Altered retinoid homeostasis catalyzed by a nicotine metabolite: Implications in macular degeneration and normal development (original) (raw)
Abstract
Retinoids (vitamin A) serve two distinct functions in higher animals: light absorption for vision and gene regulation for growth and development. Cigarette smoking is a contributing factor for diseases that affect vision such as age-related macular degeneration and increases the risk of birth defects; however, altered retinoid homeostasis has received little attention as a potential mechanism for smoking-associated toxicities. Herein, we demonstrate that nornicotine, a nicotine metabolite and component of cigarette smoke, catalyzes the _Z_-to-E alkene isomerization of unsaturated aldehydes and ketones, including retinals. Despite the recent explosion in the use of organic compounds as chemical catalysts, minimal effort has been devoted to biologically relevant organocatalysis. Our study demonstrates a system in which a lowest unoccupied molecular orbital-lowering intermediate similar to the endogenous protein rhodopsin effectively catalyzes isomerization under biologically relevant conditions. The product of retinal isomerization is _all-E_-retinal, which in the eye is a biosynthetic precursor to _N_-retinylidene-_N_-retinylethanolamine, a hallmark of age-related macular degeneration. Furthermore, 9-_Z_- and _all-E_-retinal isomers are biosynthetic precursors to 9-_Z_- and _all-E_-retinoic acids, ligands that mediate specific cellular responses by binding to transcriptional regulatory proteins critical in growth and development. Strict maintenance of retinal isomer composition is essential for proper transcriptional regulation. Nornicotine-catalyzed retinal isomerization implies an underlying molecular mechanism for age-related macular degeneration, the birth defects associated with smoking, and other smoking-associated abnormalities that stem from disruption of retinoid metabolism.
Keywords: organocatalysis, retinal isomerization
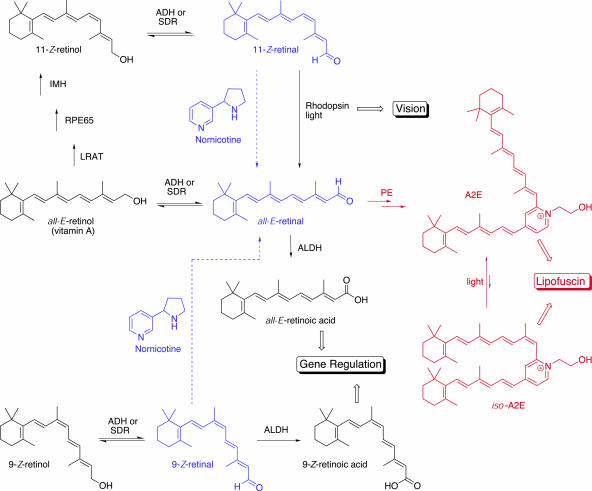
Vitamin A (_all-E_-retinol) and provitamin A carotenoids are metabolized into key retinoid intermediates that play a critical role in vision, bone growth, reproduction, cell division, and cell differentiation (1–4). These essential nutrients also help to prevent infection by maintaining the integrity of the skin and mucous membranes (5–8). Furthermore, vitamin A assists in the regulation of the immune system by stimulating lymphocyte production (2, 4, 6, 9). Retinoids are involved in numerous physiological functions, and the molecular processes can be divided into two main categories. In vision, 11-_Z_-retinal is the essential light-absorbing component of the protein rhodopsin, whereas all other nonvisual cellular responses are thought to be mediated by ligand activation of transcriptional regulatory proteins (Fig. 1) (10, 11).
Fig. 1.
Retinoid metabolism and proposed nornicotine-organocatalyzed pathways. Black, retinoid metabolism and proper cellular function; red, by-products of the vision cycle that lead to lipofuscin; blue, proposed nornicotine-catalyzed interference pathways. ADH, retinoid alcohol dehydrogenase family of enzymes; SDR, retinoid short-chain dehydrogenase/reductase family of enzymes; ALDH, retinoid aldehyde dehydrogenase family of enzymes; LRAT, lecithin retinol acyltransferase; RPE65, RPE protein 65; IMH, isomerohydrolase; PE, phosphatidylethanolamine.
In the visual cycle, _all-E_-retinol is converted to 11-_Z_-retinal through a multistep enzymatic process. The photoreceptor protein rhodopsin is an iminium-ion complex of 11-_Z_-retinal and a lysine residue of the protein opsin. Complexation effectively lowers the lowest unoccupied molecular orbital of 11-_Z_-retinal and allows for isomerization to _all-E_-retinal when rhodopsin absorbs a photon of light. A subsequent protein conformational change initiates the electrochemical signal that ultimately leads to vision. Disruption of this tightly controlled process has pathological implications. For example, accumulation of _all-E_-retinal feeds the _N_-retinylidene-_N_-retinylethanolamine (A2E) biosynthetic pathway, which is formed by sequential condensation of phosphatidylethanolamine with two molecules of _all-E_-retinal and is an undigestable by-product of the visual cycle (12). A2E and its photoisomers (e.g., _iso_-A2E) have detergent-like effects on cellular membranes (13, 14), alter lysosomal function (15, 16), and release apoptotic proteins from mitochondria (17), all of which lead to lipofuscin of retinal pigmented epithelial (RPE) cells (18–20). Lipofuscin of RPE cells degenerates the photoreceptors in the eye, results in aging, and is a characteristic of the disease states age-related macular degeneration and Stagardt's disease, an inherited form of juvenile macular degeneration (21).
In receptor-mediated nonvisual functions, the retinoid metabolites _all-E_-retinoic acid and 9-_Z_-retinoic acid serve as gene regulators by binding to two families of transcriptional regulatory proteins, the retinoic acid receptor (RAR) and the retinoid X receptor (RXR) (22–24). RXR homodimers and RAR/RXR heterodimers are the functional complexes that regulate gene expression by binding to specific retinoid response elements on DNA. RXR and RAR signaling is implicated in numerous cellular and physiological processes (see above), most notably cellular differentiation during embryonic development. Recent studies have shown that retinoic acid signaling is involved in embryonic cardiomyocyte production (25) and controls embryonic bilateral symmetry (26–29) in vertebrate animal development. Both depletion and surplus of key retinoid intermediates during gestation lead to birth defects; therefore, strict homeostatic control is essential for proper growth and development (10).
Cigarette smoking is known to contribute to diseases that affect vision and cause birth defects. Although recent genomic studies have shown that a genetic predisposition for age-related macular degeneration is manifested in complement factor H polymorphisms (30–32), the primary environmental factor contributing to age-related macular degeneration is cigarette smoking (33–35). Indeed, mice administered nicotine as a dietary supplement were shown to have an increased incidence of age-related macular degeneration (36).
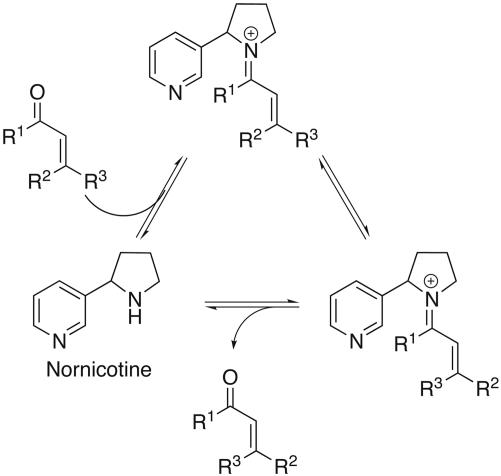
We have initiated a program aimed at studying the chemistry of long-lived nicotine metabolites and found that nornicotine, a minor metabolite of nicotine (37) and a component of cigarette smoke (38), can catalyze aldol reactions in aqueous buffer (39–41) and cause aberrant protein glycation in vivo (42, 43) through an enamine intermediate (40, 41). Iminium-ion formation is a mechanistic precursor to enamines and is the key intermediate in numerous organocatalytic transformations (e.g., Diels–Alder, cycloaddition, Friedel–Crafts alkylation) (44). Therefore, nornicotine was envisioned to serve as an organocatalyst in the alkene isomerization of unsaturated aldehydes and ketones, including the retinals, in a biomimetic fashion by a lowest unoccupied molecular orbital-lowering mechanism (Fig. 2). Nornicotine-based iminium chemistry is fundamentally plausible and is an intriguing mechanism for the pathology of key smoking-associated diseases that may stem from disrupted retinoid metabolism.
Fig. 2.
Nornicotine-catalyzed _Z_-to-E isomerization. R1, R2, R3 = H or alkyl.
Materials and Methods
General Procedures. Unless otherwise stated, all reactions were performed under an inert atmosphere with dry reagents, solvents, and oven-dried glassware. All starting material and reagents were purchased from Acros (Geel, Belgium), Sigma-Aldrich, Fisher, or Toronto Research Chemicals (Downsview, ON, Canada) and used as received. 1H NMR and 13C NMR spectra were recorded on a Varian INOVA-400 spectrometer at 400 and 100 MHz, respectively. Electrospray ionization MS experiments were performed on an API 100 PerkinElmer SCIEX single-quadrupole mass spectrometer. HPLC assays were performed on a Hitachi (Tokyo) HPLC (model L-7200) equipped with an autosampler (model L-7200), column oven (model L-7300), and UV detector (model L-7400) using Vydac (Hesperia, CA) RP-C18 columns.
Synthesis of N-[4-(1-hydroxybut-2-ynyl)phenyl]acetamide (4). To an anhydrous tetrahydrofuran solution (≈100 ml) containing 4-acetamidobenzaldehyde (3) (1.2 g, 7.4 mmol) was added a 0.5 M solution of 1-propynylmagnesium bromide in tetrahydrofuran (31 ml, 15.5 mmol) under argon at -20°C over 15 min. As the resulting mixture was allowed to warm to room temperature, the starting material was completely consumed as monitored by TLC. A saturated (sat.) solution of NH4Cl was added; then, water and then ethyl acetate (EtOAc) were added. The layers were separated, and the combined aqueous layers were extracted further with EtOAc (2×). The combined organic layers were washed with sat. NaCl, dried over anhydrous Na2SO4, and then filtered and concentrated in vacuo to yield 1.2 g (78%) of a white gum. 1H NMR (400 MHz, acetone-_d_6): δ 9.21 (s, 1H), 7.61 (m, 2H), 7.42 (m, 2H), 5.35 (m, 1H), 4.75 (d, J = 5.9 Hz, 1H), 2.07 (s, 3H) 1.83 (d, J = 1.8, 3H); 13C NMR (100 MHz, acetone-_d_6): δ 172.5, 143.3, 142.0, 131.3, 123.2, 85.3, 84.9, 67.7, 27.8, 6.9. Electrospray ionization+ MS calculated for C12H14NO2: 204.1019 (M+H)+; observed: 204.1014 (M+H)+.
Synthesis of N-(4-but-2-ynoylphenyl)acetamide (5). To an anhydrous solution of DMSO (≈100 ml) charged with 4 (1.0 g, 5.1 mmol) was added 2-iodoxybenzoic acid (2.14 g, 7.65 mmol) under argon at room temperature. The resulting slurry was stirred for 3 h, in which time the solution became homogeneous and no starting material remained (TLC). A solution of sat. NaHCO3 was added, then H2O was added, and the resulting aqueous mixture was extracted with EtOAc/Et2O (1:1, vol/vol) (3×). The combined organic layers were washed sequentially with sat. NaHCO3,H2O, and sat. NaCl, dried over anhydrous Na2SO4, filtered, and concentrated in vacuo to yield 400 mg (40%) of a tan solid. 1H NMR (400 MHz, acetone-_d_6): δ 9.57 (s, 1H), 8.06 (m, 2H), 7.80 (m, 2H), 2.16 (s, 3H), 2.13 (s, 3H); 13C NMR (100 MHz, acetone-_d_6): δ 177.8, 170.5, 146.6, 133.8, 132.3, 120.1, 93.5, 80.2, 25.4, 4.7. Electrospray ionization+ MS calculated for C12H12NO2: 202.0863 (M+H)+; observed: 202.0869 (M+H)+.
Synthesis of (Z)-N-(4-but-2-enoylphenyl)acetamide (1). A flask containing 5 (34 mg, 0.17 mmol) and 5% Pd/BaSO4 (2 mg, catalytic) was charged with anhydrous MeOH (≈15 ml) and quinoline (4 μl, 0.03 mmol). The flask was stirred under H2 at room temperature and was stopped after 2.5 h when formation of the reduced alkane appeared as monitored by HPLC [isocratic mobile phase: acetonitrile/water (15:85) with 0.1% trifluoroacetic acid, 1 ml·min-1; UV detection at 292 nm; retention times: _E_-alkene, 12.2 min; _Z_-alkene, 14.3 min; alkane, 20.2 min]. The reaction mixture was filtered, concentrated, and redissolved in 5 ml of 40% DMSO (aqueous) and isolated by preparative HPLC [isocratic mobile phase: acetonitrile/water (20:80) with 0.1% trifluoroacetic acid, 10 ml·min-1; UV detection at 292 nm; retention times: _E_-alkene, 28.4 min; _Z_-alkene, 33.0 min; alkane, 47.4 min]. Fractions were collected at 0.5-min intervals in test tubes containing 1 ml of EtOAc and 100 μl of sat. NaHCO3. The fractions containing 1 were combined, separated, and further extracted with EtOAc (2×). The organic layers were combined and dried over anhydrous Na2SO4, filtered, and concentrated in vacuo to yield 14 mg (43% yield, 5% _E_-alkene impurity as determined by 1H NMR) of white solid. 1H NMR (400 MHz, acetone-_d_6): δ 9.48 (s, 1H), 7.94 (m, 2H), 7.77 (m, 2H), 6.94 (dq, J = 11.6, 1.8 Hz, 1H), 6.42 (dq, J = 11.6, 7.2 Hz, 1H), 2.09 (dd, J = 7.2, 1.8 Hz, 3H) 2.12 (s, 3H); 13C NMR (100 MHz, acetone-_d_6): δ 191.6, 170.4, 145.5, 144.4, 135.2, 131.2, 126.9, 120.1, 25.4, 17.2. Electrospray ionization MS calculated for C12H14NO2: (M+H)+; observed: 204.1021 (M+H)+.
HPLC Assay for Z-to-E Isomerization of 1. The rate constants for nornicotine-catalyzed _Z_-to-E isomerization were determined by the method of initial rates under pseudo-first-order conditions. Reactions were initiated by adding a solution of 1 (125–1,000 μM) to phosphate buffer (200 mM, pH 6.5, 7.4, or 8.0) containing nornicotine (100 μM) to a final volume of 250 μl (5% DMSO). The reaction vessels were incubated in the dark at room temperature, and time points (20 μl) were removed and injected onto the previously described HPLC system, which was equipped with a guard column [isocratic mobile phase of acetonitrile/water with 0.1% trifluoroacetic acid (15:85), solvent flow rate of 1 ml·min-1; UV detection at 292 nm]. Each assay was performed in duplicate, and the composition of E and Z isomers were quantified by measuring peak areas relative to each other.
HPLC Assay for Z-to-E Isomerization of Retinals. The rate constants for nornicotine-catalyzed _Z_-to-E isomerization were determined by the method of initial rates under pseudo-first-order conditions. Reactions were initiated by adding a solution of _Z_-retinal (100–500 μM final) to phosphate buffer (20 mM, pH 7.4 or 8.0) containing nornicotine (300 μM) to a final volume of 1 ml (20% DMSO). Aliquots (200 μl) were placed in glass vials in the dark and degassed by a freeze-pump-thaw cycle (2×). The samples were incubated at 37°C, and time points (20 μl) were removed and injected onto the previously described HPLC system equipped with a guard column and two RP-C18 Vydac HPLC columns in series [isocratic mobile phase of acetonitrile/0.1 M NH4OAc (63:37) for 13-_Z_-retinal and acetonitrile/0.5 M NH4OAc (50:50) for 9-_Z_-retinal, solvent flow rate of 1.6 ml·min-1; UV detection at 380 nm (45)]. Before injection, the 9-_Z_-retinal samples were irradiated on a white-light transilluminator (3.4 mW/cm2) for various amounts of time. The column temperature was 45°C for 9-_Z_-retinal and room temperature for 13-_Z_-retinal. Each assay was performed in duplicate, and increase in _all-E_-retinal was determined by interpolation of peak heights relative to a standard curve.
Results and Discussion
The toxicities associated with tobacco use are well documented clinically; however, many of the underlying molecular mechanisms remain enigmatic. Nornicotine, a nicotine metabolite and component of cigarette smoke, undergoes enamine-based chemistry under biologically relevant conditions that may have ramifications in smoking-associated toxicities (39–43). Based on our understanding of this chemistry, we proposed that nornicotine can catalyze the alkene isomerization of key retinal intermediates through iminium-ion formation and disrupt proper retinoid homeostasis, revealing an underlying molecular mechanism for tobacco-dependent pathologies, particularly age-related macular degeneration and birth defects. It is interesting to note that iminium-ion lowest unoccupied molecular orbital-lowering intermediates have been exploited in an array of organocatalytic reactions for asymmetric synthesis in organic solvents (44). Recently, independent studies by List and coworkers (46) and MacMillan and coworkers (47) reported the metal-free organocatalytic asymmetric reductions of α,β-unsaturated aldehydes using iminium-ion intermediates in a biologically inspired reaction. It is interesting to note that both groups of investigators found that regardless of the E/Z isomeric composition of the α,β-unsaturated aldehyde, the iminium-ion intermediate isomerizes to the more stable E isomer before reduction.
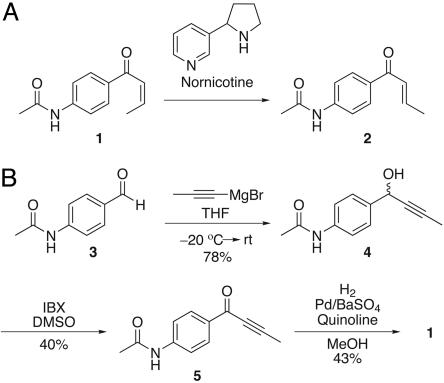
Nornicotine-Catalyzed Isomerization in a Model System. Because of the relative instability and limited aqueous solubility of retinals, a model system was required for initial investigations of nornicotine-catalyzed alkene isomerization. (Z)-_N_-(4-but-2-enoylphenyl)acetamide (1) was envisioned as a suitable model with sufficient water solubility (Fig. 3_A_) while possessing a phenylacetamide chromophore for ease of detection in an HPLC assay. Starting from 4-acetamidobenzaldehyde (3), 1 was synthesized in three steps (Fig. 3_B_). Grignard addition with 1-propynylmagnesium bromide provided substituted alcohol 4 that was subsequently oxidized with 2-iodoxybenzoic acid to yield ketone 5. Selective reduction with Lindlar's catalyst provided the _Z_-alkene (1) in acceptable yield.
Fig. 3.
Initial study of nornicotine-catalyzed isomerization. (A) Model system to examine nornicotine-catalyzed _Z_-to-E isomerization. (B) Synthesis of model compound 1. THF, tetrahydrofuran; rt, room temperature; IBX, 2-iodoxybenzoic acid.
Light energy is essential for rhodopsin isomerization of 11-_Z_-retinal; therefore, different light sources were examined in the isomerization of 1. As expected, the overall rate of isomerization increased with increasing light energy (dark < white light < UV light). Under all tested conditions, nornicotine was found to catalyze the isomerization of _Z_-alkene 1 to the _E_-alkene 2, but surprisingly, additional light energy was not required for rate enhancement. In fact, the greatest enhancement over the corresponding uncatalyzed reaction was in the dark (UV light < white light < dark). Consequently, kinetic analysis of the reaction was performed in the dark (Table 1), and the nornicotine-catalyzed reactions were found to be 10–30 times faster than the corresponding uncatalyzed reactions at different pH values. The pH-rate profile can be readily explained in terms of the structure of the catalyst. The active catalyst is the free amine, and the pyrrolidine nitrogen of nornicotine has a pKa ≈ 9 (48, 49); therefore, the amount of available catalyst is low at physiological pH and increases with increasing pH. Consequently, _k_obs in the model system increased with increasing pH in part because there is more available catalyst at higher pH.
Table 1. Pseudo-first-order rate constants for _Z_-to-E isomerization of 1 and retinals.
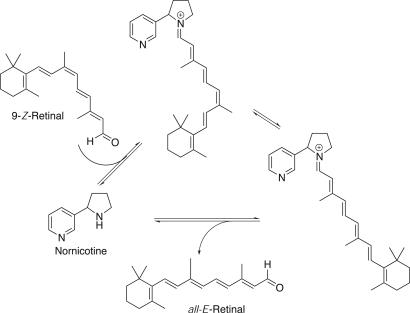
Nornicotine-Catalyzed Retinal Isomerization. The retinal isomer that is most similar to our model system is 13-_Z_-retinal, and as such, this compound was used to begin the examination of retinal isomerization (Fig. 2). It is well established that the highly conjugated system of the retinals are susceptible to oxidation; therefore, samples were degassed to minimize degradation (50). Nornicotine was found to catalyze 13-_Z_-retinal isomerization to _all-E_-retinal with a _k_obs of 5.2 × 10-4 min-1 at pH 7.4 and 37°C in the dark (Table 1). The uncatalyzed reaction was immeasurable during the same time frame (3 h), and increasing the time of the assay only lead to increased formation of degradation products.
The ability of nornicotine to catalyze the isomerization of α,β-unsaturated aldehydes and ketones is encouraging; however, 11-_Z_- and 9-_Z_-retinoids are the isomers that are known to play significant biological roles. 11-_Z_-retinal is an extremely unstable compound with the iminium-ion derivative isomerizing in visible light in a matter of femtoseconds (51); therefore, the more stable 9-_Z_-retinal was used to demonstrate that the potential of nornicotine catalyzed retinal isomerization in a biologically important system (Fig. 4). Although analytical separation of _all-E_-retinal and 9-_Z_-retinal has been reported (45), more demanding conditions for the reverse-phase HPLC separation of retinal isomers were required; accordingly, the column was warmed to 45°C. It was notable that 9-_Z_-retinal was more stable than 13-_Z_-retinal, and no additional oxidative degradation products were observed during the assay; however, no isomerization occurred in the dark at 37°C and pH 7.4. The introduction of light energy by exposure on a white-light transilluminator (3.4 mW/cm2) at 4°C and pH 7.4 lead to measurable isomerization. However, under these conditions the rate of isomerization of the samples containing nornicotine (300 μM) was minimally faster than the uncatalyzed reaction. Based on the model system and the understanding that the active nornicotine catalyst is the free amine (see above), the pH was increased to 8.0 to enhance the difference between the uncatalyzed and the nornicotine-catalyzed reaction. When examined under these assay conditions, the _k_obs for the nornicotine-catalyzed reaction was 8.0 × 10-1 min-1 at 37°C and pH 8.0 when samples were irradiated with white light. This result corresponds to a 1.4-fold rate increase over the corresponding uncatalyzed reaction (_k_obs = 5.8 × 10-1 min-1), demonstrating the potential for nornicotine catalysis with a biologically essential retinoid.
Fig. 4.
Nornicotine-catalyzed alkene isomerization of 9-_Z_-retinal.
Previous studies from our laboratories revealed that nornicotine can undergo enamine chemistry under physiological conditions. This study expands the reactivity of this tobacco alkaloid to include _Z_-to-E alkene isomerization of conjugated aldehydes and ketones, particularly retinals. Both 11-_Z_- and 9-_Z_-retinoids are metabolic intermediates in vision and gene regulation, respectively, and strict homeostatic control is essential for proper function. Although oxidative stress and nicotine receptor activation may also play a role in the development of tobacco-associated pathologies (36), the discovery that a nicotine metabolite catalyzes the isomerization of _Z_-retinals to _all-E_-retinal provides chemical evidence for an unrecognized mechanism of smoking toxicity. Specifically, the accumulation of _all-E_-retinal feeds the A2E biosynthetic pathway and leads to lipofuscin of RPE cells and ultimately age-related macular degeneration. Furthermore, recent studies have identified an additional _all-E_-retinal dimer that is a fluorescent component of RPE lipofuscin (52). Clearly, buildup of _all-E_-retinal has significant consequences in the eye, and in this context, nornicotine-catalyzed isomerization may provide a molecular mechanism underlying the clinical finding that smoking is the primary environmental determinant of age-related macular degeneration.
Additionally, the well defined role of retinoic acids as ligands for the RAR and RXR transcriptional activators suggests a second role for nornicotine-catalyzed retinal isomerization. Both _all-E_-retinoic acid and 9-_Z_-retinoic acid are the natural high-affinity ligands for RAR, whereas RXR is specifically activated by 9-_Z_-retinoic acid. Biosynthetically, the precursors to _all-E_-retinoic acid and 9-_Z_-retinoic acid are _all-E_-retinal and 9-_Z_-retinal, respectively. Specific RXR and RAR signaling is involved in numerous biological processes (see above), most notably in differentiation during embryonic development. In this context, accumulation of _all-E_-retinal from nornicotine-catalyzed isomerizations could perturb retinoic acid concentrations and result in improper gene regulation and birth defects.
It is interesting to note that the difference between the nornicotine-catalyzed rate of isomerization and the uncatalyzed rate is much less when the carbonyl moiety is distal from the _Z_-alkene, as is the case for 9-_Z_-retinal. Furthermore, the addition of light energy was required for any measurable isomerization to occur. When combined, these observations imply differing chemical mechanisms for the alkene isomerization of α,β-unsaturated carbonyl compounds and the isomerization of unsaturated systems with increasing conjugation between the _Z_-alkene and the carbonyl. Multicellular organisms use 11-_Z_- and 9-_Z_-retinoids to signal specific cellular processes rather than 13-_Z_-retinoids, as is the case for the light-harvesting ion pump bacteriorhodopsin in bacteria. Our results suggest that this selection against 13-_Z_-retinal may, in part, be a function of the thermal reactivity of this compound for isomerization after reaction with a suitable amine. The abundance of reactive amino moieties found on proteins would lead to an inherent rate of retinal isomerization that would require protective mechanisms for proper biological function. Although the significance of nornicotine-catalyzed isomerizations in vivo remains unproven, our study clearly illustrates the potential of physiologically relevant organocatalysis in a biological setting with profound implications for tobacco-associated diseases.
Acknowledgments
We thank Jason A. Moss, Young Soo Kim, and Jason Chang for assistance with preparative HPLC experiments. Financial support for this study was provided by The Skaggs Institute for Chemical Biology.
Abbreviations: A2E, _N_-retinylidene-_N_-retinylethanolamine; RPE, retinal pigmented epithelial; RAR, retinoic acid receptor; RXR, retinoid X receptor; sat., saturated; EtOAc, ethyl acetate.
References
- 1.Futoryan, T. & Gilchrest, B. A. (1994) Nutr. Rev. 52**,** 299-310. [DOI] [PubMed] [Google Scholar]
- 2.Gerster, H. (1997) Int. J. Vitam. Nutr. Res. 67**,** 71-90. [PubMed] [Google Scholar]
- 3.Hinds, T. S., West, W. L. & Knight, E. M. (1997) J. Clin. Pharmacol. 37**,** 551-558. [DOI] [PubMed] [Google Scholar]
- 4.Ross, A. C. & Gardner, E. M. (1994) Adv. Exp. Med. Biol. 352**,** 187-200. [DOI] [PubMed] [Google Scholar]
- 5.Stephens, D., Jackson, P. L. & Gutierrez, Y. (1996) Pediatr. Nurs. 22**,** 377-389. [PubMed] [Google Scholar]
- 6.Semba, R. D. (1998) Nutr. Rev. 56**,** S38-S48. [DOI] [PubMed] [Google Scholar]
- 7.Ross, D. A. (1998) Proc. Nutr. Soc. 57**,** 159-165. [DOI] [PubMed] [Google Scholar]
- 8.Harbige, L. S. (1996) Nutr. Health 10**,** 285-312. [DOI] [PubMed] [Google Scholar]
- 9.Ross, A. C. & Stephensen, C. B. (1996) FASEB J. 10**,** 979-985. [PubMed] [Google Scholar]
- 10.Duester, G. (2000) Eur. J. Biochem. 267**,** 4315-4324. [DOI] [PubMed] [Google Scholar]
- 11.Rando, R. R. (2001) Chem. Rev. (Washington, D.C.) 101**,** 1881-1896. [DOI] [PubMed] [Google Scholar]
- 12.Sakai, N., Decatur, J., Nakanishi, K. & Eldred, G. E. (1996) J. Am. Chem. Soc. 118**,** 1559-1560. [Google Scholar]
- 13.Sparrow, J. R., Parish, C. A., Hashimoto, M. & Nakanishi, K. (1999) Invest. Ophthalmol. Visual Sci. 40**,** 2988-2995. [PubMed] [Google Scholar]
- 14.De, S. & Sakmar, T. P. (2002) J. Gen. Physiol. 120**,** 147-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holz, F. G., Schutt, F., Kopitz, J., Eldred, G. E., Kruse, F. E., Volcker, H. E. & Cantz, M. (1999) Invest. Ophthalmol. Visual Sci. 40**,** 737-743. [PubMed] [Google Scholar]
- 16.Finnemann, S. C., Leung, L. W. & Rodriguez-Boulan, E. (2002) Proc. Natl. Acad. Sci. USA 99**,** 3842-3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suter, M., Reme, C., Grimm, C., Wenzel, A., Jaattela, M., Esser, P., Kociok, N., Leist, M. & Richter, C. (2000) J. Biol. Chem. 275**,** 39625-39630. [DOI] [PubMed] [Google Scholar]
- 18.Eldred, G. E. & Lasky, M. R. (1993) Nature 361**,** 724-726. [DOI] [PubMed] [Google Scholar]
- 19.Eldred, G. E. & Katz, M. L. (1988) Exp. Eye Res. 47**,** 71-86. [DOI] [PubMed] [Google Scholar]
- 20.Sparrow, J. R., Fishkin, N., Zhou, J., Cai, B., Jang, Y. P., Krane, S., Itagaki, Y. & Nakanishi, K. (2003) Vision Res. 43**,** 2983-2990. [DOI] [PubMed] [Google Scholar]
- 21.Sparrow, J. R. (2003) Proc. Natl. Acad. Sci. USA 100**,** 4353-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kastner, P., Grondona, J. M., Mark, M., Gansmuller, A., LeMeur, M., Decimo, D., Vonesch, J. L., Dolle, P. & Chambon, P. (1994) Cell 78**,** 987-1003. [DOI] [PubMed] [Google Scholar]
- 23.Lohnes, D., Mark, M., Mendelsohn, C., Dolle, P., Dierich, A., Gorry, P., Gansmuller, A. & Chambon, P. (1994) Development (Cambridge, U.K.) 120**,** 2723-2748. [DOI] [PubMed] [Google Scholar]
- 24.Mendelsohn, C., Lohnes, D., Decimo, D., Lufkin, T., LeMeur, M., Chambon, P. & Mark, M. (1994) Development (Cambridge, U.K.) 120**,** 2749-2771. [DOI] [PubMed] [Google Scholar]
- 25.Keegan, B. R., Feldman, J. L., Begemann, G., Ingham, P. W. & Yelon, D. (2005) Science 307**,** 247-249. [DOI] [PubMed] [Google Scholar]
- 26.Vermot, J., Llamas, J. G., Fraulob, V., Niederreither, K., Chambon, P. & Dolle, P. (2005) Science 308**,** 563-566. [DOI] [PubMed] [Google Scholar]
- 27.Kawakami, Y., Raya, A., Raya, R. M., Rodriguez-Esteban, C. & Belmonte, J. C. I. (2005) Nature 435**,** 165-171. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka, Y., Okada, Y. & Hirokawa, N. (2005) Nature 435**,** 172-177. [DOI] [PubMed] [Google Scholar]
- 29.Vermot, J. & Pourquie, O. (2005) Nature 435**,** 215-220. [DOI] [PubMed] [Google Scholar]
- 30.Klein, R. J., Zeiss, C., Chew, E. Y., Tsai, J. Y., Sackler, R. S., Haynes, C., Henning, A. K., SanGiovanni, J. P., Mane, S. M., Mayne, S. T., et al. (2005) Science 308**,** 385-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haines, J. L., Hauser, M. A., Schmidt, S., Scott, W. K., Olson, L. M., Gallins, P., Spencer, K. L., Kwan, S. Y., Noureddine, M., Gilbert, J. R., et al. (2005) Science 308**,** 419-421. [DOI] [PubMed] [Google Scholar]
- 32.Edwards, A. O., Ritter, R., III, Abel, K. J., Manning, A., Panhuysen, C. & Farrer, L. A. (2005) Science 308**,** 421-424. [DOI] [PubMed] [Google Scholar]
- 33.Christen, W. G., Glynn, R. J., Manson, J. E., Ajani, U. A. & Buring, J. E. (1996) J. Am. Med. Assoc. 276**,** 1147-1151. [PubMed] [Google Scholar]
- 34.Seddon, J. M., Willett, W. C., Speizer, F. E. & Hankinson, S. E. (1996) J. Am. Med. Assoc. 276**,** 1141-1146. [PubMed] [Google Scholar]
- 35.Tomany, S. C., Wang, J. J., Van Leeuwen, R., Klein, R., Mitchell, P., Vingerling, J. R., Klein, B. E., Smith, W. & De Jong, P. T. (2004) Ophthalmology 111**,** 1280-1287. [DOI] [PubMed] [Google Scholar]
- 36.Suner, I. J., Espinosa-Heidmann, D. G., Marin-Castano, M. E., Hernandez, E. P., Pereira-Simon, S. & Cousins, S. W. (2004) Invest. Ophthalmol. Visual Sci. 45**,** 311-317. [DOI] [PubMed] [Google Scholar]
- 37.Crooks, P. A., Li, M. & Dwoskin, L. P. (1997) Drug Metab. Dispos. 25**,** 47-54. [PubMed] [Google Scholar]
- 38.Kisaki, T. & Tamaki, E. (1961) Arch. Biochem. Biophys. 94**,** 252-256. [DOI] [PubMed] [Google Scholar]
- 39.Dickerson, T. J. & Janda, K. D. (2002) J. Am. Chem. Soc. 124**,** 3220-3221. [DOI] [PubMed] [Google Scholar]
- 40.Dickerson, T. J., Lovell, T., Meijler, M. M., Noodleman, L. & Janda, K. D. (2004) J. Org. Chem. 69**,** 6603-6609. [DOI] [PubMed] [Google Scholar]
- 41.Rogers, C. J., Dickerson, T. J., Brogan, A. P. & Janda, K. D. (2005) J. Org. Chem. 70**,** 3705-3708. [DOI] [PubMed] [Google Scholar]
- 42.Dickerson, T. J. & Janda, K. D. (2003) Proc. Natl. Acad. Sci. USA 100**,** 8182-8187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dickerson, T. J. & Janda, K. D. (2002) Proc. Natl. Acad. Sci. USA 99**,** 15084-15088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dalko, P. I. & Moisan, L. (2004) Angew. Chem. Int. Ed. 43**,** 5138-5175. [DOI] [PubMed] [Google Scholar]
- 45.Cia, D., Bonhomme, B., Azim, E. M., Wada, A., Doly, M. & Azais-Braesco, V. (1999) J. Chromatogr. A 864**,** 257-262. [DOI] [PubMed] [Google Scholar]
- 46.Yang, J. W., Hechavarria Fonseca, M. T. & List, B. (2004) Angew. Chem. Int. Ed. 43**,** 6660-6662. [DOI] [PubMed] [Google Scholar]
- 47.Ouellet, S. G., Tuttle, J. B. & MacMillan, D. W. C. (2005) J. Am. Chem. Soc. 127**,** 32-33. [DOI] [PubMed] [Google Scholar]
- 48.Yamamoto, I., Kamimura, H., Yamamoto, R., Sakai, S. & Goda, M. (1962) Agric. Biol. Chem. 26**,** 709-716. [Google Scholar]
- 49.Fujita, T., Nakajima, M., Soeda, Y. & Yamamoto, I. (1971) Pestic. Biochem. Physiol. 1**,** 151-162. [Google Scholar]
- 50.Nagao, A. (2004) J. Nutr. 134, 237S-240S. [DOI] [PubMed] [Google Scholar]
- 51.Kandori, H., Katsuta, Y., Ito, M. & Sasabe, H. (1995) J. Am. Chem. Soc. 117**,** 2669-2670. [Google Scholar]
- 52.Fishkin, N. E., Sparrow, J. R., Allikmets, R. & Nakanishi, K. (2005) Proc. Natl. Acad. Sci. USA 102**,** 7091-7096. [DOI] [PMC free article] [PubMed] [Google Scholar]