Homozygous WNT3 Mutation Causes Tetra-Amelia in a Large Consanguineous Family (original) (raw)
Abstract
Tetra-amelia is a rare human genetic disorder characterized by complete absence of all four limbs and other anomalies. We studied a consanguineous family with four affected fetuses displaying autosomal recessive tetra-amelia and craniofacial and urogenital defects. By homozygosity mapping, the disease locus was assigned to chromosome 17q21, with a maximum multipoint LOD score of 2.9 at markers D17S931, D17S1785, D17SS1827, and D17S1868. Further fine mapping defined a critical interval of ∼8.9 Mb between D17S1299 and D17S797. We identified a homozygous nonsense mutation (Q83X) in the WNT3 gene in affected fetuses of the family. WNT3, a human homologue of the Drosophila wingless gene, encodes a member of the WNT family known to play key roles in embryonic development. The Q83X mutation truncates WNT3 at its amino terminus, suggesting that loss of function is the most likely cause of the disorder. Our findings contrast with the observation of early lethality in mice homozygous for null alleles of Wnt3. To our knowledge, this is the first report of a mutation in a WNT gene associated with a Mendelian disorder. The identification of a WNT3 mutation in tetra-amelia indicates that WNT3 is required at the earliest stages of human limb formation and for craniofacial and urogenital development.
Mutations in genes involved in limb development have been identified in various malformation syndromes (Tickle 2002). However, the genetic basis of limb agenesis is still unknown.
Tetra-amelia (MIM 273395 and MIM 301090), the complete absence of the extremities, occurs extremely rarely. To date, it has been described in four families, where it appears to follow an autosomal recessive mode of inheritance. In all families, tetra-amelia was associated with craniofacial, nervous system, pulmonary, skeletal, and urogenital anomalies (Zimmer et al. 1985; Gershoni-Baruch et al. 1990; Rosenak et al. 1991; Zlotogora et al. 1993; Başaran et al. 1994).
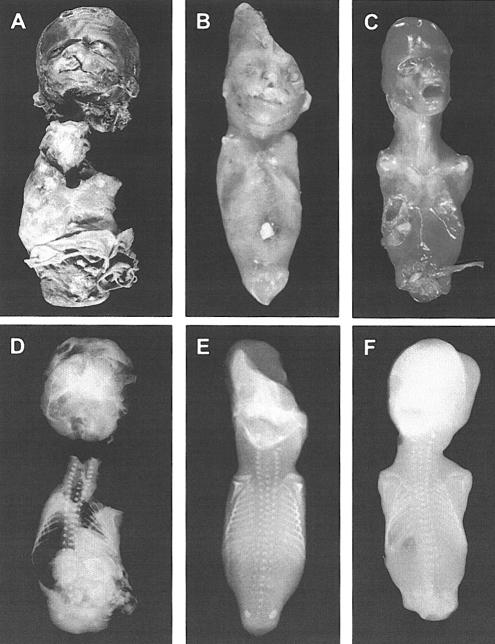
We studied a consanguineous Turkish family of Aramaic descent with autosomal recessive tetra-amelia (fig. 1). In eight pregnancies, tetra-amelia was diagnosed four times prenatally, and pregnancies were terminated between the 13th and 20th wk of gestation. Cytogenetic studies showed that three of the affected fetuses were female, and one was male. Autopsy was possible in three of the four affected fetuses (fetuses IV:1, IV:4, and IV:7) and revealed various anomalies in addition to complete absence of all four limbs (fig. 2). In the first affected fetus (fetus IV:1; fig. 2A and 2D), a female, these findings were cleft lip with cleft palate; gastroschisis; diaphragmatic defect with malposition of the right lung (which was bilobular); agenesis of the left kidney, left supra-adrenal gland, and spleen; a malformed uterus, with only a right, rudimentary ovary and salpinx; and only one umbilical artery. In the second affected fetus (fetus IV:4; fig. 2_B_ and 2E), a female, findings were a protrusion and cataract of the left eye, microphthalmia of the right eye, cleft lip with cleft palate, malformed nose with a single naris and choanal atresia, hypoplasia of the pelvis, and atresia of the urethra, vagina, and anus. In the third affected fetus (fetus IV:7; fig. 2C and 2F), a male, findings were agenesis of the left kidney and left supra-adrenal gland, hypoplasia of the pelvis, persistence of cloaca, and no external genitalia.
Figure 1.
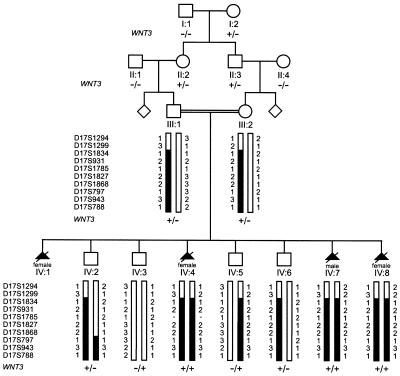
Pedigree, haplotype, and WNT3_-mutation status of tetra-amelic family. Individuals’ numbers are listed below the pedigree symbols. Genotyped markers from the chromosome 17q21 region are shown to the left of generations III and IV, and individuals’ allele numbers for each marker are given next to the bar. Black bars represent haplotype segregating with the tetra-amelia gene. WNT3 is located between markers D17S1299 and D17S1834. The mutation genotypes of WNT3 are shown below individuals’ numbers (generations I and II) or below haplotypes (generations III and IV). A plus sign (+_) indicates mutation Q83X; a minus sign (−) indicates wild-type allele.
Figure 2.
Complete limb agenesis, urogenital defects, and other anomalies in affected fetuses. Postmortem photographs (A–C) and radiographs (D–F) demonstrate complete absence of all four limbs, without defects of scapulae and clavicles. A and D, Tetra-amelic fetus IV:1 (female) at 19 wk of gestation, crown-to-rump length 17 cm. Note cleft lip with cleft palate. The separation of the head from the trunk and the multiple lesions on the fetus were iatrogenic. The poorly preserved caudal region did not allow for assessment of external genitalia. B and E, Tetra-amelic fetus IV:7 (female) at 16 wk of gestation, crown-to-rump length 10.5 cm. Note protrusion and cataract of the left eye, microphthalmia of the right eye, malformed nose, hypoplasia of the pelvis, and undefined vaginal and anal regions, with atresia of the urethra, vagina, and anus. Parts of the head were damaged during abortion. C and F, Tetra-amelic fetus IV:7 (male) at 20 wk of gestation, crown-to-rump length 15.5 cm. Note hypoplasia of the pelvis, persistence of the cloaca, and no external genitalia.
Mice in which both copies of the Fgf10 gene have been disrupted by gene targeting fail to develop limbs (Sekine et al. 1999). On the basis of the phenotypic similarities between _Fgf10_−/− mice and the affected subjects in the family in our study, FGF10 appeared to be an attractive candidate gene for limb agenesis. Sequence analysis of all three FGF10 exons in an affected subject, however, did not reveal a mutation.
We then performed homozygosity mapping with 378 autosomal polymorphic markers with an average spacing of ∼10 cM (Center for Medical Genetics [screening set version 10]) for individuals III:1, III:2, IV:2, IV:3, IV:5, IV:6, IV:7, and IV:8. Informed consent was obtained from all family members involved in this study, and ethical approval was given by the local ethics committee. Following the initial genome screen, individual markers were genotyped in fetus IV:4, from whom formalin-fixed paraffin sections were available (fig. 1). Under the assumption of an autosomal recessive mode of inheritance, selected informative markers were analyzed for fetus IV:4 when identical alleles were shared homozygously by fetuses IV:7 and IV:8, when one copy of this allele was present in both parents, and when no more than one copy of the allele was found in the unaffected individuals. This approach did not identify a shared homozygous haplotype by the above criteria in fetuses IV:4, IV:7, and IV:8, and it ruled out chromosome 5p12, to which FGF10 has been assigned. At marker D17S1299, fetuses IV:4, IV:7, and IV:8 were heterozygous for the same alleles (fig. 1), and the MLINK program of the LINKAGE software package (Lathrop and Lalouel 1984) had yielded a positive two-point LOD score. We subsequently typed additional markers surrounding marker D17S1299. For multipoint LOD score analysis, we used the HOMOZ linkage program (Kruglyak et al. 1995) and assumed autosomal recessive inheritance with full penetrance, a disease gene frequency of 0.0001, and uniform marker allele frequencies. A maximum LOD score at θ=2.9 was found at markers D17S931, D17S1785, D17SS1827, and D17S1868 (table 1). Further fine mapping and haplotype examination narrowed the tetra-amelia locus to a critical region of ∼8.9 Mb of homozygous sequence between markers D17S1299 and D17S797 on chromosome 17q21 (fig. 1).
Table 1.
LOD Scores for Markers at 17q21
| LOD at θ=0 | ||||
|---|---|---|---|---|
| Marker | Genetic Distancea(cM) | Physical Locationb(Mb) | Multipoint | Two-Point |
| D17S1294c | 50.74 | 28.2 | −∞ | −∞ |
| D17S1299c | 62.01 | 38.9 | −.097 | 1.102 |
| D17S1834 | 64.16 | 45.3 | 2.733 | 1.210 |
| D17S931 | 66.85 | 45.3 | 2.900 | 1.177 |
| D17S1785 | 66.85 | 46.3 | 2.901 | 1.102 |
| D17S1827 | 66.85 | 46.9 | 2.902 | 2.908 |
| D17S1868 | 64.16 | 47.5 | 2.902 | 2.908 |
| D17S797 | 66.85 | 47.8 | −∞ | −∞ |
| D17S943 | 68.44 | 48.2 | −∞ | −∞ |
| D17S788 | 73.62 | 50.6 | −1.145 | .604 |
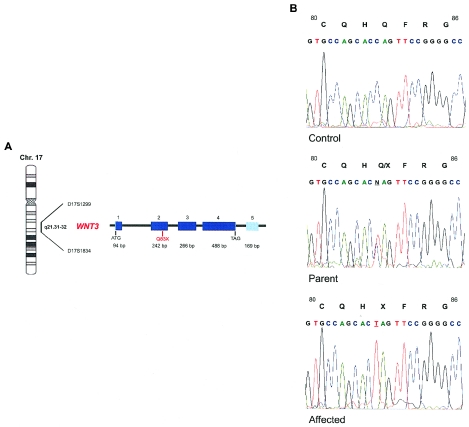
Obvious candidate genes in the critical interval were the HOXB cluster, WNT3, and WNT9B. The gene encoding WNT3 appeared particularly interesting. WNT genes, the vertebrate relatives of the Drosophila wingless gene (Klingensmith and Nusse 1994), encode a family of highly conserved, secreted signaling molecules that play major roles in many developmental processes (Wodarz and Nusse 1998). WNTs act as ligands for the frizzled family of transmembrane receptors. In mice, Wnt3 signaling has been implicated in the formation of the primary body axis and has recently been shown to be critical for limb growth (Liu et al. 1999; Barrow et al. 2003). WNT3 consists of five exons and encodes a protein of 355 amino acids. Sequence analysis of the WNT3 coding region in the affected subjects revealed a nonsense mutation, Q83X (C→T transition at position 366 [GenBank accession number NM_030753]) (fig. 3). The analyzed fetuses (fetuses IV:7 and IV:8) had homozygous mutations, whereas both parents and the unaffected subjects IV:2, IV:5, and IV:6 had heterozygous mutations. The mutation was also observed in individual I:2, who introduced the mutation into the family, and in individuals II:2 and II:3 but not in individuals I:1, II:1, and II:4. Furthermore, this mutation was absent in 170 chromosomes from the German population and was absent in 176 chromosomes from the Turkish population. The nonsense mutation at codon 83 of WNT3 in the family in our study creates a premature stop codon. This stop codon putatively results in a truncated protein of only 82 amino acids (including the signal peptide of 21 amino acids), with no other known functional domains, instead of 355 amino acids of the mature peptide. Hence, loss of function of both copies of WNT3 in the affected subjects of the family in our study is the most likely mechanism of the mutation.
Figure 3.
WNT3 nonsense mutation associated with tetra-amelia. A, Location of WNT3 on chromosome 17q21.31-32 and genomic organization of WNT3. The dark boxes represent exons, with the coding region illustrated in dark blue and the 5′ and 3′ UTRs in light blue. Positions of the initiation codon, the termination codon, and the nonsense mutation in exon 2 are indicated. B, Electropherograms of WNT3 exon 2 in a control individual (top), one parent (middle), and an affected fetus (bottom). DNA sequence analysis revealed a homozygous C→T substitution at nt 366 (from the translation start site) in the tetra-amelic fetus, causing a Q83X mutation. The parent is heterozygous with respect to the Q83X mutation.
The established models of limb initiation in vertebrates have stressed the role of two members of the fibroblast growth factor (FGF) superfamily; that is, FGF8 and FGF10 (Cohn et al. 1995; Martin 1998). It has been proposed that limb outgrowth depends on an FGF8/FGF10 positive regulatory loop. According to this model, FGF10 produced in the prospective limb mesoderm induces Fgf8 expression in cells of the surface ectoderm that later form the apical ectodermal ridge (AER) (Johnson and Tabin 1997; Niswander 2003). The AER, running along the distal margin of the limb bud, is critical for the outgrowth of the limb. Once the limb bud has developed, FGF8 produced by cells of the AER maintains Fgf10 expression in the underlying mesenchyme, which in turn maintains Fgf8 expression. This model has been tentatively expanded by work in chickens that postulates that the FGF8/FGF10 loop is mediated by Wnt/β-catenin signaling, both upstream and downstream of FGF10. First, Wnt signaling through β-catenin appears to be required for the restriction and maintenance of Fgf10 expression in the limb mesoderm and, hence, is needed for limb induction. Second, FGF10 is suggested to induce Wnt3a signaling through the β-catenin pathway in the ectoderm; this leads to activation of Fgf8 expression (Kawakami et al. 2001). Recently, this view has been supported by studies in mice in which the conditional removal of Wnt3 in the limb ectoderm leads to failure of AER formation and to limb defects that, in the most-severe cases, cause limb agenesis (Barrow et al. 2003). Our work on human tetra-amelia disorder supports and extends a role for Wnt3/WNT3 signaling in the initiation of limb development.
Our data are consistent with a model in which WNT3 is required in the limb ectoderm for initiation of AER formation. Moreover, our work may indicate that WNT3 also acts within the mesoderm. Loss of Wnt3 in the mouse ectoderm leads to variable effects, ranging from normal limbs to agenesis, whereas loss of β-catenin in the ectoderm has a more consistent and severe effect (Barrow et al. 2003). This may indicate redundancy in Wnt function in the limb ectoderm. The complete penetrance and complete absence of all four limbs, as well as the pelvic hypoplasia observed upon loss of WNT3 function in humans, lead to the suggestion that WNT3 may also be required earlier within the mesoderm to restrict and maintain FGF10 expression. Our discovery of WNT3 as a limb-inducing gene in humans provides new ways to understand the earliest stages of limb development (Martin 2001; Tickle and Münsterberg 2001).
It is intriguing that gastrulation occurs normally upon loss of human WNT3, whereas mice homozygous for null alleles of Wnt3 fail to gastrulate and thus never develop limbs (Liu et al. 1999). Under the assumption of functional inactivity of the truncated protein, this suggests that other WNT proteins act to regulate gastrulation and axis formation of the human embryo and that the phenotypic differences between the _Wnt3_−/− mice and the affected subjects in the family in our study are species specific. Different members of the Wnt family may exert identical roles in different species. Indeed, the key ligands that are postulated to regulate the activation of Fgf8 in the limb ectoderm differ between mice (Wnt3) and chickens (Wnt3a) (Kawakami et al. 2001; Barrow et al. 2003). Our work indicates that WNT3 is the key signal in the human limb. Because of the early gastrulation defect in _Wnt3_−/− mice and the lack of other conditional knockout mutants, the role of Wnt3 in tissues other than the limbs has not yet been studied. Loss of WNT3 function in the tetra-amelic fetuses causes urogenital, craniofacial, and skeletal malformations, demonstrating that WNT3 signaling is required for the embryonic development of these organs and structures. The urogenital anomalies in the affected fetuses included uterine and ovarian malformations, agenesis of kidney, and genital defects. In fact, two (fetuses IV:4 and IV:7) of the three autopsied fetuses displayed defects of the external genitalia. This could not be analyzed in the damaged fetus (fetus IV:1). The presence of urogenital defects in the limbless fetuses provides novel evidence that WNT signaling is involved in the earliest steps of limb formation and urogenital development in humans. Wnt signaling has been studied in mouse urogenital development, and Wnt4 and Wnt11 were shown to play key roles in kidney development (Stark et al. 1994; Majumdar et al. 2003). It is possible that, in humans, WNT3 signaling may regulate functions of urogenital development that are controlled by other Wnt ligands in mice.
To our knowledge, this is the first report of a mutation in a member of the WNT superfamily associated with a Mendelian disorder and the first description of a limb-inducing gene in humans. The discovery of the molecular basis of tetra-amelia demonstrates a pivotal role for WNT3 in several aspects of human embryonic development. It will be important to explore whether other mutations in WNT3 cause other human malformation syndromes.
Acknowledgments
We are grateful to the members of the family for their participation in this study. We thank S. Grussner, for ultrasonography; A. Köhler, for cytogenic studies; and T. Papadakis, for technical assistance.
Electronic-Database Information
The accession number and URLs for data presented herein are as follows:
- Center for Medical Genetics, http://research.marshfieldclinic.org/genetics/ (for marker screening set version 10)
- GenBank, http://www.ncbi.nlm.nih.gov/Genbank/ (for the human WNT3 genomic sequence [accession number NM_030753])
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for tetra-amelia)
- University of California Santa Cruz Genome Bioinformatics, http://genome.ucsc.edu/
References
- Barrow JR, Thomas KR, Boussadia-Zahui O, Moore R, Kemler R, Capecchi MR, McMahon AP (2003) Ectodermal Wnt3/β-catenin signaling is required for the establishment and maintenance of the apical ectodermal ridge. Genes and Dev 17:394–409 10.1101/gad.1044903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Başaran S, Yüksel A, Ermiş H. Kuseyri F, Ağan M, Yüksel-Apak M (1994) Tetra-amelia, lung hypo-/aplasia, cleft lip-palate, and heart defect: a new syndrome. Am J Med Genet 51:77–80 [DOI] [PubMed] [Google Scholar]
- Cohn MJ, Izpisúa-Belmonte JC, Abud H, Heath JK, Tickle C (1995) Fibroblast growth factors induce additional limb development from the flank of chick embryos. Cell 80:739–746 [DOI] [PubMed] [Google Scholar]
- Gershoni-Baruch R, Drugan A, Bronshtein M, Zimmer EZ (1990) Roberts syndrome or “X-linked amelia”? Am J Med Genet 37:569–572 2260610 [DOI] [PubMed] [Google Scholar]
- Johnson RL, Tabin CJ (1997) Molecular models of vertebrate limb development. Cell 90:979–990 [DOI] [PubMed] [Google Scholar]
- Kawakami Y, Capdevilla J, Büscher D, Itoh T, Esteban CR, Belmonte JCI (2001) WNT signals control FGF-dependent limb initiation and AER induction in the chick embryo. Cell 104:891–900 [DOI] [PubMed] [Google Scholar]
- Klingensmith J, Nusse R (1994) Signaling by wingless in Drosophila. Dev Biol 166:396–414 7813765 [DOI] [PubMed] [Google Scholar]
- Kruglyak L, Daly MJ, Lander ES (1995) Rapid multipoint linkage analysis of recessive traits in nuclear families, including homozygosity mapping. Am J Hum Genet 56:519–527 [PMC free article] [PubMed] [Google Scholar]
- Lathrop GM, Lalouel JM (1984) Easy calculations of LOD scores and genetic risks on small computers. Am J Hum Genet 36:460–465 [PMC free article] [PubMed] [Google Scholar]
- Liu P, Wakamiya M, Shea MJ, Albrecht U, Behringer RR, Bradley A (1999) Requirement for Wnt3 in vertebrate axis formation. Nat Genet 22:361–365 10.1038/11932 [DOI] [PubMed] [Google Scholar]
- Majumdar A, Vainio S, Kispert A, McMahon J, McMahon AP (2003) Wnt11 and Ret/Gdnf pathways cooperate in regulating ureter branching during metanephric kidney development. Development 130:3175–3185 10.1242/dev.00520 [DOI] [PubMed] [Google Scholar]
- Martin G (2001) Making a vertebrate limb: new players enter from the wings. Bioessays 23:865–868 10.1002/bies.1126 [DOI] [PubMed] [Google Scholar]
- Martin GR (1998) The roles of FGFs in the early development of vertebrate limbs. Genes and Dev 12:1571–1586 [DOI] [PubMed] [Google Scholar]
- Niswander L (2003) Pattern formation: old models out on a limb. Nat Rev Genet 4:133–143 10.1038/nrg1001 [DOI] [PubMed] [Google Scholar]
- Rosenak D, Ariel I, Arnon J, Diamant AYZ, Chetrit AB, Nadjari M, Zilberman R, Yaffe H, Cohen T, Ornoy A (1991) Recurrent tetraamelia and pulmonary hypoplasia with multiple malformations in sibs. Am J Med Genet 38:25–28 [DOI] [PubMed] [Google Scholar]
- Sekine K, Ohuchi H, Fujiwara M, Yamasaki M, Yoshizawa T, Sato T, Yagishita N, Matsui D, Koga Y, Itoh N, Kato S (1999) Fgf10 is essential for limb and lung formation. Nat Genet 21:138–141 10.1038/5096 [DOI] [PubMed] [Google Scholar]
- Stark K, Vainio S, Vassileva G, McMahon AP (1994) Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature 372:679–683 10.1038/372679a0 [DOI] [PubMed] [Google Scholar]
- Tickle C (2002) Molecular basis of vertebrate limb patterning. Am J Med Genet 112:250–255 10.1002/ajmg.10774 [DOI] [PubMed] [Google Scholar]
- Tickle C, Münsterberg A (2001) Vertebrate limb development: the early stages in chick and mouse. Curr Opin Genet Dev 11:476–481 10.1016/S0959-437X(00)00220-3 [DOI] [PubMed] [Google Scholar]
- Wodarz A, Nusse R (1998) Mechanisms of Wnt signaling in development. Annu Rev Cell Dev Biol 14:59–88 9891778 [DOI] [PubMed] [Google Scholar]
- Zimmer EZ, Taub E, Sova Y, Divon MY, Pery M, Peretz BA (1985) Tetra-amelia with multiple malformations in six male fetuses of one kindred. Eur J Pediatr 144:412–414 [DOI] [PubMed] [Google Scholar]
- Zlotogora J, Sagi M, Shabany YO, Jarallah RY (1993) Syndrome of tetraamelia with pulmonary hypoplasia. Am J Med Genet 47:570–571 [DOI] [PubMed] [Google Scholar]