The adjuvant activity of CpG DNA requires T-bet expression in dendritic cells (original) (raw)
Abstract
Treatment with synthetic oligodeoxynucleotides containing CpG motifs (CpG ODNs) is remarkably protective against otherwise lethal infection. Here, we describe an essential role for the transcription factor T-bet in mediating the protective function of CpG ODNs. Loss of T-bet in conventional CD11chi dendritic cells (DCs) and in plasmacytoid DCs impaired production of IFNs. Strikingly, in contrast to _Rag2_-/- mice, _Rag2_-/- mice that also lacked T-bet (DKO) could not be rescued from lethal Listeria monocytogenes infection by prior treatment with CpG ODN. Rescue was achieved by adoptive transfer of CD11chi DCs from WT, but not _T-bet_-/-, CpG ODN-treated donor mice. We conclude that T-bet in DCs is required for the adjuvant activity of CpG ODN in infection, revealing its vital role in innate immunity.
Keywords: IFN-γ, oligodeoxynucleotide, plasmacytoid dendritic cells
In the 19th century, crude extracts known as “Coley's toxins” were used for the successful treatment of patients with advanced malignancy, and extracts from Mycobacterium bovis bacillus Calmette–Guérin (BCG) continue to be a useful immunotherapy for bladder cancer (1, 2). DNA is the active component in BCG that induces antitumor immune responses, and it is the presence of CpG dinucleotides in particular base contexts in DNA that is required (1, 2). Vertebrate and bacterial genomic DNAs differ significantly in their CpG content and structural composition: vertebrate genomes show suppression of CpG dinucleotides and are usually methylated, abolishing their immune stimulatory effect (1, 2), which is characterized by activation of multiple cell types and the secretion of IL-12 and IFNγ, leading to a type 1 cytokine milieu (3).
Recent studies using CpG oligodeoxynucleotides (ODNs) as adjuvants have demonstrated additional therapeutic effects for bacterial, parasitic, and viral infections in mouse models (3). Listeria monocytogenes, a food-borne human pathogen, is a Gram-positive facultative intracellular bacterium that causes significant disease in immunocompromised and pregnant individuals (4, 5). The innate immune response plays a significant role in controlling the spread of infection in the host as demonstrated by studies of Listeria infection in severe combined immunodeficient and _Rag2_-/- mice (6). Treatment of naïve mice with CpG ODN protects from lethal doses of L. monocytogenes within 48 h (1, 2, 7).
Toll-like receptor 9 is the main receptor for CpG ODN and activates the transcription factor NFκB (8). Because the NFκB signaling pathway is shared by all IL-1/toll-like receptor family members, additional signaling pathways account for the protective effects unique to CpG ODN. Interestingly, CpG ODN was reported to mediate direct up-regulation of T-bet in B cells, suggesting this transcription factor as an alternative signaling pathway (9).
T-bet, a member of the evolutionarily conserved T-box transcription factor family, controls T helper 1 (Th1) lineage commitment. Mice that lack T-bet have impaired type 1 immunity characterized by a pronounced decrease in IFNγ and elevated levels of Th2 cytokines (10). As a consequence, these mice failed to control a Th1-dependent protozoan infection (Leishmania major), were resistant to the development of autoimmune diseases, but developed spontaneous asthma (11). Recently, we demonstrated that T-bet also influences the generation of type 1 immunity by controlling IFNγ gene transcription and effector function in CD8, natural killer (NK), and dendritic cells (DCs) (12–14). Further, _T-bet_-/- DCs were impaired in their ability to activate the Th1 program, an effect that may be partly attributed to reduced IFNγ production (14). Altogether, these observations indicated a requirement for T-bet to drive type 1 immunity through both the adaptive and the innate immune systems. In this study, we demonstrate that T-bet expression in DCs is required for the protective effect of CpG treatment against death from infection with lethal doses of L. monocytogenes.
Materials and Methods
Mice. C57BL/6 (B6), BALB/c, _Rag2_-/-/BALB/c, 129/SV mice, and _Stat1_-/- mice (4–8 weeks old) were purchased from Taconic Farms. The generation and screening of T-bet-deficient mice have been described in refs. 10 and 13, and mice used here have been backcrossed at least six generations onto the B6 and BALB/c backgrounds. T-bet-deficient mice on a BALB/c background were backcrossed to BALB/c _Rag2_-/- animals. All mice were housed in a pathogen-free facility at the Harvard School of Public Health, and all animal studies were performed according to institutional and National Institutes of Health guidelines for animal use and care.
Cell Lines. The melanoma cell line B16-FLT3L was provided by G. Dranoff (Harvard Medical School).
ODNs. The sequences of phosphorothioate ODNs (Qiagen, Valencia, CA) are as follows: TCCATGACGTTCCTGATGCT for stimulatory CpG ODN and TTCATGAGCTTCCTGATGCT for control (non-CpG) ODN. ODNs were synthesized at the Center for Biologics Evaluation and Research (Rockville, MD) core facility. Immune stimulation was obtained by administering two CpG ODNs (GCTAGACGTTAGCGT and TCAACGTTGA). The CpG motifs (underlined) were switched to TpG or GpC in control ODNs (GCTAGATGTTAGGCT and TCAAGCTTGA).
Purification, Isolation, and Adoptive Transfer of DCs. Classical CD11chi DCs were isolated by collagenase treatment (14); enriched by centrifugation in a cell separation medium (Accudenz, Accurate Chemicals); T, B, and NK cells were depleted by using coated magnetic beads (anti-CD90, anti-CD3, and anti-CD19, respectively); and DCs were further enriched with anti-CD11c magnetic beads (Miltenyi Biotec, Auburn CA) prior to FACS-sorting. In some experiments, DCs were first positively selected with anti-CD11c magnetic beads from collagenase-treated spleen. All DCs were FACS-sorted into subpopulations by staining with FITC-MHC II (I-E/I-A), PE-DX5, and APC-CD11c (Pharmingen). Plasmacytoid DCs (pDCs) were obtained from mice injected with 106 B16-FLT3L (14). After 7 days, spleens were treated with collagenase. After T, B, and NK cell depletion, pDCs (B220hi, CD11cint, and DX5neg) were FACS-sorted by using FITC-B220, PE-CD11c, and CyC-DX5. For adoptive transfers, WT and _T-bet_-/- mice (20 donors per genotype) were injected i.p. with CpG ODN (50 μg per mouse) for 72 h, and DCs were purified as above. FACS-sorting was performed to obtain the classical mature DC (CD11chi/MHChi/DX5neg) population. Splenic B cells were purified by positive selection with anti-CD19-coated magnetic beads.
DC and B Cell Stimulation. DCs, pDCs, and B cells were cultured in DMEM-10 at 1 × 106/ml, stimulated with mouse recombinant IL-12 (10 ng/ml, R & D Systems), IFNγ (10 ng/ml, Peprotech, Rocky Hill, NJ), CpG ODN (1 μg/ml), and control ODN.
Real-Time PCR, ELISA, and Western Blot Analysis. RNA isolated with TRIzol (Sigma) was treated with DNase I (Invitrogen), and cDNA synthesis was performed with 1 μg of total RNA by using SuperScript II RNase H- reverse transcriptase (Invitrogen). Real-time RT-PCR and sequences of primers and TaqMan probe for T-bet, IFNγ, TNFα, IL-12 subunits p40 and p35, and other inflammatory cytokines were performed as described in ref. 14. Expression levels are reported relative to β-actin abundance. Protein levels of IFNγ, TNFα, and IL-12p40 were detected by ELISA (Pharmingen). ELISA for IFNα was done by using 5 μg/ml capture mAb, 200 units/ml secondary polyclonal Ab (PBL Biomedical Laboratories, Piscataway, NJ), and a 1/1,000 dilution of alkaline phosphatase-conjugated anti-rabbit IgG (Jackson ImmunoResearch). Statistical significance was evaluated by using Student's t test.
Bacteria and Growth Conditions. L. monocytogenes strain EGD (American Type Culture Collection) was grown in modified Mueller–Hinton broth (Difco). BALB/c WT, _T-bet_-/-, and _T-bet_-/-/_Rag2_-/- mice were treated i.p. with 50 μg of CpG ODN, control ODN, or PBS only. Three days later, mice were challenged with 1,000 or 250 LD50 of L. monocytogenes (i.p). Cumulative survival rates were monitored on a daily basis for 14 or 21 days. All immunization and bacterial challenge experiments were performed on a minimum of 5–10 mice per group. Statistical significance was evaluated by using a Kaplan–Meier survival analysis.
Results and Discussion
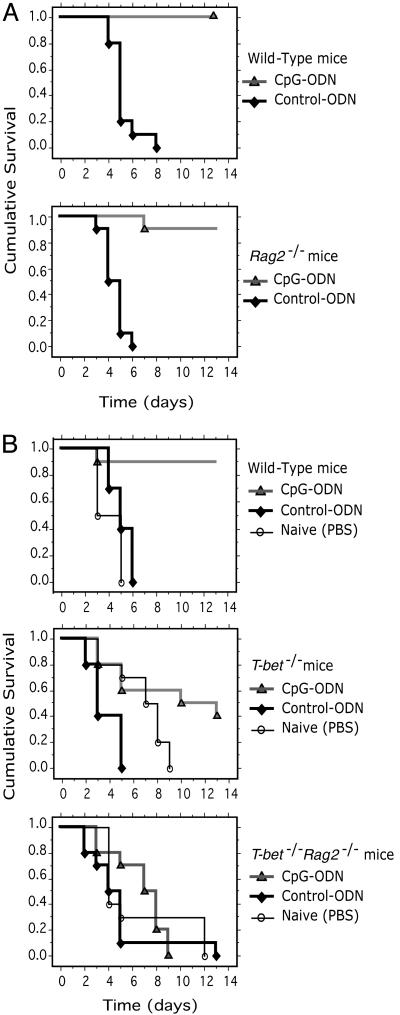
The Adjuvant Activity of CpG ODN Requires T-bet Expression in the Innate Immune System. CpG ODN pretreatment protects immunocompetent WT mice from lethal L. monocytogenes infection (3). In initial experiments (Fig. 1_B_ Middle), we observed that immunocompetent _T-bet_-/- mice were only partially protected from lethal (1,000 LD50) L. monocytogenes infection by pretreatment with CpG ODN, compared with WT mice (9 of 10 WT vs. 4 of 10 _T-bet_-/- mice survived, not statistically significant; P < 0.07). To specifically interrogate the function of T-bet in innate immunity and in the protection mediated by CpG ODN, we first determined whether CpG ODN pretreatment rescued _Rag2_-/- as well as WT mice from lethal L. monocytogenes. B6 WT or _Rag2_-/- mice were treated with CpG ODN, control ODN, or PBS at 72 h before challenge with a lethal dose of live L. monocytogenes. As discussed above, 10 of 10 CpG ODN-treated WT mice survived a lethal dose of L. monocytogenes over a 13-day time course (Fig. 1 A Upper). Interestingly, 9 of 10 CpG ODN-treated _Rag2_-/- mice also survived infection with L. monocytogenes during this time course (Fig. 1 A Lower). In contrast, as expected, 10 of 10 WT or _Rag2_-/- mice treated with either control ODN or PBS died before day 6 from L. monocytogenes infection (Fig. 1 A, P < 0.0001 when compared to all CpG-ODN-treated groups). This experiment provides direct evidence that CpG ODN primes the innate immune system to resist lethal challenge from L. monocytogenes, even in the absence of an adaptive immune response.
Fig. 1.
_Rag2_-/- mice deficient in T-bet (double-knockout, DKO) succumb to infection with L. monocytogenes despite treatment with CpG ODN. (A) Survival curves of B6 WT and _Rag2_-/- mice. (B) Survival curves of BALB/c WT, _T-bet_-/-, and _Rag2_-/-_T-bet_-/- (DKO) mice. All mice were treated (i.p.) with 50 μg of CpG ODN, control ODN, or PBS only. Three days later, mice were challenged with 1,000 LD50 of Listeria (i.p.). Cumulative survival rates were monitored on a daily basis for 14 days. All immunizations and bacterial challenge experiments were performed on a minimum of 10 mice per group. Statistical significance was evaluated by using a Kaplan–Meier survival analysis.
The T-bet mutant allele was bred onto the _Rag2_-/- background (DKO). CpG ODN treatment rescued 9 of 10 BALB/c WT mice from L. monocytogenes infection (Fig. 1_B_ Upper), but in contrast to _Rag2_-/- mice, completely failed to rescue BALB/c _T-bet_-/- _Rag2_-/- DKO mice from death. All DKO mice died before day 9 after challenge (Fig. 1_B_ Lower, P < 0.001 when compared to CpG-ODN-treated WT groups). All mice treated with control ODN or PBS died rapidly after challenge (Fig. 1_B_, P < 0.0001 when compared to all CpG-ODN-treated groups). These experiments demonstrate that T-bet expression in innate immune system cells is required for the adjuvant activity of CpG ODN against lethal infection with L. monocytogenes. We have compared the B6 and BALB/c RAG strains in this model and find no difference in their ability to be rescued from lethal L. monocytogenes infection by CpG treatment. At the high challenge doses administered, essentially all animals of both strains succumb to infection, and with the same mean time to death.
T-bet Expression in DCs. CpG ODN is a potent DC activator (1). We investigated whether CpG ODN induced T-bet expression in DC subsets. Classical DCs (CD11chi/MHChi/DX5neg) were cultured for different time periods with CpG ODN or control ODN, and RNA was isolated for real-time PCR analysis. Low levels of T-bet transcripts were present at baseline (Fig. 2_A_ Left). A modest induction of T-bet mRNA, minimally augmented by IL-12, was observed after 16 h (Fig. 2_A_ Left). IFNγ is the major inducer of T-bet mRNA in CD11chi DCs and myeloid DCs (14, 15), and we observed a marked T-bet induction by IFNγ in DCs peaking at 3 h, remaining high up to 6 h, and declining after 16 h (Fig. 2 A Left), but no synergistic effect between IFNγ and CpG ODN was detected (Fig. 2 A Left). To establish definitively whether T-bet induction by CpG ODN was IFNγ-dependent, we examined IFNγ-/- DCs. T-bet mRNA was not induced by CpG ODN in the absence of endogenous IFNγ at 16 h (Fig. 2 A Right). We conclude that CpG and IL-12 act indirectly through their induction of IFNγ to up-regulate T-bet expression in classical DCs.
Fig. 2.
T-bet expression in DCs is dependent on IFNγ. (A and B) Classical DCs (DX5neg/CD11chi/MHC-IIhi) (A) and pDCs (DX5neg/CD11cInt/B220hi) (B) were FACS-sorted (purity >95%) from the spleens of B6 and 129S/v WT, 129S/v _Stat1_-/-, and B6 IFNγ-/- mice. (C) B cells were positively selected with anti-CD19-coated magnetic beads from the spleens of WT and IFNγ-/- mice and stimulated with CpG ODN (1 μg/ml) alone or in combination with exogenous IL-12 (10 ng/ml) and IFNγ (10 ng/ml). At the indicated times, mRNA was prepared from each sample for real-time PCR analysis. T-bet mRNA is expressed as copies per 10,000 mRNA copies of β-actin.
The expression of T-bet has not previously been examined in the pDC subset. Purified splenic pDCs were stimulated for 6 h with CpG ODN, an early time point to avoid secondary effects due to CpG-induced IL-12 secretion. Fig. 2_B_ Upper shows that CpG ODN alone failed to up-regulate T-bet mRNA in pDCs, and no synergistic effect between CpG ODN and IL-12 was observed. Treatment of pDCs with CpG ODN and IFNγ or control ODN and IFNγ did induce T-bet mRNA transcripts and, as for classical DCs, there was no synergistic effect between CpG ODN and IFNγ (Fig. 2_B_ Upper). IFNγ induction of T-bet mRNA in classical DCs is dependent on Stat1 (14). Fig. 2_B_ Lower shows that up-regulation of T-bet mRNA in pDCs by both CpG ODN and by IFNγ also was dependent on Stat1, because _Stat1_-/ - pDCs failed to increase T-bet transcripts. Thus, induction of T-bet by CpG and IL-12 is dependent on signaling through IFNγ, the IFNγ receptor, and the Stat1 transcription factor.
Liu et al. (9) demonstrated that T-bet mRNA induction in B cells by CpG ODN was Stat1- and IFNγ-independent. It was possible that the control of T-bet expression differed between B cells and DCs. As shown in Fig. 2_C_, we confirmed the induction of T-bet at 24 h by CpG ODN and IL-12 as well as by IFNγ. However, induction of T-bet mRNA was absolutely dependent on IFNγ, because IFNγ-/- B cells failed to induce T-bet in response to CpG ODN and IL-12 (Fig. 2_C_). The expression of T-bet mRNA was restored by the addition of exogenous IFNγ in the presence of CpG ODN or control ODN (Fig. 2_C_). We have no obvious explanation for the discrepancy between our results and those of Liu et al. (9).
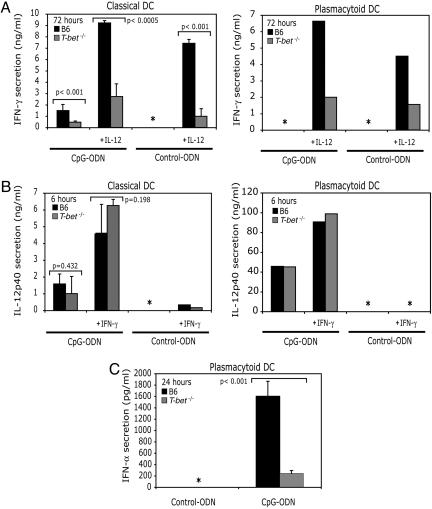
T-bet Is Important for the Optimal Production of Type I/II IFNs by DCs. Previously, we reported an important role for T-bet in IL-12-dependent IFNγ production from CD11chi DCs (14). We investigated a role for T-bet in IFNγ production in DCs (CD11chi/MHChi/DX5neg) and pDCs upon treatment with CpG ODN. Stimulation of DCs with CpG ODN alone, or in combination with IL-12 for 72 h, a time point that mimics the kinetics of IFNγ production in vivo by CpG ODN (3), resulted in 1.5 and 9.2 ng/ml IFNγ from WT DCs (Fig. 3_A_ Left) but only 0.45 and 2.7 ng/ml from _T-bet_-/- DCs (Fig. 3_A_ Left). IFNγ production was IL-12-dependent, because similar levels of IFNγ were produced upon treatment of DCs with IL-12 alone (Fig. 3_A_ Left). Although pDCs are not well known for their capacity to produce type II IFNs, we investigated a role for T-bet in IFNγ production in pDCs. WT pDCs do not produce IFNγ after CpG ODN stimulation alone (Fig. 3_A_ Right) but require the addition of exogenous IL-12. _T-bet_-/- pDCs produced significantly less IFNγ than WT pDCs (Fig. 3_A_ Right).
Fig. 3.
T-bet is required for optimal production of type I/II IFNs. (A) IFNγ production from classical DCs (DX5neg/CD11chi/MHC-IIhi) and pDCs (DX5neg/CD11cInt/B220hi). (B) IL-12p40 production from classical and pDCs. (C) IFNα production from pDCs. DCs were FACS-sorted (purity >95%) from WT and _T-bet_-/- spleens and stimulated with CpG ODN (1 μg/ml) alone or in combination with exogenous IL-12 (10 ng/ml) and IFNγ (10 ng/ml). At the indicated times, supernatants were harvested for ELISA to measure IFNγ, IL-12p40, and IFNα. These results represent at least three independent experiments, except for pDC production of IFNγ and IL-12, which represents two independent experiments. *, not detected.
CpG ODN stimulates the early production and release of IL-12p40 (16). It was possible that IFNγ production from pDCs was the consequence of IL-12 signaling rather than directly downstream of toll-like receptor 9. However, both classical DCs and pDCs from WT and _T-bet_-/- mice produced equivalent amounts of IL-12p40 in response to CpG ODN at 6 h (Fig. 3_B_); thus the defect in IFNγ was not secondary to impaired IL-12 production in the absence of T-bet. The combination of CpG ODN and exogenous IFNγ resulted in a significant increase in the production of IL-12p40 by pDCs from both WT and _T-bet_-/- mice. However, IFNγ alone failed to elicit IL-12p40 secretion from classical and pDCs (Fig. 3_B_).
CpG ODN triggers the production of IFNα from pDCs (17, 18). Fig. 3_C_ demonstrates that T-bet plays an essential role in IFNα production, because CpG ODN-induced IFNα secretion was greatly diminished in the absence of T-bet. In the light of recent reports from three laboratories demonstrating a pathogenic, rather than a protective, role for IFNα in handling Listeria infection (19–21), it was unlikely that the requirement for T-bet we observed above (Fig. 1) could be attributed to its function in driving IFNα production. Although we are not ruling out a role for pDCs in the phenotype we observed, the above data suggested that the function of T-bet in conventional DCs might be more critical. We were not able to measure IFNγ, IFNα, or a broader range of cytokines in vivo. Because Listeria is highly infectious to humans, the Center for Biologics Evaluation and Research animal regulations barred the collection or processing of blood or body fluids from these mice, which have very high levels of infectious bacteria.
Adoptive Transfer of CpG ODN-Treated WT DCs Restores Resistance of _Rag2_-/-_T-bet_-/- DKO Mice to Infection with L. monocytogenes. Adoptive transfer of antigen-presenting cell (APC) fractions revealed a critical role of IFNγ in resistance to L. monocytogenes infection; reconstitution with WT and IFNγR-/- but not IFNγ-/- APCs increased the resistance of γc-/-_Rag2_-/- mice to L. monocytogenes infection (6), and several groups have provided evidence for a function of DCs rather than other components of the innate immune system in L. monocytogenes infection (22–27).
Therefore, we performed adoptive transfer experiments where naïve (not treated with CpG ODN) _Rag2_-/- or _Rag2_-/-_T-bet_-/- DKO mice received splenic DCs (CD11chi/MHC-IIhi/DX5neg) from WT or _T-bet_-/- mice vaccinated 72 h prior with CpG ODN. In the absence of CpG treatment, there is no protection from donor spleen cells (data not shown). Fig. 4 Upper shows that the adoptive transfer of DCs (7 × 105 per mouse) from CpG ODN-treated WT mice conferred resistance to _Rag2_-/- mice against L. monocytogenes infection up to 21 days (7 of 10 mice survived, P ≤ 0.0001). All PBS-treated naïve _Rag2_-/- mice died after 12 days of L. monocytogenes infection (Fig. 4 Upper). Strikingly, adoptive transfer of DCs from CpG ODN-treated WT mice also rescued _Rag2_-/-_T-bet_-/- DKO mice (Fig. 4 Lower). After 21 days of infection, 7 of 10 mice survived (P ≤ 0.0001, compared with PBS treatment). By contrast, the adoptive transfer of DCs from CpG ODN-treated _T-bet_-/- mice failed to provide protection to _T-bet_-/-_Rag2_-/- DKO mice against L. monocytogenes (Fig. 4 Lower): all recipient mice died (10 of 10) within the first 18 days after infection, similar to PBS treatment (P < 0.001, compared with adoptive transfer of WT DCs). Some small protection was afforded by _T-bet_-/- DCs, as evidenced by a small increase in survival times, but this was not statistically significant.
Fig. 4.
Adoptive transfer of DCs from CpG ODN-treated WT but not _T-bet_-/- mice restores resistance of _Rag2_-/-_T-bet_-/- DKO mice to lethal L. monocytogenes. WT and _T-bet_-/- donor mice were treated with CpG ODN for 72 h. Classical DCs (DX5neg/CD11chi/MHC-IIhi) were FACS-sorted (purity >95%) from donor mice and adoptively transferred to _Rag2_-/- and DKO mice (7 × 105 per mouse) that were challenged with L. monocytogenes (250 LD50) a day later. Survival was monitored for 21 days. Statistical significance between groups ranged from P < 0.001 to P < 0.0001.
Although we cannot rule out that other innate immune system cells directly or indirectly play a role, in the CpG protection observed, we do not believe this to be the case. In our hands, transfer of NK-depleted spleen cells from CpG-treated donors did confer protection to Listeria, suggesting that, in normal mice, NK cells are not critical (D.M.K, unpublished observations). Further, transfer of CpG-primed WT or _T-bet_-/- DCs into WT hosts resulted in equivalent recruitment of NK cells to spleen (G.L.-V. and L.H.G., unpublished observations). We did not examine macrophages directly, because T-bet is not expressed in this cell type (14).
A recent study (28) reported no difference in survival of _T-bet_-/- mice to low-dose (0.3–1.0 LD50) L. monocytogenes and suggested that the immune system might be sufficient to handle this pathogen at low infectivity in the absence of T-bet. We also have noted situations in which production of IFNγ is T-bet-independent (29). The studies described here, however, address a different question and required the use of lethal (at least 1,000-fold higher) doses of L. monocytogenes. The focus of our studies is thus not on understanding the role of T-bet in immunity to Listeria, but rather in the apparent protection against death that is induced by treatment of infected mice with CpG ODNs. Although DCs are known to be critical for the immune response to Listeria (27, 30), our results provide evidence that DCs are responsible for the protective effect of CpG ODN treatment against lethal L. monocytogenes. Recently, Serbina et al. (22) have identified a cell population with features of DCs that are recruited to the spleen of _L. monocytogenes_-infected mice in a chemokine receptor 2-dependent fashion (27). These cells (Tip DCs) are the predominant source of TNF and inducible nitric-oxide synthase during L. monocytogenes infection and may orchestrate the innate immune response against this pathogen. Although we have not yet been successful in determining the expression of T-bet in Tip DCs, our experiments demonstrate that T-bet expression in conventional DCs (CD11chi/MHC-IIhi/DX5neg) is required for the adjuvant activity of CpG ODN against L. monocytogenes. Diminished IL-12-dependent production of IFNγ on CpG ODN treatment may contribute to the failure of _T-bet_-/- DCs to mediate protection against this intracellular pathogen.
There is great interest in investigating whether the immune responses elicited by CpG ODN can be harnessed for immune therapy of human cancer, allergy, and infectious disease. We believe that identifying a requirement for T-bet in the adjuvant protection afforded by CpG treatment is an important advance in efforts to maximize type 1 immunity against foreign agents. Studies that define the genetic regulatory networks instigated by T-bet in DCs and in pDCs should provide additional therapeutic targets.
Acknowledgments
We thank N. Iwakoshi, A. Erlebacher, and J. Wang for thoughtful review of the manuscript and valuable technical advice; L. Kangaloo, B. Tang, and J. Ramirez for excellent technical assistance and animal care; and L. de Elizalde for expert manuscript preparation. This work was supported by a National Science Foundation Graduate Fellowship (to G.L.-V.), National Institutes of Health Grant P01AI056296 (to L.H.G.), and an Ellison Medical Foundation Grant (to L.H.G.).
Author contributions: G.L.-V., D.M.K., and L.H.G. designed research; G.L.-V. and S.I. performed research; D.M.K. and L.H.G. analyzed data; and G.L.-V and L.H.G. wrote the paper.
Abbreviations: ODN, oligodeoxynucleotide; DC, dendritic cell; pDC; plasmacytoid DC; NK, natural killer.
References
- 1.Krieg, A. M. (2002) Annu. Rev. Immunol. 20**,** 709-760. [DOI] [PubMed] [Google Scholar]
- 2.Krieg, A. M. (2001) Trends Microbiol. 9**,** 249-252. [DOI] [PubMed] [Google Scholar]
- 3.Ishii, K. J., Gursel, I., Gursel, M. & Klinman, D. M. (2004) Curr. Opin. Mol. Ther. 6**,** 166-174. [PubMed] [Google Scholar]
- 4.Edelson, B. T. & Unanue, E. R. (2000) Curr. Opin. Immunol. 12**,** 425-431. [DOI] [PubMed] [Google Scholar]
- 5.Pamer, E. G. (2004) Nat. Rev. Immunol. 4**,** 812-823. [DOI] [PubMed] [Google Scholar]
- 6.Suzue, K., Asai, T., Takeuchi, T. & Koyasu, S. (2003) Eur. J. Immunol. 33**,** 2666-2675. [DOI] [PubMed] [Google Scholar]
- 7.Klinman, D. M. (2004) Expert Opin. Biol. Ther. 4**,** 937-946. [DOI] [PubMed] [Google Scholar]
- 8.Takeshita, F., Gursel, I., Ishii, K. J., Suzuki, K., Gursel, M. & Klinman, D. M. (2004) Semin. Immunol. 16**,** 17-22. [DOI] [PubMed] [Google Scholar]
- 9.Liu, N., Ohnishi, N., Ni, L., Akira, S. & Bacon, K. B. (2003) Nat. Immunol. 4**,** 687-693. [DOI] [PubMed] [Google Scholar]
- 10.Szabo, S. J., Sullivan, B. M., Stemmann, C., Satoskar, A. R., Sleckman, B. P. & Glimcher, L. H. (2002) Science 295**,** 338-342. [DOI] [PubMed] [Google Scholar]
- 11.Szabo, S. J., Sullivan, B. M., Peng, S. L. & Glimcher, L. H. (2003) Annu. Rev. Immunol. 21**,** 713-758. [DOI] [PubMed] [Google Scholar]
- 12.Sullivan, B. M., Juedes, A., Szabo, S. J., von Herrath, M. & Glimcher, L. H. (2003) Proc. Natl. Acad. Sci. USA 100**,** 15818-15823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Townsend, M., Weinmann, A. S., Matsuda, J., Saloman, R., Farnham, P., Biron, C. A., Gapin, L. & Glimcher, L. H. (2004) Immunity 20**,** 477-494. [DOI] [PubMed] [Google Scholar]
- 14.Lugo-Villarino, G., Maldonado-López, R., Possemato, R., Peñaranda, C. & Glimcher, L. H. (2003) Proc. Natl. Acad. Sci. USA 100**,** 7749-7754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lighvani, A. A., Frucht, D. M., Jankovic, D., Yamane, H., Aliberti, J., Hissong, B. D., Nguyen, B. V., Gadina, M., Sher, A., Paul, W. E. & O'Shea, J. J. (2001) Proc. Natl. Acad. Sci. USA 98**,** 15137-15142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takeshita, F. & Klinman, D. M. (2000) Eur. J. Immunol. 30**,** 1967-1976. [DOI] [PubMed] [Google Scholar]
- 17.Wang, Y. H. & Liu, Y. J. (2004) Immunity 21**,** 1-2. [DOI] [PubMed] [Google Scholar]
- 18.Rothenfusser, S., Tuma, E., Endres, S. & Hartmann, G. (2002) Hum. Immunol. 63**,** 1111-1119. [DOI] [PubMed] [Google Scholar]
- 19.O'Connell, R. M., Saha, S. K., Vaidya, S. A., Bruhn, K. W., Miranda, G. A., Zarnegar, B., Perry, A. K., Nguyen, B. O., Lane, T. F., Taniguchi, T., et al. (2004) J. Exp. Med. 200**,** 437-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Auerbuch, V., Brockstedt, D. G., Meyer-Morse, N., O'Riordan, M. & Portnoy, D. A. (2004) J. Exp. Med. 200**,** 527-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carrero, J. A., Calderon, B. & Unanue, E. R. (2004) J. Exp. Med. 200**,** 535-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Serbina, N. V., Salazar-Mather, T. P., Biron, C. A., Kuziel, W. A. & Pamer, E. G. (2003) Immunity 19**,** 59-70. [DOI] [PubMed] [Google Scholar]
- 23.Pron, B., Boumaila, C., Jaubert, F., Berche, P., Milon, G., Geissmann, F. & Gaillard, J. L. (2001) Cell Microbiol. 3**,** 331-340. [DOI] [PubMed] [Google Scholar]
- 24.Jung, S., Unutmaz, D., Wong, P., Sano, G., De los Santos, K., Sparwasser, T., Wu, S., Vuthoori, S., Ko, K., Zavala, F., et al. (2002) Immunity 17**,** 211-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andersson, A., Dai, W. J., Di Santo, J. P. & Brombacher, F. (1998) J. Immunol. 161**,** 5600-5606. [PubMed] [Google Scholar]
- 26.Barber, E. M. & Pollard, J. W. (2003) J. Immunol. 171**,** 37-46. [DOI] [PubMed] [Google Scholar]
- 27.Tam, M. A. & Wick, M. J. (2004) Trends Immunol. 25**,** 335-339. [DOI] [PubMed] [Google Scholar]
- 28.Way, S. S. & Wilson, B. W. (2004) J. Immunol. 173**,** 5918-5922. [DOI] [PubMed] [Google Scholar]
- 29.Peng, S. L., Szabo, S. J. & Glimcher, L. H. (2002) Proc. Natl. Acad. Sci. USA 99**,** 5545-5550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sundquist, M., Johansson, C. & Wick, M. J. (2003) APMIS 111**,** 715-724. [DOI] [PubMed] [Google Scholar]