Kidney stone disease (original) (raw)
Abstract
About 5% of American women and 12% of men will develop a kidney stone at some time in their life, and prevalence has been rising in both sexes. Approximately 80% of stones are composed of calcium oxalate (CaOx) and calcium phosphate (CaP); 10% of struvite (magnesium ammonium phosphate produced during infection with bacteria that possess the enzyme urease), 9% of uric acid (UA); and the remaining 1% are composed of cystine or ammonium acid urate or are diagnosed as drug-related stones. Stones ultimately arise because of an unwanted phase change of these substances from liquid to solid state. Here we focus on the mechanisms of pathogenesis involved in CaOx, CaP, UA, and cystine stone formation, including recent developments in our understanding of related changes in human kidney tissue and of underlying genetic causes, in addition to current therapeutics.
Clinical aspects of stone disease
Stone passage
Nonobstructing stones produce no symptoms or signs apart from hematuria. Stone passage produces renal colic that usually begins as a mild discomfort and progresses to a plateau of extreme severity over 30–60 minutes. If the stone obstructs the uretero-pelvic junction, pain localizes to the flank; as the stone moves down the ureter, pain moves downward and anterior. Stones at the uretero-vesicular junction often cause dysuria and urinary frequency mistaken for infection. Colic is independent of body position or motion and is described as a boring or burning sensation associated with nausea and vomiting. Stones less than 5 mm in diameter have a high chance of passage; those of 5–7 mm have a modest chance (50%) of passage, and those greater than 7 mm almost always require urological intervention. Ideally, stone analysis is performed by infrared spectroscopy or x-ray diffraction. Renal stone burden is best gauged using CT radiographs taken with 5-mm cuts, without infusion of contrast agents. The radiographic appearance and density of stones as measured by CT is a guide to their composition (1).
Urological management of stones
Extracorporeal shock wave lithotripsy (ESWL), in which sound waves are used to break the stone into small pieces that can more easily pass into the bladder, is widely used and valuable for small stones (2). Modern instruments facilitate passage of endoscopes up the ureter into the kidney pelvis and permit local stone disruption with high-powered lasers (3). Percutaneous stone removal via instruments introduced into the kidney through a small flank incision permits disruption and removal of even very large stones (4).
Renal function of stone forming people is reduced
Within the National Health and Nutrition Examination Survey III data set, subjects with a BMI greater than or equal to 27 who had kidney stones had lower estimated glomerular filtration rates than non–stone formers (non-SFs) matched for age, sex, race, and BMI (5). SFs also have higher blood pressures than non-SFs (6). Obstruction of the urinary tract, sequelae of urological interventions, and the processes that cause stone formation may all injure renal tissue, reduce renal function, and raise blood pressure.
Determinants of phase change
Supersaturation
Stones result from a phase change in which dissolved salts condense into solids, and all phase changes are driven by supersaturation (SS), which is usually approximated for such salts by the ratio of their concentration in the urine to their solubilities (7) and calculated by computer algorithms. At SS values less than 1, crystals of a substance will dissolve; at SS values greater than 1, crystals can form and grow. As expected, the composition of stones that patients form correlates with SS values from the urine they produce (8). Although increasing urine volume is an obvious way to lower SS, patients examined in a variety of practice settings have been found to be able to increase their urine volume by an average of only 0.3 l/d (9). Moreover, for unclear reasons, sodium intake and urinary calcium excretion has been found to increase with increased urine volume, partly offsetting the fall in SS. Along with urine volume, urine calcium and oxalate concentrations are the main determinants of calcium oxalate (CaOx) SS; urine calcium concentration and pH are the main determinants of calcium phosphate (CaP) SS; and urinary pH is the main determinant of uric acid (UA) SS.
The upper limit of metastability
Urine with SS greater than 1 is referred to as metastable because the excess dissolved material, being present at a concentration above its solubility, must eventually precipitate. One can add oxalate or calcium to urine and note the SS needed to produce a solid phase of CaOx or CaP. That value, called the upper limit of metastability (ULM), varies with urine SS (10) and is lower among patients with stones than in matched control subjects (11). This suggests that mechanisms that normally protect against solid-phase development are less effective in patients with stones than healthy individuals. Urine contains molecules that retard the formation of solid CaOx and CaP phases (12), and it is precisely this retardation that can permit the transient existence of SS. The measurement of ULM is not currently used in clinical practice.
Modulators of the ULM, crystal growth, and aggregation
Urine citrate reduces SS by binding calcium and inhibits nucleation and growth of calcium crystals (13); it is measured clinically, and low levels are treated as a cause of stones. Osteopontin (14), prothrombin F1 fragment (15), the inter-α-trypsin inhibitor molecule (16), calgranulin (17), Tamm Horsfall glycoprotein (18), as well as albumin, RNA and DNA fragments, and glycosaminoglycans have all been identified as urine inhibitors of CaOx and CaP crystallization (12). They have in common long stretches of polyanion chains that can bond with surface calcium atoms and prevent crystal growth. Such blockade prevents measurable phase changes and raises the ULM because newly formed crystals cannot grow beyond extremely minute dimensions. These molecules also prevent clumping of small particles into larger ones (aggregation). Because we do not know which, if any, of these molecules contribute to stone pathogenesis, they are presently of great research interest.
CaOx stones
The vast majority of CaOx SFs suffer from no systemic disease and as such are described as idiopathic CaOx SFs. Some have primary hyperparathyroidism or other disorders of calcium metabolism, and others present with hyperoxaluria because of bowel disease (enteric hyperoxaluria) and genetic disorders of oxalate metabolism (primary hyperoxaluria [PH]). We discuss these groups separately below.
Pathogenesis of idiopathic CaOx stones
Stones form on interstitial apatite plaque.
CaOx stones form on the surfaces of the renal papillae over collections of interstitial suburothelial CaP particles (19) named Randall plaque. The number of CaOx stones formed, adjusted for duration of stone formation, varies directly with plaque surface coverage (20), as would be expected if plaque were a surface that promotes CaOx overgrowth.
Plaque begins in the basement membranes of the thin Henle loops.
Basement membrane (BM) plaque comprises a myriad of particles in which crystal and organic layers alternate (Figure 1, A and B); the outer surface of all particles is the organic layer (Figure 1, B and C). The crystal in plaque is always biological apatite, the mineral phase found in bone. Outside the BM, in the interstitium, plaque particles coalesce so that islands of crystal float in an organic sea (Figure 2). It is this coalescent material that extends to the suburothelial region and over which CaOx stones grow. The identity of the organic molecules surrounding apatite in plaque are unknown except for osteopontin, which coats the surface of apatite and positions itself (Figure 1D) precisely at the apatite organic layer interface (21). We envision that plaque forms in the thin limb BM and extends to the suburothelial space and that CaOx stones then form over the organic coating of apatite particles.
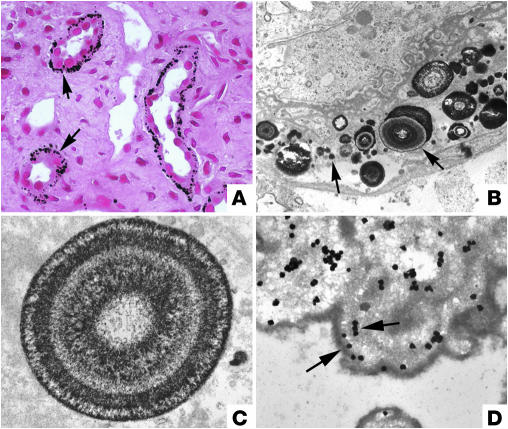
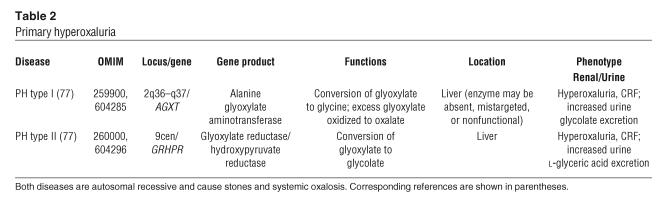
Figure 1.
Initial sites of crystal deposition and localization of osteopontin. The initial sites of calcium deposits in the deep papillary tissue of an idiopathic CaOx SF are shown in light (A) and transmission electron microscopic (TEM) images (B–D). In A, Yasue-stained (the Yasue stain detects calcium) biopsy tissue reveals sites of crystal deposits (arrows) within the BM of thin Henle loops and not in nearby inner medullary CDs. By TEM (B and C), the crystal deposits appear as single spheres with a multi-laminated (6–7 layers) internal morphology consisting of a central light region of crystalline material surrounded by a dark layer of matrix material (arrows). Note that the cells lining this Henle loop appear morphologically normal. Osteopontin (D) localizes on either side of the apatite layers, sometimes forming with a clear “tram track”–like appearance (arrows). Magnification: ×1,800 (A); ×20,000 (B); ×35,000 (C); ×37,000 (D). A and B reprinted from ref. 19. C and D reprinted with permission from Kidney International (21).
Figure 2.
Accumulation of interstitial crystal deposits as seen in light and TEM images in a papillary biopsy from an idiopathic CaOx SF. (A) Light microscopy reveals extensive accumulation of crystalline deposits (green arrow) shown around the Henle loops and nearby vascular bundles and inner medullary CDs. This progressive accumulation of crystalline material in the interstitium results in the formation of incomplete to complete cuffs of plaque. (B) TEM reveals a normal thin Henle loop surrounded by a complete cuff of interstitial plaque. (C) TEM shows a site of plaque located in the interstitial space, away from a tubular wall. Note that single crystal deposits appear embedded in a sea of matrix. Magnification: ×1,500 (A); ×13,000 (B); ×13,000 (C). A reprinted from ref. 19. B reprinted with permission from Urological Research (123). C reprinted with permission from Kidney International (21).
Crystals are not found in tubule lumens of the deep or outer medulla or cortex.
The lining cells of the tubules are normal in appearance (Figure 2) even when deposits of plaque fill the BM; no lumen crystals have to date been found in tissue from idiopathic CaOx SFs. For this reason, we do not review here the large literature on attachment of CaOx and CaP crystals to renal epithelial cells in culture (22), which does not appear to be relevant to human idiopathic CaOx stone disease. Interstitial cells also show no evident pathology.
Mechanisms of plaque and stone formation.
The driving force for CaOx overgrowth on plaque is urine CaOx SS. But the forces that create the plaque are not so clear. The fraction of papillary surface covered by plaque in idiopathic CaOx SFs correlates directly with urine calcium level and inversely with urine volume and pH (23). Given that the initial formation is in the thin Henle loops BM, efficient water extraction in the collecting duct (CD) combined with high deliveries of calcium as a result of idiopathic hypercalciuria (IH) may increase tubule and interstitial calcium concentrations and by as-yet-undetermined mechanisms stimulate apatite deposition in the thin-limb BM.
Clinical implications.
High fluid intake may be beneficial not only to prevent CaOx overgrowth, but also to reduce plaque formation itself. Thiazide diuretics, which lower urine calcium, may reduce plaque as well as urine CaOx SS, whereas measures that reduce urine oxalate concentration are important in prevention of overgrowth but have no apparent role with regard to plaque formation. As new technologies permit visualization and quantification of plaque in the kidneys of patients, effects of treatments on plaque may become a matter for prospective trials.
Idiopathic hypercalciuria
Urinary calcium excretion as measured on random diets varies in humans (24), and excretion rates among idiopathic CaOx SFs cluster at the high end of this distribution (25). Lowering urinary calcium levels prevents stone recurrence (12). Conventional upper limits of 250 mg/d (for women) and 300 mg/d (for men) are near the 95th percentiles for our published data sets (26) and are commonly used as diagnostic cutpoints. We believe SFs in the 70th percentiles (170 mg/d for women and 210 mg/d for men) may well benefit from lower calcium excretion rates. Data linking calcium excretion to stone risk (27) are supportive of the idea that it is a graded risk factor.
Diagnosis of IH requires exclusion of hypercalcemia, vitamin D excess, hyperthyroidism, malignant neoplasm, and sarcoidosis. Pathogenesis and systemic manifestations of IH are well described in standard textbooks (12). About 50% of first-degree relatives of people whose urinary calcium excretion level exceeds the 95th percentile also surpass these limits, establishing the familial and therefore inherited nature of IH. We emphasize here the most salient features and new research concerning this condition.
On average, patients with IH have higher than normal serum levels of 1,25(OH)2D3 (calcitriol) and increased intestinal absorption of calcium (28), and vitamin D receptor expression on circulating blood monocytes may also be increased. High intestinal calcium absorption can raise the load of filtered calcium presented to the renal tubules. The reabsorption of filtered calcium by the renal tubules may also be reduced. Either mechanism can increase urinary calcium level, which can be further increased by glucose or sucrose loads (29), high sodium intake (30), and a high-protein diet (31). Although dietary calcium is the main contributor to high urinary calcium levels in IH, low-calcium diets are not widely used clinically because they are not of proven effectiveness in stone prevention (32) and may predispose to reduced bone mineral content (33). Thiazide diuretics lower urinary calcium levels and promote positive bone mineral balances (34), and multiple prospective trials have documented their effectiveness for preventing calcium stones (see “Treatment trials: stone recurrence outcomes”).
Monogenic disorders that cause hypercalciuria and stones
These rare disorders illustrate links among genetic variation, urinary calcium excretion, and calcium stones. Two recent reviews (35, 36) have detailed all known mutations that can cause hypercalciuria in humans or animals; here we discuss only those that cause stones in humans. We have excluded mere gene associations with the presence of stones, with the single exception of a cytosolic soluble adenylyl cyclase.
Dent disease.
This X-linked hypercalciuric stone-forming disorder (Table 1) is due to mutations in the gene that codes for the voltage-gated endosomal chloride channel 5 (CLCN5) (37–39). Resultant low-molecular-weight proteinuria includes parathyroid hormone (PTH) loss (40, 41). As in IH, hypercalciuria results from increased serum calcitriol levels (42), and thiazide treatment lowers urine calcium concentrations (43). Because some patients with Dent disease lack CLCN5 (44) mutations, other genetic defects may also contribute to this disease. CLCN5 mutations are rarely found in IH patients (45). Gene KO studies in mice suggest that defective proximal tubule (PT) endocytosis increases PT lumen PTH levels, which activates 1α-hydroxylase and raises serum calcitriol levels, causing hypercalciuria (46, 47). In one study of _Clcn5_-KO mice (48), urinary 25OHD3 levels were high, serum levels of 25OHD3 and calcitriol were low, and urine calcium concentration was normal; urinary loss of 25OHD3 was thought to limit the increase in calcitriol from high lumen PTH.
Table 1.
Monogenic hypercalciuric stone-forming diseases
Bartter syndromes.
Of the 5 major genetic variants associated with Bartter syndrome (49), cortical and medullary renal calcifications (50) are found in patients with Bartter syndrome types I and II (Table 1), which arise from transport defects that reduce thick ascending limb (TAL) NaCl and calcium reabsorption, creating hypercalciuria. Defects in a TAL basolateral membrane chloride channel, CLCNKB, produce only variable hypercalciuria and uncommon crystal deposits (type III). Defects in a chloride channel subunit known as Barttin, encoded by BSND, produce Bartter syndrome type IV, in which deafness, but neither stones nor nephrocalcinosis, are observed. Loop diuretic treatment of premature babies, a pharmacological analog of the reduced TAL reabsorption seen in Bartter syndromes, can cause renal calcifications, 50% of which regress over time (51).
Abnormalities of the calcium-sensing receptor.
The calcium-sensing receptor (CaSR) regulates PTH secretion in response to blood calcium levels (52) and is also present on the apical membranes of PT and medullary CD principal cells and the basal membranes of TAL and distal convoluted tubule (DCT) cells, where it regulates transport functions. Gain-of-function mutations of the CaSR reduce serum PTH levels and TAL and DCT calcium reabsorption (53, 54), causing variably symptomatic hypocalcemia (55–57) with low serum PTH levels. Stones usually result from calcium and vitamin D administered in order to raise serum calcium levels. One particular mutation causes multigland primary hyperparathyroidism (58). A severe gain-of-function defect of the CaSR causes Bartter syndrome type V (Table 1), and its clinical symptoms are ascribed to CaSR activation–mediated downregulation of a renal outer medullary potassium channel (ROMK) that is mutated in type II Bartter syndrome (59, 60).
Familial hypomagnesemia with hypercalciuria and nephrocalcinosis.
Paracellin-1, a member of the claudin family (61, 62) found only in the TAL and DCT, is essential for normal, voltage-driven paracellular magnesium reabsorption in the TAL. Patients with paracellin-1 defects develop hypomagnesemia, hypercalciuria, polyuria, nephrocalcinosis, stones, distal renal tubular acidosis (dRTA), and renal failure (Table 1). A striking incidence of hypercalciuria and stones has been found in heterozygous family members (63).
Soluble adenylyl cyclase.
Among selected IH patients with reduced vertebral bone mineral density, Reed et al. (64) identified linkage to a locus on chromosome 1 (1q23.3–q24). In a specific gene (human soluble adenylyl cyclase [_hsAC_]) (65), 4 base changes segregated with IH, and bone mineral density decreased as the number of base changes increased. hsAC is a cytosolic enzyme sensitive to bicarbonate concentration in the presence of divalent cations and could explain bicarbonate sensitivity of renal and bone cells (66).
Primary hyperparathyroidism
Clinicians readily distinguish this stone-forming condition from IH because blood calcium levels are high in primary hyperparathyroidism and normal in IH. The two disorders share hypercalciuria and generally elevated serum levels of calcitriol. Diagnosis, pathogenesis, treatment approaches, and the genes related to gland enlargement have been well covered elsewhere (67).
Hyperoxaluria
Urine oxalate concentration affects CaOx SS exactly as does urine calcium concentration (68); therefore, any conditions that increase oxalate absorption from food or lead to increased oxalate production can cause CaOx stone formation.
Dietary hyperoxaluria in idiopathic CaOx SFs.
Whereas the 95th percentiles for urinary oxalate excretion for females and males are 45 mg/d and 55 mg/d, respectively, and the corresponding 70th percentiles are 31 mg/d and 41 mg/d (12), values up to approximately 80 mg/d are common among idiopathic CaOx SFs and are often unexplained by a systemic cause (69). A low-calcium diet that reduces CaOx crystallization in the gut lumen, thereby facilitating oxalate ion absorption, is easily identified and corrected (70). High protein or oxalate intake are other causes of this condition (71, 72). Oxalate absorption from food may depend upon gastrointestinal transporters (73, 74), and genetic variations in oxalate transporting proteins in red blood cells have been linked to CaOx stone formation (73).
Enteric hyperoxaluria.
If colon is present and receiving small bowel effluent, fat malabsorption of any cause (e.g., small bowel disease, resection or bypass, or exocrine pancreatic insufficiency) results in increased colon oxalate absorption and urine oxalate excretion in the range of 80–140 mg/d (69). Undigested fatty acids promote colon oxalate absorption (75). Diarrheal fluid losses and low urine pH and citrate levels resulting from stool bicarbonate losses increase urine CaOx SS and UA SS; stones usually contain 1 or both of these phases (69).
Among patients with enteric hyperoxaluria resulting from small bowel bypass for the treatment of obesity (19), some terminal CDs are plugged with apatite crystals (Figure 3) and show epithelial cell death and surrounding interstitial inflammation. How the apatite plugs form remains unknown; possibly high oxalate concentrations in tubule fluid injure epithelial cells and disturb pH regulation so that lumen pH increases. Plugging and consequent nephron damage may account for renal function loss in such patients and may benefit from urine dilution.
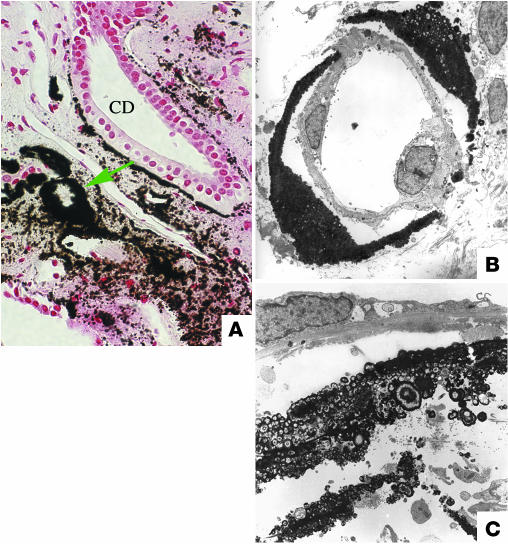
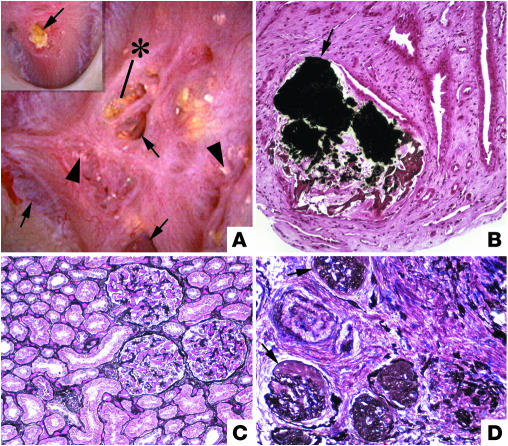
Figure 3.
Endoscopic and histological images from a SF following small bowel bypass. The papillary surface (A) shows small, round nodular structures (arrowheads) near the openings of the Bellini ducts; distinct sites of Randall plaque material are not found. (B) Biopsy through a region that contained nodules reveals crystal deposition in the lumens of a few CDs as far down as the Bellini ducts (indicate by the asterisk). Note dilated CDs (arrows) with cast material in regions of fibrosis around crystal deposit–filled CDs. (C) A single CD is shown to be completely filled with crystals with injured lining cells. Magnification: ×100 (B); ×550 (C). Reprinted with permission from ref. 19.
Treatment involves high fluid intake and reducing dietary oxalate and fat intake to the extent that nutritional requirement guidelines permit. Calcium supplements (500–1,000 mg) taken with each meal can bind food oxalate and hinder absorption. Cholestyramine (2–4 g with each meal) binds oxalate and fatty acids and reduces urine oxalate excretion. If stones contain UA, potassium alkali is needed to increase urine pH (76). No formal trials have validated these treatment approaches.
Primary hyperoxaluria.
Mutations in 1 of 2 genes — AGXT or GRHPR (77) — leads to oxalate overproduction and urine oxalate excretion rates above 100 mg/d or even 200 mg/d, resulting in PH (Table 2). When gene alterations divert active enzyme into mitochondria, where it cannot function effectively, pyridoxine may reduce urine oxalate concentration by increasing enzyme activity (78). When loss-of-function mutations inactivate enzymes, actively increasing urine volume and perhaps treatment with orthophosphate supplements may be advised (79). In type I PH, renal failure is frequent and presently treated by combined segmental liver and renal transplantation (80). Type II PH, being milder, seldom causes renal failure.
Table 2.
Primary hyperoxaluria
Hypocitraturia
The 30th percentiles for urine citrate excretion in normal women and men, 424 mg/d and 384 mg/d (26), respectively, correspond well with usual criteria for hypocitraturia (less than 500 mg/d for women and less than 350 mg/d for men). We have already alluded to the role of citrate in binding calcium and inhibiting calcium crystallization (see “Modulators of the ULM, crystal growth, and aggregation”), so it is reasonable to presume that low urine citrate levels could permit CaOx stones.
Regulation of urine citrate.
Urine citrate concentration is determined mainly by tubule reabsorption (81), which is increased by acid loads and reduced by alkali loads. Therefore, states of alkali loss such as intestinal malabsorption with diarrhea or any cause of metabolic acidosis reduce urine citrate level (82, 83). Potassium depletion, a common consequence of thiazide intake, also lowers urine citrate level, which can be raised by potassium administration (84). However, in most patients who excrete subnormal amounts of citrate in their urine, no apparent cause can be found, and the underlying mechanisms are presently unknown.
Clinical management.
Idiopathic CaOx SFs with low urine citrate levels can benefit from potassium citrate salt administration, which increases urine citrate level and reduces stone recurrence (see “Treatment trials: stone recurrence outcomes”). Suggested dosages of potassium citrate salts range from 20–60 mEq/d, achieved in 2 or 3 doses. Alkali treatment does not reliably reduce urine calcium levels (85, 86) in IH. If hypercalciuria is present and does not abate with alkali, addition of thiazide is prudent to prevent an increase in CaP SS and development of CaP stones.
Hyperuricosuria
High urine UA excretion in men and women (above 800 mg/d and 750 mg/d, respectively) is associated with the formation of idiopathic CaOx stones (12). Dissolved UA salts appear to reduce the solubility of CaOx (87–89), fostering stones. Although hyperuricosuria results from high dietary intake of beef, poultry, and fish and can be abolished by dietary changes, no clinical trials document the effectiveness of this approach. A single trial has validated the use of allopurinol, an inhibitor of xanthine oxidase, which reduces UA production, as effective in reducing stone recurrence (see “Treatment trials: stone recurrence outcomes”).
CaP stones
In a majority of kidney stones, CaOx is the main constituent and CaP is present in amounts ranging from 1% to 10% (90). When CaP becomes the main constituent (>50%) of stones, the stones are called CaP stones, and patients who form CaP stones are referred to as CaP SFs. CaP is present in urinary stones as either apatite (the principal constituent of bones and teeth) or brushite (calcium monohydrogen phosphate). Brushite, but not apatite, stones are physically resistant to ESWL, so repeated treatments may be needed (91). Brushite stones are associated with a distinctive renal disease (92). What drives the development of brushite versus apatite stones is not known.
CD plugging and brushite stones
In patients whose stones contain brushite (92), plugs of apatite fill the lumens of the terminal CD (Figure 4A). Epithelial cells are damaged or destroyed (Figure 4B). Around affected tubules, the interstitium is inflamed and scarred. Idiopathic CaOx SFs do not have intratubular crystals, whereas all brushite SFs we have biopsied to date have exhibited CD plugging. Stone type is therefore a clue to renal pathology. In the cortex of the 10 patients studied thus far (92), glomerular obsolescence and interstitial fibrosis exceeds that in cortical tissue from non-SFs and idiopathic CaOx SFs (Figure 4, C and D). Serum creatinine level was generally higher and 24-hour urine creatinine clearance was lower in the brushite SFs than in healthy controls. In our large clinical series (93), we could not find reduced renal function in brushite versus CaOx SFs, so these biopsied patients may have unusually severe renal disease. Compared with idiopathic CaOx SFs, and adjusted for sex, numbers of stones, and duration of stone disease, brushite SFs require a greater number of ESWL treatments, and this may contribute to their renal injury. Although potassium citrate salts are effective as a treatment for CaOx stones with hypocitraturia, they, along with ESWL, may promote the formation of CaP stones, whose prevalence has risen for the past 3 decades. Use of citrates requires attention in order to avoid increasing CaP SS excessively via high urine pH.
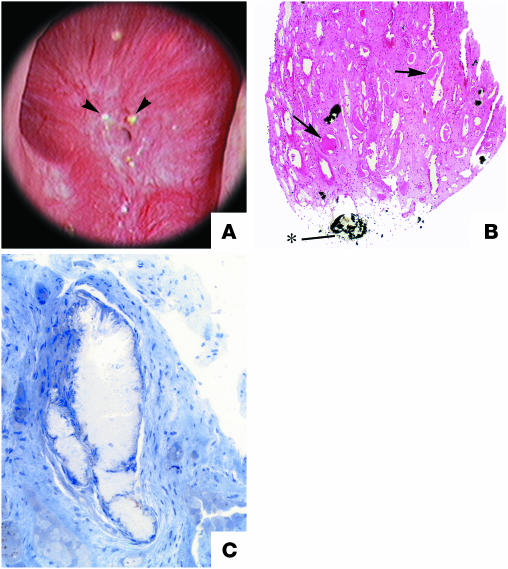
Figure 4.
Endoscopic and histological images from a brushite SF. (A) Papilla from a brushite SF that was video recorded at the time of stone removal shows depressions (arrows) near the papillary tip and flattening, a phenomenon not seen in CaOx SFs. Like CaOx SFs, the papilla possessed sites of Randall plaque (arrowheads), though lesser in number. In addition, papillae possess sites of a yellowish crystalline deposit at the openings of Bellini ducts (indicated by the asterisk). These ducts were occasionally enlarged and filled with a crystalline material that protruded from the duct (inset, arrow) that might serve as a site for stone attachment. (B) Deposits in the lumens of an individual inner medullary CD (arrow) and in an occasional nearby Henle loop are shown. The crystal deposits greatly expanded the lumen of these tubules, and cell injury to the degree of complete cell necrosis was found. A cuff of interstitial inflammation and fibrosis accompanied sites of intraluminal disposition. (C and D) A cortical sample from a normal human kidney (C) compared with that of a brushite SF (D) that reveals advanced glomerulosclerosis (arrows), moderate tubular atrophy, and interstitial fibrosis — changes not seen in CaOx SFs. Magnification: ×1,400 (B); ×1,000 (C and D). A and B reprinted with permission from Kidney International (92). C and D reprinted with permission from Urological Research (123).
High urine pH as a main risk factor for CaP stones
Idiopathic CaOx SFs and CaP SFs both are hypercalciuric because of IH, but CaP SFs produce urine of higher pH, which favors CaP crystallization (93) by increasing the abundance of urine monohydrogen phosphate (pKa ∼6.7), the ion that combines with calcium. Urine pH rises progressively with increasing CaP percentage in stones (93). The mechanism of increased urinary pH in CaP SFs is unknown. Blood bicarbonate levels are normal, and net acid excretion is not low. High ammonia production and excretion could raise urinary pH (94) but was not obvious in our patients (93).
Management of CaP SFs
CaP SFs are usually treated with fluids and thiazide diuretics to lower urine calcium excretion. Urine citrate excretion can be reduced, as in idiopathic CaOx SFs, but because potassium citrate salts can increase urine pH and CaP SS, careful follow-up is needed. No clinical trials have documented treatment outcomes for CaP SFs.
Hereditary dRTA
dRTA is a syndrome of metabolic acidosis and hypokalemia with alkaline urine pH, low urine citrate excretion, hypercalciuria, and often bone disease (95). Stones are common and usually ascribed to the combination of hypercalciuria, low urine citrate, and high urine pH. If given an acid load, patients cannot reduce urine pH below 5.5, the conventional benchmark (95). Trials have yet to document stone treatment outcomes.
Autosomal dominant dRTA (Table 3) is caused by mutations in a chloride-bicarbonate exchanger encoded by SLC4A1 on the basolateral surface of type A intercalated cells (96). The abnormal transporter is not targeted to the basolateral cell surface (97) and may form hetero-oligomers with the normal protein, reducing its function (98). Although dRTA is mild, stones and nephrocalcinosis are common and lead to the diagnosis, often in adult life. Autosomal recessive dRTA with sensory hearing loss (Table 3) causes dehydration and growth failure, beginning in infancy or early childhood (99). Hypercalciuria and high urine pH cause nephrocalcinosis. Autosomal recessive dRTA without hearing loss (Table 3) also causes growth failure and dehydration; some patients develop late-onset hearing loss (100). Autosomal recessive dRTA with osteopetrosis and cerebral calcification, caused by carbonic anhydrase II deficiency, does not cause nephrocalcinosis or stones (101).
Table 3.
Distal renal tubular acidosis
Acquired and “incomplete” dRTA
Acquired forms of dRTA with stone formation are usually associated with the autoimmune disease Sjögren syndrome and/or use of the carbonic anhydrase inhibitor acetazolamide (102, 103). “Incomplete” dRTA is used as a diagnostic term when urine citrate excretion rate is low despite high urinary pH (pH >6.5) and blood chemistries are normal; given an oral acid load, such patients may fail to lower urinary pH below 5.5 (104, 105). Treatment of the low urine citrate is as already described for CaOx and CaP SFs. Stone treatment outcomes are not known for this condition.
Management of dRTA
Supplemental alkali in the form of potassium citrate salts is required to correct the metabolic acidosis and hypokalemia. Dosage for adults should range between 1 and 3 mEq of alkali per kilogram body weight (95). No trials document stone outcomes with alkali treatment.
UA stones
UA stones form because urine pH is abnormally low. Only 90 mg/l of undissociated UA dissolves in human urine at 37°C; therefore, at a pH of 5.35 (the pKa for the dissociation of the N9 proton of UA), only 180 mg/l of total urate species can be dissolved, whereas the total urate concentration of healthy individuals and UA SFs approximates 500 mg/l of urine (12). Low urine pH is due in part to low ammonia excretion (106, 107). Low urinary pH and UA stones are common in patients with gout, diabetes mellitus, and the metabolic syndrome, perhaps as a result of insulin resistance that may reduce renal ammonia excretion (108). Urinary pH falls with increasing body weight (109), probably because of insulin resistance. Chronic diarrhea also lowers urinary pH (69) and causes UA stones.
UA stones are not easily seen on kidney, ureter, or bladder x-rays. On CT images, they resemble calcium stones, from which they can be distinguished by their lower density (1). On an i.v. pyelogram radiograph, UA stones appear as filling defects. UA gravel can occlude ureters and produce acute anuric renal failure; UA stones can fill the entire renal collecting system. UA gravel and stones are often orange or red, having adsorbed uricine, a bilirubin breakdown pigment. Prevention and even dissolution of UA stones depends upon the ability to increase urine pH above 6.0, which is accomplished by administration of 20–30 mEq potassium alkali, 2 or 3 times daily. Allopurinol is not usually required or indicated but can be used if alkali does not suffice and urine UA level is increased.
Cystinuria
Genetic abnormalities
Mutations in renal epithelial cell transporters result in reduced reabsorption and increased urine excretion of the dibasic amino acids, including cystine. High cystine SS because of overexcretion causes cystine stones (Table 4). The main apical resorption system for cystine in the kidney is a heterodimer transporter composed of a light chain catalytic subunit, b0,+ amino acid transporter (b0,+AT), and rBAT (related to b0,+AT), a heavy chain subunit (110). rBAT is responsible for targeting b0,+AT to the brush border membrane, and it is b0,+AT that acts as the transporting assembly. Although cystinuria can be subtyped genetically (Table 4), patients of either type A or B are clinically indistinguishable (111). In all forms of cystinuria, urine cystine excretion ranges from 350–500 mg/d.
Table 4.
Known gene defects in cysteinuria
Clinical management of cystinuria
Treatment of cystinuria aims to reduce SS below 1. Cystine solubility approximates 1 mM (243 mg/l) and rises with urine pH but is variable and higher in urine of cystinuric patients as compared with CaOx SFs (112) and therefore is best measured directly (113) rather than estimated via a pH nomogram. Management (114) begins with achieving a daily urine volume of 3.5–5 liters, which can dissolve the cystine excreted by some patients. Others also require administration of 40–80 mEq/d of potassium alkali to raise pH. Reduction of sodium and protein intake reduces urine cystine excretion. Those measures failing, D-penicillamine, α- mercaptopropionylglycine, or captopril, all of which form soluble heterodimers with cysteine, can be used. Blood pressure reduction can limit captopril dosing so the drug has little practical utility. D-Penicillamine and α-mercaptopropionylglycine have side effects including loss of taste, fever, proteinuria, serum sickness–type reactions, and even frank nephrotic syndrome. Increasing fluid and alkali intake are therefore preferred therapeutic modalities in as many cases as possible.
Treatment trials: stone recurrence outcomes
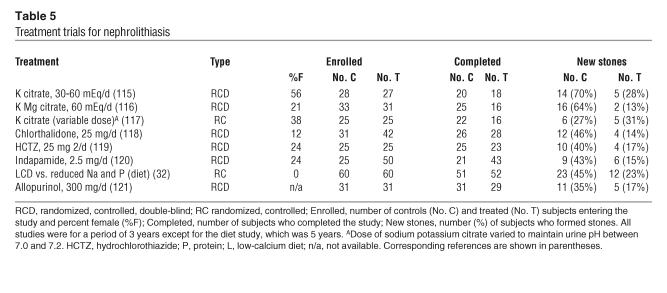
To date, 7 of 8 prospective trials, each lasting at least 3 years, have shown that selective treatments have a distinct benefit for idiopathic CaOx SFs (Table 5). Table 5 makes clear that dropout rates were high, and we are not aware that any of the investigators followed up with those individuals that did not complete the trial in order to ascertain their stone status. Therefore, additional trials may not be altogether without merit. Even so, and despite these imperfections, it is difficult to conclude that treatments are without important benefits.
Table 5.
Treatment trials for nephrolithiasis
Thiazide and citrate: recurrent calcium SFs.
Three prospective trials of potassium citrate salts have been performed to date, and 2 (115, 116) indicate therapeutic benefit (Table 5). The third (117) lacks a double-blind design, and its control subjects formed fewer stones than those in the other studies. All subjects were CaOx SFs with hypocitraturia. Thiazide diuretic agents have been studied in 3 randomized, controlled, double-blind trials. Chlorthalidone (118) at doses of 25 mg/d or 50 mg/d gave equivalent results that are pooled (Table 5). Hydrochlorothiazide gave therapeutic effects equivalent to chlorthalidone (119), as did indapamide (120).
Dietary changes for recurrent calcium SFs.
Borghi et al. (32) compared a low-calcium diet to a normal calcium diet reduced in protein and sodium in a 5-year randomized trial (Table 5). The 2 diets gave comparable results during the first 3 years, but by 5 years, the normal calcium, reduced sodium, and protein diet proved superior in that only 23% of patients had formed a new stone.
Allopurinol: recurrent calcium SFs.
Allopurinol (121) has been shown to reduce new CaOx stone formation in patients with hyperuricosuria (Table 5).
Patients who have formed only 1 calcium stone.
Over a 5-year period, 20% of patients who maintained a urine volume above 2.5 l/d had stone recurrence (122) compared with approximately 50% of patients who were counseled to avoid dehydration and to observe moderate salt and protein intakes. Counseled controls did not actually increase urine volume. We do not combine this trial with the others, as it concerns only SFs with a single stone.
A final word about stones
It is difficult to accept recurrent stone formation as incidental in any patient and allow it to continue without efforts to understand its causes and offer such treatments as seem appropriate. Available trials offer physicians excellent treatment strategies for prevention of calcium stones, and since UA stones are a consequence of low urine pH, physicians can treat them confidently despite the lack of prospective trials for additional therapeutics. Even cystine stones can be prevented, albeit with imperfect remedies. But treatments may pose their own problems. Although potassium citrate salts are effective, they, along with ESWL, may promote the formation of CaP stones, the prevalence of which continues to rise with time. Possibly this means that the use of citrates requires special attention to avoid increasing CaP SS excessively via high urine pH. Although we treat urine SS, the tissue processes of stone formation are complex, not as yet obviously related to solution chemistry within specific nephron segments, and not well understood. This is a significant area of interest that requires new research. Abnormal urine pH and calcium excretion rate are predominant findings in SFs that play a major role in the pathogenesis of stone formation. Their biologies are therefore also of particular research importance. Perhaps most important in the long run will be uncovering the links between genetic variability and urine calcium excretion and pH, for these seem at the very center of the problem of kidney stone disease.
Footnotes
Nonstandard abbreviations used: b0,+AT, b0,+ amino acid transporter; BM, basement membrane; CaOx, calcium oxalate; CaP, calcium phosphate; CaSR, calcium-sensing receptor; CD, collecting duct; CLCN5, chloride channel 5; DCT, distal convoluted tubule; dRTA, distal renal tubular acidosis; ESWL, extracorporeal shock wave lithotripsy; IH, idiopathic hypercalciuria; PH, primary hyperoxaluria; PT, proximal tubule; PTH, parathyroid hormone; SF, stone former; SS, supersaturation; rBAT, related to b0,+AT; TAL, thick ascending limb; UA, uric acid; ULM, upper limit of metastability.
Conflict of interest: F.L. Coe has a financial interest in Litholink Corp., a provider of kidney stone testing services. The remaining authors have declared that no conflict of interest exists.
References
- 1.Zarse CA, et al. Helical computed tomography accurately reports urinary stone composition using attenuation values: in vitro verification using high-resolution micro-computed tomography calibrated to fourier transform infrared microspectroscopy. Urology. 2004;63:828–833. doi: 10.1016/j.urology.2003.11.038. [DOI] [PubMed] [Google Scholar]
- 2.Lingeman JE, Kim SC, Kuo RL, McAteer JA, Evan AP. Shockwave lithotripsy: anecdotes and insights. J. Endourol. 2003;17:687–693. doi: 10.1089/089277903770802191. [DOI] [PubMed] [Google Scholar]
- 3.Bagley DH. Expanding role of ureteroscopy and laser lithotripsy for treatment of proximal ureteral and intrarenal calculi. Curr. Opin. Urol. 2002;12:277–280. doi: 10.1097/00042307-200207000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Clayman RV. Percutaneous nephrolithotomy: an update [comment] J. Urol. 2005;173:1199. doi: 10.1097/01.ju.0000155177.75597.66. [DOI] [PubMed] [Google Scholar]
- 5.Gillen DL, Worcester EM, Coe FL. Decreased renal function among adults with a history of nephrolithiasis: a study of NHANES III. Kidney Int. 2005;67:685–690. doi: 10.1111/j.1523-1755.2005.67128.x. [DOI] [PubMed] [Google Scholar]
- 6.Madore F, Stampfer MJ, Rimm EB, Curhan GC. Nephrolithiasis and risk of hypertension. Am. J. Hypertens. 1998;11:46–53. doi: 10.1016/s0895-7061(97)00371-3. [DOI] [PubMed] [Google Scholar]
- 7.Brown, C.M., and Purich, D.L. 1992. Physical-chemical processes in kidney stone formation. In Disorders of bone and mineral metabolism. F.L. Coe and M.J. Favus, editors. Raven Press. New York, New York, USA. 613–624.
- 8.Parks JH, Coward M, Coe FL. Correspondence between stone composition and urine supersaturation in nephrolithiasis. Kidney Int. 1997;51:894–900. doi: 10.1038/ki.1997.126. [DOI] [PubMed] [Google Scholar]
- 9.Parks JH, Goldfischer ER, Coe FL. Changes in urine volume accomplished by physicians treating nephrolithiasis. J. Urol. 2003;169:863–866. doi: 10.1097/01.ju.0000044922.22478.32. [DOI] [PubMed] [Google Scholar]
- 10.Asplin JR, Parks JH, Coe FL. Dependence of upper limit of metastability on supersaturation in nephrolithiasis. Kidney Int. 1997;52:1602–1608. doi: 10.1038/ki.1997.491. [DOI] [PubMed] [Google Scholar]
- 11.Asplin JR, Parks JH, Nakagawa Y, Coe FL. Reduced crystallization inhibition by urine from women with nephrolithiasis. Kidney Int. 2002;61:1821–1829. doi: 10.1046/j.1523-1755.2002.00307.x. [DOI] [PubMed] [Google Scholar]
- 12.Coe, F.L., and Parks, J.H. 2000. Pathogenesis and treatment of nephrolithiasis. In The kidney. D. Seldin and G. Giebisch, editors. Lippincott Williams & Wilkins. Philadelphia, Pennsylvania, USA. 1841–1867.
- 13.Coe, F.L., Parks, J.H., and Nakagawa, Y.N. 2002. Inhibitors and promoters of calcium oxalate crystallization: their relationship to the pathogenesis of nephrolithiasis. In Disorders of bone and mineral metabolism. F.L. Coe and M.J. Favus, editors. Lippincott Williams & Wilkins. Philadelphia, Pennsylvania. 741–775.
- 14.Asplin JR, Arsenault D, Parks JH, Coe FL, Hoyer JR. Contribution of human uropontin to inhibition of calcium oxalate crystallization. Kidney Int. 1998;53:194–199. doi: 10.1046/j.1523-1755.1998.00739.x. [DOI] [PubMed] [Google Scholar]
- 15.Stapleton AM, Ryall RL. Blood coagulation proteins and urolithiasis are linked: crystal matrix protein is the F1 activation peptide of human prothrombin. Br. J. Urol. 1995;75:712–719. doi: 10.1111/j.1464-410x.1995.tb07377.x. [DOI] [PubMed] [Google Scholar]
- 16.Marengo SR, Resnick MI, Yang L, Churchill PC. Differential expression of urinary inter-alpha-trypsin inhibitor trimers and dimers in normal compared to active calcium oxalate stone forming men. J. Urol. 1998;159:1444–1450. doi: 10.1097/00005392-199805000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Pillay SN, Asplin JR, Coe FL. Evidence that calgranulin is produced by kidney cells and is an inhibitor of calcium oxalate crystallization. Am. J. Physiol. 1998;275:F255–F261. doi: 10.1152/ajprenal.1998.275.2.F255. [DOI] [PubMed] [Google Scholar]
- 18.Hess B, Nakagawa Y, Parks JH, Coe FL. Molecular abnormality of Tamm Horsfall glycoprotein in calcium oxalate nephrolithiasis. Am. J. Physiol. 1991;29:F569–F578. doi: 10.1152/ajprenal.1991.260.4.F569. [DOI] [PubMed] [Google Scholar]
- 19.Evan AP, et al. Randall’s plaque of patients with nephrolithiasis begins in basement membranes of thin loops of Henle. J. Clin. Invest. 2003;111:607–616. doi:10.1172/JCI200317038. doi: 10.1172/JCI17038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim SC, et al. Stone formation is proportional to papillary surface coverage by Randall’s plaque. J. Urol. 2005;173:117–119. doi: 10.1097/01.ju.0000147270.68481.ce. [DOI] [PubMed] [Google Scholar]
- 21.Evan AP, et al. Apatite plaque particles in inner medulla of kidneys of calcium oxalate stone formers: osteopontin localization. Kidney Int. 2005;68:145–154. doi: 10.1111/j.1523-1755.2005.00388.x. [DOI] [PubMed] [Google Scholar]
- 22.Sheng X, Jung T, Wesson JA, Ward MD. Adhesion at calcium oxalate crystal surfaces and the effect of urinary constituents. Proc. Natl. Acad. Sci. U. S. A. 2005;102:267–272. doi: 10.1073/pnas.0406835101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuo RL, et al. Urine calcium and volume predict coverage of renal papilla by Randall’s plaque. Kidney Int. 2003;64:2150–2154. doi: 10.1046/j.1523-1755.2003.00316.x. [DOI] [PubMed] [Google Scholar]
- 24.Marshall RW, Cochran M, Hodgkinson A. Relationships between calcium and oxalic acid intake in the diet and their excretion in the urine of normal and renal-stone-forming subjects. Clin. Sci. 1972;43:91–99. doi: 10.1042/cs0430091. [DOI] [PubMed] [Google Scholar]
- 25.Lemann J, Jr, Worcester EM, Gray RW. Hypercalciuria and stones [review] Am. J. Kidney Dis. 1991;17:386–391. doi: 10.1016/s0272-6386(12)80628-7. [DOI] [PubMed] [Google Scholar]
- 26.Parks JH, Coe FL. A urinary calcium-citrate index for the evaluation of nephrolithiasis. Kidney Int. 1986;30:85–90. doi: 10.1038/ki.1986.155. [DOI] [PubMed] [Google Scholar]
- 27.Curhan GC, Willett WC, Speizer FE, Stampfer MJ. Twenty-four-hour urine chemistries and the risk of kidney stones among women and men. Kidney Int. 2001;59:2290–2298. doi: 10.1046/j.1523-1755.2001.00746.x. [DOI] [PubMed] [Google Scholar]
- 28.Favus MJ, Karnauskas AJ, Parks JH, Coe FL. Peripheral blood monocyte vitamin D receptor levels are elevated in patients with idiopathic hypercalciuria. J. Clin. Endocrinol. Metab. 2004;89:4937–4943. doi: 10.1210/jc.2004-0412. [DOI] [PubMed] [Google Scholar]
- 29.Lemann J, Jr, Lennon EJ, Piering WF, Prien EL, Jr, Ricanati ES. Evidence that glucose ingestion inhibits net renal tubular reabsorption of calcium and magnesium in man. J. Lab. Clin. Med. 1970;75:578–585. [PubMed] [Google Scholar]
- 30.Breslau NA, Sakhaee K, Pak CY. Impaired adaptation to salt-induced urinary calcium losses in postmenopausal osteoporosis. Trans. Assoc. Am. Physicians. 1985;98:107–115. [PubMed] [Google Scholar]
- 31.Hess B, Ackermann D, Essig M, Takkinen R, Jaeger P. Renal mass and serum calcitriol in male idiopathic calcium renal stone formers: role of protein intake. J. Clin. Endocrinol. Metab. 1995;80:1916–1921. doi: 10.1210/jcem.80.6.7775641. [DOI] [PubMed] [Google Scholar]
- 32.Borghi L, et al. Comparison of two diets for the prevention of recurrent stones in idiopathic hypercalciuria. N. Engl. J. Med. 2002;346:77–84. doi: 10.1056/NEJMoa010369. [DOI] [PubMed] [Google Scholar]
- 33.Asplin JR, et al. Bone mineral density and urine calcium excretion among subjects with and without nephrolithiasis. Kidney Int. 2003;63:662–669. doi: 10.1046/j.1523-1755.2003.00763.x. [DOI] [PubMed] [Google Scholar]
- 34.Coe FL, Parks JH, Bushinsky DA, Langman CB, Favus MJ. Chlorthalidone promotes mineral retention in patients with idiopathic hypercalciuria. Kidney Int. 1988;33:1140–1146. doi: 10.1038/ki.1988.122. [DOI] [PubMed] [Google Scholar]
- 35.Gambaro G, et al. Genetics of hypercalciuria and calcium nephrolithiasis: from the rare monogenic to the common polygenic forms. Am. J. Kidney Dis. 2004;44:963–986. doi: 10.1053/j.ajkd.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 36.Moe OW, Bonny O. Genetic hypercalciuria. J. Am. Soc. Nephrol. 2005;16:729–745. doi: 10.1681/ASN.2004100888. [DOI] [PubMed] [Google Scholar]
- 37.Thakker RV. Pathogenesis of Dent’s disease and related syndromes of X-linked nephrolithiasis. Kidney Int. 2000;57:787–793. doi: 10.1046/j.1523-1755.2000.00916.x. [DOI] [PubMed] [Google Scholar]
- 38.Gunther W, Luchow A, Cluzeaud F, Vandewalle A, Jentsch TJ. ClC-5, the chloride channel mutated in Dent’s disease, colocalizes with the proton pump in endocytotically active kidney cells. Proc. Natl. Acad. Sci. U. S. A. 1998;95:8075–8080. doi: 10.1073/pnas.95.14.8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luyckx VA, et al. Intrarenal and subcellular localization of rat CLC5. Am. J. Physiol. 1998;275:F761–F769. doi: 10.1152/ajprenal.1998.275.5.F761. [DOI] [PubMed] [Google Scholar]
- 40.Reinhart SC, et al. Characterization of carrier females and affected males with x-linked recessive nephrolithiasis. J. Am. Soc. Nephrol. 1994;5:S54–S58. doi: 10.1681/ASN.V571451. [DOI] [PubMed] [Google Scholar]
- 41.Nakazato H, et al. Mutations in the CLCN5 gene in Japanese patients with familial idiopathic low-molecular-weight proteinuria. Kidney Int. 1997;52:895–900. doi: 10.1038/ki.1997.410. [DOI] [PubMed] [Google Scholar]
- 42.Scheinman SJ. X-linked hypercalciuric nephrolithiasis: clinical syndromes and chloride channel mutations. Kidney Int. 1998;53:3–17. doi: 10.1046/j.1523-1755.1998.00718.x. [DOI] [PubMed] [Google Scholar]
- 43.Raja KA, et al. Responsiveness of hypercalciuria to thiazide in Dent’s disease. J. Am. Soc. Nephrol. 2002;13:2938–2944. doi: 10.1097/01.asn.0000036869.82685.f6. [DOI] [PubMed] [Google Scholar]
- 44.Hoopes RR, Jr, et al. Evidence for genetic heterogeneity in Dent’s disease. Kidney Int. 2004;65:1615–1620. doi: 10.1111/j.1523-1755.2004.00571.x. [DOI] [PubMed] [Google Scholar]
- 45.Scheinman SJ, et al. Isolated hypercalciuria with mutation in CLCN5: relevance to idiopathic hypercalciuria. Kidney Int. 2000;57:232–239. doi: 10.1046/j.1523-1755.2000.00774.x. [DOI] [PubMed] [Google Scholar]
- 46.Wang SS, et al. Mice lacking renal chloride channel, CLC-5, are a model for Dent’s disease, a nephrolithiasis disorder associated with defective receptor-mediated endocytosis. Hum. Mol. Genet. 2000;9:2937–2945. doi: 10.1093/hmg/9.20.2937. [DOI] [PubMed] [Google Scholar]
- 47.Silva IV, et al. The ClC-5 knockout mouse model of Dent’s disease has renal hypercalciuria and increased bone turnover. J. Bone Miner. Res. 2003;18:615–623. doi: 10.1359/jbmr.2003.18.4.615. [DOI] [PubMed] [Google Scholar]
- 48.Gunther W, Piwon N, Jentsch TJ. The ClC-5 chloride channel knock-out mouse — an animal model for Dent’s disease. Pflugers Arch. 2003;445:456–462. doi: 10.1007/s00424-002-0950-6. [DOI] [PubMed] [Google Scholar]
- 49.Naesens M, Steels P, Verberckmoes R, Vanrenterghem Y, Kuypers D. Bartter’s and Gitelman’s syndromes: from gene to clinic. Nephron Physiol. 2004;96:65–78. doi: 10.1159/000076752. [DOI] [PubMed] [Google Scholar]
- 50.Taugner R, et al. The juxtaglomerular apparatus in Bartter’s syndrome and related tubulopathies. An immunocytochemical and electron microscopic study. Virchows Arch. A. Pathol. Anat. Histol. 1988;412:459–470. doi: 10.1007/BF00750580. [DOI] [PubMed] [Google Scholar]
- 51.Pope JC, et al. The natural history of nephrocalcinosis in premature infants treated with loop diuretics. J. Urol. 1996;156:709–712. doi: 10.1097/00005392-199608001-00039. [DOI] [PubMed] [Google Scholar]
- 52.Brown EM, Pollak M, Hebert SC. The extracellular calcium-sensing receptor: its role in health and disease. Annu. Rev. Med. 1998;49:15–29. doi: 10.1146/annurev.med.49.1.15. [DOI] [PubMed] [Google Scholar]
- 53.Ba J, Friedman PA. Calcium-sensing receptor regulation of renal mineral ion transport. Cell Calcium. 2004;35:229–237. doi: 10.1016/j.ceca.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 54.Hebert SC, Brown EM, Harris HW. Role of the Ca(2+)-sensing receptor in divalent mineral ion homeostasis. J. Exp. Biol. 1997;200:295–302. doi: 10.1242/jeb.200.2.295. [DOI] [PubMed] [Google Scholar]
- 55.Pearce SH, et al. A familial syndrome of hypocalcemia with hypercalciuria due to mutations in the calcium-sensing receptor. N. Engl. J. Med. 1996;335:1115–1122. doi: 10.1056/NEJM199610103351505. [DOI] [PubMed] [Google Scholar]
- 56.Okazaki R, et al. A novel activating mutation in calcium-sensing receptor gene associated with a family of autosomal dominant hypocalcemia. J. Clin. Endocrinol. Metab. 1999;84:363–366. doi: 10.1210/jcem.84.1.5385. [DOI] [PubMed] [Google Scholar]
- 57.Yamamoto M, Akatsu T, Nagase T, Ogata E. Comparison of hypocalcemic hypercalciuria between patients with idiopathic hypoparathyroidism and those with gain-of-function mutations in the calcium-sensing receptor: is it possible to differentiate the two disorders? J. Clin. Endocrinol. Metab. 2000;85:4583–4591. doi: 10.1210/jcem.85.12.7035. [DOI] [PubMed] [Google Scholar]
- 58.Carling T, et al. Familial hypercalcemia and hypercalciuria caused by a novel mutation in the cytoplasmic tail of the calcium receptor. J. Clin. Endocrinol. Metab. 2000;85:2042–2047. doi: 10.1210/jcem.85.5.6477. [DOI] [PubMed] [Google Scholar]
- 59.Watanabe S, et al. Association between activating mutations of calcium-sensing receptor and Bartter’s syndrome. Lancet. 2002;360:692–694. doi: 10.1016/S0140-6736(02)09842-2. [DOI] [PubMed] [Google Scholar]
- 60.Vargas-Poussou R, et al. Functional characterization of a calcium-sensing receptor mutation in severe autosomal dominant hypocalcemia with a Bartter-like syndrome. J. Am. Soc. Nephrol. 2002;13:2259–2266. doi: 10.1097/01.asn.0000025781.16723.68. [DOI] [PubMed] [Google Scholar]
- 61.Muller D, et al. A novel claudin 16 mutation associated with childhood hypercalciuria abolishes binding to ZO-1 and results in lysosomal mistargeting. Am. J. Hum. Genet. 2003;73:1293–1301. doi: 10.1086/380418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weber S, et al. Familial hypomagnesaemia with hypercalciuria and nephrocalcinosis maps to chromosome 3q27 and is associated with mutations in the PCLN-1 gene. Eur. J. Hum. Genet. 2000;8:414–422. doi: 10.1038/sj.ejhg.5200475. [DOI] [PubMed] [Google Scholar]
- 63.Weber S, et al. Novel paracellin-1 mutations in 25 families with familial hypomagnesemia with hypercalciuria and nephrocalcinosis. J. Am. Soc. Nephrol. 2001;12:1872–1881. doi: 10.1681/ASN.V1291872. [DOI] [PubMed] [Google Scholar]
- 64.Reed BY, Heller HJ, Gitomer WL, Pak CY. Mapping a gene defect in absorptive hypercalciuria to chromosome 1q23.3-q24. J. Clin. Endocrinol. Metab. 1999;84:3907–3913. doi: 10.1210/jcem.84.11.6155. [DOI] [PubMed] [Google Scholar]
- 65.Reed BY, et al. Identification and characterization of a gene with base substitutions associated with the absorptive hypercalciuria phenotype and low spinal bone density. J. Clin. Endocrinol. Metab. 2002;87:1476–1485. doi: 10.1210/jcem.87.4.8300. [DOI] [PubMed] [Google Scholar]
- 66.Geng W, et al. Cloning and characterization of the human soluble adenylyl cyclase. Am. J. Physiol. Cell Physiol. 2005;288:C1305–C1316. doi: 10.1152/ajpcell.00584.2004. [DOI] [PubMed] [Google Scholar]
- 67.Heller, H.J., and Pak, C.Y.C. 2002. Primary hyperparathyroidism. In Disorders of bone and mineral metabolism. F.L. Coe and M.J. Favus, editors. Lippincott Williams & Wilkins. Philadelphia, Pennsylvania, USA. 516–534.
- 68.Pak CY, et al. Rapid communication: relative effect of urinary calcium and oxalate on saturation of calcium oxalate. Kidney Int. 2004;66:2032–2037. doi: 10.1111/j.1523-1755.2004.00975.x. [DOI] [PubMed] [Google Scholar]
- 69.Parks JH, Worcester EM, O’Connor RC, Coe FL. Urine stone risk factors in nephrolithiasis patients with and without bowel disease. Kidney Int. 2003;63:255–265. doi: 10.1046/j.1523-1755.2003.00725.x. [DOI] [PubMed] [Google Scholar]
- 70.von Unruh GE, Voss S, Sauerbruch T, Hesse A. Dependence of oxalate absorption on the daily calcium intake. J. Am. Soc. Nephrol. 2004;15:1567–1573. doi: 10.1097/01.asn.0000127864.26968.7f. [DOI] [PubMed] [Google Scholar]
- 71.Holmes RP, Goodman HO, Assimos DG. Contribution of dietary oxalate to urinary oxalate excretion. Kidney Int. 2001;59:270–276. doi: 10.1046/j.1523-1755.2001.00488.x. [DOI] [PubMed] [Google Scholar]
- 72.Bataille P, Presne C, Fournier A. Prevention of recurrent stones in idiopathic hypercalciuria. N. Engl. J. Med. 2002;346:1667–1669. [PubMed] [Google Scholar]
- 73.Baggio B, et al. Evidence of a link between erythrocyte band 3 phosphorylation and anion transport in patients with ‘idiopathic’ calcium oxalate nephrolithiasis. Miner. Electrolyte Metab. 1993;19:17–20. [PubMed] [Google Scholar]
- 74.Baggio B, et al. An inheritable anomaly of red-cell oxalate transport in “primary” calcium nephrolithiasis correctable with diuretics. N. Engl. J. Med. 1986;314:599–604. doi: 10.1056/NEJM198603063141002. [DOI] [PubMed] [Google Scholar]
- 75.Dobbins JW, Binder HJ. Importance of the colon in enteric hyperoxaluria. N. Engl. J. Med. 1997;296:298–301. doi: 10.1056/NEJM197702102960602. [DOI] [PubMed] [Google Scholar]
- 76.Worcester EM. Stones from bowel disease. Endocrinol. Metab. Clin. North Am. 2002;31:979–999. doi: 10.1016/s0889-8529(02)00035-x. [DOI] [PubMed] [Google Scholar]
- 77.Danpure CJ, Rumsby G. Molecular aetiology of primary hyperoxaluria and its implications for clinical management. Expert Rev. Mol. Med. 2004;2004:1–16. doi: 10.1017/S1462399404007203. [DOI] [PubMed] [Google Scholar]
- 78.Asplin JR. Hyperoxaluric calcium nephrolithiasis. Endocrinol. Metab. Clin. North Am. 2002;31:927–949. doi: 10.1016/s0889-8529(02)00030-0. [DOI] [PubMed] [Google Scholar]
- 79.Milliner DS, et al. Results of long-term treatment with orthophosphate and pyridoxine in patients with primary hyperoxaluria. N. Engl. J. Med. 1994;331:1553–1558. doi: 10.1056/NEJM199412083312304. [DOI] [PubMed] [Google Scholar]
- 80.Jamieson NV. The results of combined liver/kidney transplantation for primary hyperoxaluria (PH1) 1984-1997. The European PH1 transplant registry report. European PH1 Transplantation Study Group. J. Nephrol. 1998;11(Suppl. 1):36–41. [PubMed] [Google Scholar]
- 81.Brennan S, Hering-Smith K, Hamm LL. Effect of pH on citrate reabsorption in the proximal convoluted tubule. Am. J. Physiol. 1988;255:F301–F306. doi: 10.1152/ajprenal.1988.255.2.F301. [DOI] [PubMed] [Google Scholar]
- 82.Sakhaee K, et al. Alkali absorption and citrate excretion in calcium nephrolithiasis. J. Bone Miner. Res. 1993;8:789–794. doi: 10.1002/jbmr.5650080703. [DOI] [PubMed] [Google Scholar]
- 83.Donnelly S, Kamel KS, Vasuvattakul S, Narins RG, Halperin ML. Might distal renal tubular acidosis be a proximal tubular cell disorder? Am. J. Kidney Dis. 1992;19:272–281. doi: 10.1016/s0272-6386(13)80009-1. [DOI] [PubMed] [Google Scholar]
- 84.Pak CY, et al. Correction of hypocitraturia and prevention of stone formation by combined thiazide and potassium citrate therapy in thiazide-unresponsive hypercalciuric nephrolithiasis. Am. J. Med. 1985;79:284–288. doi: 10.1016/0002-9343(85)90305-5. [DOI] [PubMed] [Google Scholar]
- 85.Lemann J, Jr, Pleuss JA, Gray RW, Hoffmann RG. Potassium administration reduces and potassium deprivation increases urinary calcium excretion in healthy adults. Kidney Int. 1991;39:973–983. doi: 10.1038/ki.1991.123. [DOI] [PubMed] [Google Scholar]
- 86.Lemann J, Jr, Gray RW, Pleuss JA. Potassium bicarbonate, but not sodium bicarbonate, reduces urinary calcium excretion and improves calcium balance in healthy men. Kidney Int. 1989;35:688–695. doi: 10.1038/ki.1989.40. [DOI] [PubMed] [Google Scholar]
- 87.Grover PK, Ryall RL, Marshall VR. Effect of urate on calcium oxalate crystallization in human urine: evidence for a promotory role of hyperuricosuria in urolithiasis. Clin. Sci. (Lond.). 1990;79:9–15. doi: 10.1042/cs0790009. [DOI] [PubMed] [Google Scholar]
- 88.Grover PK, Ryall RL, Potezny N, Marshall VR. The effect of decreasing the concentration of urinary urate on the crystallization of calcium oxalate in undiluted human urine. J. Urol. 1990;143:1057–1061. doi: 10.1016/s0022-5347(17)40183-2. [DOI] [PubMed] [Google Scholar]
- 89.Grover PK, Ryall RG, Marshall VR. Calcium oxalate crystallization in urine: role of urate and glycosaminoglycans. Kidney Int. 1992;41:149–154. doi: 10.1038/ki.1992.20. [DOI] [PubMed] [Google Scholar]
- 90.Mandel NS, Mandel GS. Urinary tract stone disease in the United States veteran population. II. Geographical analysis of variations in composition. J. Urol. 1989;142:1516–1521. doi: 10.1016/s0022-5347(17)39145-0. [DOI] [PubMed] [Google Scholar]
- 91.Klee LW, Brito CG, Lingeman JE. The clinical implications of brushite calculi. J. Urol. 1991;145:715–718. doi: 10.1016/s0022-5347(17)38432-x. [DOI] [PubMed] [Google Scholar]
- 92.Evan AP, et al. Crystal-associated nephropathy in patients with brushite nephrolithiasis. Kidney Int. 2005;67:576–591. doi: 10.1111/j.1523-1755.2005.67114.x. [DOI] [PubMed] [Google Scholar]
- 93.Parks JH, Worcester EM, Coe FL, Evan AP, Lingeman JE. Clinical implications of abundant calcium phosphate in routinely analyzed kidney stones. Kidney Int. 2004;66:777–785. doi: 10.1111/j.1523-1755.2004.00803.x. [DOI] [PubMed] [Google Scholar]
- 94.Carlisle EJ, Donnelly SM, Halperin ML. Renal tubular acidosis (RTA): recognize the ammonium defect and pHorget the urine pH. Pediatr. Nephrol. 1991;5:242–248. doi: 10.1007/BF01095965. [DOI] [PubMed] [Google Scholar]
- 95.DuBose, T.D., Jr. 2004. Acid-base disorders. In The kidney. B.M. Brenner, editor. Saunders. Philadelphia, Pennsylvania, USA. 922–996.
- 96.Karet FE, et al. Mutations in the chloride-bicarbonate exchanger gene AE1 cause autosomal dominant but not autosomal recessive distal renal tubular acidosis. Proc. Natl. Acad. Sci. U. S. A. 1998;95:6337–6342. doi: 10.1073/pnas.95.11.6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Devonald MA, Smith AN, Poon JP, Ihrke G, Karet FE. Non-polarized targeting of AE1 causes autosomal dominant distal renal tubular acidosis. Nat. Genet. 2003;33:125–127. doi: 10.1038/ng1082. [DOI] [PubMed] [Google Scholar]
- 98.Quilty JA, Cordat E, Reithmeier RA. Impaired trafficking of human kidney anion exchanger (kAE1) caused by hetero-oligomer formation with a truncated mutant associated with distal renal tubular acidosis. Biochem. J. 2002;368:895–903. doi: 10.1042/BJ20020574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Karet FE, et al. Mutations in the gene encoding B1 subunit of H+-ATPase cause renal tubular acidosis with sensorineural deafness. Nat. Genet. 1999;21:84–90. doi: 10.1038/5022. [DOI] [PubMed] [Google Scholar]
- 100.Stehberger PA, et al. Localization and regulation of the ATP6V0A4 (a4) vacuolar H+-ATPase subunit defective in an inherited form of distal renal tubular acidosis. J. Am. Soc. Nephrol. 2003;14:3027–3038. doi: 10.1097/01.asn.0000099375.74789.ab. [DOI] [PubMed] [Google Scholar]
- 101.Shah GN, Bonapace G, Hu PY, Strisciuglio P, Sly WS. Carbonic anhydrase II deficiency syndrome (osteopetrosis with renal tubular acidosis and brain calcification): novel mutations in CA2 identified by direct sequencing expand the opportunity for genotype-phenotype correlation. Hum. Mutat. 2004;24:272. doi: 10.1002/humu.9266. [DOI] [PubMed] [Google Scholar]
- 102.Cohen EP, et al. Absence of H+ ATPase in cortical collecting tubules of a patient with Sjogren’s syndrome and distal renal tubular acidosis. J. Am. Soc. Nephrol. 1992;3:264–271. doi: 10.1681/ASN.V32264. [DOI] [PubMed] [Google Scholar]
- 103.Ahlstrand C, Tiselius HG. Urine composition and stone formation during treatment with acetazolamide. Scand. J. Urol. Nephrol. 1987;21:225–228. doi: 10.3109/00365598709180326. [DOI] [PubMed] [Google Scholar]
- 104.Gault MH, et al. Comparison of patients with idiopathic calcium phosphate and calcium oxalate stones. Medicine. 1991;70:345–358. doi: 10.1097/00005792-199111000-00001. [DOI] [PubMed] [Google Scholar]
- 105.Osther PJ, Bollerslev J, Hansen AB, Engel K, Kildeberg P. Pathophysiology of incomplete renal tubular acidosis in recurrent renal stone formers: evidence of disturbed calcium, bone and citrate metabolism. Urol. Res. 1993;21:169–173. doi: 10.1007/BF00590032. [DOI] [PubMed] [Google Scholar]
- 106.Sakhaee K, Adams-Huet B, Moe OW, Pak CY. Pathophysiologic basis for normouricosuric uric acid nephrolithiasis. Kidney Int. 2002;62:971–979. doi: 10.1046/j.1523-1755.2002.00508.x. [DOI] [PubMed] [Google Scholar]
- 107.Pak CY, Sakhaee K, Peterson RD, Poindexter JR, Frawley WH. Biochemical profile of idiopathic uric acid nephrolithiasis. Kidney Int. 2001;60:757–761. doi: 10.1046/j.1523-1755.2001.060002757.x. [DOI] [PubMed] [Google Scholar]
- 108.Abate N, Chandalia M, Cabo-Chan AV, Jr, Moe OW, Sakhaee K. The metabolic syndrome and uric acid nephrolithiasis: novel features of renal manifestation of insulin resistance. Kidney Int. 2004;65:386–392. doi: 10.1111/j.1523-1755.2004.00386.x. [DOI] [PubMed] [Google Scholar]
- 109.Maalouf NM, et al. Association of urinary pH with body weight in nephrolithiasis. Kidney Int. 2004;65:1422–1425. doi: 10.1111/j.1523-1755.2004.00522.x. [DOI] [PubMed] [Google Scholar]
- 110.Palacin M, et al. The genetics of heteromeric amino acid transporters. Physiology (Bethesda). 2005;20:112–124. doi: 10.1152/physiol.00051.2004. [DOI] [PubMed] [Google Scholar]
- 111.Font-Llitjos M, et al. New insights into cystinuria: 40 new mutations, genotype-phenotype correlation, and digenic inheritance causing partial phenotype. J. Med. Genet. 2005;42:58–68. doi: 10.1136/jmg.2004.022244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nakagawa Y, Asplin JR, Goldfarb DS, Parks JH, Coe FL. Clinical use of cystine supersaturation measurements. J. Urol. 2000;164:1481–1485. [PubMed] [Google Scholar]
- 113.Coe FL, Clark C, Parks JH, Asplin JR. Solid phase assay of urine cystine supersaturation in the presence of cystine binding drugs. J. Urol. 2001;166:688–693. [PubMed] [Google Scholar]
- 114.Shekarriz B, Stoller ML. Cystinuria and other noncalcareous calculi. Endocrinol. Metab. Clin. North Am. 2002;31:951–977. doi: 10.1016/s0889-8529(02)00054-3. [DOI] [PubMed] [Google Scholar]
- 115.Barcelo P, Wuhl O, Servitge E, Rousaud A, Pak CY. Randomized double-blind study of potassium citrate in idiopathic hypocitraturic calcium nephrolithiasis. J. Urol. 1993;150:1761–1764. doi: 10.1016/s0022-5347(17)35888-3. [DOI] [PubMed] [Google Scholar]
- 116.Ettinger B, et al. Potassium-magnesium citrate is an effective prophylaxis against recurrent calcium oxalate nephrolithiasis. J. Urol. 1997;158:2069–2073. doi: 10.1016/s0022-5347(01)68155-2. [DOI] [PubMed] [Google Scholar]
- 117.Hofbauer J, Hobarth K, Szabo N, Marberger M. Alkali citrate prophylaxis in idiopathic recurrent calcium oxalate urolithiasis — a prospective randomized study. Br. J. Urol. 1994;73:362–365. doi: 10.1111/j.1464-410x.1994.tb07597.x. [DOI] [PubMed] [Google Scholar]
- 118.Ettinger B, Citron JT, Livermore B, Dolman LI. Chlorthalidone reduces calcium oxalate calculous recurrence but magnesium hydroxide does not. J. Urol. 1988;139:679–684. doi: 10.1016/s0022-5347(17)42599-7. [DOI] [PubMed] [Google Scholar]
- 119.Laerum E, Larsen S. Thiazide prophylaxis of urolithiasis: a double-blind study in general practice. Acta Med. Scand. 1984;215:383–389. [PubMed] [Google Scholar]
- 120.Borghi L, Meschi T, Guerra A, Novarini A. Randomized prospective study of a nonthiazide diuretic, indapamide, in preventing calcium stone recurrences. J. Cardiovasc. Pharmacol. 1993;22(Suppl. 6):S78–S86. [PubMed] [Google Scholar]
- 121.Ettinger B, Tang A, Citron JT, Livermore B, Williams T. Randomized trial of allopurinol in the prevention of calcium oxalate calculi. N. Engl. J. Med. 1986;315:1386–1389. doi: 10.1056/NEJM198611273152204. [DOI] [PubMed] [Google Scholar]
- 122.Borghi L, et al. Urinary volume, water and recurrences of idiopathic calcium nephrolithiasis: a 5-year randomized prospective study. J. Urol. 1996;155:839–843. [PubMed] [Google Scholar]
- 123.Evan, A.P., Coe, F.L., Lingeman, J.E., and Worcester, E. 2005. Insights on the pathology of kidney stone formation. Urol. Res. doi:10.1007/s00240-005-0488-0. [DOI] [PubMed]