Toll-Like Receptor 2 Regulates Interleukin-1β-Dependent Cardiomyocyte Hypertrophy Triggered by Trypanosoma cruzi (original) (raw)
Abstract
Trypanosoma cruzi, the intracellular protozoan parasite that causes Chagasic cardiomyopathy, elicits a robust hypertrophic response in isolated cardiomyocytes. Previous studies established that _T. cruzi_-elicited cardiomyocyte hypertrophy is mediated by interleukin-1β produced by infected cardiomyocyte cultures. Here, we define key upstream signaling events leading to cardiomyocyte hypertrophy in response to T. cruzi infection, to be dependent on Toll-like receptor 2 and NF-κB. Furthermore, we demonstrate that cardiomyocyte hypertrophy, which is initiated by live infective T. cruzi trypomastigotes or stimulation of isolated myocytes with secreted/released trypomastigote molecules, is a common outcome of the cardiomyocyte recognition of pathogen-associated molecular patterns by intrinsic Toll-like receptors. This study is the first to link pathogen recognition by intrinsic Toll-like receptors to cardiomyocyte hypertrophy.
Chagasic cardiomyopathy, a leading cause of heart disease in South and Central America, results from chronic infection with the intracellular protozoan pathogen Trypanosoma cruzi (45). Establishment of myocardial infection by T. cruzi and the ensuing host response to this pathogen are considered to be critical events influencing the pathogenesis and progression of Chagas' disease (47). Cardiomyocytes are terminally differentiated cells that are not readily replaced in the myocardium. Consequently, they respond to stressful conditions by initiating adaptive strategies, including hypertrophy and inhibition of apoptosis (14, 40, 46). Cardiomyocyte hypertrophy involves the reactivation of an embryonic pattern of gene expression and increased expression of contractile proteins, followed by an increase in cell size (14). The biochemical and physiologic changes that accompany a hypertrophic response result in increased myocardial contractility and output, which facilitate the maintenance of cardiac function in the short term. If the hypertrophic state is prolonged, it is frequently associated with apoptotic cell death, fibrosis, and dilated cardiomyopathy, a leading predictor of failure (14).
A recent study that examined early host responses to T. cruzi infection in isolated cardiomyocytes revealed a significant fraction of hypertrophic cells in populations containing infected cardiomyocytes, where hypertrophy was demonstrated in both parasite-containing and noninfected cells in culture (35). The hypertrophic response in _T. cruzi_-infected cardiomyocytes was characterized by reexpression of the gene encoding a fetal contractile protein, myosin heavy chain β; increased expression of α-actinin; and increased cell size (35), consistent with a standard definition of hypertrophy (14). Further characterization of this response identified the proinflammatory cytokine, interleukin-1β (IL-1β), as the primary mediator of _T. cruzi_-induced cardiomyocyte hypertrophy. This was demonstrated by the addition of a specific cytokine trap to infected cardiomyocyte cultures, which blocked the activity of IL-1β and significantly diminished the overall hypertrophic response (35). These unexpected results revealed the potential for effectors of the host innate immune response to alter cardiomyocyte physiology early in the T. cruzi infective process.
Toll-like receptors (TLRs), a family of type I transmembrane proteins that recognize a wide range of exogenous ligands, are critical players in initiation of the innate immune response to pathogens (3, 27). TLR ligands include a growing list of pathogen-associated molecular patterns (PAMPs), as well as endogenous molecules such as heat shock proteins or products of oxidative stress that alert the surrounding tissue to stress or damage (12, 20, 31). Ligand-bound TLRs transduce signals via the cytoplasmic Toll/IL-1R domain following the recruitment of cytosolic adaptor molecules, including MyD88, which in turn facilitate the assembly of signaling complexes (1, 28). These events often lead to the activation of NF-κB, synthesis of proinflammatory cytokines, and induction of the costimulatory molecules required for initiation of the adaptive immune response (3). Recent studies demonstrate that Toll-like receptors are key upstream regulators of the early proinflammatory cytokine response triggered by infective T. cruzi trypomastigotes in macrophages and function as important components of the overall mechanism of host resistance to this pathogen (5, 6, 32). Experimental infection of mice deficient in functional TLRs or MyD88 reveal that the early proinflammatory cytokine response to T. cruzi is complex and likely involves input from several TLRs (6, 32). Given this complexity, studies carried out in vitro with isolated macrophages and reporter cell lines have been instrumental in providing mechanistic insights into this process (5, 32). Studies to date have identified two classes of lipid-containing T. cruzi molecules that exhibit proinflammatory properties. Glycophosphatidylinositol (GPI)-linked mucins trigger proinflammatory cytokine expression in a TLR2-dependent manner (5), and glycoinositolphospholipids (GIPLs) engage TLR4-dependent signaling pathways (32). In addition, it is possible that other parasite-shed molecules capable of engaging TLRs (33) also participate in the overall proinflammatory response to T. cruzi in the host.
The mechanism of proinflammatory cytokine induction by T. cruzi has been well characterized in macrophages (5, 6); however, cellular pathways initiating a similar response in cardiac myocytes (26) have yet to be elucidated. Of the growing family of Toll-like receptors, TLR2, -3, -4, and -6 are expressed by rat neonatal cardiomyocytes (12). While it is recognized that cardiomyocytes participate in monitoring conditions of stress in the heart via intrinsic Toll-like receptors (11-13), a role for Toll-like receptor activation in cardiac hypertrophy has not been demonstrated. In this study, we exploit isolated rat neonatal cardiomyocytes, an in vitro culture system that has been extremely valuable for probing the molecular basis of cardiomyocyte hypertrophy elicited by various stimuli (4, 10, 17, 18, 37, 39, 41, 42), to examine the role of Toll-like receptors in _T. cruzi_-elicited cardiomyocyte hypertrophy. We demonstrate that TLR2 functions as the main upstream regulator of hypertrophy triggered in isolated cardiomyocytes by T. cruzi. Moreover, we demonstrate that cardiomyocytes exposed to lipopolysaccharide (LPS) or the 2-kDa macrophage-activating lipopeptide (MALP-2) also undergo hypertrophy in a TLR-dependent manner. Overall, our findings suggest that intrinsic cardiomyocyte Toll-like receptors play a role in the rapid response to pathogens and their products and that cardiomyocyte hypertrophy may be an important early consequence of engagement of this innate system of pathogen recognition in the heart.
MATERIALS AND METHODS
Isolation of neonatal rat ventricular myocytes.
Primary cultures of ventricular cardiomyocytes from neonatal Sprague-Dawley rats (Charles River Laboratories) were prepared as described previously (35). This procedure was in compliance with Harvard University animal use committee-approved protocols.
Parasite infection.
Trypanosoma cruzi (Y strain) was propagated in monolayers of LLC-MK2 cells and infective trypomastigotes harvested as described previously (35). For infection, cardiomyocytes were incubated with trypomastigotes at 2 × 107 parasites/ml for 2 h at 37°C in 5% CO2. Extracellular parasites were then aspirated, fresh medium was added to cultures, and incubations were carried out for a total of 2, 24, or 48 h. Where indicated, cardiomyocytes were pretreated with SN50 (50 μg/ml) or a scrambled control peptide, SN50 M (50 μg/ml) (Calbiochem), for 1 h prior to infection. Blocking antibodies to TLR2 (10 μg/μl) or TLR4 (20 μg/μl) (Serotec) were used at concentrations indicated by the manufacturer and their references to block signaling. These antibodies or control mouse immunoglobulin G2 (IgG2; Sigma) were incubated with cardiomyocytes for 1 h at 37°C prior to and during infection. LPS (5 ng/ml; Sigma) or Mycoplasma fermentans MALP-2 (200 nmol/liter; Apotech) were used to activate TLR4 and TLR2/6, respectively.
Parasite-conditioned medium (PCM).
Freshly harvested and washed trypomastigotes were suspended at 5 × 106 parasites/ml and incubated overnight at 37°C in 5% CO2. Medium containing secreted/shed trypomastigote molecules was clarified by centrifugation at 1,000 × g for 10 min and filtered through a 0.2-μm-thick membrane (Millipore) which removed all parasites from the medium.
Flow cytometry.
Trypsinized cardiomyocytes fixed in 2% paraformaldehyde diluted in phosphate-buffered saline were washed in phosphate-buffered saline, and a minimum of 10,000 cells were analyzed for each condition (FACScalibur; Becton Dickinson). Cardiomyocyte hypertrophy was detected in populations of isolated cardiomyocytes using flow cytometry to measure relative changes in forward scatter (FSC) as a proxy for cell size (35). As defined previously, a ≥2-fold increase in the geometric mean of FSC correlates with increased cell size and independent markers of hypertrophy: intracellular α-actinin staining and increased expression of the fetal myosin heavy chain β gene (35). Fluorescence-activated cell sorter data were analyzed using Cell Quest software (Becton Dickinson).
ELISA.
Five hundred microliters of culture supernatant collected from experimental samples 48 h post-treatment was clarified by centrifugation at 14,000 × g for 10 min. IL-1β levels were measured by enzyme-linked immunosorbent assay (ELISA; R&D), and plates were read using a Spectra Max 190 ELISA reader (Molecular Devices).
Cardiomyocyte transfection and luciferase assay.
Cardiomyocytes plated at a density of 1 × 105 cells/ml on laminin-coated 24-well plates were transfected with 0.33 μg DNA (for each construct) by lipofection (FuGENE6; Roche). Constructs used included NF-κB firefly luciferase and Renilla luciferase (Promega), TLR2-pFLAG and TLR6-YFP (wild type), TLR2P681H and TLR6P691H (dominant-negative [DN]-TLR2 and DN-TLR6; provided by D. Golenbock, University of Massachusetts), wild-type TLR4 and DN-TLR4 (TLR4P712H; provided by E. Kurt-Jones, University of Massachusetts). The relative activity of NF-κB luciferase, normalized to the activity of Renilla luciferase, was assessed using the dual-luciferase reporter assay (Promega).
Statistical analysis.
Student's t test with Welsh correction for multiple comparisons was used for comparison between control and experimental samples. In circumstances of non-Gaussian distribution, a Wilcoxon rank-sum (Mann-Whitney) test was used. P values of <0.05 were considered significant.
RESULTS
TLR2-dependent signaling is required for _T. cruzi_-induced cardiomyocyte hypertrophy.
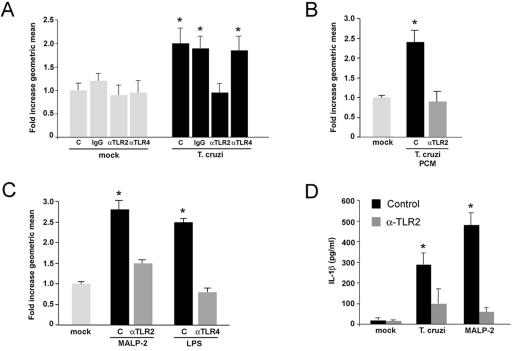
Infection of isolated primary cardiomyocytes with the intracellular pathogen Trypanosoma cruzi results in a measurable hypertrophic response that is mediated by the proinflammatory cytokine IL-1β (35). As signaling through the Toll-like receptors TLR2 and TLR4 is primarily responsible for proinflammatory cytokine production in macrophages following exposure to T. cruzi trypomastigotes (5, 32), we sought to examine the role of these TLRs as potential upstream activators of cytokine-dependent cardiomyocyte hypertrophy triggered by T. cruzi. We first tested the effect of specific TLR-blocking antibodies on the ability of T. cruzi to induce hypertrophy in infected cardiomyocytes. Consistent with previous findings (35), T. cruzi infection of cardiomyocytes results in a twofold increase in the geometric mean of forward scatter as measured by flow cytometry, indicating a significant increase in the mean cell size (Fig. 1A, T. cruzi, C) compared to mock-treated control populations (Fig. 1A, mock, C). Pretreatment of cardiomyocytes with antibodies to TLR2 that block signaling through this receptor (24) resulted in complete abrogation of the _T. cruzi_-elicited hypertrophic response where the geometric mean of forward scatter does not differ statistically from the mock-treated control (Fig. 1A, T. cruzi, αTLR2). In contrast, incubation of cardiomyocytes with TLR4-blocking antibodies (Fig. 1A, T. cruzi, αTLR4) or control IgG (Fig. 1A, T. cruzi, IgG) failed to inhibit parasite-dependent hypertrophy. Antibody treatments did not alter parasite infection (data not shown).
FIG. 1.
TLR activation triggers cytokine production and hypertrophy in isolated cardiomyocytes. (A) Cardiomyocytes incubated with medium alone (control [C]), control IgG (10 μg/μl) (IgG), anti-TLR2 IgG (10 μg/μl) (αTLR2), or anti-TLR4 IgG (20 μg/μl) (αTLR4) were mock-treated or infected with T. cruzi for 48 h. Hypertrophic cells were detected by flow cytometry, where a ≥2-fold change in the geometric mean of FSC indicates a significant increase in cell size for cardiomyocyte populations. FSC data from all treatments and controls were normalized to mock-treated cells incubated in medium alone (i.e., mock, C), for which the average FSC value was set at 1.0. Data are represented as the averages for five independent experiments carried out in duplicate ± the standard deviation. Asterisks denote a significant change from mock-treated controls (i.e., mock, C) at a P of <0.05 via Student's t test with Welch correction for multiple comparisons. (B and C) Cardiomyocytes were mock-treated or stimulated with PCM (B) or MALP-2 (10−7 U/ml) and LPS (5 ng/ml) (C) in the presence or absence of TLR-blocking antibodies. (D) ELISA analysis of IL-1β in medium harvested from mock-treated (mock) cardiomyocytes or cells exposed to T. cruzi MALP-2 trypomastigotes for 48 h in the absence (Control) or presence (αTLR2) of TLR2-blocking antibody. Data represents an average of three independent experiments carried out in triplicate ± the standard deviation. Asterisks denote a significant change from mock-treated cells (P < 0.05) via Student's t test with Welch correction for multiple comparisons.
Several surface glycoconjugates shed by infective T. cruzi trypomastigotes activate TLR2, triggering the expression of proinflammatory cytokines in macrophages (5, 33). We therefore tested the ability of PCM, which contains a complex mixture of shed trypomastigote surface molecules, to trigger hypertrophy in isolated cardiomyocytes. Similar to the response elicited by live T. cruzi trypomastigotes, exposure of cardiomyocytes to PCM caused cells to undergo hypertrophy in a TLR2-dependent manner (Fig. 1B). Pretreatment of myocytes with TLR2-blocking antibody prior to PCM treatment prevented cells from undergoing hypertrophy, as reflected in the values for geometric mean of FSC, which do not vary significantly from mock-treated controls (Fig. 1B). As a positive control for TLR activation and to confirm the efficacy of the blocking antibodies, cardiomyocytes were stimulated with MALP-2 or LPS to activate TLR2 (23) and TLR4, respectively. Stimulation of cardiomyocytes with either of these PAMPs caused a significant increase in the mean cell size of cardiomyocyte populations compared to mock-treated controls (Fig. 1C). When these PAMPs were added to cardiomyocytes in the presence of specific blocking antibodies (i.e., MALP-2 with anti-TLR2 and LPS with anti-TLR4), changes in the geometric mean of FSC were not significantly different from mock-treated controls, indicating that antibody treatments were effective in blocking TLR-dependent hypertrophy.
IL-1β was previously demonstrated to be the primary mediator of _T. cruzi_-elicited hypertrophy (35). We therefore predicted that a block of TLR2-dependent signaling, which abrogates the hypertrophic response to T. cruzi, should have a similar effect on IL-1β production. Cardiomyocyte infection with T. cruzi or stimulation with MALP-2 resulted in robust IL-1β production that was diminished by the inclusion of TLR2-blocking antibody (Fig. 1D). Together, the data represented in Fig. 1 indicate that cardiomyocyte TLR2 functions as an upstream signaling component in the pathway leading to IL-1β expression and cardiomyocyte hypertrophy triggered by T. cruzi. The sensitivity of _T. cruzi_-elicited cardiomyocyte hypertrophy to TLR2 inhibition, but not to interference with TLR4, indicates specificity for TLR2 in this pathogen-driven physiologic response. Significantly, however, the observation that both MALP-2 and LPS are capable of inducing hypertrophy in isolated cardiomyocytes highlights the broader nature of this response and the potential for different pathogens and their products to initiate signaling events through TLRs in the heart.
Functional TLR2/6 expression in cardiomyocytes mediates NF-κB activation in response to T. cruzi.
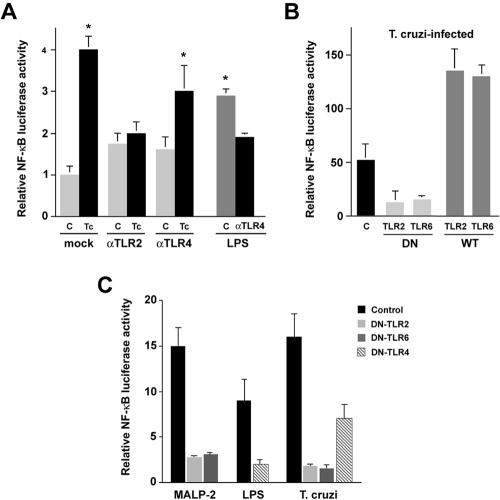
Since TLR2 functions upstream of the _T. cruzi_-elicited hypertrophic response, we predicted that NF-κB would operate as a downstream regulator of this pathway. We first tested the ability of live trypomastigotes to activate NF-κB in cardiomyocytes following transient transfection with an NF-κB luciferase reporter construct. As shown in Fig. 2A, a fourfold increase in reporter activation was observed in _T. cruzi_-infected cells within the first 2 h of exposure to parasites. In contrast, the ability of T. cruzi to activate the reporter in cardiomyocyte cultures pretreated with TLR2-blocking antibody was significantly impaired as luciferase activity did not vary statistically from mock-infected controls (Fig. 2A). Unexpectedly, antibodies to TLR4 appeared to exert a partial inhibitory effect on the _T. cruzi_-dependent activation of NF-κB (Fig. 2A). While statistical analysis (Student's t test) revealed that this decrease was not significant when compared to the luciferase activity generated in response to parasites in the absence of antibody (Fig. 2A), it should be noted that this effect was observed in several independent experiments. In contrast, the inhibitory effect of TLR4-blocking antibodies on the LPS-mediated activation of NF-κB luciferase was statistically significant (Fig. 2A). For reasons that are unclear, incubation of cardiomyocytes with TLR2 or TLR4 antibody alone caused a 1.5- to 2-fold increase in NF-κB reporter activation. Because this could potentially confound the results, a transfection approach was adopted to examine the effects of dominant interfering TLRs on the ability of T. cruzi to activate the NF-κB luciferase reporter.
FIG. 2.
_T. cruzi_-dependent activation of NF-κB in cardiomyocytes involves signaling through TLR2/6. Cardiomyocytes transiently transfected with NF-κB luciferase and control Renilla luciferase reporters were treated as follows prior to determination of luciferase activity. (A) Cells were incubated with medium alone (bar C) or blocking antibodies specific for TLR2 or TLR4 prior to T. cruzi infection or mock infection for 2 h. LPS stimulation of cells was carried out for 2 h following incubation of cells in the presence or absence (bar C) of TLR4-blocking antibodies. (B) Cardiomyocytes were transfected with NF-κB luciferase alone (bar C) or cotransfected with DN or wild-type (WT) TLR2 or TLR6 prior to infection with T. cruzi infection for 2 h. (C) Cells cotransfected with DN-TLR4, DN-TLR2, or DN-TLR6 were stimulated with LPS (5 ng/ml) or infected with T. cruzi for 2 h. Data are represented as NF-κB luciferase activity in treated cells compared to mock-treated controls normalized to Renilla luciferase for triplicate samples ± the standard deviation. Asterisks denote a significant change from mock-treated controls (P < 0.05) via Student's t test with Welch correction (A and C) or the Wilcoxon rank-sum (Mann-Whitney) test (B).
TLR2 can form functional heterodimers with TLR6 to facilitate recognition of a broader repertoire of PAMPs (34). Ectopic expression of DN-TLR2 or DN-TLR6 in cardiomyocytes prevents activation of the NF-κB luciferase reporter in response to T. cruzi, whereas expression of wild-type TLR2 or TLR6 results in a 2.5-fold increase in _T. cruzi_-induced NF-κB reporter activity over that of parasite-infected cells expressing reporter alone (Fig. 2B). In Fig. 2C, we demonstrate that in addition to T. cruzi, MALP-2-dependent activation of the luciferase reporter requires signaling through TLR2/6. However, a partial, but significant, reduction in reporter activation was observed when cardiomyocytes expressing DN-TLR4 were infected with T. cruzi. As DN-TLR4 expression exerted a more complete inhibitory effect on NF-κB activation mediated by the TLR4 ligand, LPS (Fig. 2C), these findings suggest that the engagement of cardiomyocyte TLR4 by T. cruzi ligand(s) contributes to the overall activation of NF-κB in cardiomyocytes, as suggested by the antibody-blocking experiments (Fig. 2A). The inability, however, of TLR4-blocking antibodies to inhibit _T. cruzi_-mediated cardiomyocyte hypertrophy (Fig. 1A), while effectively abolishing LPS-mediated hypertrophy (Fig. 1C), indicates that TLR4 activation by T. cruzi contributes minimally to the hypertrophic response elicited by this pathogen.
NF-κB activation is required for _T. cruzi_-induced cardiomyocyte hypertrophy.
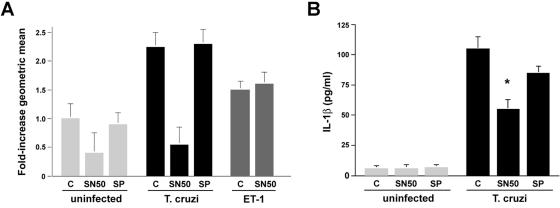
Pretreatment of cardiomyocytes with SN50, a cell-permeable peptide inhibitor of NF-κB, prevented hypertrophy in _T. cruzi_-infected (Fig. 3A, T. cruzi, SN50), whereas inclusion of a scrambled control peptide had no measurable effect on hypertrophy (Fig. 3A, T. cruzi, SP). As expected, cardiomyocyte hypertrophy elicited by endothelin 1, a prohypertrophic peptide that signals through an NF-κB-independent pathway (4), was not inhibited by SN50 (Fig. 3A, ET-1). SN50 pretreatment of cardiomyocytes resulted in a significant reduction of _T. cruzi_-triggered IL-1β production compared to treatment with the scrambled control peptide (Fig. 3B). Combined with data demonstrating that interference with TLR2-dependent signaling using specific blocking antibodies or dominant-negative constructs inhibits _T. cruzi_-dependent activation of the NF-κB luciferase reporter, these data suggest that NF-κB is activated downstream of TLR2 in response to T. cruzi and that NF-κB is involved in the pathway of parasite-elicited IL-1β production and cardiomyocyte hypertrophy.
FIG. 3.
Inhibition of NF-κB blocks _T. cruzi_-induced IL-1β production and cardiomyocyte hypertrophy. (A) Cardiomyocytes were pretreated with medium alone (bar C), SN50 (50 μg/ml), or scrambled peptide SN50 M (50 μg/ml; SP) and either mock infected, T. cruzi infected, or treated with ET-1 (10−7 mol/liter) for 48 h prior to analysis by flow cytometry. (B) Measurement of IL-1β in medium from cardiomyocyte cultures treated as described for panel A. Data are represented as an average of three experiments ± the standard deviation. Asterisks denote a significant change compared to _T. cruzi_-infected cells in the absence of inhibitor (P < 0.05) (Student's t test with Welch correction for multiple comparisons).
DISCUSSION
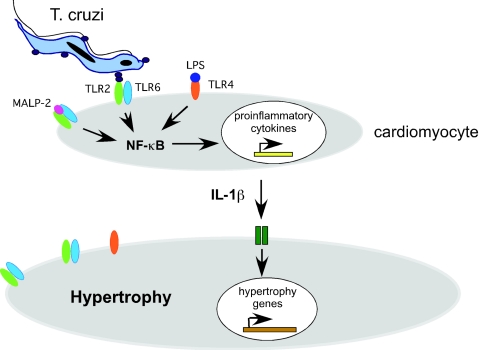
Cardiomyocytes represent an important cell type for T. cruzi infection and pathology in the host, where invasion, intracellular replication, and persistence are key factors contributing to the pathogenesis of Chagas' disease. In addition to the innate and adaptive immune responses mounted by the host, physiologic responses intrinsic to cardiomyocytes are likely to influence the progression of disease. Previously, using isolated neonatal rat cardiomyocytes as a model system to examine early host cell responses to T. cruzi infection, we established that T. cruzi infection of isolated cardiomyocytes causes a rapid and robust hypertrophic response that is dependent on the proinflammatory cytokine IL-1β (35). In the present study, we demonstrate that TLR2 and NF-κB activation occurs upstream of IL-1β production and cardiomyocyte hypertrophy elicited by T. cruzi. These events are summarized in the model outlined in Fig. 4. Here, we propose that a TLR2-dependent signaling cascade is initiated when T. cruzi ligand(s), which can be secreted or released from the surface of infective trypomastigotes, binds to TLR2/6 heterodimers expressed on the surfaces of cardiomyocytes. This event triggers the activation of NF-κB and induction of the proinflammatory cytokine IL-1β (Fig. 4). Secreted IL-1β activates a hypertrophic response program in infected and surrounding noninfected cells in culture. A prediction of this model is that any exogenous stimulus capable of inducing sufficient levels of proinflammatory cytokines through TLR activation should elicit hypertrophy (18, 19). Our finding that cardiomyocyte hypertrophy is triggered by the activation of TLR2 or TLR4 on the surfaces of cardiomyocytes by MALP-2 and LPS, respectively, supports the notion that cardiac hypertrophy may be a frequent early outcome of the innate recognition of pathogen products by cardiomyocyte TLRs. Our experimental findings strongly support a role for TLR2 as an initiator of cardiomyocyte hypertrophy triggered in response to T. cruzi as well as downstream events such as NF-κB activation and IL-1β production.
FIG. 4.
Model of TLR-dependent induction of cardiomyocyte hypertrophy. Data presented in this study indicate that the primary signaling event triggered by T. cruzi, leading to cardiomyocyte hypertrophy, involves the activation of intrinsic TLR2 molecules. This signaling cascade is initiated when T. cruzi and/or ligands secreted or released from infective trypomastigotes bind to TLR2/6 heterodimers on the cardiomyocyte surface, triggering activation of NF-κB and expression of the proinflammatory cytokine IL-1β. Secreted IL-1β activates a hypertrophic response program in both infected and surrounding noninfected cells in culture (35). Similarly, engagement of TLR2 or TLR4 by defined PAMPs is sufficient to trigger cardiomyocyte hypertrophy, likely through the induction of proinflammatory cytokines such as IL-1β.
While cardiomyocytes are not normally associated with a role in host defense, accruing evidence indicates that these cells participate in cardiac surveillance, responding rapidly to endogenous indicators of stress through the activation of intrinsic Toll-like receptors (11, 12, 38). Our results highlight the potential for intrinsic cardiomyocyte TLRs to play a role in initiating a hypertrophic response following exposure to pathogens, pathogen products, or endogenous signals emanating from damaged cardiac muscle (11, 12, 38). The rapid onset of hypertrophy observed in cultured myocytes, within 24 to 48 h, suggests that physiologic changes in cardiomyocytes initiated by engagement of intrinsic TLRs in the heart, either locally or globally, may precede an inflammatory response. Certainly, in rodent models of acute Chagas' disease, proinflammatory cytokine expression is detected in cardiac tissue at very early stages of infection (8, 44). Clearly, more detailed studies are required to examine the kinetics and global nature of a hypertrophic response following cardiac exposure to T. cruzi and other pathogens in vivo.
Engagement of innate host defense mechanisms early in the infective process, including activation of phagocytes with their production of cytokines and nitric oxide, is essential for the control T. cruzi replication in tissue and for triggering adaptive immune responses (9, 21, 25, 29, 30). The cumulative findings of several studies suggest that the innate immune response and regulation of proinflammatory cytokine production in _T. cruzi_-infected mice involves input from several TLRs (2, 5, 6, 32, 44). Infection of TLR4- or MyD88-deficient mice demonstrates the importance of TLR4/MyD88-dependent signaling pathways in mounting a proinflammatory cytokine response to T. cruzi GIPLs and for controlling parasite infection in vivo (6, 32). In addition to GIPL, GPI anchors associated with many T. cruzi surface glycoproteins, including the large GPI-mucin family, were shown to exhibit immunomodulatory activity whereby the stimulation of proinflammatory cytokine expression and release of nitric oxide from macrophages occurs in a TLR2-dependent manner (5). Whereas mice lacking functional TLR4 or MyD88 are more susceptible to infection by T. cruzi, surprisingly, no differences in parasitemia or mortality were noted following T. cruzi infection of TLR2-deficient mice in controlled experiments (6, 32). These findings suggest that the initiation of signaling events through TLR2, while likely contributing to the overall cytokine response driven by live T. cruzi trypomastigotes in the host, is not required for protection against this organism. By extrapolation of these findings to the present study, we predict that T. cruzi infection of TLR2-deficient mice would fail to reveal differences in cardiomyocyte hypertrophy in response to this pathogen. Moreover, given the redundancy that exists with respect to potential triggers of cardiac hypertrophy in experimental T. cruzi infection (proinflammatory cytokines, endothelin 1, cardiotrophin 1 [7, 22]), we predict that cardiomyocyte hypertrophy would be observed even in the absence of Toll signaling and diminished proinflammatory cytokine synthesis in MyD88-deficient animals. In an isolated culture setting, T. cruzi and soluble TLR ligands are capable of causing rapid changes in cardiomyocyte physiology, presumably in a cytokine-dependent manner (35). In vivo, however, macrophages infiltrating the infected tissue might be expected to contribute significantly higher levels of proinflammatory cytokines in the heart than the myocytes themselves.
Additional studies are needed to elucidate the role of cardiomyocyte TLRs in Chagasic cardiomyopathy as the significance of this early cardiomyocyte response is presently unclear. One intriguing possibility is that in addition to initiating an early hypertrophic response that in itself may be beneficial to the host, it has been demonstrated that myocardial exposure to low levels of proinflammatory cytokines, for example, following ischemia, triggers a prosurvival response in myocytes that enable them to survive the effects of an ensuing and greater ischemic challenge (15, 16, 36, 43). Thus, the rapid induction of proinflammatory cytokines in cardiomyocytes following T. cruzi infection could function in a protective capacity to precondition the myocardium and shield against the potentially damaging effects of inflammation associated with acute Chagas' disease. While this hypothesis has yet to be tested, it is noteworthy that <1% of individuals infected with T. cruzi present clinical signs of cardiac perturbation during acute infection when the parasite load and inflammation are relatively abundant (45).
In summary, findings presented in this paper support the growing literature that cardiomyocytes assume an important role in surveillance, where they respond rapidly to the presence of pathogens or pathogen products by eliciting proinflammatory cytokine production through the engagement of Toll-like receptors (12). In this study, we have demonstrated that a significant outcome of this innate response is the induction of cardiomyocyte hypertrophy. The observation that MALP-2 and LPS, PAMPs that signal in a TLR2- and TLR4-dependent manner, respectively, also promote hypertrophy in isolated cardiomyocytes indicates that this is a general intrinsic response of cardiomyocytes to pathogens and pathogen products. Given that cardiac hypertrophy is often maladaptive (14), our finding that Toll-like receptors, key components of innate immune function, are linked to hypertrophy has important implications for the pathogenesis of cardiac disease with both infectious and noninfectious etiologies.
Acknowledgments
We thank T. Donaghey, Department of Environment Health, Harvard School of Public Health, for materials used during neonatal rat cardiomyocyte isolation. We are grateful to P. Thomas and C. Hooper for assistance with fluorescence-activated cell sorter analysis and to D. Golenbock and E. Kurt-Jones for providing TLR constructs for this work. We acknowledge D. Harn and M. Grigg for critical reading of the manuscript.
This research was supported by NIH grant K08 AI50803 awarded to C.A.P. B.A.B. was supported by NIH grant R01 AI47960.
REFERENCES
- 1.Akira, S., and K. Takeda. 2004. Functions of toll-like receptors: lessons from KO mice. C. R. Biol. 327**:**581-589. [DOI] [PubMed] [Google Scholar]
- 2.Almeida, I. C., M. M. Camargo, D. O. Procopio, L. S. Silva, A. Mehlert, L. R. Travassos, R. T. Gazzinelli, and M. A. Ferguson. 2000. Highly purified glycosylphosphatidylinositols from Trypanosoma cruzi are potent proinflammatory agents. EMBO J. 19**:**1476-1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barton, G. M., and R. Medzhitov. 2002. Toll-like receptors and their ligands. Curr. Top. Microbiol. Immunol. 270**:**81-92. [DOI] [PubMed] [Google Scholar]
- 4.Bogoyevitch, M. A., P. E. Glennon, M. B. Andersson, A. Clerk, A. Lazou, C. J. Marshall, P. J. Parker, and P. H. Sugden. 1994. Endothelin-1 and fibroblast growth factors stimulate the mitogen-activated protein kinase signaling cascade in cardiac myocytes. The potential role of the cascade in the integration of two signaling pathways leading to myocyte hypertrophy. J. Biol. Chem. 269**:**1110-1119. [PubMed] [Google Scholar]
- 5.Campos, M. A., I. C. Almeida, O. Takeuchi, S. Akira, E. P. Valente, D. O. Procopio, L. R. Travassos, J. A. Smith, D. T. Golenbock, and R. T. Gazzinelli. 2001. Activation of Toll-like receptor-2 by glycosylphosphatidylinositol anchors from a protozoan parasite. J. Immunol. 167**:**416-423. [DOI] [PubMed] [Google Scholar]
- 6.Campos, M. A., M. Closel, E. P. Valente, J. E. Cardoso, S. Akira, J. I. Alvarez-Leite, C. Ropert, and R. T. Gazzinelli. 2004. Impaired production of proinflammatory cytokines and host resistance to acute infection with Trypanosoma cruzi in mice lacking functional myeloid differentiation factor 88. J. Immunol. 172**:**1711-1718. [DOI] [PubMed] [Google Scholar]
- 7.Chandrasekar, B., P. C. Melby, D. Pennica, and G. L. Freeman. 1998. Overexpression of cardiotrophin-1 and gp130 during experimental acute Chagasic cardiomyopathy. Immunol. Lett. 61**:**89-95. [DOI] [PubMed] [Google Scholar]
- 8.Chandrasekar, B., P. C. Melby, D. A. Troyer, J. T. Colston, and G. L. Freeman. 1998. Temporal expression of pro-inflammatory cytokines and inducible nitric oxide synthase in experimental acute Chagasic cardiomyopathy. Am. J. Pathol. 152**:**925-934. [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, L., T. Watanabe, H. Watanabe, and F. Sendo. 2001. Neutrophil depletion exacerbates experimental Chagas' disease in BALB/c, but protects C57BL/6 mice through modulating the Th1/Th2 dichotomy in different directions. Eur. J. Immunol. 31**:**265-275. [DOI] [PubMed] [Google Scholar]
- 10.Chien, K. R., K. U. Knowlton, H. Zhu, and S. Chien. 1991. Regulation of cardiac gene expression during myocardial growth and hypertrophy: molecular studies of an adaptive physiologic response. FASEB J. 5**:**3037-3046. [DOI] [PubMed] [Google Scholar]
- 11.de Kleijn, D., and G. Pasterkamp. 2003. Toll-like receptors in cardiovascular diseases. Cardiovasc. Res. 60**:**58-67. [DOI] [PubMed] [Google Scholar]
- 12.Frantz, S., R. A. Kelly, and T. Bourcier. 2001. Role of TLR-2 in the activation of nuclear factor kappaB by oxidative stress in cardiac myocytes. J. Biol. Chem. 276**:**5197-5203. [DOI] [PubMed] [Google Scholar]
- 13.Frantz, S., L. Kobzik, Y. D. Kim, R. Fukazawa, R. Medzhitov, R. T. Lee, and R. A. Kelly. 1999. Toll4 (TLR4) expression in cardiac myocytes in normal and failing myocardium. J. Clin. Investig. 104**:**271-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frey, N., and E. N. Olson. 2003. Cardiac hypertrophy: the good, the bad, and the ugly. Annu. Rev. Physiol. 65**:**45-79. [DOI] [PubMed] [Google Scholar]
- 15.Ghosh, S., L. L. Ng, S. Talwar, I. B. Squire, and M. Galinanes. 2000. Cardiotrophin-1 protects the human myocardium from ischemic injury. Comparison with the first and second window of protection by ischemic preconditioning. Cardiovasc. Res. 48**:**440-447. [DOI] [PubMed] [Google Scholar]
- 16.Ginis, I., R. Jaiswal, D. Klimanis, J. Liu, J. Greenspon, and J. M. Hallenbeck. 2002. TNF-alpha-induced tolerance to ischemic injury involves differential control of NF-kappaB transactivation: the role of NF-kappaB association with p300 adaptor. J. Cereb. Blood Flow Metab. 22**:**142-152. [DOI] [PubMed] [Google Scholar]
- 17.Gopalan, S. M., C. Flaim, S. N. Bhatia, M. Hoshijima, R. Knoell, K. R. Chien, J. H. Omens, and A. D. McCulloch. 2003. Anisotropic stretch-induced hypertrophy in neonatal ventricular myocytes micropatterned on deformable elastomers. Biotechnol. Bioeng. 81**:**578-587. [DOI] [PubMed] [Google Scholar]
- 18.Harada, E., O. Nakagawa, M. Yoshimura, M. Harada, M. Nakagawa, Y. Mizuno, Y. Shimasaki, M. Nakayama, H. Yasue, K. Kuwahara, Y. Saito, and K. Nakao. 1999. Effect of interleukin-1 beta on cardiac hypertrophy and production of natriuretic peptides in rat cardiocyte culture. J. Mol. Cell. Cardiol. 31**:**1997-2006. [DOI] [PubMed] [Google Scholar]
- 19.Higuchi, Y., K. Otsu, K. Nishida, S. Hirotani, H. Nakayama, O. Yamaguchi, Y. Matsumura, H. Ueno, M. Tada, and M. Hori. 2002. Involvement of reactive oxygen species-mediated NF-kappa B activation in TNF-alpha-induced cardiomyocyte hypertrophy. J Mol. Cell. Cardiol. 34**:**233-240. [DOI] [PubMed] [Google Scholar]
- 20.Hoffman, R. W., T. Gazitt, M. F. Foecking, R. A. Ortmann, M. Misfeldt, R. Jorgenson, S. L. Young, and E. L. Greidinger. 2004. U1 RNA induces innate immunity signaling. Arthr. Rheum. 50**:**2891-2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holscher, C., G. Kohler, U. Muller, H. Mossmann, G. A. Schaub, and F. Brombacher. 1998. Defective nitric oxide effector functions lead to extreme susceptibility of _Trypanosoma cruzi_-infected mice deficient in gamma interferon receptor or inducible nitric oxide synthase. Infect. Immun. 66**:**1208-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang, H., M. Yanagisawa, Y. Y. Kisanuki, L. A. Jelicks, M. Chandra, S. M. Factor, M. Wittner, L. M. Weiss, R. G. Pestell, V. Shtutin, J. Shirani, and H. B. Tanowitz. 2002. Role of cardiac myocyte-derived endothelin-1 in chagasic cardiomyopathy: molecular genetic evidence. Clin. Sci. (London) 103(Suppl. 48)**:**263S-266S. [DOI] [PubMed] [Google Scholar]
- 23.Into, T., K. Kiura, M. Yasuda, H. Kataoka, N. Inoue, A. Hasebe, K. Takeda, S. Akira, and K. Shibata. 2004. Stimulation of human Toll-like receptor (TLR) 2 and TLR6 with membrane lipoproteins of Mycoplasma fermentans induces apoptotic cell death after NF-kappa B activation. Cell. Microbiol. 6**:**187-199. [DOI] [PubMed] [Google Scholar]
- 24.Lien, E., T. J. Sellati, A. Yoshimura, T. H. Flo, G. Rawadi, R. W. Finberg, J. D. Carroll, T. Espevik, R. R. Ingalls, J. D. Radolf, and D. T. Golenbock. 1999. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J. Biol. Chem. 274**:**33419-33425. [DOI] [PubMed] [Google Scholar]
- 25.Lima, E. C., I. Garcia, M. H. Vicentelli, P. Vassalli, and P. Minoprio. 1997. Evidence for a protective role of tumor necrosis factor in the acute phase of Trypanosoma cruzi infection in mice. Infect. Immun. 65**:**457-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Machado, F. S., G. A. Martins, J. C. Aliberti, F. L. Mestriner, F. Q. Cunha, and J. S. Silva. 2000. Trypanosoma cruzi-infected cardiomyocytes produce chemokines and cytokines that trigger potent nitric oxide-dependent trypanocidal activity. Circulation 102**:**3003-3008. [DOI] [PubMed] [Google Scholar]
- 27.Medzhitov, R., P. Preston-Hurlburt, and C. A. Janeway, Jr. 1997. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388**:**394-397. [DOI] [PubMed] [Google Scholar]
- 28.Medzhitov, R., P. Preston-Hurlburt, E. Kopp, A. Stadlen, C. Chen, S. Ghosh, and C. A. Janeway, Jr. 1998. MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol. Cell 2**:**253-258. [DOI] [PubMed] [Google Scholar]
- 29.Michailowsky, V., N. M. Silva, C. D. Rocha, L. Q. Vieira, J. Lannes-Vieira, and R. T. Gazzinelli. 2001. Pivotal role of interleukin-12 and interferon-gamma axis in controlling tissue parasitism and inflammation in the heart and central nervous system during Trypanosoma cruzi infection. Am. J. Pathol. 159**:**1723-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyahira, Y., M. Katae, S. Kobayashi, T. Takeuchi, Y. Fukuchi, R. Abe, K. Okumura, H. Yagita, and T. Aoki. 2003. Critical contribution of CD28-CD80/CD86 costimulatory pathway to protection from Trypanosoma cruzi infection. Infect. Immun. 71**:**3131-3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohashi, K., V. Burkart, S. Flohe, and H. Kolb. 2000. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J. Immunol. 164**:**558-561. [DOI] [PubMed] [Google Scholar]
- 32.Oliveira, A. C., J. R. Peixoto, L. B. de Arruda, M. A. Campos, R. T. Gazzinelli, D. T. Golenbock, S. Akira, J. O. Previato, L. Mendonca-Previato, A. Nobrega, and M. Bellio. 2004. Expression of functional TLR4 confers proinflammatory responsiveness to Trypanosoma cruzi glycoinositolphospholipids and higher resistance to infection with T. cruzi. J. Immunol. 173**:**5688-5696. [DOI] [PubMed] [Google Scholar]
- 33.Ouaissi, A., E. Guilvard, Y. Delneste, G. Caron, G. Magistrelli, N. Herbault, N. Thieblemont, and P. Jeannin. 2002. The Trypanosoma cruzi Tc52-released protein induces human dendritic cell maturation, signals via Toll-like receptor 2, and confers protection against lethal infection. J. Immunol. 168**:**6366-6374. [DOI] [PubMed] [Google Scholar]
- 34.Ozinsky, A., D. M. Underhill, J. D. Fontenot, A. M. Hajjar, K. D. Smith, C. B. Wilson, L. Schroeder, and A. Aderem. 2000. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc. Natl. Acad. Sci. USA 97**:**13766-13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petersen, C. A., and B. A. Burleigh. 2003. Role for interleukin-1β in _Trypanosoma cruzi_-induced cardiomyocyte hypertrophy. Infect. Immun. 71**:**4441-4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sack, M. N., R. M. Smith, and L. H. Opie. 2000. Tumor necrosis factor in myocardial hypertrophy and ischaemia—an anti-apoptotic perspective. Cardiovasc. Res. 45**:**688-695. [DOI] [PubMed] [Google Scholar]
- 37.Sano, M., K. Fukuda, H. Kodama, J. Pan, M. Saito, J. Matsuzaki, T. Takahashi, S. Makino, T. Kato, and S. Ogawa. 2000. Interleukin-6 family of cytokines mediate angiotensin II-induced cardiac hypertrophy in rodent cardiomyocytes. J. Biol. Chem. 275**:**29717-29723. [DOI] [PubMed] [Google Scholar]
- 38.Satoh, M., M. Nakamura, T. Akatsu, J. Iwasaka, Y. Shimoda, I. Segawa, and K. Hiramori. 2003. Expression of Toll-like receptor 4 is associated with enteroviral replication in human myocarditis. Clin. Sci. (London) 104**:**577-584. [DOI] [PubMed] [Google Scholar]
- 39.Schaub, M. C., M. A. Hefti, B. A. Harder, and H. M. Eppenberger. 1997. Various hypertrophic stimuli induce distinct phenotypes in cardiomyocytes. J. Mol. Med. 75**:**901-920. [DOI] [PubMed] [Google Scholar]
- 40.Schwartz, S. M., J. Y. Duffy, J. M. Pearl, and D. P. Nelson. 2001. Cellular and molecular aspects of myocardial dysfunction. Crit. Care Med. 29**:**S214-S219. [DOI] [PubMed] [Google Scholar]
- 41.Sheng, Z., K. Knowlton, J. Chen, M. Hoshijima, J. H. Brown, and K. R. Chien. 1997. Cardiotrophin 1 (CT-1) inhibition of cardiac myocyte apoptosis via a mitogen-activated protein kinase-dependent pathway. Divergence from downstream CT-1 signals for myocardial cell hypertrophy. J. Biol. Chem. 272**:**5783-5791. [DOI] [PubMed] [Google Scholar]
- 42.Smith, R. M., J. McCarthy, and M. N. Sack. 2001. TNF alpha is required for hypoxia-mediated right ventricular hypertrophy. Mol. Cell. Biochem. 219**:**139-143. [DOI] [PubMed] [Google Scholar]
- 43.Smith, R. M., N. Suleman, J. McCarthy, and M. N. Sack. 2002. Classic ischemic but not pharmacologic preconditioning is abrogated following genetic ablation of the TNFalpha gene. Cardiovasc. Res. 55**:**553-560. [DOI] [PubMed] [Google Scholar]
- 44.Talvani, A., C. S. Ribeiro, J. C. Aliberti, V. Michailowsky, P. V. Santos, S. M. Murta, A. J. Romanha, I. C. Almeida, J. Farber, J. Lannes-Vieira, J. S. Silva, and R. T. Gazzinelli. 2000. Kinetics of cytokine gene expression in experimental chagasic cardiomyopathy: tissue parasitism and endogenous IFN-gamma as important determinants of chemokine mRNA expression during infection with Trypanosoma cruzi. Microbes Infect. 2**:**851-866. [DOI] [PubMed] [Google Scholar]
- 45.Tanowitz, H. B., L. V. Kirchhoff, D. Simon, S. A. Morris, L. M. Weiss, and M. Wittner. 1992. Chagas' disease. Clin. Microbiol. Rev. 5**:**400-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Empel, V. P., and L. J. De Windt. 2004. Myocyte hypertrophy and apoptosis: a balancing act. Cardiovasc. Res. 63**:**487-499. [DOI] [PubMed] [Google Scholar]
- 47.Zhang, L., and R. L. Tarleton. 1999. Parasite persistence correlates with disease severity and localization in chronic Chagas' disease. J. Infect. Dis. 180**:**480-486. [DOI] [PubMed] [Google Scholar]