Nef Enhances Human Immunodeficiency Virus Type 1 Infectivity Resulting from Intervirion Fusion: Evidence Supporting a Role for Nef at the Virion Envelope (original) (raw)
Abstract
The human immunodeficiency virus type 1 (HIV-1) accessory protein Nef stimulates viral infectivity by facilitating an early event in the HIV-1 life cycle. Although no structural or biochemical defects in Nef-defective HIV-1 particles have been demonstrated, the Nef protein is incorporated into HIV-1 particles. To localize the function of Nef within the virus particle, we developed a novel technology involving fusion of enveloped donor HIV-1 particles bearing core defects with envelope-defective target virions bearing HIV-1 receptors. Although neither virus alone was capable of infecting CD4+ target cells, the incubation of donor and target virions prior to addition to target cells resulted in infection. This effect, termed “virion transcomplementation,” required a functional Env protein on the donor virus and CD4 and an appropriate coreceptor on target virions. To provide evidence for intervirion fusion as the mechanism of complementation, experiments were performed using dual-enveloped HIV-1 particles bearing both HIV-1 and ecotropic murine leukemia virus (E-MLV) Env proteins as donor virions. Infection of CD4-negative target cells bearing E-MLV receptors was prevented by HIV-1 entry inhibitors when added before, but not after, incubation of donor and target virions prior to the addition to cells. When we used Nef+ and Nef− donor and target virions, Nef enhanced infection when present in donor virions. In contrast, no effect of Nef was detected when present in the target virus. These results reveal a potential mechanism for enhancing HIV-1 diversity in vivo through the rescue of defective viral genomes and provide a novel genetic system for the functional analysis of virion-associated proteins in HIV-1 infection.
Nef is a highly conserved accessory protein encoded by human immunodeficiency virus type 1 (HIV-1), HIV-2, and simian immunodeficiency virus (SIV) and plays a crucial role in primate lentiviral virulence. Nef-defective SIV is strongly attenuated in adult rhesus macaques (16). Furthermore, some long-term nonprogressors of HIV-1 infection harbor viruses bearing defective nef genes, suggesting that Nef is required for HIV-1 pathogenesis (17, 23, 29, 30). Finally, in a transgenic mouse model of HIV-1 disease, Nef was the sole determinant of pathogenesis (14).
In cell culture, Nef accelerates HIV-1 replication, downregulates cell surface CD4 and class I major histocompatibility complex expression, and influences T-cell activation (for reviews, see references 12 and 28). Although the relative importance of each of these activities in AIDS pathogenesis has not been established, the ability of Nef to enhance HIV-1 replication is of obvious interest, but the mechanism by which this occurs is unclear. Nef-defective particles are approximately 10-fold less infectious than wild-type HIV-1 when tested in single-cycle infection assays (10, 24). The reduction in infectivity has been localized to an early postentry defect of Nef mutant virions in target cells (2, 9, 32). Although no structural or biochemical defects in Nef− HIV-1 particles have been detected, the Nef protein itself is present within virions and is cleaved by the viral protease (4, 27, 36). However, cleavage of Nef is not required for the enhancement of infectivity (8). A significant fraction of virion-associated Nef localizes to the HIV-1 core, suggesting that Nef may facilitate postentry events by modifying the core (18). Nef also can be packaged into heterologous retroviruses such as MLV, suggesting that Nef is passively incorporated into HIV-1 particles at the time of budding due to its localization to the plasma membrane of infected cells (8). Consistent with this hypothesis, the amino-terminal membrane binding domain of Nef, containing a myristylation signal and a stretch of basic residues, is sufficient to mediate virion incorporation of a heterologous reporter protein (35). Although Nef localizes to the HIV-1 core, it is not known whether virion incorporation of Nef is required for infectivity enhancement.
Infection by enveloped viruses requires interactions between a fusion protein on the virion surface and receptors on the plasma membrane of the target cell. Engagement of the receptor induces conformational changes in the viral Env proteins, resulting in exposure of a fusion peptide and the mixing of viral and cellular lipids, thereby catalyzing membrane fusion events required for viral entry. Recently, it was reported that retroviral Env proteins can be replaced by their cognate receptors, resulting in particles that specifically infect cells expressing the viral Env proteins (3, 13). Infection by these particles, termed “receptor pseudotypes,” demonstrates that the orientation of the fusion protein-receptor interaction can be functionally reversed, suggesting that a specific directionality of the fusion reaction is not an absolute requirement for productive virus entry. Based on these reports, we hypothesized that receptor-pseudotyped virions should also be capable of fusing with enveloped virions and that the fusion products would be infectious on target cells bearing appropriate viral receptors. This system, termed “virion transcomplementation,” would allow investigation of the mechanism of action of virion-associated proteins such as Nef. In this study, we detected intervirion fusion events by assaying infection resulting from incubation of enveloped particles (“donor virions”) containing defective cores with receptor-pseudotyped target virions. Infection was enhanced by Nef only when produced with the donor virions. These results demonstrate that infectivity enhancement by Nef does not require its presence during viral core formation.
MATERIALS AND METHODS
Cells and viruses.
293T cells were used for production of HIV-1 particles and were cultured at 37°C and 5% CO2 in Dulbecco modified Eagle medium supplemented with fetal bovine serum (10%), penicillin (50 IU/ml), and streptomycin (50 μg/ml) (D10 medium). Work with infectious HIV-1 was performed in a biosafety level 3 laboratory under P3 containment conditions. Viruses were produced from 293T cells cultured in 100-mm dishes using a high-efficiency calcium phosphate transfection procedure and cloned proviral DNA (7). For production of receptor pseudotyped HIV-1 particles, Env-defective HIV-1 proviral DNA (10 μg) was cotransfected with the tail-less CD4 expression vector CMX-44X (2 μg) (22) and either pC-Fusin or pC-CCR5 (8 μg) (11). For production of dual-enveloped donor HIV-1 particles carrying both HIV-1 Env and either vesicular stomatitis virus (VSV) or Ecotropic MLV (E-MLV) Env proteins, core-defective, Env-competent HIV-1 proviral clones (10 μg) were cotransfected with either pHCMV-G (40) or pSV-E-MLV-Env (19), respectively (10 μg each plasmid). Viruses were collected from the culture supernatants 2 days after transfection and were frozen in aliquots at −80°C after passage through 0.45-μm (pore-size) filters to remove cellular debris. P4 and Z24 cells (5) are HeLa-derived HIV-1 indicator target cells expressing or lacking human CD4, respectively, and were cultured at 37°C and 5% CO2 in D10 medium. To produce Z24 cells expressing the E-MLV receptor Rec-1 (Z24-Rec-1), the Rec-1 cDNA was first cloned into the MLV retroviral vector pBABE-puro, and the vector was packaged into pantropic MLV particles by cotransfection of 293T cells with the MLV gag-pol expression vector pSVψ−MLV-_env_− (19) and pHCMV-G. Two days after transfection, viral supernatant was harvested and used to infect Z24 cells in the presence of Polybrene (8 μg/ml) (Sigma). Transduced cells were selected in puromycin (2 μg/ml) (Sigma), and the resistant population of cells was used as targets in quantitative infection assays of HIV-1 particles pseudotyped by the E-MLV envelope proteins. P4 cells expressing CCR5 were produced by a similar procedure using the pBABE.CCR5 retroviral vector (11).
Viral clones.
The Env-defective proviral clone, designated R9ΔE, was created by end filling the _Nde_I site in the wild-type infectious HIV-1 clone R9, resulting in a frameshift at residue 61 of Env. Virions produced from this clone are noninfectious but can be pseudotyped by cotransfection with plasmids encoding HIV-1, MLV, or VSV Env proteins. R9ΔEΔN, lacking functional Env and Nef genes, was created by transferring the _Sal_I-_Bam_HI fragment from R9ΔE into the Nef-defective proviral clone R9ΔN. CA5 and CA6, encoding HIV-1 particles containing nonfunctional cleavage sites between CA and NC, were provided by H.-G. Krausslich and have been described elsewhere (39). CA5-BaL was created by substituting the _Sal_I-_Bam_HI fragment, from the CCR5-tropic HIV-1 isolate BaL, for the corresponding region in CA5, thereby conferring CCR5 tropism to CA5. The unpublished HIV-1 proviral clone R9.R18AN21A, encoding two point mutations in CA, was provided by W. Sundquist. This construct produces HIV-1 particles that are noninfectious due to formation of aberrant HIV-1 cores (U. von Schwedler and W. Sundquist, personal communication). Nef-defective versions of R9ΔE, CA5, and R9.R18AN21A (R9ΔEΔN, CA5ΔN, and R18AN21AΔN, respectively) were created by closing the Nef open reading frame by end filling of the unique _Xho_I site, resulting in a frameshift at codon 33 and expression of a truncated, nonfunctional Nef protein.
Infection assays.
The P4 cell line, a HeLa cell clone engineered to express CD4 and an integrated LTR-_lac_Z reporter construct (5), was used to detect HIV-1 infection as previously described, with the following modifications. HIV-1 stocks were diluted serially in D10 medium, and samples (0.125 ml) were added to P4 target cells plated 1 day prior (20,000 cells per well in 48-well plates). After 2 h, cultures were fed with an additional 0.5 ml of D10 medium and cultured for an additional 48 h prior to X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) staining to detect infected cells. Infected cells were quantified by counting stained cells using NIH Image software analysis of images captured with a charge-coupled device camera equipped with a macro lens.
For virion transcomplementation assays, equal quantities (equivalent to 100 ng of p24) of donor and target virions were mixed and incubated at 37°C for 1 h prior to titration on indicator target cells. In experiments where inhibitors were added after the mixing of viruses, donor and target virions were first mixed and incubated at 37°C for 1 h, followed by the addition of the soluble CD4 or 2G12 anti-gp120 monoclonal antibody and incubation for an additional 30 min. Infectivity was subsequently determined by titration on P4 or Z24-Rec-1 indicator target cells.
For analysis of HIV-1 infection by flow cytometry, an _env_-defective HIV-1 proviral clone containing the gene encoding green fluorescent protein (GFP) (15) was used for producing receptor pseudotyped target virions. Two days after infection of Z24 target cells, the cells were washed with phosphate-buffered saline (PBS), detached by trypsinization, and fixed in PBS containing 4% paraformaldehyde. Cells were analyzed for GFP expression by flow cytometry using a Becton-Dickinson FACScalibur instrument after dead cells and debris were gated out using scatter parameters.
Virus capture assays.
To detect HIV-1 incorporation of CD4, CXCR4, and CCR5, Immunlon II 96-well enzyme-linked immunosorbent assay (ELISA) plates (Dynex) were coated with anti-CD4, anti-CXCR4, anti-CCR5, or anti-gp120-specific antibodies (5 μg/ml; 0.1 ml per well) overnight at 37°C. After three washes with PBS, the wells were subsequently blocked by incubation with PBS containing 1% bovine serum albumin for 1 h at 37°C. The wells were then washed twice with PBS, and receptor-pseudotyped or control HIV-1 particles were added (50 ng of p24 in 0.1 ml) and incubated at 37°C for 2 h. Unbound virions were removed by three washes with PBS, and captured HIV-1 particles were removed by the addition of p24 ELISA sample diluent (PBS containing 5% donor calf serum and 1% Triton X-100), and viral CA antigen was quantified by p24 ELISA as previously described (34).
RESULTS
Experimental strategy for detecting intervirion fusion.
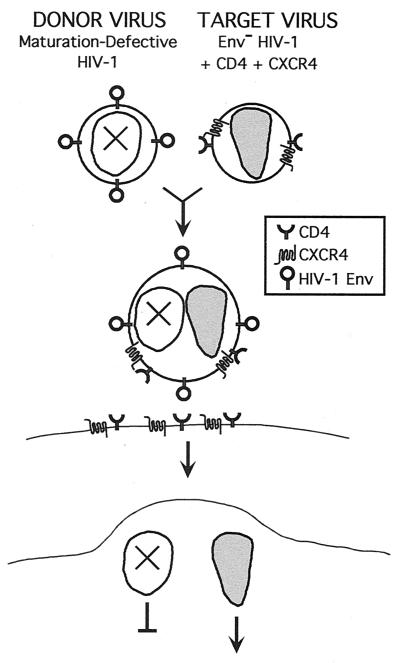
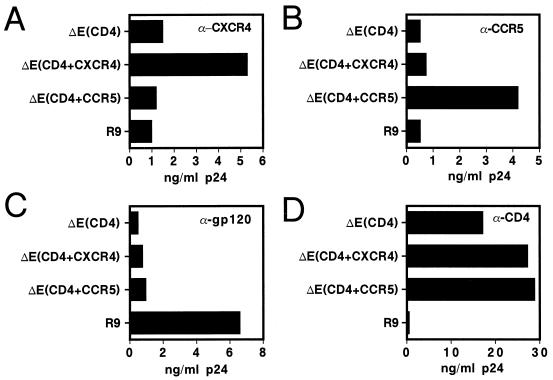
Based on a previous report that HIV-1 particles bearing CD4 and a coreceptor can infect cells expressing the cognate HIV-1 Env proteins (13), we reasoned that these receptor-pseudotyped virions might also be capable of fusing with enveloped HIV-1 particles. To test this idea, we designed a sensitive complementation assay to detect intervirion fusion products (Fig. 1). In this system, HIV-1 particles bearing defective cores were produced from proviral clones (CA5 and CA6) containing multiple mutations in the Pr55Gag cleavage sites between CA and NC, thereby blocking recognition by the viral protease and preventing core maturation. The resultant virions carry HIV-1 Env proteins on their surface and may function as fusion-competent donor virions. The receptor-pseudotyped HIV-1 particles (“target virions”) used in this system lacked HIV-1 Env proteins but contain CD4 and the HIV-1 coreceptor, CXCR4. These particles were produced by cotransfection of 293T cells with an Env-defective HIV-1 proviral clone and plasmids encoding CD4 and CXCR4. To ensure high-level CD4 expression in producer cells, a truncated form of CD4 lacking the cytoplasmic domain was used to avoid downregulation by Nef in the producer cells. Virus capture assays using antisera specific for CD4, CXCR4, and CCR5 demonstrated that these proteins were present on the virion surface only if the virus was produced from cells overexpressing these proteins (Fig. 2).
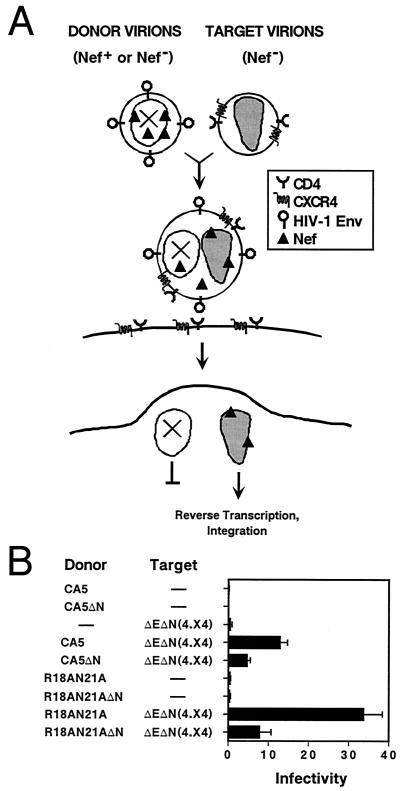
FIG. 1.
Strategy for detecting fusion between core-defective HIV-1 particles and receptor-pseudotyped HIV-1 particles. Defective HIV-1 particles, lacking functional cores due to mutations in Gag cleavage sites, are incubated with Env-defective HIV-1 particles pseudotyped by CD4 and CXCR4. While neither virus is infectious itself, fusion of the two virions is predicted to result in hybrid particles bearing a functional core and HIV-1 Env proteins. The fused virions should be capable of infecting CD4+ target cells bearing an appropriate coreceptor.
FIG. 2.
Virus capture assay for CXCR4 and CCR5 on the surface of HIV-1 particles. ELISA plates were coated with the indicated antibodies (A, α-CXCR4; B, α-CCR5; C, α-gp120; and D, α-CD4), blocked with bovine serum albumin, and virus samples were added and allowed to bind. After a washing to remove unbound virions, captured HIV-1 was removed by addition of 0.1 ml of lysis buffer and was quantified by p24 ELISA. R9, wild-type HIV-1 particles; ΔE(CD4), Env-defective particles bearing CD4; ΔE(CD4+CXCR4), Env-defective HIV-1 particles bearing CD4 and CXCR4; ΔE(CD4+CCR5), Env-defective HIV-1 particles bearing CD4 and CCR5. The results shown are representative of two independent experiments.
In agreement with a previous report (13), control experiments demonstrated that these receptor-pseudotyped HIV-1 particles were competent for infection of cells expressing HIV-1 Env proteins in a coreceptor-dependent manner (data not shown). Whereas neither donor nor target virus alone was capable of infecting CD4+ target cells, fusion of the virions was predicted to yield hybrid particles containing a functional core (from the target virion) and Env proteins (from the donor virion) at their surface. These particles should therefore be capable of infecting CD4+ target cells and be scored in single-cycle infection assays that detect Tat expression in target cells.
Infection of CD4+ target cells upon mixing of core-defective HIV-1 particles with receptor-pseudotyped HIV-1 particles.
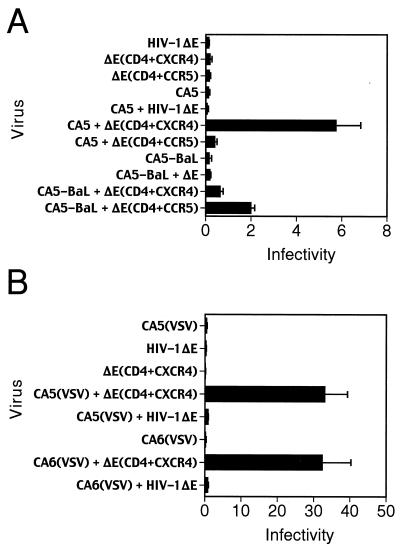
Assays of CA5 and CA6 particles alone demonstrated that these viruses were poorly infectious on CD4+ target cells, as were receptor-pseudotyped target virions. However, upon mixing of donor and target virions, a dramatic enhancement of infection (16- to 100-fold) was observed (Table 1). Infection by mixing of core-defective and receptor-pseudotyped particles, termed “virion transcomplementation,” required the presence of CD4 and a coreceptor on target virions, as demonstrated by the inability of Env-defective particles produced in the absence of these proteins (HIV-1ΔE) to serve as functional target virions (Fig. 3A). Using CXCR4-tropic donor virions, significant infection was observed only when target virions contained CXCR4, but not with target virions bearing CCR5. Similarly, mixing of core-defective, CCR5-tropic HIV-1 particles (CA5-BaL) with target virions resulted in infection that was enhanced by the presence of CCR5 on target virions (Fig. 3A). We conclude that mixing of enveloped HIV-1 particles with receptor-pseudotyped virions permits infection of CD4+ target cells in a manner that requires an appropriate Env-coreceptor interaction on donor and target virions, respectively.
TABLE 1.
Complementation of envelope-defective, receptor-pseudotyped HIV-1 particles upon mixing with core-defective virions
| Target virus | Mean infectious U ± SD/ng of p24 with donor virusa | ||
|---|---|---|---|
| None | CA5 | CA6 | |
| None | 0 | 0.68 ± 0.174 | 0.045 ± 0.011 |
| ΔE(CD4+CXCR4) | 0.032 ± 0.025 | 11.3 ± 0.74 | 5.2 ± 0.93 |
FIG. 3.
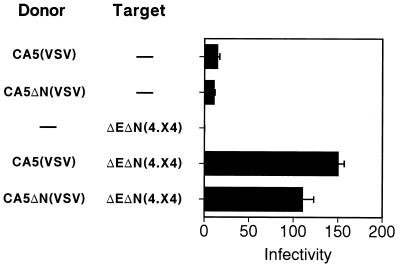
Virion transcomplementation requires a matched HIV-1 envelope-coreceptor pair on donor and target virions. (A) Infection assays were performed by mixing donor particles bearing CXCR4-tropic (CA5) and CCR5-tropic (CA5-BaL) Env proteins with receptor-pseudotyped target virions, and infection was tested using target cells bearing both coreceptors. To demonstrate the requirement for CD4 and CXCR4 on target virions, Env-defective HIV-1 particles lacking receptors (HIV-1ΔE) were used as target virions. Appreciable levels of infection were observed only when the target virions contained CD4 and an appropriate coreceptor. (B) Requirement of the HIV-1 Env proteins on donor virions and CD4 on target virions for infection of CD4-negative target cells by intervirion fusion using dual-enveloped donor particles. Core-defective particles bearing both HIV-1 and VSV Env proteins virions [CA5(VSV) or CA6(VSV)] were mixed with receptor-pseudotyped target virions, and the virus mixtures were used to infect HeLa target cells lacking CD4 (Z24 cells). The mean values of triplicate infections are shown; error bars represent one standard deviation. Results are representative of at least two independent experiments.
Virion transcomplementation occurs through intervirion fusion.
In light of these findings, we hypothesized that virion transcomplementation occurs through the fusion of donor and target virions prior to fusion with target cells. An alternative hypothesis is that infection occurs through a two-step mechanism in which enveloped donor virions fuse with target cells, depositing HIV-1 Env proteins on the cell surface and allowing subsequent fusion of receptor pseudotyped target virions. To distinguish between these mechanisms, we created core-defective donor virions carrying both HIV-1 and VSV Env proteins by transfection of either CA5 or CA6 clones with a VSV–G-protein (VSV-G) expression construct. The resulting particles, though noninfectious themselves, resulted in infection of CD4-negative HeLa cells (Z24 cells) upon mixing with receptor pseudotyped virions (Fig. 3B). Infection of CD4-negative cells indicated that entry was mediated through the receptor for VSV-G. However, the presence of CD4 and coreceptor on target virions was required for infection, as demonstrated by inability of Env-defective HIV-1 particles lacking these receptors to serve as targets (Fig. 3B). Fusion of HIV-1(VSV) pseudotyped particles requires exposure to the low-pH environment of endosomes, implying internalization through endocytosis for infection by these particles (1). Therefore, it is unlikely that infection was due to prior fusion of the enveloped particles with target cells at the plasma membrane and subsequent fusion of CD4-pseudotyped HIV-1 particles. Furthermore, the lack of infection observed in the absence of CD4 and coreceptor on target virions indicated that virion transcomplementation was not mediated by the VSV-G protein. These results support intervirion fusion as the most probable mechanism of infection.
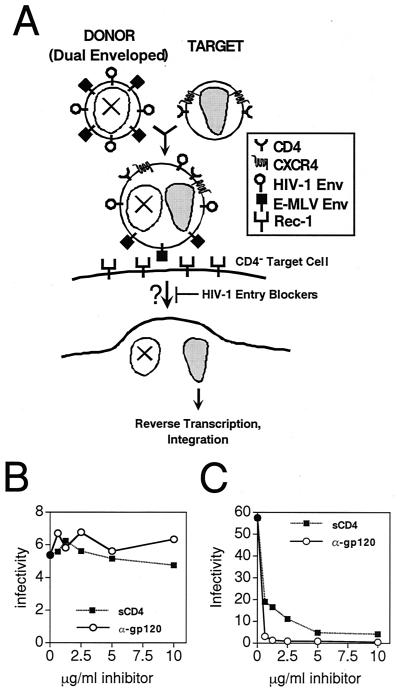
To further probe the mechanism of virion transcomplementation, we hypothesized that fusion of dual enveloped donor virions with receptor-pseudotyped target virions would result in infectious particles that no longer require the activity of the HIV-1 Env proteins (Fig. 4). To test this hypothesis, we created donor particles bearing both HIV-1 and E-MLV envelope proteins, and mixed these particles with CD4-pseudotyped HIV-1 virions. The E-MLV envelope, rather than VSV-G, was specifically chosen for this purpose to avoid the possibility of incorporation of its cognate receptor, which is not expressed in human cells, into target virions. The mixed virions infected target cells expressing the E-MLV receptor Rec-1 but lacking CD4, while neither donor nor target virus alone was infectious (data not shown).
FIG. 4.
Evidence for intervirion fusion as the mechanism of complementation. (A) Strategy for infection of CD4-negative, Rec-1 expressing target cells by virion transcomplementation using donor virions bearing both HIV-1 and E-MLV Env proteins. Virion transcomplementation is predicted to result in particles that no longer require the function of the HIV-1 Env proteins for infection of target cells expressing the E-MLV receptor Rec-1. Infection should therefore acquire resistance to specific inhibitors of HIV-1 entry. (B and C) Resistance of intervirion fusion products to neutralization by soluble CD4 (■) and anti-gp120 monoclonal antibody 2G12 (○). Inhibitors were added at the indicated concentrations after premixing of the two viruses. Infectivity was measured using CD4− target cells expressing Rec-1, the E-MLV receptor, and is represented in units of infected cells per nanogram of p24 in the sample. (B) Infection by mixing of donor and target virions. (C) Infection by wild-type HIV-1.
To determine whether infection was resistant to HIV-1 entry inhibitors, donor and target were mixed and preincubated at 37°C to permit intervirion fusion. Subsequently, either soluble CD4 or the gp120-specific HIV-1 neutralizing antibody 2G12 was titrated into parallel samples. After further incubation to allow inhibitor binding, the samples were assayed on Rec-1-expressing target cells to allow infection using the E-MLV envelope protein. Neither inhibitor exhibited a significant reduction of infection by the mixed virions (Fig. 4B). In contrast, infection of CD4-expressing cells by wild-type HIV-1 particles was efficiently blocked by low concentrations of either inhibitor (Fig. 4C). Preincubation of the E-MLV-pseudotyped donor virus with sCD4 prior to mixing with target virions resulted in 98% inhibition of infection, demonstrating the effectiveness of the inhibitor and further establishing the requirement for a functional HIV-1 Env protein for complementation (data not shown). These results demonstrate that infection in this system acquires resistance to inhibitors of HIV-1 entry, providing evidence for intervirion fusion prior to infection of target cells.
Nef enhances infection resulting from virion transcomplementation.
Nef enhances the infectivity of cell-free HIV-1 particles when tested in single cycle assays, but the mechanism by which Nef mediates this function is poorly understood. Infectivity enhancement by Nef requires its expression in producer cells at the time of HIV-1 particle formation, and although the Nef protein is incorporated into HIV-1 particles, a functional role for virion-associated Nef has not been demonstrated (2, 25, 27). To explore the potential role of virion-associated Nef in HIV-1 infection, we employed the virion transcomplementation system using Nef+ and Nef− donor virions (Fig. 5A). In this approach, core-defective HIV-1 donor particles, containing or lacking Nef, are mixed with CD4-pseudotyped, Nef-defective HIV-1 target virions. If Nef functions within the virion, then transfer of Nef from donor particles to target virions via intervirion fusion might result in enhanced infection, assuming that Nef localized to its functional target within the hybrid particle.
FIG. 5.
Effect of Nef on infection by virion transcomplementation. (A) Strategy for detecting an effect of Nef in the donor virus. Core-defective HIV-1 particles carrying Nef are incubated with Nef-defective, receptor-pseudotyped target virions prior to the addition to target cells. If Nef is functional within the HIV-1 particle, then transfer of Nef by intervirion fusion is predicted to enhance infection. (B) Results of intervirion fusion assays for a functional role of virion-associated Nef. Two core-defective HIV-1 clones (CA5, and the CA double point mutant R18AN21A) were rendered Nef defective (ΔN) and used to produce donor virions. These particles were incubated with Env-defective, Nef-defective virions pseudotyped with CD4 and CXCR4 [ΔEΔN(4.X4)], and the mixtures were assayed for infectivity on HeLa-CD4 target cells. The mean infectivity values of triplicate determinations are shown as the number of infectious units per nanogram of p24, with error bars representing one standard deviation. The results demonstrated an approximately threefold enhancement by Nef in the donor virions and are representative of three independent experiments.
To test this idea, we employed two core-defective donor viruses. The CA double point mutant R18AN21A forms aberrant cores, probably due to altered CA-CA interactions (U. von Schwedler and W. Sundquist, personal communication). HIV-1 proviral clones encoding these mutations, as well as the Pr55Gag cleavage site mutant CA5, were rendered Nef defective by closing the Nef open reading frame at codon 36. Donor virions were produced from mutant proviruses encoding functional or defective nef genes, and equal quantities of Nef+ or Nef− donor particles, as determined by p24 ELISA, were added to Nef-defective target virions [ΔEΔN(4.X4)]. After incubation at 37°C for 1 h, the mixtures were titrated on HeLa-CD4 target cells to quantify infectivity. For both core-defective mutants, infection upon mixing with receptor-pseudotyped, Nef-defective target virions was enhanced by approximately threefold when Nef was present within donor virus particles (Fig. 5B). To examine the dose dependence of this effect, we repeated these experiments and varied the ratio of donor to target virions. At a 4:1 ratio, the effect of Nef increased to approximately fivefold (Table 2). Collectively, these results demonstrate that Nef can enhance HIV-1 infectivity even when absent during the time of viral core formation.
TABLE 2.
Effect of altered ratios of donor to target virus on infectivity enhancement by Nef
| Donor virus | Mean infectious U ± SD/ng of p24 at donor-to-target-virus particle ratioa | ||
|---|---|---|---|
| 1:1 | 2:1 | 4:1 | |
| CA5 | 9.8 ± 1.3 | 18.6 ± 1.6 | 27.0 ± 2.3 |
| CA5ΔN | 2.9 ± 0.6 | 4.7 ± 1.4 | 5.0 ± 0.5 |
Infectivity of virion transcomplementation by Nef depends on the pathway of entry into the target cell.
Based on previous results from HIV-1 entry and pseudotyping experiments, it has generally been assumed that Nef affects a step in the HIV-1 life cycle following virus-cell fusion (2, 25, 32). However, pseudotyping HIV-1 particles by VSV-G relieves the requirement for Nef (1, 20), probably due to a difference in the pathway of entry of HIV-1(VSV) pseudotyped particles (6). To determine whether Nef present in the donor virus might enhance the efficiency of intervirion fusion, we tested whether donor virions containing both HIV-1 and VSV envelope proteins exhibited a dependence on Nef in the virion transcomplementation assay system. Nef+ and Nef− donor particles bearing HIV-1 and VSV Env proteins were incubated with Nef-defective target virions at 37°C in medium buffered to pH 7.4 with HEPES, thereby preventing VSV-G-induced intervirion fusion. The virus mixtures were then assayed for infection of CD4-negative target cells. In contrast to donor virions containing only the HIV-1 envelope, intervirion fusion products were not significantly enhanced by Nef when infection required entry catalyzed by VSV-G (Fig. 6). Experiments employing a target virus encoding GFP and flow cytometric analysis for detection of GFP expression in target cells confirmed this result and demonstrated that the integrated provirus is derived from the target virus genome (Table 3).
FIG. 6.
Virion transcomplementation is minimally dependent on Nef when donor virions are pseudotyped by VSV-G. Core-defective virions containing or lacking Nef were produced by contransfection with or without a VSV-G expression vector, resulting in particles bearing VSV and/or HIV-1 Env proteins. These particles were incubated with receptor-pseudotyped target virions prior to addition to CD4-negative target cells, allowing infection only through the VSV-G receptor. The mean infectivity values of triplicate determinations are shown as the number of infectious units per nanogram of p24, with error bars representing one standard deviation. In contrast to the results with nonpseudotyped donor virions (Fig. 5), only a relatively small enhancement by Nef was observed in this system.
TABLE 3.
Flow cytometric analysis of HIV-1 infection via virion transcomplementation
| Donor virus | % GFP-positive cells with target virusa | |
|---|---|---|
| None | ΔE-GFP(4.X4) | |
| None | 0.01 | 0.01 |
| CA5 | ND | 5.3 |
| CA5ΔN | ND | 0.83 |
| CA5(VSV) | 0.01 | 77.8 |
| CA5ΔN(VSV) | 0.02 | 70.5 |
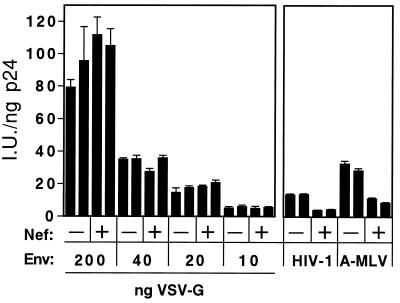
We have previously shown that pseudotyping by VSV-G results in dramatically enhanced infectivity of HIV-1 particles (1). The virion transcomplementation assays using VSG-G pseudotyped donor virions also resulted in much higher levels of infection. Therefore, to test whether the minimal contribution of Nef to infection by the VSV-G pseudotyped donor virions resulted from the relatively higher infectivity of these virions, we repeated the experiment using donor virions containing reduced quantities of VSV-G. Reduction of VSV-G on donor virions resulted in decreased infectivity upon mixing with target virions without increasing the effect of Nef in this system (Fig. 7). As an additional control, pseudotyped donor virions bearing both HIV-1 and A-MLV Env proteins were tested. Like the nonpseudotyped donor virions, the infectivity resulting from the use of the CA5(A-MLV) donor virions was enhanced by approximately threefold by Nef. These results argue against a role of Nef on the efficiency of intervirion fusion itself, and demonstrate that the requirement for Nef in infection by virion transcomplementation exhibits the same envelope dependence as enhancement of normal HIV-1 infection by Nef.
FIG. 7.
The minimal effect of Nef on virion transcomplementation using VSV-G-pseudotyped donor virions is not a consequence of overall enhancement by VSV-G. Nef+ and Nef− donor virions (CA5 and CA5ΔN, respectively) were produced by cotransfection with the indicated quantities of VSV-G expression plasmid and mixed with receptor-pseudotyped target virions. Infection was assayed as in Fig. 6. As controls, nonpseudotyped donor virions (HIV-1) and A-MLV pseudotyped virions were used as donor virions, and infection was scored on CD4+ and CD4− cells, respectively. The duplicate bars represent independent preparations of donor viruses assayed in parallel.
The ability of Nef to enhance infection through virion transcomplementation is specific to donor virus particles.
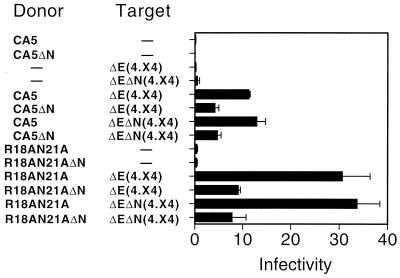
To determine whether the effect of Nef was specific to donor or target virus particles, virion transcomplementation assays were performed using Nef+ and Nef− target virus particles. Two donor virus pairs, containing either the CA5 or R18AN21A mutations, respectively, were used in these experiments. Mixing of donor and target virions resulted in levels of infection that were enhanced by approximately threefold when the donor virus expressed Nef (Fig. 8). Surprisingly, the efficiency of infection was not significantly affected by the Nef status of the target virus. Control experiments demonstrated that HIV-1 particles produced from the Env-defective proviral clones were strongly dependent on Nef when pseudotyped with either HIV-1 or amphotropic MLV envelope glycoproteins, confirming the Nef status of the proviral clones used in these experiments (data not shown).
FIG. 8.
Nef enhances infection through a specific effect on the donor virus. Equal quantities of Nef+ and Nef− donor viruses were mixed with Nef+ and Nef− target viruses [ΔE(4.X4) and ΔEΔN(4.X4), respectively], and infection was scored on CD4+ target cells. The mean values of triplicate infections are shown, with error bars representing one standard deviation. The results are representative of three independent experiments.
DISCUSSION
In this report, we describe a novel genetic assay to detect fusion events involving Env-defective HIV-1 particles bearing the HIV-1 receptor complex and core-defective HIV-1 particles bearing functional Env proteins. Neither virus was independently capable of infecting CD4+ target cells. However, mixing of the virions dramatically enhanced infection to levels that represent approximately 5% of the wild-type HIV-1 infectivity. Although the overall efficiency of virion transcomplementation was relatively low, intervirion fusion events were readily detected using a sensitive single-cycle assay of infection. Infection required CD4 and an appropriate coreceptor on target virions and a functional Env protein complex on donor virions. In a related study, Sparacio et al. also reported infection upon mixing of receptor-pseudotyped particles and enveloped particles (33). However, in the latter study, alternative mechanisms of complementation not involving intervirion fusion were not excluded. Here we demonstrate that virion transcomplementation results in acquisition of resistance to inhibitors of HIV-1 entry when the donor virions carry an additional heterologous Env protein. These results provide strong evidence for intervirion fusion as the mechanism of infection in this system.
The finding that intervirion fusion occurs in vitro has important implications for understanding the mechanism of HIV-1 entry. For some enveloped viruses, fusion systems have been reconstituted using artificial liposomes or planar lipid bilayers and virus particles, allowing precise control of the composition of the target membranes (21, 37, 38). For these viruses, fusion has been demonstrated in the absence of a target cell, indicating that cytoskeletal components or molecules other than the known viral receptor are not required for fusion. For HIV-1, it has been shown that receptor-pseudotyped particles can fuse with target cells expressing the viral Env proteins, demonstrating the lack of a strict orientation dependence of the virus-cell fusion machinery (13). Our finding that virions are capable of fusing with each other in the absence of a target cell extends these observations by demonstrating that specific cellular structures, such as an intact cytoskeleton, are not absolute requirements for HIV-1-catalyzed membrane fusion.
Virion transcomplementation also represents a potential mechanism for enhancing HIV-1 diversity in vivo. HIV-1 persistence is likely promoted by the rapid evolution of viral variants. Viral evolution represents a major obstacle for vaccine design and for drug therapy due to the ease in which viral escape mutants are generated. The observation that intervirion fusion results in temporary rescue of _env_-defective genomes suggests that this mechanism might increase the probability of reversion through the acquisition of additional mutations, thereby facilitating viral evolution in vivo. In our experiments, target virions were produced in cells overexpressing both CD4 and HIV-1 coreceptors. Whether virion transcomplementation can be detected using target virions produced in primary cells remains to be determined.
In this study, we employed the intervirion fusion system to probe the mechanism by which Nef enhances the infectivity of HIV-1 particles. Infection was enhanced when Nef was present at the time of donor virus production. Studies in our laboratory and others have concluded that _nef_-defective particles are competent for entry into target cells but are inhibited at a postentry step prior to, or concomitant with, initiation of reverse transcription, such as viral uncoating (2, 9, 32). The ability of VSV-G, which targets HIV-1 infection to an endocytic route, to alleviate the requirement for Nef in HIV-1 infection, is consistent with this conclusion (1, 20). No structural or biochemical differences between wild-type and _nef_-defective HIV-1 particles have been reported other than the presence of Nef itself. However, a functional role of virion-associated Nef in HIV-1 infection remains elusive. Our observation that Nef enhances infection resulting from virion transcomplementation indicates that the virion modification induced by Nef can be transferred from donor to target virions during intervirion fusion. The modification could be the presence of Nef itself or a Nef-associated molecule. Alternatively, Nef may modify another virion component during assembly and/or maturation.
A surprising outcome of this study is that expression of Nef during target virus production had no effect on infection resulting from virion transcomplementation. This result suggests that Nef does not act by modifying the viral core but may alter the function of the viral Env protein complex. Schaeffer et al. have recently reported that Nef promotes the cytoplasmic delivery of HIV-1 cores into target cells, suggesting that Nef enhances virus-cell fusion (31). Although Nef may act at multiple stages in HIV-1 infection, our results are more consistent with a mechanism involving the enhancement of postentry events. Using donor virus particles containing both HIV-1 Env and VSV-G, we observed a minimal effect of Nef on infection of CD4-negative target cells. However, infection required both CD4 and an appropriate coreceptor on target virions, demonstrating that intervirion fusion was not significantly enhanced by VSV-G. We conclude that Nef does not enhance the fusion of donor and target virions. Infectivity enhancement by Nef is not unique to the HIV-1 Env glycoproteins, as the infectivity of HIV-1(A-MLV) pseudotyped particles is also strongly stimulated by Nef. We have recently observed that Nef does not enhance the infectivity of A-MLV-based retroviral vector particles, indicating that the viral Env protein complex is not sufficient for Nef-mediated infectivity enhancement (J. Zhou and C. Aiken, unpublished observations).
How could Nef modify the function of the viral envelope without affecting fusion? HIV-1 budding occurs from glycolipid-enriched microdomains of the plasma membrane (26). Nef may alter the lipid composition and structure of the envelope so as to enhance release of the core into the cytoplasm following fusion. Additional studies will be needed to define precisely the mechanism by which Nef stimulates HIV-1 infection.
ACKNOWLEDGMENTS
We thank Lorraine Albritton, Uta von Schwedler, Wes Sundquist, Hans-Georg Krausslich, and Dana Gabuzda for plasmids and Dean Ballard, Terry Dermody, Sebastian Joyce, Paul Spearman, and members of the Aiken laboratory for constructive suggestions. The following reagents were obtained through the NIH AIDS Research and Reference Reagent Program: pSV-E-MLV-env, pSVψ−MLV-_env_− and pBABE.CCR5 from Ned Landau; recombinant soluble CD4 from Ray Sweet, SmithKline Beecham; and HIV-1 gp120 monoclonal antibody 2G12 from Hermann Katinger.
This work was supported by grants AI40364 and AI47056 from the National Institutes of Health.
REFERENCES
- 1.Aiken C. Pseudotyping human immunodeficiency virus type 1 (HIV-1) by the glycoprotein of vesicular stomatitis virus targets HIV-1 entry to an endocytic pathway and suppresses both the requirement for Nef and the sensitivity to cyclosporin A. J Virol. 1997;71:5871–5877. doi: 10.1128/jvi.71.8.5871-5877.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aiken C, Trono D. Nef stimulates human immunodeficiency virus type 1 proviral DNA synthesis. J Virol. 1995;69:5048–5056. doi: 10.1128/jvi.69.8.5048-5056.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balliet J W, Bates P. Efficient infection mediated by viral receptors incorporated into retroviral particles. J Virol. 1998;72:671–676. doi: 10.1128/jvi.72.1.671-676.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bukovsky A, Dorfman T, Weimann A, Gottlinger H G. Nef association with human immunodeficiency virus type 1 virions and cleavage by the viral protease. J Virol. 1997;71:1013–1018. doi: 10.1128/jvi.71.2.1013-1018.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Charneau P, Alizon M, Clavel F. A second origin of DNA plus-strand synthesis is required for optimal human immunodeficiency virus replication. J Virol. 1992;66:2814–2820. doi: 10.1128/jvi.66.5.2814-2820.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chazal N, Singer G, Aiken C, Hammarskjold M-L, Rekosh D. Human immunodeficiency virus type 1 particles pseudotyped by envelope proteins that fuse at low pH no longer require Nef for optimal infectivity. J Virol. 2001;75:4014–4018. doi: 10.1128/JVI.75.8.4014-4018.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen C, Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987;7:2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y-L, Trono D, Camaur D. The proteolytic cleavage of human immunodeficiency virus type 1 Nef does not correlate with its ability to stimulate virion infectivity. J Virol. 1998;72:3178–3184. doi: 10.1128/jvi.72.4.3178-3184.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chowers M Y, Pandori M W, Spina C A, Richman D D, Guatelli J C. The growth advantage conferred by HIV-1 nef is determined at the level of viral DNA formation and is independent of CD4 downregulation. Virology. 1995;212:451–457. doi: 10.1006/viro.1995.1502. [DOI] [PubMed] [Google Scholar]
- 10.Chowers M Y, Spina C A, Kwoh T J, Fitch N J S, Richman D D, Guatelli J C. Optimal infectivity in vitro of human immunodeficiency virus type 1 requires an intact nef gene. J Virol. 1994;68:2906–2914. doi: 10.1128/jvi.68.5.2906-2914.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 12.Emerman M, Malim M. HIV-1 regulatory/accessory genes: keys to unraveling viral and host cell biology. Science. 1998;280:1880–1884. doi: 10.1126/science.280.5371.1880. [DOI] [PubMed] [Google Scholar]
- 13.Endres M J, Jaffer S, Haggarty B, Turner J D, Doranz B J, O'Brien P J, Kolson D L, Hoxie J A. Targeting of HIV- and SIV-infected cells by CD4-chemokine receptor pseudotypes. Science. 1997;278:1462–1464. doi: 10.1126/science.278.5342.1462. [DOI] [PubMed] [Google Scholar]
- 14.Hanna Z, Kay D G, Rebai N, Guimond A, Jothy S, Jolicoeur P. Nef harbors a major determinant of pathogenicity for an AIDS-like disease induced by HIV-1 in transgenic mice. Cell. 1998;95:163–175. doi: 10.1016/s0092-8674(00)81748-1. [DOI] [PubMed] [Google Scholar]
- 15.He J, Chen Y, Farzan M, Choe H, Ohagen A, Gartner S, Busciglio J, Yang X, Hofmann W, Newman W, Mackay C R, Sodroski J, Gabuzda D. CCR3 and CCR5 are co-receptors for HIV-1 infection of microglia. Nature. 1997;385:645–649. doi: 10.1038/385645a0. [DOI] [PubMed] [Google Scholar]
- 16.Kestler H W, III, Ringler D J, Mori K, Panicali D L, Sehgal P K, Daniel M D, Desrosiers R C. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell. 1991;65:651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- 17.Kirchhoff F, Greenough T C, Brettler D B, Sullivan J L, Desrosiers R C. Brief report: absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N Engl J Med. 1995;332:228–232. doi: 10.1056/NEJM199501263320405. [DOI] [PubMed] [Google Scholar]
- 18.Kotov A, Zhou J, Flicker P, Aiken C. Association of Nef with the human immunodeficiency virus type 1 core. J Virol. 1999;73:8824–8830. doi: 10.1128/jvi.73.10.8824-8830.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Landau N R, Littman D R. Packaging system for rapid production of murine leukemia virus vectors with variable tropism. J Virol. 1992;66:5110–5113. doi: 10.1128/jvi.66.8.5110-5113.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo T, Douglas J L, Livingston R L, Garcia J V. Infectivity enhancement by HIV-1 Nef is dependent on the pathway of virus entry: implications for HIV-based gene transfer systems. Virology. 1998;241:224–233. doi: 10.1006/viro.1997.8966. [DOI] [PubMed] [Google Scholar]
- 21.Maeda T, Kawasaki K, Ohnishi S. Interaction of influenza virus hemagglutinin with target membrane lipids is a key step in virus-induced hemolysis and fusion at pH 5.2. Proc Natl Acad Sci USA. 1981;78:4133–4137. doi: 10.1073/pnas.78.7.4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mangasarian A, Foti M, Aiken C, Chin D, Carpentier J-L, Trono D. The HIV-1 Nef protein acts as a connector with sorting pathways in the Golgi and at the plasma membrane. Immunity. 1997;6:67–77. doi: 10.1016/s1074-7613(00)80243-5. [DOI] [PubMed] [Google Scholar]
- 23.Mariani R, Kirchhoff F, Greenough T C, Sullivan J L, Desrosiers R C, Skowronski J. High frequency of defective nef alleles in a long-term survivor with nonprogressive human immunodeficiency virus type 1 infection. J Virol. 1996;70:7752–7764. doi: 10.1128/jvi.70.11.7752-7764.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller M D, Warmerdam M T, Gaston I, Greene W C, Feinberg M B. The human immunodeficiency virus-1 nef gene product: a positive factor for viral infection and replication in primary lymphocytes and macrophages. J Exp Med. 1994;179:101–113. doi: 10.1084/jem.179.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller M D, Warmerdam M T, Page K A, Feinberg M B, Greene W C. Expression of the human immunodeficiency virus type 1 (HIV-1) nef gene during HIV-1 production increases progeny particle infectivity independently of gp160 or viral entry. J Virol. 1995;69:570–584. doi: 10.1128/jvi.69.1.579-584.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nguyen D H, Hildreth J E K. Evidence for budding of human immunodeficiency virus type 1 selectively from glycolipid-enriched membrane lipid rafts. J Virol. 2000;74:3264–3272. doi: 10.1128/jvi.74.7.3264-3272.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pandori M W, Fitch N J S, Craig H M, Richman D D, Spina C A, Guatelli J C. Producer-cell modification of human immunodeficiency virus type 1: Nef is a virion protein. J Virol. 1996;70:4283–4290. doi: 10.1128/jvi.70.7.4283-4290.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peter F. HIV Nef: the mother of all evil? Immunity. 1998;9:433–437. doi: 10.1016/s1074-7613(00)80626-3. [DOI] [PubMed] [Google Scholar]
- 29.Premkumar D R, Ma X Z, Maitra R K, Chakrabarti B K, Salkowitz J, Yen-Lieberman B, Hirsch M S, Kestler H W. The nef gene from a long-term HIV type 1 nonprogressor. AIDS Res Hum Retrovir. 1996;12:337–345. doi: 10.1089/aid.1996.12.337. [DOI] [PubMed] [Google Scholar]
- 30.Salvi R, Garbuglia A R, Di Caro A, Pulciani S, Montella F, Benedetto A. Grossly defective nef gene sequences in a human immunodeficiency virus type 1-seropositive long-term nonprogressor. J Virol. 1998;72:3646–3657. doi: 10.1128/jvi.72.5.3646-3657.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schaeffer E, Geleziunas R, Greene W C. Human immunodeficiency virus type 1 Nef functions at the level of virus entry by enhancing cytoplasmic delivery of virions. J Virol. 2001;75:2993–3000. doi: 10.1128/JVI.75.6.2993-3000.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwartz O, Marechal V, Danos O, Heard J-M. Human immunodeficiency virus type 1 Nef increases the efficiency of reverse transcription in the infected cell. J Virol. 1995;69:4053–4059. doi: 10.1128/jvi.69.7.4053-4059.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sparacio S, Zeilfelder U, Pfeiffer T, Henzler T, Bosch V. Membrane fusion between retroviral particles: host-range extension and vaccine prospects. Virology. 2000;271:248–252. doi: 10.1006/viro.2000.0294. [DOI] [PubMed] [Google Scholar]
- 34.Wehrly K, Chesebro B. p24 antigen capture assay for quantification of human immunodeficiency virus using readily available inexpensive reagents. Methods. 1997;12:288–293. doi: 10.1006/meth.1997.0481. [DOI] [PubMed] [Google Scholar]
- 35.Welker R, Harris M, Cardel B, Krausslich H-G. Virion incorporation of human immunodeficiency virus type 1 Nef is mediated by a bipartite membrane-targeting signal: analysis of its role in enhancement of viral infectivity. J Virol. 1998;72:8833–8840. doi: 10.1128/jvi.72.11.8833-8840.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Welker R, Kottler H, Kalbitzer H R, Krausslich H-G. Human immunodeficiency virus type 1 Nef protein is incorporated into virus particles and specifically cleaved by the viral proteinase. Virology. 1996;219:228–236. doi: 10.1006/viro.1996.0240. [DOI] [PubMed] [Google Scholar]
- 37.White J, Helenius A. pH-dependent fusion between the Semliki Forest virus membrane and liposomes. Proc Natl Acad Sci USA. 1980;77:3273–3277. doi: 10.1073/pnas.77.6.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.White J, Kartenbeck J, Helenius A. Membrane fusion activity of influenza virus. EMBO J. 1982;1:217–222. doi: 10.1002/j.1460-2075.1982.tb01150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wiegers K, Rutter G, Kottler H, Tessmer U, Hohenberg H, Krausslich H-G. Sequential steps in human immunodeficiency virus particle maturation revealed by alterations of individual Gag polyprotein cleavage sites. J Virol. 1998;72:2846–2854. doi: 10.1128/jvi.72.4.2846-2854.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yee J K, Friedmann T, Burns J C. Generation of high-titer pseudotyped retroviral with very broad host range. Methods Cell Biol. 1994;43:99–112. doi: 10.1016/s0091-679x(08)60600-7. [DOI] [PubMed] [Google Scholar]