Erythropoietin prevents motor neuron apoptosis and neurologic disability in experimental spinal cord ischemic injury (original) (raw)
Abstract
The cytokine erythropoietin (EPO) possesses potent neuroprotective activity against a variety of potential brain injuries, including transient ischemia and reperfusion. It is currently unknown whether EPO will also ameliorate spinal cord injury. Immunocytochemistry performed using human spinal cord sections showed abundant EPO receptor immunoreactivity of capillaries, especially in white matter, and motor neurons within the ventral horn. We used a transient global spinal ischemia model in rabbits to test whether exogenous EPO can cross the blood–spinal cord barrier and protect these motor neurons. Spinal cord ischemia was produced in rabbits by occlusion of the abdominal aorta for 20 min, followed by saline or recombinant human (rHu)-EPO (350, 800, or 1,000 units/kg of body weight) administered intravenously immediately after the onset of reperfusion. The functional neurological status of animals was better for rHu-EPO-treated animals 1 h after recovery from anesthesia, and improved dramatically over the next 48 h. In contrast, saline-treated animals exhibited a poorer neurological score at 1 h and did not significantly improve. Histopathological examination of the affected spinal cord revealed widespread motor neuron injury associated with positive terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling in control but not in rHu-EPO-treated animals. These observations suggest both an acute as well as a delayed beneficial action of rHu-EPO in ischemic spinal cord injury. Because rHu-EPO is currently used widely with an excellent safety profile, clinical trials evaluating its potential to prevent motor neuron apoptosis and the neurological deficits that occur as a consequence of ischemic injury are warranted.
Thoracoabdominal aortic surgery is sometimes associated with significant spinal cord injuries that have potentially devastating neurological outcomes. This complication has been attributed to temporary or permanent cord ischemia secondary to aortic clamping during the operation (1, 2). Neuronal injury arising from spinal cord ischemia is believed to result from diverse but interrelated processes such as glutamate-mediated excitotoxicity, nitric oxide overproduction, inflammation, apoptosis, and free radical generation (2). Various agents and techniques that ameliorate these different processes have been tested in experimental model of spinal cord ischemia (1, 2). Despite recent improvements in anesthetic and surgical techniques, currently there are no efficacious therapies (3). An ideal treatment would be one administered systemically and without significant side effects. One currently untested therapeutic agent is recombinant human erythropoietin (rHu-EPO), which has been shown by several research groups to possess potent neuroprotective properties in experimental cerebral ischemia (4–7).
EPO has recently been shown to play roles in the nervous system in both normal and pathological conditions (8). EPO and its receptor (EPO-R) are expressed in the central and peripheral nervous systems, and the expression of both proteins is modulated by hypoxia (4, 8, 9). Recent in vitro studies employing neurons or neuron-like cells have confirmed a protective action for EPO against various insults such as excitotoxicity, serum deprivation, and growth-factor deprivation (7, 10). Results from in vivo studies using experimental models of global and focal ischemia, blunt trauma, immune-mediated inflammation, excitotoxin-elicited seizures, subarachnoidal hemorrhage, and toxin-induced Parkinsonism suggest that EPO might be a beneficial agent in these situations (4–6, 9, 11–13). Although the exact mechanism of the EPO's neuroprotective effect is not fully known, promotion of cell survival signaling cascades (7, 14–16), attenuation of intracellular calcium and nitric oxide production (13, 17, 18), and antioxidative (19, 20) and anti-inflammatory actions (5, 7) have been implicated as possible mechanisms.
Current data suggest that EPO may exert similar neuroprotective effects in spinal cord ischemia. EPO and EPO-R are expressed by human neurons and glial cells in fetal spinal cord (21). Recombinant human EPO rescues fetal spinal motoneurons from brain-derived neurotrophic factor deprivation or kainic acid-induced cell death in vitro (7). Neurotrophic actions of EPO also have been suggested by these investigators. However, the putative neuroprotective effects of EPO have never been tested in spinal cord ischemia in vivo for which the principal early targets are motor neurons in the ventral horn.
The aim of this study was to investigate the possible neuroprotective and ameliorating effect of rHu-EPO in an experimental transient global spinal cord ischemia model in rabbits. Our results indicate that normal ventral horn motor neurons highly express EPO-R and that rHu-EPO given intravenously remarkably ameliorates the ischemia-induced neurological disability that results from motor neuron apoptosis.
Materials and Methods
Animals.
Twenty-seven New Zealand White rabbits (8–12 months old, male) weighing 1.5–2.5 kg were used in this study. Animals were housed under standard conditions in the Animal Research Laboratory at Dokuz Eylül University. The study protocol was approved by the Animal Research Committee of Dokuz Eylül University.
Surgical Procedures.
The animals were fasted for 12 h and humanely restrained. Anesthesia was induced by using 3% halothane in 100% oxygen and maintained with 0.5–1.5% halothane in a mixture of 50% oxygen and 50% air. End-tidal concentrations of halothane and CO2 were continuously measured by using a monitor (Anesthesia Gas Monitoring 1304, Bruel and Kjaer, Naerum, Denmark) by means of nasopharyngeal sampling. An i.v. catheter (22 gauge) was placed in the left ear vein. Ringer's lactate was infused at a rate of 4 ml/kg of body weight (bw) per h during the surgical procedure. Preoperatively, cefazoline 10 mg/kg bw was administered intravenously for prophylaxis of infection. The right ear artery was also cannulated for continuous monitoring of mean arterial pressure and arterial blood gases. Rectal temperatures were maintained at 38.5°C by exposing the animal to a heat lamp until recovery from anesthesia.
The animals were placed in the right lateral decubitus position. The skin was prepared with povidone iodine and infiltrated with bupivacaine (0.25%), and a flank skin incision was made parallel to the spine at the 12th costal level. After incision of the skin and s.c. thoracolumbar fascia, the longissimus lumborum and iliocostalis lumborum muscles were retracted. The abdominal aorta was exposed via a left retroperitoneal approach and mobilized just inferior to the left renal artery. A piece of PE-60 tubing was looped around the aorta immediately distal to the left renal artery, and both ends were passed through a larger rubber tube. By pulling on the polyethylene tubing, the aorta was nontraumatically occluded. Heparin (400 units) was administrated as an i.v. bolus before aortic occlusion. After 20 min of occlusion, the tube and catheter were removed and the incision was closed. The animals were monitored until full recovery, and were then returned to their cages.
Experimental Design.
Twenty-seven animals were randomly divided into five groups. Animals in a sham group (n = 3) underwent the surgical procedure but the aorta was not occluded. In a control group, animals (n = 6) received normal saline intravenously immediately after release of aortic occlusion. Treatment groups received rHu-EPO (Eprex, Cilag, Zug, Switzerland) administered intravenously immediately after the onset of the reperfusion at the doses of 350 (n = 6), 800 (n = 6), and 1,000 units/kg bw (n = 6).
Heart rate, mean arterial pressure, and rectal temperature were continuously recorded (Biopac MP 30 and Biopac BSL pro v. 3.6.5, Biopac Systems, Santa Barbara, CA), as were respiration rate and end tidal CO2. Arterial blood samples for partial pressure of CO2 (PaCO2), partial pressure of oxygen (PaO2), and pH, and venous blood samples, for hematocrit and glucose measurements were obtained. Arterial blood gases measurements were performed with a Radiometer ABL 700 Series (Copenhagen) before starting the surgical procedure, before the aortic occlusion, and after restoration of aortic perfusion.
Motor function was assessed according to the criteria of Drummond and Moore (22) by an investigator blind to the treatment at 1, 24, and 48 h after reperfusion. A score of 0 to 4 was assigned to each animal as follows: 0 = paraplegic with no evident lower extremity motor function; 1 = poor lower extremity motor function, weak antigravity movement only; 2 = moderate lower extremity function with good antigravity strength but inability to draw legs under body; 3 = excellent motor function with the ability to draw legs under body and hop, but not normally; and 4 = normal motor function. The urinary bladder was evacuated manually in paraplegic animals twice a day.
Histopathology.
Forty-eight hours after reperfusion, the animals were killed by using thiopental (120 mg/kg, i.p.), and the spinal cords were removed and fixed in 10% formalin in phosphate buffer. After fixation, transverse sections of spinal cord at the L5 level were embedded in paraffin, cut into 5-μm thick sections, and stained by using hematoxylin and eosin. Neuronal injury was evaluated at 40×, 100×, 200×, and 400× magnification by a pathologist blind to the treatment groups by using three sections per animal. Ischemic neurons were identified by cytoplasmic eosinophilia with loss of Nissl substance and pyknotic nuclei. Sections were assigned according to the presence and relative abundance of ischemic neurons in ventral gray matter area into three injury groups: mild injury (<5% of motor neurons injured); moderate injury (5–20% neuronal injury); and severe injury (>20% of neurons affected).
Terminal Deoxynucleotidyltransferase-Mediated dUTP Nick End-Labeling (TUNEL).
Ten-micrometer sections were also cut from paraffin-embedded sections of spinal cord and deparaffinized. These sections were then treated with proteinase K (20 μg/ml in 10 mM Tris⋅Cl, pH 7.6, for 15 min at room temperature), blocked in 3% H2O2 in methanol for 10 min, permeabilized for 2 min in 0.1% Triton X-100/sodium citrate at 4°C, and treated with TUNEL reaction mixture according to the manufacturer's protocol (In Situ Cell Death Detection kit_,_ Roche Diagnostics). Positive neurons were identified after development for 15 min in diaminobenzidine, dehydrated, and coverslipped. Terminal transferase was omitted as a negative control. Positive controls were generated by treatment with DNase 1 (30 units/ml in 40 mM Tris⋅Cl pH 7.6/6 mM MgCl2/2 mM CaCl2 for 30 min).
Immunochemistry.
Human spinal cord was obtained from fresh autopsy material obtained from a 60-year-old male who had died from nonneurologic causes and was immediately postfixed by 5% acrolein in 0.1 M phosphate buffer (pH 7.4) for 3 h. Sections for histological analyses were cut with a vibrating microtome (Vibratome, Ted Paella, Inc., Redding, CA) at 40-μm thickness. Immunohistochemical staining was performed as we have described (5) using free-floating sections and the indirect antibody peroxidase–antiperoxidase method using a 1:500 dilution EPO-R and EPO antiserum (C-20 and N-19; Santa Cruz Biotechnology). Endogenous peroxidase activity was quenched by pretreatment of tissue sections with hydrogen peroxide (3% in methanol for 30 min). Tissue controls were also carried out by primary antibody omission and by using the appropriate blocking peptide (Santa Cruz Biotechnology) to confirm that staining was specific for EPO-R and EPO.
Analysis.
Statistical analysis was carried out by using SPSS FOR WINDOWS software program version 8.0. Results are presented as means. All variables were analyzed by using two-tailed nonparametric testing; physiological variables were interpreted by using the Kruskal–Wallis test and Drummond–Moore scores, and histopathological scoring by means of the Kruskal–Wallis test followed by the Mann–Whitney U test when indicated. A P value less than 0.05 was considered significant.
Results
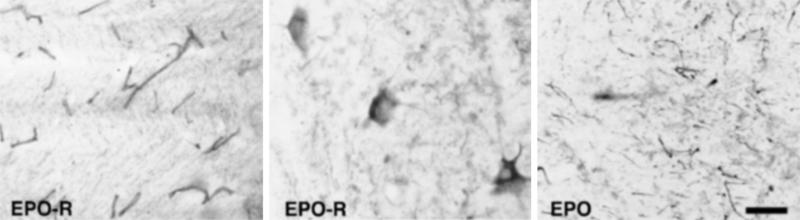
Immunohistochemical staining of normal human spinal cord revealed abundant reaction product for EPO-R localized on capillaries, especially within the white matter (Fig. 1 Left), and neurons of gray matter. Motor neurons in the ventral horn were labeled extensively, with immunoreactivity localized to the somata and proximal dendrites (Fig. 1 Center). Within the surrounding ventral horn, many processes with the morphology of dendrites exhibited EPO immunoreactivity (Fig. 1 Right). The cellular origin of these processes is unclear, as no EPO-positive cell bodies were observed within the ventral horn of comparable (adjacent) sections. The distribution of EPO and its receptor appears, therefore, to be of an autocrine/paracrine type, with the ligand in close proximity to its target.
Figure 1.
Immunocytochemical localization of EPO-R and EPO within normal human spinal cord shows that motor neurons are targets for EPO. Anti-EPO-R immunoreactivity was especially dense within capillaries in white matter (Left) as well as in motor neurons of the ventral horn (Center). Reaction product in the neurons appeared restricted to the somata and the proximal dendrite. EPO immunoreactivity with gray matter occurred within the neuropil surrounding motor neurons in a pattern consistent with dendrites. Thus, within the gray matter, EPO and EPO-R are regionally colocalized (Right). (Scale bar = 50 μm.)
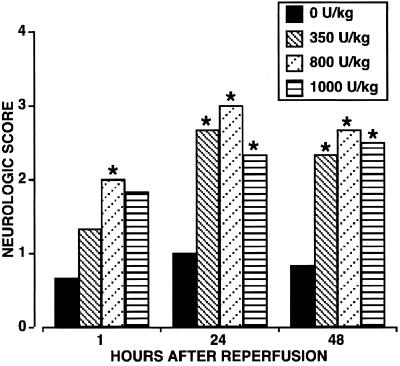
The physiological parameters of the animals obtained at baseline and throughout the surgical procedure were not significantly different (Table 1). Of note, mean arterial blood pressure was comparable at all times among the different treatment groups. All rabbits survived without major nonneurologic complications. Twenty minutes of ischemia produced an immediate and severe motor deficit in hind limbs of all animals (Fig. 2; 1-h assessment) except of those subjected to a sham procedure (clinical score of 4; not shown in the Fig.). Neurological evaluation performed after 1 h of reperfusion indicated that rHu-EPO-treated groups exhibited a better neurological score, with the 800 units/kg bw group having significantly better residual motor function than the saline control group (neurological score of 2.0 vs. 0.7; P < 0.03). When assessed after a further 24 and 48 h of recovery, all rHu-EPO-treated groups exhibited comparable and significantly improved neurological function when compared with the saline group, for which the neurological score remained unchanged (Fig. 2).
Table 1.
Physiological parameters
| Variable | Group | ||||
|---|---|---|---|---|---|
| Sham | Control | EPO 350 units/kg | EPO 800 units/kg | EPO 1000 units/kg | |
| HR, beats/min | |||||
| BS | 299 ± 31 | 326 ± 39 | 300 ± 44 | 311 ± 25 | 321 ± 40 |
| BO | — | 314 ± 24 | 297 ± 32 | 303 ± 24 | 327 ± 17 |
| AO20 | — | 318 ± 37 | 275 ± 20 | 303 ± 29 | 325 ± 34 |
| R20 | — | 298 ± 35 | 282 ± 10 | 279 ± 35 | 298 ± 55 |
| MAP, mmHg | |||||
| BS | 73 ± 14 | 73 ± 7 | 77 ± 14 | 80 ± 13 | 90 ± 13 |
| BO | — | 65 ± 9 | 74 ± 10 | 72 ± 9 | 80 ± 11 |
| AO20 | — | 77 ± 10 | 80 ± 11 | 86 ± 6 | 92 ± 12 |
| R20 | — | 69 ± 7 | 77 ± 12 | 77 ± 7 | 83 ± 10 |
| Temperature, °C | |||||
| BS | 38.6 ± 0.2 | 38.7 ± 0.2 | 38.5 ± 0.2 | 38.8 ± 0.2 | 38.6 ± 0.2 |
| BO | — | 38.7 ± 0.3 | 38.5 ± 0.2 | 38.8 ± 0.2 | 38.6 ± 0.2 |
| AO20 | — | 38.6 ± 0.1 | 38.6 ± 0.1 | 38.8 ± 0.1 | 38.6 ± 0.2 |
| R20 | — | 38.6 ± 0.2 | 38.7 ± 0.2 | 38.7 ± 0.1 | 38.9 ± 0.1 |
| Hematocrit, % | |||||
| BS | 35 ± 6 | 34 ± 5 | 32 ± 8 | 30 ± 11 | 38 ± 5 |
| BO | — | 32 ± 5 | 31 ± 7 | 30 ± 4 | 32 ± 5 |
| AO20 | — | 34 ± 3 | 27 ± 8 | 31 ± 5 | 34 ± 3 |
| R20 | — | 34 ± 6 | 30 ± 4 | 32 ± 5 | 34 ± 3 |
| pH | |||||
| BS | 7.38 ± 0.13 | 7.31 ± 0.05 | 7.28 ± 0.04 | 7.35 ± 0.08 | 7.33 ± 0.06 |
| BO | — | 7.36 ± 0.05 | 7.28 ± 0.14 | 7.31 ± 0.08 | 7.34 ± 0.08 |
| AO20 | — | 7.44 ± 0.05 | 7.34 ± 0.09 | 7.42 ± 0.06 | 7.43 ± 0.06 |
| R20 | — | 7.45 ± 0.05 | 7.40 ± 0.03 | 7.41 ± 0.07 | 7.48 ± 0.06 |
| PaCO2, mmHg | |||||
| BS | 35 ± 10 | 41 ± 10 | 41 ± 9 | 36 ± 7 | 39 ± 4 |
| BO | — | 36 ± 15 | 50 ± 27 | 42 ± 8 | 42 ± 7 |
| AO20 | — | 33 ± 7 | 39 ± 18 | 36 ± 5 | 39 ± 9 |
| R20 | — | 29 ± 8 | 32 ± 8 | 35 ± 6 | 32 ± 8 |
| PaO2, mmHg | |||||
| BS | 253 ± 43 | 252 ± 36 | 224 ± 43 | 259 ± 98 | 260 ± 67 |
| BO | — | 260 ± 58 | 260 ± 35 | 231 ± 112 | 254 ± 65 |
| AO20 | — | 258 ± 79 | 242 ± 44 | 253 ± 88 | 235 ± 62 |
| R20 | — | 168 ± 99 | 148 ± 24 | 169 ± 73 | 155 ± 57 |
| Glucose, mg/dl | |||||
| BS | 120 ± 4 | 133 ± 16 | 113 ± 17 | 112 ± 33 | 114 ± 25 |
| BO | — | 129 ± 19 | 118 ± 26 | 114 ± 29 | 114 ± 29 |
| AO20 | — | 146 ± 28 | 92 ± 35 | 118 ± 21 | 134 ± 27 |
| R20 | — | 124 ± 16 | 111 ± 17 | 114 ± 11 | 121 ± 29 |
Figure 2.
EPO administered at the time of spinal cord reperfusion is associated with a significant improvement in the neurological score within 1 h after reperfusion. Whereas saline treated animals did not significantly improve over the following 48 h, rHu-EPO treated animals improved further compared with the neurological score obtained in the immediate postoperative period. *, P < 0.05
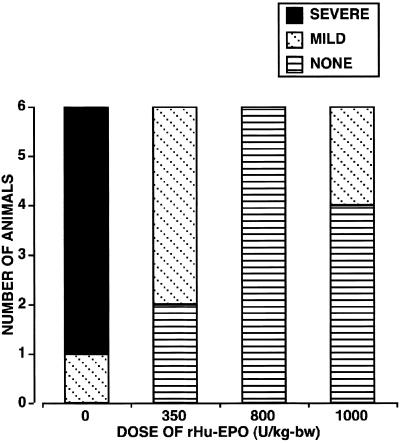
Histopathological scoring performed 48 h after reperfusion (Fig. 3) showed highly variable tissue injury depending on the treatment group. For saline-treated animals, most neurons within the gray matter were shrunken and pyknotic or necrotic. The surrounding neuropil was grossly disrupted and infiltrated by abundant mononuclear inflammatory cells. A majority of the spinal cords obtained from animals receiving only saline possessed extremely friable gray matter and sectioned poorly despite adequate fixation, consistent with extensive tissue necrosis. In contrast to gray matter, although the general architecture of the white matter was grossly retained with relatively few inflammatory cells present, moderate swelling of myelin sheaths was observed. Sections obtained from rHu-EPO-treated animals appeared very different, often lacking histological evidence of injury, despite exhibiting moderate neurological disability (mean neurological score ≈2.5). Occasional sections from rH-EPO-treated animals exhibited mild ischemic injury characterized by cytoplasmic eosinophilia and pyknotic nuclei, but lacked a prominent inflammatory infiltrate.
Figure 3.
Histopathological scoring of spinal cord tissue sections shows that rHu-EPO treatment is associated with markedly reduced cellular injury when compared with saline-treated animals. Based on this pathological analysis, 800 units/kg bw rHu-EPO appeared to protect better than the other two dosages of rHu-EPO tested, despite similar neurological scores.
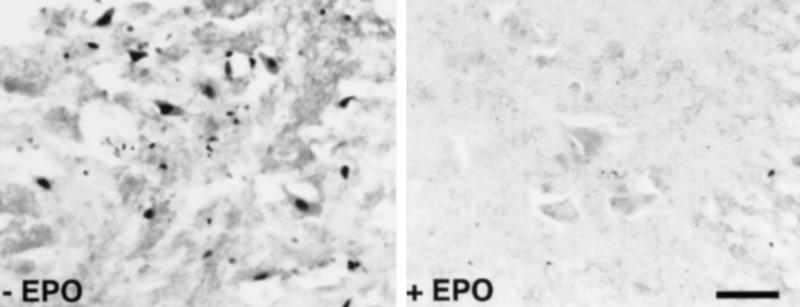
TUNEL labeling performed on adjacent spinal cord sections showed many positive large positive motor neurons contained within the ventral gray (Fig. 4 Left), as well as associated smaller cells of uncertain morphology. In contrast, ischemic spinal cords from animals that had received rHu-EPO treatment generally were devoid of TUNEL staining (Fig. 4 Right), with only very rare motor neuron reaction product observed. No significant TUNEL reaction product was observed within the white matter of either rH-EPO- or saline-treated animals.
Figure 4.
Saline-treated animals exhibit TUNEL-positive motor neurons (Left) in contrast to TUNEL-negative sections after rHu-EPO treatment (Right). White matter was devoid of TUNEL-positive cells (not shown). (Scale bar = 80 μm.)
Discussion
Histochemistry performed on adult human spinal cord sections revealed an intense immunoreactivity for EPO-R localized to capillaries, especially within the white matter. In contrast, much lower or undetectable levels were evident on larger vessels, such as arterioles. These findings are similar to those we and others have reported for the brain (5). Ultrastructural examination using electron microscopy will be required to identify which microdomains are responsible for the dense capillary staining. Based on our previous findings in the brain, much of the reaction product likely resides within astrocytic endfeet surrounding capillaries, with a smaller fraction residing within the endothelial cell or on its surface (5). Recently, the transport of rHu-EPO through an intact blood–brain barrier has been reported to occur to a degree sufficient to protect against diverse injury, including focal ischemia (5) and subarrachnoid hemorrhage (23). It is reasonable to hypothesize that the transport of EPO within the circulation across the blood–spinal cord barrier would occur to a similar degree and if so, would be neuroprotective of ischemia.
Another prominent anti-EPO-R staining pattern was of the somata and proximal dendrites of large motor neurons of the ventral horn, within a dense plexus of EPO-immunoreactive fibers. These findings suggest the existence of a local network of EPO and EPO-R involving the motor neuron. The limited location of EPO-R immunoreactivity to within the cell body and proximal dendrites could serve multiple functions, including EPO-dependent control of gene expression, modulation of afferent electrical input, or excitatory neurotransmitter release (7, 10, 18, 24, 25). Each of these processes could potentially have important implications for the response of neurons to potential ischemic injury.
Results of the present study show that rHu-EPO administered intravenously immediately after restoration of spinal cord vascular perfusion markedly improves the neurological outcome of rabbits subjected to transient global spinal ischemia. This model has proven to be a useful tool with which to evaluate potential therapeutic interventions with the goal of ameliorating spinal cord ischemic injury (26). The rabbit model of spinal cord ischemia is especially useful because of the unique segmental arterial blood supply to the spinal cord from the infrarenal aorta, which prevents bowel and kidney ischemia and thus largely avoids nonneurological complications.
Histopathological results obtained from examination of the spinal cords of the animals in this study confirm the neuroprotective effect of rHu-EPO on spinal motoneurons against ischemic insult in vivo and exhibited a parallelism with the observed neurological outcomes. The TUNEL labeling present in ventral horn motor neurons in the saline-treated group, which was almost entirely absent in the rHu-EPO-treated groups, is strongly supportive of an effect of EPO in blocking the induction of an apoptotic program in this class of neurons. Recent studies using this rabbit model have shown that within the first few days following transient spinal cord global ischemia the motor neurons undergo apoptosis (27, 28). The results observed here, therefore, confirm these observations as well as demonstrate that rHu-EPO can prevent ischemia-induced programmed cell death.
The distinctive lack of inflammatory infiltrate we observed in rHu-EPO-treated animals is consistent with earlier observations derived from a model of focal stroke (P. Villa, P. Ghezzi, T. Laragione, D. Agnello, T. Mennini, P. Bigini, A. Cagnotto, B. Viviani, M. Marinovich, A.C. and M.B., unpublished data) as well as experimental allergic encephalitis (P. Ghezzi, T. Mennini, P. Bigini, D. Agnello, P. Villa, C. Cerami, M.B., and A.C., unpublished data). The results of these studies suggest that the anti-inflammatory effects of EPO arises from its inhibition of apoptosis and/or inhibition of a proximal member of the inflammatory cascade, e.g., caspase-1.
In the current study, we observed an ameliorating effect of rHu-EPO on neurological status in this study within 1 h after reperfusion. This observation suggests that EPO acts by means of mechanisms other than its known gene-regulating effects that underlie the neuroprotective effects of EPO in in vitro models of acute brain ischemia (10). The biological basis of an immediate beneficial effect of EPO could involve modulation of the release of potentially neurotoxic compounds such as glutamate, as has been described to occur the hippocampus after EPO administration (25) or by direct electrical effects (24). Other potential mechanisms to explain the observed short latency efficacy of EPO could include vascular and metabolic effects. Vascular smooth muscle expresses EPO-R, through which rHu-EPO has a direct vasoconstrictive effects (29), including reducing postischemic vasodilation (30), which, if present, would tend to worsen injury through a vascular steal phenomenon.
In addition to its effects on the apoptosis of motor neurons, there may be other neuroprotective effects of Epo in the ischemic spinal cord that have not been assessed in this work. EPO may modulate several other processes that have been attributed in the pathogenesis of cerebral or spinal cord ischemia leading to cellular necrosis and inflammation, such as excitatory amino acid excitotoxicity, free radical overproduction, and nitric oxide overproduction. Further, long-term restorative properties of EPO based on its neurotrophic properties have not been assessed (8).
In conclusion, rHu-EPO systemically administered after a brief ischemic event that is similar to what can occur during aortic surgery protects against ischemic spinal cord injury. Because EPO has been widely used with very few adverse effects, human trials evaluating the efficacy of rHu-EPO the treatment of spinal cord ischemia, which remains one of the major perioperative complications of thoracoabdominal aortic surgery, are warranted. These findings also suggest that further studies are necessary to determine whether rHu-EPO possesses any restorative properties when administered during the subacute recovery period. A further question is whether EPO can also ameliorate injury resulting from spinal cord mechanical trauma, a pathophysiologic process that includes components of ischemia, traumatic cellular necrosis, inflammation, and apoptosis.
Abbreviations
EPO
erythropoietin
EPO-R
EPO receptor
rHu-EPO
recombinant human EPO
TUNEL
terminal deoxynucleotidyltransferase-mediated dUTP nick end-labeling
bw
body weight
References
- 1.Gharagozloo F, Larson J, Dausmann M J, Neville R F, Jr, Gomes M N. Chest. 1996;109:799–809. doi: 10.1378/chest.109.3.799. [DOI] [PubMed] [Google Scholar]
- 2.Qayumi A K, Janusz M T, Jamieson W R, Lyster D M. J Thorac Cardiovasc Surg. 1992;104:256–261. [PubMed] [Google Scholar]
- 3.Kouchoukos N T, Dougenis D. N Engl J Med. 1997;336:1876–1888. doi: 10.1056/NEJM199706263362606. [DOI] [PubMed] [Google Scholar]
- 4.Bernaudin M, Marti H H, Roussel S, Divoux D, Nouvelot A, MacKenzie E T, Petit E. J Cereb Blood Flow Metab. 1999;19:643–651. doi: 10.1097/00004647-199906000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Brines M L, Ghezzi P, Keenan S, Agnello D, de Lanerolle N C, Cerami C, Itri L M, Cerami A. Proc Natl Acad Sci USA. 2000;97:10526–10531. doi: 10.1073/pnas.97.19.10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakanaka M, Wen T C, Matsuda S, Masuda S, Morishita E, Nagao M, Sasaki R. Proc Natl Acad Sci USA. 1998;95:4635–4640. doi: 10.1073/pnas.95.8.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siren A L, Fratelli M, Brines M, Goemans C, Casagrande S, Lewczuk P, Keenan S, Gleiter C, Pasquali C, Capobianco A, et al. Proc Natl Acad Sci USA. 2001;98:4044–4049. doi: 10.1073/pnas.051606598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dame C, Juul S E, Christensen R D. Biol Neonate. 2001;79:228–235. doi: 10.1159/000047097. [DOI] [PubMed] [Google Scholar]
- 9.Sadamoto Y, Igase K, Sakanaka M, Sato K, Otsuka H, Sakaki S, Masuda S, Sasaki R. Biochem Biophys Res Commun. 1998;253:26–32. doi: 10.1006/bbrc.1998.9748. [DOI] [PubMed] [Google Scholar]
- 10.Morishita E, Masuda S, Nagao M, Yasuda Y, Sasaki R. Neuroscience. 1997;76:105–116. doi: 10.1016/s0306-4522(96)00306-5. [DOI] [PubMed] [Google Scholar]
- 11.Buemi M, Grasso G, Corica F, Calapai G, Salpietro F M, Casuscelli T, Sfacteria A, Aloisi C, Alafaci C, Sturiale A, et al. Eur J Pharmacol. 2000;392:31–34. doi: 10.1016/s0014-2999(00)00081-9. [DOI] [PubMed] [Google Scholar]
- 12.Calapai G, Marciano M C, Corica F, Allegra A, Parisi A, Frisina N, Caputi A P, Buemi M. Eur J Pharmacol. 2000;401:349–356. doi: 10.1016/s0014-2999(00)00466-0. [DOI] [PubMed] [Google Scholar]
- 13.Genc S, Kuralay F, Genc K, Akhisaroglu M, Fadiloglu S, Yorukoglu K, Fadiloglu M, Gure A. Neurosci Lett. 2001;298:139–141. doi: 10.1016/s0304-3940(00)01716-x. [DOI] [PubMed] [Google Scholar]
- 14.Bittorf T, Buchse T, Sasse T, Jaster R, Brock J. Cell Signal. 2001;13:673–681. doi: 10.1016/s0898-6568(01)00189-9. [DOI] [PubMed] [Google Scholar]
- 15.Campana W M, Myers R R. FASEB J. 2001;15:1804–1806. doi: 10.1096/fj.00-0857fje. [DOI] [PubMed] [Google Scholar]
- 16.Digicaylioglu M, Lipton S A. Nature (London) 2001;412:641–647. doi: 10.1038/35088074. [DOI] [PubMed] [Google Scholar]
- 17.Assandri R, Egger M, Gassmann M, Niggli E, Bauer C, Forster I, Gorlach A. J Physiol (London) 1999;516:343–352. doi: 10.1111/j.1469-7793.1999.0343v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koshimura K, Murakami Y, Sohmiya M, Tanaka J, Kato Y. J Neurochem. 1999;72:2565–2572. doi: 10.1046/j.1471-4159.1999.0722565.x. [DOI] [PubMed] [Google Scholar]
- 19.Kristal B, Shurtz-Swirski R, Shasha S M, Manaster J, Shapiro G, Furmanov M, Hassan K, Weissman I, Sela S. Nephron. 1999;81:406–413. doi: 10.1159/000045324. [DOI] [PubMed] [Google Scholar]
- 20.Sela S, Shurtz-Swirski R, Sharon R, Manaster J, Chezar J, Shkolnik G, Shapiro G, Shasha S M, Merchav S, Kristal B. Nephron. 2001;88:205–210. doi: 10.1159/000045991. [DOI] [PubMed] [Google Scholar]
- 21.Juul S E, Anderson D K, Li Y, Christensen R D. Pediatr Res. 1998;43:40–49. doi: 10.1203/00006450-199801000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Drummond J C, Moore S S. Anesthesiology. 1989;70:64–70. doi: 10.1097/00000542-198901000-00014. [DOI] [PubMed] [Google Scholar]
- 23.Grasso G. J Neurosurg Sci. 2001;45:7–14. [PubMed] [Google Scholar]
- 24.Kawakami M, Iwasaki S, Sato K, Takahashi M. Biochem Biophys Res Commun. 2000;279:293–297. doi: 10.1006/bbrc.2000.3926. [DOI] [PubMed] [Google Scholar]
- 25.Kawakami M, Sekiguchi M, Sato K, Kozaki S, Takahashi M. J Biol Chem. 2001;276:39469–39475. doi: 10.1074/jbc.M105832200. [DOI] [PubMed] [Google Scholar]
- 26.Zivin J A, DeGirolami U. Stroke. 1980;11:200–202. doi: 10.1161/01.str.11.2.200. [DOI] [PubMed] [Google Scholar]
- 27.Hayashi T, Sakurai M, Abe K, Sadahiro M, Tabayashi K, Itoyama Y. Stroke. 1998;29:1007–1013. doi: 10.1161/01.str.29.5.1007. [DOI] [PubMed] [Google Scholar]
- 28.Mackey M E, Wu Y, Hu R, DeMaro J A, Jacquin M F, Kanellopoulos G K, Hsu C Y, Kouchoukos N T. Stroke. 1997;28:2012–2017. doi: 10.1161/01.str.28.10.2012. [DOI] [PubMed] [Google Scholar]
- 29.Heidenreich S, Rahn K H, Zidek W. Kidney Int. 1991;39:259–265. doi: 10.1038/ki.1991.31. [DOI] [PubMed] [Google Scholar]
- 30.Buemi M, Allegra A, Lagana A, Aloisi C, Morabito N, Baldari S, Privitera M, Palella S, Imbesi D, Villari C, et al. Int Angiol. 1994;13:75–77. [PubMed] [Google Scholar]