Reduced PU.1 expression causes myeloid progenitor expansion and increased leukemia penetrance in mice expressing PML-RARα (original) (raw)
Abstract
PU.1 is a member of the ETS family of transcription factors that is known to be important for hematopoietic development. Recently, haploinsufficiency for PU.1 has been shown to cause a shift in myelomonocytic progenitor fate toward the myeloid lineage. We have previously shown that transgenic mice expressing PML-RARα (PR) and RARα-PML frequently develop acute promyelocytic leukemia (APL) in association with a large (>20 Mb) interstitial deletion of chromosome 2 that includes PU.1. To directly assess the relevance of levels of expression of PU.1 for leukemia progression, we bred hCG-PR mice with PU.1+/- mice and assessed their phenotype. Young, nonleukemic hCG-PR × PU.1+/- mice developed splenomegaly because of the abnormal expansion of myeloid cells in their spleens. hCG-PR × PU.1+/- mice developed a typical APL syndrome after a long latent period, but the penetrance of disease was 84%, compared with 7% in hCG-PR × PU.1+/+ mice (P < 0.0001). The residual PU.1 allele in hCG-PR × PU.1+/- APL cells was expressed, and complete exonic resequencing revealed no detectable mutations in nine of nine samples. However, PR expression in U937 myelomonocytic cells and primary murine myeloid bone marrow cells caused a reduction in PU.1 mRNA levels. Therefore, the loss of one copy of PU.1 through a deletional mechanism, plus down-regulation of the residual allele caused by PR expression, may synergize to expand the pool of myeloid progenitors that are susceptible to transformation, increasing the penetrance of APL.
Keywords: acute promyelocytic leukemia, mouse model, transcription factor, chromosome 2
PU.1 is a member of the ETS family of transcription factors. Its importance for hematopoietic development was established by the study of PU.1-deficient mice, which have severe defects in both lymphoid and myelomonocytic development (1, 2). Although a haploinsufficient phenotype was not originally appreciated in these animals, Simon and coworkers (3) recently demonstrated that PU.1+/- mice have a subtle defect in myelomonocytic progenitor fate that favors myeloid development. The discovery that some human acute myeloid leukemia (AML) samples have an acquired loss-of-function mutation in one copy of PU.1 suggested that this transcription factor might sometimes be involved with the pathogenesis of AML in human patients (4).
To further explore this hypothesis, Tenen and coworkers (5) decided to create a mutation that would reduce PU.1 expression in mice to a level <50% that of normal but not to the null state, which disrupts myeloid development completely. They mutated an enhancer of the PU.1 gene, which reduced expression of the gene by ≈80%. Homozygous mice bearing this hypomorphic mutation developed AML, suggesting that reducing the expression of PU.1 below haploinsufficient levels contributes to AML pathogenesis (5).
Recently published experiments from Adams and colleagues (6) and Silver and colleagues (35) have extended this paradigm. For many years, it has been known that irradiated mice frequently develop AML after a long latent period; nearly all of these tumors contain an interstitial deletion of one copy of chromosome 2 that includes the PU.1 gene (7-12). Adams' group evaluated the sequence of the residual PU.1 allele in AML tumors arising in irradiated mice and found that 87% contained mutations in the ETS-binding domain located within exon 5 (6). Similar findings were reported by Silver and colleagues (35). These single amino acid substitutions were shown to alter the function of PU.1 by reducing its transactivating activity. These data strongly suggest that PU.1 acts in a fashion similar to that of a tumor suppressor in myeloid progenitors and that a deletion of one allele, followed by a reduction in function of the other, is relevant for AML pathogenesis in this model.
We have previously shown that 10-20% of transgenic mice expressing PML-RARα (PR) in their early myeloid cells (hCG-PR mice) develop acute promyelocytic leukemia (APL) after a long latent period (13). The penetrance of APL development increases substantially when RARα-PML is coexpressed in early myeloid cells (14) or when young mice are sublethally irradiated (15): In both scenarios, the vast majority of APL tumors contain a large (>20 Mb) interstitial deletion of one copy of chromosome 2 that invariably includes the PU.1 gene (15, 16). Although many potential candidate genes for AML progression lie on this interval, PU.1 appeared to be a promising candidate because of its known importance for myeloid development. We therefore crossed our hCG-PR mice with PU.1+/- mice and learned that young, nonleukemic mice rapidly develop splenomegaly caused by an abnormal expansion of the myeloid pool. PR × PU.1+/- mice developed typical APL with a long latent period, but the penetrance of the disease increased by >10-fold. Additional experiments revealed that PR itself may reduce expression of the residual PU.1 allele, providing a second “hit” that reduces PU.1 levels below a critical threshold. Collectively, these studies strongly suggest that down-regulation of PU.1, mediated through a variety of genetic mechanisms, is an important progression factor for AML in the mouse. Because PU.1 is sometimes mutated or deleted in human AML syndromes (4) and because both AML-ETO (17) and PR can reduce the function of PU.1, this gene is also likely to be important for the development of AML in humans.
Materials and Methods
Mice. We have previously described the generation of the human cathepsin G (hCG)-PR transgene and mice (in a C3H × C57BL/6 background) (referred to as PR) (13, 18). PU.1 heterozygous mice (PU.1+/-) have been described (in a C57BL/6 background) (1). PR-expressing mice were bred to PU.1+/- mice to generate two independent intercrossed cohorts for the study. Animals were bred until 52 animals were obtained in the first cohort and 144 animals in the second cohort, with each cohort containing WT, PU.1+/-, PR, and doubly transgenic PR × PU.1+/- mice (PRxPU.1+/-) in approximately equal proportions, as expected. The Washington University Animal Studies Committee approved all animal experiments.
Analysis of APL Cells. Leukemic mice and leukemic spleen cells from PR and PRxPU.1+/- transgenic animals were analyzed as described in ref. 14 and Supporting Text, which is published as supporting information on the PNAS web site.
Hematopoietic Progenitor Assay. Bone marrow cells from individual mice were counted by using a hemocytometer and plated, in duplicate, in 1.3 ml of methylcellulose medium containing 10 ng·ml-1 IL-6, 10 ng·ml-1 IL-3, and 50 ng·ml-1 stem cell factor (SCF) (MethoCult M3534, StemCell Technologies, Vancouver). Cells were plated at 8.3 × 103/ml. Colonies with >30 cells were counted on day 8. Twenty individual colonies were picked from two separate experiments for each genotype, and cytospins of cells were scored as colony-forming unit granulocytic (CFU-G) macrophage (CFU-M), or granulocytic and macrophage (CFU-GM) colonies after staining with May-Grunwald Giemsa. A separate cytospin was prepared for each genotype by mixing all of the colonies from a duplicate culture plate.
PU.1 PCR and Sequencing. PU.1 total exonic DNA sequence, including ≈800 bases of the 5′ flanking region and ≈500 bases of the 3′ UTR, was sequenced by using methods described in ref. 19. Please see Supporting Text for a complete list of sequencing primers.
Western Blotting. Cell lysates were prepared in RIPA buffer as described in ref. 20 and Supporting Text.
Transfection, Cell Sorting, and Quantitative RT-PCR. U937 cells were electroporated with plasmid DNA and harvested by flow-sorting as described in ref. 21 and Supporting Text. Total RNA was isolated from the GFP+ U937 samples and quantitative RT-PCR was performed as described in ref. 15 and Supporting Text.
Generation of Promyelocyte-Enriched Populations. Promyelocyte-enriched populations were generated as described in refs. 15 and 21 and Supporting Text.
Results
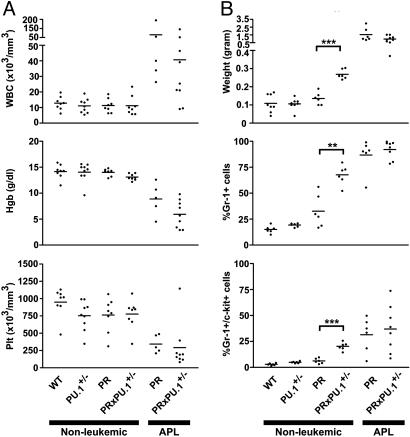
Nonleukemic PU.1+/- Mice that Express PR Developed Splenomegaly and an Expanded Myeloid Compartment. PU.1+/- mice were bred with a previously characterized hCG-PR transgenic line (13) to generate cohorts of animals that were WT, PU.1 heterozygous (PU.1+/-), PR, or PR × PU.1+/- (PRxPU.1+/-). Evaluation of whole bone marrow RNA revealed that loss of one copy of PU.1 did not significantly alter expression levels from the hCG-PR transgene, which contains a putative PU.1-binding site at position -371 (data not shown). Two- to 5-month-old WT, PU.1+/-, PR, and PRxPU.1+/- mice had normal peripheral blood counts (Fig. 1_A_). Age matched, nonleukemic PRxPU.1+/- mice developed significant splenomegaly, compared with all other genotypes (Figs. 1_B_ and 2_A_, P < 0.0001). In addition, spleen cells from PU.1+/-, PR, and PRxPU.1+/- mice contained an abnormal population of Gr-1+ cells that were also _c_-kit+, as compared with WT mice (Fig. 1_B_, P = 0.005, P = 0.02, and P < 0.0001, respectively, Fig. 2_D_). This abnormal myeloid population was associated with the appearance of a high side-scatter population in the spleens from PU.1+/-, PR, and PRxPU.1+/- mice (Fig. 2_D_).
Fig. 1.
Peripheral blood and spleen analysis of nonleukemic and leukemic mice. (A) Comparison of peripheral blood counts. Blood was obtained from four cohorts of healthy age-matched, genotyped littermates at 2-5 months of age. Data points represent individual animals, and bars indicate the mean value. Two cohorts of mice with APL are displayed at the right of each graph. WBC, white blood cell; Hgb, hemoglobin; Plt, platelet. (B) Comparison of spleen weights and the percentage of spleen cells stained for the myeloid surface antigen Gr-1 and the early hematopoietic marker _c_-kit. Data points represent individual animals, and bars indicate the mean value. **, P ≤ 0.001; ***, P < 0.0001.
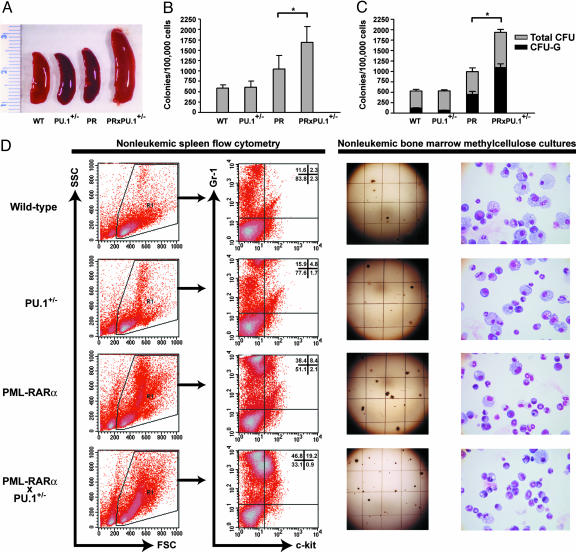
Fig. 2.
Phenotype of nonleukemic mice. (A) Spleens from littermate WT, PU.1 heterozygous (PU.1+/-), PR, and PU.1+/- mice expressing PR (PRxPU.1+/-) (scale in centimeters). (B) Duplicate aliquots of 1.1 × 104 bone marrow cells were plated in MethoCult (StemCell Technologies) containing IL-3, IL-6, and SCF, and total colonies were counted on day 8 (n = 4 for each). (*, P = 0.04.) (C) Two mice from each genotype had 20 colonies picked from separate culture plates. Scores were assigned for the number and type of individual colonies produced, based on inspection of cytospins after May-Grunwald Giemsa staining (CFU-G, granulocytic, n = two for each). (*, P = 0.02.) (D) Flow-cytometric plots, methylcellulose culture plates, and cytospins of pooled colonies from an entire methylcellulose plate. Cells in the R1 gate express the myeloid surface antigen Gr-1 (Gr-1PE) and the early hematopoietic marker _c_-kit (_c_-kitFITC). Numbers represent the percentage of cells in each quadrant. Grid lines on methylcellulose plates are spaced ≈5 mm apart.
Although the bone marrow myeloid differentials were similar for all genotypes (n = 3 mice per genotype, data not shown), the average number of total bone marrow cells harvested from the femurs and tibias of PRxPU.1+/- mice was significantly increased over all other genotypes (n = 7 per genotype, WT = 58.7 × 106 ± 19.2, PU.1+/- = 55.7 × 106 ± 9.3, PR = 68.3 × 106 ± 7.4, PRxPU.1+/- = 88.8 × 106 ± 20.1, P < 0.037 for PRxPU.1+/- mice, compared with all other genotypes). Bone marrow cells were harvested from 16 mice (4 WT, 4 PU.1+/-, 4 PR, and 4 PRxPU.1+/-), counted, and resuspended in 1.3 ml of methylcellulose medium containing IL-6, IL-3, and SCF at 8.3 × 104 cells per ml. Each sample was evaluated separately with duplicate cultures, and all colonies were counted on day 8. Cultures from PRxPU.1+/- mice contained significantly more myeloid bone marrow progenitor colonies than any other genotype (Fig. 2_B_, P ≤ 0.04). Representative culture plate images are shown for each genotype in Fig. 2_D_. Only a portion of each plate is shown, and individual colonies appear as dots. The PRxPU.1+/- culture plate contained the largest number of colonies (Fig. 2_D_). Bone marrow from PR mice also contained significantly more progenitors than that from WT mice, and there was a trend toward more progenitors from PR mice compared with PU.1+/- mice (Fig. 2_B_, P = 0.036 and P = 0.055, respectively). Eight samples (two independent samples from each genotype) had 20 individual colonies picked and scored as granulocytic (CFU-G), macrophage (CFU-M), or granulocytic and macrophage (CFU-GM), after cytospins were stained with May-Grunwald Giemsa. Bone marrow from PRxPU.1+/- mice had more CFU-G progenitors, compared with all other genotypes (Fig. 2_C_, P ≤ 0.02). All colonies from a duplicate culture plate were mixed, and a cytospin was prepared. Representative images from the pooled colonies are shown in Fig. 2_D_. The PRxPU.1+/- sample contained the largest percentage of mature myeloid cells compared with all other genotypes (Fig. 2_D_). These results correlate closely with the number of CFU-G colonies, as shown in Fig. 2_C_.
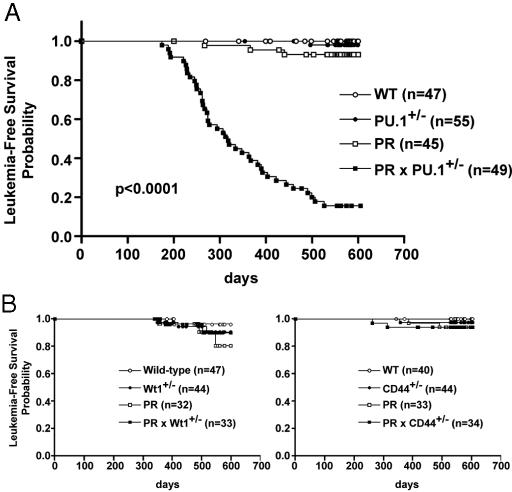
The Penetrance of APL Was Greatly Increased in PRxPU.1+/- Mice. Two independent cohorts were generated by breeding hCG-PR transgenic mice with PU.1+/- mice to obtain approximately equal numbers of WT, PU.1+/-, PR, and PRxPU.1+/- mice. In the first completed intercross, AML developed in 0 of 18 WT animals, 0 of 9 PU.1+/- animals, 0 of 13 PR animals, and 10 of 12 PRxPU.1+/- animals, after 18 months of observation. In the second intercross, AML developed in 0 of 29 WT animals, 1 of 46 PU.1+/- animals, 3 of 32 PR animals, and 31 of 37 PRxPU.1+/- animals, after 18 months of observation. The two independent cohorts were not significantly different from one another. There was a significant increase in the penetrance of APL in PRxPU.1+/- mice vs. PR mice (pooled cohorts, 84.4% vs. 6.9%, respectively, Fig. 3_A_, P < 0.0001), without a change in average latency (328 ± 102 days vs. 358 ± 87 days, respectively, Fig. 3_A_, P = 0.6).
Fig. 3.
Kaplan-Meier analysis for leukemia-free survival. The percentage of surviving mice (y axis) is plotted with respect to time, in days (x axis). The number of animals per group is indicated. (A) This plot represents pooled data from two independent cohorts of mice. The cumulative probability of death resulting from APL is significantly higher in PRxPU.1+/- animals compared with PR animals (P < 0.0001). (B Left) There was no significant increase in the penetrance of APL in PRx_Wt1_+/- (Wilms' tumor homologue) animals vs. PR animals (10% vs. 20% respectively, P = 0.58). (Right) There was no significant increase in the penetrance of APL in PRxCD44+/- animals vs. PR animals (6% vs. 6%, respectively, P = 0.98).
Wilms' tumor homologue (Wt1) and CD44 are two additional genes located on the del(2) interval that have been implicated in cancer and hematopoiesis (22, 23). To test whether heterozygous loss of these genes could contribute to leukemia development, we also bred PR-expressing mice with mice heterozygous for null mutations of either Wt1 or CD44, as described above for PU.1 (24, 25). The incidence of leukemia in PRxWt1+/- and PRxCD44+/- mice was not significantly different from that of PR mice at 18 months of followup for both cohorts (10% vs. 20% and 6% vs. 6%, respectively) (Fig. 3_B_). These results further support the importance of PU.1 as a critical gene for leukemia development on the del(2) interval.
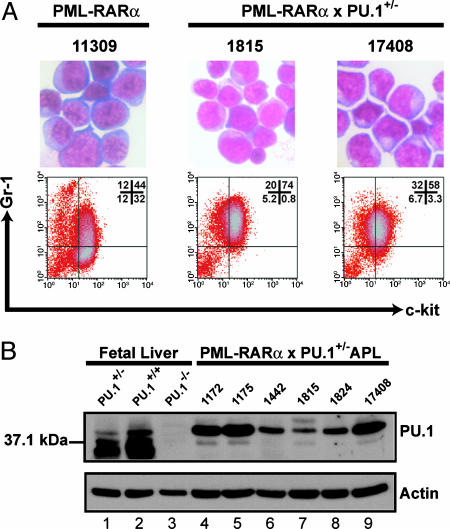
The Phenotype of Leukemic Cells Derived from PRxPU.1+/- Mice Was Similar to That of PR Mice. Peripheral blood from nine PRxPU.1+/- leukemic mice (obtained from both intercrosses) and six PR leukemic animals (previously cryopreserved) was analyzed. The average total white blood cell counts were similarly elevated, and the hemoglobin concentrations and platelet counts were similarly reduced (Fig. 1 A). Spleen weights from both PR and/or PRxPU.1+/- leukemic animals were significantly enlarged, compared with nonleukemic WT, PU.1+/-, PR, and PRxPU.1+/- mice (Fig. 1_B_). Leukemic spleen cells from PR and PRxPU.1+/- mice were uniformly positive for the myeloid differentiation marker Gr-1 (Fig. 1_B_). In addition, six of six PR APL and nine of nine PRxPU.1+/- leukemic spleens contained an abnormal population of Gr-1+ cells that was also _c_-kit+ (Figs. 1_B_ and 4_A_). We i.p. injected 1 × 106 cryopreserved spleen cells from three PRxPU.1+/- leukemic mice into four to five C3HxC57Bl/6 F1 animals for each tumor. PRxPU.1+/- APL spleen cells were fatal in 100% of naïve recipients by 74 days, similar to previously described PR APL spleen cells (data not shown). Cryopreserved leukemic spleen cells from five of five PRxPU.1+/- mice demonstrated in vitro differentiation in response to 1 μM all-trans retinoic acid treatment (data not shown).
Fig. 4.
Characterization of APL cells. (A) Morphology by May-Grunwald Giemsa staining and flow-cytometric analysis for Gr-1 and _c_-kit is shown for one splenic tumor arising in a PR mouse and in two independent PU.1+/- mice expressing PR (PRxPU.1+/-). Numbers represent the percentage of cells in each quadrant. (B) Expression of PU.1 protein in PR × PU.1+/- leukemic spleen cells is shown. Western blots were performed for PU.1 and actin on fetal liver cells and APL cells. Lanes 1-3 contain total extracts from fetal liver cells harvested from Sfpi1/PU.1 heterozygous (PU.1+/-), PU.1 WT (PU.1+/+), or PU.1 homozygous knock-out (_PU.1_-/-) day-15 littermate embryos. Lanes 4-9 contain total extracts from PRxPU.1+/- APL cells. Shown on the left is the position of a 37.1-kDa marker.
The Residual PU.1 Gene Was Expressed in PRxPU.1+/- APL Cells and Did Not Contain Mutations in the Coding Regions. To determine whether the residual PU.1 allele was expressed in PRxPU.1+/- APL cells, we performed Western blotting with PU.1 and actin-specific antibodies. The specificity of the PU.1 antibody was verified by using fetal liver cells harvested from day-15 embryos deficient for this protein (Fig. 4_B_). There is no detectable PU.1 protein in homozygous PU.1 knock-out fetal liver cells (_PU.1_-/-) and a reduced level in heterozygous cells (PU.1+/-) (Fig. 4_B_). PRxPU.1+/- APL samples contain PU.1 protein by Western blotting, although heterogeneity exists in expression levels among these samples (Fig. 4_B_).
Amplicons including the five exons of PU.1 (and the 5′ and 3′ flanking regions) were generated from the genomic DNA of nine PRxPU.1+/- APL samples by using PCR. Each sample was sequenced on both the coding and noncoding DNA strands. A total of 7,178 bases of sequence data was generated for each sample. The PCR and sequencing primers were designed to include ≈100 bp upstream and downstream from each exon, so that all splice junctions could be evaluated for mutations that might reduce expression or change function. No mutation was detected within or near exons 1-4 from both PU.1 alleles or from the residual WT exon 5 PU.1 allele of any PRxPU.1+/- APL sample.
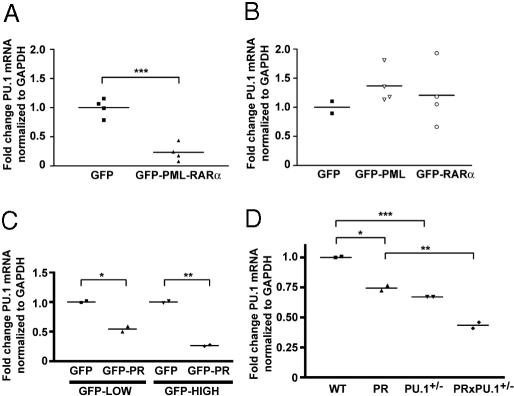
PR Overexpression in U937 Cells Caused Decreased PU.1 mRNA Abundance. Although heterozygous PU.1 mutations are present in a subset of AML patients, no mutations have been identified in the 51 t(15;17) APL samples that have been reported, thus far (4, 19, 26). These data, coupled with the observations reported above, suggested that PR might directly target PU.1 expression or function. To test this idea, we transiently transfected U937 cells (early myelomonocytic) with plasmids containing GFP, a GFP-PR fusion, a GFP-PML fusion, or a GFP-RARα fusion cDNA. PML oncogenic domains are fully disrupted in U937 cells expressing the GFP-PR cDNA, demonstrating that the fusion protein is functional in this system (21). Eight hours after transfection, GFP+ U937 cells were harvested with a high-speed cell sorter, RNA was extracted, and quantitative RT-PCR was performed with primers specific for endogenous PU.1. mRNA abundance was normalized, based on GAPDH mRNA levels. We collected 3-10 × 105 GFP+ cells per transfection, and 17-34% of cells in a stringent “live” gate (based on forward and side scatter) were GFP+ (data not shown). Eight hours after transfection, <1.8% of cells in the live gate were 7-actinomycin-D (7-AAD) positive (data not shown). Four separate transfections were performed with each construct. GFP-PR expression in U937 cells was associated with a 77% decrease (P = 0.0004) in endogenous PU.1 mRNA after 8 h, compared with the GFP vector (Fig. 5_A_); GFP-PML and GFP-RARα did not significantly alter endogenous PU.1 levels (Fig. 5_B_). To determine whether PR levels correlate with PU.1 mRNA abundance, we transfected U937 cells with GFP or GFP-PR plasmids and flow-sorted GFP+ “low” (geometric mean fluorescence: GFP = 39.2 ± 0.38, GFP-PR = 37.2 ± 0.18, gate range = 16.6-77.7) and GFP+ “high” (geometric mean fluorescence: GFP = 1,507.7 ± 49.3, GFP-PR = 1,217.8 ± 22.3, gate range = 523.3-9,646.6) cells 8 h after transfection, isolated RNA, and performed quantitative RT-PCR for PU.1 and GAPDH, as described above. U937 cells expressing low levels of GFP-PR displayed a 46% reduction in PU.1 mRNA abundance, compared with control cells; cells expressing high GFP-PR levels displayed a 74% decrease in PU.1 mRNA levels, compared with controls (n = 2 transfections per condition, P = 0.04 and P = 0.001, respectively) (Fig. 5_C_). The half-life of PU.1 mRNA in U937 cells was 7.3 h (data not shown), similar to the half-life in murine erythroid leukemia cells (27), suggesting that PU.1 transcription was rapidly repressed by a dominant gain-of-function property of PR in this system. This effect was not due to random RNA degradation, because mRNA levels of many genes increased with transient transfection of GFP-PR in this system (W. Yuan and T.J.L., unpublished work). For example, we detected increased abundance of Klf6 (3.68-fold increase) and Pld1 (1.98-fold increase) mRNA, compared with GFP alone (n = 4 transfections per condition, P < 0.0001 and P = 0.029, respectively); GFP-PML and GFP-RARα did not significantly alter endogenous Klf6 and Pld1 levels (data not shown).
Fig. 5.
PR expression alters PU.1 mRNA levels in U937 cells and primary early myeloid bone marrow cells. (A) U937 cells were electroporated with GFP or GFP-PR. Live GFP+ U937 cells were purified by flow sorting 8 h after transfection and quantitative RT-PCR was performed for PU.1 and GAPDH mRNA. PU.1 mRNA fold change was normalized to cells transfected with the GFP plasmid. Each data point represents an independent experiment. (***, P = 0.0004). (B) U937 cells were electroporated with GFP, GFP-PML, or GFP-RAR α. Live GFP+ U937 cells were purified by flow-sorting 8 h after transfection, and quantitative RT-PCR was performed and normalized, as described above. Each data point represents an independent experiment. (C) U937 cells were electroporated with GFP or GFP-PR (GFP-PR). GFP+ low or GFP+ high U937 cells were purified by flow-sorting 8 h after transfection and quantitative RT-PCR was performed and normalized, as described above. Each data point represents an independent experiment. (*, P = 0.04; **, P = 0.001). (D) Total RNA was isolated from bone marrow derived, promyelocyte-enriched, day-2 cultures from WT, PR, PU.1+/-, and PRxPU.1+/- mice, as described in refs. 15 and 21. Quantitative RT-PCR was performed for PU.1 and GAPDH mRNA. PU.1 mRNA fold change was normalized to WT cells. Each data point represents an independent experiment from a pool of mice (n = 3-4 mice per pool), and bars indicate the mean value. (*, P = 0.04; **, P = 0.01; ***, P = 0.008.)
PR Expression in Primary Early Myeloid Bone Marrow Cells Caused Decreased PU.1 mRNA Abundance. To test whether PR expression reduces PU.1 mRNA abundance in primary early myeloid cells, we compared PU.1 mRNA levels in enriched promyelocytes derived from young nonleukemic mice from all four genotypes. We have previously shown that enriched early hematopoietic progenitors undergo a synchronized “wave” of myeloid differentiation under the influence of granulocyte-colony-stimulating factor (G-CSF) and SCF (see Materials and Methods). On day 0 of G-CSF/SCF treatment, cells are predominantly myeloblasts, whereas, on day 2, cells are predominantly promyelocytes (15, 21). Using progenitors from PR mice, we previously showed that PR is significantly activated between days 0 and 2 (21). We therefore harvested the day-2 promyelocyte-enriched cells from WT, PR, PU.1+/-, and PRxPU.1+/- mice and performed quantitative RT-PCR for GAPDH, PU.1, and PR mRNA. PR mRNA levels were similar in PR and PRxPU.1+/- day-2 cells (data not shown). PU.1 mRNA abundance was reduced 33% in PU.1+/- day-2 cells (P = 0.008), 26% in PR-expressing day-2 cells (P = 0.04), and 57% in PRxPU.1+/- day-2 cells (P = 0.02) (Fig. 5_D_).
Discussion
In this article, we describe the consequences of reduced PU.1 expression in transgenic mice expressing a PR fusion cDNA under the control of an early myeloid-specific promoter. In PU.1+/- mice expressing PR, early myeloid progenitors expand in number, leading to early splenomegaly caused by the accumulation of abnormal young myeloid cells. The penetrance of APL is dramatically increased in these mice, but the phenotype of the disease is the same. The long latent period persists, suggesting that additional genetic events are required for leukemia progression. Our data show that PR expression causes the down-regulation of PU.1, an effect that may cooperate with the loss of one copy of PU.1 to facilitate APL development. Our results strongly suggest that PU.1 is relevant for myeloid leukemia syndromes that develop in association with interstitial deletions of mouse chromosome 2.
Even though PU.1 deficiency causes a severe myeloid differentiation block, no haploinsufficient phenotype had been detected until Simon and colleagues (3) carefully examined PU.1+/- mice in the setting of myeloid stress. The subtle shift in myelomonocytic progenitor fate that is caused by PU.1 haploinsufficiency is also easily detected in mice that have a strong myeloid proliferation “hit” caused by the expression of PR in early myeloid cells. Although PU.1+/- mice have no detectable alterations in myeloid progenitor frequency, a dramatic increase in the number of bone marrow CFU-G is detected at an early age in PRxPU.1+/- mice, which leads to the accumulation of abnormal young myeloid cells in the spleen. The precise molecular mechanisms that lead to the expansion of the myeloid progenitor pool are not yet clear, but the consequence is a much larger population of cells that may be uniquely susceptible to the transforming properties of PR (28). The long latent period associated with APL development in hCG-PR mice is not altered in PU.1+/- mice, supporting the hypothesis that additional genetic hits are required for leukemia progression. Together, these data suggest that a major effect of the reduction in PU.1 expression in hCG-PR mice is to expand the early myeloid progenitor pool and increase the likelihood that APL will develop in these mice because of an expanded number of transformable cells.
Several mouse models of AML have now been shown to contain leukemic cells that acquire an interstitial deletion of chromosome 2 that appears to invariably include the PU.1 gene. Interstitial deletions of chromosome 2 have been described in AML syndromes induced by x-irradiation (11), benzene (29), expression of PR under the control of the MRP-8 promoter (30-32), overexpression of Hox genes in bone marrow progenitors (33), the coexpression of hCG-PR and hCG-RARα-PML (RP) (PRxRP) (16), and sublethal irradiation (XRT) of hCG-PR mice (PR+XRT) (15). Our data suggest that the increased APL penetrance observed in our PRxRP and PR+XRT models is predominantly mediated by the acquisition of del(2), resulting in the deletion of one copy of PU.1; the heterozygous PU.1 loss-of-function mutation (PU.1+/-) recapitulates the highly penetrant APL associated with the facilitators of del(2) formation (RP expression and irradiation).
As noted above, PU.1 haploinsufficiency per se does not lead to the development of AML. Therefore, in all of these model systems, AML development appears to require additional genetic hits. In two recent studies, it has become clear that one of those additional hits can actually occur in the residual PU.1 allele, strongly suggesting that PU.1 has tumor-suppressor-like properties in early myeloid cells. In the first of these studies, Tenen and colleagues (5) showed that an 80% reduction in PU.1 expression was associated with the development of AML. More recently, Adams and Silver and colleagues (6, 35) have shown that the residual PU.1 allele is mutated in most AML tumors arising in irradiated mice. Together, these data prompted us to examine the residual PU.1 allele in our PRxPU.1+/- mice to determine whether that allele was expressed or whether it contained mutations that could alter its expression or function.
We measured PU.1 protein levels in nine APL samples that developed in PRxPU.1+/- mice and found that the residual PU.1 allele was expressed in all nine. The expression levels were variable, probably because of the heterogeneity of APL tumors arising in distinct animals. We have previously shown that APL tumors arising in different PR mice exhibit considerable cellular heterogeneity and differences in maturity (14); this heterogeneity probably reflects the tumor-specific genetic events that contribute to progression. Because all of the residual PU.1 alleles were expressed, we resequenced all five exons (and their boundaries) and the flanking regions of the PU.1 gene from DNA derived from these tumors. No acquired mutations were found. Importantly, none of the exon 5 mutations described in refs. 6 and 35 were present in these nine tumors. Because most of the residual PU.1 alleles were mutated in AML tumors arising in irradiated mice, it is highly unlikely that this mutational mechanism is operating to reduce PU.1 function in our transgenic model of APL. For this reason, we decided to determine whether PR (which can act as a transcriptional repressor) might down-regulate expression of PU.1, providing the further reduction in PU.1 function that appears to be required for leukemia progression.
We therefore developed a system that allowed us to directly examine PU.1 expression in a pure population of cells expressing a GFP-PR fusion cDNA that retains all known functions of PR (21). Using this approach, we were able to show that overexpression of PR in U937 myelomonocytic cells results in a rapid, dose-dependent reduction in endogenous PU.1 mRNA levels, suggesting that PR may directly (or indirectly) repress PU.1 transcription; additional experiments will be required to fully define the mechanism. Similarly, Koeffler and colleagues (34) have shown that PU.1 is expressed 8.7-fold less abundantly in induced PR9 cells (U937 cells stably transfected with a zinc-inducible PR) than in parental U937 cells. Importantly, the expression of PR in this system is not associated with toxicity, because these cells do not express neutrophil elastase (21).
We also showed that the expression of PR in primary early myeloid cells reduces endogenous PU.1 mRNA levels and that levels are further reduced in PRxPU.1+/- cells. Collectively, these data show that PR can down-regulate PU.1 expression, which may create the second PU.1 hit in cells that are already missing one copy of this gene. These data strongly suggest that PU.1 acts as a unique kind of tumor suppressor, because a complete loss of its function results in a collapse of myeloid development, but a loss >50% is associated with leukemia progression.
Because PU.1 is sometimes directly mutated in human AML samples (4) and because it is down-regulated by PR expression, it may well be an important target gene that is relevant for some human AML syndromes, including APL. In addition, PU.1 is targeted by a different mechanism by the fusion protein AML-ETO, which binds to PU.1 and reduces its function (17). Collectively, these data strongly suggest that PU.1 is relevant for both mouse and human AML progression and that restoration of its function could be considered as a potential therapeutic strategy in selected AML syndromes.
Supplementary Material
Supporting Text
Acknowledgments
We thank Harinder Singh (University of Chicago, Chicago) for the kind gift of the PU.1+/- mice; Jordan Kreidberg (Children's Hospital, Boston) for the Wt1+/- mice; Mieke Hoock for excellent colony management; the Siteman Cancer Center's High-Speed Cell Sorter Core for flow-sorting studies; Ginger Fewell, Cami Jeliti, and Henry F. Bauer for assistance with sequencing PU.1; and Nancy Reidelberger for expert editorial assistance. This work was supported by National Institutes of Health Grants CA83962 and CA101937, the Bakewell Cancer Research Fund, and the Buder Charitable Foundation (T.J.L.), and a Fellowship grant from the Leukemia Research Foundation (to M.J.W.).
Author contributions: M.J.W. and T.J.L. designed research; M.J.W., J.S.P., R.E.R., S.K.L., M.M., and S.J. performed research; M.J.W., M.M., R.K.W., E.R.M., and T.J.L. analyzed data; and M.J.W. and T.J.L. wrote the paper.
Abbreviations: AML, acute myeloid leukemia; APL, acute promyelocytic leukemia; CFU, colony-forming unit; PR, PML-RARα; SCF, stem cell factor.
References
- 1.Scott, E. W., Simon, M. C., Anastasi, J. & Singh, H. (1994) Science 265**,** 1573-1577. [DOI] [PubMed] [Google Scholar]
- 2.McKercher, S. R., Torbett, B. E., Anderson, K. L., Henkel, G. W., Vestal, D. J., Baribault, H., Klemsz, M., Feeney, A. J., Wu, G. E., Paige, C. J., et al. (1996) EMBO J. 15**,** 5647-5658. [PMC free article] [PubMed] [Google Scholar]
- 3.Dahl, R., Walsh, J. C., Lancki, D., Laslo, P., Iyer, S. R., Singh, H. & Simon, M. C. (2003) Nat. Immunol. 4**,** 1029-1036. [DOI] [PubMed] [Google Scholar]
- 4.Mueller, B. U., Pabst, T., Osato, M., Asou, N., Johansen, L. M., Minden, M. D., Behre, G., Hiddemann, W., Ito, Y. & Tenen, D. G. (2002) Blood 100**,** 998-1007. [DOI] [PubMed] [Google Scholar]
- 5.Rosenbauer, F., Wagner, K., Kutok, J. L., Iwasaki, H., Le Beau, M. M., Okuno, Y., Akashi, K., Fiering, S. & Tenen, D. G. (2004) Nat. Genet. 36**,** 624-630. [DOI] [PubMed] [Google Scholar]
- 6.Cook, W. D., McCaw, B. J., Herring, C., John, D. L., Foote, S. J., Nutt, S. L. & Adams, J. M. (2004) Blood 104**,** 3437-3444. [DOI] [PubMed] [Google Scholar]
- 7.Hayata, I., Seki, M., Yoshida, K., Hirashima, K., Sado, T., Yamagiwa, J. & Ishihara, T. (1983) Cancer Res. 43**,** 367-373. [PubMed] [Google Scholar]
- 8.Mole, R. H., Papworth, D. G. & Corp, M. J. (1983) Br. J. Cancer 47**,** 285-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trakhtenbrot, L., Krauthgamer, R., Resnitzky, P. & Haran-Ghera, N. (1988) Leukemia 2**,** 545-550. [PubMed] [Google Scholar]
- 10.Haran-Ghera, N., Peled, A., Krautghamer, R. & Resnitzky, P. (1992) Leukemia 6**,** 689-695. [PubMed] [Google Scholar]
- 11.Silver, A., Moody, J., Dunford, R., Clark, D., Ganz, S., Bulman, R., Bouffler, S., Finnon, P., Meijne, E., Huiskamp, R., et al. (1999) Genes Chromosomes Cancer 24**,** 95-104. [PubMed] [Google Scholar]
- 12.Rithidech, K. N., Cronkite, E. P. & Bond, V. P. (1999) Blood Cells Mol. Dis. 25**,** 38-45. [DOI] [PubMed] [Google Scholar]
- 13.Grisolano, J. L., Wesselschmidt, R. L., Pelicci, P. G. & Ley, T. J. (1997) Blood 89**,** 376-387. [PubMed] [Google Scholar]
- 14.Pollock, J. L., Westervelt, P., Kurichety, A. K., Pelicci, P. G., Grisolano, J. L. & Ley, T. J. (1999) Proc. Natl. Acad. Sci. USA 96**,** 15103-15108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walter, M. J., Park, J. S., Lau, S. K., Li, X., Lane, A. A., Nagarajan, R., Shannon, W. D. & Ley, T. J. (2004) Mol. Cell. Biol. 24**,** 10882-10893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zimonjic, D. B., Pollock, J. L., Westervelt, P., Popescu, N. C. & Ley, T. J. (2000) Proc. Natl. Acad. Sci. USA 97**,** 13306-13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vangala, R. K., Heiss-Neumann, M. S., Rangatia, J. S., Singh, S. M., Schoch, C., Tenen, D. G., Hiddemann, W. & Behre, G. (2003) Blood 101**,** 270-277. [DOI] [PubMed] [Google Scholar]
- 18.Grisolano, J. L., Sclar, G. M. & Ley, T. J. (1994) Proc. Natl. Acad. Sci. USA 91**,** 8989-8993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ley, T. J., Minx, P. J., Walter, M. J., Ries, R. E., Sun, H., McLellan, M., DiPersio, J. F., Link, D. C., Tomasson, M. H., Graubert, T. A., et al. (2003) Proc. Natl. Acad. Sci. USA 100**,** 14275-14280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lane, A. A. & Ley, T. J. (2003) Cell 115**,** 305-318. [DOI] [PubMed] [Google Scholar]
- 21.Lane, A. A. & Ley, T. J. (2005) Mol. Cell. Biol. 25**,** 23-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alberta, J. A., Springett, G. M., Rayburn, H., Natoli, T. A., Loring, J., Kreidberg, J. A. & Housman, D. (2003) Blood 101**,** 2570-2574. [DOI] [PubMed] [Google Scholar]
- 23.Schmits, R., Filmus, J., Gerwin, N., Senaldi, G., Kiefer, F., Kundig, T., Wakeham, A., Shahinian, A., Catzavelos, C., Rak, J., et al. (1997) Blood 90**,** 2217-2233. [PubMed] [Google Scholar]
- 24.Kreidberg, J. A., Sariola, H., Loring, J. M., Maeda, M., Pelletier, J., Housman, D. & Jaenisch, R. (1993) Cell 74**,** 679-691. [DOI] [PubMed] [Google Scholar]
- 25.Protin, U., Schweighoffer, T., Jochum, W. & Hilberg, F. (1999) J. Immunol. 163**,** 4917-4923. [PubMed] [Google Scholar]
- 26.Vegesna, V., Takeuchi, S., Hofmann, W. K., Ikezoe, T., Tavor, S., Krug, U., Fermin, A. C., Heaney, A., Miller, C. W. & Koeffler, H. P. (2002) Leuk. Res. 26**,** 451-457. [DOI] [PubMed] [Google Scholar]
- 27.Hensold, J. O., Stratton, C. A., Barth, D. & Galson, D. L. (1996) J. Biol. Chem. 271**,** 3385-3391. [DOI] [PubMed] [Google Scholar]
- 28.Westervelt, P. & Ley, T. J. (1999) Blood 93**,** 2143-2148. [PubMed] [Google Scholar]
- 29.Rithidech, K., Dunn, J. J., Bond, V. P., Gordon, C. R. & Cronkite, E. P. (1999) Mutat. Res. 428**,** 33-39. [DOI] [PubMed] [Google Scholar]
- 30.Kogan, S. C., Brown, D. E., Shultz, D. B., Truong, B. T., Lallemand-Breitenbach, V., Guillemin, M. C., Lagasse, E., Weissman, I. L. & Bishop, J. M. (2001) J. Exp. Med. 193**,** 531-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Le Beau, M. M., Bitts, S., Davis, E. M. & Kogan, S. C. (2002) Blood 99**,** 2985-2991. [DOI] [PubMed] [Google Scholar]
- 32.Le Beau, M. M., Davis, E. M., Patel, B., Phan, V. T., Sohal, J. & Kogan, S. C. (2003) Blood 102**,** 1072-1074. [DOI] [PubMed] [Google Scholar]
- 33.Fischbach, N. A., Rozenfeld, S., Shen, W., Fong, S., Chrobak, D., Ginzinger, D., Kogan, S. C., Radhakrishnan, A., Le Beau, M. M., Largman, C., et al. (2005) Blood 105**,** 1456-1466. [DOI] [PubMed] [Google Scholar]
- 34.Park, D. J., Vuong, P. T., de Vos, S., Douer, D. & Koeffler, H. P. (2003) Blood 102**,** 3727-3736. [DOI] [PubMed] [Google Scholar]
- 35.Suraweera, N., Meijne, E., Moody, J., Carvahal-Carmona, L. G., Yoshida, K., Pollard, P., Fitzgibbon, J., Riches, A., vanLaar, T., Huiskamp, R., et al. (2005) Oncogene 19**,** 3678-3683. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Text