Lipopolysaccharide from Nonvirulent Ara+ Burkholderia pseudomallei Isolates Is Immunologically Indistinguishable from Lipopolysaccharide from Virulent Ara− Clinical Isolates (original) (raw)
Abstract
Different lines of evidence suggest that a discrepancy between the distribution of Burkholderia (Pseudomonas) pseudomallei in the environment and the distribution of the disease melioidosis is attributable, at least in part, to phenotypic differences between clinical and some environmental isolates. Two antigenically and biochemically distinct biotypes have been described, only one of which is virulent. In this study, lipopolysaccharides (LPSs) were extracted by the proteinase K digestion method from a total of 214 B. pseudomallei isolates, and their immunoreactivities with sera from patients with different clinical spectra and with other infections were evaluated. With the exception of 4 isolates from a total of 214 tested, the sodium dodecyl sulfate-polyacrylamide gel electrophoresis silver-staining profiles of the LPSs from the two biotypes showed identical ladder patterns that were typical for smooth LPSs from other gram-negative bacteria. The 210 isolates with typical LPS patterns (119 Ara− clinical, 13 Ara− soil, 70 Ara+ soil, and 8 reference National Type Culture Collection strains) also exhibited similar immunoblot profiles against pooled sera from patients with melioidosis and hyperimmune mouse sera. Concordant findings were noted in the indirect enzyme-linked immunosorbent assay with Ara− and Ara+ LPSs to coat the microtiter plates. The LPSs of the different B. pseudomallei biotypes appear antigenically indistinguishable. It is, therefore, unlikely that this component is related to the virulence and pathogenicity of B. pseudomallei.
Burkholderia (Pseudomonas) pseudomallei is the causative agent of melioidosis, a potentially fatal human infection in the tropics (3, 4, 28). The disease has diverse clinical manifestations, from localized infection to acute fatal septicemia. Subclinical infections, which are defined by the detection of hemagglutinating antibody in people residing in areas of endemicity, e.g., northeastern Thailand and northern Australia, are very common. Antibody to lipopolysaccharide (LPS) is believed to be a major contributor to seropositivity, since the indirect hemagglutination assay routinely used for screening in a diagnostic laboratory (28) is biased toward the detection of anti-LPS. In Thailand, melioidosis is largely restricted to the northeast, yet B. pseudomallei has been recovered from the environment, e.g., from soil throughout the country (23, 27). Clinical isolates from different geographical locations, from Thailand and Malaysia to Australia, appeared to have similar morphological and biochemical characteristics (28). Although B. pseudomallei has been reported to possess two structurally different forms of LPS (16), a previous study by Pitt et al. (18) showed the LPSs from a limited number of clinical isolates, presumably belonging to the Ara− biotype described in the present communication, to be rather homogeneous with regard to their immunoreactivities with human and animal sera.
It was shown subsequently (27) that all clinical B. pseudomallei isolates failed to utilize l-arabinose (Ara−), whereas some soil isolates from the areas of endemic infection could utilize this carbohydrate (Ara+). It has been demonstrated very recently that the Ara− biotype is virulent in experimental animals (1, 22). Differences in the virulence of B. pseudomallei could result from differences in the LPS molecules, which are a known toxic component of various gram-negative organisms. However, the results presented in this study showed that the LPSs from Ara− and Ara+ biotypes were indistinguishable from one another with regard to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) profiles and immunoreactivities with immune sera.
MATERIALS AND METHODS
Bacterial isolates.
A total of 123 clinical B. pseudomallei isolates (Ara−) were from patients admitted to University Hospital (Khon Kaen University) in Khon Kaen province and Sappasitprasong Hospital in Ubon Ratchatani province in the northeastern part of Thailand, where infection is endemic. Thirteen Ara− soil isolates and 70 Ara+ soil isolates used were collected from different geographical locations (Table 1). All isolates were originally identified as B. pseudomallei based on their biochemical characteristics, colonial morphologies on selective media, antibiotic sensitivity profiles, and reactions with polyclonal antibody (27, 28). They were classified subsequently into Ara+ or Ara− biotypes based on their abilities to utilize l-arabinose (27). In addition to these 206 local isolates, eight reference B. pseudomallei strains (NTCC 1688, NTCC 4845, NTCC 4846, NTCC 6700, NTCC 7131, NTCC 7383, NTCC 8016, and NTCC 8707) were also included in the analysis.
TABLE 1.
Sources of B. pseudomallei isolates used for LPS preparation
| Source | Total no. of isolates | No. of isolates that were: | |
|---|---|---|---|
| Ara− | Ara+ | ||
| Clinical (humans) | 123 | 123 | 0 |
| Environmental (soil) | |||
| Thailand | |||
| Endemic (northeast) | 20 | 9 | 11 |
| Nonendemic (central) | 11 | 0 | 11 |
| Vietnam | 52 | 4 | 48 |
| NTCCa | 8 | 8 | 0 |
| Total | 214 | 144 | 70 |
Serum specimens.
The sera from patients with culture-proven melioidosis and other septicemias caused by gram-negative organisms used in this study (Table 2) were the same as those described in the previous report (21). Normal sera were collected from healthy individuals in areas of endemicity and nonendemicity. Positive and negative reference sera used throughout this study were pooled from four to five specimens from each group, aliquoted, and kept frozen at −20°C.
TABLE 2.
ELISA specificities for LPSs from Ara+ and Ara− B. pseudomallei biotypes
| Serum source (n) | OD490 for the following antigens for coatinga: | ||
|---|---|---|---|
| Ara+ LPS | Ara− LPS | Affinity purified | |
| Patients with melioidosis (20) | 0.804 ± 0.341 | 0.730 ± 0.300 | 0.680 ± 0.380 |
| Septicemic (10) | 0.780 ± 0.330 | 0.720 ± 0.290 | 0.547 ± 0.336 |
| Localized (10) | 0.826 ± 0.366 | 0.740 ± 0.330 | 0.818 ± 0.394 |
| Patients with other infections (10) | 0.079 ± 0.083 | 0.060 ± 0.075 | 0.075 ± 0.099 |
| Normal individuals | |||
| Nonendemic (20) | 0.020 ± 0.013 | 0.022 ± 0.074 | 0.011 ± 0.007 |
| Endemic (20) | 0.020 ± 0.017 | 0.033 ± 0.023 | 0.036 ± 0.016 |
Production of mouse anti-LPS antibody.
Six-week-old female BALB/c mice were immunized intraperitoneally with the Ara− LPS prepared by the phenol-chloroform-petroleum ether method (10). Each animal received an initial injection of 100 μg of LPS in complete Freund’s adjuvant followed 1 month later by another injection of the same amount of LPS in incomplete Freund’s adjuvant. The animals were bled 3 weeks later, and the antibody titer of pooled serum tested by indirect enzyme-linked immunosorbent assay (ELISA) against purified LPS was higher than 1:200,000.
Preparation of B. pseudomallei LPS.
LPS was extracted from individual B. pseudomallei isolates by either the proteinase K (15) or the phenol-chloroform-petroleum ether (10) method. The two preparations were similar to each other with regard to SDS-PAGE profiles and immunoreactivities with immune sera (unpublished data). Unless otherwise specified, the data presented in this study were obtained with the proteinase K product because of its simplicity and higher yield. Briefly, clinical and soil isolates of both biotypes were grown individually on trypticase agar at 37°C for 48 h and washed with phosphate-buffered saline at pH 7.2, and then the bacterial suspensions were adjusted to contain approximately 109 CFU/ml, based on turbidimetric measurement. After centrifugation at 5,000 × g for 15 min, the supernatant fluid was discarded, the pellet was resuspended in an equal volume of 4% mercaptoethanol in 6 M urea, and the suspension was boiled for 20 min. Then, 1/10 volume of proteinase K (2.5 mg/ml) was added and digestion was allowed to proceed at 50°C for 16 h. The enzymatic reaction was terminated by adding protease inhibitor (phenylmethylsulfonyl fluoride) at a final concentration of 20 μg/ml. The digest was then dialyzed for 2 days against several changes of distilled water and centrifuged at 5,000 × g for 30 min to remove bacterial debris. The LPS solution was lyophilized, and its carbohydrate content was determined by the orcinol-sulfuric acid method (26). The LPS prepared as described was used in indirect ELISA. The LPS to be used for SDS-PAGE and immunoblot experiments was extracted in a similar manner, with the exception that proteinase K digestion was carried out in the presence of 2% SDS–4% mercaptoethanol–10% glycerol in 1 M Tris buffer (pH 6.8) instead of 6 M urea.
SDS-PAGE and immunoblots.
SDS-PAGE was performed according to the method described by Laemmli (12) as described in detail in our previous report (21). Each lane of the 12% gel was loaded with 10 μg of LPS, and after electrophoresis was terminated, the separated components were detected with a modified silver stain (5). For the immunoblots, the electrophoresed components were electrotransferred immediately onto a nitrocellulose membrane (19) and then probed with a reference positive human serum or immune mouse serum. The optimal serum dilutions were 1:7,000 for human serum and 1:500 for mouse serum. The reactions were detected with horseradish peroxidase-conjugated rabbit anti-human immunoglobulin G (IgG) or anti-mouse immunoglobulin (Dako A/S, Copenhagen, Denmark), respectively. Pooled sera from healthy individuals resident in areas of nonendemicity and preimmunized mouse sera, respectively, were used as negative controls. Molecular weights were calculated as described by Weber and Osborn (25).
ELISA.
Indirect ELISA was performed to compare the immunoreactivities of Ara− and Ara+ LPSs with sera from different groups of individuals as originally described for the detection of IgG antibody to affinity-purified antigen (21). The LPS preparations for coating the microtiter plates were both used at a concentration of 2.5 μg/ml. Affinity-purified antigen prepared as described in our previous report (21), which was used at a concentration of 0.7 μg/ml, was included for comparison. All sera were used at an optimal dilution of 1:2,000, and reactions were detected with horseradish peroxidase-conjugated rabbit anti-human IgG.
Other techniques.
Protein concentrations were determined as described by Lowry et al., with bovine serum albumin as the standard (13). Quantitation of LPS was based on its carbohydrate content, which was measured by an orcinol-sulfuric acid method (26).
RESULTS
SDS-PAGE profile of LPSs extracted from Ara− and Ara+ B. pseudomallei isolates.
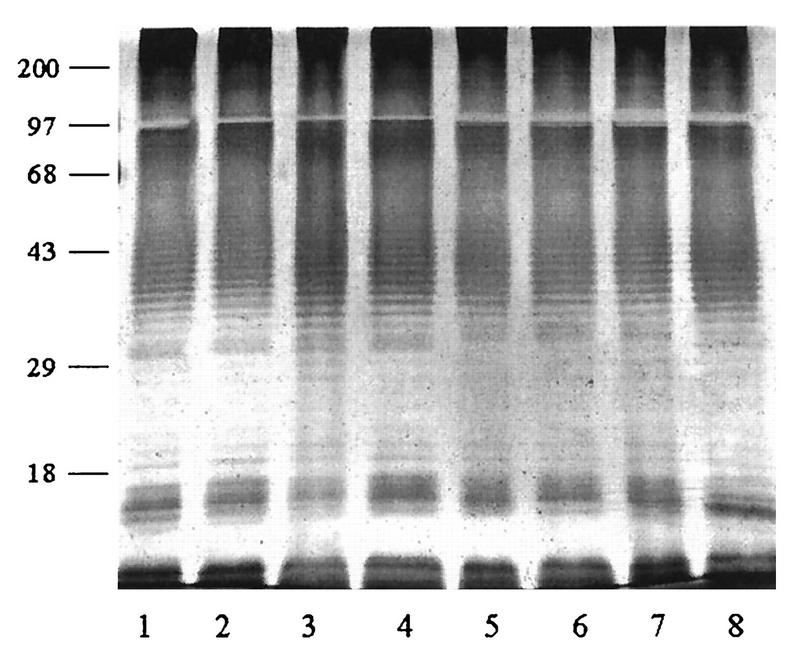
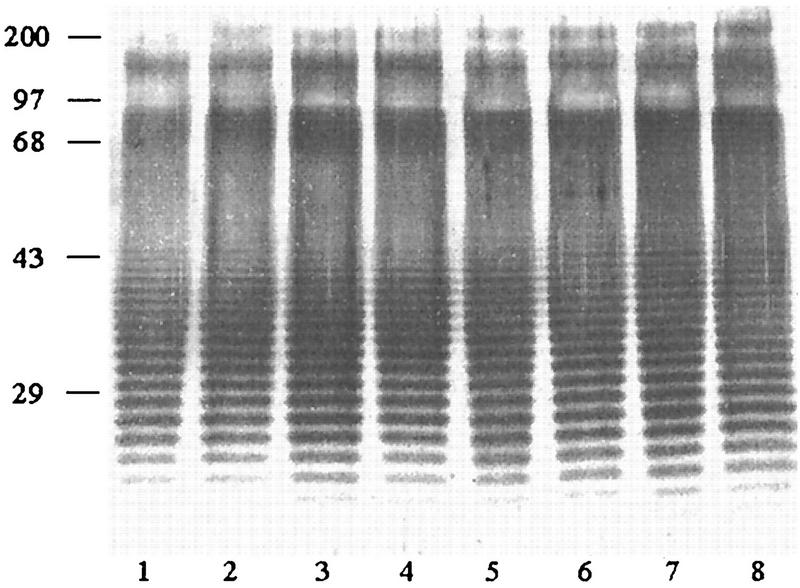
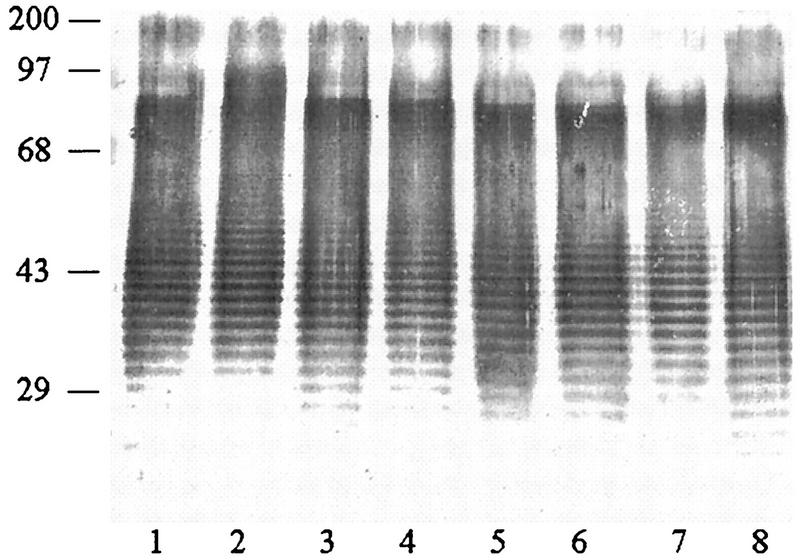
The proteinase K-extracted LPSs from both Ara− and Ara+ soil isolates and Ara− clinical isolates were subjected to SDS-PAGE, and their silver-staining profiles were compared. Representative patterns from the three groups are shown in Fig. 1. With the exception of four clinical isolates, the remaining 210 isolates (140 Ara− and 70 Ara+ isolates) exhibited identical staining profiles, showing the distinctive ladder pattern typical for LPSs from other gram-negative bacteria. The results typically showed at least 20 to 30 repeating bands with uniform spacing, which was particularly obvious in the region between 29 and 43 kDa. The atypical LPS pattern exhibited by two of the four clinical isolates is shown in Fig. 2. However, a densely stained high-molecular-mass region (68- to 97-kDa position) was present in all extracts. In this region, the staining could not be resolved into a distinct ladder as in the lower region (Fig. 1 and 2). The silver-staining profiles of clinical and soil isolates from Thailand and Vietnam were identical and were similar to those exhibited by the eight reference strains (data not shown).
FIG. 1.
Representative typical silver-stained SDS-PAGE profiles of LPSs extracted from 214 Ara− and Ara+ B. pseudomallei isolates. Lanes: 1 and 2, Ara− clinical isolates; 3 and 4, Ara− soil isolates; 5 to 8, Ara+ soil isolates. Numbers on the left are molecular weight markers (in thousands).
FIG. 2.
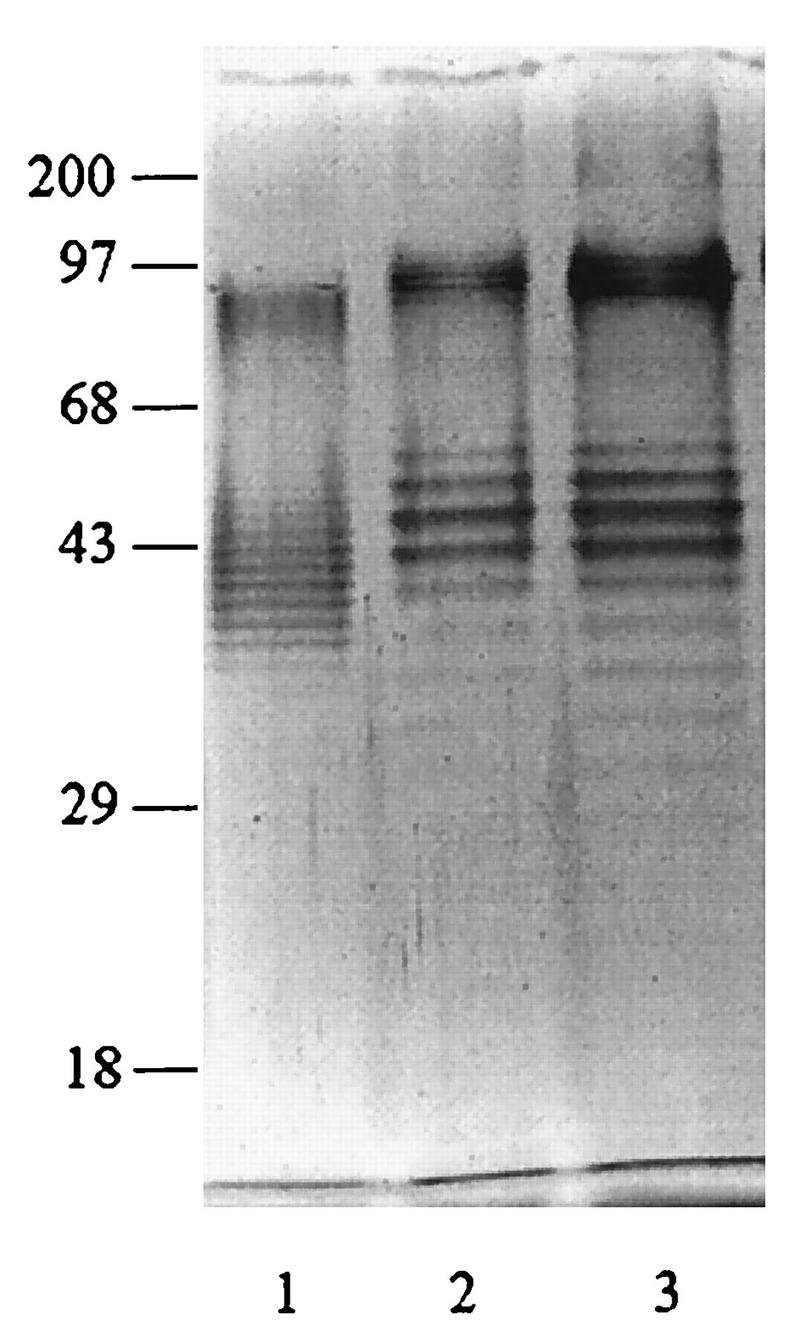
Atypical silver-stained SDS-PAGE profiles of LPSs extracted from two clinical Ara− isolates (lanes 2 and 3). A representative pattern of typical B. pseudomallei isolates is shown for comparison (lane 1). Numbers on the left are molecular weight markers (in thousands).
Immunoblot profiles of different LPS extracts with anti-B. pseudomallei sera.
Representative profiles from the three groups of B. pseudomallei, namely, Ara− clinical, Ara− soil, and Ara+ soil, are shown in Fig. 3 and 4. When the isolates were probed with human melioidosis serum (Fig. 3), the immunoreactive patterns of all isolates from the three groups were indistinguishable from one another. The characteristic LPS ladders could be readily observed. In addition to these ladders, all extracts exhibited patterns of unresolved immunoreactive banding in the 68- to 97-kDa region similar to the ones noted with the silver staining shown in Fig. 2. However, unlike the results with silver staining, with the immunoblots another dense unresolved, immunoreactive component at the 97- to 200-kDa position could also be detected in all LPS extracts. Essentially identical immunoblot patterns were observed when the reactions were developed with mouse anti-LPS serum (Fig. 4). Rabbit antisera to Ara+ or Ara− whole-cell antigens reacted equally well with the LPS from either biotype in the ELISA and the immunoblotting assay (data not shown).
FIG. 3.
Representative immunoblot profiles of LPSs from Ara− and Ara+ B. pseudomallei isolates against sera pooled from patients with culture-proven melioidosis. The serum was used at a 1:7,000 dilution. Lanes: 1 and 2, Ara− clinical isolates; 3 and 4, Ara− soil isolates; 5 to 8, Ara+ soil isolates. Numbers on the left are molecular weight markers (in thousands).
FIG. 4.
Representative immunoblot profiles of LPSs from Ara− and Ara+ B. pseudomallei isolates against immune mouse serum. The serum was used at a dilution of 1:500. Lanes: 1 and 2, Ara− clinical isolates; 3 and 4, Ara− soil isolates; 5 to 8, Ara+ soil isolates. Numbers on the left are molecular weight markers (in thousands).
Immunoreactivities of Ara− and Ara+ LPSs with different groups of human sera.
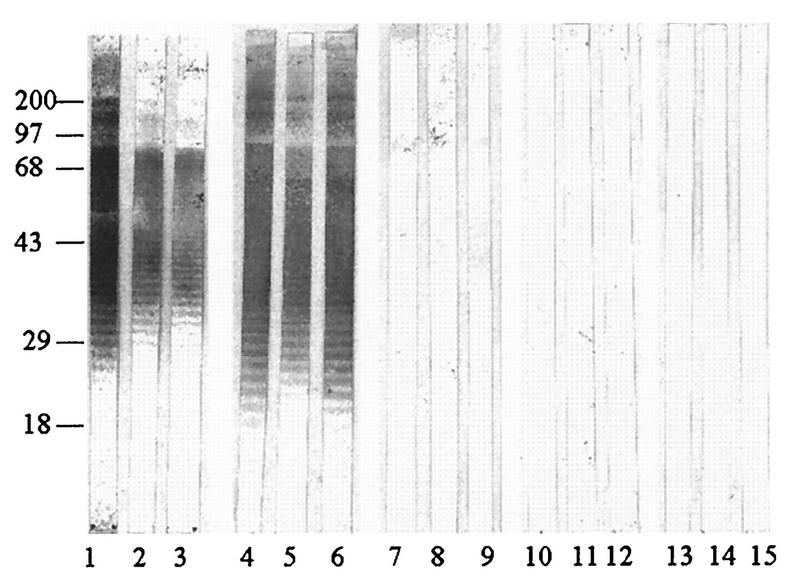
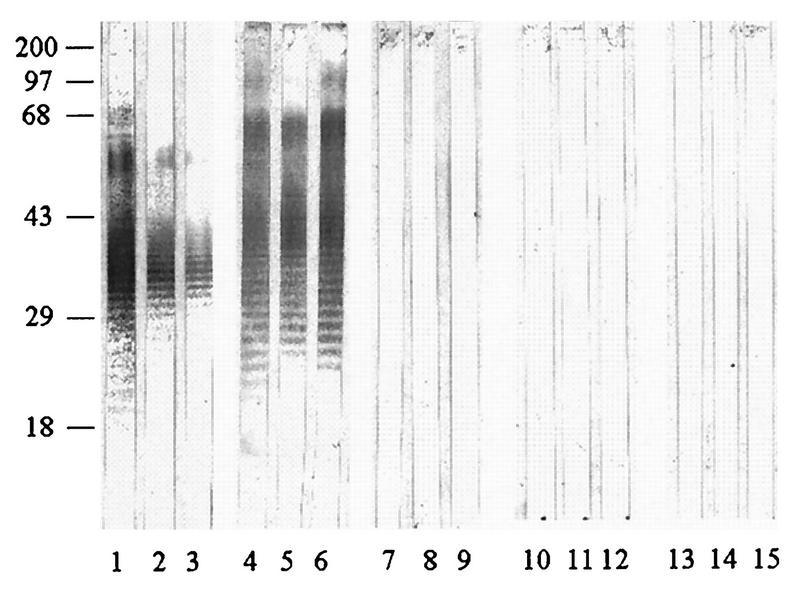
To analyze the anti-LPS responses in melioidosis patients with different clinical spectra and possible immunological cross-reactivities with sera from patients with other infections, both the immunoblot assays (Fig. 5 and 6) and the indirect ELISA (Table 2) for IgG antibody were employed. To avoid bias results possibly arising from individual strain variation, at least four respective individual LPS preparations from either the Ara− or the Ara+ biotype were pooled and used in the experiment. Representative immunoreactive profiles shown in Fig. 5 and 6 are consistent with those presented earlier (Fig. 3), demonstrating typical LPS ladder appearance. Sera from patients with septicemia (Fig. 5 and 6, lanes 1 to 3) and localized infections (lanes 4 to 6) exhibited similar anti-LPS patterns, regardless of the type of LPS used in the analyses. Under the same conditions, almost all sera from patients with other infections (lanes 7 to 9) and healthy individuals (lanes 10 to 15) failed to give positive immunoblots. However, a few serum specimens from these individuals reacted weakly, particularly when the experiment was performed with LPS extracted from the Ara+ B. pseudomallei biotype.
FIG. 5.
Immunoreactivities of LPSs from the Ara+ biotype. Sera were from patients with septicemic melioidosis (lanes 1 to 3), melioidosis patients with localized infections (lanes 4 to 6), and patients with other infections caused by gram-negative organisms (lanes 7 to 9) and from normal individuals from areas where infection is endemic (lanes 10 to 12) and nonendemic (lanes 13 to 15). All sera were used at a 1:7,000 dilution. Numbers on the left are molecular weight markers (in thousands).
FIG. 6.
Immunoreactivities of pooled LPS extracted from Ara− B. pseudomallei. See legend to Fig. 5 for details.
The LPS preparations from both biotypes coating the microtiter plates reacted equally well in the indirect ELISA with sera from melioidosis patients with and without septicemia (Table 2). The ELISA data with these two LPS preparations resembled those of the affinity-purified antigen simultaneously analyzed and shown in Table 2 for comparison (r = 0.689). In terms of specificity and potential diagnostic value, either one of these LPS preparations compared favorably with or was slightly better than the affinity-purified antigen reported earlier (21).
DISCUSSION
The results presented in this study show that the LPSs from the Ara− clinical and Ara− soil isolates were indistinguishable and were also identical to those of the Ara+ soil isolates with regard to SDS-PAGE profiles (Fig. 1) and immunoreactivities with the melioidosis sera (Fig. 3 and 4). With the exception of 4 isolates, the 210 remaining isolates, regardless of biotype or source of origin, gave similar proteinase K-digested SDS-PAGE profiles, showing characteristic ladder patterns with identical spacings and positions of bands. The observation presented in our study is consistent with the earlier report of Pitt et al., which demonstrated that 12 clinical strains of B. pseudomallei, presumably of the Ara− biotype, from Southeast Asia and Australia isolated over a period of 70 years exhibited identical LPS moieties (18). The antigenic similarity between the pathogenic (Ara−) and nonpathogenic (Ara+) biotypes reported in our study probably explains the discrepancy between seroprevalence and disease prevalence in melioidosis. People may be inoculated with the nonvirulent biotype outside the disease-endemic area and produce antibodies against LPS. These antibodies are considered the major component of the current indirect hemagglutination assay for melioidosis. Altogether, it appears that the LPS of B. pseudomallei is both highly conserved and constant. The detection of 4 clinical isolates with atypical LPS patterns (among the 123 analyzed) is not unexpected, in view of the fact that this has been previously noted by two other groups (11, 16). These earlier investigators presented structural studies indicating the possible presence of two different forms of LPS in B. pseudomallei clinical isolates. The simultaneous production of more than one structurally different form of LPS by a gram-negative bacterium is known to occur. The unexpected observation is that two of the four clinical isolates with atypical LPS patterns were from patients experiencing a relapse of melioidosis. The relapsing status of the patients from whom the two remaining atypical isolates were taken could not be determined because one died after bacterial identification and the other had just recovered from the initial infection. The significance of this observation remains to be investigated.
The similarity of LPSs from Ara− (virulent) and Ara+ (nonvirulent) B. pseudomallei isolates in the present study was unexpected, since in general this immunodominant component of gram-negative bacteria possesses different antigenic carbohydrate side chains. The latter not only confer species specificity but also determine virulence. This raises the question of the role of LPS in the virulence and pathogenicity of B. pseudomallei. For many years, this area of investigation has given inconsistent and controversial results (2, 6–9, 14, 17, 20, 24). Observations by Iwasa et al. (8) and more recently by Matsuura et al. (14) suggested that the LPSs of B. pseudomallei clinical isolates appeared to be less toxic than those from other gram-negative bacteria. Our findings are consistent with this conclusion, since we have shown here that the LPS from the nonvirulent Ara+ isolates was indistinguishable from that of its virulent Ara− counterpart. We have data showing that unlike the situation with the LPSs, the antigenic compositions of Ara+ and Ara− biotypes were different from each other, based on their immunoreactivities with polyclonal mouse and rabbit antisera and mouse monoclonal antibody (unpublished data). Ho et al. recently demonstrated that anti-LPS antibodies could mediate phagocytic killing of B. pseudomallei by polymorphonuclear leukocytes (7). Additional investigation should give some insight that would be most valuable in understanding the pathogenicity of B. pseudomallei and in development of a vaccine for melioidosis.
ACKNOWLEDGMENTS
This work was supported by the Chulabhorn Foundation, the National Science and Technology Development Agency of Thailand, and the Thailand Research Fund. V.W. and N.J.W. were supported by the Wellcome–Mahidol University–Oxford Tropical Medicine Research Programme funded by the Wellcome Trust of Great Britain.
We are grateful to Surasakdi Wongratanacheewin (Department of Microbiology, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand) for some of the clinical B. pseudomallei isolates used in this study.
REFERENCES
- 1.Brett P J, Deshazer D, Woods D E. Characterization of Burkholderia pseudomallei and Burkholderia pseudomallei-like strains. Epidemiol Infect. 1997;118:137–148. doi: 10.1017/s095026889600739x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bryan L E, Wong S, Woods D E, Dance D A B, Chaowagul W. Passive protection of diabetic rats with antisera specific for the polysaccharide portion of the lipopolysaccharide from Pseudomonas pseudomallei. Can J Infect Dis. 1994;5:170–178. doi: 10.1155/1994/856850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaowagul W, White N J, Dance D A B, Wattanagoon Y, Naigowit P, Davis T M E, Looareesuwan S, Pitakwatchara N. Melioidosis: a major course of community-acquired septicemia in northeastern Thailand. J Infect Dis. 1989;159:890–899. doi: 10.1093/infdis/159.5.890. [DOI] [PubMed] [Google Scholar]
- 4.Dance D A B. Melioidosis: the tip of the iceberg? Clin Microbiol Rev. 1991;4:52–60. doi: 10.1128/cmr.4.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fomsgaard A, Freundenberg M A, Galanos C. Modification of the silver staining technique to detect lipopolysaccharide in polyacrylamide gels. J Clin Microbiol. 1990;28:2627–2631. doi: 10.1128/jcm.28.12.2627-2631.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gotoh N, White N J, Chaowagul W, Wood D E. Isolation and characterization of the outer-membrane proteins of Burkholderia (Pseudomonas) pseudomallei. Microbiology. 1994;140:797–805. doi: 10.1099/00221287-140-4-797. [DOI] [PubMed] [Google Scholar]
- 7.Ho M, Schollaardt T, Smith M D, Perry M B, Brett P J, Chaowagul W, Bryan L E. Specificity and functional activity of anti-Burkholderia pseudomallei polysaccharide antibodies. Infect Immun. 1997;65:3648–3653. doi: 10.1128/iai.65.9.3648-3653.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iwasa S, Petkanchanapong V, Naigowit P. Endotoxic property of Pseudomonas pseudomallei as detected by the body-weight decreasing reaction in mice. A comparison with P. cepacia and P. aeruginosa. Jpn J Med Sci Biol. 1992;45:33–47. doi: 10.7883/yoken1952.45.35. [DOI] [PubMed] [Google Scholar]
- 9.Jayanetra P, Vorachit M, Costerton J W. Proceedings of the 5th European Congress of Clinical Microbiology and Infectious Diseases. 1991. Role of glycocalyx in melioidosis; pp. 55–61. [Google Scholar]
- 10.Kawahara K, Dejsirilert S, Danbara H, Ezaki T. Extraction and characterization of lipopolysaccharide from Pseudomonas pseudomallei. FEMS Microbiol Lett. 1992;96:129–133. doi: 10.1016/0378-1097(92)90392-2. [DOI] [PubMed] [Google Scholar]
- 11.Knirel Y A, Paramonov N A, Shashkov A S, Kochetkov N K, Yarullin R G, Farber S M, Efremenko V I. Structure of the polysaccharide chains of Pseudomonas pseudomallei lipopolysaccharide. Carbohydr Res. 1992;233:185–193. doi: 10.1016/s0008-6215(00)90930-3. [DOI] [PubMed] [Google Scholar]
- 12.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 13.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 14.Matsuura M, Kawahara K, Ezaki T, Nakano M. Biological activities of lipopolysaccharide of Burkholderia (Pseudomonas) pseudomallei. FEMS Microbiol Lett. 1996;137:79–83. doi: 10.1111/j.1574-6968.1996.tb08086.x. [DOI] [PubMed] [Google Scholar]
- 15.Michael A, Apicella J, Griffiss M, Schneider H. Isolation and characterization of lipopolysaccharides, lipooligosaccharides and lipid A. Methods Enzymol. 1994;235:242–252. doi: 10.1016/0076-6879(94)35145-7. [DOI] [PubMed] [Google Scholar]
- 16.Perry M B, Maclean L L, Schollaardt T, Bryan L E, Ho M. Structural characterization of the lipopolysaccharide O antigens of Burkholderia pseudomallei. Infect Immun. 1995;63:3348–3352. doi: 10.1128/iai.63.9.3348-3352.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phung L V, Tran T B, Hotta H, Yabuuchi E, Yano I. Cellular lipid and fatty acid compositions of Burkholderia pseudomallei strains isolated from human and environment in Viet Nam. Microbiol Immunol. 1995;39:105–116. doi: 10.1111/j.1348-0421.1995.tb02176.x. [DOI] [PubMed] [Google Scholar]
- 18.Pitt T L, Aucken H, Dance D A B. Homogeneity of lipopolysaccharide antigens in Pseudomonas pseudomallei. J Infect. 1992;25:139–146. doi: 10.1016/0163-4453(92)93920-l. [DOI] [PubMed] [Google Scholar]
- 19.Pyle S W, Schill W B. Rapid serological analysis of bacterial lipopolysaccharides by electrotransfer to nitrocellulose. J Immunol Methods. 1985;85:371–382. doi: 10.1016/0022-1759(85)90146-2. [DOI] [PubMed] [Google Scholar]
- 20.Rapaport F T, Miller J W, Ruch J. Endotoxic properties of Pseudomonas pseudomallei. Arch Pathol. 1961;71:429–436. [PubMed] [Google Scholar]
- 21.Rugdech P, Anuntagool N, Sirisinha S. Monoclonal antibodies to Pseudomonas pseudomallei and their potential for diagnosis of melioidosis. Am J Trop Med Hyg. 1995;52:231–235. doi: 10.4269/ajtmh.1995.52.231. [DOI] [PubMed] [Google Scholar]
- 22.Smith, M. D., B. J. Angus, V. Wuthiekanun, and N. J. White. Arabinose assimilation defines a nonvirulent biotype of Burkholderia pseudomallei. Infect. Immun. **65:**4319–4321. [DOI] [PMC free article] [PubMed]
- 23.Smith M D, Wuthiekanun V, Walsh A L, White N J. Quantitative recovery of Burkholderia pseudomallei from soil in Thailand. Trans R Soc Trop Med Hyg. 1995;89:488–490. doi: 10.1016/0035-9203(95)90078-0. [DOI] [PubMed] [Google Scholar]
- 24.Velianov D, Naidenski K. Virulence and susceptibility to phagocytosis of Pseudomonas pseudomallei R- and S-forms for ground squirrels (Citellus citellus L.) Acta Microbiol Bulgarica. 1993;30:11–16. [PubMed] [Google Scholar]
- 25.Weber K, Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969;244:4406–4412. [PubMed] [Google Scholar]
- 26.White C A, Kennedy J F. Oligosaccharides. In: Chaplin M F, Kennedy J F, editors. Carbohydrate analysis: a practical approach. Oxford, United Kingdom: IRL Press; 1986. pp. 37–54. [Google Scholar]
- 27.Wuthiekanun V, Smith M D, Dance D A B, Walsh A L, Pitt T L, White N J. Biochemical characteristics of clinical and environmental isolates of Burkholderia pseudomallei. J Med Microbiol. 1996;45:408–412. doi: 10.1099/00222615-45-6-408. [DOI] [PubMed] [Google Scholar]
- 28.Yabuuchi E, Arakawa M. Burkholderia pseudomallei and melioidosis: be aware in temperate area. Microbiol Immunol. 1993;37:823–836. doi: 10.1111/j.1348-0421.1993.tb01712.x. [DOI] [PubMed] [Google Scholar]