Characterization of protein kinase C β isoform's action on retinoblastoma protein phosphorylation, vascular endothelial growth factor-induced endothelial cell proliferation, and retinal neovascularization (original) (raw)
Abstract
Retinal neovascularization is a major cause of blindness and requires the activities of several signaling pathways and multiple cytokines. Activation of protein kinase C (PKC) enhances the angiogenic process and is involved in the signaling of vascular endothelial growth factor (VEGF). We have demonstrated a dramatic increase in the angiogenic response to oxygen-induced retinal ischemia in transgenic mice overexpressing PKCβ2 isoform and a significant decrease in retinal neovascularization in PKCβ isoform null mice. The mitogenic action of VEGF, a potent hypoxia-induced angiogenic factor, was increased by 2-fold in retinal endothelial cells by the overexpression of PKCβ1 or β2 isoforms and inhibited significantly by the overexpression of a dominant-negative PKCβ2 isoform but not by the expression of PKC α, δ, and ζ isoforms. Association of PKCβ2 isoform with retinoblastoma protein was discovered in retinal endothelial cells, and PKCβ2 isoform increased retinoblastoma phosphorylation under basal and VEGF-stimulated conditions. The potential functional consequences of PKCβ-induced retinoblastoma phosphorylation could include enhanced E2 promoter binding factor transcriptional activity and increased VEGF-induced endothelial cell proliferation.
Protein kinase C (PKC) activation enhances angiogenesis by participating in the intracellular signaling of vascular endothelial growth factor (VEGF) in endothelial cells (1–3). VEGF, a potent angiogenic factor that is induced by hypoxia plays a central role in the development of neovascularization in multiple diseases including tumor growth (4, 5), rheumatoid arthritis (6), and proliferative diabetic retinopathy (7, 8). VEGF can activate several PKC isoforms including α, β1 and β2, and δ isoforms (2, 7). Involvement of PKC activation in VEGF action has also been suggested by the use of a PKCβ isoform selective inhibitor, which decreased VEGF-induced endothelial cell proliferation and retinal vascular permeability (2, 7). However, definitive in vivo evidence on the role of specific PKC isoforms with regard to hypoxia-induced angiogenesis is not available. In addition, the potential signaling mechanism by which PKC isoforms are acting to enhance VEGF-induced endothelial cell proliferation is unknown.
Materials and Methods
Mice.
Linearized PEP8-PKCβ2 DNA was used to derive transgenic mice as described (9–11). PKCβ2 cDNA probe was used to examine the incorporation of the transgene. Transgene expression was confirmed by Northern blot and immunoblot on heart, aorta, and retina. Founders were bred with C57BL/6 mice and F1 mice were used for experiments. PKCβ null mice have been described previously (12).
Ischemia-Induced Proliferative Retinopathy.
The study adhered to the Association for Research in Vision and Ophthalmology statement for the Use of Animals in Ophthalmic and Vision Research. Postnatal day 7 (P7) mice and their nursing mothers were exposed to 75 ± 2% oxygen for 5 days to induce and then returned to room air inducing retinal vaso-obliteration. Maximum neovascularization was observed at P17. Flat-mounted, fluorescein-conjugated dextran-perfused retinas were examined to assess the retinal vasculature (see refs. 13 and 14) .
Quantitation of Neovascularization.
Mice at P17 were killed and eyes enucleated, fixed, and embedded in paraffin. Fifty serial sections (6 μm) starting at the optic nerve head were placed on microscope slides. After staining with periodic acid/Schiff reagent and hematoxylin, 10 intact sections of equal length, each 30 μm apart, were evaluated for a span of 300 μm. All retinal vascular cell nuclei anterior to the internal limiting membrane were counted in each section by a fully masked protocol. The mean of all 10 counted sections yielded average neovascular cell nuclei per 6-μm section per eye (see refs. 13 and 14).
Northern and Western Blot Analysis.
Total RNA or protein from 8–10 retinas at P14 was assessed by Northern (15) or Western (16) blot analysis as described.
Cell Culture.
Primary cultures of bovine retinal endothelial cells (BREC) were isolated and cultured as described (17). Only cells from passages 2–7 were used for the experiments.
Recombinant Adenoviruses.
cDNA of dominant-negative PKCs was constructed as described (18–21). The replication-deficient recombinant adenoviruses were constructed by homologous recombination between the parental virus genome and shuttle vector as described (22). The dominant-negatives of PKCβ2 isoform were constructed by converting threonine-500 to valine which caused the PKC isoform to become kinase-inactive. The adenoviruses were applied at a concentration of 1 × 108 plaque-forming units/ml, and adenoviruses with the same parental genome carrying enhanced green fluorescent protein (GFP) gene (CLONTECH) were used as controls. Infection efficiency was monitored by fluorescence which showed expression in >80% of cells. Expression of each recombinant protein was confirmed by Western blot analysis of each PKC isoform. Both the wild types and their respective dominant-negative isoforms exhibited increases 8- to 10-fold above the endogenous isoforms by protein levels.
Cell Growth Assay.
BREC were plated onto 12-well culture plates and incubated overnight in DMEM containing 10% calf serum, after which the cells were infected with adenovirus (23). After incubation for 4 days at 37°C with or without VEGF (0.6 nM), the cells were lysed in 0.1% SDS, and the DNA content was measured by means of Hoechst-33258 dye and a fluorometer (model TKO-100, Hoefer). E2 promoter binding factor (E2F) decoys were used as described (24).
Cell Migration Assay.
A modified Boyden chamber migration assay was performed by using BREC (25). The top and bottom surface of the chamber membrane was coated with collagen I. Serum-starved BREC overexpressing each PKC isoform were induced to migrate toward VEGF (0.6 nM) placed in the bottom chamber and harvested after 4 h. Cells that migrated to the bottom of the chamber were enumerated by counting SYTOX green (Molecular Probes) nucleic acid-stained cells.
In Vitro Phosphorylation of Retinoblastoma (Rb) Protein.
In vitro phosphorylation of Rb protein by recombinant PKC (Upstate Biotechnology, Lake Placid, NY) was performed as described (26) with recombinant Rb protein (QED Bioscience, San Diego) as a substrate.
Luciferase Assay.
Luciferase reporter constructs (Mercury Cell Cycle Profiling System, CLONTECH) were introduced into cells with LipofectAMINE reagent (Life Technologies, Rockville, MD) as instructed by the manufacturer. Luciferase activity was measured by using the Dual-Luciferase Reporter Assay system (Promega).
Results
Role of PKCβ in Ischemia-Induced Retinal Neovascularization.
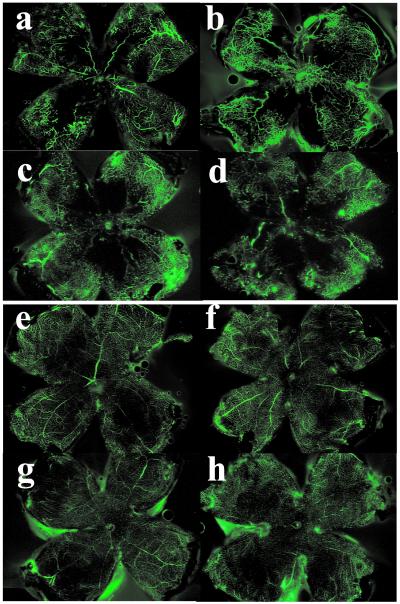
The effects of PKCβ isoforms on ischemia-induced retinal neovascularization were studied in the oxygen-induced retinopathy-of-prematurity model by using PKCβ null mice (12) (PKCβ KO mice) or transgenic mice overexpressing PKCβ2 isoform (PKCβ Tg) in vascular tissues under preproendothelin promoter (9, 10), which exhibited a 9-fold increase in PKC activity. Five days after oxygen treatment, both wild-type and PKCβ Tg mice developed retinal neovascularization as observed by fluorescein-perfused retinal flat mount (Fig. 1). However, a more extensive network of neovascularization was observed in PKCβ Tg mice (Fig. 1b) than with the wild type (B6/FVB) (Fig. 1a). In contrast, much less neovascularization was observed in the retina of PKCβ KO mice (Fig. 1d) than with their respective control mice (B6/129) (Fig. 1c). No retinal neovascularization or significant morphological differences were observed in the vasculature of any groups not receiving oxygen treatment (Fig. 1 _e_– h). No differences were observed in the extent of retinal capillary occlusion in mice with oxygen treatment at postnatal day 12 (P12) (data not shown).
Figure 1.
Retinal vascular pattern by fluorescein-perfused retinal flat mount of (a) wild-type and (b) PKCβ2 transgenic mice (FVB × C57BL/6); (c) wild type and (d) PKCβ null mice (129sv × C57BL/6), with (a–d) and without (e–h) oxygen treatment. Five days after oxygen treatment (P17), fluorescence perfusion showed that the vasculature was increased substantially in PKC β2 transgenic mice and reduced in knockout mice as compared with wild-type mice. No retinal neovascularization or morphological differences in the vasculature were observed in the following groups without oxygen treatment; (e) wild-type and (f) PKCβ2 transgenic mice; (g) wild-type and (h) PKCβ knockout mice.
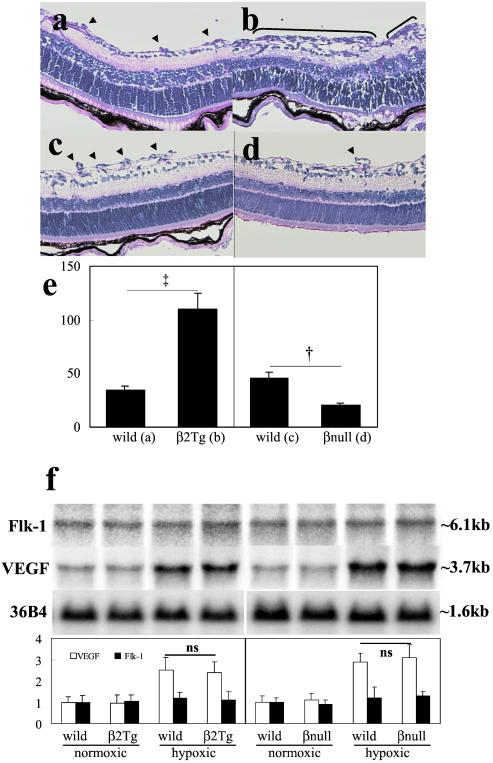
Analysis of retinal cross-sections demonstrated that the number of endothelial cell nuclei anterior to the internal limiting membrane (a measure of the extent of neovascularization) decreased by 55% in PKCβ KO mice (Fig. 2c control, 2d-KO mice) and increased by 3.2-fold in PKCβ Tg mice (Fig. 2 a, control, and b, Tg mice) as compared with their respective controls. These data suggested that activation of PKCβ2 isoform contributed significantly to the development of neovascularization in response to hypoxia. However, PKCβ2 isoform is not essential for the angiogenic process during embryonic development or growth, because both of these processes are normal in PKCβ Tg and KO mice.
Figure 2.
Cross-sectional analysis of retina from (a) wild-type and (b) PKCβ2 transgenic mice; (c) wild-type and (d) PKCβ knockout mice. After retinal neovascularization was induced by oxygen treatment (P17), sections of the eyes were evaluated for neovascularization (parenthesis or arrowhead). (e) Quantification of retinal neovascularization (P17) by counting the neovascular cell nuclei. Vertical axis, number of nuclei anterior to the internal limiting membrane per 6-μm cross section (mean ± standard error of mean; data were calculated from 50 sections of five eyes). Symbols indicate significant differences at P < 0.01 (†) and P < 0.001 (‡). (f) Results were derived from Northern blot analysis of total RNA isolated from the retina of animals 2 days after oxygen treatment and from age-matched normal controls (P14). Northern blots with 36B4 control (Upper) and quantification (Lower). Results were calculated from three separate experiments.
VEGF has been reported to be one of the main growth factors inducing neovascularization in the oxygen-induced retinopathy model (13). Thus, mRNA levels of VEGF and Flk1, a tyrosine kinase receptor primarily mediating VEGF's mitogenic actions in endothelial cells, were measured by Northern blot analysis. No differences were detected in the mRNA levels of VEGF or Flk1 in either PKCβ transgenic mice or knockout mice as compared with their individual controls on postnatal day 14 (Fig. 2f). These data suggested that the angiogenic effects of PKCβ isoform are potentially caused by enhancement of VEGF's intracellular action rather than by increasing the concentration of VEGF or Flk1.
Role of PKC Isoforms in VEGF-Induced Angiogenic Responses.
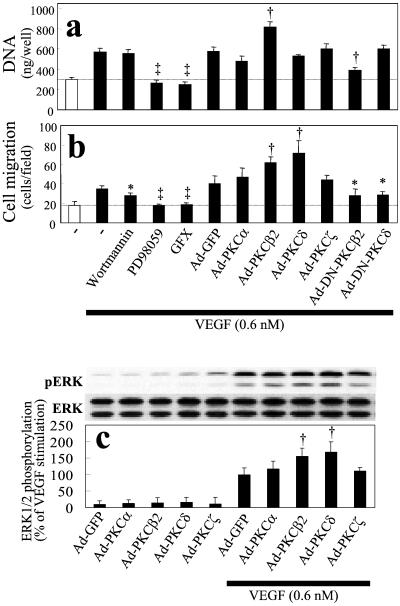
The effects of various PKC isoforms on VEGF-induced cellular proliferation and migration were correlated with several intracellular signaling pathways in BREC. VEGF (0.6 nM) induced a 2-fold increase in BREC total DNA content of BREC after 4 days. The addition of GFX (a general PKC inhibitor) and PD98059 (a MEK inhibitor) blocked VEGF's mitogenic activity completely (Fig. 3a) (2). The effects of each PKC isoform were determined by infecting BREC with nonreplicating adenoviral vectors containing either the wild-type or kinase-inactive dominant-negative mutants of PKC α, β1 and 2, δ, and ζ isoforms. (PKCβ1 and β2 had similar effects in all studies with BREC.) Only PKCβ isoforms enhanced VEGF-induced growth in BREC, resulting in an 86% increase. Overexpression of dominant-negative of PKCβ2 isoform inhibited PKCβ activity by 90% and decreased VEGF-induced growth by 68% (Fig. 3a), suggesting that VEGF's mitogenic activity in BREC depended in part on PKCβ isoform and mitogen-activated protein kinase pathway activation.
Figure 3.
Role of PKC, PI3-kinase, ERK1/2, and specific PKC isoforms on VEGF-induced growth and migration (mean ± standard deviation). VEGF (0.6 nM)-induced increases in (a) cell growth and (b) migration. (a) Cells were incubated with vehicle, GFX (1 μM), wortmannin (100 nM), PD98059 (20 μM), or infected with adenovirus (1.0 × 108 plaque-forming units/ml). After incubation for 4 days at 37°C with or without VEGF (0.6 nM), the cells were lysed in 0.1% SDS, and the DNA content was measured by means of Hoechst-33258 dye and a fluorometer (model TKO-100) to determine cell growth. (b) To evaluate cell migration, a modified Boyden chamber migration assay with BREC was performed. The top and bottom surface of the chamber membrane was coated with collagen I. Serum-starved BREC overexpressing each PKC isoform were induced to migrate toward VEGF (0.6 nM) placed in the bottom chamber and harvested after 4 h. Cells that migrated to the bottom of the chamber were enumerated by counting SYTOX green (Molecular Probes) nucleic acid-stained cells. Overexpression of PKCβ1 had the same results as PKCβ2 (data not shown). (c) Effects of individual PKC isoform on VEGF-induced ERK phosphorylation. Cells overexpressing each PKC isoform were incubated with VEGF (0.6 nM) for 10 min, and total proteins were separated by SDS-PAGE and transferred to nitrocellulose membrane. Immunoblots were probed with an antibody to phosphorylated ERK (Upper) and reprobed with antibody to total ERK (Lower). Symbols indicate significant differences at P < 0.05 (*), P < 0.01 (†), and P < 0.001 (‡) vs. VEGF alone. Results were calculated from three separate experiments and are presented as mean ± standard deviation.
PKC activation also enhanced VEGF-induced BREC migration. VEGF (0.6 nM) increased migration of BREC 2-fold, an effect inhibited by GFX, PD98059, and partially (41%) by wortmannin [a phosphatidylinositol 3-kinase (PI3-kinase) inhibitor]. Overexpression of PKC α, β2, δ, and ζ isoforms by adenoviral vectors increased protein levels of these PKC isoforms by more than 10-fold; however, only PKCβ2 and δ isoforms enhanced VEGF-induced migration, by 100% and 145%, respectively. Overexpression of dominant-negative PKCβ2 and δ isoforms, as described above, inhibited VEGF-induced BREC migration by 55% and 32%, respectively (Fig. 3b). In addition, overexpression of PKCβ2 and PKCδ isoforms increased VEGF-induced mitogen-activated protein kinase (extracellular signal-regulated kinase; ERK1/2) phosphorylation by 61% and 74%, respectively (Fig. 3c), but did not increase VEGF-induced PI3-kinase–Akt activation (data not shown). These data indicated that both PKCβ and PKCδ isoforms mediated VEGF-induced ERK1/2 activation and migration, but only PKCβ isoform enhanced VEGF-induced endothelial cell proliferation, suggesting that these PKC isoforms have both common and distinct targets for their downstream actions.
Role of PKCβ in VEGF-Induced Rb-E2F Pathway Activation.
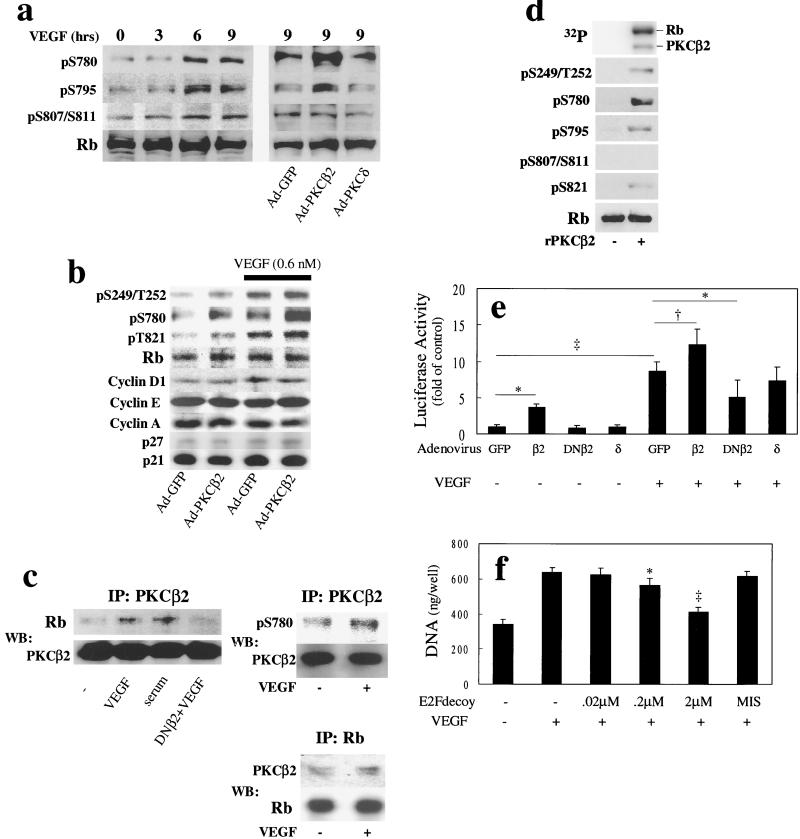
Because PKC activation, particularly the β isoforms, enhanced VEGF's mitogenic activity in BREC, we characterized the effect of overexpressing PKCβ2 isoform on various signaling molecules that regulate the progression of the cell cycle. VEGF and overexpression of PKCβ2, but not PKCδ isoforms increased basal phosphorylation of Rb (Fig. 4), a tumor suppressor that can regulate cellular proliferation, differentiation, and death by binding and inactivating the E2F transcriptional factor family (27). As shown in Fig. 4 a and b, VEGF increased phosphorylation of Rb in a time-dependent manner by up to 3-fold at all phosphorylation sites as quantified by various phospho-specific antibodies (S249/T252, S780, S795, S807/S811, and S821). Overexpression of PKCβ2 isoform by adenoviral vectors increased basal- and VEGF-induced Rb phosphorylation at S780 and S795 (Fig. 4a) by more than 3-fold, but did not change significantly the phosphorylation at S249/T252, S807/S811, or S821 and did not alter the expression of cyclins or cyclin-dependent kinase inhibitors (Fig. 4b). Overexpression of PKCδ isoform did not significantly alter VEGF-induced phosphorylation of Rb.
Figure 4.
Effects of PKCβ2 isoform activation and VEGF on Rb phosphorylation and actions. (a) Time course of VEGF's (0.6 nM) stimulation or the overexpression of PKCβ2 or δ isoforms. Phosphorylation of S780, S795, and S807/S811 were quantified by phospho-specific antibodies. After the addition of VEGF, the cells were harvested at each time point and total protein was separated by SDS-PAGE, transferred to nitrocellulose membrane, and analyzed with phospho-specific and total Rb antibodies by immunoblot. (b) Effects of PKCβ2 overexpression on basal and VEGF-induced Rb phosphorylation at S780, S249/T252, and S821, and the pattern of expression of cyclin D1, E, A, and cyclin-dependent kinase inhibitors p27 and p21 were characterized after the addition of VEGF (0.6 nM) for 24 h. (c) Interaction of PKCβ2 and Rb protein in vivo (Left and Right Upper). BREC overexpressing PKCβ2 were incubated with VEGF (0.6 nM) or 10% calf serum for 24 h. Proteins were immunoprecipitated with anti-PKCβ2 antibodies and fractionated by SDS-PAGE. Immunoblots were probed with an antibody to Rb or phosphoserine 780 (Top) and reprobed with antibody to PKCβ2 (Bottom) to determine whether PKCβ2 can associate directly with Rb (Right Lower). Cells were incubated with VEGF (0.6 nM) for 24 h, and proteins were immunoprecipitated with anti-Rb antibodies and fractionated by SDS-PAGE. Immunoblots were probed with an antibody to PKCβ2 (Top) and reprobed with antibody to Rb (Bottom) to determine whether Rb can associate directly with PKCβ2. (d) In vitro phosphorylation of Rb by recombinant PKCβ2. PKCβ2 (0.2 μM) was incubated with Rb protein (1.2 μM) for 15 min at 30°C in the presence of Ca2+ (1 mM), phosphatidylserine (100 μg/ml), and diacylglycerol (1.3 μg/ml). The reaction was initiated by the addition of ATP (30 μM) with [γ-32P]ATP (30 μCi/100 nmol). After 15 min, the reaction was terminated by the addition of Laemmli's sample buffer. Samples were separated by SDS-PAGE, dried, and exposed to PhosphorImager (Top), or transferred to nitrocellulose membrane, and analyzed with phospho-specific and total Rb antibodies by immunoblot. (e) Effects of VEGF and PKC on E2F promoter activity. Cells were transfected with reporter constructs 24 h after adenovirus infection containing each PKC isoform or mutant, and stimulated by VEGF (0.6 nM) for 9 h. Cells were harvested, and luciferase activity was measured. (f) Cells were incubated for 4 days at 37°C with or without VEGF (0.6 nM) after introduction of E2F (14 nucleotide base) decoy or mismatch control decoy. The cells were lysed in 0.1% SDS and the DNA content was measured. Symbols indicate significant differences at P < 0.05 (*), P < 0.01 (†), and P < 0.001 (‡). Results were calculated from three separate experiments and plotted as mean ± standard deviation.
To determine whether PKCβ2 isoform can associate and phosphorylate Rb directly in BREC, immunoprecipitation studies were performed with anti-PKCβ2 antibody, and Rb protein was detected by immunoblot analysis. At basal conditions, some association of PKCβ2 isoform and Rb protein was observed. The addition of VEGF and serum increased the association of PKCβ2 with Rb by 2.2- and 2.6-fold, respectively. Overexpression of PKCβ2 dominant-negative mutant did not associate with Rb (Fig. 4c). The same complex of PKCβ2 and Rb were characterized with antibodies to pS780 of Rb, which showed a basal phosphorylation with the overexpression of PKCβ2. The addition of VEGF (0.6 nM) increased phosphorylated Rb levels by 2- to 3-fold (Fig. 4c). Total cell lysates were immunoprecipitated with antibodies to Rb protein and immunoblotted with antibodies to PKCβ2. At basal conditions some associations of PKCβ2 and Rb were observed, but the addition of VEGF (0.6 nM) increased the amount of PKCβ2- associated Rb protein by 1.7-fold (Fig. 4c). In vitro studies demonstrated that recombinant PKCβ2 isoform phosphorylated Rb protein in the presence of diacylglycerol and phosphatidylserine at S249/T252, S780, S795, and S821 sites (Fig. 4d). These data suggested that PKCβ2 isoform, when activated, could phosphorylate Rb protein at multiple, but specific sites in an isoform-selective manner.
To determine whether PKCβ2 isoform could increase E2F activity by the phosphorylation of Rb protein, the effect of overexpressing PKCβ2 isoform on VEGF-induced E2F activation was evaluated by luciferase assay in BREC. VEGF and overexpression of PKCβ2 independently increased E2F activity by 8.6- and 3.7-fold, respectively. Overexpression of PKCβ2 increased VEGF's effect by 49% (Fig. 4e). Again, overexpression of PKCδ isoform was not effective, whereas overexpression of PKCβ2 dominant-negative inhibited VEGF's effect. Finally, inhibition of E2F by E2F double-structured DNA decoy decreased VEGF-induced BREC proliferation in a dose-dependent manner, with a maximum inhibition of 76% (Fig. 4f).
Discussion
These studies have demonstrated that activation of the β isoforms of PKC can selectively enhance VEGF's mitogenic effects on endothelial cells and hypoxia-induced retinal neovascularization. VEGF's effect on endothelial cell growth differed from its effect on migration, which involved PKCδ isoform and PI3-kinase pathways. The mechanism of the mitogenic activity of PKCβ isoforms on BREC includes the activation of ERK and phosphorylation of Rb protein. These data have identified Rb protein as a potential isoform-selective target of the activated PKCβ2 isoform in the endothelial cells. The isoform selectivity of PKCβ isoforms could be caused by their subcellular localization that can translocate to the nuclear membrane when activated (28).
VEGF clearly can phosphorylate multiple serine and threonine sites on the Rb protein which are potential phosphorylation sites of cyclin D and E kinases in retinal capillary endothelial cells (29, 30). Phosphorylation of Ser-780, Ser-795, Ser-807, Ser-811, and Thr-821 decreases the binding of Rb to E2F, which would permit endothelial cells to progress from G1 to later stages of the cell cycle (29–32). Activation of PKCβ2 isoform also phosphorylated these serine and threonine sites, except Ser-807 and Ser-811 on the Rb in vitro. However, overexpression of PKCβ2 isoform, but not the PKCδ or the PKCβ2 dominant-negative isoforms, increased only the phosphorylation of Ser-780 and Ser-795 at the C-terminal of Rb but did not phosphorylate other sites induced by PKCβ isoform in vitro or by VEGF. Brown et al. (31) has reported that the loss of Ser-781 and the Ser-788 sites did not affect the binding of Rb to E2F-DNA. However, the phosphorylation of these sites combined with other phosphorylation sites in the N and C terminals affected by VEGF can alter the binding and the promoter activities of E2F on transcription (31, 32).
The results of the coprecipitation studies suggested that the activated PKCβ2 but not its kinase-inactive dominant-negative isoform can directly bind and phosphorylate Rb. It is also possible that PKCβ2 isoform could indirectly phosphorylate Ser-780 and Ser-785 of Rb by binding to cyclin E kinase, which has been reported to bind and phosphorylate the same serine sites of Rb (29). Further studies will be needed to decipher the potential interactions of PKCβ isoforms with cyclin E kinase and the definitive role of Ser-780 and Ser-795 phosphorylation on Rb in mediating the enhancement of VEGF-induced endothelial cell proliferation and retinal neovascularization. However, these results have established that the activation of PKCβ isoforms can selectively enhance the mitogenic action of VEGF and hypoxia-induced retinal neovascularization potentially by directly binding to and phosphorylating Rb and subsequently permitting activation of E2F to mediate transcription and cell cycle progression in retinal endothelial cells.
Acknowledgments
We appreciate the suggestions provided by Dr. Edward P. Feener, and for technical assistance by Mr. Edward Boschetti. cDNA of PKCs and preproendothelin promoter were kindly provided by Dr. Douglas Ways (Lilly Research Laboratories, Indianapolis) and Dr. Hiroki Kurihara (Tokyo University, Tokyo), respectively. This work was supported by National Institutes of Health Grants R01 EY5110 (to G.L.K.) and EY10827 (to L.P.A.). K.S. is a recipient of the Mary K. Iacocca Fellowship, and L.P.A. is a recipient of the Research to Prevent Blindness Dolly Green Scholarship.
Abbreviations
PKC
protein kinase C
VEGF
vascular endothelial growth factor
P_n_
postnatal day n
Rb
retinoblastoma
BREC
bovine retinal endothelial cells
Tg
transgenic
KO
null
PI3-kinase
phosphatidylinositol 3-kinase
E2F
E2 promoter binding factor
ERK
extracellular signal-regulated kinase
References
- 1.Wu L W, Mayo L D, Dunbar J D, Kessler K M, Baerwald M R, Jaffe E A, Wang D, Warren R S, Donner D B. J Biol Chem. 2000;275:5096–5103. doi: 10.1074/jbc.275.7.5096. [DOI] [PubMed] [Google Scholar]
- 2.Xia P, Aiello L P, Ishii H, Jiang Z Y, Park D J, Robinson G S, Takagi H, Newsome W P, Jirousek M R, King G L. J Clin Invest. 1996;98:2018–2026. doi: 10.1172/JCI119006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takahashi T, Ueno H, Shibuya M. Oncogene. 1999;18:2221–2230. doi: 10.1038/sj.onc.1202527. [DOI] [PubMed] [Google Scholar]
- 4.Kim K J, Li B, Winer J, Armanini M, Gillett N, Phillips H S, Ferrara N. Nature (London) 1993;362:841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 5.Plate K H, Breier G, Weich H A, Risau W. Nature (London) 1992;359:845–848. doi: 10.1038/359845a0. [DOI] [PubMed] [Google Scholar]
- 6.Firestein G S. J Clin Invest. 1999;103:3–4. doi: 10.1172/JCI5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aiello L P, Bursell S E, Clermont A, Duh E, Ishii H, Takagi C, Mori F, Ciulla T A, Ways K, Jirousek M, et al. Diabetes. 1997;46:1473–1480. doi: 10.2337/diab.46.9.1473. [DOI] [PubMed] [Google Scholar]
- 8.Aiello L P, Avery R L, Arrigg P G, Keyt B A, Jampel H D, Shah S T, Pasquale L R, Thieme H, Iwamoto M A, Park J E, et al. N Engl J Med. 1994;331:1480–1487. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- 9.Harats D, Kurihara H, Belloni P, Oakley H, Ziober A, Ackley D, Cain G, Kurihara Y, Lawn R, Sigal E. J Clin Invest. 1995;95:1335–1344. doi: 10.1172/JCI117784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohashi Y, Kawashima S, Hirata K, Yamashita T, Ishida T, Inoue N, Sakoda T, Kurihara H, Yazaki Y, Yokoyama M. J Clin Invest. 1998;102:2061–2071. doi: 10.1172/JCI4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wakasaki H, Koya D, Schoen F J, Jirousek M R, Ways D K, Hoit B D, Walsh R A, King G L. Proc Natl Acad Sci USA. 1997;94:9320–9325. doi: 10.1073/pnas.94.17.9320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leitges M, Schmedt C, Guinamard R, Davoust J, Schaal S, Stabel S, Tarakhovsky A. Science. 1996;273:788–791. doi: 10.1126/science.273.5276.788. [DOI] [PubMed] [Google Scholar]
- 13.Aiello L P, Pierce E A, Foley E D, Takagi H, Chen H, Riddle L, Ferrara N, King G L, Smith L E. Proc Natl Acad Sci USA. 1995;92:10457–10461. doi: 10.1073/pnas.92.23.10457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith L E, Wesolowski E, McLellan A, Kostyk S K, D'Amato R, Sullivan R, D'Amore P A. Invest Ophthalmol Visual Sci. 1994;35:101–111. [PubMed] [Google Scholar]
- 15.Takagi H, King G L, Aiello L P. Diabetes. 1996;45:1016–1023. doi: 10.2337/diab.45.8.1016. [DOI] [PubMed] [Google Scholar]
- 16.Jiang Z Y, Lin Y W, Clemont A, Feener E P, Hein K D, Igarashi M, Yamauchi T, White M F, King G L. J Clin Invest. 1999;104:447–457. doi: 10.1172/JCI5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.King G L, Goodman A D, Buzney S, Moses A, Kahn C R. J Clin Invest. 1985;75:1028–1036. doi: 10.1172/JCI111764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uberall F, Giselbrecht S, Hellbert K, Fresser F, Bauer B, Gschwendt M, Grunicke H H, Baier G. J Biol Chem. 1997;272:4072–4078. doi: 10.1074/jbc.272.7.4072. [DOI] [PubMed] [Google Scholar]
- 19.Orr J W, Newton A C. J Biol Chem. 1994;269:27715–27718. [PubMed] [Google Scholar]
- 20.Li W, Yu J C, Shin D Y, Pierce J H. J Biol Chem. 1995;270:8311–8318. doi: 10.1074/jbc.270.14.8311. [DOI] [PubMed] [Google Scholar]
- 21.Uberall F, Hellbert K, Kampfer S, Maly K, Villunger A, Spitaler M, Mwanjewe J, Baier-Bitterlich G, Baier G, Grunicke H H. J Cell Biol. 1999;144:413–425. doi: 10.1083/jcb.144.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He T C, Zhou S, da Costa L T, Yu J, Kinzler K W, Vogelstein B. Proc Natl Acad Sci USA. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thieme H, Aiello L P, Takagi H, Ferrara N, King G L. Diabetes. 1995;44:98–103. doi: 10.2337/diab.44.1.98. [DOI] [PubMed] [Google Scholar]
- 24.Morishita R, Gibbons G H, Horiuchi M, Ellison K E, Nakama M, Zhang L, Kaneda Y, Ogihara T, Dzau V J. Proc Natl Acad Sci USA. 1995;92:5855–5859. doi: 10.1073/pnas.92.13.5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakamoto T, Ishibashi T, Kimura H, Yoshikawa H, Spee C, Harris M S, Hinton D R, Ryan S J. Invest Ophthalmol Visual Sci. 1995;36:1076–1083. [PubMed] [Google Scholar]
- 26.Ishii H, Jirousek M R, Koya D, Takagi C, Xia P, Clermont A, Bursell S E, Kern T S, Ballas L M, Heath W F, et al. Science. 1996;272:728–731. doi: 10.1126/science.272.5262.728. [DOI] [PubMed] [Google Scholar]
- 27.Kaelin W G. BioEssays. 1999;21:950–958. doi: 10.1002/(SICI)1521-1878(199911)21:11<950::AID-BIES7>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 28.Fields A P, Pettit G R, May W S. J Biol Chem. 1988;263:8253–8260. [PubMed] [Google Scholar]
- 29.Connell-Crowley L, Harper J W, Goodrich D W. Mol Biol Cell. 1997;8:287–301. doi: 10.1091/mbc.8.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zarkowska T, Mittnacht S. J Biol Chem. 1997;272:12738–12746. doi: 10.1074/jbc.272.19.12738. [DOI] [PubMed] [Google Scholar]
- 31.Brown V D, Phillips R A, Gallie B L. Mol Cell Biol. 1999;19:3246–3256. doi: 10.1128/mcb.19.5.3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knudsen E S, Wang J Y. Mol Cell Biol. 1997;17:5771–5783. doi: 10.1128/mcb.17.10.5771. [DOI] [PMC free article] [PubMed] [Google Scholar]