ATPase/Helicase Activities of p68 RNA Helicase Are Required for Pre-mRNA Splicing but Not for Assembly of the Spliceosome (original) (raw)
Abstract
We have previously demonstrated that p68 RNA helicase, as an essential human splicing factor, acts at the U1 snRNA and 5′ splice site (5′ss) duplex in the pre-mRNA splicing process. To further analyze the function of p68 in the spliceosome, we generated two p68 mutants (motif V, RGLD to LGLD, and motif VI, HRIGR to HLIGR). ATPase and RNA unwinding assays demonstrated that the mutations abolished the RNA-dependent ATPase activity and RNA unwinding activity. The function of p68 in the spliceosome was abolished by the mutations, and the mutations also inhibited the dissociation of U1 from the 5′ss, while the mutants still interacted with the U1-5′ss duplex. Interestingly, the nonactive p68 mutants did not prevent the transition from prespliceosome to the spliceosome. The data suggested that p68 RNA helicase might actively unwind the U1-5′ss duplex. The protein might also play a role in the U4.U6/U5 addition, which did not require the ATPase and RNA unwinding activities of p68. In addition, we present evidence here to demonstrate the functional role of p68 RNA helicase in the pre-mRNA splicing process in vivo. Our experiments also showed that p68 interacted with unspliced but not spliced mRNA in vivo.
mRNA precursors (pre-mRNA) are spliced in a large RNA-protein complex, the spliceosome (12, 30, 42). Spliceosome assembly requires precise recognition of each of the splice sites, namely, the 5′ splice site (5′ss), branch point, and 3′ splice site. Assembly of a functional spliceosome proceeds through an ordered addition of four small nuclear ribonucleoprotein particles (snRNPs) (U1, U2, U4/U6, and U5) as well as many non-snRNP proteins. This pathway leads to the formation of several intermediate spliceosome complexes. Recognition of the 5′ss by the U1 snRNP, along with binding of the polypyrimidine tract and the branch point by U2AF65 and SF1, results in the formation of the commitment complex. Recruitment of the U2 snRNP to the commitment complex leads to the formation of complex A, or the prespliceosome. At this point, the preformed U4.U6/U5 tri-snRNPs will join into the prespliceosome, leading to the formation of the spliceosome (1, 14, 29). After an extensive rearrangement, a two-step chemical reaction is catalyzed by the spliceosome to remove the intron from the pre-mRNA.
Pre-mRNA splicing is remarkably accurate. The splicing accuracy is achieved by inspection of the individual splice site multiple times by multiple factors (33). Recognition of the 5′ splice site is an early event in the pre-mRNA splicing process. The 5′ss is recognized by 5- to 7-base-pair interactions between the 5′ss and 5′ end of the U1 snRNA (44). Recent studies suggest that prior to the recognition of the 5′ss by the U1-5′ss RNA-RNA base pair interactions the 5′ss is recognized by a protein factor, the U1 snRNP U1C. It is believed that binding of U1C to the 5′ss helps the base pair interactions between U1 and the 5′ss (3, 48). The U1-5′ss duplex is unwound to expose the same 5′ss sequence for pairing with the U6 snRNA prior to the first-step chemical reaction of splicing (29). However, before the U1-5′ss unwinding, the U4.U6/U5 tri-snRNP must be added to the prespliceosome (20). Presumably, addition of the tri-snRNP, unwinding of the U1-5′ss duplex, and formation of the U6-5′ss duplex must be tightly coupled.
The multiple-step procedure of recognition of a splice site in the spliceosome involves the formation and remodeling of a number of RNA-RNA and RNA-protein interactions (20, 43, 44). It is generally believed that remodeling of the complex RNA-RNA and RNA-protein interactions in the spliceosome is catalyzed by a family of DEAD/DExH box putative RNA helicases (39, 47). The RNA helicases unwind RNA-RNA base pairing (44) and RNA-protein interactions (43, 44) at the expense of the energy derived from ATP hydrolysis. To date, eight yeast splicing factors and six mammalian proteins that are homologues to the superfamily of RNA helicases have been implicated in pre-mRNA splicing (10, 28, 39, 47). Many of these proteins have demonstrated RNA unwinding activities in vitro (22, 36, 41, 45). These putative RNA helicases are involved in every step of the pre-mRNA splicing process, including unwinding the U1-5′ss duplex, unwinding the U4/U6 RNA helixes, dissociation of the protein-RNA interaction at the branch point to promote the U2-branch point interactions, and dissociation of the spliced mRNA from the spliceosome.
The nuclear p68 RNA helicase was first identified by cross-reaction with a monoclonal antibody, PAb204, that was originally raised against simian virus 40 large T antigen two decades ago (4, 23). The protein is a prototypical member of the DEAD box family of RNA helicases. As an early example of a cellular RNA helicase, the ATPase and the RNA unwinding activities of p68 RNA helicase were documented with the protein that was purified from human 293 cells (6, 13, 17). It has been suggested that p68 RNA helicase might be involved in transcription regulation (5, 7, 38, 46) and DNA damage repair pathways (18). Most recently, the experiments carried out in our laboratory demonstrated that p68 RNA helicase is an essential human splicing factor in vitro that plays a role in unwinding the transient U1-5′ splice site duplex (25, 26). Consistently, by large-scale proteomic analyses of the human spliceosome, other research laboratories also suggested the existence of p68 RNA helicase in the human spliceosome (9, 11, 15, 19, 37, 52).
In this report, we used two p68 mutants that lack ATPase and RNA unwinding activities. The in vitro splicing assays show that the function of p68 in the spliceosome is abolished by the mutations. Our experiments also show that the mutations inhibit the dissociation of U1 from the 5′ss, while the mutants still interact with the U1-5′ss duplex. Our results strongly suggest that p68 RNA helicase unwinds the transient U1-5′ss duplex during the spliceosome assembly process. Interestingly, the nonactive p68 mutants do not prevent the transition from prespliceosome to the spliceosome. The mutants are also successfully assembled into both the prespliceosome and the spliceosome. Given our previous observation that depletion of p68 RNA helicase from HeLa nuclear extracts inhibited the prespliceosome-to-spliceosome transition, our data indicated that p68 RNA helicase may play a role in the U4.U6/U5 addition, which does not require the ATPase and RNA unwinding activities of p68. Although previous data demonstrated the functional role of p68 in the in vitro pre-mRNA splicing process, whether p68 also plays a role in the pre-mRNA splicing process in vivo remains a question. We examined the pre-mRNA splicing efficiency in HeLa cells where p68 RNA helicase was knocked down by RNA interference (RNAi). Our data demonstrated that p68 RNA helicase plays a role in pre-mRNA splicing in vivo.
MATERIALS AND METHODS
Expression of recombinant p68 RNA helicase and mutants.
Expression and purification of recombinant p68 were carried out by the same experimental procedures as previously described (16).
Cell culture and RNA interference.
HeLa S3 and HT-29 cells were purchased from the American Type Culture Collection and were grown according to the vendor's instructions. All cell culture medium contained penicillin (100 U/ml) and streptomycin (100 μg/ml). All DNA or RNA oligonucleotide transfections were performed using Lipofectamine 2000 by following the manufacturer's instructions (Invitrogen). For the small interfering RNA (siRNA) experiments, cells were grown to 50% confluence and transfected with siRNA (100 pmol). The duplex RNA oligonucleotides for targeting p68 RNA helicase were purchased from Dharmacon siGENOME and SMARTpool. For transient expression of wild-type or mutant p68 in p68 knockdown cells, four nucleotides in the siRNA target sequence were mutated to avoid RNAi targeting. The mutations did not change the amino acid sequence. The cells were transfected with the indicated plasmid DNA 24 h after the cells were transfected with duplex siRNA and harvested after an additional 48-h incubation. The cell extracts were prepared immediately after harvest.
Luciferase reporter assays.
HT-29 cells were appropriately treated (indicated in figures). The cells were transfected with 0.5 μg of reporter plasmid pTN23 (31) and 0.5 μg of pHM6-p68 wild type (wt)/mutant. The total amount of plasmid DNA was adjusted with pHM6-blank vector. Firefly luciferase and β-galactosidase activities present in cellular lysates were assayed using a Dual-light reporter system (Biosystem). Light emission was measured using a Sirius luminometer (Berthold Technologies). Data were represented as firefly luciferase activity divided by β-galactosidase activity. The values plotted were the average ± standard error of triplicate samples from typical experiments.
Dephosphorylation of recombinant p68 RNA helicase.
Dephosphorylation of recombinant p68 RNA helicase was carried out with PP2A and/or protein tyrosine phosphatase 1B (PTP1B) by following the same procedure of our previous report (49).
ATP binding, ATPase, and RNA unwinding assays.
ATP binding by wild-type p68 RNA helicase or its mutants was analyzed by UV-induced photo-cross-linking. Briefly, 2 μl of ∼200 ng of dephosphorylated recombinant p68 RNA helicase or mutants was incubated with 0.5 mM ATP, including 5 μCi [α-32P]ATP, in a total of 10 μl at 30°C for 5 min in the ATP binding buffer (50 mM Tris-HCl, pH 7.5, 50 mM NaCl, 0.5 mM dithiothreitol, 0.5 mM EDTA). The protein-ATP mixtures were then brought under a UV cross-linker. The samples were placed on ice about 4 cm below the light source, which is five 8-W UV light bulbs with a λmax of 254 nm. The samples were irradiated for 25 min. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) loading dye (5 μl) was added to the samples. The samples were subsequently analyzed by 12% SDS-PAGE followed by autoradiography.
ATPase activities were determined by measuring the released inorganic phosphate during ATP hydrolysis using a direct colorimetric assay (2, 16, 35). The same experimental procedure described in our previous report was followed (16). The concentrations of inorganic phosphate were determined by matching the _A_630 in a standard curve of _A_630 versus known phosphate concentrations. The ATPase activity was expressed as μM of inorganic phosphate per mg of p68.
RNA unwinding activities were determined by a method similar to that described in our previous report (16). Briefly, the RNA unwinding reactions were carried out in 20-μl reaction volumes containing 25 fmol of partial double-stranded RNA (dsRNA) and 2 μl of dephosphorylated helicase or mutants (∼200 ng). The unwinding reactions were incubated at 37°C for 60 min. The unwinding reaction mixtures were directly loaded onto 12% SDS-PAGE, and the gel was subjected to autoradiography.
In vitro pre-mRNA splicing and spliceosome complex assembly.
Splicing reactions were carried out with pPIP10A in 40% HeLa nuclear extracts. The dephosphorylated His-tagged p68 RNA helicase or mutants were added to 40% nuclear extracts to a final concentration of ∼15 ng/μl. The mixture was incubated at 30°C for 15 min under normal in vitro splicing conditions. About 25 fmol of pre-mRNA pPIP10A was then added to 10 μl of preincubated extracts, and the splicing reaction was incubated at 30°C for appropriate times. The splicing products were analyzed by 12% urea-PAGE.
For assembly of the spliceosome complexes, splicing reactions were performed with pPIP10A in 20 to 25% intact HeLa nuclear extracts or the reconstituted nuclear extracts (described in the above paragraph). After incubation for 30 min, heparin was added to the reaction mixture to a final concentration of 0.5 mg/ml. The reactions were incubated at 30°C for an additional 5 min. The reaction products were analyzed by 4% (80:1 acrylamide:bis-acrylamide) native PAGE. The gel was either subjected to autoradiography or transferred to a membrane for Western blotting.
Trioxsalen cross-linking and methylene blue cross-linking.
The experimental procedure for trioxsalen cross-linking was similar to our previous report (25).
Methylene blue cross-linking experiments were carried out as described in our previous report (27). The cross-linking reactions were subjected to RNasemix digest. The cross-linked p68 was analyzed by 10% SDS-PAGE and subjected to autoradiography. The recombinant His-p68 wt/mutant was detected by immunoblotting with anti-His antibody.
RNA immunoprecipitations and RT-PCR.
RNA immunoprecipitation (RNA-IP) was performed using essentially the same procedure as that described by Gilbert and colleagues (8). Briefly, 48 h posttransfection (indicated), HT-29 cells were fixed in 1% formaldehyde for 30 min at room temperature. The cells were lysed in chromatin immunoprecipitation lysis buffer (Active motif) containing RNase inhibitor (50 U/500 μl). The cell nuclei were released by homogenization. The collected nuclei were further lysed by FA buffer (50 mM HEPES-KOH [pH 7.5], 140 mM NaCl, 1 mM EDTA, 1% Triton X-100, 0.1% sodium deoxycholate, protease inhibitors) containing RNase inhibitor. The resultant samples were treated by extensive sonication followed by treatment with RNase-free DNase I at 37°C for 30 min. Immunoprecipitation using antihemagglutinin (anti-HA) antibody (Upstate) was carried out with the samples. The immunoprecipitates were adjusted to 200 mM NaCl and incubated at 65°C overnight. The samples were further treated with 20 μg proteinase K and phenol-chloroform extraction followed by ethanol precipitation at −80°C. Reverse transcription-PCR (RT-PCR) (Titan kit, Roche) was performed using 1/100 RNA of precipitates or 1/2,000 RNA of input. RT-PCR results were analyzed on a 2% agarose gel.
RT-PCRs were performed with 1 μg of total RNA using a one-step RT-PCR kit (Titan kit, Roche) by following the manufacturer's instruction. RT-PCR results were analyzed on a 2% agarose gel. The primers for all RT-PCRs or PCRs are listed in Table 1.
TABLE 1.
RT-PCR primers used in this study
| Name | Sequence | Location |
|---|---|---|
| ActinF | CACGGCCGAGCGGGAAAT | Exon 4 |
| Actin4R | CGGGAGACAGTCTCCAC | Intron 4 |
| Actin5R | TGCATCCTGTCGGCAATGC | Exon 5 |
| GAPDHF | TGTTCCAATATGATTCCACCC | Exon 4 |
| GAPDH4R | AAGGGAGCCACACCATCCT | Intron 4 |
| GAPDH5R | CTTCTCCATGGTGGTGAAGA | Exon 5 |
| HistoneF | CCAGTGTACCTGGCGGCA | |
| HistoneR | GTACTCCTGGGAGGCCTG | |
| CEBPF | AGCACCACGACTTCCTCTC | |
| CEBPR | GGGTGCAGGGGCGCGAA |
RESULTS
p68 mutants lack ATPase and RNA unwinding activities.
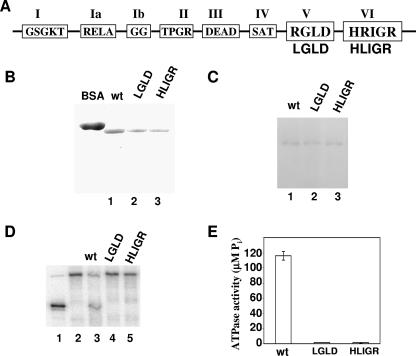
p68 RNA helicase is an essential human splicing factor in HeLa nuclear extracts (25). The protein was detected interacting with the transient U1-5′ splice site duplex (26). It is thus suggested that p68 RNA helicase functions to unwind the RNA duplex during the spliceosome assembly process. If p68 actively unwinds the U1-5′ss duplex, we reasoned that ATPase and RNA unwinding activities must be essential for the function of the protein in the spliceosome. To test this conjecture, two p68 RNA helicase mutants were generated (Fig. 1A). The first mutant carried a mutation at the consensus sequence motif V, RGLD, in the helicase-core region. The first R was changed to an L (referred to as LGLD). The second mutant carried a mutation at the conserved sequence motif VI, HRIGRXXR. The second R was changed to an L (referred to as HLIGR). Wild-type p68 and two mutants were expressed in Escherichia coli bacteria. Three recombinant proteins were purified by a two-column procedure (see Materials and Methods) (Fig. 1B). ATP cross-linking, ATPase, and RNA unwinding assays were carried out to characterize the expressed wild type and p68 mutants. Since the bacterially expressed recombinant p68 and mutants were phosphorylated at serine/threonine and tyrosine residues (50), the proteins were dephosphorylated by PP2A and PTP1B prior to the ATP cross-linking, ATPase, and RNA unwinding analyses. Western blotting with antibodies against the specific phosphoamino acids phosphoserine, phosphothreonine, and phosphotyrosine showed that dephosphorylation of p68 was complete to an undetectable level (data not shown). ATP cross-linking with dephosphorylated wild-type and mutant p68 showed that ATP binding was not affected by the mutations (Fig. 1C). ATPase assays demonstrated that these two mutations completely abolished the ATPase activity of p68 RNA helicase (Fig. 1E). The RNA unwinding activity of wild-type p68 and mutants was examined with a partial dsRNA containing a short RNA duplex (∼22 bp in length) and long 186-nt and 88-nt 3′ overhangs on both sides (16). The experiments indicated that wild-type p68 unwound the dsRNA. However, the two mutants were unable to unwind the dsRNA (Fig. 1D).
FIG. 1.
(A) Schematic illustration of the sequence motifs in the helicase core of DEAD box RNA helicase and the p68 LGLD and HLIGR mutants. (B) Coomassie blue staining of SDS-PAGE of recombinant p68 wt (lane 1), LGLD mutant (lane 2), or HILGR mutant (lane 3) that were expressed and purified from bacterial E. coli. (C) Cross-links of p68 wt (lane 1), LGLD mutant (lane 2), or HILGR mutant (lane 3) to [α-32P]ATP were analyzed by SDS-PAGE followed by autoradiography. (D) RNA unwinding by p68 wt (lane 3), LGLD mutant (lane 4), or HILGR mutant (lane 5) was analyzed by SDS-PAGE followed by autoradiography. Lane 1 is duplex RNA denatured by heating to 95°C. Lane 2 is the duplex RNA. (E) ATPase activities of p68 wt/mutant (indicated) were measured by colorimetric assay. The ATPase activity (y axis) is expressed as μM Pi/mg of p68 or mutants.
p68 mutants that lack ATPase and helicase activities do not support pre-mRNA splicing.
Next, we examined the effects of the mutations on the function of p68 in the pre-mRNA splicing process. We previously demonstrated that the bacterially expressed recombinant p68 RNA helicase was phosphorylated at serine/threonine and tyrosine residues (50). We observed that only the tyrosyl phosphorylation affected the function of p68 in pre-mRNA splicing (51). To obtain the recombinant p68 and mutants without tyrosine phosphorylations, the bacterially expressed recombinant proteins were dephosphorylated by PTP1B. Dephosphorylation of p68 was complete as indicated by Western blotting with monoclonal antibody PY20 (data not shown). The dephosphorylated proteins were separated from the added protein phosphatase by Ni-nitrilotriacetic acid micro column. After elution and microdialysis, the dephosphorylated proteins were added to HeLa nuclear extracts in which p68 was immunodepleted. Splicing activity of the reconstituted HeLa nuclear extracts was examined with splicing substrate pPIP10A. It was evident that the splicing activity of the p68-depleted HeLa nuclear extracts was restored by addition of wild-type recombinant p68 (Fig. 2, lane 3). On the other hand, the splicing activity was not recovered by addition of the two p68 mutants to the HeLa extracts (Fig. 2, lanes 4 and 5). The results indicated that the mutations that abolished the ATPase and RNA unwinding activities of p68 also abolished the function of the protein in the pre-mRNA splicing process.
FIG. 2.
Splicing of transcript pPIP10A in HeLa nuclear extracts in which the endogenous p68 is mock depleted (lane 2) or depleted by antibody against p68 (lanes 3 to 5). The dephosphorylated recombinant p68 (Dp), protein buffer (lane 2), wild type (lane 3), mutant HLIGR (lane 4), or mutant LGLD (lane 5) was added to the p68-depleted extracts. Lane 1 is the pre-mRNA (pPIP10A) without splicing.
p68 mutants that lack ATPase and RNA unwinding activities interact with the U1-5′ss duplex but do not support the dissociation of U1 from the 5′ splice site.
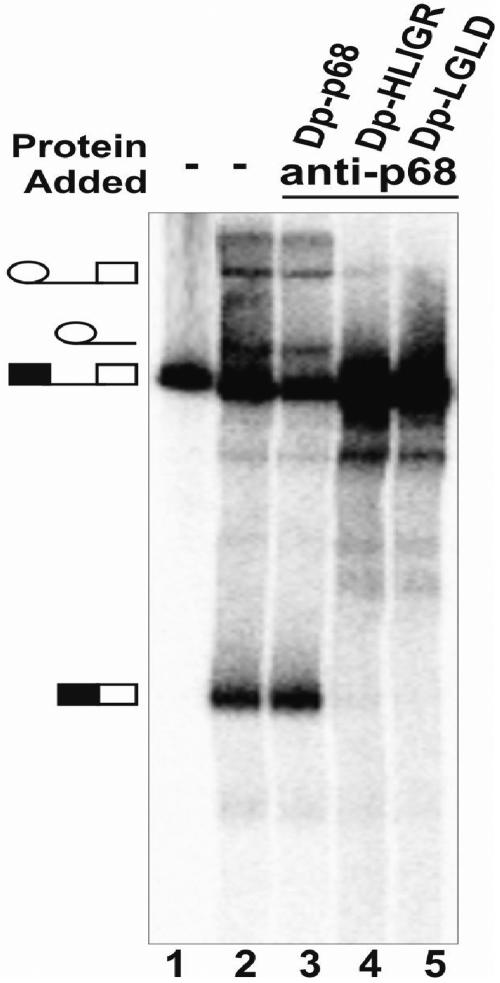
In a previous report, we demonstrated that depletion of p68 RNA helicase from HeLa nuclear extracts inhibited the dissociation of U1 from the 5′ss (25). We reasoned that if p68 RNA helicase is involved in unwinding the U1-5′ss duplex, the ATPase and RNA unwinding activities of the protein must be required for this action. To test this conjecture, we monitored the U1-5′ss RNA-RNA interactions in the p68-depleted HeLa nuclear extracts supplemented with wild-type p68 or the two mutants. The trioxsalen cross-linking experiment similar to that described in our previous report was employed to analyze the U1-5′ss interactions (25). The splicing substrate pPIP10A was used for our in vitro splicing. Cross-links of the U1 snRNA to the pre-mRNA occurred in the intact HeLa nuclear extracts at the 15-min time point (Fig. 3, lane 3). The cross-linking signal completely disappeared after 180 min of splicing (Fig. 3, lane 2). When the p68-depleted HeLa nuclear extracts were supplemented with wild-type recombinant p68 RNA helicase, the U1-5′ss cross-link signal was very weak, even at the 5-min time point (Fig. 3, lane 11), and almost completely disappeared after 90 min (Fig. 3, lane 14). In contrast, the pre-mRNA-U1 snRNA cross-links remained almost constant in the same splicing time course in the HeLa nuclear extracts that were supplemented with the p68 mutants (Fig. 3, lanes 6 to 10 and 16 to 20). The data suggested that the dissociation of U1 from the 5′ss was inhibited by the mutations of p68 RNA helicase that abolished the ATPase and RNA unwinding activities of the protein.
FIG. 3.
Trioxsalen cross-linking of the U1 snRNA to pre-mRNA in HeLa nuclear extracts. The U1-pre-mRNA cross-linking band is indicated. The cross-linking reactions were carried out in the intact extracts (lanes 2 to 5), p68-depleted extracts (lanes 6 to 20), and p68-depleted extracts to which wild-type p68 (lanes 11 to 15), mutant HLIGR (lanes 6 to 10), or mutant LGLD (lanes 16 to 20) was added. The splicings were carried out for the indicated times before the trioxsalen was added to the splicing reactions. Lane 4 is cross-linked RNAs that are further treated with RNase H in the presence of DNA oligonucleotide αU1 that is complementary to U1 64-75 (αU164-75). Lane 5 is the cross-linked RNAs that are further treated with RNase H in the presence of random sequence DNA oligonucleotide Act1.
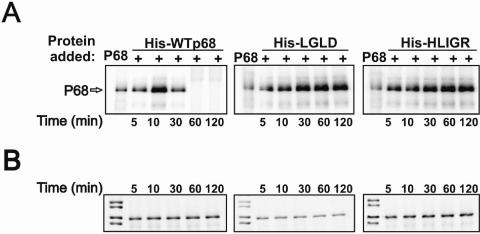
p68 RNA helicase was first detected interacting with the transient U1-5′ss duplex by a methylene blue-mediated RNA-protein cross-linking method (26, 27). The recombinant protein also cross-linked to this short RNA duplex during the spliceosome assembly process (25). We questioned whether these two p68 mutants that did not support splicing would interact with the U1-5′ss duplex during spliceosome assembly. To this end, we employed the same methylene blue (MB) cross-linking method to examine the interaction of the p68 mutants with the U1-5′ss duplex. Since the strongest p68 cross-linking signals were obtained with the splicing substrate GC+DX (a derivative from α-tropomyosin), the splicing substrate GC+DX was used in our experiments. Similar to our previous observations, a cross-linking band that comigrated at about 65 kDa was detected in the p68-depleted HeLa nuclear extracts supplemented with wild-type or mutant p68 (Fig. 4A). The cross-links to the wt p68 reached maximum at about 10 min and decreased thereafter (Fig. 4A, left panel). However, the cross-links to two mutants did not decrease over a 180-min time course (Fig. 4A, middle and right panels). Since the cross-linking band was precipitated by Ni-nitrilotriacetic acid column, this indicated that recombinant p68 cross-linked to the U1-5′ss duplex (data not shown). The identity of this cross-linking band was further verified by immunoprecipitation of this cross-linking band with an antibody against His6 tag (data not shown). To exclude the possibility that the different MB cross-links were due to the different amounts of recombinant p68s (wt/mutant) added to the extracts, we immunoblotted the same MB cross-linking SDS-PAGE using anti-His antibody. The results showed that roughly the same amount of p68 (wt/mutant) was added to the cross-linking extracts (Fig. 4B).
FIG. 4.
(A) MB cross-linking of wild-type (left panel), mutant LGLD (middle panel), or mutant HLIGR (right panel) His-p68 to the radiolabeled transcript pGC+DX in endogenous p68-depleted HeLa nuclear extracts. The splicings are carried out for the indicated times before the methylene blue is added to the splicing reactions. The lane marked p68 is the MB cross-linking carried out in intact HeLa nuclear extracts without addition of recombinant p68. (B) The amount of p68 in each cross-linking reaction was determined by immunoblotting of the same MB cross-link SDS-PAGE with anti-His antibody.
The ATPase and RNA unwinding activities of p68 are not required for assembly of the spliceosome.
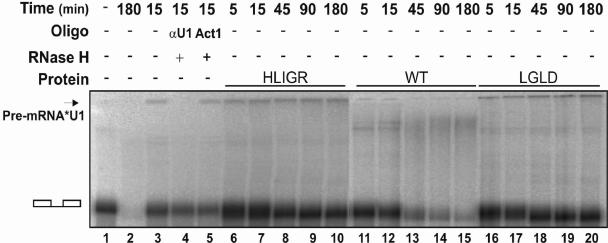
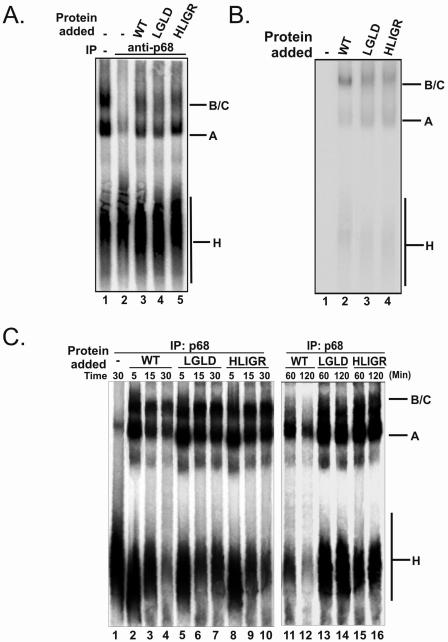
In the spliceosome assembly pathway, unwinding the U1-5′ss duplex must be tightly coupled with the addition of the U4.U6/U5 tri-snRNP. In our previous report, we demonstrated that depletion of p68 RNA helicase from HeLa nuclear extracts blocked the transition from prespliceosome to spliceosome (25). In this study, we endeavored to examine the effects of p68 mutations on spliceosome assembly. We employed native gel electrophoresis to analyze spliceosome complex formation in the p68-depleted HeLa nuclear extracts supplemented with recombinant p68 wt/mutant. The spliceosome complexes were assembled on the splicing substrate pPIP10A. It was evident that the spliceosome complexes A and B/C were assembled normally in intact HeLa nuclear extracts (Fig. 5A, lane 1). Consistent with our previous report, the formation of the B/C complex was inhibited by p68 depletion (Fig. 5A, lane 2). Addition of wt p68 to the p68-depleted extracts restored the spliceosome complex assembly (Fig. 5A, lane 3). Interestingly, assembly of A and B/C complexes was not affected in the presence of p68 mutants that lacked ATPase and RNA unwinding activities (Fig. 5A, lanes 4 and 5). To further elucidate the effects of mutation of p68 on spliceosome assembly, we probed the assembly of the recombinant p68 wt/mutant in the spliceosome complexes by immunoblotting. Since the recombinant proteins carried a His tag, a commercially available monoclonal antibody against His6 was used in the immunoblotting experiments. Our experiments showed that the recombinant wild-type p68 and mutants were assembled into the A and B/C complexes (Fig. 5B, lanes 2, 3, and 4).
FIG. 5.
(A) Electrophoretic separation of spliceosome complexes. Splicing reactions were carried out with splicing substrate pPIP10A in the extracts: untreated (lane 1), p68 was depleted with antibody against p68 (lane 2), p68 was depleted and recombinant wt p68 was added (lane 3), p68 was depleted and recombinant LGLD mutant was added (lane 4), and p68 was depleted and recombinant HLIGR mutant was added (lane 5). All of the splicing reactions were incubated at 30°C for 30 min. (B) Assembly of wt p68 (lane 2), the LGLD mutant (lane 3), or the HLIGR mutant (lane 4) into spliceosome complexes in p68-depleted extracts was detected by immunoblotting of recombinant p68 using antibody against His6 tag. The recombinant p68s are dephosphorylated before adding to the splicing reactions. Lane 1 is the complexes assembled in the extracts without addition of recombinant p68. (C) Spliceosome complex assembly in p68-depleted HeLa nuclear extracts to which wt p68 was added (lanes 2 to 4 and 11 and 12), mutant LGLD was added (lanes 5 to 7 and 13 and 14), or mutant HLIGR was added (lanes 8 to 10 and 15 and 16). The splicing reactions were carried out for the time indicated.
To further analyze the effects of the mutations of p68 on spliceosome complex assembly, we monitored complex assembly in p68-depleted HeLa nuclear extracts. The p68 (wt or mutant) was added to the p68-depleted extracts. The spliceosome complexes were assembled on PIP10A in a time course and analyzed by native PAGE. It was evident that the assembly of the A and B/C complexes was not significantly affected in the presence p68, wt or mutant, in the time course of 30 min (Fig. 5C, lanes 2 to 10), with a slightly faster kinetics in the presence of wild-type p68 than in the presence of mutant p68 (data not shown and Fig. 5C). However, it was clear that there were significant decreases in the H, A, and B/C complexes in the presence of wild-type p68 (Fig. 5C, lanes 11 and 12) compared to that in the presence of mutant at the 120-min time point (Fig. 5C, lanes 13 to 16). A decrease in the H complex but not A and B/C complexes was observed in the presence of mutant (Fig. 5C, lanes 13 to 16). The data again indicated that the mutations of p68 did not affect the assembly of the spliceosome but affected the subsequent steps of the pre-mRNA splicing process.
p68 RNA helicase affects pre-mRNA splicing in vivo.
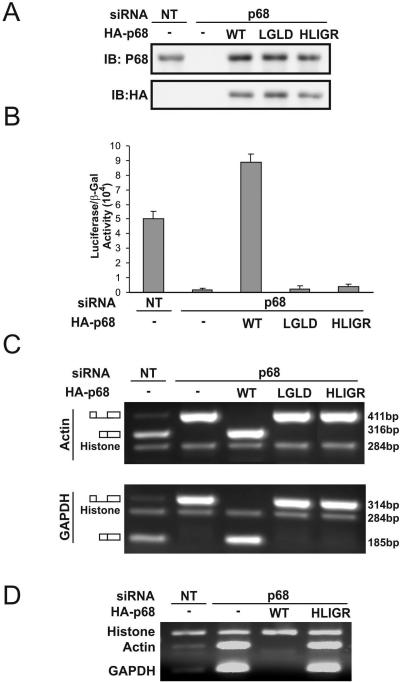
p68 was shown to be an essential splicing factor in HeLa nuclear extracts. To analyze the functional role of p68 RNA helicase in the pre-mRNA splicing process in vivo, we employed the RNAi technique to knock down the endogenous p68 in HT-29 cells, a colon cancer cell line. Immunoblotting demonstrated that the cellular level of p68 was reduced by over 90% by the RNAi knockdown (Fig. 6A). The pre-mRNA splicing activity in p68 knockdown cells was examined. We used a construct developed by Nasim and colleagues (31). This construct used a double reporter system to assay the changes in the ratio of spliced and total mRNA in mammalian cells. It was evident that the ratio of spliced/total mRNA was dramatically reduced in p68 knockdown cells (Fig. 6B). Exogenous expression of wild-type p68 in the p68 knockdown HT-29 cells completely recovered the splicing (Fig. 6B). However, expression of the HLIGR or LGLD mutant did not result in any splicing activity recovery (Fig. 6B).
FIG. 6.
Functional role of p68 in the pre-mRNA splicing process in vivo. (A) Knockdown of p68 by RNA interference and exogenous expression of HA-p68 wt/mutant in HT-29 cells were analyzed by immunoblotting using anti-p68 antibody (upper panel) or anti-HA antibody (bottom panel). NT means the cells were treated with nonspecific siRNA duplex (control). (B) Double reporter assays for the ratio of spliced/total pre-mRNA in HT-29 cells in which p68 was knocked down and wt or mutant p68 (indicated) was expressed. The spliced/total pre-mRNA ratio is expressed as the luciferase activity divided by β-galactosidase activity (31). (C and D) RT-PCR probe of the spliced/unspliced mRNA of β-actin and GAPDH genes in HT-29 cells in which p68 was knocked down and wt or mutant p68 (indicated) was expressed. A pair of primers spanning exon 4 and exon 5 (C) or exon 4 and intron 4 (D) was used in the RT-PCRs.
The effects of p68 RNA helicase on the pre-mRNA splicing process in cells were further examined by probing the spliced or unspliced mRNA of endogenous β-actin and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) genes in HT-29 cells where the p68 RNA helicase was knocked down and p68 wt/mutant was expressed. The spliced or unspliced mRNA was probed by RT-PCR using a primer targeting exon 4 and intron 4 or exon 4 and exon 5 of both genes. To eliminate the effects other than pre-mRNA splicing, we used intronless histone H2A gene as a control to normalize the RT-PCR products under different conditions. It was clear that knockdown of p68 RNA helicase led to the accumulation of large amounts of unspliced mRNA of both β-actin and GAPDH genes (Fig. 6C and D). Under overexposure conditions, a very faint band corresponding to spliced mRNA could be visualized in the p68 knockdown cells (data not shown). There was a larger accumulation of unspliced mRNA in HT-29 cells in which the nonactive p68 mutant LGLD or HLIGR was expressed in p68 knockdown cells (Fig. 6C and D). However, the accumulation of unspliced mRNA disappeared in the p68 knockdown cells in which wild-type p68 was expressed. In fact, expression of wild-type p68 in the p68 knockdown cells promoted the pre-mRNA splicing to some degree (Fig. 6C and D). The result was consistent with the observations of the above double reporter assay.
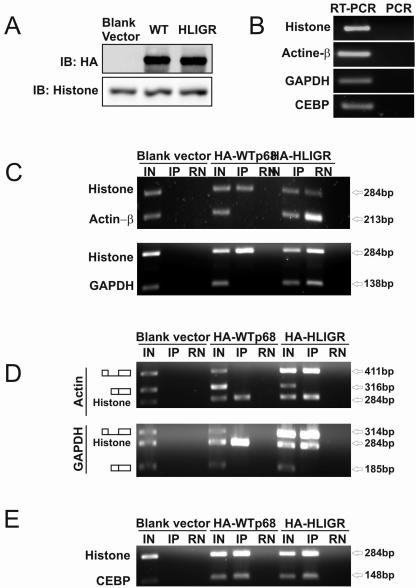
Furthermore, we analyzed the effects of p68 RNA helicase on the pre-mRNA splicing process by RNA-IP (8). The HA-p68 (wild type or HLIGR mutant) was expressed in HT-29 cells (Fig. 7A). The RNAs were precipitated by HA antibody. It was clear that significant amounts of unspliced mRNA (both β-actin and GAPDH) coprecipitated with the nonactive p68 mutant HLIGR, while no detectable unspliced mRNA precipitated with wild-type HA-p68 in both the β-actin and GAPDH cases (Fig. 7C). On the other hand, the p68 (wt or HLIGR) did not interact with spliced mRNA as demonstrated by RT-PCR of RNA-IP using primers crossing exon 4 and exon 5 of both genes (Fig. 7D). The results provided the in vivo evidence that p68 RNA helicase interacted with pre-mRNA in both β-actin and GAPDH cases.
FIG. 7.
RNA-IP of spliced/unspliced β-actin and GAPDH mRNA in HT-29 cells in which wt or mutant HA-p68 (indicated) is expressed. RNAs were precipitated by anti-HA antibody. (A) Expression of HA-p68 wt/mutant was detected by immunoblotting with anti-HA antibody. (C) The immunoprecipitated RNAs were detected by RT-PCR using a pair of primers crossing exon 4 and intron 4 of β-actin (upper panel) or GAPDH (bottom panel) genes. (D) The immunoprecipitated RNAs were detected by RT-PCR using primers crossing both exon 4 and intron 4 or exon 4 and exon 5 (indicated) of β-actin (upper panel) or GAPDH (bottom panel) genes. (E) The immunoprecipitated RNAs were detected by RT-PCR using a pair of primers annealed to histone 2B or CEBP (Table 1) genes. IN indicates input, where the RT-PCRs were performed with total RNA extracts without IP. IP indicates that the RT-PCRs were performed with the RNAs that were coprecipitated with anti-HA antibody. RN indicates the immunoprecipitated mixtures were treated with RNase before performing RT-PCRs. (B) The immunoprecipitated RNAs were detected by RT-PCR or PCR using the same primers as in panel C or D.
Interestingly, we repeatedly observed the coprecipitation of wild-type and mutant p68 with histone mRNA in the RNA-IP experiments (Fig. 7C and D). There was no significant difference in the precipitation of histone mRNA by HA-p68, wild type or mutant. To determine whether the coprecipitation of p68 with histone mRNA was histone mRNA specific, we carried out the RNA-IP experiments with another intronless gene, CEBP, with a pair of RT-PCR primers within the coding region (Table 1). The same experimental procedure was employed. It was clear that the CEBP mRNA was also precipitated with p68 RNA helicase, wt and HLIGR mutant (Fig. 7E).
DISCUSSION
p68 RNA helicase was shown to be an essential splicing factor in vitro that acted at the U1-5′ss duplex (25). In this report, we further demonstrated the functional role of p68 RNA helicase in vivo. We showed that the ATPase activity was required for the function of the protein in the spliceosome. We showed here that the p68 mutants that lacked ATPase and RNA unwinding activities still interacted with the U1-5′ss duplex. Nevertheless, the U1-5′ss duplex was not unwound in the HeLa nuclear extracts supplemented with the p68 mutants. The requirement of the ATPase activity for the function of p68 in the spliceosome and for the dissociation of the U1-5′ss duplex strongly argued that p68 RNA helicase actively unwound the transient RNA duplex in the spliceosome. p68 RNA helicase could directly unwind the U1-5′ss duplex RNA. Alternatively, p68 could also actively act on the protein factor(s) which stabilizes the duplex. Given that p68 itself cross-linked to the RNA duplex and that the mutants also interacted with the RNA, it is most likely that the RNA helicase unwinds the RNA duplex. Interaction of p68 with the U1-5′ss duplex and assembly of p68 to the spliceosome complexes do not require ATPase/helicase activities, indicating that the ATPase/helicase activities of p68 are not required for the interaction of the helicase with the spliceosome machinery. We generated specific p68 mutants that lack dsRNA or single-stranded RNA binding properties (data not shown). It will be interesting to test whether or not these p68 mutants will be assembled to the spliceosome and interact with the U1-5′ss duplex.
During the spliceosome assembly process, the dissociation of U1 from the 5′ splice site is tightly coupled to the addition of the U4.U6/U5 tri-snRNPs to the prespliceosome. In our experiments, assembly of the spliceosome B/C complex was not affected by the p68 mutations that abolished ATPase/helicase activities. Further, the U1-5′ss duplex was not unwound in the spliceosome containing p68 mutants. The experiments demonstrated an excellent example that the addition of the U4.U6/U5 tri-snRNP was uncoupled with the unwinding of the U1-5′ss duplex in the spliceosome assembly process. Uncoupling of these two events by disrupting the biochemical activities of p68 certainly suggested a role of p68 in the communication between addition of the tri-snRNPs to the prespliceosome and the U1-5′ss duplex unwinding. This is consistent with our previous observations that depletion of p68 inhibited the prespliceosome-to-spliceosome transition (25). Taking together all of our experimental observations (25, 26), we propose a hypothetical model for the function of p68 RNA helicase in the pre-mRNA splicing process. p68 actively unwinds the U1-5′ss duplex by direct strand displacement of the RNA duplex or by destabilizing the protein factor(s) that stabilizes the duplex. The protein also plays a role in the addition of the tri-snRNP to the prespliceosome. p68 may fulfill the role by interacting with both the 5′ss and the U4.U6/U5 tri-snRNP. The interaction may be direct or may act through other proteins (21). The ATPase activity of p68 is not required for this interaction. This model is consistent with the observations of other laboratories that p68 RNA helicase is detected in the prespliceosome as well as the matured spliceosome (11, 19, 32). The dual functions of p68 RNA helicase in the human spliceosome are reminiscent of the case of Prp22 in the yeast spliceosome. It was demonstrated that Prp22 plays two distinct roles. The protein plays an important role in the second catalytic step of pre-mRNA splicing. Prp22 is essential for releasing the matured mRNA from the spliceosome (40). Thus, it may be a general phenomenon that some DEAD/DExH box RNA helicases not only function in unwinding the target but also coordinate the events upstream and/or downstream.
The function(s) of p68 RNA helicase in the pre-mRNA splicing process remains an intriguing question. Our previous experiments demonstrated an essential role of the protein in the in vitro pre-mRNA splicing in HeLa nuclear extracts (25, 26). Consistently, other research laboratories have detected p68 RNA helicase in the mammalian spliceosome that is assembled in HeLa nuclear extracts as a constitutive component (11, 19, 32). On the other hand, Guil and colleagues observed that p68 helicase plays a role in regulating c-H-ras alternative splicing (9). All of these experiments suggested a functional role of p68 in the pre-mRNA splicing process. However, these experiments also raised an important question. Is p68 a general human pre-mRNA splicing factor in vivo or does the protein function only in splicing a subset of pre-mRNA? We presented experimental results here to show that p68 RNA helicase functioned in the splicing of two housekeeping genes, β-actin and GAPDH, in HT-29 cells. In addition, the intron that was tested in the double reporter was derived from late transcripts of adenovirus. It would be expected that the efficiency of splicing of these introns should reflect the efficiency of the general pre-mRNA splicing process in cells. Thus, we believe that p68 RNA helicase is a general splicing factor. In supporting our conclusion, we observed that the growth rate of p68 knockdown cells was reduced by over threefold (data not shown). The growth of the cells in which the endogenous p68 was knocked down and the HLIGR/LGLD mutant was exogenously expressed was almost completely inhibited, and the cells were eventually dead after several days (data not shown). It is also possible that, although p68 is an essential splicing factor, its function could be replaced by another RNA helicase in cells when p68 is absent. p72 could be a candidate for this redundant function. It was recently demonstrated that p68 and p72 exist as a heterodimer in cells (34). Furthermore, p72 was shown to associate with the U1 snRNP (24), and the protein plays a role in regulating the splicing of alternative exons containing AC-rich exon enhancer elements (15).
Coprecipitation of p68 RNA helicase (mutant) with pre-mRNA in the RNA-IP experiments demonstrated more evidence for the functional role of the protein in the pre-mRNA splicing process. The experiments also suggested the interaction of p68 with pre-mRNA in vivo, which is consistent with our previous observations with HeLa nuclear extracts (25, 26). Coprecipitation of mRNAs of the intronless genes histone H2A and CEBP with p68 RNA helicase, both wild type and mutant, is an interesting observation. At the current stage, we do not know whether the precipitated RNAs are the mRNA precursor, matured mRNA, or both. It will be interesting to determine any differences in the RNAs precipitated by wild-type or mutant p68. Unlike many mRNA precursors, the intronless histone or CEBP mRNA precursors are not spliced. Therefore, one potential explanation for the observation is that p68 RNA helicase is associated with all mRNA precursors. p68 participates the pre-mRNA splicing. After the splicing, the protein is removed from mRNA with the disassociation of components of the spliceosome. Without the splicing, p68 may “stay” with the transcripts. However, this explanation opens several interesting questions. (i) How is p68 deposited at all mRNA precursors? (ii) Does p68 play a potential role in the processing of the intronless mRNA precursors? (iii) How does p68 eventually dissociate from the intronless mRNAs before the export of the mRNA?
Acknowledgments
We thank Roger Bridgeman for antibody p68-rgg production. We are grateful to I. C. Eperon for providing the vector of double reporter pTN23 for pre-mRNA splicing. The manuscript was greatly improved by critical comments from April Ellis, Christie Carter, and Heena Dey.
This work was supported in part by research grants from the National Institutes of Health (GM063874) and the Georgia Cancer Coalition to Z.-R.L.
REFERENCES
- 1.Abovich, N., and M. Rosbash. 1997. Cross-intron bridging interactions in the yeast commitment complex are conserved in mammals. Cell 89**:**403-412. [DOI] [PubMed] [Google Scholar]
- 2.Chan, K.-M., D. Delfert, and K. D. Junger. 1986. A direct colorimetric assay for Ca2+-stimulated ATPase activity. Anal. Biochem. 157**:**375-380. [DOI] [PubMed] [Google Scholar]
- 3.Chen, J. Y., L. Stands, J. P. Staley, R. R. Jackups, Jr., L. J. Latus, and T. H. Chang. 2001. Specific alterations of U1-C protein or U1 small nuclear RNA can eliminate the requirement of Prp28p, an essential DEAD box splicing factor. Mol. Cell 7**:**227-232. [DOI] [PubMed] [Google Scholar]
- 4.Crawford, L., K. Leppard, D. Lane, and E. Harlow. 1982. Cellular proteins reactive with monoclonal antibodies directed against simian virus 40 T-antigen. J. Virol. 42**:**612-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Endoh, H., K. Maruyama, Y. Masuhiro, Y. Kobayashi, M. Goto, H. Tai, J. Yanagisawa, D. Metzger, S. Hashimoto, and S. Kato. 1999. Purification and identification of p68 RNA helicase acting as a transcriptional coactivator specific for the activation function 1 of human estrogen receptor alpha. Mol. Cell. Biol. 19**:**5363-5372. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Ford, M. J., I. A. Anton, and D. P. Lane. 1988. Nuclear protein with sequence homology to translation initiation factor eIF-4A. Nature 332**:**736-738. [DOI] [PubMed] [Google Scholar]
- 7.Fujita, T., Y. Kobayashi, O. Wada, Y. Tateishi, L. Kitada, Y. Yamamoto, H. Takashima, A. Murayama, T. Yano, T. Baba, S. Kato, Y. Kawabe, and J. Yanagisawa. 2003. Full activation of estrogen receptor alpha activation function-1 induces proliferation of breast cancer cells. J. Biol. Chem. 278**:**26704-26714. [DOI] [PubMed] [Google Scholar]
- 8.Gilbert, C., A. Kristjuhan, G. S. Winkler, and J. Q. Svejstrup. 2004. Elongator interactions with nascent mRNA revealed by RNA immunoprecipitation. Mol. Cell 14**:**457-464. [DOI] [PubMed] [Google Scholar]
- 9.Guil, S., R. Gattoni, M. Carrascal, J. Abián, J. Stévenin, and M. Bach-Elias. 2003. Roles of hnRNP A1, SR proteins, and p68 helicase in c-H-ras alternative splicing regulation. Mol. Cell. Biol. 23**:**2927-2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamm, J., and A. I. Lamond. 1998. Spliceosome assembly: the unwinding role of DEAD-box proteins. Curr. Biol. 8**:**R532-R534. [DOI] [PubMed] [Google Scholar]
- 11.Hartmuth, K., H. Urlaub, H. P. Vornlocher, C. L. Will, M. Gentzel, M. Wilm, and R. Luhrmann. 2002. Protein composition of human prespliceosomes isolated by a tobramycin affinity-selection method. Proc. Natl. Acad. Sci. USA 99**:**16719-16724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hastings, M. L., and A. R. Krainer. 2001. Pre-mRNA splicing in the new millennium. Curr. Opin. Cell Biol. 13**:**302-309. [DOI] [PubMed] [Google Scholar]
- 13.Hirling, H., M. Scheffner, T. Restle, and H. Stahl. 1989. RNA helicase activity associated with the human p68 protein. Nature 339**:**562-564. [DOI] [PubMed] [Google Scholar]
- 14.Hodges, P. E., and J. D. Beggs. 1994. RNA splicing. U2 fulfils a commitment. Curr. Biol. 4**:**264-267. [DOI] [PubMed] [Google Scholar]
- 15.Honig, A., D. Auboeuf, M. M. Parker, B. W. O'Malley, and S. M. Berget. 2002. Regulation of alternative splicing by the ATP-dependent DEAD-box RNA helicase p72. Mol. Cell. Biol 22**:**5698-5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang, Y., and Z. R. Liu. 2002. The ATPase, RNA unwinding, and RNA binding activities of recombinant p68 RNA helicase. J. Biol. Chem. 277**:**12810-12815. [DOI] [PubMed] [Google Scholar]
- 17.Iggo, R. D., and D. P. Lane. 1989. Nuclear protein p68 is an RNA-dependent ATPase. EMBO J. 8**:**1827-1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jost, J. P., S. Schwarz, D. Hess, H. Angliker, F. V. Fuller-Pace, H. Stahl, S. Thiry, and M. Siegmann. 1999. A chicken embryo protein related to the mammalian DEAD box protein p68 is tightly associated with the highly purified protein-RNA complex of 5-MeC-DNA glycosylase. Nucleic Acids Res. 27**:**3245-3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jurica, M. S., L. J. Licklider, S. R. Gygi, N. Grigorieff, and M. J. Moore. 2002. Purification and characterization of native spliceosomes suitable for three-dimensional structural analysis. RNA 8**:**426-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jurica, M. S., and M. J. Moore. 2003. Pre-mRNA splicing: awash in a sea of proteins. Mol. Cell 12**:**5-14. [DOI] [PubMed] [Google Scholar]
- 21.Kuhn, A. N., Z. Li, and D. A. Brow. 1999. Splicing factor Prp8 governs U4/U6 RNA unwinding during activation of the spliceosome. Mol. Cell 3**:**65-75. [DOI] [PubMed] [Google Scholar]
- 22.Laggerbauer, B., T. Achsel, and R. Luhrmann. 1998. The human U5-200kD DEXH-box protein unwinds U4/U6 RNA duplices in vitro. Proc. Natl. Acad. Sci. USA 95**:**4188-4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lane, D. P., and W. K. Hoeffler. 1980. SV40 large T shares an antigenic determinant with a cellular protein of molecular weight 68,000. Nature 288**:**167-170. [DOI] [PubMed] [Google Scholar]
- 24.Lee, C. G. 2002. RH70, a bidirectional RNA helicase, co-purifies with U1snRNP. J. Biol. Chem. 277**:**39679-39683. [DOI] [PubMed] [Google Scholar]
- 25.Liu, Z. R. 2002. p68 RNA helicase is an essential human splicing factor that acts at the U1 snRNA-5′ splice site duplex. Mol. Cell. Biol. 22**:**5443-5450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu, Z. R., B. Sargueil, and C. W. Smith. 1998. Detection of a novel ATP-dependent cross-linked protein at the 5′ splice site-U1 small nuclear RNA duplex by methylene blue-mediated photo-cross-linking. Mol. Cell. Biol. 18**:**6910-6920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu, Z. R., A. M. Wilkie, M. J. Clemens, and C. W. Smith. 1996. Detection of double-stranded RNA-protein interactions by methylene blue-mediated photo-crosslinking. RNA 2**:**611-621. [PMC free article] [PubMed] [Google Scholar]
- 28.Luking, A., U. Stahl, and U. Schmidt. 1998. The protein family of RNA helicases. Crit. Rev. Biochem. Mol. Biol. 33**:**259-296. [DOI] [PubMed] [Google Scholar]
- 29.Madhani, H. D., and C. Guthrie. 1994. Dynamic RNA-RNA interactions in the spliceosome. Annu. Rev. Genet. 28**:**1-26. [DOI] [PubMed] [Google Scholar]
- 30.Moore, M. J., C. C. Query, and P. A. Sharp. 1993. Splicing of precursors to messenger RNAs by the spliceosome, p. 303-507. In R. F. Gesteland and J. F. Atkins (ed.), RNA world. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 31.Nasim, M. T., H. M. Chowdhury, and I. C. Eperon. 2002. A double reporter assay for detecting changes in the ratio of spliced and unspliced mRNA in mammalian cells. Nucleic Acids Res. 30**:**e109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neubauer, G., A. King, J. Rappsilber, C. Calvio, M. Watson, P. Ajuh, J. Sleeman, A. Lamond, and M. Mann. 1998. Mass spectrometry and EST-database searching allows characterization of the multi-protein spliceosome complex. Nat. Genet. 20**:**46-50. [DOI] [PubMed] [Google Scholar]
- 33.Nilsen, T. W. 2003. The spliceosome: the most complex macromolecular machine in the cell? Bioessays 25**:**1147-1149. [DOI] [PubMed] [Google Scholar]
- 34.Ogilvie, V. C., B. J. Wilson, S. M. Nicol, N. A. Morrice, L. R. Saunders, G. N. Barber, and F. V. Fuller-Pace. 2003. The highly related DEAD box RNA helicases p68 and p72 exist as heterodimers in cells. Nucleic Acids Res. 31**:**1470-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pugh, G. E., S. M. Nicol, and F. V. Fuller-Pace. 1999. Interaction of the Escherichia coli DEAD box protein DbpA with 23 S ribosomal RNA. J. Mol. Biol. 292**:**771-778. [DOI] [PubMed] [Google Scholar]
- 36.Raghunathan, P. L., and C. Guthrie. 1998. RNA unwinding in U4/U6 snRNPs requires ATP hydrolysis and the DEIH-box splicing factor Brr2. Curr. Biol. 8**:**847-855. [DOI] [PubMed] [Google Scholar]
- 37.Rappsilber, J., U. Ryder, A. I. Lamond, and M. Mann. 2002. Large-scale proteomic analysis of the human spliceosome. Genome Res. 12**:**1231-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rossow, K. L., and R. Janknecht. 2003. Synergism between p68 RNA helicase and the transcriptional coactivators CBP and p300. Oncogene 22**:**151-156. [DOI] [PubMed] [Google Scholar]
- 39.Schwer, B. 2001. A new twist on RNA helicases: DExH/D box proteins as RNPases. Nat. Struct. Biol. 8**:**113-116. [DOI] [PubMed] [Google Scholar]
- 40.Schwer, B., and C. H. Gross. 1998. Prp22, a DExH-box RNA helicase, plays two distinct roles in yeast pre-mRNA splicing. EMBO J. 17**:**2086-2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schwer, B., and T. Meszaros. 2000. RNA helicase dynamics in pre-mRNA splicing. EMBO J. 19**:**6582-6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sharp, P. A. 1994. Split genes and RNA splicing. Cell 77**:**805-815. [DOI] [PubMed] [Google Scholar]
- 43.Singh, R. 2002. RNA-protein interactions that regulate pre-mRNA splicing. Gene Expr. 10**:**79-92. [PMC free article] [PubMed] [Google Scholar]
- 44.Staley, J. P., and C. Guthrie. 1998. Mechanical devices of the spliceosome: motors, clocks, springs, and things. Cell 92**:**315-326. [DOI] [PubMed] [Google Scholar]
- 45.Wang, Y., J. D. Wagner, and C. Guthrie. 1998. The DEAH-box splicing factor Prp16 unwinds RNA duplexes in vitro. Curr. Biol. 8**:**441-451. [DOI] [PubMed] [Google Scholar]
- 46.Watanabe, M., J. Yanagisawa, H. Kitagawa, K. Takeyama, S. Ogawa, Y. Arao, M. Suzawa, Y. Kobayashi, T. Yano, H. Yoshikawa, Y. Masuhiro, and S. Kato. 2001. A subfamily of RNA-binding DEAD-box proteins acts as an estrogen receptor alpha coactivator through the N-terminal activation domain (AF-1) with an RNA coactivator, SRA. EMBO J. 20**:**1341-1352. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47.Will, C. L., and R. Luhrmann. 2001. Molecular biology. RNP remodeling with DExH/D boxes. Science 291**:**1916-1917. [DOI] [PubMed] [Google Scholar]
- 48.Will, C. L., S. Rumpler, J. Klein Gunnewiek, W. J. van Venrooij, and R. Luhrmann. 1996. In vitro reconstitution of mammalian U1 snRNPs active in splicing: the U1-C protein enhances the formation of early (E) spliceosomal complexes. Nucleic Acids Res. 24**:**4614-4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang, L., and Z. R. Liu. 2004. Bacterially expressed recombinant p68 RNA helicase is phosphorylated on serine, threonine, and tyrosine residues. Protein Expr. Purif. 35**:**327-333. [DOI] [PubMed] [Google Scholar]
- 50.Yang, L., J. Yang, Y. Huang, and Z. R. Liu. 2004. Phosphorylation of p68 RNA helicase regulates RNA binding by the C-terminal domain of the protein. Biochem. Biophys. Res. Commun. 314**:**622-630. [DOI] [PubMed] [Google Scholar]
- 51.Yang, L., C. Lin, and Z.-R. Liu. Cell. Signal., in press.
- 52.Zhou, Z., L. J. Licklider, S. P. Gygi, and R. Reed. 2002. Comprehensive proteomic analysis of the human spliceosome. Nature 419**:**182-185. [DOI] [PubMed] [Google Scholar]