RBR3, a member of the retinoblastoma-related family from maize, is regulated by the RBR1/E2F pathway (original) (raw)
Abstract
Retinoblastoma-related (RBR) proteins regulate cell division in higher eukaryotes by controlling the adenovirus E2 promoter binding factor (E2F)/dimerization partner (DP) family of transcription factors that regulate expression of many genes involved in cell-cycle progression. We identified a previously undescribed member of the maize RBR family, RBR3, which has the characteristic structure and binding activities of pocket proteins, where interaction depends on a LxCxE motif in the partner proteins and a critical cysteine within the B pocket domain. Like other RBR proteins, RBR3 appears to be regulated by phosphorylation mediated by cyclin-dependent kinases. During endosperm development, RBR3 expression is restricted to the mitotic stage preceding the onset of endoreduplication. This finding suggests a role distinct from RBR1, which is constitutively expressed. Two sites in the RBR3 promoter bind to complexes containing maize E2F1 and DP proteins. Expression of wheat dwarf virus RepA protein, which blocks RBR1 activity and stimulates cell proliferation, dramatically up-regulates RBR3, but not RBR1, RNA in embryogenic maize calli. The results indicate that RBR3 expression is controlled by RBR1 through the activity of E2F/DP and that RBR3 is the maize equivalent of mammalian p107. Furthermore, maize and related grasses might have evolved a compensatory mechanism among distinct types of RBR proteins to ensure robust control of pocket protein activity.
Keywords: cell cycle, pocket protein, RepA
Specific gene expression programs are key targets for cell-cycle control. As cells advance through G1 and S phase, they sequentially up-regulate batteries of genes involved in licensing of replication origins, synthesis of G1/S-phase-specific cyclins (Cycs) and Cyc-dependent kinases (CDKs), and DNA synthesis. The adenovirus E2 promoter binding factor (E2F) family of transcription factors controls the expression of many genes required for entry into and execution of S-phase and cell-cycle progression (reviewed in refs. 1–4). Most E2F proteins require dimerization with a distantly related dimerization partner (DP) protein for activity. In humans, at least eight E2F family members have been identified that can be functionally grouped into transcriptional activators or repressors (reviewed in refs. 4 and 5), and a similar family of E2F proteins is present in plants (6–8).
The retinoblastoma-related (RBR) family of proteins (RB, p107, and p130 in mammals) primarily represses the G1/S-phase transition of the cell cycle by inhibiting, directly (through masking of the E2F transactivation domain) or indirectly (by recruiting different chromatin-remodeling complexes and thereby silencing specific chromatin regions), the E2F/DP family of transcription factors (reviewed in refs. 9 and 10). Collectively, the activity of RBR proteins results in inhibition of the expression of E2F-regulated genes in G1, effectively preventing the transition into S phase. The consensus understanding from many studies in mammals indicates that from late G1, in response to developmental or mitogenic signals, RBRs are inhibited through phosphorylation by CDKs associated with CycD, -E, and -A, which in turn relieves the block on numerous E2F-target promoters. RBR activity also can be repressed and the cell cycle can be stimulated through binding of specific inhibitors, as is the case for several oncoviral proteins, such as simian virus (SV) 40 T-antigen, adenovirus (Ad) E1A, and human papillomavirus (HPV) E7.
The first plant RBR gene, RBR1, was isolated from maize (11, 12), and subsequently this gene was identified in other species, including Arabidopsis. Plant RBRs have a molecular mass of ≈100 kDa and share ≈30% amino acid identity with animal retinoblastoma (RB) proteins over the A/B pocket domain. They interact with a range of proteins containing the RB-binding (LxCxE)-motif, including CycDs (13, 14), animal oncoviral proteins (11), and plant geminivirus proteins, such as wheat dwarf virus (WDV) RepA (11, 12, 15). Consistent with the antiproliferation properties of animal pocket proteins, plant RBRs undergo phosphorylation by CDK/Cyc complexes in a cell-cycle-dependent fashion (11, 13, 14, 16, 17) and have a negative effect on cell-cycle progression in maize, Arabidopsis, and tobacco (18–20). In agreement with the ability of WDV RepA to bind to RBR1, expression of RepA in maize calli and tobacco cell culture counteracts a RBR1-mediated cell cycle block and stimulates cell proliferation (18). Here, we report the characterization of a previously undescribed pocket protein from maize, RBR3, and show that its expression is regulated by the RBR1/E2F pathway. Based on similarities with the properties of the p107 subfamily of RBR proteins, it appears that RBR3 encodes a plant ortholog of mammalian p107. The control of RBR3 expression by RBR1 suggests that a compensatory mechanism is in place in maize cells, and probably other grasses, to ensure a robust regulation of overall pocket protein activity, which may have profound implications for the genetic manipulation of this species.
Materials and Methods
Database Search and Sequence Analyses. Sequence similarity searches of the Pioneer Hi-Bred International maize EST database were performed with the blast algorithm (21). Deduced amino acid sequences were aligned with clustalx v1.51b (22) and macaw (23). Sequence analyses were carried out with netphos 2.0 (www.cbs.dtu.dk/services/NetPhos), promoter prediction (www.cbs.dtu.dk/services/promoter), matinspector and promoter inspector (www.genomatix.de/products/index.html), dragon promoter finder (http://research.i2r.a-star.edu.sg/promoter/promoter1_5/DPF.htm), and tess (www.cbil.upenn.edu/tess) and by searching the PLACE database (www.dna.affrc.go.jp/PLACE). Oligonucleotide sequences used in this work are given in Table 2, which is published as supporting information on the PNAS web site.
Plant Materials. Maize (Zea mays L.) inbred B73 was grown in the field or greenhouse and hand-pollinated. RNA and protein analyses were performed on endosperms manually dissected from kernels harvested at different developmental stages. Shoot apices and leaf blades were isolated from seedlings for RT-PCR experiments. Calli were obtained by bombardment of immature high type II (Hi-II) embryos essentially as described in ref. 18.
RNA Extraction and Semiquantitative RT-PCR. Total RNA, free from DNA, was extracted with an Absolutely RNA RT-PCR Miniprep Kit (Stratagene). RT-PCR was performed with 25 ng of total RNA and a Titan One-Tube RT-PCR kit (Roche, Hamburg, Germany) by using gene-specific primers. RBR1 transcript was amplified with primers ZmRBF1 and RRB1–6, RBR3 transcript was amplified with RB3ABF and P12, and actin transcript was amplified with ACT1F and ACT1R. Annealing steps were at 65°C. The amplification included 0.7 μl of 10 mCi/ml (1 Ci = 37 GBq) [α-32P]dCTP (NEN); typically, 24–27 cycles were performed for the simultaneous amplification of RBR RNAs and 17 cycles for actin RNA, which resulted in DNA amplification within the exponential range. Reaction products were separated by 4% TBE (90 mM Tris/64.6 mM boric acid/2.5 mM EDTA, pH 8.3) and PAGE, dried, and exposed to a Phosphorscreen (Molecular Dynamics); signal quantification was carried out with a Storm 860 PhosphorImager (Molecular Dynamics) and imagequant image analysis software (Amersham Pharmacia). For the analysis of RNA expression in embryogenic calli, a total of 39 samples were processed in two independent experiments.
Expression of GST Fusions in Escherichia coli and Purification. Sequences encoding selected protein domains for expression in E. coli were PCR-amplified with Pfu DNA polymerase (Stratagene) and verified by sequencing. The sequence encoding amino acids 28–129 of RBR3 was amplified with primers RB3NF1 and RB3NR1 and cloned at the SmaI site of the pGEX-2TK vector (Amersham Pharmacia). The sequence encoding amino acids 2–225 of RBR1 was amplified with primers RRB1F and RRB1R, digested with EcoRI and XhoI, and cloned in pGEX-6P3 (Amersham Pharmacia). These RBR3 and RBR1 domains, proximal to their N termini, were expressed in E. coli, and the proteins were used to raise specific antibodies (see below). Primers GEXRepAF and GEXRepAR were used to amplify sequences encoding full-length wild-type RepA and mutant RepARBmut (in which the LICHE motif of RepA was changed to LIGHK) from Pioneer's plasmids PHP17008 and PHP19080, respectively, which then were digested with BamHI and cloned in pGEX-2TK. Clones encoding GST-E7, GST-E7ΔDLYC and GST-E1A, are described in ref. 11. The clone expressing GST-RBR3 pocket was obtained by PCR amplification of the sequence encoding residues 390-1010 of RBR3 with primers RB3ABF and RRB3, which was cloned at the SmaI site of pGEX-2TK. The clone encoding GST-RBR1 pocket was as described in ref. 11. GST-E2F1 and GST-DP fusions were obtained in a similar fashion (P.A.S., R.A.D., and B.A.L., unpublished data). GST fusions were expressed in E. coli strain BL21(DE3) Codonplus RIL (Stratagene) and purified according to standard procedures.
Antibodies. Affinity-purified GST fusions were separated by SDS/PAGE; gels were stained with 0.05% Coomassie blue R250 in H2O, and the regions containing antigens were excised. New Zealand White rabbits were injected four times (200 μg of protein each) at Strategic BioSolutions (Newark, DE). Affinity-purification of antibodies was performed as described in ref. 24. An IgG-enriched fraction was obtained from crude antisera by precipitation at 50% NH3SO4 saturation, which was subjected to two successive rounds of affinity chromatography (first on cross-linked GST and second on the GST-fusion antigen), and the eluted antibodies were dialyzed against PBS (pH 7.2). Antibodies against maize CycA1;3 will be described elsewhere (R.A.D., P.A.S. and B.A.L., unpublished data). Antibodies against maize E2F1 and DP proteins were raised in a similar manner to those against RBRs and will be described separately (P.A.S., R.A.D., and B.A.L., unpublished data). Mouse monoclonal anti-actin (N350) and anti-α tubulin (N356) antibodies were purchased from Amersham Pharmacia.
In Vitro Transcription/Translation. Coupled transcription/translation of RBR3 wild type and C788G mutant pockets (amino acids 357-1010) was performed with a TNT T7 Quick for PCR DNA (Promega) according to the manufacturer's instructions in the presence of l-[35S]methionine (Amersham Pharmacia). The C788G mutation was generated by PCR with a QuikChange mutagenesis kit (Stratagene) using primers C788GF and C788GR.
Protein Extracts, Immunoblotting, Immunoprecipitation, and Kinase Assays. Endosperm cell extracts were prepared according to procedures described in ref. 25. Nuclear and cytosolic fractions were obtained based on methods described in ref. 26, except that dissected endosperms were used, the supernatant above the Percoll cushion enriched for cytosolic proteins also was collected and processed, and the lysis buffer contained 0.15 M NaCl to preserve protein complexes. Immunoblotting, immunoprecipitation, and phosphorylation assays will be described elsewhere (R.A.D., P.A.S., and B.A.L., unpublished data).
In Vitro Pull-Down Assays. All binding/washing steps were carried out on ice or at 4°C. Equimolar amounts of different GST fusion proteins (ranging from 2.5 to 5.0 μg) were used in each tube, which contained 35 μl of gluthathione–agarose beads (Sigma) slurry and 100 μl NETT buffer (20 mM Tris·HCl, pH 8.0/100 mM NaCl/20 mM Na2EDTA/0.5% (vol/vol) Triton X-100). 35S-labeled, in vitro synthesized protein (5 μl) was added, and the binding reaction was allowed to proceed for 1 h. Beads were washed six times for 15 min with 1 ml of NETT buffer and resuspended in 30 μl of 1 × SDS/PAGE sample loading buffer (pH 6.8), boiled, and separated by 10% SDS/PAGE. The gel was stained, scanned, and subjected to fixing and signal amplification according to the TNT T7 Quick for PCR DNA kit instructions. The gel was dried and exposed to an x-ray film at –80°C overnight.
Cloning of RBR3 Promoter Sequence. A sequence containing part of the RBR3 promoter was obtained by PCR amplification of a Pioneer Hi-Bred International B73 genomic library made in λZAPII (Stratagene). A reverse primer, specific to the 5′ UTR of the RBR3 cDNA, RB3PR, was used in combination with either primer flanking the vector's cloning site, M13R23 or T7–24. The RB3PR/M13R23 primer combination produced a genomic fragment extending the RBR3 5′ UTR by 414 nt into the promoter region. By using gene-specific primers, this band was recloned from B73 genomic DNA and found to be identical to the sequence in a bacterial artificial chromosome clone spanning this region of the RBR3 gene. It also matched, starting at position –757 from the translation initiation site, the genomic sequence with GenBank accession no. BZ540362.
EMSA. For each E2F-binding site tested, two complementary oligonucleotides were synthesized as follows: E2F site I, RB3PI and RB3PII; site II, RB3PIII and RB3PIV; site III, RB3PV and RB3PVI; and site IV, RB3PVII and RB3PVIII. Probes were prepared by end-labeling one oligonucleotide of each pair with T4 polynucleotide kinase and 32P and annealing to the complementary oligonucleotide. Identical unlabeled oligonucleotide pairs also were annealed to provide a specific competitor. Annealed oligonucleotides were purified by 12% PAGE and eluted from the crushed gel slice with H2O overnight. Binding reactions, carried out on ice for 30–60 min, contained a probe with 105 cpm in binding buffer containing 25 mM Hepes (pH 7.6), 40 mM KCl, 2 mM Na2EDTA, 3% (wt/vol) Ficoll 400, 1 mM DTT, 1× Complete EDTA-free Protease Inhibitor Mixture (Roche), 0.5 mM phenylmethylsulfonyl fluoride, 0.5 μg of poly(dI-dC)·poly(dI-dC) (Amersham Pharmacia), and 5 μg of nuclear extract. In particular reactions, samples were incubated with a 50-fold molar excess of unlabeled oligonucleotides as a specific competitor or 0.2 μg of pGEX-2TK plasmid as nonspecific competitor. Affinity-purified antibodies (100 ng) were preincubated with extracts for 15 min before adding the probe. The same amount of purified, preimmune IgG was used as an antibody control. In preliminary 15-min incubations, antibodies were titrated with 200 ng of either GST-E2F1 or GST-DP. An equimolar amount of GST alone was used as a control. Reaction products were separated by 10% TBE-PAGE; gels were dried and exposed to x-ray films.
Results
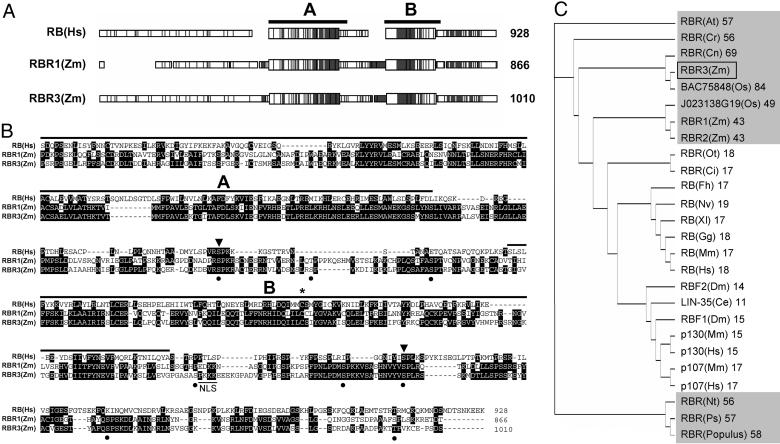
RBR3 Encodes a Previously Undescribed RBR Protein from Maize. The RBR3 cDNA was identified by querying the Pioneer Hi-Bred International EST database with the maize RBR1 sequence. It is 3,856 nt long with an ORF (nucleotides 513-3545) that encodes a polypeptide of 1,010 aa with a calculated molecular mass of 111.5 kDa and a pI of 8.68. It appears to encode a full-length polypeptide based on the presence of one upstream, in-frame stop codon, a translation initiation context around the first ATG codon that is a good match to the consensus for monocotyledonous plants, and the similarity with the N termini of several other plant RBR proteins. The RBR3 nucleotide sequence is 30% identical to that of RBR1 and 16% and 26% identical to those of RBR2a and RBR2b, respectively, which are partial cDNAs resulting from alternative splicing of the RBR2 gene (27). RBR3 shares 43–84% amino acid sequence identity with full-length plant RBR sequences and 11–19% identity with those from other species (Fig. 1_C_). RBR1 and RBR2 amino acid sequences are 89% identical, suggesting that they might result from gene duplication. They share only 43% identity with RBR3, suggesting that RBR3 represents a distinct class of RBR genes. Whereas only one RBR gene has been identified in most plant genomes, including that of Arabidopsis thaliana, at least two classes of RBR sequences are present in rice, which share 49% and 84% amino acid sequence identity with maize RBR3 (Fig. 1_C_). RBR3 protein is not predicted to have a signal peptide, but it contains two putative nuclear localization signals, KKKP and PKKK at positions 289–292 and 871–874, respectively, and is expected to localize to the nucleus. Two domains, termed A (amino acids 436–615) and B (amino acids 729–864), are conserved with other RBR proteins and constitute the functional pocket region (Fig. 1). Two additional regions with limited similarity with other RBRs are the spacer between the A and B domains and the C terminus. Close inspection of the 200 N-terminal amino acids of RBR3 revealed a sequence similar to a domain specifically conserved in p107 and p130, which has been shown to be involved in growth suppression and binding to and inhibition of CDK/CycA/E complexes (28) (Fig. 2) but not in the RB subclass of proteins. There are 10 potential CDK phosphorylation sites in RBR3 distributed around the A and B domains (positions S-382, S-394, S-673, S-693, S-712, S-870, S-902, S-915, S-946, and T-1001), with two being conserved among human RB and the maize RBR1 and RBR3 proteins (Fig. 1_B_). A cysteine residue, which is critical for the activity of human RB (29) and maize RBR1 (11, 18), also is conserved in the RBR3 sequence at position 866 (Fig. 1_B_).
Fig. 1.
RBR3 is a previously undescribed maize protein belonging to the RB family. (A) Schematic macaw alignment of maize (Zm) RBR3 and RBR1 with human (Hs) RB, showing the conserved A and B pocket domains. Conserved regions are shaded. (B) Alignment of the amino acid sequences shown in A over the pocket and C-terminal domains. Identical residues are highlighted by a black background. The A and B pocket domains are indicated. Dots indicate putative CDK phosphorylation sites in RBR3, with arrowheads pointing to conserved sites in the three sequences. An asterisk indicates the conserved cysteine in the B pocket domain critical for protein activity. A potential nuclear localization signal is underlined in the RBR3 sequence. (C) Tree illustrating the relationships among a selected, representative set of full-length RBR amino acid sequences. Sequences from higher plants are shaded in gray. The percent of identity with maize RBR3 is shown next to the sequence name. The maize RBR3 protein is indicated by a box. Species abbreviations and GenBank accession numbers are as follows: At, A. thaliana (AAF79146); Cr, Chenopodium rubrum (CAA09736); Cn, Cocos nucifera (AAM77469); Os, Oryza sativa (BAC75848 and KOME database clone number J023138G19); Zm, Zea mays (RBR3; RBR1, AAB69649; RBR2, CAC82493); Ot, Ostreococcus tauri (AAV68604); Ci, Chlamydomonas incerta (AAV41810); Fh, Fundulus heteroclitus (AAS80140); Nv, Notophthalmus viridescens (CAA70428); Xl, Xenopus laevis (A44879); Gg, Gallus gallus (NP_989750); Mm, Mus musculus (RB, NP_033055; p107, Q64701; p130, Q64700); Hs, Homo sapiens (RB, NP_000312; p107, AAA02489; p130, NP_005602); Dm, Drosophila melanogaster (RBF1, AAF45551; RBF2, AAF62399); Ce, Caenorhabditis elegans (NP_491686); Nt, Nicotiana tabacum (BAA76477); Ps, Pisum sativum (BAA88690); Populus (AAF61377).
Fig. 2.
Sequence similarity among N-terminal domains of RBR3, p107, and p130 proteins, with identical residues in RBR3 and p107 or p130 sequences shaded in black and conservative substitutions in gray.
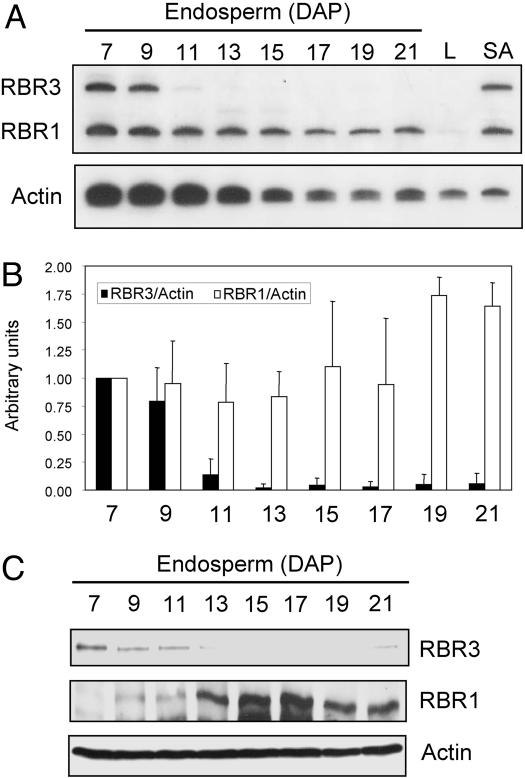
RBR3 Is Expressed Differently from RBR1. RT-PCR analysis of transcript accumulation and immunoblotting with specific antibodies revealed that RBR3 and RBR1 have different expression patterns during endosperm development (Fig. 3). Semiquantitative RT-PCR analysis of RBR3, RBR1 and actin transcripts was carried out with RNA from endosperms dissected 7–21 days-after-pollination (DAP), as well as leaf blades (nondividing) and meristematic shoot apices. During endosperm development, expression of both RBR1 and RBR3 RNAs was highest at 7 DAP, when endosperm is mitotically active. However, RBR1 RNA declined gradually during development, whereas RBR3 RNA was dramatically reduced after 9–11 DAP as endosperm cells begin to undergo endoreduplication. Both RNAs were highly transcribed in the shoot apex, but only RBR1 RNA was detectable in leaf blade cells. Actin RNA was present in all samples analyzed, and its accumulation decreased gradually in 7- to 21-DAP endosperms. Because massive transcription of genes encoding zein storage proteins and starch biosynthetic enzymes takes place in endosperms after 10 DAP, we analyzed RNA levels of RBR3 and RBR1 relative to those of actin to minimize dilution effects (Fig. 3_B_). This analysis confirmed the marked decrease in RBR3 RNA after 9 DAP and showed that the relative level of RBR1 RNA was nearly constant at <17 DAP and then increased up to 0.75-fold by 19 and 21 DAP. Antibodies against regions located toward the N termini of the RBR3 and RBR1 proteins were raised and purified. Their specificity was confirmed by immunoblotting of recombinant proteins and extracts from maize mutants with altered RBR1 and RBR3 expression (P.A.S., R.A.D., and B.A.L., unpublished data). Immunoblots of 7- to 21-DAP endosperm extracts revealed a sharp decrease in the RBR3 protein after 11 DAP and an increase in RBR1 accumulation, consistent with RNA data (Fig. 3_C_).
Fig. 3.
Maize RBR3 and RBR1 have different expression patterns. (A) Semiquantitative RT-PCR analysis of RBR3, RBR1, and actin control RNAs in 7- to 21-DAP endosperms, leaf blades (L), and shoot apices (SA). (B) Histogram illustrating RBR3 and RBR1 RNA levels relative to actin RNA in developing endosperms. RNA levels at 7 DAP are considered as one expression unit. Data are from three independent experiments with error bars showing standard deviations. (C) RBR3 and RBR1 immunoblot analyses in developing endosperm extracts. Analysis of actin was carried out to confirm equal sample loadings.
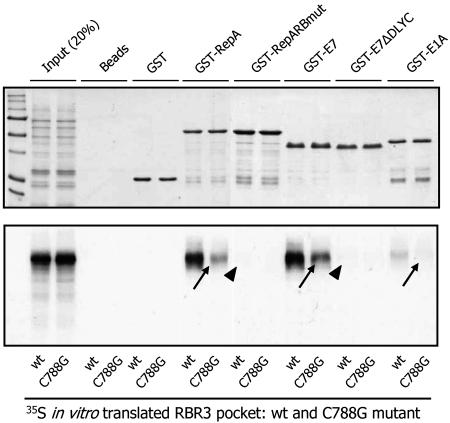
RBR3 Is a Pocket Protein. Interaction between pocket proteins of the RB family and their protein partners often depends on an intact LxCxE motif in the latter and a critical Cys residue in the B pocket domain of the former (11, 29, 30). We used an in vitro pull-down assay to test whether this characteristic was also true for RBR3 (Fig. 4 and Table 1). Three proteins known to interact with RBR through LxCxE motifs, WDV RepA, HPV E7, and Ad E1A (11, 30), were expressed in E. coli as GST fusions and used as baits in pull-down experiments. In addition, two mutants in which the critical LxCxE motif was either partially deleted (E7ΔDLYC) or mutagenized (RepARBmut), thereby preventing their interaction with pocket proteins (11), also were used. The region of RBR3 comprising the A/B pocket and C-terminal domain, hereafter referred to as “RBR3 pocket,” was synthesized and labeled with 35S in vitro and used as prey in interaction assays with the GST fusion partners. To test whether the conserved Cys-788 in RBR3 is important for these interactions, a C788G mutant was generated and tested. As shown in Fig. 4, all of the viral baits with an intact LxCxE motif (GST-RepA, GST-E7, and GST-E1A) interacted with the RBR3 pocket, whereas mutagenized versions with altered LxCxE motifs did not (indicated by arrowheads in the figure). The RBR3 pocket failed to bind agarose beads and GST in control reactions, revealing that the viral protein interactions were specific. In addition, interaction with wild-type viral proteins was dramatically reduced for the C788G RBR3 pocket mutant (arrows), indicating that Cys-788 is crucial for pocket activity. The results indicate that RBR3 has the interaction properties of pocket proteins, which depend on both Cys-788 in the B pocket domain and an intact LxCxE motif in the protein partners.
Fig. 4.
RBR3 has properties of pocket proteins. In vitro pull-down experiment shows that interaction between WDV RepA, HPV E7, Ad E1A proteins, and RBR3 depends on an intact LxCxE motif in the viral proteins and on the conserved Cys-788 residue in the B pocket of RBR3. Pull-down baits were either wild-type or LxCxE motif-mutant viral proteins, as GST fusions. Agarose beads alone and GST were used as negative controls. The region of RBR3 including the A/B pocket and C-terminal domains was synthesized and labeled in vitro with 35S and tested as a prey. Both wild-type RBR3 and a C788G mutant were assayed, as indicated. Two aliquots (20%) of the in vitro synthesized RBR3 proteins were loaded as a reference for prey inputs. (Upper) Coomassie-stained gel showing bait proteins. (Lower) Fluorography of the same gel showing labeled, pulled-down prey proteins. Arrowheads indicate abolished interactions by mutagenesis of the LxCxE motif in the viral baits. Arrows show dramatically reduced interactions as a result of the C788G mutation in RBR3. A summary listing of the key motifs and residues used that affect the interaction between viral proteins and RBR3 is shown in Table 1.
Table 1. Key residues for interaction between pocket proteins and LxCxE motif.
| Protein | Motif |
|---|---|
| LXCXE motif | LXCXE |
| Ad5 E1A | LTCHE |
| HPV-16 E7 | LYCYE |
| HPV-16 E7ΔDLYC | ---YE |
| WDV RepA | LICHE |
| WDV RepARBmut | LI**GHK** |
| HsRB | IMMCSMY |
| HsRBC706F | IMM**F**SMY |
| ZmRBR1 | LILCCLY |
| ZmRBR1C653G | LIL**G**CLY |
| ZmRBR3 | IILCSIY |
| ZmRBR3C788G | IIL**G**SIY |
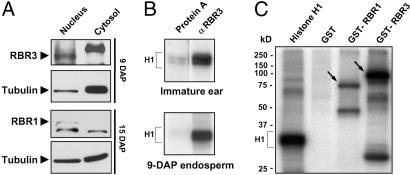
RBR3 Is a Nuclear Protein Potentially Regulated by CDKs. Because proteins of the RB family localize to the nucleus, we tested whether this characteristic was true of RBR3. RBR3 protein was identified in the nuclear, but not in the cytosolic, extracts by immunoblotting (Fig. 5_A_); in contrast, the tubulin control was mostly present in the cytosolic fraction. This result is consistent with the known nuclear localization of RBR proteins from many species, including the maize RBR1 homolog (Fig. 5_A_).
Fig. 5.
RBR3 is a nuclear protein that can be phosphorylated by CDKs in vitro. (A) Western blot analysis of nuclear and cytosolic endosperm extracts (10 μg per lane) showing the nuclear localization of RBR3 and RBR1. Antibodies against tubulin were used as a control. (B) Histone H1 (H1) kinase assay using immunoprecipitated fractions from immature ears and 9-DAP endosperms isolated with RBR3-specific antibodies. (C) Both RBR3 and RBR1 pockets are phosphorylated in vitro by immunoprecipitated maize CycA1;3/kinase complexes. The maize RBR proteins were assayed as GST-fusion substrates (arrows). Histone H1 and GST were assayed as positive and negative controls, respectively.
It is well known that the inhibitory activity of RBR proteins on mammalian cell-cycle progression is repressed through phosphorylation by CycD-, CycE-, and CycA-containing CDKs. The presence of 10 putative CDK phosphorylation sites in RBR3 prompted us to test whether RBR3 was associated with and could be phosphorylated by CDKs. Histone H1 kinase activity was immunoprecipitated from immature ears and 9-DAP endosperms, both of which are mitotically active, by using the RBR3-specific antibody. The observed kinase activity was well above the background obtained with protein A-agarose beads alone (Fig. 5_B_). We also tested whether RBR3 and RBR1 proteins can function as substrates for phosphorylation by CycA–CDK complexes. The RBR3 and RBR1 pockets were expressed in E. coli, purified, and tested in vitro for phosphorylation by CDKs immunoprecipitated from 9-DAP endosperms with antibodies specific for maize CycA1;3 (R.A.D., P.A.S., and B.A.L., unpublished data). Both GST-RBR3 and GST-RBR1 pockets were phosphorylated in this assay, as was histone H1. GST was not phosphorylated, indicating that this moiety was not a substrate of CycA1;3/CDK.
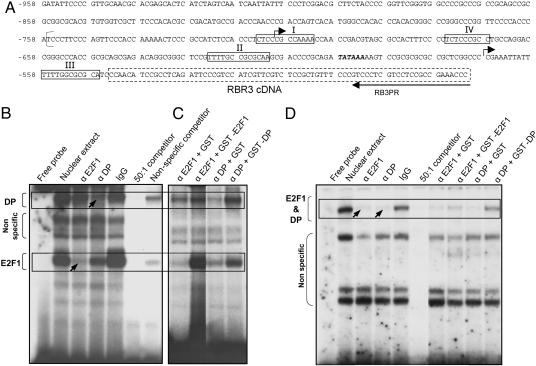
E2F Motifs in the RBR3 Promoter Interact with E2F1/DP. A 414-bp sequence of the RBR3 promoter and 5′ UTR was isolated from a maize B73 genomic library by PCR amplification (Fig. 6_A_). Analysis of this partial promoter sequence revealed four potential E2F binding sites (consensus: TTTC/GC/GCGC), two putative transcription start sites, and a TATA box. The second transcription start site (at position –548) is downstream of all of the E2F-binding sites and the TATA box and is, therefore, likely to represent the true transcription start site. The four putative E2F-binding sites were tested for binding to E2F and DP proteins by EMSA, and two of them, sites I and IV, were shown to interact (Fig. 6 B_–_D). Maize cDNAs related to mammalian E2F1 and DP were isolated, and specific antibodies were raised against the encoded proteins. Proteins in 9-DAP endosperm nuclear extracts generated retarded bands when incubated with 32P-labeled oligonucleotides corresponding to sites I (Fig. 6 B and C) and IV (Fig. 6_D_). For site I, two bands were dramatically reduced when the binding reaction was incubated with specific antibodies against maize E2F1 and DP proteins (arrows in Fig. 6_B_). Presumably, the antibodies inhibited binding of the antigen in the nuclear extract to the probe. Incubation with purified, nonimmune IgG did not alter the banding pattern, indicating that the effect of antibodies against E2F1 and DP was specific. Each of the two retarded bands appeared to contain, or be due to, either E2F1 or DP proteins. However, close inspection revealed that each antibody interfered, to different extents, with both bands, indicating the presence of both E2F1 and DP in the complex. All retarded bands could be competed out by a 50:1 molar excess of unlabeled probe sequence, but only background bands were competed out by nonspecific DNA, indicating that interaction with E2F1 and DP specifically depended on the probe sequence. Additional evidence to support the observation of a specific interaction between E2F binding site I and E2F1 and DP proteins was obtained by titrating the E2F1 and DP antibodies with recombinant GST-E2F1 or GST-DP proteins, respectively, which reconstituted, at least in part, the retarded binding pattern obtained after incubation with nuclear extract (Fig. 6_C_). Incubation with equimolar amounts of GST alone failed to titrate the antibodies, indicating that the interaction was antigen-specific. Similar analyses with E2F site IV also revealed a specific interacting band containing E2F1 and DP (arrows in Fig. 6_D_). Thus, at least two motifs in the RBR3 promoter can interact with maize E2F1 and DP proteins, suggesting that RBR3 is regulated by E2F/DP.
Fig. 6.
The RBR3 promoter contains two E2F-binding sites that interact with maize E2F1 and DP proteins. (A) Partial RBR3 promoter sequence containing four putative E2F binding sites (I–IV) (boxed residues). The binding site of the reverse RB3PR primer is shown. The 5′ region of the RBR3 cDNA sequence is indicated by a broken line. Two putative transcription initiation sites are indicated by arrows. Bold, italic letters indicate the TATA box. The bracket at position –759 indicates the beginning of the genomic sequence with GenBank accession no. BZ540362 that matches the shown sequence. Numbers on the left of the sequence indicate residue positions relative to the translation initiation in the RBR3 cDNA (numbering reflects the presence of an intron in the 5′ UTR). (B_–_D) EMSAs showing interaction between E2F-binding site I (B and C) and IV (D) and E2F1 and DP proteins in 9-DAP endosperm nuclear extracts. Interaction of site I with E2F1 and DP (B) was inhibited with anti-E2F1 and -DP antibodies (α E2F1 and α DP) (arrows). This inhibition could be titrated by incubation with an excess of recombinant GST-E2F1 and GST-DP (but not by GST alone), restoring the normal binding pattern (C). E2F1 and DP binding to E2F-binding site IV were shown by similar experiments (D). Boxes indicate specific interactions. Nonspecific bands are indicated by brackets.
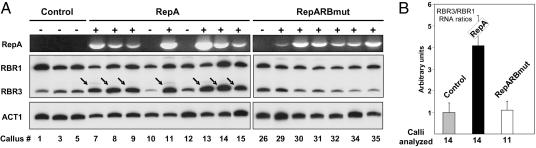
RBR3 RNA Expression Is Up-Regulated in Calli Expressing WDV RepA. The above results indicated that RBR3 expression is more closely associated with actively dividing cells than that of RBR1 and that E2F1/DP transcription factors may be involved in its regulation. Consequently, we reasoned that RBR3 expression may be controlled by RBR1 through the activity of E2F1/DP. To address this possibility, we used an assay based on the antagonistic roles of RBR1 and RepA in the cell cycle. RepA is known to stimulate cell proliferation in maize calli and tobacco cell cultures, an effect that is counteracted by overexpressing RBR1 (18). Consistent with this observation, we found that many maize homologs of known E2F-targets are up-regulated upon RepA expression in transgenic maize calli (data not shown). We hypothesized that because the E2F pathway is generally up-regulated in RepA-expressing callus, RBR3 expression also would be stimulated if this gene is a target of this pathway. Accordingly, we measured the relative levels of RBR3 and RBR1 RNAs in calli expressing either wild-type RepA, a RepA mutant with a nonfunctional LxCxE motif (RepARBmut), and control, nontransgenic calli. Calli were scored for the absence (–) or presence (+) of RepA RNA by RT-PCR (Fig. 7_A_). Noticeably, several calli initially scored as “transgenic” based on BASTA herbicide resistance did not express detectable levels of RepA or RepARBmut RNA (calli nos. 10, 12, and 26). This result was probably due to sporadic, uncoupled integration of the cobombarded selectable-marker mo-PAT gene and RepA constructs. Nevertheless, these calli represented useful internal controls. RBR1 and RBR3 RNA levels were simultaneously measured by semiquantitative RT-PCR amplification, and actin RNA was amplified from the same samples in separate reactions. Expression of RBR3 RNA in RepA+ calli was markedly increased compared with control calli, whereas that of RBR1 was mostly unchanged. However, RBR3 RNA in RepARBmut-expressing calli was similar to the control. Quantitation of the RNA levels showed that RBR3 RNA was up-regulated 4-fold as a result of RepA expression (Fig. 7_B_). For this analysis, RBR3 RNA expression was measured relative to RBR1, which provided a better standard than actin RNA because RBR1 and RBR3 RNA are similarly abundant and could be simultaneously amplified under identical conditions. Preliminary analyses revealed that RBR1 RNA, relative to actin RNA, was mostly unaffected by RepA expression (data not shown). The results show that RepA expression results in up-regulation of RBR3 expression and provide in vivo evidence in support of the hypothesis that RBR1 regulates RBR3 expression.
Fig. 7.
RBR3 RNA expression is up-regulated in transgenic calli expressing RepA, which is dependent on an intact LxCxE motif in RepA. (A) Representative RT-PCR analysis of RepA, RBR1, and RBR3 RNA expression in control calli and calli expressing wild-type RepA or a mutant (RepARBmut) in which the LICHE motif was mutagenized to LIGHK. The top row shows expression of RepA or RepARBmut RNAs; + and – indicate whether or not the callus expressed the transgene. RBR1 and RBR3 RNAs were detected by semiquantitative RT-PCR in the same reaction tube, and actin RNA signal was used as loading control. Arrows indicate increased RBR3 RNA in the RepA-expressing calli. Expression of RepARBmut did not appreciably affect RBR3 RNA levels. (B) Histogram showing RBR3/RBR1 RNA ratio as a measure of RBR3 up-regulation in RepA-expressing calli, relative to an unaffected transcript. Error bars indicate standard deviations.
Discussion
We have established that the maize RBR1/E2F pathway regulates expression of RBR3, a previously undescribed member of the RBR pocket protein family. RBR3 encodes a polypeptide that is larger than RBR1 by virtue of a unique N-terminal domain of ≈200 aa, but it contains the canonical A and B domains that constitute the conserved pocket region. RBR3 displays properties typical of pocket proteins. It binds other proteins through their LxCxE motifs, and a conserved Cys-788 in its B domain is critical for interaction. RBR3 is a nuclear protein that is associated with histone H1 kinase activity and that can be phosphorylated by CycA/CDK complexes. These data, obtained from actively dividing maize tissues, are consistent with the well-known inhibition of pocket proteins by CDKs in animal cells, which results in S-phase entry and progression (31).
Several lines of evidence prompt us to suggest that RBR3 is the maize ortholog of mammalian p107. First, RBR3 is more closely related to similar proteins from different plant species (49–84% sequence identity) than to maize RBR1 or RBR2 (43% identity), suggesting that the apparent sequence redundancy in the maize RBR family underscores distinct functional properties. It is interesting to note that two distinct classes of RBR sequences (i.e., RBR1- and RBR3-type) have been identified in maize so far, and we found a similar situation in rice. This finding contrasts with the identification of only one RBR sequence in A. thaliana and many other plants, suggesting that the RBR/E2F pathway in the Poaceae family, or perhaps monocots in general, evolved to a higher degree of complexity. Indeed, the presence of two clearly distinct EST classes for RBR genes in wheat (e.g., GenBank accession nos. TC248813 and TC269028) supports this view. Second, the N-terminal region of RBR3, unlike maize RBR1 and RBR2, shows similarity with the N-terminal sequences of p107 and p130, which have been implicated in growth suppression and inhibition of specific CDK/Cyc complexes. This finding suggests that RBR3 properties may be more closely related to those of p107/p130 than RB proteins. Third, the expression of RBR3 is limited largely to the mitotic phase of endosperm development and is remarkably different from that of RBR1, which is constitutive. This finding is reminiscent of the up-regulation of p107 in actively dividing mammalian cells (32). Human RB and plant RBR1 proteins are constitutively expressed, whereas p130 is expressed mostly in quiescent cells, and its expression drops in proliferating cells (33). Fourth, data from two sets of experiments provide evidence that RBR3 is regulated by RBR1 through the E2F/DP pathway. Two E2F binding sites were identified in the promoter of RBR3, which are a perfect match with E2F binding sites in A. thaliana (PLACE database site no. S000417) (34). Both sites were shown by EMSA to interact with nuclear proteins from 9-DAP endosperm having antigenic properties of maize E2F1 (a protein structurally related to mammalian activator E2Fs; ref. 4) and DP, suggesting that RBR3 is regulated by E2F/DP and, further upstream, by RBR1. The hypothesis that RBR3 transcription is regulated by RBR1 via E2F/DP was supported by experiments with transgenic maize calli expressing WDV RepA. RepA expression antagonizes RBR1 and stimulates cell proliferation in maize callus and tobacco BY2 cell culture (18), presumably by up-regulating E2F targets. We found that several cell cycle genes known to be controlled by the E2F pathway are up-regulated in RepA-expressing calli (P.A.S., R.A.D., and B.A.L., unpublished data). In addition, RBR3 RNA was up-regulated 4-fold in transgenic vs. control calli, whereas RBR1 RNA levels were unaffected. Stimulation of E2F target genes, including RBR3, was dependent on an intact LxCxE motif in the RepA protein, as demonstrated by the ineffective expression of a mutagenized form of RepA (RepARBmut) devoid of a functional LxCxE motif (Fig. 4 and ref. 18). Thus, the up-regulation of RBR3 in transgenic RepA calli provided in vivo evidence supporting the EMSA data.
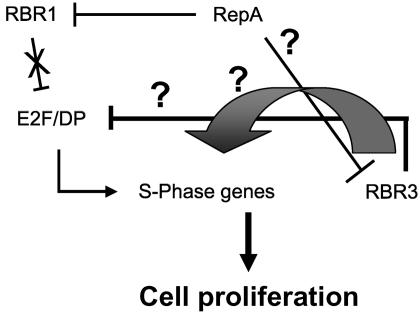
Stimulation of RBR3 expression, resulting from RepA-mediated inhibition of RBR1, resembles the up-regulation of p107 in mammals due to loss of RB function (35, 36). A model to account for this regulatory circuitry between the two maize RBR proteins is shown in Fig. 8. It proposes an inhibitory cell-cycle role for RBR3, similar to that of mammalian p107. Expression of RepA in maize cells inactivates RBR1, de-repressing E2F targets and up-regulating RBR3. Although different members of the pocket protein family in animals have clearly distinct cell-cycle roles, they also functionally overlap to a considerable extent (10). Recent evidence indicates that this redundancy provides the basis for compensatory mechanisms that maintain correct cell-cycle regulation (36).
Fig. 8.
Model illustrating the regulation of RBR3 expression by the RBR1/E2F pathway and the roles of the maize pocket proteins in cell-cycle control. In RepA-expressing callus, the block on E2F/DP is relieved as RBR1 is inhibited. As a result, S-phase genes are up-regulated, leading to cell proliferation. RBR3 is also up-regulated, but it also might be inhibited by RepA. It remains to be determined whether RBR3 can interact with E2F family members and with which specificities. The intriguing up-regulation of RBR3, as a result of RepA expression, also suggests an alternative and potentially novel positive role of RBR3 in the cell cycle (large arrow).
As with Arabidopsis, many plant species seem to possess only one RBR gene, and its inactivation or loss can result in cell-cycle deregulation in certain developmental contexts (19, 20). Our data, however, suggest that in maize and possibly other cereals, the RBR/E2F pathway has evolved to compensate for the loss of activity of one pocket protein (i.e., RBR1) by up-regulating the expression of another (i.e., RBR3). Accordingly, simultaneous inactivation/loss of both types of RBR proteins would be necessary for cell-cycle deregulation. RepA can bind RBR1 (11, 12) and RBR3 (Fig. 4), and we predict that its expression in maize callus would inactivate both pocket proteins and neutralize, at least in part, the RBR1/RBR3 compensatory mechanism, thereby stimulating cell-cycle progression. Consistent with this hypothesis, RepA expression dramatically stimulates growth of maize transformants and increases transformation frequencies (18).
The existence of a pathway that compensates for the inactivation/loss of RBR1 through up-regulating RBR3 may have far-reaching implications for plant biotechnology. Compensatory mechanisms between RBR family members in cereals that preserve pocket protein activity might explain the recalcitrance to genetic transformation and cellular plasticity typical of grass species (37). Cell-cycle progression and cell competence for proliferation profoundly influence plant transformation (18). CycDs play an important role in integrating external sucrose and hormone signaling and in mediating downstream cell-cycle responses (reviewed in ref. 38). Indeed, CycD3;1 expression is induced in response to hormone treatment, and its overexpression in Arabidopsis tissue culture triggers cell-cycle progression and results in cytokinin-autotrophic callus initiation and growth (39). Recent evidence suggests that CycD3;1 stimulates cell division by directly suppressing the RBR-dependent block on E2F target genes (40, 41). Thus, in species with a single RBR gene, such as Arabidopsis, up-regulation of certain CDK/CycD complexes may result in phosphorylation and inactivation of RBR and may be sufficient to release tissue culture cells from a RBR-dependent cell cycle block. Our results suggest the situation in maize and other cereals might be different: both RBR1 and RBR3 functions would need to be inhibited for cell-cycle stimulation to occur. This hypothesis is consistent with the RBR3/RepA in vitro interaction data, which suggest that RepA-driven cell-cycle up-regulation in maize callus occurs through simultaneous inactivation of both RBR1 and RBR3. As in the dicotyledonous plants studied in detail so far, CDK/CycD up-regulation could take place in maize embryo culture, but the resulting derepression of E2F targets, due to removal of the RBR1-dependent block, would be counteracted by increased expression of RBR3. RBR3, in turn, could inhibit G1 and/or S-phase CDK/Cyc complexes through its p107/p130-like N-terminal domain or, perhaps, could lead to silencing of a subset of E2F-responsive genes through interaction (like p107 or p130) with repressor-type E2F proteins (10). Although this hypothesis is attractive, presently there are insufficient experimental data to rule out alternative scenarios. For example, RBR3 may have a novel, positive role in inducing cell-cycle progression (Fig. 8).
Demonstration that one RBR gene, RBR1, controls the expression of another one, RBR3, has not been reported in the plant kingdom. The RBR/E2F pathway of maize, and probably other monocots as well, appears to have a level of complexity that approaches that of animals. Clearly, more research will be required in maize and related species to reach a detailed understanding of cell-cycle regulation by the RBR/E2F pathway in important cereal crops.
Supplementary Material
Supporting Table
Acknowledgments
We thank Robert Meeley for assistance with genomics, Brian Dilkes and Chungsheng Zhang for initial database searches and cDNA analysis, Joseph Ahlander for callus RNA isolation, and the members of the B.A.L. laboratory for helpful discussions. This work was supported in part by a grant from Pioneer Hi-Bred International and Department of Energy Grant DE-96ER20242. R.A.D. received a graduate scholarship from the Conselho Nacional de Desenvolvimento Científico e Tecnológico of Brazil.
Author contributions: P.A.S., W.J.G.-K., and B.A.L. designed research; P.A.S., R.A.D., and J.T.L.-N. performed research; R.J. and W.J.G.-K. contributed new reagents/analytic tools; P.A.S., R.A.D., W.J.G.-K., and B.A.L. analyzed data; P.A.S., R.A.D., and B.A.L. wrote the paper; and B.A.L. served as laboratory director.
This contribution is part of the special series of Inaugural Articles by members of the National Academy of Sciences elected on April 30, 1996.
Abbreviations: Cyc, cyclin; CDK, Cyc-dependent kinase; DAP, days after pollination; DP, dimerization partner; E2F, adenovirus E2 promoter binding factor; RB, retinoblastoma; RBR, RB-related; WDV, wheat dwarf virus.
Data deposition: The nucleotide sequences reported in this paper have been deposited in GenBank database (accession nos. DQ124423 and DQ124424).
References
- 1.Stevens, C. & La Thangue, N. B. (2003) Arch. Biochem. Biophys. 412**,** 157–169. [DOI] [PubMed] [Google Scholar]
- 2.Blais, A. & Dynlacht, B. D. (2004) Curr. Opin. Genet. Dev. 14**,** 527–532. [DOI] [PubMed] [Google Scholar]
- 3.Attwooll, C., Lazzerini Denchi, E. & Helin, K. (2004) EMBO J. 23**,** 4709–4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dimova, D. K. & Dyson, N. J. (2005) Oncogene 24**,** 2810–2826. [DOI] [PubMed] [Google Scholar]
- 5.Bracken, A. P., Ciro, M., Cocito, A. & Helin, K. (2004) Trends Biol. Sci. 29**,** 409–417. [DOI] [PubMed] [Google Scholar]
- 6.Mariconti, L., Pellegrini, B, Cantoni, R., Stevens, R., Bergounioux, C., Cella, R. & Albani, D. (2002) J. Biol. Chem. 277**,** 9911–9919. [DOI] [PubMed] [Google Scholar]
- 7.Shen, W.-H. (2002) Trends Plant Sci. 7**,** 505–511. [DOI] [PubMed] [Google Scholar]
- 8.Inze, D. (2005) EMBO J. 24**,** 657–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frolov, M. V. & Dyson, N. J. (2004) J. Cell Sci. 117**,** 2173–2181. [DOI] [PubMed] [Google Scholar]
- 10.Cobrik, D. (2005) Oncogene 24**,** 2796–2809. [DOI] [PubMed] [Google Scholar]
- 11.Grafi, G., Burnett, R. J., Helentjaris, T., Larkins, B. A., DeCaprio, J. A., Sellers, W. R. & Kaelin, W. G., Jr. (1996) Proc. Natl. Acad. Sci. USA 93**,** 8962–8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie, Q., Sanz-Burgos, A. P., Hannon, G. H. & Gutierrez, C. (1996) EMBO J. 15**,** 4900–4908. [PMC free article] [PubMed] [Google Scholar]
- 13.Nakagami, H., Sekine, M., Murakami, H. & Shinmyo, A. (1999) Plant J. 18**,** 243–252. [DOI] [PubMed] [Google Scholar]
- 14.Nakagami, H., Kawamura, K., Sugisaka, K., Sekine, M. & Shinmyo, A. (2002) Plant Cell 14**,** 1847–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xie, Q., Suarez-Lopez, P. & Gutierrez, C. (1995) EMBO J. 14**,** 4073–4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grafi, G. & Larkins, B. A. (1995) Science 269**,** 1262–1264. [DOI] [PubMed] [Google Scholar]
- 17.Boniotti, M. B. & Gutierrez, C. (2001) Plant J. 28**,** 341–350. [DOI] [PubMed] [Google Scholar]
- 18.Gordon-Kamm, W., Dilkes, B. P., Lowe, K., Hoerster, G., Sun, X., Ross, M., Church, L. Bunde, C., Farrell, J., Hill, P., et al. (2002) Proc. Natl. Acad. Sci. USA 99**,** 11975–11980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ebel, C., Mariconti, L. & Gruissem, W. (2004) Nature 429**,** 776–780. [DOI] [PubMed] [Google Scholar]
- 20.Park, J.-A., Ahn, J.-W., Kim, Y.-K., Kim, S. J., Kim, J.-K., Kim, W. T. & Pai, H.-S. (2005) Plant J. 42**,** 153–163. [DOI] [PubMed] [Google Scholar]
- 21.Altchul, S. F., Gish, W., Miller, W. & Lipman, D. J. (1990) J. Mol. Biol. 215**,** 403–410. [DOI] [PubMed] [Google Scholar]
- 22.Thompson, J. D., Higgins, D. G. & Gibson, T. J. (1994) Nucleic Acids Res. 22**,** 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schuler, G. D., Altschul, S. F. & Lipman, D. J. (1991) Proteins 9**,** 180–190. [DOI] [PubMed] [Google Scholar]
- 24.Harlow, E. & Lane, D. (1988) Antibodies: A Laboratory Manual (Cold Spring Harbor Lab. Press, Woodbury, NY).
- 25.Leiva-Neto, J. T., Grafi, G., Sabelli, P., Dante, R. A., Woo, Y. M., Maddock, S., Gordon-Kamm, W. & Larkins, B. A. (2004) Plant Cell 16**,** 1854–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmidt, R. J., Ketudat, M., Aukerman, M. J. & Hoscheck, G. (1992) Plant Cell 4**,** 689–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ach, R. A, Durfee, T., Miller, A. B., Taranto, P., Hanley-Bowdoin, L., Zambryski, P. C. & Gruissem, W. (1997) Mol. Cell. Biol. 17**,** 5077–5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Castano, E., Kleyner, Y. & Dynlacht, B. D. (1998) Mol. Cell. Biol. 18**,** 5380–5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaye F. J., Kratzke, R. A., Gerster, J. L. & Howowitz, J. M. (1990) Proc. Natl. Acad. Sci. USA 87**,** 6177–6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bayley, S. T. & Mymryk, J. S. (1994) Int. J. Oncol. 5**,** 425–444. [DOI] [PubMed] [Google Scholar]
- 31.Yang, R., Muller, C., Huynh, V., Fung, Y. K., Yee, A. S. & Koeffler, H. P. (1999) Mol. Cell. Biol. 19**,** 2400–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beijersbergen, R. L., Carlee, L., Kerkhoven, R. M. & Bernards, R. (1995) Genes Dev. 9**,** 1340–1353. [DOI] [PubMed] [Google Scholar]
- 33.Smith, E. J., Leone, G., Degregori, J., Jakoi, L. & Nevins, J. R. (1996) Mol. Cell. Biol. 16**,** 6965–6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramirez-Parra, E., Frundt, C. & Gutierrez, C. (2003) Plant J. 33**,** 801–811. [DOI] [PubMed] [Google Scholar]
- 35.Hurford, R. K., Cobrinik, D., Lee, M. H. & Dyson, N. (1997) Genes Dev. 11**,** 1447–1463. [DOI] [PubMed] [Google Scholar]
- 36.Sage, J., Miller, A. L., Perez-Mancera, P. A., Wysocki, J. M. & Jacks, T. (2003) Nature 424**,** 223–228. [DOI] [PubMed] [Google Scholar]
- 37.Potrykus, I. (1989) TIBTECH 7**,** 269–273. [Google Scholar]
- 38.Dewitte, W. & Murray, J. A. H. (2003) Annu. Rev. Plant Biol. 54**,** 235–264. [DOI] [PubMed] [Google Scholar]
- 39.Riou-Khamlichi, C., Huntley, R., Jacqmard, A. & Murray, J. A. (1999) Science 283**,** 1541–1544. [DOI] [PubMed] [Google Scholar]
- 40.Dewitte, W., Riou-Khamlichi, C., Scofield, S., Healy, J. M., Jacqmard, A., Kilby, N. J. & Murray, J. A. (2003) Plant Cell. 15**,** 79–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uemukai, K., Iwakawa, H., Kosugi, S., de Uemukai, S., Kato, K., Kondorosi, E., Murray, J. A., Ito, M., Shinmyo, A. & Sekine, M. (2005) Plant Mol. Biol. 57**,** 83–100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Table