation. Scale bar, 25 μm.
We examined whether direct binding of HSP90 to N-WASP is required for podosome formation. The binding region of N-WASP for HSP90 was first examined. 3Y1/v-Src cells expressing WT N-WASP showed normal podosome formation, whereas expression of the N-WASP ΔIQ-Basic mutant inhibited podosome formation in 72% of transfected cells (Figure 4B), suggesting that direct binding between HSP90 and N-WASP is important for podosome formation. As the N-WASP ΔIQ-Basic mutant is known to be constitutively active without binding to PIP2 or F-actin (Prehoda et al, 2000; Suetsugu et al, 2001b) and might simply sequester the Arp2/3 complex for podosome-induced actin polymerization, we prepared two N-WASP mutants, BKA1 and BKA2, with approximately 50% decreased affinity for HSP90 in comparison to WT N-WASP (Figure 2E). BKA1, in which lysine residues 183 and 187 of N-WASP were replaced by alanines, and BKA2, in which lysine residues 183, 187, 191, 194, and 197 were replaced by alanines, are distinguished from the N-WASP mutant without PIP2-binding activity as described previously (Rohatgi et al, 2000). As with WT N-WASP, the two N-WASP mutants were activated by PIP2 in in vitro actin polymerization assays (Figure 2F) and showed binding to F-actin in cosedimentation assays (Figure 2G). Overexpression of the BKA1 or BKA2 mutants in 3Y1/v-Src cells reduced the rate of podosome formation in cells with strong expression (Figure 4B).
We next expressed WT HSP90, the HSP90 ΔM3 mutant, or the M3 mutant containing only the latter part of the middle region in 3Y1/v-Src cells (Figure 4C). Expressed WT HSP90 was localized to podosomes as well as the cytoplasm and did not induce alteration in podosome formation. In contrast, 3Y1/v-Src cells overexpressing the HSP90 ΔM3 or M3 mutant showed reduced podosome formation in approximately 67 and 48% of transfected cells, respectively, thus indicating that the M3 HSP90 mutant acts as a dominant-negative form. Taken together, these results strongly indicate that direct binding between HSP90 and N-WASP is essential for podosome formation in these cells.
Similar results were obtained when we tested the effect of geldanamycin (GA), a specific inhibitor of HSP90, on podosome formation. GA binds to the N-terminal ATP-binding site of HSP90 and induces dissociation of target proteins from HSP90 (Mayer and Bukau, 1999; Neckers, 2002). Treatment of 3Y1/v-Src cells with 100 nM GA inhibited complex formation of HSP90 and N-WASP (Figure 4D) and induced significant alterations in cell morphology (Figure 4E). Podosome formation was inhibited; instead, stress fibers and focal adhesions were observed. HSP90 and N-WASP accumulations were dispersed from podosomes and localized to the cytoplasm. When we expressed a dominant-negative mutant of N-WASP, ΔVPH, lacking the actin-binding region, we also observed the effects similar to GA treatment (data not shown). These results indicate that N-WASP is essential for the podosome formation and that loss of N-WASP function causes changes in actin structure and morphology in 3Y1/v-Src cells.
Effect of HSP90 on N-WASP/Arp2/3 complex-induced in vitro actin polymerization
To identify the role of HSP90 in N-WASP-dependent podosome formation, we tested the effect of HSP90 on N-WASP-induced Arp2/3 complex-mediated in vitro actin polymerization. HSP90 did not activate the Arp2/3 complex directly without N-WASP and had little effect on actin polymerization by WT N-WASP (Figure 5A). VCA N-WASP and a constitutively active mutant of N-WASP (Δa) markedly activated Arp2/3 complex-induced actin polymerization, as described previously (Rohatgi et al, 1999; Suetsugu et al, 2001a). HSP90 did not affect VCA- or Δa-induced actin polymerization. The N-WASP ΔIQ-Basic mutant showed greater actin polymerization activity than did WT N-WASP. HSP90 had no effect on activity of the ΔIQ-Basic mutant (Figure 5B). We concluded that HSP90 alone could not release the folded N-WASP conformation.
Figure 5.
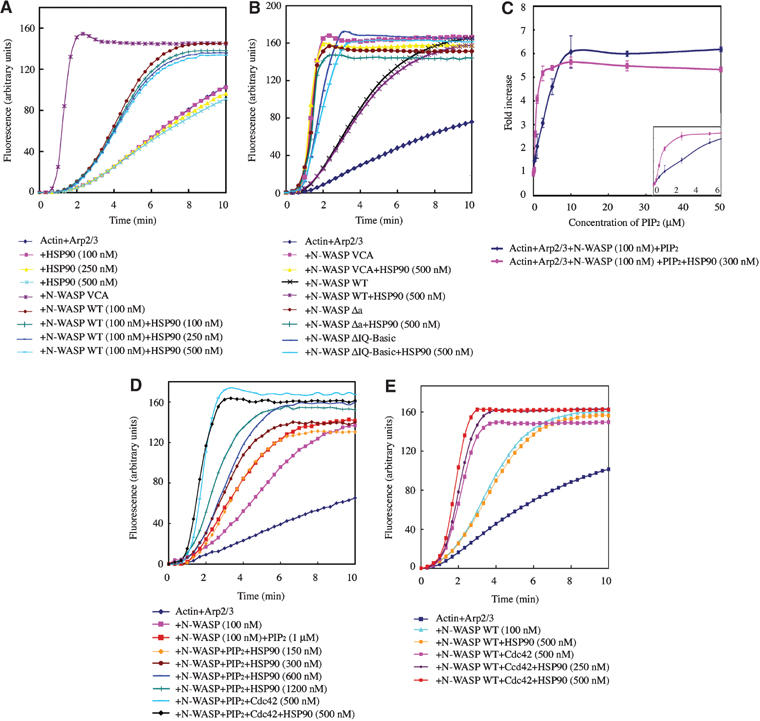
Effect of HSP90 on N-WASP-induced activation of the Arp2/3 complex. (A) Actin polymerization with Arp2/3 complex (60 nM) and N-WASP proteins (100 nM) was examined in the presence of various concentrations of HSP90. The data shown are mean values of at least three independent experiments. (B) Δa, a constitutively active mutant of N-WASP, contains only three acidic amino acids in the C-terminus of N-WASP. (C, D) N-WASP, HSP90, and/or GTPγS-Cdc42 proteins were added simultaneously to the actin polymerization mixture with 1 μM PIP2. (E) Effect of HSP90 on Cdc42-induced N-WASP activation.
HSP90 bound predominantly to the basic region of N-WASP. This region corresponds to the PIP2-binding site. We examined the effect of HSP90 on PIP2-induced N-WASP activation. If HSP90 overlaps the region necessary for PIP2 binding, N-WASP would not be further activated by PIP2. The actin polymerization activity of N-WASP in the presence of various concentrations of PIP2 was first quantified (Figure 5C). N-WASP activation increased linearly at concentrations of PIP2 from 0.5 to 5 μM and reached a maximum at 10 μM PIP2. Addition of HSP90 enhanced activation of N-WASP only under conditions in which PIP2 concentration-dependent N-WASP activation was observed, whereas, under conditions in which N-WASP was strongly activated by excess PIP2 greater than 10 μM, HSP90 did not have a considerable effect on activation of N-WASP (Figure 5C and the inset). HSP90 also enhanced the activation of N-WASP by PIP2 at a low concentration such as 1 μM in an HSP90 concentration-dependent manner (Figure 5D). The ability of HSP90 to activate N-WASP appeared to be dependent on the activation level of N-WASP. Similarly, HSP90 had little effect when N-WASP was strongly stimulated in the presence of both PIP2 and Cdc42 (Figure 5D). Taken together with the results shown in Figure 2E and F, these results indicate that the N-WASP-binding sites for HSP90 and PIP2 are not identical and HSP90 does not compete with PIP2 for N-WASP binding. The results of Figure 2C, in which concentrations of PIP2 greater than 50 μM inhibited HSP90 binding to N-WASP, appeared to be due to the fact that such high concentrations of PIP2 reduce specificity in the N-WASP-binding region and bind nonspecifically to basic charged regions of N-WASP for HSP90 binding. It is likely that HSP90 interacts predominantly with partially activated N-WASP, because HSP90 did not have an additional activation effect on inactive WT N-WASP or fully activated N-WASP.
HSP90 also did not significantly enhance activation of N-WASP by Cdc42. Only a little activation was shown in an HSP90 concentration-dependent manner (Figure 5E). Some adaptor proteins, such as Nck, act cooperatively with PIP2 or phosphorylations, not with Cdc42, to activate N-WASP (Rohatgi et al, 2001); phosphorylation of N-WASP is not significantly affected by Cdc42 (Suetsugu et al, 2002). HSP90 may provide Cdc42-independent signals to the N-WASP-dependent actin cytoskeleton.
HSP90 is known to act together with other cochaperones such as HSP70, HSP40, p23, and p50cdc37 (Scheibel et al, 1998; Young et al, 2001). When we examined the effect of cochaperones with HSP90 in N-WASP-induced actin polymerization assays, no significant effect was observed (data not shown).
HSP90 enhances phosphorylation and activation of N-WASP by v-Src
According to the results shown in Figure 1A, HSP90 bound specifically to N-WASP in 3Y1/v-Src cells. Both HSP90 and N-WASP were localized to podosomes in these cells (Figure 4A). Cytoskeletal proteins such as FAK, vinculin, and cortactin, which are known to localize to podosomes, are downstream substrates of v-Src and underlie the oncogenic transformation caused by v-src (Linder and Aepfelbacher, 2003). Thus, we examined whether N-WASP is tyrosine phosphorylated in 3Y1/v-Src cells. We found that N-WASP was tyrosine phosphorylated in these cells and that this phosphorylation was decreased when cells were treated with GA (Figure 6A). We used 100 nM GA to block the interaction of HSP90 and N-WASP, and this abolished nearly 100% of the tyrosine phosphorylation of N-WASP. HSP90 is reported to regulate the catalytic activity of v-Src (Xu and Lindquist, 1993; An et al, 2000). With this concentration of GA, tyrosine phosphorylation of v-Src was still present, although the level was reduced by approximately 40%. This is consistent with previous reports that v-Src was almost completely inhibited by concentrations of GA greater than 1 μM (Shakarjian et al, 1993; An et al, 2000). We also found that N-WASP is a major HSP90-binding protein that responded to 100 nM GA treatment by analyzing complex proteins of HSP90 formed in 3Y1/v-Src cells (Supplementary Figure 3).
Figure 6.
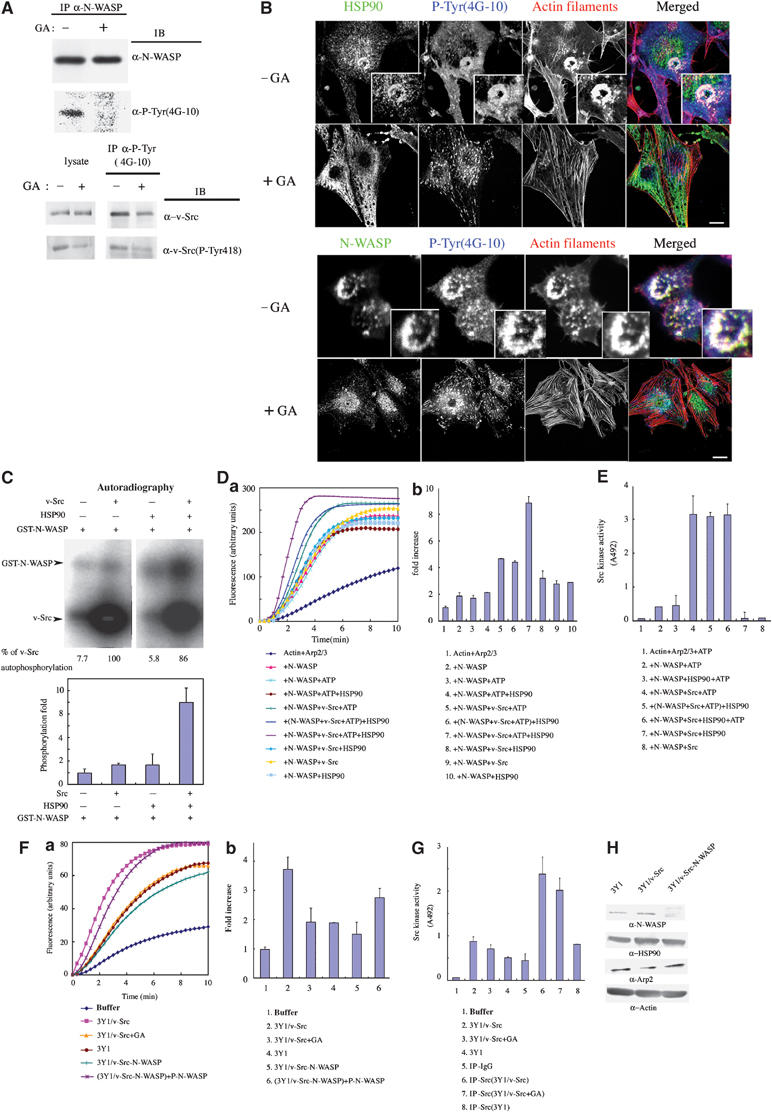
v-Src phosphorylated N-WASP. (A) In vivo phosphorylation of N-WASP. N-WASP was immunoprecipitated from 3Y1/v-Src cells cultured with or without 100 nM GA for 12 h and examined by Western blotting with the indicated antibodies. Cell lysates were also immunoprecipitated with antiphosphotyrosine antibody (4G10). Precipitates were immunoblotted with anti-v-Src antibody and antiphosphotyrosine-v-Src antibody (pTyr418) (lower panel). (B) 3Y1/v-Src cells were cultured with or without 100 nM GA for 12 h and then fixed and stained with the indicated antibody. Scale bar, 25 μm. (C) In vitro phosphorylation of N-WASP by v-Src. GST-N-WASP was mixed with v-Src and/or HSP90 and incubated with γ-32P-ATP for 15 min at 30°C. Reaction mixtures were subjected to SDS–PAGE and visualized by Coomassie Brilliant Blue staining and autoradiography. (D) N-WASP phosphorylated by v-Src (N-WASP+v-Src+ATP) was subjected to pyrene–actin assay, in which N-WASP and v-Src were 100 and 34 nM, respectively. HSP90 was added in the phosphorylation reaction (N-WASP+v-Src+ATP+HSP90) or after the phosphorylation reaction, that is, just before the addition of actin ((N-WASP+v-Src+ATP)+HSP90). N-WASP with v-Src and/or HSP90 was assayed as a negative control (N-WASP+v-Src; N-WASP+v-Src+HSP90). N-WASP without v-Src was treated by the same phosphorylation procedure in the absence or presence of HSP90 (N-WASP+ATP; N-WASP+ATP+HSP90). Da, actin polymerization curves. (Db) The initial rates of actin polymerization of (Da). Results shown are the fold increase compared to that of actin+Arp2/3 complex. (E) Src kinase activity with a synthetic random polymer substrate PGT. Src kinase activity was examined under the same conditions as those in the actin polymerization assay in (D). The linear portion of the reaction was first determined by varying incubation times prior to the experiments. (F) (Fa) 3Y1, 3Y1/v-Src, and 3Y1/v-Src cells cultured with 100 nM GA were lysed, and 10 μg of total cell lysate was added to each actin polymerization assay. In control assays, cell lysate buffer was added. For N-WASP depletion experiments, 3Y1/v-Src cell lysates were treated with anti-N-WASP antibody to deplete N-WASP (3Y1/v-Src-N-WASP). (Fb) initial ratio of actin polymerization in (Fa). (G) Tyrosine kinase assays with cell lysates were examined. In all, 10 μg of total cell lysate was added to each reaction. v-Src was also immunoprecipitated with anti-v-Src antibody from each cell lysate. (H) Western blots of N-WASP-depleted cell lysates.
Immunostaining of 3Y1/v-Src cells with anti-HSP90 or anti-N-WASP antibody was coincident with antiphosphotyrosine staining at podosomes (Figure 6B). Treatment with GA abolished the phosphotyrosine staining in podosomes, suggesting an essential role of tyrosine-phosphorylated proteins in podosome formation. Thus, we reasoned that N-WASP may be a substrate of v-Src, which is involved in the association with HSP90. To investigate this possibility, we performed in vitro phosphorylation assays (Figure 6C). A GST fusion protein of WT N-WASP was incubated with purified v-Src in the presence of γ-32P-ATP for 15 min, during which autophosphorylation of v-Src is in the linear range. v-Src phosphorylated N-WASP, although the level of phosphorylation was not marked. Addition of HSP90 to the phosphorylation reaction dramatically enhanced phosphorylation of N-WASP by v-Src without increased autophosphorylation of v-Src. To control for the potential effect of the GST protein fused to N-WASP, we also used His-fused N-WASP with similar results (data not shown). These results suggest that HSP90 is involved in v-Src-induced phosphorylation of N-WASP.
We then examined the effect of N-WASP phosphorylation by v-Src on Arp2/3 complex-mediated actin polymerization (Figure 6D). Phosphorylation of WT N-WASP by v-Src (N-WASP+v-Src+ATP) caused greater actin polymerization than did unphosphorylated N-WASP (N-WASP; N-WASP+v-Src; N-WASP+HSP90; N-WASP+v-Src+HSP90). Addition of HSP90 to the v-Src phosphorylation reaction mixture (N-WASP+v-Src+ATP+HSP90) further increased the actin polymerization activity of N-WASP. In contrast, addition of HSP90 after termination of the v-Src phosphorylation reaction ((N-WASP WT+v-Src+ATP))+HSP90) did not enhance actin polymerization by N-WASP, indicating that HSP90 activates phosphorylation of N-WASP, leading to Arp2/3 complex-mediated actin polymerization. The direct effect of HSP90 on phosphorylation of N-WASP and not on v-Src was also proved by examining the kinase activity of Src. HSP90 did not affect Src kinase-induced phosphorylation of a synthetic random polymer substrate poly-Glu-Tyr (PGT) under the same conditions described in Figure 6D (Figure 6E).
We then examined whether N-WASP is regulated in a similar fashion in 3Y1/v-Src cells where N-WASP associates with HSP90 (Figure 6F). We prepared 3Y1/v-Src cell lysates instead of purified N-WASP protein and Arp2/3 complex proteins. Addition of 3Y1/v-Src cell lysates to polymerization assays induced actin polymerization; the level of polymerization was greater than that in 3Y1 parental cell lysates or in 3Y1/v-Src cell lysates treated with GA. To determine whether this actin polymerization was due to N-WASP, we depleted N-WASP from cell lysates with anti-N-WASP antibody. Actin polymerization was decreased when cell lysates lacking N-WASP were used. In this case, levels of HSP90 and Arp2/3 complex in the cell lysates remained constant (Figure 6H). In turn, addition of v-Src-phosphorylated N-WASP to N-WASP-depleted cell lysates restored actin polymerization activity, suggesting that N-WASP is responsible for actin polymerization in these cells and that it exists in an active form via phosphorylation in 3Y1/v-Src cells.
We assayed for protein tyrosine kinase activity in each cell lysate used in experiments described in Figure 6F (Figure 6G). v-Src proteins immunoprecipitated from the respective cell lysates by anti-v-Src antibody were also subjected to the assay. 3Y1/v-Src cell lysates incubated with 100 nM GA caused only slight reduction in tyrosine kinase activity (approximately 16.5%) compared to that of control cell lysates incubated with DMSO. Similar results were obtained in assays with v-Src immunoprecipitates from these cell lysates. GA treatment induced only an 18% decrease in Src kinase activity, suggesting that GA treatment does not so much affect v-Src tyrosine kinase activity. We did not detect distinguishable differences in serine/threonine kinase activity among these cell lysates (data not shown).
HSP90 influences N-WASP-dependent neurite extension in PC12 cells
We next examined whether HSP90 has other functions, in addition to podosome formation, where phosphorylation of N-WASP is involved. Src family kinases are reported to induce neurite extension in rat pheochromocytoma PC12 cells (Alema et al, 1985). We previously reported that N-WASP is phosphorylated and activated by the Src family kinase Fyn in response to nerve growth factor (NGF) stimulation in PC12 cells and that this is essential for N-WASP-mediated neurite extension (Suetsugu et al, 2002). Thus, we examined whether HSP90 controls the phosphorylation of N-WASP necessary for neurite extension in PC12 cells. PC12 cells were serum starved in the presence or absence of GA and/or NGF, and endogenous N-WASP was immunoprecipitated. In control cells, endogenous N-WASP was weakly tyrosine phosphorylated, and NGF stimulation increased tyrosine phosphorylation of N-WASP (Figure 7A). In this case, we found that association between HSP90 and N-WASP was increased in proportion to the phosphorylation of N-WASP. In contrast, treatment of the cells with PP2, an inhibitor of Src family kinases, or GA, an inhibitor of HSP90, strongly decreased the phosphorylation of N-WASP by NGF stimulation. The association between HSP90 and N-WASP was also decreased, suggesting that HSP90 acts in the process of tyrosine phosphorylation of N-WASP by Src family kinases in response to neurite extension signals. After NGF stimulation of PC12 cells, HSP90 and N-WASP were colocalized in extending neurites, particularly at growing tips by immunostaining and by GFP-fusion protein (Figure 7B, Supplementary Figure 1B). Treatment with PP2 or GA completely blocked this N-WASP-dependent neurite extension.
Figure 7.
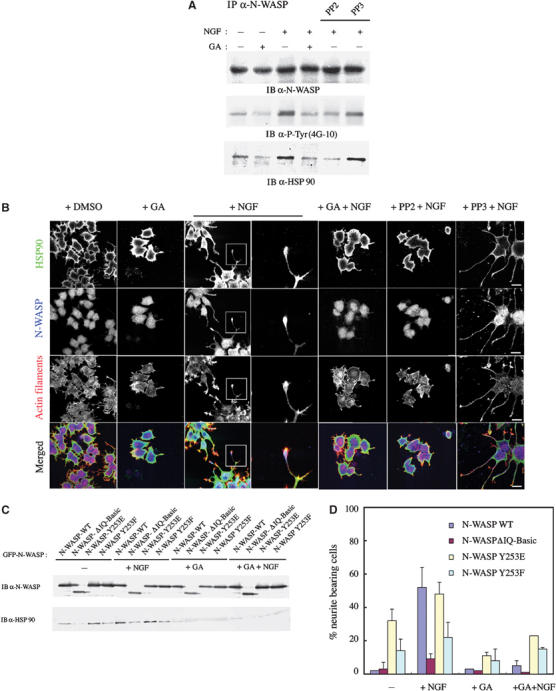
HSP90 regulates N-WASP-induced neurite extension in PC12 cells. (A) Serum-starved PC12 cells were treated with NGF (100 ng/ml) and/or GA (100 nM) in the presence or absence of PP2 (10 μM) or PP3 (10 μM) for 16 h. N-WASP was immunoprecipitated and then subjected to Western blotting with indicated antibodies. (B) PC12 cells cultured under the same conditions as those in (A) were fixed and stained with each antibody and with phalloidin. Scale bar, 25 μm. Neurites in small squares were also shown to be enlarged in right panels. (C, D) PC12 cells were transfected with plasmids expressing WT, IQ-Basic, Y253E, and Y253F mutants of N-WASP (GFP-tagged) and treated with NGF (100 ng/ml) and/or GA (100 nM).
We next compared the ability of HSP90 to associate with the constitutively active form of N-WASP and with inactive N-WASP (Figure 7C). We expressed dominant-active (Y253E) and -negative (Y253F) mutants of N-WASP, in which the tyrosine phosphorylation site of N-WASP is replaced by glutamine or phenylalanine, respectively. The ΔIQ-Basic mutant of N-WASP was also expressed to determine the importance of direct interaction between HSP90 and N-WASP in neurite extension. Under conditions of serum starvation, the level of HSP90 immunoprecipitated with Y253E N-WASP was greater than that precipitated with WT N-WASP or Y253F N-WASP. Little HSP90 co-precipitated with the ΔIQ-Basic mutant of N-WASP. After NGF stimulation, binding of WT N-WASP to HSP90 was increased to a level comparable to that of Y253E N-WASP. The Y253F and ΔIQ-Basic mutants still had less affinity for HSP90 than WT N-WASP or Y253E N-WASP had. Treatment with GA decreased association between HSP90 and all types of N-WASP, even under conditions of NGF stimulation. These results suggest that HSP90 favors active N-WASP phosphorylated by Src family kinases. Approximately 32% of cells expressing Y253E N-WASP showed neurite extension despite the absence of NGF, whereas cells expressing WT, Y253F, or the ΔIQ-Basic mutant showed neurite outgrowth in less than 10% of cells (Figure 7D). In response to NGF stimulation, WT N-WASP induced neurite extension to a degree comparable to that of Y253E N-WASP. In contrast, the ΔIQ-Basic mutant failed to induce neurite extension even under conditions of NGF stimulation, suggesting that neurite extension is induced by interaction between tyrosine-phosphorylated N-WASP and HSP90 in PC12 cells.
HSP90 stabilizes active N-WASP
As N-WASP activated by phosphorylation rapidly undergoes degradation (Suetsugu et al, 2002), we examined whether interaction of HSP90 and N-WASP affects the stabilization of N-WASP. We analyzed the degradation states of endogenous N-WASP in 3Y1/v-Src cells (Figure 8A) and PC12 cells (Figure 8B). As HSP90 enhances the phosphorylation of N-WASP, we presumed that HSP90 would result in rapid degradation of N-WASP. However, degradation of endogenous N-WASP in 3Y1/v-Src cells, where N-WASP associates with HSP90, was not significant. Rather, inhibition of HSP90 by treatment with GA increased the degradation of N-WASP. The GA-induced degradation of N-WASP was inhibited by treatment with the proteasome inhibitor MG-132, suggesting that degradation of N-WASP in 3Y1/v-Src cells depends on a proteasome system that is attenuated by HSP90. HSP90 appeared to protect activated N-WASP from proteasome-dependent degradation. Similar results were obtained in experiments with PC12 cells. As we performed all experiments with PC12 cells under conditions of serum starvation, rapid degradation of N-WASP in control cells was not present. Even under these conditions, treatment with GA induced degradation of N-WASP, indicating that HSP90 also contributes to the stabilization of N-WASP under serum-starved conditions. Incubation of the cells with MG-132 inhibited the GA-induced degradation of N-WASP, suggesting that HSP90 protects N-WASP from the proteasome degradation system in PC12 cells. NGF stimulation alone did not include the degradation of N-WASP. Treatment with NGF in the presence of GA increased the rapid degradation of N-WASP. Thus, HSP90 appears to play a role not only in the activation of N-WASP through phosphorylation but also in the process of stabilization of activated N-WASP.
Figure 8.
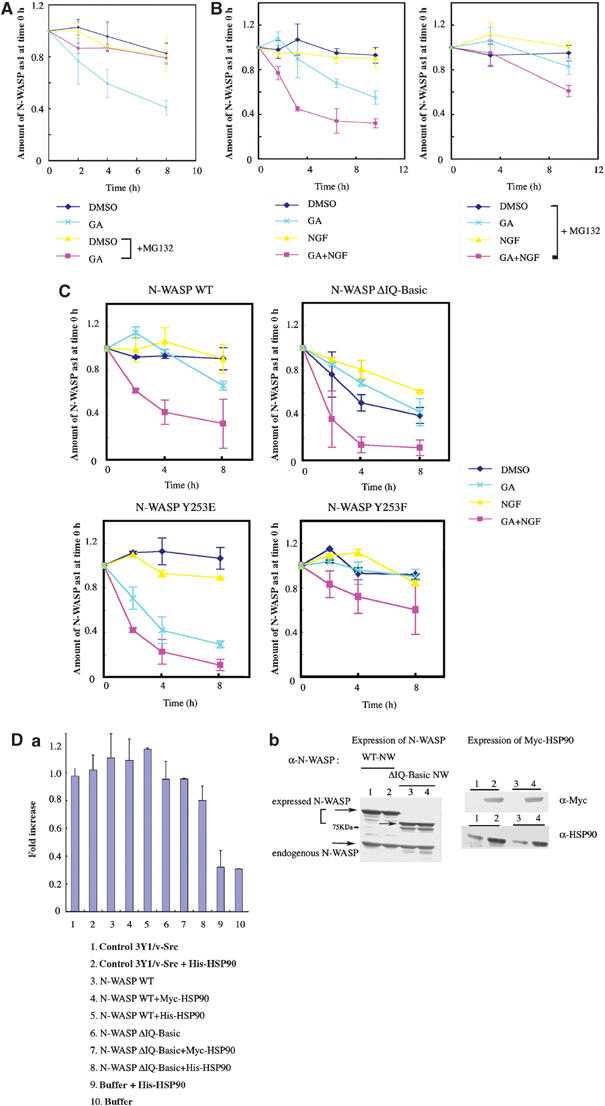
HSP90 stabilizes active N-WASP. (A) Turnover of N-WASP in 3Y1/v-Src cells. Cells were pulse-labeled with 35S-labeled methionine/cysteine and chased with unlabeled medium. N-WASP was then immunoprecipitated, analyzed by Western blotting, and subjected to autoradiography. Amounts of N-WASP immunoprecipitated and levels of radioactivity were measured by densitometry. The level of radioactivity per amount of N-WASP was considered to be 1 at time 0. Data shown are mean values of at least three independent experiments. (B) Turnover of N-WASP in PC12 cells. PC12 cells were treated with GA (100 nM) and/or NGF (100 ng/ml) in the presence or absence of MG-132 (20 μM). (C) Turnover of N-WASP mutants in PC12 cells. PC12 cells were transfected with plasmids expressing WT, IQ-Basic, Y253E, and Y253F mutants of N-WASP (GFP-tagged) and treated with NGF (100 ng/ml) and/or GA (100 nM). (D) Actin polymerization with 3Y1/v-Src cell lysates expressing WT or ΔIQ-Basic N-WASP. (a) Polymerization assays were performed with 1.2 μM pyrene-labeled actin. (b) Expression levels of WT and ΔIQ-Basic N-WASP in 3Y1/v-Src cells. Myc-tagged HSP90 was also coexpressed with N-WASP in cells.
Finally, we compared the degradation of N-WASP mutants (Figure 8C). In control PC12 cells without NGF treatment, the active Y253E mutant did not show a significant difference in degradation compared to that of WT N-WASP or the Y253F mutant. GA treatment induced more rapid degradation of the Y253E mutant than that of WT or Y253F N-WASP, suggesting that HSP90 is involved predominantly in stabilization of active Y253E compared to WT N-WASP or the inactive Y253F mutant. In NGF-treated cells, WT N-WASP was degraded in a manner similar to that of Y253E. GA treatment under this condition initiated rapid degradation of both WT and Y253E N-WASP. These results show that HSP90 contributes to the stabilization of activated N-WASP and that active N-WASP without association with HSP90 is rapidly degraded. The ΔIQ-Basic mutant, which cannot bind HSP90, was markedly degraded under all conditions. 3Y1/v-Src cell lysates expressing WT or the ΔIQ-Basic mutant of N-WASP were subjected to in vitro actin polymerization assays (Figure 8D). In contrast to the high in vitro activity of purified ΔIQ-Basic N-WASP as shown in Figure 5B, lysates from cells expressing the ΔIQ-Basic mutant showed lower activity than did lysates from cells expressing WT N-WASP. We also increased the level of HSP90 to sufficiently saturate the level of ectopically expressed N-WASP, although endogenous HSP90 was present at levels approximately 20-fold that of N-WASP (data not shown). HSP90 was overexpressed together with N-WASP. Purified HSP90 protein was added directly to the N-WASP-expressing cells. However, increased levels of HSP90 did not alter the results with N-WASP-expressing cell lysates. These results clearly indicate that direct interaction with HSP90 is responsible for stabilization of active N-WASP.
Discussion
HSP90 appears to enhance the conformational activation of N-WASP. The introduction of negative charge by phosphorylation as well as binding of PIP2 appears to release the autoinhibitory conformation of WASP family proteins. However, phosphorylation or PIP2 binding alone is not sufficient for full activation. In the case of phosphorylation of N-WASP by Fyn, addition of PIP2 substantially enhances phosphorylation and activation, suggesting that full activation of N-WASP requires some factor, such as PIP2, in addition to phosphorylation. HSP90 appears to be an efficient activator of N-WASP phosphorylation. HSP90 increased the levels of phosphorylation of N-WASP by Src family kinases, leading to further activation of N-WASP. HSP90 alone had no effect on phosphorylation of N-WASP or on N-WASP-induced actin polymerization. We propose that Src family kinases initiate phosphorylation of N-WASP in response to extracellular stimuli and that subsequent association of HSP90 and N-WASP enhances phosphorylation of N-WASP, which promotes exposure of the VCA region of N-WASP.
In vivo binding of HSP90 to N-WASP is dependent on N-WASP activation. HSP90 bound to active N-WASP in 3Y1/v-Src cells, whereas this association was not detected in parental 3Y1 cells. Similar results were obtained in experiments with PC12 cells. The basic region of N-WASP necessary for binding to HSP90 is masked in a closed conformation in the absence of stimuli. Thus, it is reasonable that HSP90 binds to the basic region of opened, active N-WASP. In in vitro pull-down assays with GST- or His-fusion proteins of N-WASP, we also found that the VCA region of N-WASP has a weak affinity for HSP90 and that this interaction occurs via the former part of the middle region of HSP90 (HSP90 M2) (Supplementary Figure 4). We did not ascertain how much the interaction of N-WASP VCA and HSP90 M2 contributes to regulation of N-WASP in cells, because of the weak affinity and because expression of ΔVCA N-WASP or VCA N-WASP mutant in cells can inhibit, independently of HSP90, podosome formation or neurite extension by direct effect to actin and the Arp2/3 complex (Mizutani et al, 2002). However, through in vitro actin polymerization assays with HSP90 M2 or the latter part of the middle region of HSP90 (M3), we confirmed the interaction of HSP90 M3 and the N-WASP basic region function mainly to activate N-WASP. The VCA region interacts with the basic region of N-WASP, resulting in a closed and inactive conformation of N-WASP. In response to extracellular stimuli, both the VCA and the basic region would be exposed. The VCA region is also known to be phosphorylated in response to extracellular activating signals (Cory et al, 2003). Thus, we cannot completely rule out the possibility that the VCA region as well as the Basic region may also be a weak binding site of HSP90 in cells, which contributes to maintaining the opened N-WASP. This may explain why a weak association between the ΔIQ-Basic mutant and endogenous HSP90 remained and why the ΔIQ-Basic mutant was somewhat sensitive to GA and NGF treatment, similar to WT N-WASP and other N-WASP mutants in PC12 cells (Figures 7C and 8C).
PIP2 as well as HSP90 bind to the basic region of N-WASP. However, it is unlikely that HSP90 competes with PIP2 for N-WASP binding. Two mutants of N-WASP, BKA1 and BKA2, could still bind to PIP2 in the absence of HSP90 binding. Thus, it is possible that HSP90 and PIP2 bind simultaneously to the basic region of N-WASP, resulting in further activation of N-WASP. Indeed, at low concentrations of PIP2 in which activation of N-WASP is not maximum, HSP90 in combination with PIP2 could activate N-WASP (Figure 5C and D). In the presence of PIP2 concentrations greater than 10 μM that activate fully N-WASP, HSP90 did not act cooperatively to activate N-WASP. Similar results were also obtained with respect to phosphorylation level-dependent N-WASP activation. The effect of HSP90 to increase phosphorylation of N-WASP was clear under conditions in which increased N-WASP phosphorylation was in the linear range (Supplementary Figure 2). Thus, we believe that HSP90 predominantly functions on partially activated N-WASP and does not exert a direct effect on activation of inactive WT N-WASP or an additional effect on fully activated N-WASP, at least in vitro. Indeed, HSP90 had no effect on constitutively active N-WASP, Δa, or inactive WT N-WASP. We observed similar results under conditions in which N-WASP was strongly activated by a combination of GTPγs-Cdc42 and PIP2 as well as by high concentrations of PIP2.
HSP90 is known to be involved in the activation and stabilization of many different kinases, including v-Src (Xu and Lindquist, 1993; Mayer and Bukau, 1999). Indeed, NGF-induced neurite extension in PC12 cells was inhibited by the Src family kinase inhibitor PP2 to an extent similar to that with GA treatment (Figure 7B). Thus, in vivo treatment with GA might affect not only the HSP90–N-WASP association but also the association of HSP90 with other target proteins such as v-Src. However, treatment of 3Y1/v-Src cells with 100 nM GA induced a reduction of approximately 40% in the tyrosine phosphorylation signal of v-Src, whereas phosphorylation of N-WASP was almost completely lost under the same conditions (Figure 6A). In kinase assays with immunoprecipitated v-Src, only an 18% reduction of the Src kinase activity was observed (Figure 6G), whereas in actin polymerization assays with cell lysates, GA treatment reduced activation of N-WASP to approximately 52% (Figure 6F). We determined that HSP90 had no direct effect on Src kinase activity, at least in vitro (Figure 6E). In most cases, treatment with 100 nM GA did not result in marked degradation of v-Src (data not shown), which differed from the significant degradation of N-WASP observed in response to GA treatment. Taken together with the results in Supplementary Figure 3, we concluded that 100 nM GA has higher specificity for HSP90 associated with N-WASP than with any other target. This conclusion is supported by our finding that overexpression of the HSP90 protein fragment responsible for N-WASP binding inhibited podosome formation and neurite extension.
A schematic of the potential mechanism by which HSP90 participates in the regulation of N-WASP is shown in Figure 9. In resting cells, N-WASP exists in a folded and inactive form. Specific extracellular stimuli lead to activation of N-WASP through various signals, including tyrosine phosphorylation by v-Src or PIP2. At this step, HSP90 binds to N-WASP, inducing further activation of N-WASP and preventing activated N-WASP from degradation, which results in N-WASP-dependent actin polymerization and podosome formation and neurite extension.
Figure 9.
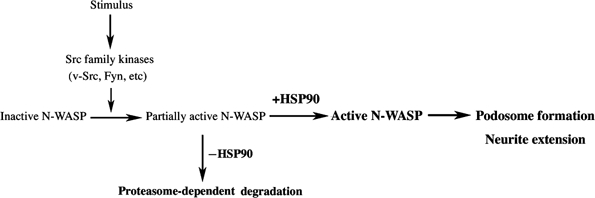
Possible role of HSP90 in N-WASP-mediated podosome formation and neurite extension. In resting cells, N-WASP exists in a folded and inactive form. An extracellular stimulus leads to release of the autoinhibitory conformation of N-WASP through a combination of various signals, including phosphorylation and PIP2. At this step, HSP90 binds to N-WASP and facilitates further activation of N-WASP, which induces reorganization of the N-WASP-regulated actin cytoskeleton. In the absence of HSP90, active N-WASP is rapidly degraded by ubiquitin-dependent proteasomes.
Materials and methods
Proteins
WT and various mutant proteins of N-WASP were produced with the Bac-to-Bac baculovirus expression system (Gibco BRL) or expressed in Escherichia coli as described previously (Suetsugu et al, 2001a, 2001b). N-WASP mutants IQKA (with K125A, K126A, K129A, R137A, and R138A), BKA1 (with K183A and K187A), and BKA2 (with K183A, K187A, K191A, R194A, and K197A) were created using the Quick-change protocol (Stratagene, La Jolla, CA). His-fusion proteins of IQKA, BKA1, and BKA2 were produced in Sf9 cells with recombinant baculoviruses. V-Src was from Upstate Biotechnology. HSP90 from bovine was purchased from Sigma. The human HSP90α (GenBank accession no. X15183) and HSP90β (GenBank accession no. M16660) was obtained by reverse transcription–PCR from HeLa cells. Each His- or GST-fusion full-length protein was produced in Sf9 cells. We determined that the His- or GST-fusion full-length HSP90β and bovine HSP90 possess ATPase activity, and that HSP90β binds to HSP70 and HSP40 in in vitro pull-down assays (data not shown). GST-fusion fragments of HSP90β were expressed in E. coli.
Antibodies and immunofluorescence microscopic analysis
Anti-N-WASP polyclonal antibody was produced as described previously (Miki et al, 1998). Anti-Arp2 antibody was produced by immunizing rabbits with synthetic oligopeptide with the sequence CEKGVRVLEKLGVTVR. Anti-HSP90 monoclonal (F8) and polyclonal (H-114) antibodies were from Santa Cruz Biotechnology. Antiphosphotyrosine monoclonal (4G10) antibody was from Upstate Biotechnology, Inc. Anti-v-Src (clone 327) and anti-phosphotyrosine-v-Src (pTyr418) antibodies were from Sigma and Biosource, respectively.
Immunofluorescence staining of 3Y1/v-Src and PC12 cells were performed as described previously (Mizutani et al, 2002; Suetsugu et al, 2002).
In vitro binding assay
GST-fusion proteins were immobilized on 20 μl of glutathione sepharose 4B beads (Amersham Pharmacia) and mixed with target proteins. The beads were washed with PBS plus 0.1% Tween 20 and then subjected to SDS–PAGE, followed by Coomassie Brilliant Blue staining or Western blot analysis.
In vitro phosphorylation of N-WASP
Phosphorylation of N-WASP was performed as follows: 3 μM N-WASP, 1 μM v-Src, and 3 μM HSP90 were mixed in a solution containing 20 mM HEPES, pH 7.2, 10 mM MgCl2, 150 mM KCl, and 1 mM ATP with or without 20 μCi/ml γ-32P-ATP. The mixture was incubated for 15 min at 30°C, analyzed by SDS–PAGE, and then subjected to autoradiography.
Phosphorylation of N-WASP for pyrene–actin assays was performed for 30 min at 30°C as described above. The mixtures of N-WASP, v-Src, or HSP90 reacted with ATP were subjected directly to pyrene–actin assay with 30-fold dilution to give final concentrations of 100 nM N-WASP, 34 nM v-Src, and 100 nM HSP 90.
Actin polymerization assay
Pyrene–actin assays were performed as described previously (Rohatgi et al, 1999). N-WASP and HSP90 were added at a final concentration of 100 nM unless indicated otherwise. For actin polymerization assays with 3Y1 and 3Y1/v-Src cell lysates, cell lysates were prepared as described previously (Yarar et al, 1999). The polymerization reaction mixture was prepared by mixing 10 μl of cell lysate, 10 μl ATP-regenerating mix, 80 μl × buffer, and 0.8 μM pyrene-labeled actin. The reaction mixture was preincubated for 10 min before fluorescence was monitored.
In vitro tyrosine kinase assay
Tyrosine kinase activity was measured using a nonradioactive protein tyrosine kinase assay kit (Sigma) according to the manufacturer's guidelines. For tyrosine kinase assays with cell lysates, the cells were lysed in 50 mM HEPES buffer, pH 7.4, containing 0.5% Triton X-100, 150 mM NaCl, 10% glycerol, 1 mM DTT, 1 mM Na3VO4, and protease inhibitors.
In vivo pulse-chase analysis
In vivo pulse-chase analysis was performed as described previously (Suetsugu et al, 2002). 3Y1/v-Src cells (1 × 106) or PC12 cells (2 × 106) were plated in 6-cm dishes and cultured overnight with or without 100 nM GA, 10 μM PP2, and 20 μM MG-132. Each dish was labeled with 50 μCi of 35S-labeled methionine/cysteine (Redivue Pro-Mix L-35S In Vitro Cell Labeling Mix, Amersham Pharmacia) for 4 h pulse with or without GA, PP2, and MG-132, washed three times with phosphate-buffered saline (PBS), and chased with normal media with or without GA, PP2, and MG-132. At each time point, cells were harvested, and N-WASP was immunoprecipitated with anti-N-WASP antibody.
Supplementary Material
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Supplementary Figure 4
References
- Alema S, Casalbore P, Agostini E, Tato F (1985) Differentiation of PC12 phaeochromocytoma cells induced by v-src oncogene. Nature 316: 557–559 [DOI] [PubMed] [Google Scholar]
- An WG, Schulte TW, Neckers LM (2000) The heat shock protein 90 antagonist geldanamycin alters chaperone association with p210bcr–abl and v-src proteins before their degradation by the proteasome. Cell Growth Differ 11: 355–360 [PubMed] [Google Scholar]
- Carlier MF, Nioche P, Broutin-L'Hermite I, Boujemaa R, Le Clainche C, Egile C, Garbay C, Ducruix A, Sansonetti P, Pantaloni D (2000) GRB2 links signaling to actin assembly by enhancing interaction of neural Wiskott–Aldrich syndrome protein (N-WASp) with actin-related protein (ARP2/3) complex. J Biol Chem 275: 21946–21952 [DOI] [PubMed] [Google Scholar]
- Cory GO, Cramer R, Blanchoin L, Ridley AJ (2003) Phosphorylation of the WASP-VCA domain increases its affinity for the Arp2/3 complex and enhances actin polymerization by WASP. Mol Cell 11: 1229–1239 [DOI] [PubMed] [Google Scholar]
- Cory GO, Garg R, Cramer R, Ridley AJ (2002) Phosphorylation of tyrosine 291 enhances the ability of WASp to stimulate actin polymerization and filopodium formation. Wiskott–Aldrich syndrome protein. J Biol Chem 277: 45115–45121 [DOI] [PubMed] [Google Scholar]
- Linder S, Aepfelbacher M (2003) Podosomes: adhesion hot-spots of invasive cells. Trends Cell Biol 13: 376–385 [DOI] [PubMed] [Google Scholar]
- Machesky LM, Mullins RD, Higgs HN, Kaiser DA, Blanchoin L, May RC, Hall ME, Pollard TD (1999) Scar, a WASp-related protein, activates nucleation of actin filaments by the Arp2/3 complex. Proc Natl Acad Sci USA 96: 3739–3744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer MP, Bukau B (1999) Molecular chaperones: the busy life of Hsp90. Curr Biol 9: R322–R325 [DOI] [PubMed] [Google Scholar]
- Miki H, Sasaki T, Takai Y, Takenawa T (1998) Induction of filopodium formation by a WASP-related actin-depolymerizing protein N-WASP. Nature 391: 93–96 [DOI] [PubMed] [Google Scholar]
- Mizutani K, Miki H, He H, Maruta H, Takenawa T (2002) Essential role of neural Wiskott–Aldrich syndrome protein in podosome formation and degradation of extracellular matrix in src-transformed fibroblasts. Cancer Res 62: 669–674 [PubMed] [Google Scholar]
- Neckers L (2002) Hsp90 inhibitors as novel cancer chemotherapeutic agents. Trends Mol Med 8: S55–S61 [DOI] [PubMed] [Google Scholar]
- Prehoda KE, Scott JA, Mullins RD, Lim LA (2000) Integration of multiple signals through cooperative regulation of the N-WASP-Arp2/3 complex. Science 290: 801–806 [DOI] [PubMed] [Google Scholar]
- Rohatgi R, Ho HY, Kirschner MW (2000) Mechanism of N-WASP activation by CDC42 and phosphatidylinositol 4, 5-bisphosphate. J Cell Biol 150: 1299–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohatgi R, Ma L, Miki H, Lopez M, Kirchhausen T, Takenawa T, Kirschner MW (1999) The interaction between N-WASP and the Arp2/3 complex links Cdc42-dependent signals to actin assembly. Cell 97: 221–231 [DOI] [PubMed] [Google Scholar]
- Rohatgi R, Nollau P, Ho HY, Kirschner MW, Mayer BJ (2001) Nck and phosphatidylinositol 4,5-bisphosphate synergistically activate actin polymerization through the N-WASP-Arp2/3 pathway. J Biol Chem 276: 26448–26452 [DOI] [PubMed] [Google Scholar]
- Scheibel T, Weikl T, Buchner J (1998) Two chaperone sites in Hsp90 differing in substrate specificity and ATP dependence. Proc Natl Acad Sci USA 95: 1495–1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakarjian MP, Eiseman E, Penhallow RC, Bolen JB (1993) 3-Hydroxy-3-methylglutaryl-coenzyme A reductase inhibition in a rat mast cell line. Impairment of tyrosine kinase-dependent signal transduction and the subsequent degranulation response. J Biol Chem 268: 15252–15259 [PubMed] [Google Scholar]
- Suetsugu S, Hattori M, Miki H, Tezuka T, Yamamoto T, Mikoshiba K, Takenawa T (2002) Sustained activation of N-WASP through phosphorylation is essential for neurite extension. Dev Cell 3: 645–658 [DOI] [PubMed] [Google Scholar]
- Suetsugu S, Miki H, Takenawa T (2001a) Identification of another actin-related protein (Arp) 2/3 complex binding site in neural Wiskott–Aldrich syndrome protein (N-WASP) that complements actin polymerization induced by the Arp2/3 complex activating (VCA) domain of N-WASP. J Biol Chem 276: 33175–33180 [DOI] [PubMed] [Google Scholar]
- Suetsugu S, Miki H, Yamaguchi H, Obinata T, Takenawa T (2001b) Enhancement of branching efficiency by the actin filament-binding activity of N-WASP/WAVE2. J Cell Sci 114: 4533–4542 [DOI] [PubMed] [Google Scholar]
- Suetsugu S, Takenawa T (2003) Translocation of N-WASP by nuclear localization and export signals into the nucleus modulates expression of HSP90. J Biol Chem 278: 42515–42523 [DOI] [PubMed] [Google Scholar]
- Svitkina TM, Bulanova EA, Chaga OY, Vignjevic DM, Kojima S, Vasiliev JM, Borisy GG (2003) Mechanism of filopodia initiation by reorganization of a dendritic network. J Cell Biol 160: 409–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres E, Rosen MK (2003) Contingent phosphorylation/dephosphorylation provides a mechanism of molecular memory in WASP. Mol Cell 11: 1215–1227 [DOI] [PubMed] [Google Scholar]
- Xu Y, Lindquist S (1993) Heat-shock protein hsp90 governs the activity of pp60v-src kinase. Proc Natl Acad Sci USA 90: 7074–7078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarar D, To W, Abo A, Welch MD (1999) The Wiskott–Aldrich syndrome protein directs actin-based motility by stimulating actin nucleation with the Arp2/3 complex. Curr Biol 9: 555–558 [DOI] [PubMed] [Google Scholar]
- Young JC, Moarefi I, Hartl FU (2001) Hsp90: a specialized but essential protein-folding tool. J Cell Biol 154: 267–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Supplementary Figure 4