A functional chaperone triad on the yeast ribosome (original) (raw)
Abstract
The chaperones RAC (ribosome-associated complex), consisting of Ssz1p and zuotin, and Ssb1/2p are associated with ribosomes of yeast. Ssb1/2p was previously shown to form a crosslink product to polypeptides trapped in ribosome-nascent chain complexes (RNCs) in vitro. Here we show that an efficient crosslink of the nascent chain to Ssb1/2p depends on the presence of functional RAC. The crosslink to Ssb1/2p was significantly diminished if (i) RAC was removed from RNCs: a process reversed by addition of purified RAC; (ii) RAC carried a mutation in the J-domain of zuotin, leading to its inactivation in vivo; (iii) RAC's Ssz1p subunit was absent because RNCs were generated in a Δ_ssz1_-derived translation extract. In vivo the same specific set of growth defects caused by the absence of any of the three chaperones was also displayed by a Δ_ssb1_/2_Δ_ssz1_Δ_zuo1 strain. The combination of in vitro and in vivo data supports a model in which Ssb1/2p, Ssz1p, and zuotin act in concert on nascent chains while they are being synthesized.
Ribosomes are molecular machines that synthesize polypeptides. They consist mainly of RNA and even the active site of the peptidyl-transferase center is almost devoid of protein. Core-ribosomal proteins seem predominantly involved in stabilizing the rRNA structure (1, 2). The term molecular chaperone describes various ubiquitous protein families, which share the ability to interact with nonnative polypeptide substrates (3). The number of proteins, which have chaperone-like properties and are dynamically and specifically associated with ribosomes, is increasing.
Nascent polypeptide-associated complex (NAC) was identified in higher eukaryotes as a ribosome-associated protein in close proximity to nascent chains (4). Like a typical chaperone, NAC interacts with nascent chains independent of their amino acid sequence (4, 5). The yeast homolog stimulates the in vitro translocation of a ribosome-bound mitochondrial precursor protein into mitochondria, providing additional evidence for the chaperone-like properties of NAC (6).
The Hsp70s and Hsp40s are two functionally interacting chaperone families (7). Common to the mechanism of Hsp70 is the interaction of its C-terminal peptide-binding domain with unfolded polypeptide substrates. The cycle of substrate binding and release is modulated by the activity of the N-terminal ATPase domain, which is stimulated by Hsp40 partner chaperones. Functional interaction between the ATPase domain of Hsp70 and the J-domain of Hsp40 is transient (8–11). In yeast, members of the Hsp70 and Hsp40 families are associated with cytosolic ribosomes. The essential Hsp40 homolog Sis1p binds to the small ribosomal subunit and is thought to be involved in translation initiation (12). There is evidence that the partner of Sis1p may be one or both of the cytosolic Hsp70 subfamilies Ssa1/2p or Ssb1/2p (13–15). Ssb1/2p itself is distributed between a ribosome-associated and a soluble pool (16, 17). Part of ribosome-associated Ssb1/2p can easily be removed by a salt wash. However, a subpopulation behaves like a core-ribosomal protein and cannot be removed without destruction of the ribosome (17). The Hsp40 homolog zuotin binds directly to ribosomes in a salt-sensitive manner (18). Yeast strains lacking either zuotin or Ssb1/2p were initially shown to display the same phenotype, suggesting that these two chaperones form a functional Hsp70/Hsp40 couple (18). Later it was found that the Hsp40 homolog zuotin forms a stable dimeric complex with the Hsp70 homolog Ssz1p that is almost entirely bound to ribosomes in vivo. This complex, termed ribosome-associated complex (RAC), was identified by its ability to stimulate translocation of a ribosome-bound mitochondrial precursor into mitochondria in vitro. RAC is so far the only example of a stable Hsp70–Hsp40 complex in the eukaryotic cell (19). The finding that yeast strains lacking Ssz1p displayed the same phenotype as strains lacking either Ssb1/2p or zuotin and the finding that zuotin and Ssz1p physically interact raises the question of whether zuotin serves as the partner chaperone for Ssz1p, Ssb1/2p, or both (19, 20).
Here we present evidence that the J-domain of zuotin functionally interacts with Ssb1/2p but that this interaction requires Ssz1p. We conclude that Ssz1p, together with Ssb1/2p and zuotin, form a chaperone triad acting on nascent chains emerging from the ribosome. Whether zuotin or another Hsp40 homolog is also the functional J-partner for Ssz1p remains to be established.
Materials and Methods
Yeast Strains and Plasmids.
Standard yeast genetic techniques were used (22). The inability to grow at room temperature (22°C) and below is defined as cold sensitivity. MH272–3f a/α (ura3/ura3, leu2/leu2, his3/his3, trp1/trp1, ade2/ade2) is the parental wild-type strain of all derivatives used in this study (19, 23). The generation of the deletion strains lacking ssz1 (IDA2: MATa, ura3, leu2, his3, trp1, ade2, ssz1_∷_LEU2), zuo1 (IDA1: MATa, ura3, leu2, his3, trp1, ade2, zuo1_∷_TRP1), or both genes (IDA12: MATα, ura3, leu2, his3, trp1, ade2, zuo1_∷_TRP1 ssz1_∷_LEU2) are described in ref. 19. Disruption of SSB1 was by replacing the entire gene with the kanMX module (24). The resulting strains, MH272–3f α _ssb1_∷kanR, IDA1 _ssb1_∷kanR, IDA2 _ssb1_∷kanR, and IDA12 _ssb1_∷kanR, were used as the parental strains for the disruption of SSB2. The 1.4-kb _Cla_I/AgeI fragment in the _SSB2_-coding region was replaced with HIS3. The resulting strains are: IDA56A (MATα; ura3, leu2, his3, trp1, ade2, _ssb1_∷kanR, ssb2_∷_HIS3), IDA156A (MATa, ura3, leu2, his3, trp1, ade2, zuo1_∷_TRP1, _ssb1_∷kanR, ssb2_∷_HIS3), IDA256A (MATa, ura3, leu2, his3, trp1, ade2, ssz1_∷_LEU2, _ssb1_∷kanR, ssb2_∷_HIS3), and IDA1256A (MATα, ura3, leu2, his3, trp1, ade2, ssz1_∷_LEU2, zuo1_∷_TRP1, ssb1_∷kanR, ssb2_∷_HIS3). The absence of Ssb1/2p in IDA56A, IDA156A, IDA256A, and IDA1256A was confirmed by Western blots using an Ab specifically recognizing Ssb1/2p (data not shown). To test SSB1, SSZ1, and ZUO1 as multicopy suppressors, the genes were cloned into YEplac195 (2μ URA3) (25). Resulting plasmids are 2μ-SSZ1, 2μ-SSB1, and 2μ-ZUO1. The J-domain mutant of zuotin was generated by a single base pair exchange leading to the substitution of His-128 to glutamine (zuotin-H128Q). For expression in IDA1 (Δ_zuo1), zuotin-H128Q was cloned into pYEPlac195 (2μ URA3) or pYCPlac33 (CEN URA3), respectively (25). RAC containing zuotin-H128Q is termed RAC-H128Q.
Purification of Wild-Type RAC, RAC-H128Q, Partial Purification of Ssb1/2p, and Generation of Abs.
RAC and RAC-H128Q were purified as described (19) with the following modification. After elution from MonoQ HR5/5 column (Amersham Pharmacia) fractions were pooled, diluted with 6 vol of 100 mM Mes-KOH pH 6.5, and loaded onto a MonoS HR5/5 column (Amersham Pharmacia). Bound RAC/RAC-H128Q was eluted with a 150–600 mM 25-ml linear KAcetate gradient in 40 mM Mes-KOH, pH 6.5. RAC/RAC-H128Q eluted at 250–350 mM KAcetate. Ssb1/2p was partly purified from the protein mixture released from ribosomes (19). Released proteins were diluted with 6 vol of 40 mM Hepes-KOH (pH 7.4) to a final concentration of 100 mM KAcetate and loaded onto a ResourceQ anion-exchange column (Amersham Pharmacia). Bound proteins were eluted with a 100–800 mM, 25 ml, linear KAcetate gradient in 40 mM Hepes-KOH, pH 7.4. Ssb1/2p containing fractions (300–500 mM KAcetate) were detected by using Ab against Ssb1/2p. Partly purified Ssb1/2p was free of RAC (data not shown). Ab specifically recognizing Ssb1/2p was generated against the Ssb1/2p peptide QIEDPSADELRKAEVC (Eurogentec, Brussels).
In Vitro Translation, Preparation of Ribosome-Nascent Chain Complexes (RNCs), Translocation Assay, and Crosslinking.
Yeast translation extract was prepared as described (26) from JK9 3d (23), YRG16 (19, 27), IDA2 (19), or IDA56A (this study). Translation reactions were performed in the presence of 35S-labeled methionine (Amersham Pharmacia). Generation of ribosome-bound yeast mitochondrial malate dehydrogenase (mdh1-t) was as described (6). Ribosome-bound nascent chains for crosslinking experiments were produced by the same method using mRNA lacking a stop codon. _p_-mdh1: (104 N-terminal aa of yeast mitochondrial malate dehydrogenase); prepro α: (87 N-terminal aa of yeast prepro α factor); and _m_-mdh1: (89 aa of yeast mitochondrial malate dehydrogenase lacking the 17-aa N-terminal presequence). Translocation reactions into mitochondria with ribosome-bound mitochondrial malate dehydrogenase as a precursor were performed as described (19). For crosslinking reactions, RNCs were isolated under high-salt or low-salt conditions, respectively. For this purpose 1 vol of translation reaction was loaded onto 2 vol of either low-salt cushion (20 mM Hepes-KOH, pH 7.4/25% sucrose/5 mM MgAcetate/2 mM DTT/0.5 mM PMSF/120 mM KAcetate, pH 7.4) or high-salt cushion (20 mM Hepes-KOH, pH 7.4/25% sucrose/5 mM MgAcetate/2 mM DTT/0.5 mM PMSF/700 mM KAcetate, pH 7.4). After centrifugation for 50 min at 200,000 × g at 4°C, ribosomal pellets were resuspended in CL buffer (20 mM Hepes-KOH, pH 7.4/5 mM MgAcetate/2 mM DTT/0.5 mM PMSF/0.6 M sorbitol 0.05 unit/μl RNase Inhibitor) corresponding to 3 vol of the original translation reaction. Crosslinking reactions (typically 40 μl of final vol) were started by the addition of the amino-reactive, homo-bifunctional crosslinker BS3 [(bis-(sulfosuccinimidyl),-suberate)] to 400 μM final concentration (Pierce, spacer length 114 nm) and were incubated for 30 min on ice. The reaction was quenched by the addition of glycyl-glycine to a final concentration of 5 mM. Reactions were analyzed on 10% Tris-Tricine gels (28) followed by autoradiography.
Immunoprecipitation.
To identify the crosslink partners of the nascent chains, immunoprecipitations were performed under denaturating conditions. Protein A-Sepharose beads (CL-4B, Amersham Pharmacia) were precoated with IgGs directed against Ssz1p, zuotin, Ssb1/2p, or with preimmune IgGs as described (29). Crosslinking reactions were supplemented with 5% trichloroacetic acid; protein pellets were collected by centrifugation, resuspended in IP buffer (4% SDS/200 mM Tris⋅HCl, pH 7.5/10 mM EDTA/100 μg/ml ovalbumine/1 mM PMSF), and incubated at 95°C for 10 min. Denatured samples were diluted 1:30 into TNTE buffer (10 mM Tris⋅HCl, pH 7.5/150 mM NaCl/5 mM EDTA/25 μg/ml ovalbumine/1% Triton X-100/0.5 mM PMSF) containing precoated protein A-Sepharose beads and were incubated at 4°C for 4 h on a shaker. The supernatant (containing unbound material) was separated from the beads by centrifugation, and aliquots of both were analyzed by autoradiography and Western blotting followed by immunodecoration. Western blotting indicated that zuotin and Ssz1p were immunoprecipitated with an efficiency of >90% and Ssb1/2p with ≈50% (data not shown).
Miscellaneous.
Ssb1/2p represents Ssb1p and Ssb2p, two 99% identical proteins with identical function and similar expression levels (21); high-salt RNCs represent ribosome nascent-chain complexes isolated in the presence of 700 mM KAcetate; low-salt RNCs represent ribosome nascent-chain complexes isolated in the presence of 120 mM KAcetate.
Protein concentrations were determined by the Bradford assay (Bio-Rad) by using BSA as a standard. 125I-labeled protein A was used to develop the immunoblots (30). Yeast cytosol was prepared as described (6). NAC was purified as described (6). Mitochondria were isolated from JK9–3dα grown on lactate-based medium and purified as described (31).
Results
Nascent Chains on Low-Salt-Washed Ribosomes Form Two Major Crosslink Products.
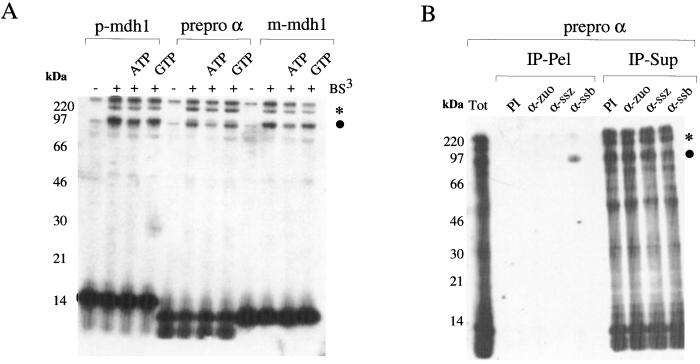
When a truncated mRNA is translated in a yeast translation system, a RNC is generated and can be isolated by centrifugation (6). A yeast translation extract was primed with truncated mRNA encoding a mitochondrial precursor protein (_p_-mdh1), an endoplasmic reticulum-targeted protein (prepro α), and a mitochondrial protein lacking the presequence (_m_-mdh1) (also see Materials and Methods). Independent of the amino acid sequence of the nascent chain, two major crosslink products of ≈120 kDa and 80 kDa were generated. At the border between stacking and separating gel, additional crosslinked material with a molecular mass of above 220 kDa was visible. Formation of the crosslink products was independent of the presence of ATP or GTP during the crosslinking reaction (Fig. 1A). A radio-labeled background band of variable intensity was observed at the same position as the 80-kDa crosslink even without addition of crosslinking reagent (Figs. 1A, 2C, and 3A, −BS3). This material could not be immunoprecipitated with Abs recognizing Ssb1p, Ssz1p, or zuotin (data not shown).
Figure 1.
Nascent chains bound to low-salt-treated ribosomes form two major crosslink products. (A) Different nascent chains form similar crosslink products. RNCs carrying the N-terminal 104 aa of mitochondrial malate dehydrogenase (_p_-mdh1), the N-terminal 87 aa of prepro α factor (prepro α), or 89 aa of the N terminus of mature malate dehydrogenase (_m_-mdh1) were produced in the presence of radiolabeled 35S-methionine and isolated under low-salt conditions. RNCs were resuspended in CL buffer (Materials and Methods) supplemented with 1 mM ATP or 1 mM GTP as indicated. Reactions were incubated either in the absence (−) or presence (+) of BS3 as described in Materials and Methods. Nascent chains run at 14 kDa (_p_-mdh1) or 10 kDa (prepro α and _m_-mdh1), respectively. (B) The 80-kDa crosslink product of the nascent chain is to Ssb1/2p. A crosslink reaction was performed by using low-salt-treated RNCs carrying prepro α factor as a nascent chain and subsequently immunoprecipitation reactions were performed as described in Materials and Methods. IP-Pel, material bound to the Ab under denaturating conditions; IP-Sup, unbound material; PI, preimmune serum; α-zuo, α-ssz, and α-ssb, Abs specifically recognizing zuotin, Ssz1p, or Ssb1/2p. Samples were run on 10% Tris-Tricine gels and subsequently analyzed by autoradiography. ●, 80-kDa crosslink product; *, 120-kDa crosslink product. The crosslinked material of >220 kDa did not enter the separating gel. This product was also partly immunoprecipitated with Abs directed against Ssb1/2p and most likely represents multiply crosslinked species (compare also Fig. 3B).
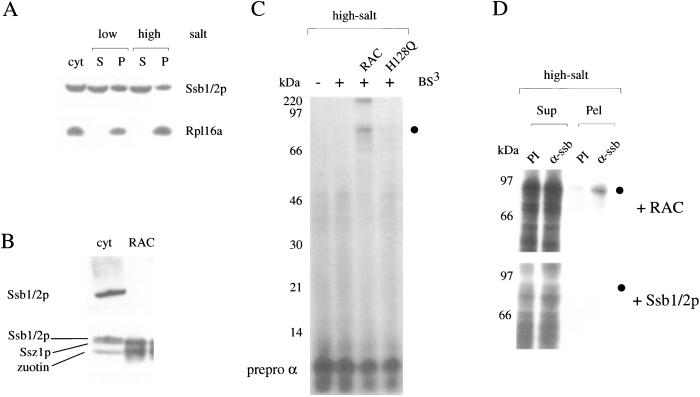
Figure 2.
Efficient formation of the crosslink to Ssb1/2p depends on the presence of functional RAC. (A) High-salt treatment does not remove Ssb1/2p from ribosomes. Ribosomes were isolated under low-salt or high-salt conditions as indicated in Materials and Methods. Aliquots were analyzed by Western blotting by using Abs specific for Ssb1/2p or the ribosomal protein Rpl16a. cyt, yeast cytosol; S, postribosomal supernatant; P, ribosomal pellet. (B) Purified RAC is free of Ssb1/2p. Yeast cytosol (cyt) and purified RAC were separated on 10% Tris-Tricine gels and transferred onto a nitrocellulose membrane. The blot was incubated with Abs specifically recognizing Ssb1/2p. After exposure (Upper) the same blot was reprobed with Abs recognizing Ssz1p and zuotin (Lower). (C) Addition of functional RAC to high-salt-treated RNCs induces the 80-kDa crosslink product. RNCs carrying prepro α factor were generated in the presence of [35S]methionine by using a wild-type translation extract, isolated under high-salt conditions, and incubated in the absence (−) or presence (+) of BS3. When indicated 100 nM purified RAC or 100 nM purified RAC-H128Q (H128Q) was added before the crosslinking reaction. ●, 80-kDa crosslink product. (D) The RAC-induced 80-kDa crosslink product can be immunoprecipitated with Abs specific for Ssb1/2p. RNCs carrying prepro α factor as a nascent chain were produced in the presence of [35S]methionine, isolated under high-salt conditions, and subsequently supplemented with either purified RAC (+RAC) or with a partial purified preparation of Ssb1/2p (+ Ssb1/2p). Crosslinking followed by immunoprecipitation under denaturating conditions was performed as described in Materials and Methods. Sup, unbound material; Pel, material bound to the Ab; PI, preimmune serum; α-ssb, Ab specifically recognizing Ssb1/2p; ●, 80-kDa crosslink product.
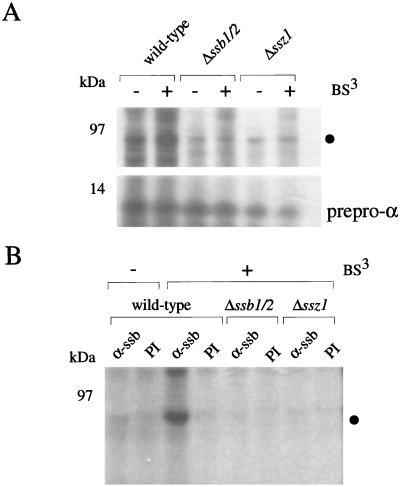
Figure 3.
Both Ssb1/2p and the Ssz1p subunit of RAC are required for the formation of the 80-kDa crosslink product. (A) RNCs carrying prepro α factor as a nascent chain were generated from either wild-type, Δ_ssb1_/2, or Δ_ssz1_ yeast translation extracts. After isolation under low-salt conditions, RNCs were incubated in the absence (−) or presence (+) of BS3. Samples were run on 10% Tris-Tricine gels and subsequently analyzed by autoradiography. ●, 80-kDa crosslink product. (B) Aliquots of the material shown in A lane 1 (wild type, −BS3), lane 2 (wild type, +BS3), lane 4 (Δ_ssb1_/2, +BS3), and lane 6 (Δ_ssz1_, +BS3) were objected to immunoprecipitation under denaturating conditions (Materials and Methods). Shown is the material bound to Protein A-Sepharose beads preincubated with either preimmune serum (PI) or Ab specifically recognizing Ssb1/2p (α-ssb). ●, 80-kDa crosslink product. The crosslink product of >220 kDa at the front between stacking and separating gel was also partly immunoprecipitated with α-ssb.
The Nascent Chain Forms an 80-kDa Crosslink with Ssb1/2p That Is Induced by RAC.
A crosslink between a nascent chain and the ribosome-associated chaperone Ssb1/2p (66 kDa) has been reported (17). Potential crosslink products to Ssz1p (62 kDa) or zuotin (49 kDa) should be somewhat smaller in size; however, mobility of crosslink products on SDS gels may vary considerably (e.g., see ref. 32). To determine whether the 80-kDa crosslink was to Ssb1/2p, Ssz1p, zuotin, or a mixture of different proteins, we performed immunoprecipitation reactions with Abs directed against Ssb1/2p, Ssz1p, or zuotin. The 80-kDa crosslink product was exclusively immunoprecipitated with α-Ssb1/2p (Fig. 1B). The result confirms that nascent chains are in close proximity to ribosome-bound Ssb1/2p. The experimental conditions did not generate a crosslink product between the nascent chain and one of the RAC subunits (Fig. 1B). A fraction of ribosome-bound Ssb1/2p was firmly bound and could not be removed from the ribosomes by treatment with high salt (Fig. 2A and ref. 17). It is this fraction of Ssb1/2p that is thought to be in close proximity to the nascent chain (17). However, after high-salt treatment of RNCs, the nascent chain did not form any prominent crosslink product (Fig. 2C, +BS3). The result suggests that either it was not the salt-resistant fraction of Ssb1/2p that formed the 80-kDa crosslink to the nascent chain or that Ssb1/2p was no longer in close proximity to the nascent chain. Addition of Ssb1/2p to high-salt-treated RNCs did not restore the 80-kDa crosslink to Ssb1/2p (Fig. 2D, +Ssb1/2p and data not shown). However, addition of highly purified RAC that was free of Ssb1/2p restored the 80-kDa crosslink (Fig. 2 B and C, +RAC). Like the 80-kDa crosslink formed on low-salt RNCs (Fig. 1B), the 80-kDa crosslink formed on high-salt RNCs after addition of purified RAC was to Ssb1/2p (Fig. 2D, +RAC).
To test the effect of the different chaperones on RNCs isolated under low-salt conditions, yeast translation extracts derived from strains lacking Ssb1/2p (Δ_ssb1_/2) or Ssz1p (Δ_ssz1_) were generated. A Δ_zuo1_ strain was not tested because Ssz1p is no longer bound to the ribosome in the absence of zuotin (19). As expected no 80-kDa crosslink was generated on RNCs from Δ_ssb1_/2 yeast translation extract (Fig. 3 A and B, Δ_ssb1_/2). Δ_ssz1-_RNCs isolated under low-salt conditions contain zuotin and associated proteins including Ssb1/2p, a fraction of which is resistant to treatment with high-salt (data not shown and ref. 19). Crosslinking of the nascent chain bound to Δ_ssz1-_RNCs did not generate an 80-kDa crosslink (Fig. 3 A and B). The result suggests that the Ssz1p subunit of RAC is required for the intimate interaction of Ssb1/2p with nascent chains.
The J-Domain of Zuotin Is Required for the Function of RAC in Vivo and in Vitro.
The zuotin subunit of RAC contains a typical J-domain (33). The HPD motif with the highly conserved tripeptide HPD is essential for the functional interaction of Hsp40 homologs with their Hsp70 partner proteins (34–36). We have generated a mutation in the J-domain of zuotin changing the signature motif HPD to QPD. The phenotype of the strain expressing zuo1-H128Q from either a low or a high copy number plasmid was identical to a Δ_zuo1_ strain (see Fig. 5A and data not shown). Although zuo1-H128Q was not functional in vivo, it was quantitatively bound to ribosomes under low-salt conditions and released from ribosomes in the presence of high concentrations of salt (data not shown). Zuotin-H128Q formed a stable complex with Ssz1p that could be purified like wild-type RAC, suggesting that it is not the J-domain of zuotin that mediates stable binding of the two RAC subunits (ref. 19 and Fig. 4A). When purified zuotin-H128Q was added to high-salt-treated RNCs, the mutant protein failed to induce the 80-kDa crosslink, indicating that a functional zuotin subunit of RAC is essential to induce the binding of Ssb1/2p to nascent chains (Fig. 2C, compare lanes +RAC and +H128Q).
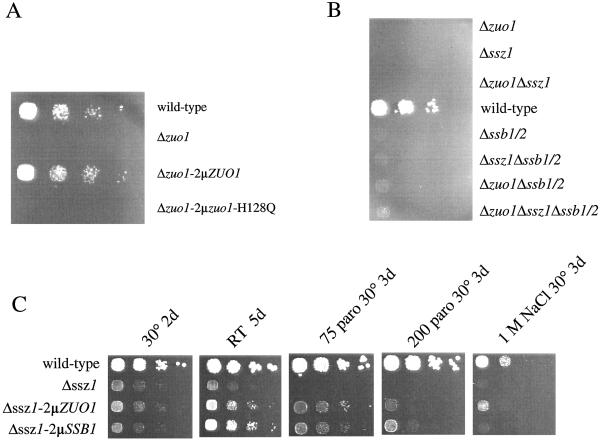
Figure 5.
Genetic interaction between the three ribosome-bound chaperones, Ssb1/2p, Ssz1p, and zuotin. Haploid yeast strains were grown to early log phase at 30°C on minimal glucose medium. Serial 10-fold dilutions containing the same number of cells were spotted from left to right onto rich glucose-containing medium [(yeast extract/peptone/dextrose (YPD)] and incubated as indicated. (A) Δ_zuo1_ (Lacking the gene encoding zuotin), Δ_zuo1_-2μ_ZUO1_ (Δ_zuo1_ overexpressing wild-type zuotin), and Δ_zuo1_-2μ_zuo1_-H128Q (Δ_zuo1_ overexpressing zuotin-H128Q) after 2 d of incubation on YPD supplemented with 75 μg/ml paromomycin at 30°C. (B) MH272 3f (wild type) and strains deleted in ssb1/2, ssz1, zuo1, and all possible combinations of these deletions after 3 d of incubation on YPD at 22°C (for details on the strains see Materials and Methods). (C) MH272 3f (wild type), Δ_ssz1_, Δ_ssz1-2μ_-ZUO1 (Δ_ssz1_ overexpressing zuotin), and Δ_ssz1-2μ_-SSB1 (Δ_ssz1_ overexpressing Ssb1p). As indicated the medium was supplemented with 75 μg/ml paromomycin, 200 μg/ml paromomycin, or 1 M NaCl. Incubation was at 30° or at 22°C (room temperature) for the time indicated.
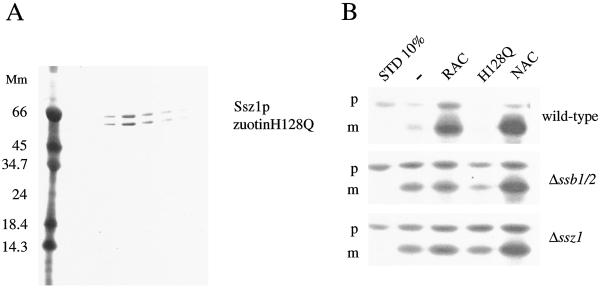
Figure 4.
RAC stimulates translocation of RNCs into mitochondria only in the presence of Ssb1/2p. (A) RAC-H128Q forms a stable complex. The last step of a RAC-H128Q purification from yeast. Shown are fractions eluted from a MonoS HR5/5 column on a Coomassie-stained Tris-Tricine gel. (B) Ribosome-bound full-length malate dehydrogenase was generated in the presence of [35S]methionine in a wild-type yeast translation (wild type) extract, a yeast translation extract generated from the Δ_ssb1_/2 strain, or the Δ_ssz1_ strain. RNCs were stripped from ribosome-bound proteins by treatment with high salt. Mitochondria were supplemented with 100 nM purified wild-type RAC, purified RAC-H128Q (H128Q), or purified NAC as indicated. Translocation reactions were started by the addition of RNCs and mitochondria were isolated after the reaction. Equal amounts of mitochondria, containing mitochondria-bound RNCs, were resuspended, precipitated with trichloroacetic acid, and analyzed on SDS/PAGE, followed by autoradiography. STD 10%, 10% of the RNCs added per reaction; p, precursor of malate dehydrogenase; and m, mature malate dehydrogenase after removal of the N-terminal presequence.
Ssb1/2p and RAC Altogether Are Involved in Chaperoning a Mitochondrial Precursor Protein.
We have previously shown that RAC, like NAC, is able to stimulate the in vitro translocation of a ribosome-bound mitochondrial precursor protein into isolated mitochondria (6, 19). Our crosslinking data raised the question whether RAC by itself was responsible for translocation stimulation or whether the presence of Ssb1/2p was required for the activity of RAC. To address this question, malate dehydrogenase lacking the terminal stop codon (mdh1-t) was generated in a wild-type, a Δ_ssb1_/2, or a Δ_ssz1_ translation extract. RNCs were isolated under high-salt conditions, removing stimulating factors, and subsequently translocation into mitochondria was tested. Translocation efficiency of high-salt-treated RNCs from wild type, Δ_ssb1_/2, and Δ_ssz1_ was ≈10% (Fig. 4B, compare STD 10% to the adjacent lane). As previously shown, translocation of high-salt-treated wild-type RNCs was stimulated by RAC and NAC (Fig. 4B, wild type). RAC-H128Q did not support translocation of mdh1-t (Fig. 4B, wild type, H128Q). When high-salt RNCs were generated by using a Δ_ssb1_/2 translation extract, the ability of RAC to stimulate translocation was strongly reduced. NAC, however, was still effective (Fig. 4B, Δ_ssb1_/2). This is in agreement with the observation that NAC and RAC act independently in the in vitro translocation system (19). When high-salt RNCs were generated by using a Δ_ssz1_ translation extract, addition of RAC did not effectively restore translocation (Fig. 4B, Δ_ssz1_). This finding suggests that efficient translocation of a mitochondrial precursor in an Ssb1/2p/RAC-dependent manner depends on functional RAC during in vitro synthesis. Consistent with this notion RAC also failed to induce the crosslink to Ssb1/2p when added to high-salt Δ_ssz1_-RNCs (M.G. and S.R., unpublished data). The combination of biochemical data from crosslinking and translocation experiments indicates that both subunits of RAC and Ssb1/2p have to function in concert to chaperone a ribosome-bound polypeptide.
RAC and Ssb1/2p Display Genetic Interaction.
Slow growth, cold sensitivity, hypersensitivity to high osmolarity, and hypersensitivity to aminoglycosides is the specific set of growth defects originally assigned to yeast lacking the Hsp70-Homolog Ssb1/2p (16). Later it was found that deletion of either zuotin or Ssz1p gives rise to the same combination of growth defects (18, 19). Here we show that all possible combinations among Δ_ssb1_/2, Δ_zuo1_, and Δ_ssz1_ lead to a similar set of phenotypes (Fig. 5B). This genetic interaction among SSB1/2, SSZ1, and ZUO1 suggests that all three chaperones are involved in the same process in vivo. We have tested the effects of overexpression of Ssb1p, Ssz1p, and zuotin in the whole set of deletion strains shown in Fig. 5B. Strains bearing deletions in zuo1 or ssb1/2 could not be suppressed by overexpression of Ssb1p/Ssz1p or Ssz1p/zuotin, respectively (data not shown and ref. 18). However, the Δ_ssz1_ strain was partly rescued by overexpression of either Ssb1p or zuotin (Fig. 5C and compare also ref. 19). Although suppression of slow growth at 30°C and room temperature was only weak, efficient suppression was observed on paromomycin-containing media. On paromomycin-containing media Ssb1p was a more effective suppressor than zuotin. Zuotin, however, was more effectively suppressing the NaCl sensitivity of Δ_ssz1_ (Fig. 5C).
Discussion
The Hsp40 homolog zuotin and the Hsp70 homolog Ssb1/2p directly bind to the yeast ribosome. Deletion of either zuo1 or ssb1/2 results in the same set of growth defects, and in addition overexpression of zuotin does not affect an Δ_ssb1_/2 strain or vice versa. This strong genetic interaction suggests that Ssb1/2p and zuotin is an Hsp70-Hsp40 chaperone pair (18). The Hsp70 homolog Ssz1p forms a unique and stable complex with zuotin (19). Deletion analysis revealed that all three ribosome-bound chaperones SSB1/2, SSZ1, and ZUO1 display genetic interaction (Fig. 5B and refs. 18 and 19). Only Δ_ssz1_, however, can be partly rescued by overexpression of either SSB1 or ZUO1 (Fig. 5C). The ability of SSB1 to suppress Δ_ssz1_ requires the presence of ZUO1 like suppression by ZUO1 requires the presence of SSB1 (data not shown). The simplest explanation for the genetic data is that Ssz1p, while not obligatory for the common function of Ssb1/2p and zuotin, is involved in the same cellular process.
Earlier crosslinking approaches, based on derivatized, photoactivatable lysyl residues, have led to the identification of eukaryotic ribosome-bound proteins that interact with nascent polypeptides (e.g., refs. 4, 17, and 32). We have here used BS3, a homo-bifunctional crosslinker reactive toward primary amino groups to study the environment of ribosome-bound nascent chains. Before the crosslinking reaction ribosomes/RNCs were isolated, a procedure that can be performed under conditions removing loosely ribosome-associated factors. The approach allowed us to study the effect of various purified proteins on nascent chain-crosslinks. Using low-salt RNCs bearing associated proteins, we were able to confirm the crosslink of nascent chains to NAC and Ssb1/2p (data not shown and Fig. 1B). In addition, we observed at least one high molecular mass crosslink product to an at yet unidentified ribosome-associated protein. Removing ribosome-associated proteins by salt stripping abolished all crosslink products. It is important to keep in mind that at the same time Ssb1/2p was still bound to the ribosome (Fig. 2A). It was suggested earlier that this salt-resistant fraction of Ssb1/2p forms the crosslink to the nascent chain (17). Our results confirm this notion. However, the presence of salt-resistant Ssb1/2p alone is not sufficient to generate a crosslink to the nascent chain. Formation of the Ssb1/2p crosslink depends on functional RAC. Two experiments strongly suggest that RAC dependence on the Ssb1/2p crosslink is not caused by sterical alterations upon salt treatment of the ribosome. First, addition of RAC, but not RAC-H128Q, to high-salt RNCs, reinduces the Ssb1/2p crosslink (Fig. 2C). Second, the Ssb1/2p crosslink is not formed on low-salt RNCs generated in a Δ_ssz1_ translation extract (Fig. 3A). Additional evidence for a functional interaction between RAC and Ssb1/2p comes from the translocation of a ribosome-bound mitochondrial precursor into mitochondria in vitro. Although this system does not truly simulate mitochondrial translocation in vivo it was successfully used as a tool for the identification of ribosome-associated proteins with chaperone-like properties (6, 19).
Our in vitro results provide biochemical evidence that the zuotin subunit of RAC and Ssb1/2p functionally interact. Despite the unique physical interaction between Ssz1p and zuotin, their functional interaction is only poorly understood. The genetic data suggest a much more stringent interaction between SSB1/2 and ZUO1. However, Ssz1p is required for the formation of the crosslink between Ssb1/2p and the nascent chain, and also for the efficient translocation of mdh1-t into mitochondria (Figs. 3 and 4B). As zuotin is bound to the ribosome independently of Ssz1p (19), Ssz1p itself influences the interaction of the nascent chain with Ssb1/2p. Possibly the nascent chain initially binds to Ssz1p and is transferred to Ssb1/2p. Fast transfer of the nascent chain from Ssz1p to the more tightly binding Ssb1/2p might account for the lack of a direct crosslink to Ssz1p (Fig. 1B and ref. 37). However, sequential binding of the nascent chain to Ssz1p and Ssb1/2p seems not essential for functionality of the chaperone triad. The unusually short C terminus of Ssz1p is dispensable for normal growth at 30°, room temperature, and on paromomycin-containing media (37). This result suggests that Ssz1p might function by modulating the ability of zuotin to act as a partner chaperone for Ssb1/2p. It also would be consistent with the idea that Ssz1p binds to a specific, possibly small, subset of nascent chains. Interestingly Ssz1p, originally named Pdr13p, was identified as a posttranslational regulator of the transcription factor Pdr1p (38). Whether Pdr1p or other newly synthesized polypeptides directly interact with Ssz1p and whether the function of Ssz1p is influenced by zuotin awaits further investigation.
Acknowledgments
We thank Betty Craig for sharing results before publication, Jeff Brodsky for plasmid pDJ100 encoding yeast prepro α factor, and Yves Dubaquié for comments on the manuscript. This study was supported by the Fonds der Chemischen Industrie (to S.R.).
Abbreviations
NAC
nascent polypeptide-associated complex
RAC
ribosome-associated complex
RNC
ribosome-nascent chain complex
BS3
(bis-(sulfosuccinimidyl),-suberate)
References
- 1.Cech T R. Science. 2000;289:878–879. doi: 10.1126/science.289.5481.878. [DOI] [PubMed] [Google Scholar]
- 2.Maguire B A, Zimmermann R A. Cell. 2001;104:813–816. doi: 10.1016/s0092-8674(01)00278-1. [DOI] [PubMed] [Google Scholar]
- 3.Frydman J. Annu Rev Biochem. 2001;70:603–647. doi: 10.1146/annurev.biochem.70.1.603. [DOI] [PubMed] [Google Scholar]
- 4.Wiedmann B, Sakai H, Davis T A, Wiedmann M. Nature (London) 1994;370:434–440. doi: 10.1038/370434a0. [DOI] [PubMed] [Google Scholar]
- 5.Bukau B, Deuerling E, Pfund C, Craig E A. Cell. 2000;101:119–122. doi: 10.1016/S0092-8674(00)80806-5. [DOI] [PubMed] [Google Scholar]
- 6.Fünfschilling U, Rospert S. Mol Biol Cell. 1999;10:3289–3299. doi: 10.1091/mbc.10.10.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bukau B, Horwich A L. Cell. 1998;92:351–366. doi: 10.1016/s0092-8674(00)80928-9. [DOI] [PubMed] [Google Scholar]
- 8.Gassler C S, Buchberger A, Laufen T, Mayer M P, Schroder H, Valencia A, Bukau B. Proc Natl Acad Sci USA. 1998;95:15229–15234. doi: 10.1073/pnas.95.26.15229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greene M K, Maskos K, Landry S J. Proc Natl Acad Sci USA. 1998;95:6108–6113. doi: 10.1073/pnas.95.11.6108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Misselwitz B, Staeck O, Matlack K E, Rapoport T A. J Biol Chem. 1999;274:20110–20115. doi: 10.1074/jbc.274.29.20110. [DOI] [PubMed] [Google Scholar]
- 11.Mayer M P, Laufen T, Paal K, McCarty J S, Bukau B. J Mol Biol. 1999;289:1131–1144. doi: 10.1006/jmbi.1999.2844. [DOI] [PubMed] [Google Scholar]
- 12.Zhong T, Arndt K T. Cell. 1993;73:1175–1186. doi: 10.1016/0092-8674(93)90646-8. [DOI] [PubMed] [Google Scholar]
- 13.Lu Z, Cyr D M. J Biol Chem. 1998;273:27824–27830. doi: 10.1074/jbc.273.43.27824. [DOI] [PubMed] [Google Scholar]
- 14.Horton L E, James P, Craig E A, Hensold J O. J Biol Chem. 2001;276:14426–14433. doi: 10.1074/jbc.M100266200. [DOI] [PubMed] [Google Scholar]
- 15.Ohba M. FEBS Lett. 1997;409:307–311. doi: 10.1016/s0014-5793(97)00535-8. [DOI] [PubMed] [Google Scholar]
- 16.Nelson R J, Ziegelhoffer T, Nicolet C, Werner-Washburne M, Craig E A. Cell. 1992;71:97–105. doi: 10.1016/0092-8674(92)90269-i. [DOI] [PubMed] [Google Scholar]
- 17.Pfund C, Lopez-Hoyo N, Ziegelhoffer T, Schilke B A, Lopez-Buesa P, Walter W A, Wiedmann M, Craig E A. EMBO J. 1998;17:3981–3989. doi: 10.1093/emboj/17.14.3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan W, Schilke B, Pfund C, Walter W, Kim S, Craig E A. EMBO J. 1998;17:4809–4817. doi: 10.1093/emboj/17.16.4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gautschi M, Lilie H, Fünfschilling U, Mun A, Ross S, Lithgow T, Rücknagel P, Rospert S. Proc Natl Acad Sci USA. 2001;98:3762–3767. doi: 10.1073/pnas.071057198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michimoto T, Aoki T, Toh-e A, Kikuchi Y. Gene. 2000;257:131–137. doi: 10.1016/s0378-1119(00)00381-4. [DOI] [PubMed] [Google Scholar]
- 21.Craig E A, Jacobsen K. Mol Cell Biol. 1985;5:3517–3524. doi: 10.1128/mcb.5.12.3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sherman F, Fink G R, Hicks J B. Methods in Yeast Genetics. Plainview, NY: Cold Spring Harbor Lab. Press; 1986. [Google Scholar]
- 23.Heitmann J, Movva N R, Hiestand P C, Hall M N. Proc Natl Acad Sci USA. 1991;88:1948–1952. doi: 10.1073/pnas.88.5.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wach A, Brachat A, Pohlmann R, Philippsen P. Yeast. 1994;10:1793–1808. doi: 10.1002/yea.320101310. [DOI] [PubMed] [Google Scholar]
- 25.Gietz R D, Sugino A. Gene. 1988;74:527–534. doi: 10.1016/0378-1119(88)90185-0. [DOI] [PubMed] [Google Scholar]
- 26.Garcia P D, Hansen W, Walter P. Methods Enzymol. 1991;194:675–682. doi: 10.1016/0076-6879(91)94049-i. [DOI] [PubMed] [Google Scholar]
- 27.George R, Beddoe T, Landl K, Lithgow T. Proc Natl Acad Sci USA. 1998;95:2296–2301. doi: 10.1073/pnas.95.5.2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schägger H, von Jagow G. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 29.Rospert S, Hallberg R L. Methods Enzymol. 1995;260:287–292. doi: 10.1016/0076-6879(95)60145-7. [DOI] [PubMed] [Google Scholar]
- 30.Haid A, Suissa M. Methods Enzymol. 1983;96:192–205. doi: 10.1016/s0076-6879(83)96017-2. [DOI] [PubMed] [Google Scholar]
- 31.Glick B S, Pon L A. Methods Enzymol. 1995;260:213–223. doi: 10.1016/0076-6879(95)60139-2. [DOI] [PubMed] [Google Scholar]
- 32.Plath K, Rapoport T A. J Cell Biol. 2000;151:167–178. doi: 10.1083/jcb.151.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang S, Lockshin C, Herbert A, Winter E, Rich A. EMBO J. 1992;11:3787–3796. doi: 10.1002/j.1460-2075.1992.tb05464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsai J, Douglas M G. J Biol Chem. 1996;271:9347–9354. doi: 10.1074/jbc.271.16.9347. [DOI] [PubMed] [Google Scholar]
- 35.Wall D, Zylicz M, Georgopoulos C. J Biol Chem. 1995;270:2139–2144. doi: 10.1074/jbc.270.5.2139. [DOI] [PubMed] [Google Scholar]
- 36.Qian Y Q, Patel D, Hartl F U, McColl D J. J Mol Biol. 1996;260:224–235. doi: 10.1006/jmbi.1996.0394. [DOI] [PubMed] [Google Scholar]
- 37.Hundley H, Eisenman H, Walter W, Evans T, Hotokezaka Y, Weidman M, Craig E. Proc Natl Acad Sci USA. 2002;99:4203–4208. doi: 10.1073/pnas.062048399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hallstrom T C, Katzmann D J, Torres R J, Sharp W J, Moye-Rowley W S. Mol Cell Biol. 1998;18:1147–1155. doi: 10.1128/mcb.18.3.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]