Cdc42 Is Not Essential for Filopodium Formation, Directed Migration, Cell Polarization, and Mitosis in Fibroblastoid Cells (original) (raw)
Abstract
Cdc42 is a small GTPase involved in the regulation of the cytoskeleton and cell polarity. To test whether Cdc42 has an essential role in the formation of filopodia or directed cell migration, we generated Cdc42-deficient fibroblastoid cells by conditional gene inactivation. We report here that loss of Cdc42 did not affect filopodium or lamellipodium formation and had no significant influence on the speed of directed migration nor on mitosis. Cdc42-deficient cells displayed a more elongated cell shape and had a reduced area. Furthermore, directionality during migration and reorientation of the Golgi apparatus into the direction of migration was decreased. However, expression of dominant negative Cdc42 in Cdc42-null cells resulted in strongly reduced directed migration, severely reduced single cell directionality, and complete loss of Golgi polarization and of directionality of protrusion formation toward the wound, as well as membrane blebbing. Thus, our data show that besides Cdc42 additional GTPases of the Rho-family, which share GEFs with Cdc42, are involved in the establishment and maintenance of cell polarity during directed migration.
INTRODUCTION
Cdc42 is an ubiquitously expressed protein that belongs to the family of Rho GTPases (Bishop and Hall, 2000). Rho GTPases control the organization of the actin and microtubule cytoskeleton, proliferation, apoptosis, membrane transport, and gene expression. They exist in an inactive GDP-bound and an active GTP-bound state. In their active conformation they can interact with more than 60 effectors regulating multiple signal transduction pathways. Activation of Rho GTPases is mediated by guanine nucleotide exchange factors (GEFs) that catalyze the replacement of GDP by GTP. Only a fraction of the more than 80 GEFs known to date have been characterized in detail, but it is clear that many GEFs can activate more than one Rho GT-Pase (Schmidt and Hall, 2002).
Expression of constitutively active Cdc42 induces the formation of actively protruding filopodia with or without concomitant lamellipodium formation depending on the cell type. Conversely, expression of dominant negative Cdc42 (dnCdc42) was described to prevent filopodium formation in Swiss 3T3 cells and primary fibroblasts (Kozma et al., 1995; Nobes and Hall, 1995, 1999). Moreover, Cdc42-deficient embryonic stem (ES) cells were reported to lack spike-like protrusions, which were readily observed in wild-type ES cells (Chen et al., 2000).
Recently, other Rho GTPases have also been reported to induce filopodia (Neudauer et al., 1998; Murphy et al., 1999; Ellis and Mellor, 2000; Vignal et al., 2000; Tao et al., 2001; Abe et al., 2003; Aspenström et al., 2004). A constitutively active form of Rif was suggested to induce filopodia in a Cdc42-independent manner, because expression of dnCdc42 did not interfere with these protrusions (Ellis and Mellor, 2000). In contrast, RhoG-induced filopodium formation was shown to be dependent on Cdc42 (Gauthier-Rouvière et al., 1998). Whether Cdc42 is required for filopodium formation induced by TC10, TCL, RhoD, or Wrch-1 is currently unknown.
A significant body of evidence has indicated a crucial role for Cdc42 in cell migration (reviewed by Fukata et al., 2003). Dominant negative inhibition of Cdc42 in migrating primary fibroblasts and astrocytes results in impaired directed migration and defective polarization of lamellipodial activity and Golgi reorientation (Nobes and Hall, 1999; Etienne-Manneville and Hall, 2001). In astrocytes, Cdc42 regulates the polarization of the microtubule cytoskeleton via Par6-protein kinase Cζ-mediated phosphorylation of GSK3β (Etienne-Manneville and Hall, 2001).
Cdc42 has cell-type specific effects on the regulation of certain signaling pathways. In several fibroblastoid cell lines, Cdc42 activation correlated with the activation of JNK, Erk, and p38 (Frost et al., 1997; Bishop and Hall, 2000). In contrast, dominant negative inhibition of Cdc42 in primary fibroblasts was shown to result in upregulation of Akt and Erk activity (Zugasti et al., 2001). In ES cells, loss of Cdc42 had no influence on basal and serum stimulated activation of Erk, JNK, and p38 (Chen et al., 2000).
Dominant negative mutant forms of the Rho GTPases have been essential for the analysis of Rho GTPase function in cells (Feig, 1999). These molecules bind the corresponding activating GEFs with higher affinity than wild-type Rho GTPases but cannot interact with effector molecules. The use of certain dominant negative mutants however may have disadvantages. The degree of inhibition is dependent on the expression level of the dominant negative mutant (Braga et al., 2000). Moreover, because many GEFs are not specific for a single Rho GTPase, dominant negative Rho GTPases may also inhibit the activation of other Rho GTPases (Schmidt and Hall, 2002).
To overcome these problems and to test whether Cdc42 is essential for filopodium formation and directed cell migration, we generated fibroblastoid cells that lack a functional Cdc42 gene. In addition, we used these cells to experimentally assess the specificity of dominant negative inhibition by transducing Cdc42-deficient cells with dominant negative Cdc42 (dnCdc42).
MATERIALS AND METHODS
Cell Lines
ES cells were modified by two rounds of homologous recombination to obtain (fl/–) and (–/–) cells using a targeting construct for a conditional inactivation of the Cdc42 gene (Wu et al., unpublished data). Cells carrying a conditional and a null allele (fl/–) were further differentiated in vitro in the presence of 0.5% dimethyl sulfoxide, immortalized by retroviral transduction of the SV40 large T antigen, and cloned (Cdc42(fl/–)). By adenoviral transduction of the cre recombinase, Cdc42-null cells were obtained and cloned (Cdc42(–/–)). These cells were then infected with a retrovirus expressing dnCdc42 (Cdc42(–/– + N17)) or wild-type Cdc42 (Cdc42(–/– + wt)) and a hygromycin resistance gene. Fibroblastoid cells were grown in DMEM, 10% fetal calf serum (FCS), and 5% CO2 at 37°C and supplemented with 100 μg/ml hygromycin B if required. Before serum induction with normal growth medium, confluent cells were starved for 12 h with DMEM, 0.5% FCS. Endodermal-like cell lines were differentiated from Cdc42(fl/–) and Cdc42 (–/–) ES cells by transfection of Gata-4 expression vector into this cells (Fujikura et al., 2002 and Wu et al., unpublished data). ES cells and the endodermal cell lines were grown in DMEM, 20% FCS, high glucose supplemented with 0.1 mM β-mercaptoethanol, nonessential amino acids, sodium pyruvate, and 1000 U/ml leukemia inhibitory factor at 5% CO2 and 37°C. For all experiments two independent clones of cells between passage 4 and 18 were used.
FACS Analysis and Adhesion Assay
To determine integrin expression levels by FACS, Cdc42(fl/–), and Cdc42(–/–) cells were trypsinized and stained with antibodies against β1, β3, α1, α2, α5, α6, and αv integrin (all BD Biosciences, Heidelberg, Germany), which were either fluorescently labeled (β1, α6), biotinylated (β3, α5, αv), or unconjugated (α1, α2). Biotinylated antibodies were detected with streptavidin-Cy5 and unconjugated antibodies by FITC-conjugated rat anti-hamster antibodies (Jackson ImmunoResearch, Soham, Cambridgeshire, United Kingdom). To assess autofluorescence and nonspecific staining cells were stained with streptavidin-Cy5 (β3, α5, αv), FITC-conjugated rat anti-hamster antibodies (α1, α2), or were measured unstained (β1, α6). Cell adhesion assays were performed using vitronectin (from human placenta; Yatohgo et al., 1988), fibronectin (Behringwerke, Marburg, Germany), laminin-1 (Paulsson et al., 1987) and collagen I (Cohesion, Palo Alto, CA) in coated 96-wells at a concentration of 0.05–50 μg/ml per well. All wells were blocked with 2% bovine serum albumin in phosphate-buffered saline (PBS), pH 7.4, containing 1 mM Ca2+ and 1 mM Mg2+. Cells were allowed to attach to the wells for 60 min at 37°C. Then nonadherent cells were washed away. Remaining cells were fixed with 70% ethanol, stained with 50 μl 5 mg/ml crystal violet in 20% methanol, washed with PBS, and extracted with 50 ml 0.1 M sodium citrate, pH 4.2. Microplates were colorimetrically evaluated at 562 nm.
Immunofluorescence, Western Blotting, TUNEL Assay, and Pulldown Analysis
Immunofluorescence and Western blotting were carried out according to standard protocols. The following antibodies were used: anti-α-tubulin (clone YL1/2; obtained from Jürgen Wehland, Braunschweig, Germany), anti-Rac1, anti-Cdc42, anti-Gsk-3β, anti-GM130, anti-β-catenin, anti-paxillin (all from BD Biosciences), anti-phosho-p38 (pTpY 180/182), anti-p38, anti-phospho-JNK1&2 (pTpY 183/185), anti-JNK1 (all from Biosource, Solingen, Germany), anti-phosho-Akt (Ser473), anti-Akt, anti-phospho-Gsk-3β (Ser21/9), anti-p44/42 MAP kinase, anti-phospho-p44/42 MAP kinase (Thr202/204; all from Cell Signaling, Beverly, MA). Cy3- or HRP-conjugated secondary antibodies were used for detection (Jackson ImmunoResearch). Phalloidin Alexa 488 was used to stain actin filaments (Molecular Probes, Karlsruhe, Germany). Nuclei were stained with DAPI. Apoptotic cells were detected by TUNEL assay (Roche, Penzberg, Germany). Fluorescent images were taken with a Leica DMRA2 microscope equipped with NPLAN 40×/(0.65 NA) objective and a Leica DMRA2 camera (Solms, Germany). Images were processed with Photoshop (Adobe, San Jose, CA). To determine the amount of active GTP-bound Rac1 and RhoA, pulldown assays were carried out as described (Zondag et al., 2000).
In Vitro Wound Healing Assay
For wound healing assays, cells were seeded at high density on six-well plates in growth medium and wounded 1 d later by scraping across the confluent monolayers. Wound closure speed was measured every 2 or 0.5 h (fibroblastoid and endodermal cells, respectively) in 10–15 randomly chosen regions of the wound (width: 300–400 or 150–250 μm for fibroblastoid and endodermal cells, respectively) and normalized to Cdc42(–/–) cells after 8 or 3.5 h (for fibroblastoid and endodermal cells, respectively). At these time points, the wound edges were just before touching. Activation of signaling molecules induced by wounding was analyzed as described (Etienne-Manneville and Hall, 2003).
Polarization of the Golgi Apparatus
Polarization of the Golgi apparatus into the direction of migration was determined as described previously (Nobes and Hall, 1999). Because a 120° sector was chosen to define polarization, 33.3% of cells will have the Golgi apparatus (detected by anti-GM130 monoclonal antibody) located within this sector in case of random polarization. In each experiment, 100–500 cells from within the first row of migrating cells were examined.
Time-lapse Microscopy
Phase-contrast and fluorescence video microscopy were performed as described (Rottner et al., 1999). For some experiments, cells were transiently transfected with constructs driving the expression of EGFP-VASP (Carl et al., 1999) or EGFP-β-actin (BD Biosciences) using FuGENE6 (Roche) and plated on fibronectin-coated (50 μg/ml, Roche) glass coverslips for high-magnification videomicroscopy. To perform single-cell analysis of migrating cells, nuclei were stained for 30 min with 1 μg/ml Hoechst 33342 (Sigma, Munich, Germany) before fluorescence videomicroscopy and tracked using MetaMorph 6.0 software (MetaSystems, Belmont, MA).
DNA Microarray Hybridization and Analysis
Quality and integrity of the total RNA isolated from 2 × 106 fibroblastoid cells for each cell line were controlled by running all samples on an Agilent Technologies 2100 Bioanalyzer (Agilent Technologies, Waldbronn, Germany). For biotin-labeled target synthesis starting from 3 μg of total RNA, reactions were performed using standard protocols supplied by the manufacturer (Affymetrix, Santa Clara, CA). Briefly, 5 μg total RNA was converted to dsDNA using 100 pmol of a T7T23V primer (Eurogentec, Seraing, Belgium) containing a T7 promotor. The cDNA was then used directly in an in vitro transcription reaction in the presence of biotinylated nucleotides.
The concentration of biotin-labeled cRNA was determined by UV absorbance. In all cases, 12.5 μg of each biotinylated cRNA preparation were fragmented and placed in a hybridization cocktail containing four biotinylated hybridization controls (BioB, BioC, BioD, and Cre) as recommended by the manufacturer. Samples were hybridized to an identical lot of Affymetrix MOE430A for 16 h. After hybridization, the GeneChips were washed, stained with SA-PE and read using an Affymetrix GeneChip fluidic station and scanner. Gene expression levels were determined by means of Affymetrix's Microarray Suite 5.0 (MAS 5.0).
Online Supplementary Material
Supplementary Figure 1 shows normal spreading kinetics in Cdc42-null cells. Supplementary Figure 2 shows quantification of experiments described in Figures 1C and 5B. Quantification of chemiluminescence signals was performed by using a CCD camera (LAS 1000, Fujifilm, Tokyo, Japan) and the software programs Image Reader LAS 1000 V1.1 and Image Gauge V3.01 (Fujifilm). Asterisks indicate significant increase of the signal (p < 0.05) between time points 0 and 5 min within a given cell line, whereas two asterisks indicate significant difference (p < 0.05) between the induction after 5 and 30 min. Supplementary Figure 3 shows β-catenin stabilization at the leading edge of migrating fibroblastoid cells, irrespective of their genotype. Supplementary Movie 1 shows normal filopodium and lamellipodium formation of Cdc42-null cells expressing GFP-actin (scale ba, 5 μm). Supplementary Movie 2 demonstrates membrane blebbing in Cdc42(–/– + N17) cells (scale bar, 50 μm). Supplementary Movies 3–5 show unstable direction of cell migration in Cdc42(–/– + N17) cells compared with Cdc42(fl/–) and Cdc42(–/–) cells (scale bar, 100 μm). Supplementary Movies 6 and 7 show filopodium formation in Cdc42(fl/–) and Cdc42(–/–) embryonic stem cells (scale bar, 10 μm).
Figure 1.
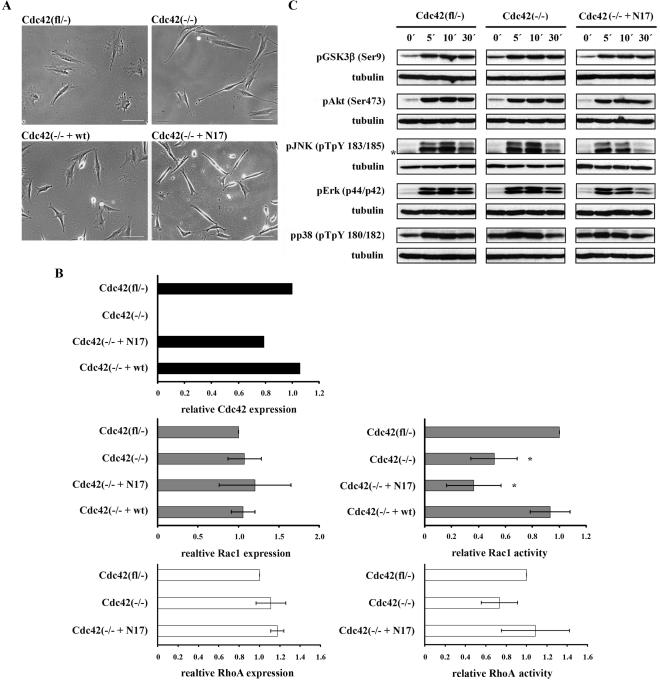
Cdc42 mutant cell lines display morphological alterations and reduced Rac1 activity. (A) Phase-contrast images of fibroblastoid cell lines studied. Note the spindle-shape morphology of Cdc42(–/–) and Cdc42(–/– + N17) mutants and rescue of the mutant morphology in Cdc42(–/– + wt) cells (scale bar, 100 μm). (B) Top: quantification of Western blot analyses confirming loss of Cdc42 expression in Cdc42(–/–) cells and reexpression of Cdc42 in Cdc42(–/– + N17) and Cdc42(–/– + wt) cell lines. Middle: Rac1 activity relative to total Rac1 as determined by pulldown experiments demonstrating decreased Rac1 activity in Cdc42(–/–) cells (n = 5), a further reduction in Cdc42(–/– + N17) cells (n = 3), and normal levels in Cdc42(–/– + wt) cells (n = 2), relative to Cdc42(fl/–) cells (n = 5). Expression levels of Rac1 were similar in all these cell lines (n = 3). *p < 0.05, compared with control cells. Bottom: RhoA activity as assessed by pulldown assay is similar in control and mutant cell lines and thus does not correlate with observed morphological alterations (n = 3). Total RhoA levels are not significantly different (n = 3). (C) Western blot analysis of starved confluent cells, stimulated with serum as indicated. Representative results of three independent experiments are shown. Asterisk denotes an unspecific band.
Figure 5.
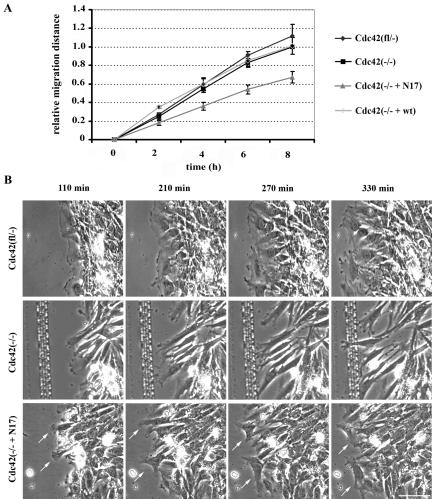
Cdc42 is not essential for directed cell migration. (A) Relative migration of Cdc42(fl/–), Cdc42(–/–), Cdc42(–/– + wt), and Cdc42(–/– + N17) cells in a wounding assay (n = 5). Only Cdc42(–/– + N17) cells show a significantly reduced directed migration. (B) Phase-contrast time-lapse microscopy of Cdc42(fl/–), Cdc42(–/–), and Cdc42(–/– + N17) cells during a scratch assay, demonstrating stable polarization of lamellipodial activity oriented toward the scratch in Cdc42(fl/–) and Cdc42(–/–) cells and instable polarization in the Cdc42(–/–+ N17) cells with lamellipodial protrusions also pointing into other directions than the scratch (arrows; scale bar, 100 μm).
Supplementary Table 1 shows quantification of cell area, elongation ratio, and tail length in Cdc42(fl/–) cells and both mutant fibroblastoid cell lines. Quantification was performed using MetaMorph 6.0 software.
RESULTS
Generation of Cdc42-deficient and Reconstituted Fibroblastoid Cell Lines
Cdc42-deficient fibroblastoid cells were generated by differentiation and immortalization of ES cells with a conditional inactivation of the Cdc42 gene (Cdc42(fl/–)) and subsequent deletion of the Cdc42 gene by adenoviral transduction of the cre recombinase (Cdc42(–/–)). Analysis of isolated clones by Southern and Western blotting confirmed the gene deletion and the absence of Cdc42 protein (Figure 1B and Wu et al., unpublished data). These Cdc42-deficient cells were then reconstituted with either wild-type Cdc42 (Cdc42(–/– + wt)) or dnCdc42 (Cdc42(–/– + N17); Figure 1B). For all experiments two independent clones of each mutant cell line that behaved virtually identical were analyzed.
Altered Morphology, but Normal Adhesion of Cdc42-null Cells
Although the parental Cdc42(fl/–) cells displayed a well-spread polygonal morphology, Cdc42-null clones and Cdc42-null clones expressing dnCdc42 (Cdc42(–/– + N17)) were less spread and more spindle-shaped (Figure 1A and Supplementary Table 1). Compared with Cdc42(fl/–) control cells (5641 ± 1423 μm2), cell area was significantly reduced in Cdc42(–/–) cells (3184 ± 1024 μm2) and even further in Cdc42(–/– + N17) cells (1544 ± 449 μm2). Cell elongation as determined by the ratio of long-to-short cell axis, increased from control (2.7 ± 1.1) to Cdc42-null (5.7 ± 1.1) and Cdc42(–/– + N17) cells (6.1 ± 3.6). Expression of wild-type Cdc42 in Cdc42-deficient cells (Cdc42(–/– + wt)) restored the parental phenotype (Figure 1A). The expression level of wt Cdc42 transduced into null cells was comparable to endogenous Cdc42 in Cdc42 (fl/–) cells, whereas that of dnCdc42 was slightly lower (Figure 1B).
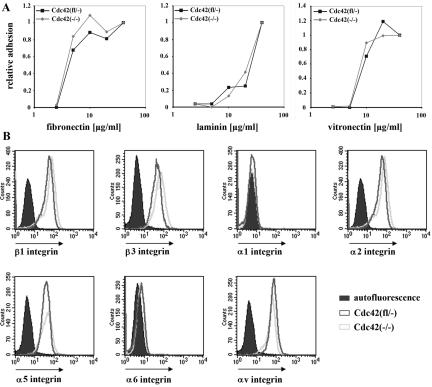
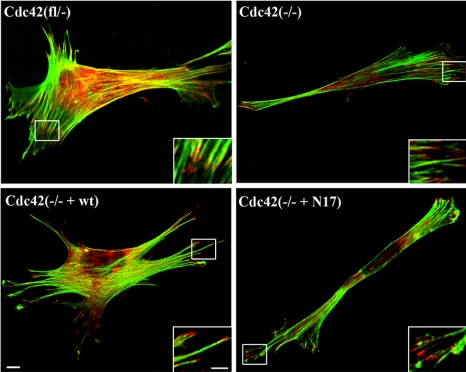
When growing close to confluency, proliferation of Cdc42(fl/–), Cdc42(–/–), Cdc42(–/– + wt), and Cdc42(–/– + N17) clones was comparable, whereas when seeded sparsely, Cdc42(–/– + N17) clones were growing more slowly than the other cell types (unpublished data). In contrast to a previous report (Chen et al., 2000), cell adhesion of Cdc42-deficient cells on laminin, fibronectin, and vitronectin was similar to the parental cells and not significantly altered (Figure 2A). Adhesion to collagen was poor in both Cdc42-null and parental cells, most presumably because of the absence of the collagen-binding integrin receptors α1β1 and α2β1 on both cell types as determined by FACS analysis (unpublished data and Figure 2B). Expression of α5, αv, β1, and β3 integrin were similar in Cdc42-deficient and control cells (Figure 2B). Analysis of paxillin by immunofluorescent staining and of the actin cytoskeleton by FITC-conjugated phalloidin revealed focal adhesion formation and stress fibers in Cdc42(fl/–), Cdc42(–/–), Cdc42(–/– + N17), and Cdc42(–/– + wt) cells (Figure 3). In Cdc42-deficient cells, nearly all stress fibers appeared to be parallel to each other and to the long axis of the cell, whereas in control cells differentially oriented groups of stress fibers could be identified.
Figure 2.
Comparable adhesion and spreading kinetics and normal integrin expression in the absence of Cdc42. (A) Cell adhesion to different ECM components normalized to the adhesion of the Cdc42(fl/–) cells (n = 2). (B) Integrin expression is not changed in the absence of Cdc42; the background staining is identical for both cell lines.
Figure 3.
Differentially organized cytoskeleton in Cdc42 mutant cells. Cdc42(–/–) and Cdc42(–/– + N17) mutants form focal contacts but display actin organization rather different from the control (fl/–) and reconstituted (–/– + wt) cell lines as determined by paxillin (red) and phalloidin (green) staining (scale bar, 10 μm). Insets show enlargements of the focal contacts at the tips of actin filaments (scale bar, 5 μm).
To assess the time course of spreading, we compared Cdc42(fl/–) and Cdc42(–/–) cells 5, 15, 30, 60, 120, 240 min, and 24 h after seeding on a plastic culture dish. After 15 min, the majority of cells of both lines was adherent (Supplementary Figure 1 and unpublished data). No obvious difference in the kinetics of spreading was observed in Cdc42-deficient fibroblastoid cells compared with controls (Supplementary Figure 1).
Defective Rac1 Activation in the Absence of Cdc42
Cdc42 was previously demonstrated to activate Erk, JNK, p38, Akt, and GSK3β, although cell type specific differences were reported (Frost et al., 1997; Chen et al., 2000; Zugasti et al., 2001). We therefore tested the basal and serum stimulated levels of Erk, JNK, p38, Akt, and GSK3β in the fibroblastoid Cdc42 (fl/–), Cdc42 (–/–), and Cdc42 (–/– + N17) cell lines. All cell lines showed significantly induced phosphorylation of Erk, JNK, Akt, GSK3β, and p38 (Figure 1C; Supplementary Figure 2A). In Cdc42(–/– + N17) cells, however, the activation of Erk decayed significantly faster than in control and null cells. The stimulation of JNK tended to decrease more quickly in both mutant cell lines. Total amounts of these proteins were comparable in control and mutant cells and did not change throughout the course of the experiments (unpublished data). These data indicate that loss or dominant negative inhibition of Cdc42 has only limited influence on serum-induced signaling pathways in fibroblastoid cells.
Cdc42 was shown to induce lamellipodia that are inhibited by dnRac1, suggesting that Cdc42 can activate Rac1 (Nobes and Hall, 1995). However, it remained unknown to what extent Rac1 activity is dependent on Cdc42. In our cell lines, loss of Cdc42 reduced the relative level of activated Rac1-GTP to 52% (Cdc42(–/–); Figure 1B). This shows that in fibroblasts a substantial part of Rac1 activity is dependent on Cdc42. Reexpression of wt Cdc42 restored Rac1-GTP levels (Cdc42(–/– + wt), but expression of dnCdc42 decreased Rac1 activity further to 36% (Cdc42(–/– + N17; Figure 1B). Rac1 expression was not altered in the absence of Cdc42 or in the presence of dnCdc42 (Figure 1B). These data suggest that dnCdc42 decreases Rac1 activation not only by blocking Cdc42-dependent activation of Rac1, but also by other pathways, most likely by the inhibition of GEFs, which can activate both Cdc42 and Rac1.
Transient overexpression of constitutively active Rac1 in Cdc42-null cells increased cell spreading and promoted a migratory phenotype with strong lamellipodium formation, thus not rescuing the phenotype (unpublished data). Expression and activity of RhoA was not altered in Cdc42(–/–) and Cdc42(–/–+ N17) compared with control cells (Figure 1B), suggesting negligible activation of GEFs, which can activate both Cdc42 or RhoA under normal growth conditions.
To reveal the expression levels of mRNAs coding for Rho GTPases expressed in the fibroblastoid cells, we performed an array expression analysis of 2 Cdc42(fl/–) and 2 Cdc42(–/–) clones (Affymetrix). All cell lines expressed high amounts of RhoA, RhoB, RhoC, Rac1, and Cdc42 (in case of Cdc42(–/–) cells, the mRNA will not result in the expression of functional protein), medium levels of TC10, Wrch-2, Rnd3, RhoG, and Miro-1, and low or no detectable levels of Rac2, Rac3, TCL, Wrch-1, RhoH, Miro-2, and RhoD. No significant change in Rho GTPase expression was found between control und mutant cell lines. Rif, Rnd1, and Rnd2 were not present on the array.
Cdc42-null Cells Have No Mitosis Defect
Dominant negative inhibition of Cdc42 in HeLa cells was demonstrated to result in a high amount of multinucleated cells, indicating a crucial role of Cdc42 in chromosome segregation (Yasuda et al., 2004). To test whether Cdc42-null cells displayed any mitosis defect, we seeded our cells at low density, stained DNA with DAPI, and counted the percentage of cells with aberrant or multiple nuclei. Of 1300 cells of each cell line counted 24 h after plating, Cdc42(fl/–) and Cdc42(–/–) cells displayed similar percentages of cells with aberrant or multiple nuclei, with 4.2 ± 1.5 and 3.5 ± 1.6%, respectively. In Cdc42(–/– + N17) the frequency of cells with aberrant nuclei increased to 6.9 ± 2.9%. This difference, however, was not significant (p > 0.05) when compared with control cells. Clearly, Cdc42 is not required for normal chromosome segregation, whereas dominant negative inhibition might lead to mitosis defects.
Loss of Cdc42 Does Not Affect the Formation of Filopodia and Lamellipodia
Expression of dnCdc42 in primary rat embryonic fibroblasts and Swiss 3T3 cells was shown to block the formation of filopodia (Kozma et al., 1995; Nobes and Hall, 1995) and Cdc42-null ES cells generated by Chen et al. (2000) were reported to be incapable of formation of any peripheral protrusion including filopodia. Surprisingly, inactivation of the Cdc42 gene in our fibroblastoid cells did not prevent the formation of filopodia or lamellipodia as determined by phase-contrast time-lapse microscopy, which most directly allows distinction between actively protruding filopodia and other nonprotrusive peripheral structures such as retraction fibers.
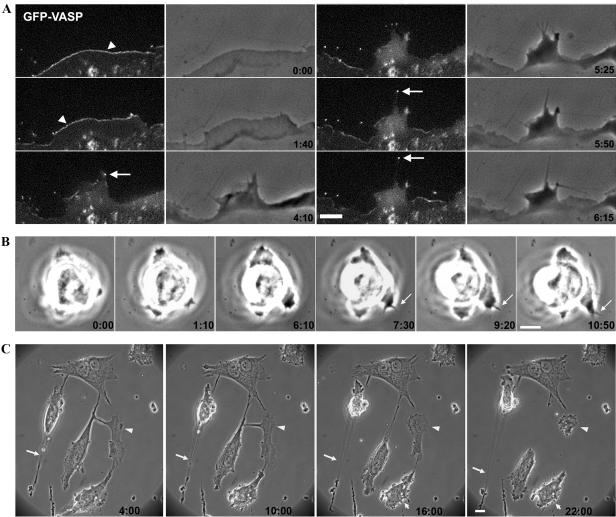
Examination of the dynamics of these protrusions by fluorescence videomicroscopy in cells expressing GFP-actin or GFP-VASP, which is present at the tips of these protrusive structures (Rottner et al., 1999), confirmed normal lamellipodium and filopodium formation in Cdc42(–/–) cells (Figure 4A; Supplementary Movie 1). In addition, these data indicated recruitment of prominent components of the actin polymerization machinery such as Ena/VASP proteins to the tips of these protrusions as in control cells.
Figure 4.
Loss of Cdc42 does not abrogate lamellipodia and filopodia protrusions. (A) Fluorescence and phase-contrast videomicroscopy (min:sec) taken from a EGFP-VASP-expressing Cdc42-null cell revealing normal recruitment of this protein to the tips of lamellipodia (arrowheads) and filopodia (arrows; scale bar, 5 μm). (B) Phase-contrast videomicroscopy (min:sec) demonstrating formation of a filopodium (arrows) in Cdc42 null ES cells (scale bar, 10 μm). (C) Phase-contrast videomicroscopy of cells expressing dnCdc42 displaying prominent membrane blebbing (small arrows). Note also the lack of proper retraction of the trailing edge, which is commonly left behind (arrows), causing the frequent formation of a-nucleated cytoplasts (arrowheads; scale bar, 10 μm).
Expression of dnCdc42 in Cdc42-null cells resulted in membrane blebbing in 47% of cells (n = 28), whereas only 18% of the Cdc42-deficient cells (n = 22) and 7% of the parental cells analyzed (n = 16) showed blebbing during 6 h of observation (Figure 4C; Supplementary Movie 2 and unpublished data). Testing subconfluently seeded Cdc42(fl/–), Cdc42(–/–), and Cdc42(–/– + N17) cells by TUNEL staining 48 h after seeding, we could not find any detectable TUNEL positive, apoptotic cells. These data suggest that the blebbing observed in Cdc42-deficient cells is not related to programmed cell death.
Defining the tail as a thin, trailing end opposite to the leading edge, we found that the tail length increased from Cdc42(fl/–) to Cdc42(–/–) to Cdc42(–/– + N17) cells (Supplementary Table 1). Because of high cell-to-cell variations, however, the difference was not significant between control and null cells, yet the tail length was significantly reduced when Cdc42(fl/–) and Cdc42(–/– + N17) cells were compared. These data indicate that loss of Cdc42 or expression of dnCdc42 may interfere with proper tail retraction. This interpretation is corroborated by the observation that dnCdc42 expressing cells frequently lost their trailing edge, because of either defective rear detachment or reduced rigidity of the cell body (Figure 4C).
In spite of these morphological alterations, filopodium formation was still observed in dnCdc42 expressing cells and lamellipodia were still present. This does not exclude that at higher amounts of dnCdc42, filopodium, or lamellipodium formation might be completely abrogated.
To confirm that filopodium formation observed in the absence of Cdc42 is not restricted to fibroblastoid cells, we analyzed protrusion formation of Cdc42(–/–) ES cells by time-lapse video-microscopy. Individual Cdc42-null ES cells attached to glass coated with collagen I had a round, smooth surface similar to control ES cells and showed frequent formation of lamellipodia and filopodia (Figure 4B and Supplementary Movies 6 and 7). These data suggest that Cdc42 is not essential for filopodium formation irrespective of cell type or transformation.
Cdc42 Is Not Crucial for Directed Migration
In previous studies, primary fibroblasts expressing dnCdc42 or WASp(201–321), which binds and inhibits Cdc42, showed a 50% reduction of wound closure in an in vitro wound closure assay, suggesting an important function of Cdc42 in directed migration (Nobes and Hall, 1999).
Fibroblastoid Cdc42(–/–) cells, however, displayed a wound closure speed highly comparable to Cdc42(fl/–) and Cdc42(–/– + wt) cells, demonstrating that Cdc42 is not crucial for directed migration (Figure 5A).
In contrast, expression of dnCdc42 in Cdc42(–/–) cells resulted in a 45% decrease of wound closure speed, revealing that Rho GTPases other than Cdc42 contribute to directed migration (Figure 5A).
After staining the nuclei of living cells with Hoechst 33342 (Haraguchi et al., 1999), we tracked the paths of individual cells and analyzed them statistically. Cdc42(fl/–) and Cdc42(–/–) cells showed similar average velocities (Cdc42(fl/–): 1.0 ± 0.28 μm/min; Cdc42(–/–): 1.0 ± 0.17 μm/min; n = 60), but Cdc42(–/– + N17) cells had a strongly reduced migration speed (0.7 ± 0.12 μm/min; n = 60, p < 0.05 vs. control and null cells).
Cdc42 Is Not Essential for Cell Polarization in Migrating Fibroblasts, but Contributes to the Reorientation of the Golgi
In primary rat fibroblasts, expression of dnCdc42 was found to result in a complete loss of cell polarization, with lamellipodial activity seen all around the cell periphery (Nobes and Hall, 1999). Furthermore, a complete loss of orientation of the Golgi apparatus into the direction of migration was observed, indicating an impaired polarization of the microtubule system, which, however, was not required for the directed migration of fibroblasts.
Time-lapse analysis of our Cdc42(fl/–) cells in an in vitro wounding assay showed cell polarization characterized by lamellipodium formation at the leading edge, but not at the trailing edge stably oriented toward the wound during 6 h of observation (Figure 5B; Supplementary Movie 3). In addition, the Golgi apparatus slowly oriented into the direction of migration (Figure 7A).
Figure 7.
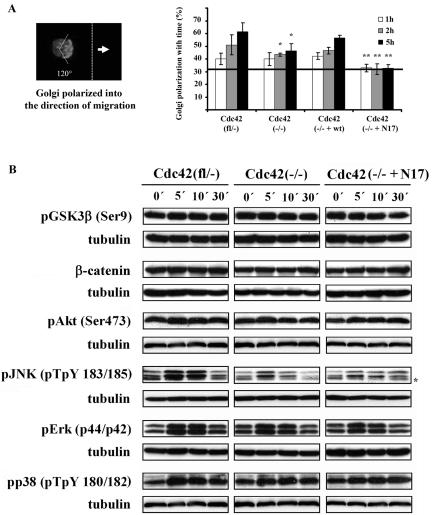
Golgi reorientation is partially dependent on Cdc42, but independent of Gsk3β phosphorylation. (A) Percentage of cells with their Golgi apparatus oriented to the direction of migration (random polarization of 33.3% is indicated by solid line). Cdc42(–/–) cells showed a 50% reduction of nonrandomly polarized cells at 5 h of migration, whereas Cdc42(–/–+ N17) cells showed no nonrandom polarization at all (n = 5). Results significantly different from control cells; *p < 0.05); results significantly different from control and null cells; **p < 0.05). (B) Western blot analysis of signal transduction in migrating Cdc42(fl/–), Cdc42(–/–), and Cdc42(–/– + N17) cells at different time points after wounding. Representative results of three independent experiments are shown. Asterisk denotes an unspecific band.
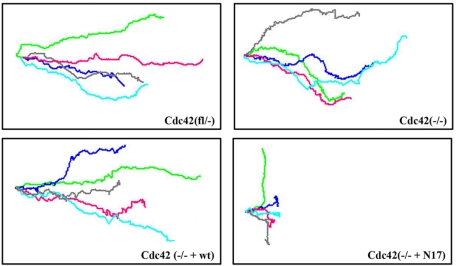
Unexpectedly however, also in the absence of Cdc42, protrusion formation was polarized with rather stable directionality (Figure 5B; Supplementary Movie 4). When overlaying the migration paths of different migrating cells, it became clear that the directionality of Cdc42(–/–) cells is slightly lower than that of control cells (Figure 6). Mutant cells seem to sense the direction toward the wound, but to be less able to maintain it. A sensitive parameter for directionality is the mean angle vector, which obtains the value of 1 for a straight line and the value of 0 for random walk. Cdc42(–/–) cells had a significantly decreased mean angle vector compared with control cells (Cdc42(fl/–): 0.82 ± 0.1; n = 60; Cdc42(–/–): 0.55 ± 0.11; n = 60, p < 0.05 vs. control cells). Nonrandom polarization of the Golgi apparatus was reduced by 50% 5 h after wounding in Cdc42-deficient cells (Figure 7A), indicating a partial role of Cdc42 in this aspect of cell polarization during migration.
Figure 6.
Cdc42 influences single-cell directionality. Single-cell tracking of migrating cells revealed stable directionality of Cdc42(fl/–) and Cdc42(–/– + wt) rescued cells, reduced directionality of Cdc42(–/–) cells and further reduction in Cdc42(–/– + N17) cells. Lines represent individual cell tracks; five representative tracks per cell line are shown (n = 60).
Videomicroscopy revealed that Cdc42(–/– + N17) cells could polarize, but that the protrusion formation was not stably oriented toward the wound (Figure 5B, arrows; Supplementary Movie 5). As revealed by overlays of the migration paths of different dnCdc42-expressing cells, these mutant cells often stopped and migrated backward or sidewards (Figure 6). This resulted in a significantly reduced migration speed of individual Cdc42(–/– + N17) cells and mean angle vector (0.38 ± 0.14; n = 60, p < 0.05 vs. control and null cells) compared with Cdc42-deficient cells. Furthermore, expression of dnCdc42 in Cdc42-deficient cells completely abrogated polarization of the Golgi apparatus, similar to the data reported for primary fibroblasts (Figure 7A).
Expression of wild-type Cdc42 in Cdc42-null cells restored reorientation of the Golgi and single-cell directionality to levels close to control cells (Figures 6 and 7A; mean angle vector: 0.71 ± 0.07; n = 60).
Golgi Reorientation Is Not Dependent on Induced Phosphorylation of GSK3β
In astrocytes, the polarization of the Golgi is reportedly controlled via Cdc42-mediated phosphorylation of GSK3β. Phosphorylation of GSK3β was also required for the stabilization of β-catenin and its translocation to the leading edge (Etienne-Manneville and Hall, 2003). However, Cdc42(fl/–) cells did not display a detectable change in GSK3β phosphorylation after wounding (Figure 7B; Supplementary Figure 2B), suggesting that in fibroblasts Golgi reorientation does not require the induction of GSK3β phosphorylation. Furthermore, there was no obvious stabilization of β-catenin (Figure 7B; Supplementary Figure 2B). Targeting of β-catenin to the leading edge occurred in all cell types tested (Supplementary Figure 3), indicating that subcellular positioning of this protein neither requires stabilization of β-catenin nor GSK3β phosphorylation. Apparently, different pathways are used to regulate the reorientation of the Golgi apparatus during directed migration of our fibroblastoid cells versus astrocytes.
To assess how the activation of known downstream effectors of Cdc42 during wounding correlates with the observed phenotypes, we determined the relative activation of Erk, p38, JNK, and Akt, at different time points after wounding. In three independent experiments, wounding of the monolayer induced phosphorylation of Erk and JNK in control and mutant cells, although the changes were not significant. Phosphorylation of Akt and p38 were not changed (Figure 7B; Supplementary Figure 2B). Activation of JNK tended to be reduced and to decrease more quickly in Cdc42(–/–) and Cdc42(–/– + N17) cells, although the relevance of these observations remains to be determined. These data do not reveal a striking correlation of the strong defects in polarization and migration observed in Cdc42(–/– + N17) cells with changes in the activation of Erk, JNK, p38, or Akt.
As described above Cdc42(–/–) and Cdc42(–/–+ N17) cells have severely reduced basal levels of active Rac1. To test whether Rac1 is induced upon migration, we wounded control Cdc42(fl/–) cells and performed pulldown assays after 5 and 30 min. We could not observe a significant change in Rac1 activity at any of the time points tested (unpublished data), indicating that either Rac1 activity is not changing during migration or more likely that the changes are locally restricted and not detectable in the total lysates.
Cdc42 Is Not Required for Efficient Wound Closure and Golgi Reorientation during Migration of Endodermal Cells
Because of the unexpected ability of Cdc42-deficient fibroblastoid cell lines to polarize, we wondered whether this observation is restricted to transformed fibroblastoid cells or could also be made with other, preferentially untransformed cell types. ES cells are not transformed, but migrate only poorly. We therefore forced the ES cells to differentiate into the endodermal lineage by transfecting them with a GATA-4 transcription factor encoding expression vector. Such untransformed endodermal cells are highly migratory, but have significantly less cell-cell contacts than fibroblasts. Western blot analysis confirmed the presence of Cdc42 in control and the absence of Cdc42 in mutant endodermal cells (Wu et al., unpublished data).
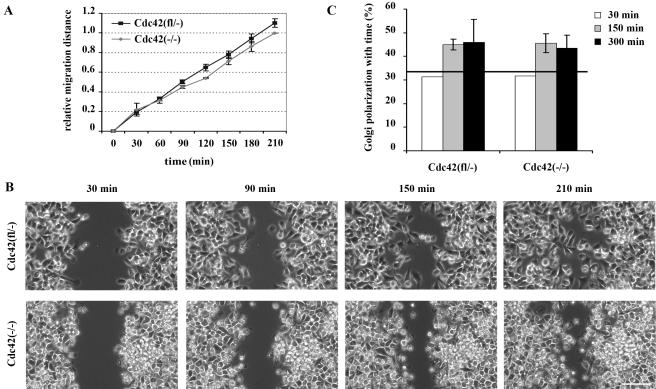
Cdc42-null endodermal cells had a morphology similar to that of Cdc42(fl/–) control cells, although in highly dense monolayers mutant cells were less attached to each other than to control cells (Wu et al., unpublished observations). After wounding, control and mutant cells closed the gap with similar speed (Figure 8, A and B). Measuring the average velocity of individually migrating cells, no significant difference was found between Cdc42(fl/–) and Cdc42(–/–) endodermal cells (Cdc42(fl/–): 1.61 ± 0.44 μm/min; Cdc42(–/–): 1.55 ± 0.46 μm/min; n = 40). Compared with the fibroblastoid cell lines, endodermal cell lines migrated ∼1.5 times as fast. Quite likely because of the reduced cell-cell contacts of endodermal cells compared with fibroblasts, the directionality of migrating endodermal cells was lower than that of fibroblastoid cells. However, taking the average mean angle vector of individually migrating cells as a very sensitive indicator, the directionality of endodermal cells lacking Cdc42 was reduced compared with Cdc42(fl/–) control cells (Cdc42(fl/–): 0.45 ± 0.14; Cdc42(–/–): 0.38 ± 0.13; n = 40, p < 0.05), similar to fibroblastoid cells. Both control and mutant cells were able to slowly reorient the Golgi into the direction of migration (Figure 8C).
Figure 8.
Cdc42 endodermal cells display migration and polarization capacity similar to control cells. (A) Similar relative migration of Cdc42(fl/–) and Cdc42(–/–) endodermal cells in a wounding assay (n = 3). (B) Phase-contrast pictures of migrating endodermal cells. Note reduced cell-cell contacts in the migrating front of both cell lines (scale bar, 100 μm). (C) Percentage of cells with their Golgi apparatus oriented in the direction of migration. Cdc42 null cells show polarization ability not significantly different from control cells at 150 and 300 min of migration. Initial polarization was assessed 30 min after wounding (n = 1) and was close to random polarization of 33.3%, indicated by solid line.
These data prove that the nonessential role of Cdc42 with respect to cell polarization during migration is not restricted to fibroblastoid cells and is not related to cell transformation.
DISCUSSION
Analysis of Cdc42-deficient fibroblasts gave important insight into Cdc42-dependent functions, the specificity of dominant negative inhibition of Cdc42 and the groups of GEFs regulating filopodium formation and the establishment of cell polarity.
Filopodia are fingerlike, active protrusions that emerge from the cell periphery in an actin polymerization-dependent manner (Small et al., 2002). Only by time-lapse microscopy can they be distinguished from retraction fibers, which result from incomplete membrane retraction. Cdc42 was shown to induce filopodia in various cell lines. Moreover, dominant negative inhibition of Cdc42 in fibroblastoid cell lines and deletion of the Cdc42 gene in ES cells was concluded to prevent the formation of filopodia and suggested an essential role for Cdc42 in this process (Nobes and Hall, 1999; Chen et al., 2000). We have analyzed Cdc42-deficient fibroblastoid and ES cells and found that both cell types are capable of constitutive filopodium and lamellipodium formation, demonstrating that Cdc42 is not required for the formation of these protrusions. DnCdc42 did not inhibit filopodium and lamellipodium formation at expression levels which abrogated Golgi reorientation during directed migration completely. This suggests that filopodium formation is regulated by GEFs different from the ones controlling cell polarization in migrating fibroblasts.
In our system, the molecular mechanism of protrusion formation seems to be independent of the mechanism defining the subcellular site where the protrusions are formed, similar to previous observations (Nobes and Hall, 1999). These data, however, are in contrast to a recent report showing that in migrating Vero cells Cdc42 and Rac1 together control both lamellipodium formation at the leading edge and reorientation of the Golgi-associated microtubule organizing center by binding to IQGAP-1 (Rios and Bornens, 2003; Watanabe et al., 2004).
Besides Cdc42, other Rho GTPases such as Rif (Ellis and Mellor, 2000), TC10 (Neudauer et al., 1998; Murphy et al., 1999; Vignal et al., 2000), TCL (Abe et al., 2003) and Wrch-1 (Tao et al., 2001) were suggested to induce filopodium formation, although only few studies used time lapse microscopy to distinguish actively protruding filopodia from retraction fibers. Furthermore, the appearance of the filopodia induced by activated mutant forms of these Rho GTPases was often rather different from Cdc42-induced protrusions and was cell type dependent (Saras et al., 2004). Only for Rif it was shown that dnCdc42 did not block filopodium formation (Ellis and Mellor, 2000). Our data prove that there are Cdc42-independent pathways of formation of filopodia and that filopodium formation by other Rho GTPases is not generally due to cross-activation of Cdc42. Cdc42 was shown previously to cross-activate Rac1 in mammalian cells, because constitutive activation of Cdc42 resulted in lamellipodium formation, which could be blocked by dnRac1 (Kozma et al., 1995; Nobes and Hall, 1995). It was, however, not clear to which extent Cdc42 contributes to active Rac1 levels. We show now that in fibroblastoid cells ∼50% of Rac1 activity derives from the presence of Cdc42, whereas 50% are independent of Cdc42. This decrease in Rac1 activity was not related to a decrease in the level of Rac1 protein, which was not changed in the absence of Cdc42. Reduction of active Rac1 levels in Cdc42-null cells by dnCdc42 expression demonstrated furthermore that dnCdc42 reduces Rac1 activity also by nonspecific inhibition, conceivably by blocking GEFs shared by Cdc42 and Rac1. In contrast, loss of Cdc42 or expression of dominant negative Cdc42 had no clear effect on RhoA activity, indicating no or very low cross-activation by Cdc42 and coactivation by GEFs shared between Cdc42 and RhoA.
The speed of wound closure and the polarized formation of lamellipodia toward the wound were not significantly affected by the loss of Cdc42, and reorientation of the Golgi was only partially reduced in our fibroblastoid cells, contrary to previous suggestions (Nobes and Hall, 1999). Also in endodermal cells Cdc42 was not essential for efficient wound closure and Golgi reorientation.
In astrocytes, increased phosphorylation of GSK3β is crucial for cell polarization during migration (Etienne-Manneville and Hall, 2001). No indication was found for an involvement of migration-induced phosphorylation of GSK3β in the establishment of cell polarity in migrating fibroblastoid cells, suggesting different effector pathways in fibroblasts and astrocytes. We were also unable to detect significant induction of p38 or Akt upon wounding, whereas activation of Erk and JNK was observed, but was not significantly changed. In case of JNK a trend for a lower induction and faster decay of the signal was observed in mutant cell lines, yet it is not clear whether this observation correlates with the observed migratory phenotypes. It is possible that Rho GTPase-dependent activation of PAK kinases might be involved in polarization and migration of fibroblastoid cells (Bokoch, 2003). Alternatively, GSK3β, Erk, JNK, p38, or Akt phosphorylation could be changed only locally to an extent not detectable in total cell lysates. Although loss of Cdc42 resulted in a pronounced spindle-shaped cell morphology, indicating a reorganization of the actin cytoskeleton, this did not correspond to an impaired polarization of protrusion formation during migration, suggesting that different Rho GTPases are involved in the regulation of these processes. Most likely, the reorientation of lamellipodium formation and the Golgi apparatus toward the wound are regulated by Cdc42 together with other Rho GTPases, because expression of dnCdc42 in Cdc42-null cells impaired all these processes, apparently by nonspecific inhibition of Rho GTPases other than Cdc42. The structurally highly related GTPases of the Cdc42 subfamily, TC10, TCL, Wrch-1, and Wrch-2, are possible candidates for Rho GTPases with redundant functions (Wennerberg and Der, 2004). Expression analysis indicated that TC10 and Wrch-2 are expressed in fibroblastoid cells at medium levels, whereas TCL and Wrch-1 were low or nondetectable. Further experiments, therefore, will focus on the role of TC10 and Wrch-2 in cell polarization during migration.
Recently, Stramer et al. (2005) reported that in Drosophila Cdc42-deficient hemocytes show a twofold increased migration speed and an inability to maintain persistent polarity in response to wound-induced cues. In contrast, mammalian fibroblastoid Cdc42-null cells demonstrated rather stable directionality and no increased migration speed when analyzed on a single-cell level. However, a certain reduction in directionality was measurable by a decrease of the value of the mean angle vector, a highly sensitive indicator of directed migration. Expression of dominant-negative Cdc42 in Cdc42-deficient cells resulted in a significantly decreased single cell velocity during wound closure, opposite to the increased speed of Cdc42-null hemocytes in Drosophila (Stramer et al., 2005), and a further reduction of the mean angle vector. Interestingly, dominant negative inhibition of Cdc42 in hemocytes resulted in the same migratory phenotype as inactivation of the Cdc42 gene, giving no evidence of other Rho GTPases with overlapping functions in Drosophila (Stramer et al., 2005).
Cdc42-mediated Rac1 activation was not required for normal wound closure speed, although Rac1 was suggested to be very important for fibroblast migration (Nobes and Hall, 1999). Furthermore, we were not able to detect in our fibroblastoid cells a wounding-induced increase in Rac1 activation. However, Rac1 might be activated locally without significantly affecting Rac1 activation levels globally.
Dominant negative inhibition of Cdc42 in HeLa cells resulted in a huge increase of multinucleated cells, which led to the suggestion that Cdc42 is essential for mitosis (Yasuda et al., 2004). Unexpectedly, Cdc42 was not required for mitosis in our fibroblastoid cells, because no increased number of binucleated cells was observed. Furthermore, also Cdc42-null keratinocytes and chondrocytes in vivo displayed no detectable increase in the percentage of multinucleated cells (Brakebusch et al., unpublished data). Clearly, the role of Cdc42 in mitosis is more subtle than proposed earlier. In a recent genome wide RNAi screen in Drosophila cells, Cdc42 was not identified as being crucial for cytokinesis (Eggert et al., 2004). Although this negative result could have technical reasons, it seems to corroborate our findings of the nonessential role of Cdc42 for mitosis.
Using Cdc42-deficient cells and dominant negative inhibition of Cdc42, we could demonstrate in this study that the establishment and maintenance of polarity during cell migration is mediated not only by Cdc42, but also by other Rho GTPases. DnCdc42 may effectively inhibit several members of the Rho-GTPase family and might be considered, therefore, a subfamily inhibitor rather than an inhibitor specific for Cdc42. Multiple knockouts or knockdowns of Rho GTPases will certainly be helpful in identifying those GTPases involved in the regulation of cell polarity in migrating fibroblastoid cells.
Supplementary Material
[Supplemental Materials]
Acknowledgments
We thank Brigitte Denker, Ursula Kuhn, and Mischa Reiter for expert technical assistance; Drs. Jürgen Wehland and Reinhard Fässler for their support and for critical reading of the manuscript; and Dr. Kathryn Rodgers for her comments. In addition we also thank Dr. Erik Danen for help in establishing the pulldown assays and Dr. Hitoshi Niwa for the Gata-4 expression vector. This work was supported by the German Research Council (DFG) and the Max Planck Society. J.v.H. has a fellowship of the Fund for Scientific Research, Flanders.
References
- Abe, T., Kato, M., Miki, H., Takenawa, T., and Endo, T. (2003). Small GTPase Tc10 and its homologue RhoT induce N-WASP-mediated long process formation and neurite outgrowth. J. Cell Sci. 116, 155–168. [DOI] [PubMed] [Google Scholar]
- Aspenström, P., Fransson, A., and Saras, J. (2004). Rho GTPases have diverse effects on the organization of the actin filament system. Biochem. J. 377, 327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop, A. L., and Hall, A. (2000). Rho GTPases and their effector proteins. Biochem. J. 348, 241–255. [PMC free article] [PubMed] [Google Scholar]
- Bokoch, G. M. (2003). Biology of the p21-activated kinases. Annu. Rev. Biochem. 72, 743–781. [DOI] [PubMed] [Google Scholar]
- Braga, V. M., Betson, M., Li, X., and Lamarche-Vane, N. (2000). Activation of the small GTPase Rac is sufficient to disrupt cadherin-dependent cell-cell adhesion in normal human keratinocytes. Mol. Biol. Cell. 11, 3703–3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carl, U. D., Pollmann, M., Orr, E., Gertler, F. B., Chakraborty, T., and Wehland, J. (1999). Aromatic and basic residues within the EVH1 domain of VASP specify its interaction with proline-rich ligands. Curr. Biol. 9, 715–718. [DOI] [PubMed] [Google Scholar]
- Chen, F. et al. (2000). Cdc42 is required for PIP2 induced actin polymerization and early development but not for cell viability. Curr. Biol. 10, 758–765. [DOI] [PubMed] [Google Scholar]
- Eggert, U. S., Kiger, A. A., Richter, C., Perlman, Z. E., Perrimon, N., Mitchison, T. J., and Field, C. M. (2004). Parallel chemical genetic and genome-wide RNAi screens identify cytokinesis inhibitors and targets. PLoS Biol. 2, e379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis, S., and Mellor, H. (2000). The novel Rho-family GTPase Rif regulates coordinated actin-based membrane rearrangements. Curr. Biol. 10, 1387–1390. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville, S., and Hall, A. (2001). Integrin-mediated activation of Cdc42 controls cell polarity in migrating astrocytes through protein kinase Cζ. Cell 106, 489–498. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville, S., and Hall, A. (2003). Cdc42 regulates GSK-3β and adenomatous polyposis coli to control cell polarity. Nature 421, 753–756. [DOI] [PubMed] [Google Scholar]
- Feig, L. A. (1999). Tools of the trade: use of dominant-inhibitory mutants of Ras-family GTPases. Nat. Cell Biol. 1, E25–E27. [DOI] [PubMed] [Google Scholar]
- Frost, J. A., Steen, H., Shapiro, P., Lewis, T., Ahn, N., Shaw, P. E., and Cobb, M. H. (1997). Cross-cascade activation of ERKs and ternary complex factors by Rho family proteins. EMBO J. 16, 6426–6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujikura, J., Yamato, E., Yonemura, S., Hosoda, K., Masui, S., Nakao, K., Miyazaki, J., and Niwa, H. (2002). Differentiation of embryonic stem cells is induced by GATA factors. Genes Dev. 16, 784–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukata, M., Nakagawa, M., and Kaibuchi, K. (2003). Roles of Rho-family GTPases in cell polarisation and directional migration. Curr. Opin. Cell Biol. 15, 590–597. [DOI] [PubMed] [Google Scholar]
- Gauthier-Rouvière, C., Vignal, E., Mériane, M., Roux, P., Montcourier, P., and Fort, P. (1998). RhoG GTPase controls a pathway that independently activates Rac1 and Cdc42Hs. Mol. Biol. Cell 9, 1379–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraguchi, T., Ding, D-Q., Yamamoto, A., Kaneda, T., Koujin, T., and Hiraoka, Y. (1999). Multiple-color fluorescence imaging of chromosomes and microtubules in living cells. Cell Struct. Funct. 24, 291–298. [DOI] [PubMed] [Google Scholar]
- Kozma, R., Ahmed, S., Best, A., and Lim, L. (1995). The Ras-related protein Cdc42Hs and bradykinin promote formation of peripheral actin microspikes and filopodia in Swiss 3T3 fibroblasts. Mol. Cell. Biol. 15, 1942–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy, G. A., Solski, P. A., Jillian, S. A., Pérez de la Ossa, P., D'Eustachio, P., Der, C. J., and Rush, M. G. (1999). Cellular functions of TC10, a Rho family GTPase: regulation of morphology, signal transduction and cell growth. Oncogene 18, 3831–3845. [DOI] [PubMed] [Google Scholar]
- Neudauer, C. L., Joberty, G., Tatsis, N., and Macara, I. G. (1998). Distinct cellular effects and interactions of the Rho-family GTPase TC10. Curr. Biol. 8, 1151–1160. [DOI] [PubMed] [Google Scholar]
- Nobes, C. D., and Hall, A. (1995). Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 81, 53–62. [DOI] [PubMed] [Google Scholar]
- Nobes, C. D., and Hall, A. (1999). Rho GTPases control polarity, protrusion, and adhesion during cell movement. J. Cell Biol. 144, 1235–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsson, M., Aumalley, M., Deutzmann, R., Timpl, R., Beck, K., and Engel, J. (1987). Laminin-nidogen complex. Extraction with chelating agents and structural characterization. Eur. J. Biochem. 166, 11–19. [DOI] [PubMed] [Google Scholar]
- Rios, R. M., and Bornens, M. (2003). The Golgi apparatus at the cell centre. Curr. Opin. Cell Biol. 15, 60–66. [DOI] [PubMed] [Google Scholar]
- Rottner, K., Behrendt, B., Small, J. V., and Wehland, J. (1999). VASP dynamics during lamellipodia protrusion. Nat. Cell Biol. 1, 321–322. [DOI] [PubMed] [Google Scholar]
- Saras, J., Wollberg, P., and Aspenström, P. (2004). Wrch1 is a GTPase-deficient Cdc42-like protein with unusual binding characteristics and cellular effects. Exp. Cell Res. 299, 356–369. [DOI] [PubMed] [Google Scholar]
- Schmidt, A., and Hall, A. (2002). Guanine nucleotide exchange factors for Rho GTPases: turning on the switch. Genes Dev. 16, 1587–1609. [DOI] [PubMed] [Google Scholar]
- Small, J. V., Stradal. T., Vignal, E., and Rottner, K. (2002). The lamellipodium: where motility begins. Trend Cell Biol. 12, 112–120. [DOI] [PubMed] [Google Scholar]
- Stramer, B., Wood, W., Galko, M. J., Redd, M. J., Jacinto, A., Parkhurst, S. M., and Martin, P. (2005). Live imaging of wound inflammation in Drosophila embryos reveals key roles for small GTPases during in vivo cell migration. J. Cell Biol. 168, 567–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao, W., Pennica, D., Xu, L., Kalejta, R.F., and Levine, A. J. (2001). Wrch-1, a novel member of the Rho gene family that is regulated by Wnt-1. Genes Dev. 15, 1796–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignal, E., De Toledo, M., Comunale, F., Ladopoulou, A., Gauthier-Rouvière, C., Blangy, A., and Fort, P. (2000). Characterization of TCL, a new GTPase of the rho family related to TC10 and Ccdc42. J. Biol. Chem. 275, 36457–36464. [DOI] [PubMed] [Google Scholar]
- Watanabe, T., Wang, S., Noritake, J., Sato, K., Fukata, M., Takefuji, M., Nakagawa, M., Izumi, N., Akiyama, T., and Kaibuchi, K. (2004). Interaction with IQGAP1 links APC to Rac1, Cdc42, and actin filaments during cell polarization and migration Dev. Cell 7, 871–883. [DOI] [PubMed] [Google Scholar]
- Wennerberg, K., and Der, C. J. (2004). Rho-family GTPases: it's not only Rac and Rho (and I like it). J. Cell Sci. 117, 1301–1312. [DOI] [PubMed] [Google Scholar]
- Yasuda, S., Oceguera-Yanez, F., Kato, T., Okamoto, M., Yonemura, S., Terada, Y., Ishizaki, T., and Narumiya, S. (2004). Cdc42 and mDia3 regulate microtubule attachment to kinetochores. Nature 428, 767–771. [DOI] [PubMed] [Google Scholar]
- Yatohgo, T., Izumi, I., Kashiwagi, H., and Hayashi, M. (1988). Novel purification of vitronectin from human plasma by heparin affinity chromatography. Cell Struct. Funct. 13, 281–292. [DOI] [PubMed] [Google Scholar]
- Zondag, G. C., Evers, E. E., ten Klooster, J. P., Janssen, L., van der Kammen, R. A., and Collard, J. G. (2000). Oncogenic Ras downregulates Rac activity, which leads to increased Rho activity and epithelial-mesenchymal transition. J. Cell Biol. 149, 775–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zugasti, O., Rul, W., Roux, P., Peyssonnaux, C., Eychene, A., Franke, T. F., Fort, P., and Hibner, U. (2001). Raf-MEK-Erk cascade in anoikis is controlled by Rac1 and Cdc42 via Akt. Mol. Cell Biol. 21, 6706–6717. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
[Supplemental Materials]