Cross-Reactivity between Hepatitis C Virus and Influenza A Virus Determinant-Specific Cytotoxic T Cells (original) (raw)
Abstract
The cellular immune response contributes to viral clearance as well as to liver injury in acute and chronic hepatitis C virus (HCV) infection. An immunodominant determinant frequently recognized by liver-infiltrating and circulating CD8+ T cells of HCV-infected patients is the HCVNS3-1073 peptide CVNGVCWTV. Using a sensitive in vitro technique with HCV peptides and multiple cytokines, we were able to expand cytotoxic T cells specific for this determinant not only from the blood of 11 of 20 HCV-infected patients (55%) but also from the blood of 9 of 15 HCV-negative blood donors (60%), while a second HCV NS3 determinant was recognized only by HCV-infected patients and not by seronegative controls. The T-cell response of these healthy blood donors was mediated by memory T cells, which cross-reacted with a novel T-cell determinant of the A/PR/8/34 influenza A virus (IV) that is endogenously processed from the neuraminidase (NA) protein. Both the HCV NS3 and the IV NA peptide displayed a high degree of sequence homology, bound to the HLA-A2 molecule with high affinity, and were recognized by cytotoxic T lymphocytes with similar affinity (10−8 M). Using the HLA-A2-transgenic mouse model, we then demonstrated directly that HCV-specific T cells could be induced in vivo by IV infection. Splenocytes harvested from IV-infected mice at the peak of the primary response (day 7 effector cells) or following complete recovery (day 21 memory cells) recognized the HCV NS3 peptide, lysed peptide-pulsed target cells, and produced gamma interferon. These results exemplify that host responses to an infectious agent are influenced by cross-reactive memory cells induced by past exposure to heterologous viruses, which could have important consequences for vaccine development.
Recovery from acute hepatitis C virus (HCV) infection has been associated with an early, multispecific helper and cytotoxic T lymphocyte (CTL) response (10, 26) that is maintained for at least 2 decades after recovery and is significantly weaker in chronically infected patients (40). Both viral and host factors have been implicated in this differential cellular immune response and outcome of infection.
Interestingly, it has recently been demonstrated that HCV-specific CD8+ T cells could also be expanded from the peripheral blood memory T-cell populations of some control persons who were not HCV infected (6, 8). Several possibilities have been discussed to explain this observation. First, these individuals may have had a self-limited HCV infection in the distant past and subsequently maintained cellular immune responses in the absence of persisting humoral responses (40). Second, healthy subjects may have been exposed to HCV occupationally (21) or via infected family members (33) and generated and maintained CD45RO+ memory T cells (33) in the absence of any detectable viremia or disease. Third, a primary HCV-specific CD8+ T-cell response may have been induced in vitro by repetitive and prolonged stimulation with HCV-specific peptides. While the last hypothesis might be compatible with the observation that HCV-specific CD8+ T cells could be expanded with individual, but not all, peptides representing HCV determinants, it does not readily explain the finding that HCV-specific CD8+ T cells could be isolated from the blood of only some and not all HCV-negative subjects (6, 8). Similar to studies with HCV-infected patients (6), HCV peptide-specific T-cell responses were eliminated when CD45RO+ memory cells were depleted from peripheral blood mononuclear cells (PBMC) of HCV-negative subjects without any history of prior HCV infection (33). This finding in humans as well as studies with rodents (34) suggested that these T cells may represent cross-reacting memory T cells that recognize other pathogens.
This study was designed to identify heterologous antigens that induce cross-reactive CD8+ T cells specific for an HLA-A2-restricted, immunodominant HCV NS3 determinant and to characterize the induction and effector function of these cross-reactive T cells in vitro and in vivo.
MATERIALS AND METHODS
Patient population.
Thirty-five HLA-A2-positive individuals were studied. Twenty patients were chronically infected with HCV and had been HCV RNA positive for at least 5 years. Fifteen HLA-A2-positive healthy blood donors without a history of hepatitis B virus (HBV) or HCV infection served as normal controls. None of the chronically infected patients had received antiviral therapy for hepatitis C. All patients had been monitored for at least 5 years and were seen twice a year in the Liver Diseases Section or the Department of Transfusion Medicine at the National Institutes of Health (NIH), Bethesda, Md. All patients and normal controls were tested for anti-HCV with a third-generation enzyme immunoassay. Nested PCR for HCV RNA and genotyping were done as previously described (23, 40). Liver biopsies were performed for 13 chronically infected patients within 3 months of lymphocyte collection for this study, and none displayed evidence of cirrhosis. Baseline characteristics, histological findings, and virological and biochemical features for the patients are summarized in Table 1. All patients were participants in studies of the natural history and therapy of hepatitis and gave informed consent for participation in this study. The details of the study were approved by the National Institute of Diabetes and Digestive and Kidney Diseases, NIH, institutional review board.
TABLE 1.
Patient characteristics and HCVNS3-1073 and HCVNS3-1406 peptide-specific cytotoxic activity of T-cell lines derived from PBMC of patients with chronic hepatitis C
| Patient | HCV genotype | HCV RNA (106copies/ml) | ALTa(U/ml) | Liver histologyb | Specific lysis (%) ofc: | ||
|---|---|---|---|---|---|---|---|
| Inflammatory score | Fibrosis score | HCVNS3-1073-loaded target cells | HCVNS3-1406-loaded target cells | ||||
| Chr-1 | 1b | 30.9 | 25 | 7 | 0 | 48 | 29 |
| Chr-2 | 2b | 6.1 | 35 | 9 | 0 | 59 | 53 |
| Chr-3 | 2b | 4.7 | 14 | NDd | ND | 19 | 5 |
| Chr-4 | 1b | 0.4 | 18 | 5 | 0 | 6 | 2 |
| Chr-5 | 2b | 43 | 25 | ND | ND | 43 | 8 |
| Chr-6 | 1a | 7.0 | 27 | 3 | 0 | 0 | 0 |
| Chr-7 | UGe | 43.1 | 32 | ND | ND | 3 | 4 |
| Chr-8 | 1b | 1.3 | 22 | ND | ND | 27 | 7 |
| Chr-9 | 1b | 0.2 | 22 | ND | ND | 44 | 12 |
| Chr-10 | 2b | 6.0 | 20 | ND | ND | 2 | 21 |
| Chr-11 | 1a | 0.3 | 60 | 6 | 0 | 42 | 42 |
| Chr-12 | 1a | 21.1 | 59 | 8 | 1 | 44 | 36 |
| Chr-13 | 1a | 1.2 | 55 | 6 | 0 | 9 | 43 |
| Chr-14 | 1a | 6.4 | 40 | 5 | 0 | 2 | 5 |
| Chr-15 | 1b | 0.5 | 50 | 7 | 1 | 3 | 3 |
| Chr-16 | 1a | 0.5 | 91 | 3 | 0 | 18 | 15 |
| Chr-17 | 1a | 2.5 | 55 | 6 | 0 | 2 | 28 |
| Chr-18 | 1a | 31.7 | 69 | ND | ND | 3 | 16 |
| Chr-19 | 1a | 6.8 | 51 | 7 | 1 | 38 | 0 |
| Chr-20 | 1a | 1.4 | 49 | 7 | 0 | 48 | 26 |
| No. of patients positive/total (% positive) | 11/20 (55) | 11/20 (50) | |||||
| Mean cytotoxicityf ± SD | 39 ± 12 | 29 ± 12 |
Synthetic peptides.
The HCVNS3-1073peptide CVNGVCWTV has previously been identified as an HLA-A2-restricted CTL determinant (6, 20) and has been used to investigate HCV-specific CD8+ T-cell responses in several studies (6–8, 20, 31, 32, 40). Its sequence is conserved among HCV genotype 1B strains, the most frequent HCV genotype in the United States. The sequence of the influenza A virus (IV) peptide IVNA-231 CVNGSCFTV is conserved between IV N1 strains and is included in vaccines. Importantly, H1N1 viruses also constituted the predominant virus isolates of major IV outbreaks during the last several years (1). The HPVL1-315 peptide HNNGICWGN is derived from the human papillomavirus (HPV) type 44 major capsid protein L1 (4). The WAG161 peptide CQNGACWTS is derived from the wheat protein agglutinin isolectin 1 precursor (46). The cytomegalovirus pp65 peptide NLVPMVATV (45) and the IV matrix peptide (IVM1-58) GILGFVFTLT (42) were used as positive controls. All peptides were synthesized at >80% purity at Research Genetics, Huntsville, Ala., or at the Facility for Biotechnology Resources, Center for Biologics Evaluation and Research, Food and Drug Administration, Bethesda, Md.
Major histocompatibility complex (MHC) binding assay.
Peptide binding assays were performed as previously described (9) with the following modification. T2 cells (transporter associated with antigen processing [TAP]-deficient human lymphoid-derived cells) were cultured for 16 h at 26°C to enhance expression of peptide-receptive cell surface molecules. After addition of decreasing amounts of synthetic peptides, cells were incubated at 37°C for 2 h to unfold HLA-A2 molecules not stabilized by peptide binding. A final concentration of 200 μM dithiothreitol was maintained during this incubation step to avoid cysteinylation and dimerization of peptides with cysteine residues (9). Cells were then washed and stained with fluorescein-conjugated anti-HLA-A2 antibody (One Lambda Inc., Los Angeles, Calif.) and 1 μg of propidium iodide per ml. Live cells were gated based on forward and side scatter and exclusion of propidium iodide-positive cells. Data were expressed as mean fluorescence intensity.
Stimulation of PBMC with synthetic peptides.
PBMC were isolated from blood and lymphopheresis samples by density gradient centrifugation and washed thrice in phosphate-buffered saline (PBS) as previously described (31). Peptide-specific T cells were expanded from PBMC in 96-well round-bottom plates. Specifically, replicate cultures of 0.4 × 106 cells/100 μl/well were stimulated with synthetic peptides (10 μg/ml), recombinant interleukin-7 (rIL-7) (10 ng/ml), and rIL-12 (100 pg/ml) (PeproTech Inc., Rocky Hill, N.J.) in RPMI 1640 (Gibco Laboratories, Grand Island, N.Y.) supplemented with 10% heat-inactivated human AB serum, l-glutamine (2 mM), penicillin (100 U/ml), and streptomycin (100 μg/ml). Cultures were restimulated with 10 μg of peptide per ml, 20 U of rIL-2 (Chiron Corp., Emeryville, Calif.) per ml, and 105 irradiated (3,000 rads) autologous PBMC as feeder cells in the presence of IL-7 on day 7 and in the absence of IL-7 on day 14. On days 3, 10, and 18, 100 μl of RPMI with 10% (vol/vol) human AB serum and rIL-2 at a 10-U/ml final concentration was added to each well. In contrast to earlier studies that employed split-well CTL assays (7, 31, 32, 40), eight cultures were pooled on days 20 to 24 and tested for CTL activity at a defined effector/target cell ratio of 30:1 to compensate for differences in the expansion of specific T cells during culture.
Cytotoxicity analysis of HCV peptide-specific T cells expanded from CD45RO+ and CD45RO− T-cell subpopulations.
To assess the HCV-specific CTL activity from CD45RO+ and CD45RO− T-cell subsets, PBMC were stained with phycoerythrin-labeled antibody against CD45RO (Becton Dickinson, San Jose, Calif.) and sorted into CD45RO+ and CD45RO−subpopulations on a Coulter flow cytometer. Purity was confirmed by analysis after sorting. CD45RO-enriched, CD45RO-depleted, or unfractionated T cells (5 × 104) were then stimulated with 105 irradiated (3,000 rads) autologous PBMC, 10 μg of HCVNS3-1073peptide per ml, 10 ng of IL-7 per ml, and 100 pg of IL-12 (PeproTech) per ml. Cells were restimulated twice at 7-day intervals with irradiated PBMC, 10 ng of IL-7 per ml, and 10 μg of HCVNS3-1073 per ml. One hundred microliters of RPMI with 10% (vol/vol) human AB serum and rIL-2 at a 10-U/ml final concentration was added on days 3, 10, and 18, and cultures were assayed for cytotoxic activity after 20 to 24 days of culture.
Infection of HLA-A2-transgenic mice and mouse CTL cultures.
Transgenic mice expressing the α1 and α2 domains from the HLA-A2.1 molecule and the α3 domain from the murine H-2Dd molecule (29), kindly provided by Victor Engelhard, University of Virginia, were bred in a specific-pathogen-free environment at the NIH animal facility. Mice were immunized intraperitoneally with ≈500 hemagglutinating units of A/PuertoRico/8/34 (PR8) IV, 107 PFU of the WR strain of vaccinia virus (VV), or 107 PFU of recombinant VV expressing the HCV NS3 protein and amino acids 1007 to 1890 of the HCV NS4 protein (NS3-VV; kindly provided by Ralf Bartenschlager, University of Mainz, Mainz, Germany) (3). Splenocytes harvested on day 7 (effector cells) or on day 21 (memory cells) after infection were either tested directly ex vivo for cytotoxic activity and gamma interferon (IFN-γ) production or cultured in T-25 flasks (3 × 107cells/flask) for 7 days with synthetic peptide (10 μg/ml) in complete mouse T-cell medium (a 1:1 mixture of RPMI 1640 and Eagle-Hanks' amino acid medium supplemented with 10% heat-inactivated fetal calf serum [Biowhittaker, Walkersville, Md.], l-glutamine [2 mM], penicillin [100 U/ml], streptomycin [100 μg/ml], and 2-mercaptoethanol [50 μM]). Rat-T-stim (10%; Collaborative Biomedical Products, Bedford, Mass.) was added on day 2.
Cytotoxicity assay.
C1R-A2 cells, i.e., the human lymphoblastoid cell line HMYC1R transfected with HLA-A2.1 (38), were used as target cells for human CTL lines. C1R-AAD cells, i.e., HMYC1R cells transfected with MHC chimeric molecules containing the α1 and α2 domains of the HLA-A2.1 molecule and the α3 domain of the murine H-2Dd molecule (29), were used as targets for murine CTL lines. Both cell lines were kindly provided by J. A. Berzofsky, National Cancer Institute. Target cells were incubated overnight with the indicated concentrations of synthetic peptide and labeled with 25 μCi of51Cr (Amersham Corp., Arlington Heights, Ill.) for 1 h. After three washes with PBS, targets were plated at 3,000 cells/well in complete medium in round-bottom 96-well plates. Unlabeled cold targets (60,000 cells/well) were added to reduce nonspecific lysis. In contrast to earlier studies (7, 31, 32, 40), effector cells were added at defined effector-to-target ratios to compensate for differences in the expansion of peptide-specific T cells during culture. Percent cytotoxicity was determined from the formula 100 × [(experimental release − spontaneous release)/(maximum release − spontaneous release)]. Maximum release was determined by lysis of 51Cr-labeled targets with 5% Triton X-100 (Sigma Chemical Co., St. Louis, Mo.). Spontaneous release was <15% of maximum release in all experiments. The specific cytotoxic activity was calculated as [(cytotoxic activity in the presence of peptide) − (cytotoxic activity in the absence of peptide)]. A specific cytotoxic activity of >10% was considered to be positive.
Enzyme-linked immunospot (Elispot) assays.
Ninety-six-well plates (Millititer; Millipore, Bedford, Mass.) were coated with anti-human IFN-γ (0.5 μg/ml; Endogen, Woburn, Mass.) or anti-mouse IFN-γ (3 μg/ml; Pharmingen, San Diego, Calif.) at 4°C overnight and washed four times with sterile PBS. The plates were blocked with RPMI–1% bovine serum albumin (Sigma) for 1 h at 25°C. Cryopreserved PBMC (3 × 105) from the same blood sample used for the CTL cultures were thawed and added in duplicate cultures in RPMI 1640–5% AB serum–2 mMl-glutamine–10 μg of MHC class I-restricted HCV peptides per ml. Mouse spleen cells were used either ex vivo, i.e., immediately after isolation, or after 7 days of in vitro stimulation and plated in serial dilutions (3 × 105, 1 × 105, 33 × 103, and 11 × 103 cells) with 105 irradiated C1R-AAD cells and 10 μg of peptide per ml. After 30 h, the plates were washed seven times and incubated overnight with 100 μl of the secondary antibody (biotin-conjugated anti-human IFN-γ [0.25 μg/ml; Endogen] or biotin-conjugated anti-mouse IFN-γ [2 μg/ml; Pharmingen]). After four washes, streptavidin-alkaline phosphatase (1:2,000; DAKO, Glostrup, Denmark) was added and left for 2 h. Finally, the plates were washed again four times with PBS and developed with freshly prepared nitroblue tetrazolium–5-bromo-4-chloro-3-indolylphosphate solution (Bio-Rad, Hercules, Calif.). The reaction was stopped by rinsing with distilled water. The number of specific spots was determined by subtracting the number of spots in the absence of antigen from the number of spots in the presence of antigen. Responses were considered positive if more than 10 specific spots were detected and if the number of spots in the presence of antigen was at least twofold greater than the number of spots in the absence of antigen. Positive controls consisted of cultures stimulated with phytohemagglutinin (1 μg/ml; Murex Biotech Limited, Dartford, England) or the HLA-A2-restricted cytomegalovirus pp65 determinant NLVPMVATV (45).
HLA typing.
HLA typing of PBMC from patients and control subjects was performed by complement-dependent microcytotoxicity using HLA typing trays purchased from One Lambda.
RESULTS
HCVNS3-1073-specific CTLs can be expanded from the blood of healthy, uninfected blood donors.
The HCVNS3-1073 peptide CVNGVCWTV is an immunodominant, endogenously processed determinant that is recognized by liver-infiltrating and circulating CTLs of HCV-infected patients (20). T-cell responses to this determinant may play a special role in the outcome of HCV infection, because it is the most frequently recognized HLA-A2-restricted determinant during acute, self-limited HCV infection (13, 25, 26) and one of only two epitopes for which virus-encoded antagonist peptides have been described for chronic hepatitis C (7, 44).
In contrast to our earlier studies, which did not detect significant responses in healthy, uninfected control persons (31, 40), in the present study we used a modified, more sensitive technique that is capable of expanding determinant-specific CTLs of a frequency of less than one in 100,000 PBMC (H. Wedemeyer and B. Rehermann, unpublished results). Specifically, addition of IL-7 and IL-12 to the peptide-stimulated T-cell cultures enriched determinant-specific CTLs to up to 20 to 40% at week 3 of the cell culture as assessed by analysis with an HLA-A2 tetramer presenting the HCV NS3 determinant (Wedemeyer and Rehermann, unpublished results). With this technique, we were able to expand HCVNS3-1703-specific CTLs not only from the blood of 11 of 20 HCV-infected patients (55%) but also from the blood of 9 of 15 HCV-negative blood donors (60%), which displayed a comparable cytotoxic activity against peptide-pulsed target cells at a high effector/target ratio of 60:1 (Tables 1 and2). The T-cell response of HCV-negative controls was specific for this peptide, since a second HCV NS3 CTL determinant, which is frequently recognized by HCV-infected patients (Table 1) (6, 7, 8, 20, 25, 31, 32), tested negative in this group of uninfected subjects (Table 2) and since HCVNS3-1073-specific T cells specifically recognized only the HCVNS3-1073 peptide and not unrelated HBV control peptides, such as HBVcore18-27 FLPSDFFPSV (not shown).
TABLE 2.
HCVNS3-1073 and HCVNS3-1406 and HCVNS3-1406 peptide-specific cytotoxic activity of T-cell lines derived from PBMC of HCV-negative, healthy blood donors
| HCV-negative blood donor | Specific lysis (%) ofa: | |
|---|---|---|
| HCVNS3-1073-loaded target cells | HCVNS3-1406-loaded target cells | |
| HD-1 | 40 | 13 |
| HD-2 | 16 | 1 |
| HD-3 | 2 | 0 |
| HD-4 | 2 | 1 |
| HD-5 | 43 | 0 |
| HD-6 | 15 | 0 |
| HD-7 | 38 | 0 |
| HD-8 | 27 | 2 |
| HD-9 | 20 | 5 |
| HD-10 | 27 | 0 |
| HD-11 | 1 | 2 |
| HD-12 | 2 | 5 |
| HD-13 | 46 | 3 |
| HD-14 | 2 | 3 |
| HD-15 | 6 | 1 |
| No. of patients positive/total (% positive) | 9/15 (60) | 1/15 (7) |
| Mean cytotoxicityb ± SD | 30 ± 11 | 2 ± 3 |
HCVNS3-1073-specific T cells observed in a subgroup of healthy, non-HCV-infected blood donors display the phenotype of memory cells.
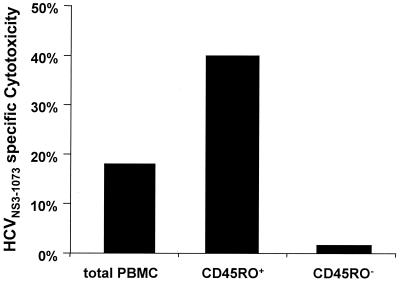
To determine whether the responding T cells of these non-HCV-infected individuals resided in the memory or naive subsets, PBMC were sorted into CD45RO+ T cells and CD45RA+ T cells and stimulated with the HCVNS3-1073 peptide. As demonstrated in Fig.1, depletion of CD45RO+ T cells prior to in vitro stimulation abolished the peptide-specific T-cell response completely. In contrast, enrichment of CD45RO+ T cells prior to in vitro stimulation enhanced HCVNS3-1073-specific cytotoxicity. Thus, the T-cell response of these healthy, HCV-negative blood donors, who had been screened to respond to the HCVNS3-1073 determinant, was mediated by memory, not by in vitro-induced, T cells.
FIG. 1.
HCVNS3-1073-specific cytotoxicity is mediated by cells expanded from the CD45RO+ memory T-cell pool.
Identification of peptides with a high degree of sequence homology to the HCVNS3-1073 determinant.
To identify cross-reactive antigens, we searched the National Center for Biotechnology Information GenBank database for peptides displaying a high degree of sequence homology with the HLA-A2-restricted HCVNS3-1073 peptide. Three 9-mer peptides, derived from the PR8 IV neuraminidase protein (designated IVNA-231), the HPV capsid protein (designated HPVL1-315), and the wheat agglutinin isolectin 1 protein (designated WAG161), were identified (Table 3). None of these peptides had previously been described as a T-cell determinant.
TABLE 3.
Peptides
| Peptide | Source | Sequencea | Reference | GenBank accession no. |
|---|---|---|---|---|
| HCVNS3-1073 | HCV (genotype 1B) NS3 protein | CVNGVCWTV | 19a | P26662 |
| IVNA-231 | IV neuraminidase | CVNGSCFTV | 4a | J02146 |
| HPVL1-315 | HPV type 44 capsid protein L1 | HNNGICWGN | 4 | P50816 |
| WAG161 | Wheat agglutinin Isolectin 1 | CONGACWTS | 38a | P10968 |
The highest degree of sequence homology is observed between the HCVNS3-1073 peptide and the IV neuraminidase peptide. First, the IVNA-231 peptide differs from the HCVNS3-1073 peptide in only two of nine amino acids, while the WAG161 peptide differs in three and the HPVL1-315 peptide differs in four of nine amino acids. Second, the IVNA-231 peptide is the only peptide with conserved amino acids in residues 2 and 9, the residues critical for binding to the HLA-A2 molecule. Finally, although two amino acids differed between the IVNA-231 peptide and the HCVNS3-1073 peptide, these amino acids belong to the same group and share certain physicochemical characteristics. Specifically, both valine and serine in position 5 are aliphatic, and both tryptophan and phenylalanine in position 7 are aromatic, nonpolar amino acids. In contrast, the amino acid residues in the HLA-A2 anchor positions 2 and 9 of peptide HPVL1-315 are polar acidic asparagines, while the corresponding residues of the peptide HCVNS3-1073 epitope are aliphatic valines. Similarly, position 2 of the WAG161 peptide has an acidic glutamine, while in the HCVNS3-1073peptide, there is an aliphatic valine.
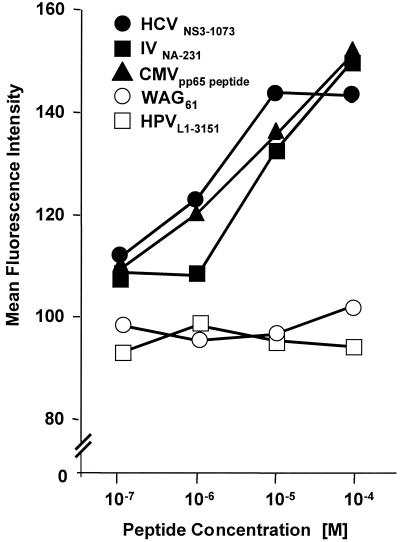
As indicated in Fig. 2, both the HCVNS3-1073 and the IVNA-231 peptides bound to the HLA-A2 molecule with high affinity, comparable to the binding of a known optimal control determinant from the cytomegalovirus pp65 protein (45). In contrast, the two peptides with amino acid substitutions in HLA-A2 binding residues (WAG61and HPVL1-3151) displayed significantly lower binding affinities even at high peptide concentrations.
FIG. 2.
MHC binding affinity. TAP-deficient T2 cells were cultured for 16 h at 26°C to enhance expression of peptide-receptive cell surface molecules and then incubated with various concentrations of individual peptides at 37°C for 2 h, washed, and stained with fluorescein-conjugated anti-HLA-A2 antibody (One Lambda Inc.) and 1 μg of propidium iodide per ml. Data express the mean fluorescence intensity of live, propidium iodide-negative cells.
Direct ex vivo analysis demonstrates circulating IV-specific T cells in the blood of patients with HCVNS3-1073-specific CTLs.
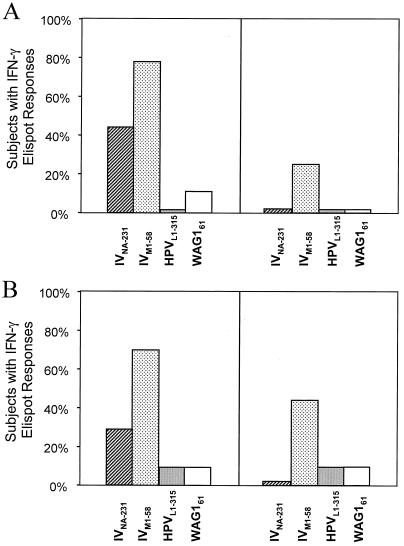
To determine whether those subjects from whom HCVNS3-1073-specific CTLs could be expanded also displayed immune responses against the homologous peptides, we performed direct ex vivo cytokine Elispot analysis of PBMC with the IVNA-231, HPVL1-315, and WAG161 peptides. As shown in Fig.3A, blood donors were divided into two groups; the first group (left panel of Fig. 3A) had HCVNS3-1073-specific CTLs that could be expanded in vitro, and the second group (right panel of Fig. 3A) did not. We then tested PBMC from each individual directly ex vivo for production of IFN-γ during a 30-h incubation with the indicated peptide. While the HPVL1-315 and WAG161peptides were recognized neither by HCV-negative blood donors with HCVNS3-1073-specific CTL responses nor by those without such responses, this was quite different for the IVNA-231 peptide. Forty-four percent of blood donors with HCVNS3-1073-specific CTL responses but none of those without HCVNS3-1073-specific CTL responses recognized the IVNA-231 peptide in a direct ex vivo IFN-γ Elispot assay. As an independent control for exposure to IV, we also tested the ex vivo IFN-γ response to a well-characterized, immunodominant IV matrix peptide, the IVM1-58 determinant (42). In accordance with its higher degree of conservation in IV strains, this peptide was even more frequently recognized by blood donors with HCVNS3-1073-specific CTL responses, evidencing exposure to IV. These results demonstrate that searching for cross-reactive epitopes to HCV led to the identification of a novel IV epitope.
FIG. 3.
(A) IFN-γ production assessed by direct ex vivo Elispot analysis of PBMC from healthy, HCV-negative blood donors. Blood donors were divided into two groups, those with (left panel) and without (right panel) HCVNS3-1073-specific CTLs. PBMC from each individual were then tested directly ex vivo for production of IFN-γ during a 30-h incubation with the indicated peptide. Responses were considered positive if more than 10 specific spots were detected and if the number of spots in the presence of antigen was at least twofold greater than the number of spots in the absence of antigen. (B) IFN-γ production assessed by direct ex vivo Elispot analysis of PBMC from HCV-infected patients. HCV-infected patients were divided into two groups, those with (left panel) and without (right panel) HCVNS3-1073-specific CTLs. PBMC from each individual were then tested directly ex vivo for production of IFN-γ during a 30-h incubation with the indicated peptide. Responses were considered positive if more than 10 specific spots were detected and if the number of spots in the presence of antigen was at least twofold greater than the number of spots in the absence of antigen.
In addition, we performed the same experiment for HCV-infected patients (Fig. 3B). Direct ex vivo IFN-γ responses against the IVNA-231 peptide were observed less frequently than in the group of healthy, uninfected blood donors. Similar to the results for healthy blood donors, however, IVNA-231-specific responses were observed only in the subgroup of patients with HCVNS3-1073-specific CTLs, and none of the patients without HCVNS3-1073-specific CTLs recognized the IVNA-231 peptide.
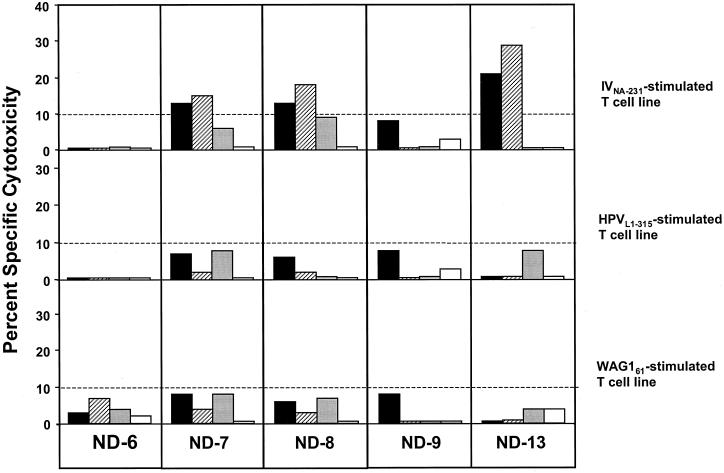
Recognition of HCVNS3-1073 by CTL lines expanded with the IVNA-231, HPVL1-315, and WAG161peptides.
To assess whether the heterologous peptides could in vitro expand CTLs that recognized the HCVNS3-1073determinant, PBMC of five healthy, HCV-negative blood donors were stimulated in vitro with the IVNA-231, HPVL1-315, or WAG161peptide and tested for specific lysis of target cells pulsed with the identical peptide or HCVNS3-1073 after 3 weeks of culture (Fig. 4). Stimulation of PBMC with the IVNA-231 peptide yielded IV-specific CTLs from three of five healthy, uninfected controls. Importantly, each of the three IVNA-231-specific CTL lines also recognized HCVNS3-1073 determinant-presenting target cells with a similar cytotoxic activity. In contrast, no significant cytotoxicity against any of the peptides could be induced when PBMC were expanded with either the HPVL1-315or the WAG161 peptide.
FIG. 4.
In vitro cross-reactivity. Cryopreserved PBMC of five HCV-seronegative, healthy controls were stimulated for 3 weeks in vitro in the presence of IL-2, IL-7, IL-12, and 10 μg of IVNA-231, HPVL1-315, or WAG61peptide per ml. Each CTL line was tested in a 6-h51Cr release assay against C1R-A2 targets pulsed overnight with 10 μg of HCVNS3-1073 (▪), IVNA-231(▨), HPVL1-315 (░⃞), or WAG161 (□) per ml. The 10% cutoff for a positive CTL assay is indicated by the horizontal line.
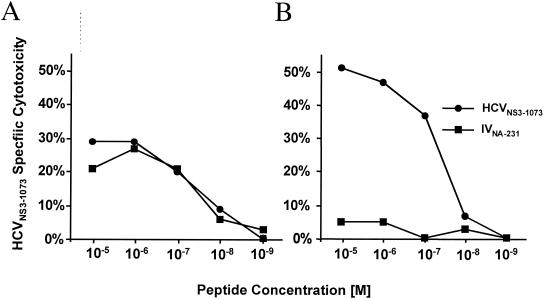
The IVNA-231 determinant expands cross-reactive, HCVNS3-1073-specific CTLs that recognize both peptides with similar affinity.
To examine this further, we in vitro stimulated CTLs from HCV-infected patient Chr-2 with each peptide. Figure 5A demonstrates that IVNA-231-stimulated CTLs recognized both the IVNA-231 and the HCVNS3-1073 peptides with precisely the same affinity (10−8 M). In contrast, HCVNS3-1073-stimulated T cells of the same patient recognized only the HCVNS3-1073determinant and not the IVNA-231determinant (Fig. 5B). Thus, both peptides were recognized with similar affinity by HCVNS3-1073-specific T cells, but only the IVNA-231 peptide could expand cross-reactive CTLs. These findings suggest that, at least for this individual, a heterogeneous T-cell population exists that possesses different stimulation requirements for T-cell expansion and cytotoxicity.
FIG. 5.
T-cell receptor affinity. PBMC of patient Chr-2 were stimulated for 3 weeks in the presence of IL-7, IL-12, and 10 μg of peptide IVNA-231 (A) or peptide HCVNS3-1073 (B) per ml. CTL lines were tested in a 6-h 51Cr release assay against target cells pulsed with the indicated concentrations of HCVNS3-1073 or IVNA-231 peptide.
HCVNS3-1073- and IVNA-231-specific T-cell lines recognize IV-infected target cells that endogenously process IVNA-231.
Because it is not known whether the IVNA-231 peptide is endogenously processed and presented by IV-infected cells, an HCVNS3-1073-specific CTL line expanded from PBMC of patient Chr-5 and an IVNA-231-specific CTL line expanded from PBMC of the healthy, HCV-negative blood donor HD-7 were tested against target cells infected with PR8 IV. Figure6A demonstrates that the HCVNS3-1073-specific CTL line recognized IV-infected target cells. In fact, the cytotoxicity of the cross-reactive HCVNS3-1073-specific CTL line was comparable to that of an IVNA-231-specific CTL line (Fig. 6B). Target cells that endogenously processed the IV determinant were also recognized (Fig. 6C).
FIG. 6.
(A) HCVNS3-1073-specific CTLs recognize IV-infected target cells. IV PR8-infected C1R-A2 cells (filled circles) or uninfected C1R-A2 cells (open circles) were labeled with51Cr for 1 h and used as target cells in a standard 6-h 51Cr release assay with the HCVNS3-1073-specific CTL line derived from patient Chr-5. (B) IVNA-231-specific CTL lines recognize IV-infected target cells. IV PR8-infected C1R-A2 cells (filled squares) or uninfected C1R-A2 cells (open squares) were labeled with51Cr for 1 h and used as target cells in a standard 6-h 51Cr release assay with IVNA-231-specific CTL effectors derived from healthy, HCV-negative blood donor HD-7. (C) Cytotoxic activity of an HCVNS3-1073-specific CTL line from patient Chr-5 against peptide-pulsed or IV-infected target cells. The specific lysis at an effector/target ratio of 33:1 is shown.
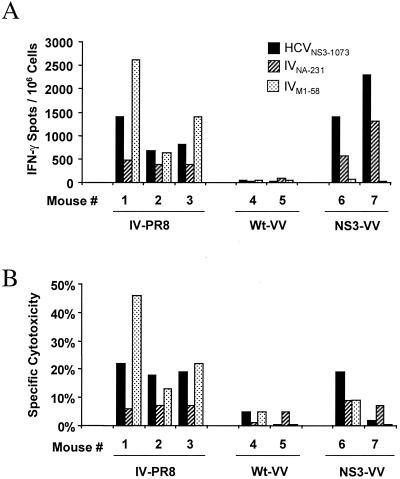
HCVNS3-1073-specific T cells can be induced by IV infection of HLA-A2-transgenic mice.
To directly demonstrate that IV infection does indeed induce HCV-specific CTLs in vivo, we infected HLA-A2-transgenic mice with IV. Splenocytes harvested at the peak of the primary response (day 7 effector cells) or following recovery (day 21 memory cells) were analyzed for IFN-γ production by IFN-γ Elispot analysis and for cytotoxicity after 1 week of in vitro stimulation with the respective peptide. As demonstrated in Fig.7, memory T cells induced by IV recognized the cross-reactive HCVNS3-1073 peptide following HCVNS3-1073 stimulation in vitro better than the cross-reactive IVNA-231 peptide following IVNA-231 stimulation as measured by the number of IFN-γ-producing cells (Fig. 7A) or by lysis of peptide-coated target cells (Fig. 7B). The immunodominant IVM1-58 peptide, used as a positive control for successful induction of IV-specific immune responses, was also recognized by all mice, although with varying strength. Similar responses against the HCVNS3-1073 and IVNA-231 peptides could be generated by infection of mice with recombinant VV expressing HCV NS3 sequences but not by infection of mice with wild-type VV (Fig. 7).
FIG. 7.
Induction of HCVNS3-1073-specific CTL by IV infection in vivo. HLA-A2-transgenic mice were infected intraperitoneally with ≈500 hemagglutinating units of PR8 IV, 107 PFU of wild-type WR strain VV (Wt-VV), or 107 PFU of recombinant VV expressing HCV-NS3 (NS3-VV). At 21 days following immunization, splenocytes were stimulated in vitro for 7 days in the presence of 10 μg of the indicated peptides per ml. (A) IFN-γ production as assessed by Elispot analysis. (B) Cytotoxicity tested in a standard 6-h 51Cr release assay. Cytotoxicity was tested against peptide-coated and noncoated target cells; the specific cytotoxicity, i.e., cytotoxicity in the presence of peptide minus cytotoxicity in the absence of peptide, is shown. Similar results for both IFN-γ production and the 51Cr release assay were observed for splenocytes harvested 7 days after infection.
DISCUSSION
The adaptive CD8+ T-cell response to infectious pathogens has been shown to target short, linear peptides of defined sequences in the binding grooves of MHC class I molecules on infected cells. Prospective studies with mice have demonstrated that the frequency of CTL precursors that recognize viral pathogens can remain stable for at least 2 years, even after clearance of the virus (18, 24, 28). Similarly, patients who have cleared HCV possess virus-specific CD8+ T cells in the blood for at least 2 decades (40).
However, if each CD8+ T cell recognized only a single peptide of a given pathogen, this would require the number of memory T cells to be larger than 1012, the total number of lymphocytes in humans. Thus, a certain flexibility and degeneracy in T-cell recognition has been proposed. Indeed, the immune response towards a single determinant of HBV, for example, can be extremely diverse at the level of T-cell receptor fine specificity and beta-chain usage (19). Vice versa, it has also been described that a single T-cell receptor of a given T-cell clone can recognize quite disparate peptides (22, 27).
Our demonstration that HCV NS3-specific memory T cells expanded from the blood of healthy, non-HCV-infected blood donors recognize a determinant of the IV neuraminidase protein supports this theory of cross-reactive T cells that was first developed by Selin et al. in studies with rodents (34, 36). In fact, our study may even underestimate the extent of cross-reactivity for at least two reasons. First, the cross-reactive peptide was identified based on sequence homology, while cross-reactivities at the level of T-cell recognition may not necessarily depend on a conserved linear sequence of several amino acids (16, 17). The fact that only 44% of HCV-negative blood donors with HCVNS3-1073-specific CTL responses displayed cross-reactivity to this IV epitope suggests that additional cross-reactivities exist or that not all individuals had been recently exposed to this particular IV strain. Second, the cross-reactive peptide was identified only by a search of known sequences, and additional, yet-unidentified cross-reactive sequences may exist.
One of the factors that influence the number of cross-reactive T cells may be the frequency of exposure to a given virus and the sequence variability of that specific virus. Although only a few reports describe cross-reactive T cells in humans, most of them relate to IV-specific T cells. First, T-cell cross-reactivity between different proteins of IV has been described. Specifically, an H-2Kd-restricted IV-specific CTL clone recognized two distinct peptides of the IV HA and NS1 proteins (22), and CTLs specific for the IV nucleoprotein lysed targets sensitized with two different IV basic polymerase 2 peptides (2). Second, a dissimilar IV matrix peptide induced HLA-A2-restricted CTLs against the human rotavirus VP4 peptide (37). Third, in the present study, we have directly shown the induction of HCV NS3-specific, HLA-A2-restricted CTLs following IV infection of HLA-A2-transgenic mice. The generation of a cross-reactive T-cell pool by IV infection may be facilitated by the fact that infection with IV induces particularly large numbers of virus-specific cytotoxic T cells in the pulmonary tissue, lymphoid organs, and peripheral blood in mice and humans (11, 12,14). Also, reexposure to variant IVs occurs frequently, and in contrast to primary responses, secondary responses against variant IV strains are characterized by a lack of strain specificity (15) and IV-specific immune responses of HCV-infected patients have been shown to be comparable to those of healthy controls (32). This may lead to a selective expansion of a cross-reactive T-cell population. In addition, the cross-reactive T cells that we have identified belong to the memory T-cell pool, and memory T cells have been described to be more susceptible to stimulation by a low-affinity T-cell antigen or cytokines than naive T cells (30, 39, 41). This may be due to the fact that enhanced expression of adhesion molecules and IL-2 receptors by memory cells is compatible with less stringent activation requirements.
In regard to the in vivo role of the observed cross-reactivity, we cannot yet assess its effects on protective immunity against HCV and/or on immunopathology and liver disease. Both possibilities have been discussed with respect to other virus infections. In regard to the outcome of infection, it has been demonstrated in the mouse model that memory immune responses to one virus modulated future primary immune responses to other viruses (35). Furthermore, heterologous virus infections quantitatively delete and qualitatively alter the memory pool of T cells specific for a previously encountered virus (34). In regard to immunopathology, it has been shown that previous infection with IV dramatically protects mice from respiratory syncytial virus-induced immunopathology (43).
These hypotheses are particularly intriguing with regard to the identified IV and HCV determinants, because immune responses against the HCVNS3-1073 determinant have been described in all studies investigating HCV infection so far (5, 8, 13, 20,25, 26, 31, 33) and because patients with acute self-limited HCV infection (25) and recovered persons (40) display a significantly stronger T-cell response to this CTL determinant than chronically HCV-infected patients. Furthermore, the HCVNS3-1073 epitope is one of the two HCV CTL determinants for which viral escape mutants have been demonstrated (7, 44). However, it also has to be taken into account that HCV-specific T-cell responses are characteristically targeted against multiple determinants, and therefore, multiple cross-reactivities must be considered. These may even reach beyond the constraints of strict sequence homology. Notably, they may extend to yet-unidentified, less conserved, and not immunodominant T-cell determinants, such as the IV determinant in this study, which was identified by searching for cross-reactive epitopes. Thus, as demonstrated in the mouse model (34, 36), the quality of the human immune response to an infectious agent should be regarded as a function of all previous infections and their influence on the memory T-cell pool.
ACKNOWLEDGMENTS
We thank Susan Leitman for blood samples from healthy blood donors, Jake Liang and Jay Hoofnagle for samples from patients with chronic hepatitis C, and Jeffery Miller for fluorescence-activated cell sorting.
H.W. was supported by grant We 2431/1 from the Deutsche Forschungsgemeinschaft, Bonn, Germany.
REFERENCES
- 1.Advisory Committee on Immunization Practices. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP) Morb Mortal Wkly Rep. 1999;48:1–28. [PubMed] [Google Scholar]
- 2.Anderson R W, Bennink J R, Yewdell J W, Maloy W L, Coligan J E. Influenza basic polymerase 2 peptides are recognized by influenza nucleoprotein-specific cytotoxic T lymphocytes. Mol Immunol. 1992;29:1089–1096. doi: 10.1016/0161-5890(92)90041-u. [DOI] [PubMed] [Google Scholar]
- 3.Bartenschlager R, Ahlborn-Laake L, Mous J, Jacobsen H. Kinetic and structural analyses of hepatitis C virus polyprotein processing. J Virol. 1994;68:5045–5055. doi: 10.1128/jvi.68.8.5045-5055.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernard H U, Chan S Y, Manos M M, Ong C K, Villa L L, Delius H, Peyton C L, Bauer H M, Wheeler C M. Identification and assessment of known and novel human papillomaviruses by polymerase chain reaction amplification, restriction fragment length polymorphisms, nucleotide sequence, and phylogenetic algorithms. J Infect Dis. 1994;170:1077–1085. doi: 10.1093/infdis/170.5.1077. [DOI] [PubMed] [Google Scholar]
- 4a.Blok J, Air G M. Comparative nucleotide sequences at the 3′ end of the neuraminidase gene from eleven influenza type A viruses. Virology. 1980;107:50–60. doi: 10.1016/0042-6822(80)90271-8. [DOI] [PubMed] [Google Scholar]
- 5.Cerny A, Ferrari C, Chisari F V. The class I restricted cytotoxic T lymphocyte response to predetermined epitopes in the hepatitis B and C viruses. In: Oldstone M B A, editor. Current topics in microbiology and immunology. Heidelberg, Germany: Springer-Verlag; 1994. pp. 169–186. [DOI] [PubMed] [Google Scholar]
- 6.Cerny A, McHutchison J G, Pasquinelli C, Brown M E, Brothers M A, Grabscheid B, Fowler P, Houghton M, Chisari F V. Cytotoxic T lymphocyte response to hepatitis C virus-derived peptides containing the HLA A2.1 binding motif. J Clin Invest. 1995;95:521–530. doi: 10.1172/JCI117694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang K M, Rehermann B, McHutchison J G, Pasquinelli C, Southwood S, Sette A, Chisari F V. Immunological significance of cytotoxic T lymphocyte epitope variants in patients chronically infected by the hepatitis C virus. J Clin Invest. 1997;100:2376–2385. doi: 10.1172/JCI119778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang K M, Thimme R, Melpolder J J, Oldach D, Pemberton J, Moorhead-Loudis J, McHutchison J G, Alter H J, Chisari F V. Differential CD4 and CD8 T-cell responsiveness in hepatitis C virus infection. Hepatology. 2001;33:267–276. doi: 10.1053/jhep.2001.21162. [DOI] [PubMed] [Google Scholar]
- 9.Chen W, Yewdell J W, Levine R L, Bennink J R. Modification of cysteine residues in vitro and in vivo affects the immunogenicity and antigenicity of major histocompatibility complex class I-restricted viral determinants. J Exp Med. 1999;189:1757–1764. doi: 10.1084/jem.189.11.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diepolder H M, Gerlach J-T, Zachoval R, Hoffmann R M, Jung M C, Wierenga E A, Scholz S, Santantonio T, Houghton M, Southwood S, Sette A, Pape G R. Immunodominant CD4+T-cell epitope within nonstructural protein 3 in acute hepatitis C virus infection. J Virol. 1997;71:6011–6019. doi: 10.1128/jvi.71.8.6011-6019.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunbar P R, Ogg G S, Chen J, Rust N, van der Bruggen P, Cerundolo V. Direct isolation, phenotyping and cloning of low-frequency antigen-specific cytotoxic T lymphocytes from peripheral blood. Curr Biol. 1998;8:413–416. doi: 10.1016/s0960-9822(98)70161-7. [DOI] [PubMed] [Google Scholar]
- 12.Flynn K J, Belz G T, Altman J D, Ahmed R, Woodland D L, Doherty P C. Virus-specific CD8+ T cells in primary and secondary influenza pneumonia. Immunity. 1998;8:683–691. doi: 10.1016/s1074-7613(00)80573-7. [DOI] [PubMed] [Google Scholar]
- 13.Gruner N H, Gerlach T J, Jung M C, Diepolder H M, Schirren C A, Schraut W W, Hoffmann R, Zachoval R, Santantonio T, Cucchiarini M, Cerny A, Pape G R. Association of hepatitis C virus-specific CD8+ T cells with viral clearance in acute hepatitis C. J Infect Dis. 2000;181:1528–1536. doi: 10.1086/315450. [DOI] [PubMed] [Google Scholar]
- 14.Haanen J B, Toebes M, Cordaro T A, Wolkers M C, Kruisbeek A M, Schumacher T N. Systemic T cell expansion during localized viral infection. Eur J Immunol. 1999;29:1168–1174. doi: 10.1002/(SICI)1521-4141(199904)29:04<1168::AID-IMMU1168>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 15.Haanen J B, Wolkers M C, Kruisbeek A M, Schumacher T N. Selective expansion of cross-reactive CD8(+) memory T cells by viral variants. J Exp Med. 1999;190:1319–1328. doi: 10.1084/jem.190.9.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hausmann S, Wucherpfennig K W. Activation of autoreactive T cells by peptides from human pathogens. Curr Opin Immunol. 1997;9:831–838. doi: 10.1016/S0952-7915(97)80186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hemmer B, Gran B, Zhao Y, Marques A, Pascal J, Tzou A, Kondo T, Cortese I, Bielekova B, Straus S E, McFarland H F, Houghten R, Simon R, Pinilla C, Martin R. Identification of candidate T-cell epitopes and molecular mimics in chronic Lyme disease. Nat Med. 1999;5:1375–1382. doi: 10.1038/70946. [DOI] [PubMed] [Google Scholar]
- 18.Hou S, Hyland L, Ryan K W, Portner A, Doherty P C. Virus-specific CD8+ T-cell memory determined by clonal burst size. Nature. 1994;369:652–654. doi: 10.1038/369652a0. [DOI] [PubMed] [Google Scholar]
- 19.Ishikawa T, Kono D, Chung J, Fowler P, Theofilopoulos A, Kakumu S, Chisari F V. Polyclonality and multispecificity of the CTL response to a single viral epitope. J Immunol. 1998;161:5842–5850. [PubMed] [Google Scholar]
- 19a.Kato N, Hijikata M, Ootsuyama Y, Nakagawa M, Ohkoshi S, Sugimura T, Shimotohno K. Molecular cloning of the human hepatitis C virus genome from Japanese patients with non-A, non-B hepatitis. Proc Natl Acad Sci USA. 1990;87:9524–9528. doi: 10.1073/pnas.87.24.9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19b.Knodell R G, Ishak K G, Black W C, Chen T S, Craig R, Kaplowitz N, Kiernan T W, Wollman J. Formulation and application of a numerical scoring system for assessing histological activity in symptomatic chronic active hepatitis. Hepatology. 1981;1:431–435. doi: 10.1002/hep.1840010511. [DOI] [PubMed] [Google Scholar]
- 20.Koziel M J, Dudley D, Afdhal N, Grakoui A, Rice C M, Choo Q L, Houghton M, Walker B D. HLA class I-restricted cytotoxic T lymphocytes specific for hepatitis C virus. Identification of multiple epitopes and characterization of patterns of cytokine release. J Clin Invest. 1995;96:2311–2321. doi: 10.1172/JCI118287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koziel M J, Wong D K H, Dudley D, Houghton M, Walker B D. Hepatitis C virus-specific cytolytic T lymphocyte and T helper cell responses in seronegative persons. J Infect Dis. 1997;176:859–866. doi: 10.1086/516546. [DOI] [PubMed] [Google Scholar]
- 22.Kuwano K, Reyes V E, Humphreys R E, Ennis F A. Recognition of disparate HA and NS1 peptides by an H-2Kd-restricted, influenza specific CTL clone. Mol Immunol. 1991;28:1–7. doi: 10.1016/0161-5890(91)90080-4. [DOI] [PubMed] [Google Scholar]
- 23.Lau D T Y, Kleiner D E, Ghany M G, Park Y, Schmid P, Hoofnagle J H. 10-Year follow-up after interferon-alpha therapy for chronic hepatitis C. Hepatology. 1998;28:1121–1127. doi: 10.1002/hep.510280430. [DOI] [PubMed] [Google Scholar]
- 24.Lau L L, Jamieson B D, Somasundaram T, Ahmed R. Cytotoxic T-cell memory without antigen. Nature. 1994;369:648–652. [PubMed] [Google Scholar]
- 25.Lechner F, Gruener N H, Urbani S, Uggeri J, Santantonio T, Kammer A R, Cerny A, Phillips R, Ferrari C, Pape G R, Klenerman P. CD8+ T lymphocyte responses are induced during acute hepatitis C virus infection but are not sustained. Eur J Immunol. 2000;30:2479–2487. doi: 10.1002/1521-4141(200009)30:9<2479::AID-IMMU2479>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 26.Lechner F, Wong D K, Dunbar P R, Chapman R, Chung R T, Dohrenwend P, Robbins G, Phillips R, Klenerman P, Walker B D. Analysis of successful immune responses in persons infected with hepatitis C virus. J Exp Med. 2000;191:1499–1512. doi: 10.1084/jem.191.9.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loftus D J, Chen Y, Covell D G, Engelhard V H, Appella E. Differential contact of disparate class I/peptide complexes as the basis for epitope cross-recognition by a single T cell receptor. J Immunol. 1997;158:3651–3658. [PubMed] [Google Scholar]
- 28.Mullbacher A. The long-term maintenance of cytotoxic T cell memory does not require persistence of antigen. J Exp Med. 1994;179:317–321. doi: 10.1084/jem.179.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Newberg M H, Smith D H, Haertel S B, Vining D R, Lacy E, Engelhard V H. Importance of MHC class 1 alpha2 and alpha3 domains in the recognition of self and non-self MHC molecules. J Immunol. 1996;156:2473–2480. [PubMed] [Google Scholar]
- 30.Pihlgren M, Dubois P M, Tomkowiak M, Sjogren T, Marvel J. Resting memory CD8+ T cells are hyperreactive to antigenic challenge in vitro. J Exp Med. 1996;184:2141–2151. doi: 10.1084/jem.184.6.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rehermann B, Chang K M, McHutchison J, Kokka R, Houghton M, Rice C M, Chisari F V. Differential cytotoxic T lymphocyte responsiveness to the hepatitis B and C viruses in chronically infected patients. J Virol. 1996;70:7092–7102. doi: 10.1128/jvi.70.10.7092-7102.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rehermann B, Chang K M, McHutchison J G, Kokka R, Houghton M, Chisari F V. Quantitative analysis of the peripheral blood cytotoxic T lymphocyte response, disease activity and viral load in patients with chronic hepatitis C virus infection. J Clin Invest. 1996;98:1432–1440. doi: 10.1172/JCI118931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scognamiglio P, Accapezzato D, Casciani A, Cacciani A, Artini M, Bruno G, Chircu M L, Sidney J, Southwood S, Abrignani S, Sette A, Barnaba V. Presence of effector CD8+ T cells in hepatitis C virus-exposed healthy seronegative donors. J Immunol. 1999;162:6681–6689. [PubMed] [Google Scholar]
- 34.Selin L K, Lin M Y, Kraemer K A, Pardoll D M, Schneck J P, Varga S M, Santolucito P A, Pinto A K, Welsh R M. Attrition of T cell memory: selective loss of LCMV epitope-specific memory CD8 T cells following infections with heterologous viruses. Immunity. 1999;11:733–742. doi: 10.1016/s1074-7613(00)80147-8. [DOI] [PubMed] [Google Scholar]
- 35.Selin L K, Nahill S R, Welsh R M. Cross-reactivities in memory cytotoxic T lymphocyte recognition of heterologous viruses. J Exp Med. 1994;179:1933–1943. doi: 10.1084/jem.179.6.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Selin L K, Varga S M, Wong I C, Welsh R M. Protective heterologous antiviral immunity and enhanced immunopathogenesis mediated by memory T cell populations. J Exp Med. 1998;188:1705–1715. doi: 10.1084/jem.188.9.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shimojo N, Maloy W L, Anderson R W, Biddison W E, Coligan J E. Specificity of peptide binding by the HLA-A2.1 molecule. J Immunol. 1989;143:2939–2947. [PubMed] [Google Scholar]
- 38.Shirai M, Arichi T, Nishioka M, Nomura T, Ikeda K, Kawanishi K, Engelhard V H, Feinstone S M, Berzofsky J A. CTL responses of HLA-A2.1-transgenic mice specific for hepatitis C viral peptides predict epitopes for CTL of humans carrying HLA-A2.1. J Immunol. 1995;154:2733–2742. [PubMed] [Google Scholar]
- 38a.Smith J J, Raikhel N V. Nucleotide sequences of cDNA clones encoding wheat germ agglutinin isolectins A and D. Plant Mol Biol. 1989;13:601–603. doi: 10.1007/BF00027321. [DOI] [PubMed] [Google Scholar]
- 39.Sprent J, Tough D F. Lymphocyte life-span and memory. Science. 1994;265:1395–1400. doi: 10.1126/science.8073282. [DOI] [PubMed] [Google Scholar]
- 40.Takaki A, Wiese M, Maertens G, Depla E, Seifert U, Liebetrau A, Miller J L, Manns M P, Rehermann B. Cellular immune responses persist, humoral responses decrease two decades after recovery from a single source outbreak of hepatitis C. Nat Med. 2000;6:578–582. doi: 10.1038/75063. [DOI] [PubMed] [Google Scholar]
- 41.Tough D F, Sprent J. Turnover of naive- and memory-phenotype T cells. J Exp Med. 1994;179:1127–1135. doi: 10.1084/jem.179.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vitiello A, Marchesini D, Furze J, Sherman L A, Chesnut R W. Analysis of the HLA-restricted influenza-specific cytotoxic T lymphocyte response in transgenic mice carrying a chimeric human-mouse class I major histocompatibility complex. J Exp Med. 1991;173:1007–1015. doi: 10.1084/jem.173.4.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walzl G, Tafuro S, Moss P, Openshaw P J, Hussell T. Influenza virus lung infection protects from respiratory syncytial virus-induced immunopathology. J Exp Med. 2000;192:1317–1326. doi: 10.1084/jem.192.9.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weiner A, Erickson A L, Kansopon J, Crawford K, Muchmore E, Hughes A L, Houghton M, Walker C M. Persistent hepatitis C virus infection in a chimpanzee is associated with emergence of a cytotoxic T lymphocyte escape variant. Proc Natl Acad Sci USA. 1995;92:2755–2759. doi: 10.1073/pnas.92.7.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wills M R, Carmichael A J, Mynard K, Jin X, Weekes M P, Plachter B, Sissons J G P. The human cytotoxic T-lymphocyte (CTL) response to cytomegalovirus is dominated by structural protein pp65: frequency, specificity and T-cell receptor usage of pp65-specific CTL. J Virol. 1996;70:7569–7579. doi: 10.1128/jvi.70.11.7569-7579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wright C S. 2.2 A resolution structure analysis of two refined N-acetylneuraminyl-lactose–wheat germ agglutinin isolectin complexes. J Mol Biol. 1990;215:635–651. doi: 10.1016/S0022-2836(05)80174-3. [DOI] [PubMed] [Google Scholar]