DNA-dependent protein kinase suppresses double-strand break-induced and spontaneous homologous recombination (original) (raw)
Abstract
DNA-dependent protein kinase (DNA-PK), composed of Ku70, Ku80, and the catalytic subunit (DNA-PKcs), is involved in repairing double-strand breaks (DSBs) by nonhomologous end-joining (NHEJ). Certain proteins involved in NHEJ are also involved in DSB repair by homologous recombination (HR). To test the effects of DNA-PKcs on DSB-induced HR, we integrated neo direct repeat HR substrates carrying the I_-Sce_I recognition sequence into DNA-PKcs-defective Chinese hamster ovary (V3) cells. The DNA-PKcs defect was complemented with a human DNA-PKcs cDNA. DSB-induced HR frequencies were 1.5- to 3-fold lower with DNA-PKcs complementation. In complemented and uncomplemented strains, all products arose by gene conversion without associated crossover, and average conversion tract lengths were similar. Suppression of DSB-induced HR in complemented cells probably reflects restoration of NHEJ, consistent with competition between HR and NHEJ during DSB repair. Interestingly, spontaneous HR rates were 1.6- to >3.5-fold lower with DNA-PKcs complementation. DNA-PKcs may suppress spontaneous HR through NHEJ of spontaneous DSBs, perhaps at stalled or blocked replication forks. Because replication protein A (RPA) is involved in both replication and HR, and is phosphorylated by DNA-PKcs, it is possible that the suppression of spontaneous HR by DNA-PKcs reflects regulation of replication-dependent HR by DNA-PKcs, perhaps by means of phosphorylation of RPA.
Homologous recombination (HR) is an important double-strand break (DSB) repair pathway (1), and HR has also been implicated in lesion bypass during replication (2). A specific type of HR is gene conversion, a conservative process that can occur with or without an associated crossover. DSB-induced gene conversion involves nonreciprocal transfer of information from an unbroken donor locus to a broken (recipient) locus. Single-strand annealing is a distinct, nonconservative HR process restricted to cases when interacting regions are arranged as direct repeats (3–5). Key HR proteins include RAD51, five RAD51 paralogs (6–10), RAD52, and RAD54 (11, 12). Several other proteins have poorly defined roles in HR, including replication protein A (RPA), p53, and ATM (13–16). In yeast, Rad50 and Mre11 have been implicated in HR, particularly in an early step involving processing of broken ends to 3′ single-stranded tails (17).
DNA-dependent protein kinase (DNA-PK) is best known for its role in nonhomologous end-joining (NHEJ), an alternative DSB repair pathway. DNA-PK is a trimeric complex of a 465-kDa catalytic subunit (DNA-PKcs), Ku70, and Ku80 (18). DNA-PKcs, ATM, and ATR are members of the phosphatidylinositol kinase family. These proteins exhibit serine–threonine protein kinase activity, and are involved in the regulation of transcription, cell cycle progression, and genomic stability (19). Defects in NHEJ proteins in mammalian cells confer marked sensitivity to ionizing radiation (IR) and radiomimetic chemicals, and defects in DNA-PKcs inactivate V(D)J recombination, conferring severe combined immune deficiency in mice (20). Other DNA-PKcs mutants are the Chinese hamster ovary (CHO) V3 cell line, and the human glioblastoma cell line MO59J (20, 21). DNA-PKcs has not previously been known or proposed to have any function in relation to HR.
Genetic evidence supports the concept of HR and NHEJ as distinct (22–24), yet competing DSB repair pathways (25, 26). However, there is also evidence for mechanistic overlap between these pathways. In addition to DNA-PK, NHEJ requires LIG4 and XRCC4, and in yeast is influenced by defects in the Rad50–Mre11–Xrs2 complex (27). MRE11–RAD50–NBS1 is the comparable complex in mammalian cells (17). Thus, the MRE11–RAD50 complex has roles in both NHEJ and HR.
In view of the potential mechanistic overlap and/or competition between HR and NHEJ, we sought to test the effects of DNA-PKcs deficiency on HR. We measured HR frequencies and product spectra in DNA-PKcs-defective CHO cells carrying chromosomal direct repeat HR substrates, and in isogenic derivatives complemented with human DNA-PKcs. We found that both spontaneous and DSB-induced HR were suppressed by DNA-PKcs. However, DSB-induced HR product spectra were similar in the presence and absence of DNA-PKcs, indicating that the effect of DNA-PKcs on DSB-induced HR is limited to the initiation step. These results are discussed with respect to the competition between HR and NHEJ in DSB repair. We also discuss potential roles for DNA-PKcs in repairing replication-associated DSBs, and in recombinational bypass at stalled replication forks.
Materials and Methods
Isogenic Cell Lines with Defective or Functional DNA-PKcs.
Cell culture and electroporation conditions have been described (28). Plasmid pMSG_neo_2S12His (28) has a simian virus 40 promoter-driven Escherichia coli gpt gene flanked by a mouse mammary tumor virus (MMTV) promoter-driven neo gene (MMTV_neo_) inactivated by an insertion with an I-_Sce_I nuclease recognition site, a promoterless copy of neo with 12 phenotypically silent restriction fragment length polymorphisms (RFLPs) called neo12, and the yeast HIS3 gene, which serves as a buffer during integration. A related plasmid, pMSGneo2SHis, lacks the RFLPs. CHO V3 derivatives containing single copies of these HR substrates (Fig. 1) were constructed and characterized as described (28). The DNA-PKcs defect was complemented by coelectroporating 10 μg each of pPur (CLONTECH) and a human DNA-PKcs expression vector (29), or colipofecting 200 ng of pPur and 2 μg of DNA-PKcs expression vector. Control cell lines were prepared by transfection with pPur only. Puromycin-resistant transfectants were seeded to replicate 24-well dishes. Radiation-resistant clones were identified in one dish from each set exposed to continuous low-dose γ irradiation for 10 days (total dose ≈12 Gy) by using a 137Cs source (J.L. Shepherd and Associates model 81–12 Dual Beam Irradiator with model 155 Attenuator), or by using fractionated doses (1 Gy per day for 10 days). Replicate (nonirradiated) cultures of radiation-resistant clones were used in subsequent experiments. To quantify radiation resistance, 1.25 × 107 cells were irradiated in 25 ml of growth medium in 50-ml tubes, seeded at appropriate dilutions into 10-cm (diameter) dishes in growth medium, and incubated for 10 days before staining colonies with 1% (wt/vol) crystal violet in methanol. To measure DNA-PKcs levels, total cell lysates were prepared from 1.5 × 107 cells by using 50 mM Tris, pH 7.5/150 mM NaCl/0.5% (vol/vol) IGEPAL CA-630 [(octylphenoxy)polyethoxyethanol], with 1 μg/ml each of the protease inhibitors aprotinin, leupeptin, and pepstatin, and 50 μg/ml PMSF added fresh before lysis. Lysate protein concentrations were determined by Bio-Rad DC assay, and 100 μg of protein was combined with Laemmli loading buffer (2× buffer: 4% SDS/120 mM Tris, pH 6.8/20% glycerol/0.02% bromophenol blue/200 mM DTT added just before use), and heated to 65°C for 5 min before loading onto a SDS/4% PAGE gel. Separated proteins were transferred to a poly(vinylidene difluoride) membrane with transfer buffer (48 mM Tris base/390 mM glycine/0.1% SDS/5% methanol). ECL reagents (Amersham Pharmacia) were used to detect DNA-PKcs on Western blots using an overnight incubation at 4°C of a 1:250 dilution [in 5% nonfat milk/TBST (10 mM Tris⋅HCl, pH 8.0/150 mM NaCl/0.05% Tween-20)] of mouse monoclonal anti-DNA-PKcs antibodies (DNA-PKcs Ab-2, Oncogene), and a 3-h incubation at room temperature of a 1:1000 dilution of secondary antibodies in TBST. Loading controls were processed in parallel except that 10 μg of protein was loaded and probed with anti-β actin antibodies (Sigma).
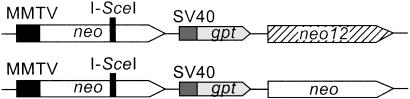
Figure 1.
HR substrates. (Upper) Two copies of a 1.4-kbp fragment carrying the neo gene (open and hatched boxes) are separated by a simian virus 40 (SV40) promoter-driven gpt gene. The upstream neo is driven by the MMTV promoter, and is inactivated by a frameshift insertion consisting of an I_-Sce_I site. The downstream copy (neo12) is inactive because it lacks a promoter. neo12 contains 12 silent, single-base mutations that create RFLPs for mapping conversion tracts (for details, see ref. 28). (Lower) HR substrate in which the downstream neo lacks RFLPs.
Recombination Assays.
DSB-induced HR was assayed essentially as described (28). Briefly, 4 × 105 cells were seeded into 3.5-cm wells, incubated for 24 h, and transfected with 2 μg of pCMV3xnls(I-_Sce_I) (26) to induce DSBs, or with 2 μg of the negative control vector pCMV(I-Sce_I−) (30) by using Lipofectamine Plus as recommended by Gibco/BRL (Gaithersburg, MD). Twenty-four hours after transfection, 2 × 104 cells were seeded to each of two 10-cm dishes. After an additional 24 h, G418 was added (600 μg/ml, 100% active). Cell viability was determined by plating appropriate dilutions into nonselective medium, and DSB-induced HR frequencies were calculated as the number of G418-resistant colonies per viable cell plated in G418 medium. Gross structures of HR products were analyzed by Southern hybridization as described (28). Gene conversion tracts were measured by restriction mapping of 1.5-kbp PCR products of MMTV_neo as described (31). Spontaneous HR rates were measured by using fluctuation analysis. For each cell line, we generated four subclones and distributed ≈20 × 106 viable cells of each subclone among 20 10-cm dishes containing G418. Cell viability was measured as above. After 12–14 days, colonies were stained and counted, and spontaneous HR rates were calculated as described by Reenan and Kolodner (32).
Results
Isogenic Cell Lines with neo Direct Repeats and Functional or Nonfunctional DNA-PKcs.
CHO V3 cells are highly sensitive to IR, reflecting the absence of functional DNA-PKcs (20), although the precise nature of the mutation is unknown. V3 radiosensitivity can be complemented with a cDNA encoding human DNA-PKcs (29). We constructed derivatives of V3 cells that each carried a single, integrated copy of one of two neo HR substrates (Fig. 1). In the first substrate, one copy of neo is driven by the MMTV promoter but is inactivated by insertion of an I_-Sce_I recognition sequence. The second neo has 12 phenotypically silent, single-base mutations at ≈100-bp intervals that create RFLPs, but is inactive because it lacks a promoter (28). The RFLPs allow detailed analysis of gene conversion tracts, including lengths, directionality, and continuity. The second HR substrate lacks the RFLPs. HR is detected by selecting for G418-resistant colonies. Six V3 derivatives were constructed, including four with RFLPs (V24, V714, V719, and V727) and two without (VD7 and VD13). Cotransfection of a human DNA-PKcs cDNA and a puromycin-resistance gene generated complemented derivatives; control strains were constructed by transfecting only the puromycin-resistance gene. Candidate DNA-PKcs-complemented transfectants were screened for increased radioresistance, and 1–3 functionally complemented derivatives of each cell line were isolated (identified by suffixes “-C1, -C2, … ”).
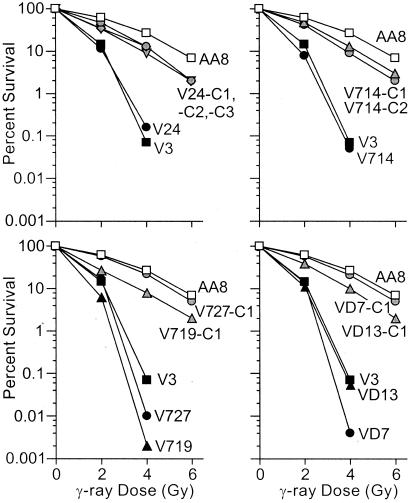
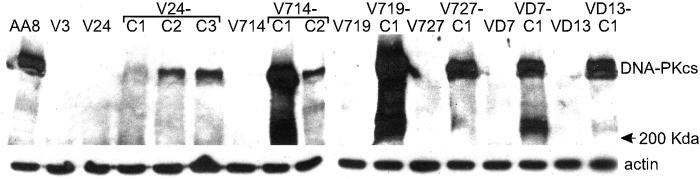
We measured cell survival after IR exposure and DNA-PKcs protein levels in each cell line. As expected, V3 and the six V3 derivatives were much more IR-sensitive than AA8, the wild-type parent of V3. All nine DNA-PKcs-complemented cell lines showed significantly increased radioresistance compared with noncomplemented lines, but only two showed essentially wild-type radioresistance (Fig. 2). The inability of human DNA-PKcs to fully restore wild-type radioresistance may reflect limitations of cross-species complementation. Western detection of DNA-PKcs showed high levels in AA8, none detectable in V3, V24, V714, V727, VD7, or VD13, and variable protein levels in the complemented cell lines (Fig. 3). There was little correlation between DNA-PKcs protein levels and radioresistance. In particular, V24-C1 had very low DNA-PKcs levels, yet it was as radioresistant as cell lines expressing much higher levels of DNA-PKcs. Several independent Western analyses produced results comparable to those in Fig. 3, indicating that the low DNA-PKcs level in V24-C1 is not an artifact of protein degradation. Previous complementation of V3 with human DNA-PKcs also showed a lack of correlation between DNA-PKcs levels and radioresistance (29). These results suggest that very low DNA-PKcs levels are sufficient to confer near-normal levels of NHEJ required for wild-type IR resistance.
Figure 2.
Functional complementation with human DNA-PKcs. Cells were irradiated with 137Cs γ-rays and the resulting colonies with at least 50 cells were counted. Data are averages of three determinations per strain. Control data (AA8 and V3) are reproduced in each panel.
Figure 3.
Western analysis of DNA-PKcs in wild-type (AA8), mutant (V), and complemented (-C) cells. Detection with anti-DNA-PKcs antibodies is shown above; anti-actin loading controls run in parallel are shown below.
DNA-PKcs Suppresses DSB-Induced HR.
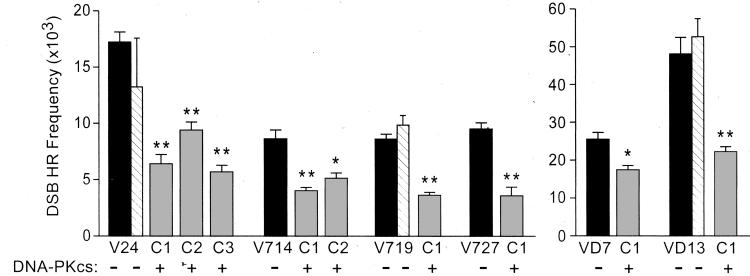
As in wild-type CHO cells (28), DSBs induced by I-_Sce_I nuclease enhanced HR frequencies by >2,000-fold in all cell lines in the present study (data not shown). DNA-PKcs complementation reduced DSB-induced HR by ≈2-fold (range: 1.5- to 3-fold; Fig. 4). In contrast, transfection of DNA-PKcs-defective cell lines with pPur alone had no significant effect on DSB-induced HR frequencies (Fig. 4; compare paired black and hatched bars). Thus, DNA-PKcs suppressed DSB-induced HR at six different chromosomal loci with substrates that were fully homologous or that had 12 heterologies. These results are consistent with the view that NHEJ mediated by DNA-PK competes with HR for repair of DSBs (see Discussion). Because IR resistance (and hence NHEJ) is restored to near wild-type levels by very low levels of DNA-PKcs, it is not surprising that HR was reduced to approximately the same degree among cell lines with different levels of DNA-PKcs expression.
Figure 4.
DSB-induced HR is suppressed by DNA-PKcs. Average HR frequencies (±SD) are shown for DNA-PKcs defective and complemented cell lines for 3–6 determinations per cell line. Value for each complemented derivative is significantly different from respective parent line (*, P < 0.01; **, _P_ < 0.001; _t_ tests). Hatched bars represent average HR frequencies of three independent pPur (control) transfectants of V24, V719, or VD13, based on three determinations per transfectant. None of the pPur values are significantly different from respective parent lines (all _P_ > 0.15, t tests).
In prior analyses in wild-type CHO cells (derived from strain K1c), 97% of DSB-induced HR products of the neo12 substrate arose by short tract gene conversion without associated crossover; the remainder arose by deletion of a copy of neo and SV_gpt_ by single-strand annealing, intrachromosomal crossovers, or unequal sister chromatid exchange (28). If DNA-PKcs complementation suppresses DSB-induced HR by increasing NHEJ, DSB-induced HR product spectra would be expected to be similar in the presence or absence of DNA-PKcs. We isolated 10 DSB-induced HR products from V24 cells and 9 products from DNA-PKcs complemented V24-C1 cells. All 19 products arose by gene conversion without associated crossover, and most tracts were <200 bp in length (data not shown). Thus, there was no apparent difference among product spectra generated from V24, V24-C1, and the prior wild-type spectrum (28). These results suggest that DNA-PKcs affects the frequency of HR initiation, but not subsequent steps.
DNA-PKcs Suppresses Spontaneous HR.
In a prior study with the neo12 HR substrate in wild-type CHO cells, the frequency of spontaneous HR was below the limit of detection (<10−7) (28). Interestingly, preliminary analysis indicated that spontaneous HR with this same substrate was readily detected in the DNA-PKcs mutant cell lines V24, V714, V719, and V727 (data not shown). Thus, it appeared that these DNA-PKcs-defective cell lines had hyper-recombination phenotypes. However, spontaneous HR frequency measurements are prone to error because of jackpots (reflecting early HR events during population expansion). To characterize the effect of DNA-PKcs on spontaneous HR, we measured spontaneous HR rates in two of these lines (V24, V714) and their respective DNA-PKcs-complemented derivatives by using fluctuation analysis (see Materials and Methods). In both cases, DNA-PKcs complementation suppressed spontaneous HR rates by ≈3-fold (or more) (Fig. 5). We characterized 14 spontaneous G418-resistance products of V24 and determined that all arose by gene conversion without associated crossovers (data not shown); spontaneous products from the complemented V24 derivatives were not characterized because too few were recovered.
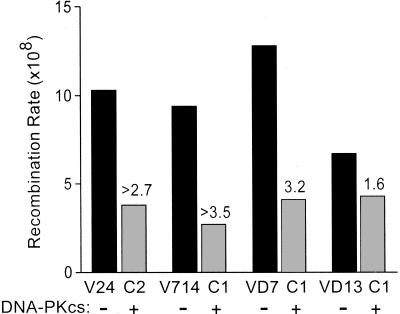
Figure 5.
Spontaneous HR is suppressed by DNA-PKcs. HR rates for V24-C2 and V714-C1 represent minimum estimates because the median number of G418-resistant colonies was zero. Fold decreases with DNA-PKcs complementation are given above bars.
In bacteria, yeast, and mammalian cells, sequence divergence (as with neo12) inhibits spontaneous HR, often by 100- to 1,000-fold (33–47), and this inhibition is largely mediated by the mismatch repair system (38–40, 43, 44, 48). Consistent with these prior studies, preliminary analysis (involving single determinations of spontaneous HR frequencies per cell line) indicated that frequencies were generally higher among 11 V3 derivatives with neo as donor than among 14 V3 derivatives with the neo12 donor (data not shown). However, HR frequencies varied widely even among cell lines carrying the same HR substrate, likely reflecting both jackpots and position effects (49). To determine whether DNA-PKcs influence on spontaneous HR depends on sequence heterology in HR substrates, we performed fluctuation analysis on two cell lines with the fully homologous neo donor (VD7 and VD13) and their complemented derivatives. As above, DNA-PKcs complementation suppressed spontaneous HR rates by 1.6- to 3.2-fold in the absence of RFLPs (Fig. 5). Thus, DNA-PKcs suppressed spontaneous HR rates at several different chromosomal loci, and with both fully homologous or diverged HR substrates.
Discussion
Competition Between NHEJ and HR.
DSBs are repaired by both HR and NHEJ in yeast and mammalian cells, but the relative contributions of the two repair pathways differ between these cell types. This difference might reflect differences in repair proteins [yeast apparently lack both poly(ADP-ribose) polymerase and DNA-PKcs] or differential regulation of a common set of repair proteins. Early genetic evidence suggested competition between HR and NHEJ (25), and this view was strengthened by findings that HR proteins (RAD51, RAD52) and NHEJ proteins (Ku70, Ku80, DNA-PKcs) bind to DNA ends at DSBs (1, 50). In Drosophila, chicken DT40 cells, and mouse, combined defects in HR (RAD54) and NHEJ (Ku70 or DNA-PKcs) led to synergistic increases in IR sensitivity, suggesting that these genes are in distinct epistasis groups (22–24), although it is clear that HR and NHEJ repair overlapping sets of DSB damage (26) as well as mitomycin C crosslinks (23). If these two pathways are in passive competition for DSBs (see below), and neither pathway is saturated, eliminating one pathway should increase the contribution of the other pathway. Consistent with the hypothesis that inactivation of NHEJ shunts DSBs to the competing HR pathway, we show here that complementation of DNA-PKcs-defective CHO cells with human DNA-PKcs suppresses DSB-induced HR by ≈2-fold. This hypothesis was also supported by our analogous study in which we showed that that DSB-induced HR was 1.25- to 1.5-fold lower in wild-type yeast compared with NHEJ-defective yku70 mutants (51). These modest decreases probably reflect the relatively minor role of NHEJ in yeast. Although a prior report suggested that DSB-induced HR frequencies in mammalian cells may not be affected by a defect in Ku80 (52), recent data show Ku80 effects comparable to those reported here (73).
I_-Sce_I-induced DSBs are known to be repaired by imprecise NHEJ and by precise NHEJ (i.e., direct ligation of cohesive ends). Although imprecise NHEJ is thought to require the end-alignment function of DNA-PK (53), precise NHEJ (not detectable in our system) may be efficient in the absence of DNA-PKcs, requiring only Ku70/Ku80, LIG4, and XRCC4. Nuclease DSBs are known to be repaired relatively efficiently by direct ligation in mammalian cells (54, 55). Thus, a defect in DNA-PKcs may shunt toward HR only that fraction of DSBs ordinarily destined for repair by imprecise end-joining. In mouse cells, 30–50% of I_-Sce_I-induced DSBs were repaired by HR (26). If the imprecise end-joining events are shunted to HR in the absence of DNA-PKcs, DSB-induced HR would increase by 2- to 3-fold, as observed here.
We can envision passive and active modes of competition between NHEJ and HR. With passive competition, the repair outcome might depend on whether HR or NHEJ proteins bind first to broken ends, and/or the availability of a homologous repair template. With active competition, the proteins involved in NHEJ and HR interact directly and influence each others' activities. Active competition is supported by the findings that the MRE11/RAD50/NBS1(XRS2) complexes have roles in both HR and NHEJ, and by the interactions between DNA-PKcs and proteins that influence HR, such as p53, ATM, and RPA. Current data do not distinguish between these models, and both may operate under specific conditions. For example, competition for nuclease DSBs might be passive, requiring relocation of repair proteins to broken ends, as with Ku in yeast (56). In contrast, active interactions between HR and NHEJ proteins may be particularly important when DNA damage is encountered during replication, as discussed below.
Does DNA-PKcs Suppress DSB-Induced HR and Spontaneous HR by Distinct Mechanisms?
HR induced by I-_Sce_I DSBs is likely to be replication-independent. Increasing evidence suggests that spontaneous HR depends on replication, but it is not clear whether DSBs are involved. Single-strand breaks, arising during nucleotide and base excision repair of spontaneous lesions, may convert to DSBs when encountered by a replication fork (reviewed in ref. 57). Many types of damage stall or block replication fork progression, and in E. coli DSBs have been observed at arrested replication forks (58). However, arrested replication can restart by a process termed recombination-dependent DNA replication, or homologous recombinational bypass, that is independent of DSB formation (2).
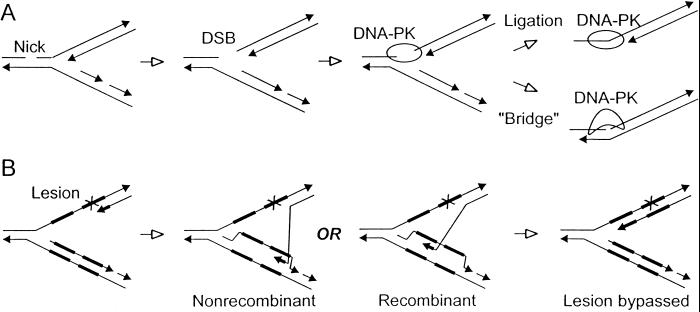
We found that spontaneous HR rates were suppressed by ≈3-fold by DNA-PKcs complementation. This finding may reflect critical roles for DNA-PKcs in the repair of replication-dependent DSBs, although it is unlikely that such a role involves error-prone imprecise end-joining. Instead, DNA-PKcs might mediate precise end-joining of these DSBs, perhaps by promoting ligation of nonoverlapping single strands as observed by Roth and Wilson (55), or by acting to bridge such sequences during DNA synthesis (Fig. 6A). In the absence of DNA-PKcs, replication-dependent DSBs may default to HR bypass (Fig. 6B). In the more general case of replication arrested by any form of template defect, DNA-PKcs might negatively regulate HR bypass by binding to the 3′ end of the newly synthesized strand, thereby impeding the formation of a RAD51-nucleoprotein filament, the initiation of strand transfer, or extension of the transferred strand. In this regard, it is interesting that DNA-PKcs has been shown to block DNA end-processing in vitro (59).
Figure 6.
Potential roles of DNA-PKcs in repair of replication-dependent DSBs or lesion bypass. (A) Nicks are converted to DSBs when encountered by a replication fork. DNA-PKcs might facilitate direct ligation of single-stranded ends, or it might transiently bridge the discontinuity, allowing replication to proceed. (B) During recombinational lesion bypass, the nascent end at a blocked replication fork invades the sister chromatid. If repeats are present (shown by thick segments), invasion can occur in misaligned or correctly aligned modes, producing recombinants or nonrecombinants, respectively. In either case, reassociation of this strand with the original template effects lesion bypass.
Alternatively, DNA-PKcs suppression of spontaneous HR may involve interactions of DNA-PKcs with (or phosphorylation of) proteins involved in NHEJ, HR, and replication. For example, Ku is a likely phosphorylation target of DNA-PKcs (20), and a defect in DNA-PKcs might affect HR by altering Ku activity. Also, p53 and ATM are potential DNA-PKcs phosphorylation targets and both influence spontaneous HR (13–15). p53 interacts with RAD51 (60, 61), and ATM interacts with BRCA1 (62), which is involved in HR (63). p53 and/or ATM might influence HR at blocked replication forks, or their effects on HR may be indirect, reflecting instead their checkpoint functions. In cells with defective checkpoints, replication continues despite DNA damage, thus increasing the probability that a replication fork will encounter template lesions, stall and/or produce DSBs.
The possibility that DNA-PKcs might modulate HR repair or bypass in conjunction with replication is further underscored by its interactions with RPA. RPA is a trimeric protein complex that binds single-stranded DNA and has important roles in both replication and HR (16). RPA is one of several proteins with accessory roles in RAD51-dependent strand transfer (64–69). RPA may also be an important in vivo phosphorylation target of DNA-PKcs. Although there have been conflicting reports in this regard (70, 71), the most recent data indicate that DNA-PKcs and RPA form a stable complex in unstressed cells, and that DNA-PKcs phosphorylates RPA in response to DNA damage specifically during DNA replication, leading to dissociation of the complex (72). In the absence of DNA-PKcs, RPA would remain nonphosphorylated, and in this form RPA might interfere with NHEJ, and thereby promote HR. Alternatively, nonphosphorylated RPA might promote the transfer of a nascent end at a blocked replication fork to a sister chromatid. Although some HR in our system may involve interactions between linked neo repeats, most events likely involve interactions between repeats on sister chromatids (12). To produce a selectable (neo+) recombinant product, the neo repeats in the two sister chromatids must be misaligned (Fig. 6B). At blocked replication forks, interactions between RPA and DNA-PKcs may suppress strand transfer between sister chromatids, and/or influence the likelihood of misalignment between repeated elements.
Acknowledgments
We thank Scott Peterson and James Halbrook for their valuable comments and Cheryl Pulaski for technical assistance. This work was supported by a Department of Defense Breast Cancer Grant and National Institutes of Health Grants CA74046 (to D.J.C.) and CA77693 (to J.A.N.).
Abbreviations
DSB
double-strand break
NHEJ
nonhomologous end-joining
DNA-PK
DNA-dependent protein kinase
DNA-PKcs
catalytic subunit of DNA-PK
HR
homologous recombination
RPA
replication protein A
IR
ionizing radiation
CHO
Chinese hamster ovary
MMTV
mouse mammary tumor virus
RFLP
restriction fragment length polymorphism
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Haber J E. Trends Biochem Sci. 2000;16:259–264. [Google Scholar]
- 2.Kogoma T. Microbiol Mol Biol Rev. 1997;61:212–238. doi: 10.1128/mmbr.61.2.212-238.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin F-L, Sperle K, Sternberg N. Mol Cell Biol. 1984;4:1020–1034. doi: 10.1128/mcb.4.6.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fishman-Lobell J, Rudin N, Haber J E. Mol Cell Biol. 1992;12:1292–1303. doi: 10.1128/mcb.12.3.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nickoloff J A, Singer J D, Hoekstra M F, Heffron F. J Mol Biol. 1989;207:527–541. doi: 10.1016/0022-2836(89)90462-2. [DOI] [PubMed] [Google Scholar]
- 6.Takata M, Sasaki M S, Sonoda E, Fukushima T, Morrison C, Abala J S, Swagemakers S M A, Kanaar R, Thompson L H, Takeda S. Mol Cell Biol. 2000;20:6476–6482. doi: 10.1128/mcb.20.17.6476-6482.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takata M, Sasaki M S, Tachiiri S, Fukushima T, Sonoda E, Schild D, Thompson L H, Takeda S. Mol Cell Biol. 2001;21:2858–2866. doi: 10.1128/MCB.21.8.2858-2866.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson R D, Liu N, Jasin M. Nature (London) 1999;401:397–399. doi: 10.1038/43932. [DOI] [PubMed] [Google Scholar]
- 9.Pierce A J, Johnson R D, Thompson L H, Jasin M. Genes Dev. 1999;13:2633–2638. doi: 10.1101/gad.13.20.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brenneman M A, Weiss A E, Nickoloff J A, Chen D J. Mutat Res. 2000;459:89–97. doi: 10.1016/s0921-8777(00)00002-1. [DOI] [PubMed] [Google Scholar]
- 11.Yamaguchi-Iwai Y, Sonoda E, Buerstedde J M, Bezzubova O, Morrison C, Takata M, Shinohara A, Takeda S. Mol Cell Biol. 1998;18:6430–6435. doi: 10.1128/mcb.18.11.6430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dronkert M L G, Beverloo H B, Johnson R D, Hoeijmakers J H J, Jasin M, Kanaar R. Mol Cell Biol. 2000;20:3147–3156. doi: 10.1128/mcb.20.9.3147-3156.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bertrand P, Rouillard D, Boulet A, Levalois C, Soussi T, Lopez B S. Oncogene. 1997;14:1117–1122. doi: 10.1038/sj.onc.1200931. [DOI] [PubMed] [Google Scholar]
- 14.Mekeel K L, Tang W, Kachnic L A, Luo C M, Defrank J S, Powell S N. Oncogene. 1997;14:1847–1857. doi: 10.1038/sj.onc.1201143. [DOI] [PubMed] [Google Scholar]
- 15.Meyn M S. Science. 1993;260:1327–1330. doi: 10.1126/science.8493577. [DOI] [PubMed] [Google Scholar]
- 16.Iftode C, Daniely Y, Borowiec J A. Crit Rev Biochem Mol Biol. 1999;34:141–180. doi: 10.1080/10409239991209255. [DOI] [PubMed] [Google Scholar]
- 17.Petrini J H J, Maser R S, Bressan D A. In: DNA Damage and Repair: Advances from Phage to Humans. Nickoloff J A, Hoekstra M F, editors. Vol. 3. Totowa, NJ: Humana; 2001. pp. 147–172. [Google Scholar]
- 18.Smith G C M, Divecha N, Lakin N D, Jackson S P. Biochem Soc Symp. 1999;64:91–104. [PubMed] [Google Scholar]
- 19.Dasika G K, Lin S J, Zhao S, Sung P, Tomkinson A, Lee E Y-H P. Oncogene. 1999;18:7883–7899. doi: 10.1038/sj.onc.1203283. [DOI] [PubMed] [Google Scholar]
- 20.Peterson S R, Kurimasa A, Oshimura M, Dynan W S, Bradbury E M, Chen D J. Proc Natl Acad Sci USA. 1995;92:3171–3174. doi: 10.1073/pnas.92.8.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lees-Miller S P, Godbout R, Chan D W, Weinfeld R, Day R D, Barron G M, Allalunis-Turner M J. Science. 1995;267:1183–1185. doi: 10.1126/science.7855602. [DOI] [PubMed] [Google Scholar]
- 22.Takata M, Sasaki M S, Sonoda E, Morrison C, Hashimoto M, Utsumi H, Yamaguchi-Iwai Y, Shinohara A, Takeda S. EMBO J. 1998;17:5497–5508. doi: 10.1093/emboj/17.18.5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Essers J, van Steeg H, de Wit J, Swagemakers S M A, Vermeij M, Hoeijmakers J H J, Kanaar R. EMBO J. 2000;19:1703–1710. doi: 10.1093/emboj/19.7.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kooistra R, Pastink A, Zonneveld J B M, Lohman P H M, Eeken J C J. Mol Cell Biol. 1999;19:6269–6275. doi: 10.1128/mcb.19.9.6269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roth D, Wilson J H. Proc Natl Acad Sci USA. 1985;82:3355–3359. doi: 10.1073/pnas.82.10.3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liang F, Han M G, Romanienko P J, Jasin M. Proc Natl Acad Sci USA. 1998;95:5172–5177. doi: 10.1073/pnas.95.9.5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Critchlow S E, Jackson S P. Trends Biochem Sci. 1998;23:394–398. doi: 10.1016/s0968-0004(98)01284-5. [DOI] [PubMed] [Google Scholar]
- 28.Taghian D G, Nickoloff J A. Mol Cell Biol. 1997;17:6386–6393. doi: 10.1128/mcb.17.11.6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurimasa A, Kumano S, Boubnov N V, Story M D, Tung C S, Peterson S R, Chen D J. Mol Cell Biol. 1999;19:3877–3884. doi: 10.1128/mcb.19.5.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choulika A, Perrin A, Dujon B, Nicolas J-F. Mol Cell Biol. 1995;15:1968–1973. doi: 10.1128/mcb.15.4.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li W H, Hesabi B, Babbo A, Pacione C, Liu J M, Chen D J, Nickoloff J A, Shen Z Y. Nucleic Acids Res. 2000;28:1145–1153. doi: 10.1093/nar/28.5.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reenan R A G, Kolodner R D. Genetics. 1992;132:975–985. doi: 10.1093/genetics/132.4.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Claverys J P, Lacks S A. Microbiol Rev. 1986;50:133–165. doi: 10.1128/mr.50.2.133-165.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen W, Jinks-Robertson S. Genetics. 1999;151:1299–1313. doi: 10.1093/genetics/151.4.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matic I, Rayssiguier C, Radman M. Cell. 1995;80:507–515. doi: 10.1016/0092-8674(95)90501-4. [DOI] [PubMed] [Google Scholar]
- 36.Rayssiguier C, Thaler D S, Radman M. Nature (London) 1989;342:396–401. doi: 10.1038/342396a0. [DOI] [PubMed] [Google Scholar]
- 37.Bailis A M, Rothstein R. Genetics. 1990;126:535–547. doi: 10.1093/genetics/126.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chambers S R, Hunter N, Louis E J, Borts R H. Mol Cell Biol. 1996;16:6110–6120. doi: 10.1128/mcb.16.11.6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Datta A, Adjiri A, New L, Crouse G F, Jinks-Robertson S. Mol Cell Biol. 1996;16:1085–1093. doi: 10.1128/mcb.16.3.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Datta A, Hendrix M, Lipsitch M, Jinks-Robertson S. Proc Natl Acad Sci USA. 1997;94:9757–9762. doi: 10.1073/pnas.94.18.9757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harris S, Rudnicki K S, Haber J E. Genetics. 1993;135:5–16. doi: 10.1093/genetics/135.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Porter G, Westmoreland J, Priebe S, Resnick M A. Genetics. 1996;143:755–767. doi: 10.1093/genetics/143.2.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Selva E M, New L, Crouse G F, Lahue R S. Genetics. 1995;139:1175–1188. doi: 10.1093/genetics/139.3.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Wind N, Dekker M, Berns A, Radman M, te Riele H. Cell. 1995;82:321–330. doi: 10.1016/0092-8674(95)90319-4. [DOI] [PubMed] [Google Scholar]
- 45.Elliott B, Richardson C, Winderbaum J, Nickoloff J A, Jasin M. Mol Cell Biol. 1998;18:93–101. doi: 10.1128/mcb.18.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Waldman A S, Liskay R M. Proc Natl Acad Sci USA. 1987;84:5340–5344. doi: 10.1073/pnas.84.15.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang D, Waldman A S. Mol Cell Biol. 1997;17:3614–3628. doi: 10.1128/mcb.17.7.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Negritto M T, Wu X, Kuo T, Chu S, Bailis A M. Mol Cell Biol. 1997;17:278–286. doi: 10.1128/mcb.17.1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bollag R J, Waldman A S, Liskay R M. Annu Rev Genet. 1989;23:199–225. doi: 10.1146/annurev.ge.23.120189.001215. [DOI] [PubMed] [Google Scholar]
- 50.Baumann P, West S C. Trends Biochem Sci. 1998;23:247–251. doi: 10.1016/s0968-0004(98)01232-8. [DOI] [PubMed] [Google Scholar]
- 51.Clikeman J A, Khalsa G J, Barton S L, Nickoloff J A. Genetics. 2000;157:579–589. doi: 10.1093/genetics/157.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liang F, Romanienko P J, Weaver D T, Jeggo P A, Jasin M. Proc Natl Acad Sci USA. 1996;93:8929–8933. doi: 10.1073/pnas.93.17.8929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hammarsten O, DeFazio L G, Chu G. J Biol Chem. 2000;275:1541–1550. doi: 10.1074/jbc.275.3.1541. [DOI] [PubMed] [Google Scholar]
- 54.Lin Y, Lukacsovich T, Waldman A S. Mol Cell Biol. 1999;19:8353–8360. doi: 10.1128/mcb.19.12.8353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roth D B, Wilson J H. Mol Cell Biol. 1986;6:4295–4304. doi: 10.1128/mcb.6.12.4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martin S G, Laroche T, Suka N, Grunstein M, Gasser S M. Cell. 1999;97:621–633. doi: 10.1016/s0092-8674(00)80773-4. [DOI] [PubMed] [Google Scholar]
- 57.Rothstein R, Michel B, Gangloff S. Genes Dev. 2000;14:1–10. [PubMed] [Google Scholar]
- 58.Michel B, Ehrlich S D, Uzest M. EMBO J. 1997;16:430–438. doi: 10.1093/emboj/16.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Calsou P, Frit P, Humbert O, Muller C, Chen D J, Salles B. J Biol Chem. 1999;274:7848–7856. doi: 10.1074/jbc.274.12.7848. [DOI] [PubMed] [Google Scholar]
- 60.Buchhop S, Gibson M K, Wang X W, Wagner P, Sturzbecher H W, Harris C C. Nucleic Acids Res. 1997;25:3868–3874. doi: 10.1093/nar/25.19.3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lim D-S, Hasty P. Mol Cell Biol. 1996;16:7133–7143. doi: 10.1128/mcb.16.12.7133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang Y, Cortez D, Yazdi Y, Neff N, Elledge S J, Qin J. Genes Dev. 2000;14:927–939. [PMC free article] [PubMed] [Google Scholar]
- 63.Moynahan M E, Chiu J W, Koller B H, Jasin M. Mol Cell. 1999;4:511–518. doi: 10.1016/s1097-2765(00)80202-6. [DOI] [PubMed] [Google Scholar]
- 64.Sigurdsson S, Trujillo K, Song B W, Stratton S, Sung P. J Biol Chem. 2001;276:8798–8806. doi: 10.1074/jbc.M010011200. [DOI] [PubMed] [Google Scholar]
- 65.McIlwraith M J, Van Dyck E, Masson J Y, Stasiak A Z, Stasiak A, West S C. J Mol Biol. 2000;304:151–164. doi: 10.1006/jmbi.2000.4180. [DOI] [PubMed] [Google Scholar]
- 66.Hays S L, Firmenich A A, Massey P, Banerjee R, Berg P. Mol Cell Biol. 1998;18:4400–4406. doi: 10.1128/mcb.18.7.4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gasior S L, Wong A K, Kora Y, Shinohara A, Bishop D K. Genes Dev. 1998;12:2208–2221. doi: 10.1101/gad.12.14.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Park M S, Ludwig D L, Stigger E, Lee S H. J Biol Chem. 1996;271:18996–19000. doi: 10.1074/jbc.271.31.18996. [DOI] [PubMed] [Google Scholar]
- 69.Sung P, Robberson D L. Cell. 1995;82:453–461. doi: 10.1016/0092-8674(95)90434-4. [DOI] [PubMed] [Google Scholar]
- 70.Fried L M, Koumenis C, Peterson S R, Green S L, van Zijl P, Allalunis-Turner M J, Chen D J, Fishel R, Giaccia A J, Brown J M, Kirchgessner C U. Proc Natl Acad Sci USA. 1996;93:13825–13830. doi: 10.1073/pnas.93.24.13825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Boubnov N V, Weaver D T. Mol Cell Biol. 1995;15:5700–5706. doi: 10.1128/mcb.15.10.5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shao R G, Cao C X, Zhang H, Kohn K W, Wold M S, Pommier Y. EMBO J. 1999;18:1397–1406. doi: 10.1093/emboj/18.5.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pierce A J, Hu P, Han M G, Ellis N, Jasin M. Genes Dev. 2001;15:3237–3242. doi: 10.1101/gad.946401. [DOI] [PMC free article] [PubMed] [Google Scholar]