The G/G Genotype of a Resistin Single-Nucleotide Polymorphism at −420 Increases Type 2 Diabetes Mellitus Susceptibility by Inducing Promoter Activity through Specific Binding of Sp1/3 (original) (raw)
Abstract
Insulin resistance is a major cause of type 2 diabetes mellitus (T2DM). Resistin, an adipocyte-secreted hormone, antagonizes insulin. Transgenic mice that overexpress the resistin gene (Retn) in adipose tissue are insulin-resistant, whereas Retn (−/−) mice show lower fasting blood glucose, suggesting that the altered Retn promoter function could cause diabetes. To determine the role of RETN in human T2DM, we analyzed polymorphisms in its 5′ flanking region. We found that the −420G/G genotype was associated with T2DM (397 cases and 406 controls) (_P_=.008; adjusted odds ratio = 1.97 [by logistic regression analysis]) and could accelerate the onset of disease by 4.9 years (_P_=.006 [by multiple regression analysis]). Meta-analysis of 1,888 cases and 1,648 controls confirmed this association (_P_=.013). Linkage disequilibrium analysis revealed that the −420G/G genotype itself was a primary variant determining T2DM susceptibility. Functionally, Sp1 and Sp3 transcription factors bound specifically to the susceptible DNA element that included −420G. Overexpression of Sp1 or Sp3 enhanced RETN promoter activity with −420G in Drosophila Schneider line 2 cells that lacked endogenous Sp family members. Consistent with these findings, fasting serum resistin levels were higher in subjects with T2DM who carried the −420G/G genotype. Therefore, the specific recognition of −420G by Sp1/3 increases RETN promoter activity, leading to enhanced serum resistin levels, thereby inducing human T2DM.
Type 2 diabetes mellitus (T2DM [MIM #125853]), a common disease that affects ∼5% of adults, is characterized by insulin resistance (DeFronzo et al. 1992). Major genetic factors for T2DM remain to be determined, although its association with some polymorphisms has been reported (Altshuler et al. 2000; Horikawa et al. 2000; McCarthy and Froguel 2002). Resistin (resistance to insulin) (MIM 605565), an adipocyte-secreted hormone, antagonizes insulin (Steppan et al. 2001; Pravenec et al. 2003; Rajala et al. 2003; Banerjee et al. 2004; Steppan and Lazar 2004). Transgenic mice that overexpress the resistin gene (Retn) in adipose tissue are insulin-resistant (Pravenec et al. 2003), whereas Retn (−/−) mice show lower fasting blood glucose (Banerjee et al. 2004), suggesting that altered Retn promoter function could cause diabetes. Despite the established role of Retn in rodents (Steppan et al. 2001; Pravenec et al. 2003; Rajala et al. 2003; Banerjee et al. 2004), a link between RETN and human T2DM remains to be elucidated (Engert et al. 2002; Ma et al. 2002; Cho et al. 2004; Steppan and Lazar 2004).
We initially sequenced an ∼1-kb upstream region of RETN in 24 Japanese patients with T2DM (see online-only appendix C for a description of our methods), since we reported that SNPs in the exons and introns of RETN were not associated with T2DM (Osawa et al. 2002). We identified five common SNPs: −1093A→G, −638G→A, −537A→C, −420C→G, and −358G→A. When the regions encompassing these SNPs were sequenced in 176 additional cases and 200 controls, two additional SNPs, −1082C→T and −821G→A, were found. We sequenced the regions that included all these SNPs, in a total of 200 cases and 200 controls (see panel 1 in table 1). These SNPs were in Hardy-Weinberg equilibrium in controls. Of these SNPs, only the allele frequency of −420C→G tended to be increased in patients with T2DM compared with controls (χ2=3.84; _P_=.05) (table 2). When the frequency of the −420G/G genotype was compared with that of the C/C genotype, the G/G genotype was associated with T2DM (_P_=.018; odds ratio [OR] = 2.26; 95% CI 1.14–4.47) (see panel 1 in table 3). The frequencies of the C/G and C/C genotypes did not differ between cases and controls, suggesting that only homozygotes of −420G are associated with T2DM.
Table 1.
Characteristics of the Study Populations[Note]
| No. of Subjectswith Genotype | Mean Age ± SD(Years) | |||||||
|---|---|---|---|---|---|---|---|---|
| Panel andSubject Group | No. of Subjects(Male/Female) | C/C | C/G | G/G | At Testing | At Onset of Diabetesa | BMI(kg/m2) | HbA1C(%) |
| Panel 1: | ||||||||
| Patients with T2DM | 200 (96/104) | 86 | 84 | 30 | 60 ± 12 | 48 ± 12 | 23 ± 4 | 7.6 ± 1.7 |
| Controls | 200 (107/93) | 97 | 88 | 15 | 54 ± 8 | NA | 23 ± 3 | 4.8 ± .3 |
| Panel 2: | ||||||||
| Patients with T2DM | 197 (119/78) | 82 | 85 | 30 | 60 ± 11 | 50 ± 12 | 24 ± 4 | 8.6 ± 2.1 |
| Controls | 206 (108/98) | 87 | 98 | 21 | 63 ± 6 | NA | 23 ± 3 | 5.0 ± .3 |
| Panel 3: | ||||||||
| Patients with T2DM | 149 (101/48) | 48 | 85 | 16 | 56 ± 14 | 49 ± 13 | 25 ± 4 | 7.6 ± 1.7 |
| Controls | 158 (71/87) | 63 | 83 | 12 | 67 ± 9 | NA | 24 ± 3 | 4.9 ± .3 |
Table 2.
Allele Frequencies of SNPs in the 5′ Flanking Region of RETN for 200 Patients with T2DM and 200 Control Subjects in Panel 1[Note]
| No. of Alleles(Frequency [%]) in | ||||
|---|---|---|---|---|
| SNPa | Patients with T2DM | Controls | χ2 | P |
| −1093A→G | 41 (10.3) | 29 (7.3) | 2.25 | .13 |
| −1082C→T | 0 (0) | 1 (.3) | … | … |
| −821G→A | 1 (.3) | 0 (0) | … | … |
| −638G→A | 89 (22.3) | 77 (19.3) | 1.10 | .30 |
| −537A→C | 24 (6.0) | 14 (3.5) | 2.76 | .10 |
| −420C→G | 144 (36.0) | 118 (29.5) | 3.84 | .05 |
| −358G→A | 89 (22.3) | 77 (19.3) | 1.10 | .30 |
Table 3.
Association of the RETN −420G/G Genotype with T2DM[Note]
| Panel(s) (No. of Cases/Controls)and Genotype Comparisona | χ2 | P | OR | 95% CI |
|---|---|---|---|---|
| Panel 1 (200/200): | ||||
| G/G vs. C/C | 5.59 | .018 | 2.26 | 1.14–4.47 |
| C/G vs. C/C | .12 | .728 | 1.08 | .71–1.63 |
| G/G vs. C/C + C/G | 5.63 | .018 | 2.18 | 1.13–4.19 |
| Panels 1 and 2 (397/406): | ||||
| G/G vs. C/C | 6.59 | .010 | 1.83 | 1.15–2.90 |
| C/G vs. C/C | .00 | .974 | 1.00 | .74–1.34 |
| G/G vs. C/C + C/G | 7.44 | .006 | 1.83 | 1.18–2.84 |
| Panels 1, 2, and 3 (546/564): | ||||
| G/G vs. C/C | 8.38 | .004 | 1.81 | 1.21–2.71 |
| C/G vs. C/C | .36 | .548 | 1.08 | .84–1.39 |
| G/G vs. C/C + C/G | 8.18 | .004 | 1.74 | 1.19–2.55 |
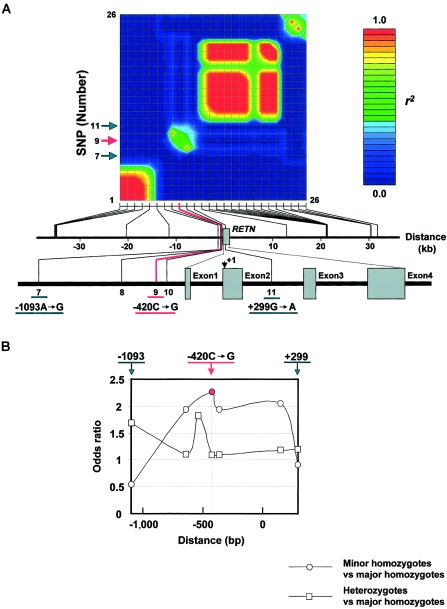
To assess the possibility that −420C→G in RETN is a primary variant that determines susceptibility to T2DM, we first examined the pattern of linkage disequilibrium (LD) around RETN by typing 26 frequent SNPs selected from the ∼70-kb region (fig. 1_A_). The LD between −420C→G and its nearby SNPs existed in a quite restricted area. In this area, the LD of −420C→G with its adjacent SNPs—namely, −638G→A or −358G→A—was strong, whereas that of −420C→G with distant SNPs—namely, −1093A→G or +299G→A—was weak. Thus, the LD of −420C→G did not extend beyond −1093A→G or +299G→A, suggesting that the association of −420C→G with T2DM is not caused by LD of an unidentified susceptibility variant around RETN with −420C→G.
Figure 1.
LD and OR results for RETN −420C→G, a primary variant associated with susceptibility to T2DM. A, The LD of −420C→G did not extend beyond −1093A→G or +299G→A. The pairwise LD of 26 frequent SNPs, as measured by r 2 , is shown in the upper panel (see online-only appendix F for a description of our methods). The physical positions of these SNPs (counted from the translation start site of RETN as +1) are shown in the lower panel. Arrows indicate a restricted LD area around −420C→G. Five SNPs are labeled in the figure: −1093A→G (7), −638G→A (8), −420C→G (9), −358G→A (10), and +299G→A (11). The other SNPs are summarized in table C2 (online only). B, The OR of minor-allele homozygotes to major-allele homozygotes was highest at −420C→G, and this OR was significantly larger than 1 among the seven frequent SNPs that have minor-allele frequencies >5% and that are located between −1093 and +299 in RETN. The ORs of minor-allele homozygotes to major-allele homozygotes and of heterozygotes to major-allele homozygotes were calculated by use of panel 1 subjects, for each of the following variants: −1093A→G, −638G→A, −537A→C, −420C→G, −358G→A, +157C→T, and +299G→A. The distance was counted from the translation initiation site.
We next calculated, using panel 1, the ORs of minor-allele homozygotes to major-allele homozygotes and those of heterozygotes to major-allele homozygotes for seven frequent SNPs that have minor-allele frequencies >5% and are located between −1093 and +299 (fig. 1_B_). The OR of minor-allele homozygotes to major-allele homozygotes was highest at −420C→G and was lower for SNPs distant from −420C→G. Of all the ORs, only the OR of −420G/G to −420C/C was significantly larger than 1 (_P_=.018). When haplotype frequencies defined by these seven SNPs were estimated for cases and controls, frequencies of any particular haplotypes including −420C→G were not significantly increased in patients with T2DM (table 4 [online only]). Collectively, all these findings suggest that the SNP −420C→G itself is the primary variant that determines susceptibility to T2DM.
Table 4.
No Association of Specific Haplotypes Including −420C→G with T2DM[Note]
| Nucleotide at | Frequency in | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| MajorHaplotype | −1093 | −638 | −537 | −420 | −358 | +157 | +299 | Patientswith T2DM(n = 200) | Controls(n = 200) |
| 1 | A | A | A | G | A | C | A | .164 | .151 |
| 2 | A | G | A | C | G | C | A | .105 | .148 |
| 3 | A | G | A | C | G | C | G | .503 | .537 |
| 4 | A | G | C | G | G | C | G | .048 | .030 |
| 5 | G | G | A | G | G | T | A | .049 | .042 |
| Total frequency | .869 | .908 |
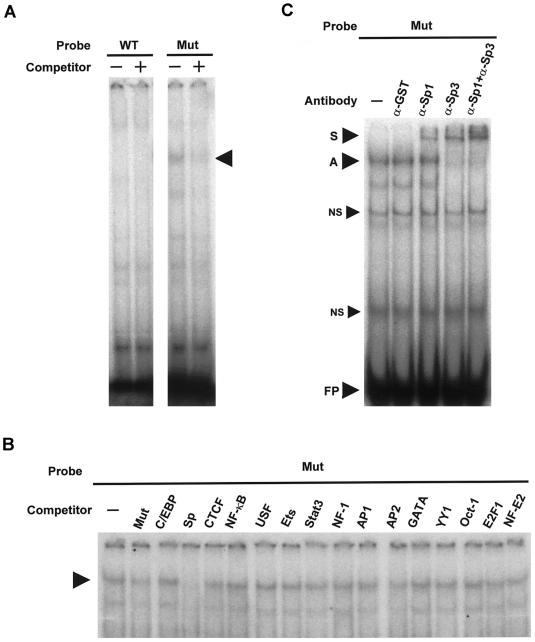
To identify specific transcription factors that bind to the DNA element, we examined whether one base substitution (C→G at −420) affects the specific binding of proteins to the DNA element (fig. 2_A_). An electrophoretic mobility shift assay (EMSA), by use of nuclear extracts from 3T3-L1 adipocytes, indicated that proteins bound specifically to the susceptible DNA element that included G at −420 (mutant probe) (third lane from the left in fig. 2_A_) but not to the wild-type element that included C (wild-type probe) (first lane in fig. 2_A_). A mutant competitor inhibited protein binding to the mutant probe (fourth lane in fig. 2_A_).
Figure 2.
Identification of Sp1 and Sp3 as major transcription factors binding only to the diabetes-susceptibility DNA element with −420G in RETN. A, Specific protein binding to the diabetes-susceptibility DNA element was analyzed. For the EMSA, nuclear extracts prepared from differentiated 3T3-L1 adipocytes were incubated with 32P-labeled double-stranded oligonucleotide probes that corresponded to the region (−434/−406) of RETN, as described in appendix D (online only). The wild-type (WT) probe includes C at −420, and the mutant (Mut) probe includes G at −420. Unlabeled double-stranded oligonucleotides for WT and Mut sequences (a 200-fold molar excess) were used as competitors for WT and Mut probes, respectively. An arrow indicates complexes with specific nuclear factors. B, Only an Sp transcription factor consensus binding site competes with the diabetes-susceptibility DNA element for specific protein binding. For a competition analysis, a 200-fold molar excess of unlabeled double-stranded oligonucleotides for consensus binding sites for a variety of transcription factors was added. Cold oligonucleotides for the mutant (Mut) DNA element (positive control [_second lane from the left_]) and the Sp consensus binding site (fourth lane) competed with the protein binding. An arrow indicates complexes with specific nuclear factors. C, Sp1 and Sp3 are major transcription factors binding specifically to the diabetes-susceptibility DNA element. Antibodies against Sp1, Sp3, and GST (negative control) were added to the reaction. S = supershifted complex with specific nuclear factors and antibodies; A = complex with specific nuclear factors; NS = complex with nonspecific binding nuclear proteins; FP = free probes. These data represent at least three independent experiments.
To determine which consensus DNA element shares binding proteins with this susceptible DNA element, we added oligonucleotides of each binding site for various transcription factors as competitors (fig. 2_B_). Only oligonucleotides for Sp binding sites reduced specific protein binding (fourth lane from the left in fig. 2_B_), suggesting that the susceptible DNA element binds specifically to Sp transcription factors. A positive-control competitor also reduced the specific protein binding (second lane in fig. 2_B_ [Mut]).
To identify which Sp factors bind to the susceptible DNA element, we examined the effects of anti-Sp1 and anti-Sp3 antibodies on protein-DNA binding (fig. 2_C_). Whereas the anti-Sp1 antibody weakly affected protein binding (third lane from the left in fig. 2_C_), the anti-Sp3 antibody strongly supershifted the complex (fourth lane in fig. 2_C_). When the anti-Sp1 and anti-Sp3 antibodies were added together, the complex was completely supershifted (fifth lane in fig. 2_C_). A negative-control anti-GST antibody had no effect (second lane in fig. 2_C_). Therefore, we provisionally identified Sp1 and Sp3 as major transcription factors binding specifically to the susceptible DNA element.
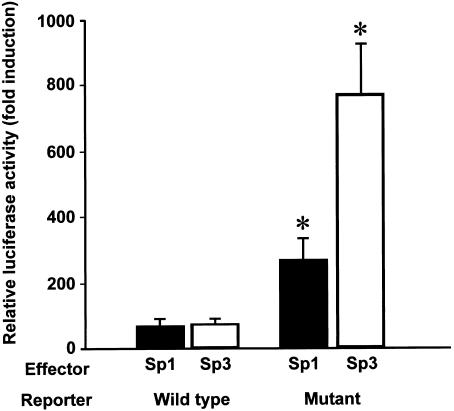
We next tested whether the C→G substitution at −420 affects RETN promoter activity through the specific binding of Sp1 and Sp3 (fig. 3). To assess isolated effects of Sp1 and Sp3, we employed Drosophila Schneider line 2 (SL2) cells, which lack endogenous Sp family transcription factors. When Sp1 or Sp3 was overexpressed in SL2 cells, the RETN promoter activity with G at −420 (mutant) was significantly enhanced, compared with the activity with C at −420 (wild type). Thus, Sp1 and Sp3 are major transcription factors enhancing RETN promoter activity by binding specifically to the DNA element with −420G.
Figure 3.
Activation of the RETN promoter with the diabetes-susceptibility SNP −420G binding specifically to Sp1/3. Either the wild-type (with −420C) or mutant (with −420G) RETN promoter reporter was transiently transfected into SL2 cells with effectors—namely, pPac (control), pPac-Sp1, or pPac-USp3, and the internal control pPac-βGal. Luciferase activity was measured as described in appendix E (online only). Relative luciferase activities are shown as the mean fold induction ± SE, relative to the activity of each reporter with a control effector. The data represent four independent experiments with duplicate wells for each condition. The asterisk (*) indicates P<.05, compared with the wild-type promoter activity with the same effector (ANOVA).
To evaluate the association between the −420G/G genotype and T2DM in a larger sample size, we further sequenced only the regions containing −420C→G for the 197 cases and 206 controls in panel 2 (table 1), which was collected from the same geographic area as panel 1. When the G/G genotype was compared with the C/C genotype in the combined data of panels 1 and 2, the G/G genotype was associated with T2DM (_P_=.010; _OR_=1.83; 95% CI 1.15–2.90) (table 3). The adjusted ORs of the G/G and C/G genotypes were estimated by use of multiple logistic regression analysis, adjusted for age, sex, and maximum BMI (see online-only appendix F for a description of our methods). The adjusted OR of G/G was significantly high (two-sided _P_=.008; _OR_=1.97; 95% CI 1.19–3.26). Since the adjusted OR of C/G was 1.08 (_P_=.65; 95% CI 0.78–1.49), we conclude that the −420G/G genotype increases the risk of T2DM. Multiple regression analysis, adjusted for sex and maximum BMI, of 397 subjects with T2DM revealed that the age at onset of disease for patients with the G/G genotype was 4.9 years younger than for patients with the C/C genotype (_P_=.006; 95% CI 1.39–8.41). When panel 3 (table 1), which was collected from another area in Japan, was added, the association of the G/G genotype with T2DM was consistent (_P_=.004; _OR_=1.81; 95% CI 1.21–2.71) (see panels 1, 2, and 3 in table 3). Therefore, the −420G/G genotype is associated with T2DM in a large number of Japanese subjects.
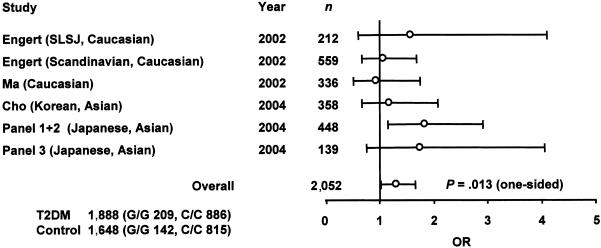
Although the association of −420C→G with T2DM has been examined in several ethnic populations (Engert et al. 2002; Ma et al. 2002; Cho et al. 2004), individual studies did not have sufficient power, probably because of limited sample size or a lower frequency of −420C→G than that found in Japanese subjects. To obtain evidence that was more conclusive (Lohmueller et al. 2003), we conducted a meta-analysis of results from previous studies (Engert et al. 2002; Ma et al. 2002; Cho et al. 2004) and the present study, in which the OR was used as the metric of association (fig. 4). The random-effects OR estimate for the risk of developing T2DM was significantly higher in subjects with −420G/G compared with subjects with −420C/C (one-sided _P_=.013; _OR_=1.31; 95% CI 1.03–1.66), and no evidence of between-study heterogeneity was found in the meta-analysis (_P_=.470). Therefore, the G/G genotype is associated with susceptibility to T2DM, which supports a common-disease/common-variant hypothesis (Lander 1996). Since the OR of the G/G genotype appears to be higher in Japanese subjects, racial differences may exist.
Figure 4.
Meta-analysis confirming association of the −420G/G genotype with T2DM. The data from Engert et al. (2002), Ma et al. (2002), and Cho et al. (2004) were combined with data from the present study. Samples from different geographic locations were analyzed separately (panels 1 and 2 and panel 3). The total number of subjects typed was 1,888 patients with T2DM and 1,648 controls. ORs of G/G genotypes to C/C genotypes were estimated, as described in appendix F (online only). Circles indicate ORs; bars represent 95% CIs; n = number of subjects with G/G and C/C genotypes. SLSJ = Saguenay–Lac-Saint-Jean region of Quebec.
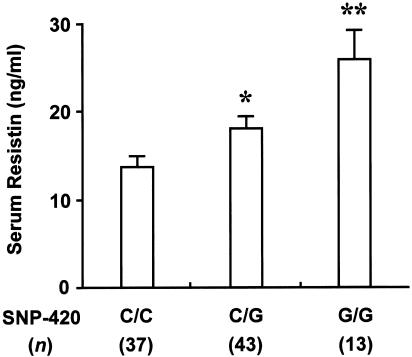
Finally, to examine whether the enhanced RETN promoter activity with −420G is associated with enhanced RETN expression in humans, we measured fasting serum resistin levels by use of 93 samples available from subjects with T2DM in panels 1 and 2 (fig. 5). Consistent with our genetic and molecular data, subjects with the G/G genotype had higher serum resistin levels than subjects with the C/C genotype (_P_=.0018). Subjects with the C/G genotype also had higher resistin levels than those with the C/C genotype, although this difference was smaller. All these findings suggest that the G/G genotype of a functional RETN promoter SNP at −420 determines human T2DM susceptibility—probably through enhanced RETN expression.
Figure 5.
Increased fasting serum resistin levels in subjects with T2DM and a −420G/G genotype. Serum resistin levels were measured by use of a human resistin ELISA kit (LINCO Research), as described in appendix B (online only). We analyzed 93 fasting serum samples available from subjects with T2DM in panels 1 and 2. Data represent mean ± SE. Student’s t test was used for statistical analysis. The asterisk (*) indicates _P_=.012; two asterisks (**) indicate _P_=.0018, compared with subjects with the C/C genotype.
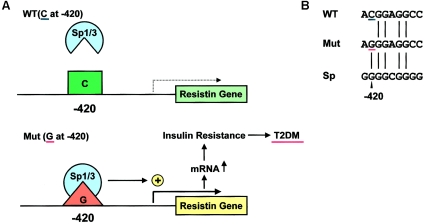
Our in vitro data are supported by previous studies (Smith et al. 2003; Cho et al. 2004). A DNA element that includes −420G binds to unidentified factors in nuclear extracts of 3T3-L1 adipocytes (Cho et al. 2004). The RETN promoter activity with −420G (described as −180) is enhanced to 400% of that with −420C in 3T3-L1 adipocytes, without manipulation of expression of Sp transcription factors (Smith et al. 2003). In humans, obese subjects with the G/G genotype have higher resistin mRNA levels in their abdominal subcutaneous fat (Smith et al. 2003). Cho et al. (2004) reported that subjects with the G/G genotype have higher serum resistin levels, and our results support this finding. Taken together, these results lead us to propose that one base substitution from C to G at −420 activates RETN transcription by specific Sp1/3 binding, which could induce insulin resistance associated with T2DM (fig. 6 [online only]). By recognizing specific sequences in regulatory regions, ubiquitous factors could affect disease susceptibility. In the RETN promoter with −420G, Sp3 acted as a stronger activator than Sp1, whereas Sp3 has been shown to act as a transcriptional activator or an Sp1-mediated transcription repressor (Bouwman and Philipsen 2002).
Figure 6.
Proposed model for the induction of T2DM susceptibility by RETN −420G. A, Schematic representation of the possible role of the diabetes-susceptibility RETN SNP −420G and the Sp1 and Sp3 transcription factors in human T2DM. In subjects with C at −420 (the wild-type [WT] allele), Sp1/3 cannot bind to this DNA region, and the RETN promoter is not activated. In subjects with G at −420 (mutant diabetes-susceptibility [Mut] allele), Sp1/3 can bind specifically to this DNA element, which activates the promoter. This enhanced RETN expression in adipocytes—and possibly in macrophages—could increase T2DM susceptibility by inducing whole-body insulin resistance. B, Comparison of sequences between wild-type (WT) and mutant (Mut) resistin DNA elements and Sp-factor consensus sequence (Sp). Only one base substitution (C→G) induces a specific capacity for binding Sp factors.
Controversy exists about whether an increase in serum resistin levels is associated with human T2DM and obesity (Lee et al. 2003; McTernan et al. 2003; Cho et al. 2004; Fujinami et al. 2004; Steppan and Lazar 2004). Serum resistin probably exists as a hexamer (major form) or a trimer (a more biologically active form) (Patel et al. 2004), which may affect the assay results. Serum resistin levels were higher in subjects with T2DM who had the −420G/G genotype than in those who had other genotypes, a finding that is supported by Cho et al. (2004), suggesting that the discrepancy may be resolved by considering the different genotypes at −420. It should be noted that the main source of human serum resistin remains unknown, because it is most highly expressed in macrophages (Nagaev and Smith 2001; Savage et al. 2001; Wellen and Hotamisligil 2003; Banerjee et al. 2004).
In summary, the −420G/G genotype in RETN is associated with susceptibility to T2DM. Sp1 and Sp3 bind specifically to the DNA element with −420G and enhance the promoter activity. This provides evidence for a link between an RETN promoter SNP and human T2DM, encouraging further detailed studies in rodents (Steppan et al. 2001; Pravenec et al. 2003; Rajala et al. 2003; Banerjee et al. 2004). Functional SNPs in regulatory regions could represent promising candidates for susceptibility genes in other common diseases as well; in fact, a similar mechanism has been recently reported in an organic cation transporter gene, SLC22A4, and in the gene encoding Runt-related transcription factor 1 (RUNX1) for rheumatoid arthritis (Tokuhiro et al. 2003).
Descriptions of the methods used in this study are available in appendices A–F (online only).
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research on Priority Areas (C) “Medical Genome Science” (12204007) from the Ministry of Education, Culture, Science, Sports, and Technology of Japan. We thank Dr. T. Noguchi, for providing SL2 cells; Dr. G. Suske, for providing the pPac, pPac-Sp1, and pPac-USp3; and Dr. T. F. Osborne, for providing the pPac-βGal. We also thank F. Tanabe and M. Murase, for technical assistance and suggestions, and Drs. K. Kato, K. Ono, O. Ebisui, Y. Kusunoki, and T. Egashira, for collecting clinical data and samples.
Appendix A: Subjects
All subjects analyzed in the present study were unrelated and native Japanese. The study involved an initial screening of 24 outpatients with T2DM from the Ehime University Hospital and the Ehime Prefectural Hospital. These 24 subjects were selected on the basis of typical characteristics of T2DM—namely, an age at onset of between 40 and 60 years, treatment with diet alone or oral hypoglycemic agents, and the presence of first-degree relatives with T2DM. Diabetes mellitus was diagnosed on the basis of the American Diabetes Association criteria, as reported in 1998 (Expert Committee on the Diagnosis and Classification of Diabetes Mellitus 2003). We initially sequenced ∼1 kb of the 5′ flanking region of RETN in these 24 patients with T2DM. This screening of 24 subjects permitted the detection of an allele that has a frequency of 3.3% with a power of 80% and 4.7% with a power of 90%. For the association study, the regions containing the identified polymorphisms were sequenced in 176 additional subjects with T2DM and in 200 nondiabetic control subjects. These control subjects were selected on the basis of having no history of diabetes, no evidence of diabetes among first-degree relatives, and a normal glucose tolerance, as evidenced by a 75-g oral glucose tolerance test. The age of the control subjects was significantly younger than the age at onset for subjects with T2DM. We increased the sample size when any significant association was found at the significant level of 5% (P<.05) in the analysis of panel 1 (a total of 200 cases and 200 controls). For the extension study, samples from 206 additional nondiabetic controls and 197 subjects with T2DM were collected as panel 2. The control subjects were chosen on the basis of being >55 years old and having HbA1C levels <5.6, fasting plasma-glucose levels <110 mg/dl, no history of diabetes, and no evidence of diabetes among first-degree relatives. From a different area of Japan (Chiba), an additional 149 cases and 158 controls were selected (panel 3) on the basis of the same criteria (except controls were >60 years old). The clinical characteristics of these subjects are summarized in table 1. All patients and controls were told the purpose of the study, and informed consent was obtained. The study was approved by the ethics committees of the Ehime University Hospital and Ehime Prefectural Hospital.
Appendix B: Measurement of Serum Resistin Levels
Serum resistin levels were measured by use of a human resistin ELISA kit (LINCO Research), in accordance with the manufacturer’s protocol. According to the manufacturer's data, the limit of detection is 0.16 ng/ml and the intra-assay coefficient of variation (CV) is ⩽4% for low levels and ⩽7% for high levels. The interassay CV is ⩽8% for low and high levels. The recovery rate is >90%. We confirmed that linearity was maintained below 0.16 ng/ml, and intra-assay CVs and recovery rates were comparable to the manufacturer's data: 1.7%–8.1% and 88%–100%, respectively. The antibodies in this ELISA have no detectable crossreactivity to mouse resistin or other cytokines in human serum.
Appendix C: PCR Direct Sequencing
The reference sequence was determined on the basis of the human sequence for RETN on chromosome 19 (GenBank accession number NT_077812) and its cDNA sequence (GenBank accession number NM_020415). PCR direct sequencing was performed by use of the primers listed in table C1 (online only), as described elsewhere (Osawa et al. 2002, 2003), with the following modifications: 50 ng of genomic DNA were amplified in a 25-μl reaction mixture, which included 0.625 U Taq DNA polymerase (Takara Shuzo), 5 pmol of each primer, and 5 nmol of each dNTP. For amplification of region 1, 5% DMSO was also included. After an initial denaturing for 3 min at 94°C, PCR was performed for 35 cycles, with denaturing at 94°C for 30 s, annealing at 55°C for 30 s, and extension at 72°C for 1 min, with a final extension period of 3 min.
These PCR products were sequenced by use of the forward and/or reverse primers listed in table C1 (online only), as described elsewhere (Osawa et al. 2002, 2003). Both strands of the entire ∼1 kb of the RETN 5′ flanking region were sequenced for the initial screening of the 24 subjects with T2DM to detect any unknown polymorphisms. Sequences of plus strands of regions, where the identified polymorphisms were located, were then checked for the association study by use of pr1F and pr2F primers, since these strands allow the polymorphisms to be identified more precisely. The other strand was also sequenced, when required. SNPs between −379 and +1177 (except +691 to +854) had been systematically searched and analyzed elsewhere (Osawa et al. 2002). The other 21 SNPs used to assess the pattern of LD around RETN and their PCR conditions are summarized in tables C2 and C3 (online only), respectively.
Table C1.
Primers Used for PCR and Sequencing of the 5′ Flanking Region of RETN
| Amplified Region and Primer (5′→3′) | Primer Namea | Primer Use | 5′ PrimerPositionb |
|---|---|---|---|
| 1: | |||
| GAGAAGTGGTCTTGCTCTG | pr1F | PCR and sequencing | −705 |
| GGCTTGGCTAATAAGTCCC | pr1R | PCR and sequencing | −294 |
| 2: | |||
| GTATAACCATTCCAAGCTCATGGGTGGTCC | pr2F | PCR | −1232 |
| TACCCTCTCGGTGGGCTCAGCTAACCAAAT | pr2R | PCR | −203 |
| GTATAACCATTCCAAGCTCATGGGTGGTCC | pr2F | Sequencing | −1232 |
| CCACCACGCCCAGTTAATT | pr3F | Sequencing | −951 |
| TGGCTCACGCCTGTAATCC | pr3R | Sequencing | −814 |
| CTACTAAGGAGGCTGACGT | pr4R | Sequencing | −593 |
Table C2.
SNPs Analyzed for LD around RETN in 52 Subjects with T2DM[Note]
| SNP or_RETN_ Exon | Position | NucleotideChange | dbSNP ID | Minor-AlleleFrequency(%) |
|---|---|---|---|---|
| 1 | 303201 | C→T | rs3745364 | 18.3 |
| 2 | 303288 | A→G | rs4804758 | 18.3 |
| 3 | 303343 | C→T | rs4804217 | 18.3 |
| 4 | 303418 | G→A | rs4804218 | 18.3 |
| 5 | 317503 | T→C | rs7471841 | 22.1 |
| 6 | 326839 | G→T | … | 47.1 |
| 7a | 337116 | A→G | … | 10.6 |
| 8b | 337571 | G→A | … | 17.3 |
| 9c | 337789 | C→G | … | 31.7 |
| 10d | 337851 | G→A | … | 17.3 |
| 11e | 338507 | G→A | … | 36.5 |
| 12 | 351289 | T→G | rs6952 | 26.9 |
| 13 | 359465 | T→C | rs2277995 | 26.9 |
| 14 | 359468 | G→T | … | 26.9 |
| 15 | 359474 | T→C | … | 26.9 |
| 16 | 359475 | G→A | … | 26.9 |
| 17 | 359478 | A→G | … | 26.9 |
| 18 | 359481 | G→A | … | 26.9 |
| 19 | 359482 | C→T | … | 18.3 |
| 20 | 359514 | A→G | rs2277994 | 26.9 |
| 21 | 359567 | G→A | rs2277993 | 26.9 |
| 22 | 359578 | A→G | rs2277992 | 26.9 |
| 23 | 368545 | 4Cs→5Cs | rs3214456 | 86.5 |
| 24 | 368737 | G→A | rs2287867 | 73.1 |
| 25 | 368768 | T→C | rs2287863 | 73.1 |
| 26 | 370270 | T→C | … | 87.5 |
| Exon 1 | 337968–338003 | … | … | … |
| Exon 2 | 338199–338326f | … | … | … |
| Exon 3 | 338703–338780 | … | … | … |
| Exon 4 | 339101–339336 | … | … | … |
Table C3.
Primers Used for PCR and the Sequencing of SNPs around RETN[Note]
| SNP(s) and Primer (5′→3′) | Primer Namea | Primer Use | 5′ PrimerPosition | AnnealingTemperature |
|---|---|---|---|---|
| 1–4: | ||||
| GGAACAGGAGGCGAGACTC | −30KA-F | PCR and sequencing | −35158 | 60°C or TDb |
| GCAGGAGGTATCACATGAAC | −30KA-R | PCR and sequencing | −34709 | 60°C or TDb |
| 5: | ||||
| AAGGCTGGTGGCGTGCAAG | −20KA-F | PCR and sequencing | −20851 | 55°Cc or TDb |
| AGTCTGTGGTACTTTGTTA | −20KA-R | PCR and sequencing | −20528 | 55°Cc or TDb |
| 6: | ||||
| CCCCACCTCTACTAAAAAT | −10KA-F | PCR and sequencing | −11414 | TDb |
| GCAAGAGGTAGCAAGAACC | −10KA-R | PCR and sequencing | −11050 | TDb |
| 12: | ||||
| GCCTTCGCACTGCTGTTCT | +10KC-FN | PCR and sequencing | +13009 | 55°Cc |
| GCCCTTGACGAAGAGCAAC | +10KC-RN | PCR and sequencing | +13200 | 55°Cc |
| 13–22: | ||||
| GTGGACCCACTGCTTGGTG | +20KB-F | PCR and sequencing | +21143 | 60°C |
| GGTGTTTGTGGAGATGCTG | +20KB-R | PCR and sequencing | +21524 | 60°C |
| 23–25: | ||||
| CACCAGTCCCTTTCTTAGA | +30KA-F | PCR | +30281 | TDb |
| ATCAGCAAATGGCCCCTGA | +30KA-R | PCR | +30621 | TDb |
| GTCCCTTTCTTAGAAATTC | +30KA-FN | Sequencing | +30286 | TDb |
| CAAATGGCCCCTGAGTGCC | +30KA-RN | Sequencing | +30616 | TDb |
| 26: | ||||
| GGGGTGTGGCGCGGCCAGGCTCTGATTAGC | +37KA-LF | PCR | +31921 | 55°C |
| GGACAAATGACTTACCCTCTTGGAACCTCA | +37KA-LR | PCR | +32228 | 55°C |
| AGGCTCTGATTAGCGGCAG | +37KA-F | Sequencing | +31937 | 55°C |
| TCTTGGAACCTCAGTTCCA | +37KA-R | Sequencing | +32211 | 55°C |
Appendix D: EMSA
Preparation of nuclear extracts and the EMSA were performed as described elsewhere (Yamada et al. 1997, 2000). Oligonucleotides corresponding to the −434/−406 region of RETN with the 5′ _Kpn_I and 3′ _Bgl_II sites were synthesized for probes and competitors. Wild-type oligonucleotides contain C at −420, and mutant oligonucleotides contain G at −420. The nucleotide sequences of the other oligonucleotides used as competitors have been described elsewhere (Shou et al. 2003). For a competition analysis, a 200-fold molar excess of competitor DNAs was added to the binding mixture. For supershift assays, antibodies were mixed with the nuclear extracts on ice for 30 min, and a 32P-labeled probe was then added to the mixture, followed by another 30 min of incubation. After completion of the binding, the mixture was subjected to electrophoresis on a 6% polyacrylamide gel (ratio of acrylamide to bis-acrylamide, 19:1) in 44.5 mM Tris-HCl (pH 8.0), 44.5 mM boric acid, and 1 mM EDTA at 200 volts for 1 h. The gels were dried and exposed to a Fujix imaging plate. Signals were detected with the Fujix BAS 2000 image analyzing system.
Appendix E: DNA Transfections
The 5′ flanking region between −450 and −206 of RETN was amplified by PCR by use of DNA from either a subject homozygous for a wild-type allele (−420C) or a subject homozygous for a mutant allele (−420G). Each PCR product was ligated into the _Kpn_I/_Bgl_II sites of pGL3-Basic Vector (Promega). The generated wild-type and mutant constructs are referred to as “pGL-b-resistin 2A wt” and “pGL-b-resistin 2A −420G,” or, more simply, wild-type (WT) and mutant (Mut) reporters, respectively. The sequence of each strand was confirmed by DNA sequencing. The pPac, pPac-Sp1, and pPac-USp3 were provided by Dr. Guntram Suske (Philipps-Universität, Marburg, Germany), and pPac-βGal was provided by Dr. Timothy F. Osborne (University of California, Irvine) (Courey et al. 1988; Hagen et al. 1994; Dooley et al. 1998). All plasmids used for the transfection were prepared by use of a Qiagen plasmid kit, followed by CsCl2 density-gradient ultracentrifugation.
SL2 cells, a Drosophila cell line, were a gift from Dr. Tamio Noguchi (Nagoya University, Nagoya, Japan). The cells were grown in Schneider’s medium supplemented with 10% fetal bovine serum at 25°C. Cells were plated at a density of 1 × 106 cells/60-mm dish. After 24 h, 2 μg of luciferase reporter plasmid, 100 ng of pPac-βGal, and 100 ng of pPac-derived expression plasmids were transfected into the SL2 cells by use of a calcium phosphate method (Di Nocera and Dawid 1983). The cells were harvested 48 h after transfection and were subjected to luciferase and β-galactosidase assays (Kikkawa et al. 2001).
Appendix F: Statistical Analysis
The χ2 test was used to assess an association between RETN polymorphisms and T2DM, unless otherwise indicated. To avoid a false-positive result due to deviation from Hardy-Weinberg equilibrium (HWE) in the controls, an HWE test was performed for each SNP site. The frequencies of haplotypes, consisting of two or seven SNPs, were estimated on the basis of the expectation-maximization algorithm (Excoffier and Slatkin 1995) by use of the LDfinder program (Omi et al. 2003) and the SNPAlyze software, version 3.2 Pro (Dynacom), respectively. Pairwise LD between two polymorphic sites was measured by use of the r 2 statistic on the basis of the estimated two-SNP haplotype frequency. The results of pairwise r 2 were visualized by the GOLD program (Abecasis and Cookson 2000). The frequencies of seven-SNP haplotypes were compared between patients with T2DM and controls by permutation tests (Fallin et al. 2001) by use of the SNPAlyze software, version 3.2 Pro (Dynacom).
To estimate ORs of −420G/G and −420C/G genotypes, ORs were adjusted simultaneously for potentially confounding variables by multiple logistic regression analysis for panels 1 and 2. The variables considered in this model were age, sex, maximum BMI, and −420 genotype (three types: −420G/G, −420C/G, and −420C/C). To examine the effect of −420G/G genotype on the age at onset, multiple regression analyses, adjusted for sex and maximum BMI, were performed for 397 patients with T2DM.
A meta-analysis was performed to examine the relationship between the −420G/G genotype and susceptibility to T2DM. The examined genotype contrast was −420G/G versus −420C/C. The OR was used as the metric of association and was calculated by use of data from three previous studies (Engert et al. 2002; Ma et al. 2002; Cho et al. 2004) and the present study (panels 1, 2, and 3). When data from an individual study were reported separately for populations of similar racial descent but from different geographic locations, the division of data was sustained. In the present study, panels 1 and 2 and panel 3 were analyzed separately. Between-study heterogeneity was examined by use of the χ2-based Q statistic (Lau et al. 1997). Heterogeneity was considered significant at a P value <.1. Study-specific data were combined by use of a general variance-based random-effects method. This model incorporates an estimate of between-study variance. P values are two-tailed.
Electronic-Database Information
Accession numbers and URLs for data presented herein are as follows:
- dbSNP Home Page, http://www.ncbi.nlm.nih.gov/SNP/ (for −1093A→G [accession number rs3760678], −420C→G [accession number rs1862513], −358G→A [accession number rs3219175], +157C→T [accession number rs3219177], and +299A→G [accession number rs3745367])
- GenBank, http://www.ncbi.nlm.nih.gov/Genbank/ (for genomic structure of the RETN gene [accession number NT_077812] and RETN cDNA [accession number NM_020415])
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for T2DM and resistin)
References
- Abecasis G, Cookson W (2000) GOLD: graphical overview of linkage disequilibrium. Bioinformatics 16:182–183 10.1093/bioinformatics/16.2.182 [DOI] [PubMed] [Google Scholar]
- Altshuler D, Hirschhorn JN, Klannemark M, Lindgren CM, Vohl MC, Nemesh J, Lane CR, Schaffner SF, Bolk S, Brewer C, Tuomi T, Gaudet D, Hudson TJ, Daly M, Groop L, Lander ES (2000) The common PPARγ Pro12Ala polymorphism is associated with decreased risk of type 2 diabetes. Nat Genet 26:76–80 10.1038/79216 [DOI] [PubMed] [Google Scholar]
- Banerjee R, Rangwala S, Shapiro J, Rich A, Rhoades B, Qi Y, Wang J, Rajala M, Pocai A, Scherer P, Steppan C, Ahima R, Obici S, Rossetti L, Lazar M (2004) Regulation of fasted blood glucose by resistin. Science 303:1195–1198 10.1126/science.1092341 [DOI] [PubMed] [Google Scholar]
- Bouwman P, Philipsen S (2002) Regulation of the activity of Sp1-related transcription factors. Mol Cell Endocrinol 195:27–38 10.1016/S0303-7207(02)00221-6 [DOI] [PubMed] [Google Scholar]
- Cho Y, Youn B, Chung S, Kim K, Lee H, Yu K, Park H, Shin H, Park K (2004) Common genetic polymorphisms in the promoter of resistin gene are major determinants of plasma resistin concentrations in humans. Diabetologia 47:559–565 [DOI] [PubMed] [Google Scholar]
- Courey AJ, Holtzman DA, Jackson SP, Tjian R (1988) Synergistic activation by the glutamine-rich domains of human transcription factor Sp1. Cell 59:827–836 10.1016/0092-8674(89)90606-5 [DOI] [PubMed] [Google Scholar]
- DeFronzo RA, Bonadonna RC, Ferrannini E (1992) Pathogenesis of NIDDM: a balanced overview. Diabetes Care 15:318–368 [DOI] [PubMed] [Google Scholar]
- Di Nocera P, Dawid I (1983) Transient expression of genes introduced into cultured cells of Drosophila. Proc Natl Acad Sci USA 80:7095–7098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley KA, Millinder S, Osborne TF (1998) Sterol regulation of 3-hydroxy-3-methylglutaryl-coenzyme A synthase gene through a direct interaction between sterol regulatory element binding protein and the trimeric CCAAT-binding factor/nuclear factor Y. J Biol Chem 273:1349–1356 10.1074/jbc.273.3.1349 [DOI] [PubMed] [Google Scholar]
- Engert JC, Vohl MC, Williams SM, Lepage P, Loredo-Osti JC, Faith J, Dore C, Renaud Y, Burtt NP, Villeneuve A, Hirschhorn JN, Altshuler D, Groop LC, Despres JP, Gaudet D, Hudson TJ (2002) 5′ flanking variants of resistin are associated with obesity. Diabetes 51:1629–1634 [DOI] [PubMed] [Google Scholar]
- Excoffier L, Slatkin M (1995) Maximum-likelihood estimation of molecular haplotype frequencies in a diploid population. Mol Biol Evol 12:921–927 [DOI] [PubMed] [Google Scholar]
- Expert Committee on the Diagnosis and Classification of Diabetes Mellitus (2003) Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care Suppl 26:S5–S20 [DOI] [PubMed] [Google Scholar]
- Fallin D, Cohen A, Essioux L, Chumakov I, Blumenfeld M, Cohen D, Schork N (2001) Genetic analysis of case/control data using estimated haplotype frequencies: application to APOE locus variation and Alzheimer’s disease. Genome Res 11:143–151 10.1101/gr.148401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujinami A, Obayashi H, Ohta K, Ichimura T, Nishimura M, Matsui H, Kawahara Y, Yamazaki M, Ogata M, Hasegawa G, Nakamura N, Yoshikawa T, Nakano K, Ohta M (2004) Enzyme-linked immunosorbent assay for circulating human resistin: resistin concentrations in normal subjects and patients with type 2 diabetes. Clin Chim Acta 339:57–63 10.1016/j.cccn.2003.09.009 [DOI] [PubMed] [Google Scholar]
- Hagen G, Muller S, Beato M, Suske G (1994) Sp1-mediated transcriptional activation is repressed by Sp3. EMBO J 13:3843–3851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horikawa Y, Oda N, Cox NJ, Li X, Orho-Melander M, Hara M, Hinokio Y, Lindner TH, Mashima H, Schwarz PE, del Bosque-Plata L, Oda Y, Yoshiuchi I, Colilla S, Polonsky KS, Wei S, Concannon P, Iwasaki N, Schulze J, Baier LJ, Bogardus C, Groop L, Boerwinkle E, Hanis CL, Bell GI (2000) Genetic variation in the gene encoding calpain-10 is associated with type 2 diabetes mellitus. Nat Genet 26:163–175 10.1038/79876 [DOI] [PubMed] [Google Scholar]
- Kikkawa E, Hinata M, Keng VW, Myint Z, Sato A, Yamada K, Tanaka T, Noguchi T (2001) Sp family members stimulate the transcription of Hex gene via interactions with GC boxes. J Biochem (Tokyo) 130:885–891 [DOI] [PubMed] [Google Scholar]
- Lander E (1996) The new genomics: global views of biology. Science 274:536–539 10.1126/science.274.5287.536 [DOI] [PubMed] [Google Scholar]
- Lau J, Ioannidis J, Schmid C (1997) Quantitative synthesis in systematic reviews. Ann Intern Med 127:820–826 [DOI] [PubMed] [Google Scholar]
- Lee J, Chan J, Yiannakouris N, Kontogianni M, Estrada E, Seip R, Orlova C, Mantzoros C (2003) Circulating resistin levels are not associated with obesity or insulin resistance in humans and are not regulated by fasting or leptin administration: cross-sectional and interventional studies in normal, insulin-resistant, and diabetic subjects. J Clin Endocrinol Metab 88:4848–4856 10.1210/jc.2003-030519 [DOI] [PubMed] [Google Scholar]
- Lohmueller K, Pearce C, Pike M, Lander E, Hirschhorn J (2003) Meta-analysis of genetic association studies supports a contribution of common variants to susceptibility to common disease. Nat Genet 33:177–182 10.1038/ng1071 [DOI] [PubMed] [Google Scholar]
- Ma X, Warram JH, Trischitta V, Doria A (2002) Genetic variants at the resistin locus and risk of type 2 diabetes in Caucasians. J Clin Endocrinol Metab 87:4407–4410 10.1210/jc.2002-020109 [DOI] [PubMed] [Google Scholar]
- McCarthy MI, Froguel P (2002) Genetic approaches to the molecular understanding of type 2 diabetes. Am J Physiol Endocrinol Metab 283:E217–E225 [DOI] [PubMed] [Google Scholar]
- McTernan P, Fisher F, Valsamakis G, Chetty R, Harte A, McTernan C, Clark P, Smith S, Barnett A, Kumar S (2003) Resistin and type 2 diabetes: regulation of resistin expression by insulin and rosiglitazone and the effects of recombinant resistin on lipid and glucose metabolism in human differentiated adipocytes. J Clin Endocrinol Metab 88:6098–6106 10.1210/jc.2003-030898 [DOI] [PubMed] [Google Scholar]
- Nagaev I, Smith U (2001) Insulin resistance and type 2 diabetes are not related to resistin expression in human fat cells or skeletal muscle. Biochem Biophys Res Commun 285:561–564 10.1006/bbrc.2001.5173 [DOI] [PubMed] [Google Scholar]
- Omi K, Ohashi J, Patarapotikul J, Hananantachai H, Naka I, Looareesuwan S, Tokunaga K (2003) CD36 polymorphism is associated with protection from cerebral malaria. Am J Hum Genet 72:364–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osawa H, Onuma H, Murakami A, Ochi M, Nishimiya T, Kato K, Shimizu I, Fujii Y, Ohashi J, Makino H (2002) Systematic search for single nucleotide polymorphisms in the resistin gene: the absence of evidence for the association of three identified single nucleotide polymorphisms with Japanese type 2 diabetes. Diabetes 51:863–866 [DOI] [PubMed] [Google Scholar]
- ——— (2003) Systematic search for single nucleotide polymorphisms in the FOXC2 gene: the absence of evidence for the association of three frequent single nucleotide polymorphisms and four common haplotypes with Japanese type 2 diabetes. Diabetes 52:562–567 [DOI] [PubMed] [Google Scholar]
- Patel S, Rajala M, Rossetti L, Scherer P, Shapiro L (2004) Disulfide-dependent multimeric assembly of resistin family hormones. Science 304:1154–1158 10.1126/science.1093466 [DOI] [PubMed] [Google Scholar]
- Pravenec M, Kazdova L, Landa V, Zidek V, Mlejnek P, Jansa P, Wang J, Qi N, Kurtz T (2003) Transgenic and recombinant resistin impair skeletal muscle glucose metabolism in the spontaneously hypertensive rat. J Biol Chem 278:45209–45215 10.1074/jbc.M304869200 [DOI] [PubMed] [Google Scholar]
- Rajala M, Obici S, Scherer P, Rossetti L (2003) Adipose-derived resistin and gut-derived resistin-like molecule-β selectively impair insulin action on glucose production. J Clin Invest 111:225–230 10.1172/JCI200316521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage D, Sewter C, Klenk E, Segal D, Vidal-Puig A, Considine R, O’Rahilly S (2001) Resistin/Fizz3 expression in relation to obesity and peroxisome proliferator-activated receptor-γ action in humans. Diabetes 50:2199–2202 [DOI] [PubMed] [Google Scholar]
- Shou Z, Yamada K, Inazu T, Kawata H, Hirano S, Mizutani T, Yazawa T, Sekiguchi T, Yoshino M, Kajitani T, Okada K, Miyamoto K (2003) Genomic structure and analysis of transcriptional regulation of the mouse zinc-fingers and homeoboxes 1 (ZHX1) gene. Gene 302:83–94 10.1016/S0378-1119(02)01093-4 [DOI] [PubMed] [Google Scholar]
- Smith S, Bai F, Charbonneau C, Janderova L, Argyropoulos G (2003) A promoter genotype and oxidative stress potentially link resistin to human insulin resistance. Diabetes 52:1611–1618 [DOI] [PubMed] [Google Scholar]
- Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA (2001) The hormone resistin links obesity to diabetes. Nature 409:307–312 10.1038/35053000 [DOI] [PubMed] [Google Scholar]
- Steppan CM, Lazar MA (2004) The current biology of resistin. J Intern Med 255:439–447 [DOI] [PubMed] [Google Scholar]
- Tokuhiro S, Yamada R, Chang X, Suzuki A, Kochi Y, Sawada T, Suzuki M, Nagasaki M, Ohtsuki M, Ono M, Furukawa H, Nagashima M, Yoshino S, Mabuchi A, Sekine A, Saito S, Takahashi A, Tsunoda T, Nakamura Y, Yamamoto K (2003) An intronic SNP in a RUNX1 binding site of SLC22A4, encoding an organic cation transporter, is associated with rheumatoid arthritis. Nat Genet 35:341–348 10.1038/ng1267 [DOI] [PubMed] [Google Scholar]
- Wellen K, Hotamisligil G (2003) Obesity-induced inflammatory changes in adipose tissue. J Clin Invest 112:1785–1788 10.1172/JCI200320514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Tanaka T, Miyamoto K, Noguchi T (2000) Sp family members and nuclear factor-Y cooperatively stimulate transcription from the rat pyruvate kinase M gene distal promoter region via their direct interactions. J Biol Chem 275:18129–18137 10.1074/jbc.M001543200 [DOI] [PubMed] [Google Scholar]
- Yamada K, Tanaka T, Noguchi T (1997) Members of the nuclear factor 1 family and hepatocyte nuclear factor 4 bind to overlapping sequences of the L-II element on the rat pyruvate kinase L gene promoter and regulates its expression. Biochem J 324:917–925 [DOI] [PMC free article] [PubMed] [Google Scholar]