γδ T cell homeostasis is established in competition with αβ T cells and NK cells (original) (raw)
Abstract
γδ T cells are a diverse population of lymphocytes that play an important role in immune regulation. The size of the γδ T cell pool is tightly regulated, comprising only 1-10% of total lymphoid T cells in mice and humans. We examined the homeostatic regulation of γδ T cells using a model of lymphopenia-induced homeostatic expansion. We found that IL-15 and, to a lesser extent, IL-7 play an important role in lymphoid γδ T cell homeostasis. Moreover, γδ T cell homeostatic expansion was limited not only by γδ T cells themselves but also by natural killer cells and αβ T cells. Our results suggest that CD8+ αβ T cells are the most potent inhibitors of γδ T cell homeostasis and exert their effect by competing for IL-15.
Keywords: CD4 T cells, CD8 T cells, homeostatic proliferation
The γδ T cells are a diverse population of lymphocytes that have been shown to play an important role in immune regulation (1). Consequently, strict control of γδ T cell function and population size is likely essential for the generation of an optimal immune response. Maintenance of lymphocyte populations, or lymphocyte homeostasis, is achieved by balancing the generation of new cells and clonal expansion with cell death. Uncovering the mechanisms responsible for this process has contributed to our understanding of the immune system and is essential for the future success of cellular immunotherapy. Lymphocyte populations have been shown to undergo spontaneous expansion when adoptively transferred into lymphopenic hosts (2-10). Characterization of this phenomenon, termed homeostatic proliferation, has provided insight into the mechanisms normally responsible for lymphocyte homeostasis and shaping the lymphocyte repertoire.
The mechanisms responsible for regulating lymphocyte homeostasis vary between lymphocyte subsets. For example, whereas IL-7 is essential for naïve αβ T cell homeostasis (11-15), IL-15 plays a key role in regulating the CD8+ memory αβ T cells (16-18), natural killer (NK) cells (7, 16, 18-21), and NK T cells (5, 8). Both IL-7 and IL-15 have been implicated in γδ T cell biology and might play a role in γδ T cell homeostasis. IL-7 is essential for rearrangement of the TCRγ gene during γδ T cell development (22, 23) and may also increase γδ T cell life span in the periphery (24). Although IL-15 is not required for γδ T cell development in general, IL-15-/- mice lack Vγ5+ epidermal γδ T cells (25) and Thy1- splenic and intestinal intraepithelial γδ T cells (16, 26).
To investigate the mechanisms responsible for regulating lymphoid γδ T cell homeostasis, we developed a model of lymphopenia-induced γδ T cell homeostatic expansion. Our studies reveal that γδ T cell homeostasis is regulated not only by γδ T cells themselves but also by αβ T cells and NK cells. The effect of αβ T cells appears to be mediated primarily by CD8+ αβ T cells and results, at least in part, from the ability of αβ T cells to monopolize IL-15 resources.
Materials and Methods
Mice. Wild-type C57BL/6, C57BL/6 TCRβ-/-, C57BL/6 TCRδ-/-, C57BL/6 CD8α-/-, C57BL/6 β2m-/-, and C57BL/6 TCRβ-/-/δ-/- mice were purchased from The Jackson Laboratory and were maintained and bred in our facility. Both male and female mice were used in these experiments at 6-12 weeks of age. For any given experiment, mice were matched for both age and sex.
In Vivo γδ T Cell Proliferation. Donor γδ T cells were isolated from either C57BL/6 wild-type or TCRβ-/- mice. Single-cell suspensions were prepared from donor spleens. After red blood cell lysis with Gey's solution, cell suspensions were passed over nylon wool columns to enrich for T lymphocytes. αβ T cells were depleted from C57BL/6 samples by using the magnetic-activated cell sorting (MACS) system (biotinylated anti-TCRβ (27) plus streptavidin-coated magnetic microbeads, Miltenyi Biotec, Auburn, CA). Cell purity was determined by flow cytometry (see below) before adoptive transfer, and γδ T cell number was determined by multiplying the total cell number after nylon wool purification by the percentage of live cells and by the percentage of TCRδ+/CD3+ cells. C57BL/6-derived γδ T cells comprised 20% of the final cell suspension. TCRβ-/--derived γδ T cells were enriched to 50% of the final cell suspension after nylon wool purification. The contaminating cells were composed of mainly B cells and NK cells. Enriched populations were resuspended in PBS at 2 × 106 cells per ml and labeled with 0.1 μM 5-(and 6)-carboxyfluorescein diacetate succinimidyl ester (CFSE, Molecular Probes) at 37°C for 15 min. Cells were washed twice with 50 ml of PBS and resuspended in injection saline. 1 × 106 CFSE-labeled γδ T cells were injected intravenously into recipient mice. Spleens were harvested at various time points after adoptive transfer, T cells were isolated as described above, and cell proliferation was determined by flow cytometry. For cotransfer experiments, splenic αβ T cells were isolated from TCRδ-/- donors, enriched by nylon wool purification, and injected i.v. with γδ T cells at the designated ratios into TCRβ-/-/δ-/- recipients. C57BL/6 β2m-/- and CD8-/- recipients were exposed to 600 cGy whole-body irradiation 1 day before cell transfer.
Listeria Infection. Mice were injected intravenously with Listeria monocytogenes (strain EGD, 2.5 × 103 colony-forming units in 200 μl) or saline alone 24 h after γδ T cell adoptive transfer and harvested on day 4 of the infection.
Isolation of Purified αβ T Cell Subsets and Long-Term Reconstitution Studies. After treatment with Gey's solution to lyse red blood cells, C57BL/6 spleen cell suspensions were passed over nylon wool columns. The enriched T cell population was divided into three samples to generate total αβ T cells, CD4+ αβ T cells, or CD8+ αβ T cells. Cells were resuspended in PBS/0.5% BSA (200 μl/1 × 107 cells) and preincubated with unlabeled 2.4G2 mAb (28) to reduce nonspecific mAb binding by Fc receptors. All populations were incubated with a biotinylated anti-TCRδ antibody (GL3). CD8+ and CD4+ T cell preparations were also incubated with biotinylated anti-CD4 (GK1.5) and anti-CD8 (53.6.7) antibodies, respectively. After antibody binding, cells were incubated with streptavidin-coated magnetic microbeads, passed over a miniMACS separation column (Miltenyi Biotec), and the nonadherent cells were collected. Purity was determined by flow cytometry before adoptive transfer (<1% contaminating CD4+ or CD8+ αβ T cells). TCRβ-/--derived γδ T cells (5 × 105) (see above) were injected i.v., either alone or in combination with 5 × 105 MACS-purified αβ T cells, into TCRβ-/-/δ-/- mice.
Antibody-Mediated Depletions. αβ T cells and NK cells were depleted from recipient TCRδ-/- and TCRβ-/-/δ-/- mice by using antibodies targeted against the TCRβ chain (H57-597) or NK1.1 (PK136), respectively. Mice received 200 μg of control antibody (hamster IgG or mouse IgG, Jackson ImmunoResearch) or cell-specific antibody i.v. 3 days before adoptive transfer. The extent of cell depletion was measured by flow cytometry. To ensure that αβ T cells and NK cells were truly depleted, we stained for alternative surface markers. αβ T cell depletion was verified by staining with anti-CD3, anti-CD4, and anti-CD8 antibodies. Depletion of NK cells was verified by the loss of the IL-2Rβhi/CD3- population.
Cytokine Injections. Recombinant IL-7 and IL-15 were purchased from PeproTech (Rocky Hill, NJ). Cytokines were resuspended in sterile PBS and stored at -70°C. TCRβ-/-/δ-/- mice received PBS or 1 μg of cytokine per i.v. injection.
Cytokine-Blocking Experiments. Anti-IL-7 (M25) (29), anti-IL-7Rα (A7R34) (30), anti-IL-2Rβ (TMβ1) (31), anti-IL-2 (S4B6) (32), and anti-IL-2Rα (PC-61, American Type Culture Collection) antibodies were isolated from _in vitro_-cultured hybridomas. Rat IgG was used as a nonspecific control antibody. TCRβ-/-/δ-/- mice received 300 μg of total antibody (150 μg of each antibody) per i.v. injection. Anti-IL-2Rβ was injected with 150 μg of rat IgG to control for total protein levels.
Flow Cytometry. Nylon-wool-enriched T cells were resuspended in staining buffer (Hanks' BSS/2% FBS/0.1% sodium azide, pH 7.1) and aliquoted into round-bottom 96-well polystyrene tissue culture plates (105-106 cells per well). Cells were preincubated with 2.4G2 to avoid nonspecific antibody binding by Fc receptors. γδ T cells were identified by staining with biotinylated or phycoerythrin (PE)-conjugated anti-TCRδ antibodies (GL3, BD Pharmingen) in combination with anti-CD3-PE-Cy5 (145-2C11, BD Pharmingen). αβ T cells were identified by staining with anti-CD3-PE-Cy5, anti-CD3-FITC (KT3) (33), anti-TCRβ-PE (H57-597, BD Pharmingen), anti-CD8α-PE (53.6.7, BD Pharmingen), and anti-CD4-PE (GK1.5, BD Pharmingen) antibodies. Anti-NK1.1-PE (PK136, eBioscience, San Diego, CA) was used to identify NK cells. Anti-IL-2Rβ (5H4, eBioscience), anti-IL-7Rα (A7R34, eBioscience), and anti-IL15Rα-biotin (R & D Systems) antibodies were used to assess cytokine receptor expression levels on gated T cell populations. Biotinylated antibodies were detected with streptavidin-PE (BioSource International, Camarillo, CA) or streptavidin-PE-Cy5 (BD Pharmingen). Cells were fixed with 1% paraformaldehyde in PBS and analyzed for immunofluorescence on a FACScan analyzer (Becton Dickinson). Collected data were analyzed by using flowjo 4.4.3 software. Briefly, live γδ T cells or αβ T cells were gated and assessed for proliferation or surface-marker expression. Percent proliferation was determined as the percentage of total T cells that had undergone at least one cell division at the time of harvest. With each cell division, CFSE fluorescence is reduced by 50%, allowing the determination of proliferation by flow cytometry (34). Total T cell number was determined by multiplying the total number of cells recovered after nylon wool enrichment by the percentage of live cells and by the percentage of each cell type as determined by flow cytometry.
Statistical Analysis. Each experiment in this study was performed at least three times. In general, three mice were used per condition in each experiment. To control for potential variability in donor cells, sham or untreated mice that received the same donor cells were included in relevant experiments and were analyzed at the same time point. Data shown are either representative of numerous experiments or display the combined data for multiple experiments. For combined data sets, the mean value is shown ±SEM. The statistical relevance of our studies was determined by using the Mann-Whitney t test for nonparametric data, with a 95% confidence interval.
Results
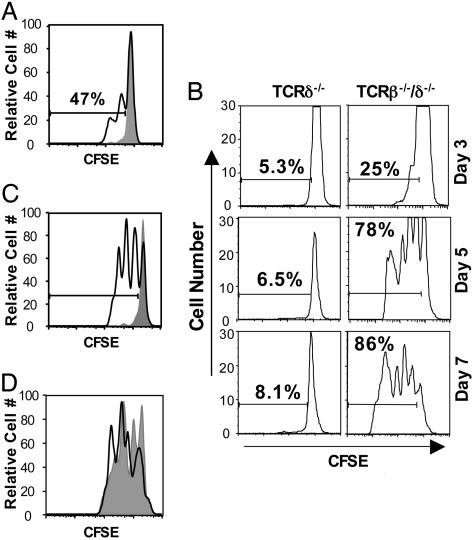
γδ T Lymphocytes Undergo Homeostatic Expansion in T Cell-Deficient Mice. To assess the homeostatic potential of γδ T cells, splenic γδ T cells were obtained from TCRβ-/- (35) donors, labeled with CFSE, and adoptively transferred into either TCRδ-/- (36) recipients, which lack endogenous γδ T cells, or TCRβ-/-/δ-/- (36, 37) mice, which lack both αβ and γδ T cells. Despite their ability to undergo proliferation in TCRδ-/- mice after infection with L. monocytogenes, donor γδ T cells did not proliferate in uninfected TCRδ-/- recipients (Fig. 1_A_). In contrast, γδ T cells underwent extensive homeostatic proliferation after transfer into TCRβ-/-/δ-/- recipients (Fig. 1_B_). Cell division was apparent 3 days after cell transfer, and ≈80% of the donor cells had undergone at least one division by day 5. The transferred γδ T cells population continued to expand over time in TCRβ-/-/δ-/- mice, such that, 2 months after cell transfer, total γδ T cell numbers were similar to that observed in TCRβ-/- mice (1-2 × 106 γδ T cells per spleen, data not shown). Of note, γδ T cells did not proliferate when adoptively transferred into TCRβ-/- mice, which contain γδ T cells but lack αβ T cells (Fig. 1_C_). Because γδ T cell development might be altered in the TCRβ-/- mice that we used as donors (38), we also tested the ability of donor γδ T cells isolated from wild-type C57BL/6 mice to expand in TCRβ-/-/δ-/- recipients and obtained similar results (Fig. 1_D_). Thus, γδ T cells undergo homeostatic expansion only in the absence of both γδ T cells and αβ T cells, suggesting that both γδ T cells and αβ T cells regulate γδ T cell homeostasis.
Fig. 1.
γδ T cells are capable of homeostatic expansion in the absence of endogenous γδ T cells and αβ T cells. Splenic γδ T lymphocytes obtained from TCRβ-/- donors were labeled with CFSE and injected into uninfected (shaded) and _Listeria_-infected (black line) TCRδ-/- mice (A), TCRδ-/- and TCRβ-/-/δ-/- mice (B), or TCRβ-/-/δ-/- (black line) and TCRβ-/- (shaded) mice (C). (D) Homeostatic expansion of TCRβ-/--derived γδ T cells (shaded) was compared with that of C57BL/6-derived γδ T cells (black line) in TCRβ-/-/δ-/- recipients. Recipient splenocytes were harvested at the designated time points (B) or day 5 (A, C, and D) after adoptive transfer, enriched for T cells by nylon wool purification, and analyzed by flow cytometry.
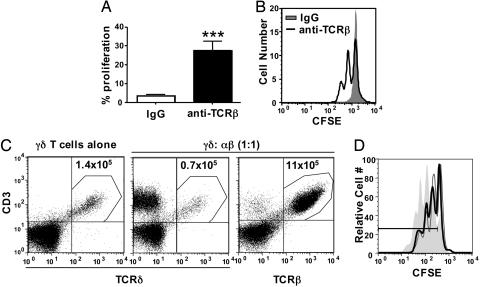
Antibody-Mediated Depletion of αβ T Cells from TCRδ-/- Mice Allows γδ T Cell Homeostatic Expansion. To test whether αβ T cell deficiency is sufficient to allow for γδ T cell homeostatic expansion in the absence of endogenous γδ T cells, we depleted TCRδ-/- mice of αβ T cells by using a pan-specific anti-TCRβ antibody before γδ T cell adoptive transfer. This treatment resulted in a 4-fold reduction in αβ T cell number at the time of harvest (data not shown). Although γδ T cells did not undergo expansion in TCRδ-/- mice treated with a control nondepleting antibody, mice treated with the anti-TCRβ antibody became permissive for homeostatic expansion of donor γδ T cells (Fig. 2 A and B). On average, ≈30% of the donor γδ T cells had undergone at least one division in anti-TCRβ-treated TCRδ-/- mice 5 days after cell transfer.
Fig. 2.
αβ T cells inhibit γδ T cell homeostasis. (A) TCRδ-/- mice were treated with hamster IgG control antibody or an antibody against TCRβ 3 days before adoptive transfer. Splenic γδ T cells obtained from TCRβ-/- donor mice were labeled with CFSE and injected into antibody-treated TCRδ-/- mice. Splenocytes were recovered from recipient mice 5 days after adoptive transfer, T cells were enriched by nylon wool, and γδ T cell proliferation was assessed by flow cytometry (***, P < 0.001). (B) Representative histogram of γδ T cell proliferation in antibody-treated mice. (C) Splenic γδ T cells obtained from TCRβ-/- donor mice were injected alone or in the presence of αβ T cells (wild-type, C57BL/6) at a 1:1 ratio into TCRβ-/-/δ-/- mice. Splenocytes were recovered from recipient mice 1 month after adoptive transfer, T cells were enriched by nylon wool, and γδ T cell (anti-TCRδ+/CD3+) or αβ T cell (anti-TCRβ+/CD3+) reconstitution was determined by flow cytometry. (D) CFSE-labeled donor γδ T cells from TCRβ-/- mice were transferred alone (filled histogram) or in the presence of unlabeled γδ T cells (gray line) or unlabeled αβ T cells (black line) from TCRδ-/- mice into TCRβ-/-/δ-/- recipients. γδ T cell homeostatic expansion was assessed 5 days after adoptive transfer (percent proliferation: γδ alone, 86.9%; γδ plus unlabeled γδ, 62.4%; γδ plus αβ, 67.4%).
αβ T Cells Inhibit γδ T Cell Homeostatic Expansion When Cotransferred into TCRβ-/-/δ-/- Mice. To assess whether αβ T cells could inhibit γδ T cell homeostasis when cotransferred into TCRβ-/-/δ-/- recipients, splenic αβ T cells were injected with γδ T cells at a 1:1 ratio into TCRβ-/-/δ-/- recipients. One month after cell transfer, the number of γδ T cells was reduced approximately 2-fold in the presence of αβ T cells, compared with the number recovered from mice that received only γδ T cells (Fig. 2_C_). Surprisingly, αβ T cells displayed an obvious homeostatic advantage over γδ T cells. Although TCRβ-/-/δ-/- recipients received an equal number of γδ T cells and αβ T cells, αβ T cells were at least 17-fold more abundant than γδ T cells in TCRβ-/-/δ-/- recipient spleens after 1 month (Fig. 2_C_). As shown in Fig. 2_D_, the negative regulatory effect of αβ T cells is due, at least in part, to early inhibition of γδ T cell homeostatic expansion, because cotransfer of either excess γδ T cells or αβ T cells reduced γδ T cell proliferation to a similar extent 5 days after cell transfer.
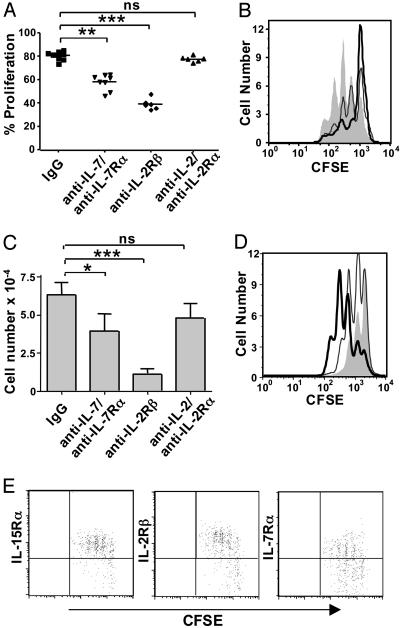
IL-15 Plays a Dominant Role in γδ T Cell Homeostatic Expansion. To determine whether the inhibitory effect of αβ T cells on γδ T cell homeostasis results from their superior ability to compete for key growth or survival factors, we next investigated the role of IL-7 and IL-15. Here, we used cytokine and cytokine-receptor-specific antibodies to block the effects of endogenous cytokines (Fig. 3 A and B). TCRβ-/-/δ-/- mice received a combination of anti-IL-7 and anti-IL-7Rα antibodies to inhibit endogenous IL-7, or anti-IL-2Rβ antibody to block IL-15. Because IL-2Rβ is also a component of the IL-2 receptor complex, we used antibodies against both IL-2 and IL-2Rα to assess the role of IL-2 in our model. γδ T cell homeostatic proliferation was somewhat reduced in mice that received anti-IL-7 plus anti-IL-7Rα and markedly diminished in TCRβ-/-/δ-/- mice that received anti-IL-2Rβ treatment. Importantly, blocking IL-2 alone by anti-IL-2/anti-IL-2Rα treatment did not inhibit γδ T cell expansion, indicating that the blocking effect of anti-IL-2Rβ is due to inhibition of IL-15 rather than IL-2. The number of γδ T cells recovered from antibody-treated mice was also reduced in anti-IL-7/anti-IL-7Rα-treated mice and greatly decreased in mice that received anti-IL-2Rβ treatment (Fig. 3_C_). Consistent with these findings, treating mice with recombinant IL-7 enhanced γδ T cell homeostatic proliferation in TCRβ-/-/δ-/- mice, and recombinant IL-15 had an even stronger effect (Fig. 3_D_). These data suggest that both IL-15 and IL-7 contribute to γδ T cell homeostasis, and that IL-15 may play a dominant role. In support of this hypothesis, increased expression of both IL-15Rα and IL-2Rβ, but not IL-7Rα, correlate with γδ T cell homeostatic proliferation after transfer into TCRβ-/-/δ-/- mice (Fig. 3_E_).
Fig. 3.
IL-15 plays a dominant role in γδ T cell homeostasis. (A) TCRβ-/-/δ-/- mice were treated with the designated antibodies on days -2, 0, and 2, relative to transfer of CFSE-labeled γδ T cells. Recipient splenocytes were harvested 5 days after adoptive transfer. (B) Representative histograms of γδ T cell proliferation in mice treated with control IgG (shaded), anti-IL-7/anti-IL-7Rα (gray line), or anti-IL-2Rβ (black line). (C) γδ T cell number was determined by flow cytometry 5 days after cell transfer. (D) TCRβ-/-/δ-/- recipients received PBS (shaded), IL-7 (gray line), or IL-15 (black line) on days 0, 1, and 2, relative to γδ T cell transfer, and splenocytes were harvested on day 3. (E) γδ T cell cytokine receptor expression was determined by flow cytometry 5 days after cell transfer. ns, not statistically significant; *, P < 0.1; **, P < 0.01; ***, P < 0.001.
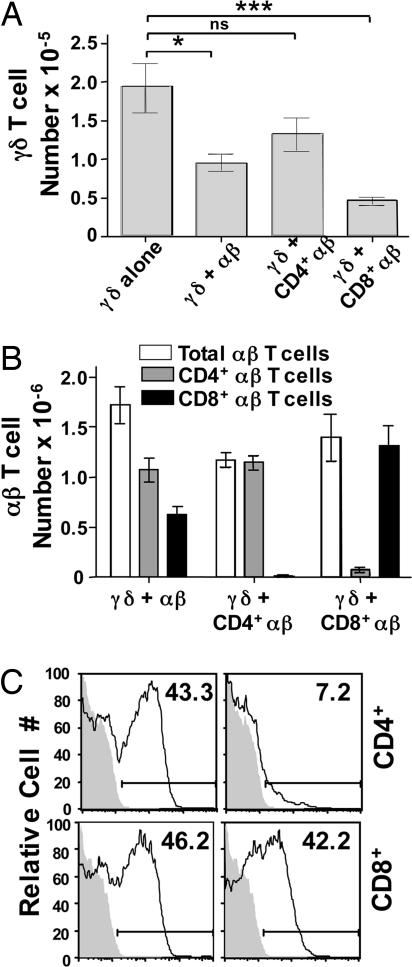
CD8+/IL-2Rβ+ αβ T Cells Are Potent Inhibitors of γδ T Cell Reconstitution. In our model, >90% of CD8+ αβ T cells but only 50% of CD4+ αβ T cells have undergone cell division in TCRβ-/-/δ-/- recipients by day 5 (data not shown), and similar results have been reported in other models (12, 39, 40). Thus, the robustly proliferating CD8+ subset of αβ T cells might play a predominant inhibitory role. To test this possibility, we assessed the effects of purified CD4+ and CD8+ αβ T cells on γδ T cell reconstitution in TCRβ-/-/δ-/- mice (Fig. 4_A_). Compared with mice that received only γδ T cells, cotransfer of purified CD4+ αβ T cells resulted in only a slight reduction in γδ T cell number, which was not statistically significant. In contrast, γδ T cell recovery was considerably reduced in the presence of purified CD8+ αβ T cells. Importantly, the total number of αβ T cells recovered 1 month after adoptive transfer was similar whether mice received unfractionated αβ T cells, purified CD4+ αβ T cells, or purified CD8+ αβ T cells (Fig. 4_B_), even though their initial proliferation rates differ. Of interest, at the time of harvest, CD4+ and CD8+ αβ T cell populations expressed similar levels of IL-7Rα. In contrast, >40% of the CD8+ population expressed IL-2Rβ, whereas <10% of the CD4+ population was positive for IL-2Rβ (Fig. 4_C_). These data suggest that IL-15-responsive (IL-2Rβ+) CD8+ αβ T cells are largely responsible for the inhibitory effect of αβ T cells on γδ T cell homeostasis.
Fig. 4.
Increased levels of IL-2Rβhi CD8+ αβ T cells coincide with poor γδ T cell reconstitution. Splenic γδ T cells obtained from TCRβ-/- donor mice were injected alone or in the presence of total αβ T cells, purified CD4+ αβ T cells, or purified CD8+ αβ T cells at a 1:1 ratio into TCRβ-/-/δ-/- mice. Splenocytes were recovered from recipient mice 1 month after adoptive transfer, T cells were enriched by nylon wool, and γδ T cell (A) and αβ T cell (B) reconstitution was determined by flow cytometry. (C) Expression of IL-7Rα (Left) and IL-2Rβ (Right) was assessed on CD4+/TCRβ+ and CD8+/TCRβ+ cells recovered from TCRβ-/-/δ-/- mice that were originally reconstituted with either γδ T cells plus purified CD4+ αβ T cells or γδ T cells plus purified CD8+ αβ T cells, respectively. Shaded histograms represent the background fluorescence of each population. The percent of T cells that express each cell surface protein is shown. *, P = 0.0071; ns, not statistically significant; ***, P = 0.0001.
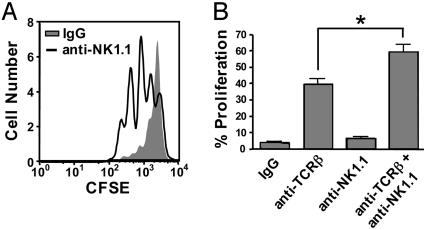
γδ T Cell Homeostatic Expansion Is Enhanced After NK Cell Depletion. If competition for IL-15 is at least partly responsible for αβ T cell-mediated inhibition of γδ T cell homeostasis, it follows that NK cells, which also rely on IL-15, might inhibit γδ T cell homeostasis. To test this hypothesis, TCRβ-/-/δ-/- mice were treated with an anti-NK1.1 antibody 3 days before γδ T cell adoptive transfer, resulting in a 30-fold reduction of endogenous NK cells, compared with mice that received mouse IgG control antibody (data not shown). As predicted, γδ T cell homeostatic expansion was substantially increased in mice that received anti-NK1.1 (Fig. 5_A_). On average, 70% of the donor γδ T cells had undergone at least one division 3 days after adoptive transfer into anti-NK1.1-treated TCRβ-/-/δ-/- mice, whereas only 20% had divided in control mice. Similarly, γδ T cell homeostatic expansion was enhanced in TCRδ-/- mice that received both anti-TCRβ and anti-NK1.1, compared with those treated with anti-TCRβ alone (Fig. 5_B_). In contrast, γδ T cells did not undergo significant homeostatic expansion in TCRδ-/- mice treated with anti-NK1.1 (Fig. 5_B_). These data suggest that, in the absence of αβ T cells, NK cells are capable of inhibiting γδ T cell homeostatic proliferation.
Fig. 5.
Antibody-mediated depletion of NK cells results in enhanced γδ T cell homeostatic expansion in the absence of αβ T cells. (A) TCRβ-/-/δ-/- mice were treated with control mouse IgG or anti-NK1.1 antibody 3 days before adoptive transfer of CFSE-labeled γδ T cells. (B) TCRδ-/- mice were treated with control IgG, anti-TCRβ, anti-NK1.1, or anti-TCRβ plus anti-NK1.1 3 days before adoptive transfer of CFSE-labeled γδ T cells. Recipient splenocytes were harvested 3 (A) or 5 (B) days after adoptive transfer. γδ T cell proliferation was determined by flow cytometry. *, P < 0.1.
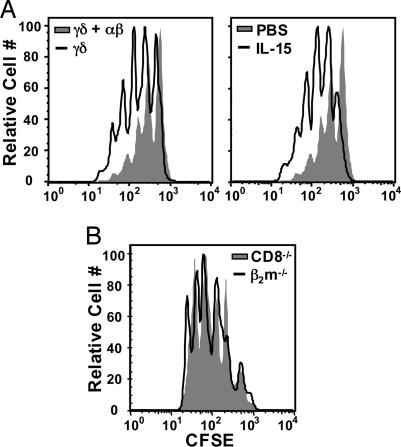
Exogenous IL-15 Restores γδ T Cell Homeostatic Expansion in the Presence of αβ T Cells. The inhibitory effects of CD8+ αβ T cells and NK cells may stem from their ability to outcompete γδ T cells for endogenous IL-15. To assess this possibility, we tested whether exogenous IL-15 could alleviate the inhibitory effect of αβ T cells on γδ T cell homeostatic expansion in TCRβ-/-/δ-/- recipients (Fig. 6_A_). As expected, γδ T cell proliferation was reduced in mice that also received αβ T cells (54 ± 1.8%), compared with mice that received only γδ T cells (73 ± 0.9%). However, γδ T cell proliferation was restored to near or above the levels achieved in the absence of αβ T cells when recipient mice also received injections of recombinant IL-15 (83 ± 0.7%). Thus, αβ T cells did not inhibit short-term γδ T cell proliferation in the presence of excess IL-15.
Fig. 6.
γδ T cells and αβ T cells compete for IL-15 but not MHCI during homeostatic expansion. (A) Donor γδ T cells from TCRβ-/- mice were labeled with CFSE and transferred alone (Left, black line) or in the presence of αβ T cells (Left, shaded) from TCRδ-/- mice into TCRβ-/-/δ-/- recipients. Cotransfer recipients received PBS (Right, shaded) or IL-15 (black line, Right) on days 0, 1, 2, 3, and 4, relative to γδ T cell transfer, and splenocytes were harvested on day 5. γδ T cell homeostatic expansion was assessed by flow cytometry. (B) CFSE-labeled γδ T cells from TCRβ-/- mice were transferred into sublethally irradiated CD8-/- or β2m-/- recipients. Splenocytes were harvested on day 6.
γδ T Cells Do Not Require MHCI Molecules During Homeostatic Expansion. Although the role of MHC in γδ T cell biology remains controversial, MHCI molecules could provide an additional source of competition between CD8+ αβ T cells and γδ T cells during the establishment of homeostasis. To address the role of MHCI in γδ T cell homeostasis, we compared γδ T cell proliferation after adoptive transfer into sublethally irradiated CD8-/- (41) and β2m-/- (42) recipients, both of which lack a normal CD8+ αβ T cell compartment. Consistent with previous studies (2), γδ T cell proliferation was comparable in β2m-/- and CD8-/- mice (Fig. 6_B_), suggesting that, overall, MHCI does not influence γδ T cell homeostatic proliferation. Consequently, MHCI molecules do not likely provide a source of competition between γδ T cells and CD8+ αβ T cells.
Discussion
In the last decade, lymphocyte homeostasis has been the subject of extensive study. Here, we report the ability of γδ T cells to undergo homeostatic expansion in a T cell-deficient environment (TCRβ-/-/δ-/-). In support of our findings, recent work by Baccala et al. (2) revealed that γδ T cells also undergo expansion in irradiated and Rag-/- recipients. Our studies provide direct evidence that αβ T cells, in addition to γδ T cells, regulate γδ T cell homeostasis. This finding is consistent with earlier observations that γδ T cell number is increased in C57BL/6 TCRβ-/- mice, compared with wild-type C57BL/6 mice (37, 43) and may also explain why mice expressing a mutant form of linker for activation of T cells (LAT) (LATY7/8/9F) develop a γδ T cell lymphoproliferative disorder (44). Although the authors suggest that LAT plays a negative regulatory role in γδ T cell homeostasis, the αβ T cell population is dramatically reduced in LATY7/8/9F mice.
Many studies have focused on defining the relationship between distinct subsets within a major lymphocyte population in maintaining homeostasis. αβ T cell subsets appear to overlap, to some extent, in their homeostatic requirements, such that the CD8+ population is expanded in CD4-/- mice (45), and memory CD8+ αβ T cells are capable of inhibiting the homeostatic expansion of both CD4+ and CD8+ naïve αβ T cells (46). Similarly, follicular B cells may inhibit the homeostatic expansion of both mature and immature B cells (4). In contrast, T cells and B cells are thought to constitute independent homeostatic pools (47, 48). Despite the apparent autonomy of B cell and T cell populations, recent data suggest that competition between heterologous lymphocytes may also play a role in lymphocyte homeostasis. Specifically, NK T cell homeostatic expansion is enhanced in the absence of NK cells (5, 8). Here, we show that both NK cells and CD8+ αβ T cells influence γδ T cell homeostasis. Because γδ T cells have been shown to regulate both αβ T cell and NK cell function (49, 50), it is intriguing that these cells inversely play a role in regulating γδ T cell homeostasis.
The ability of αβ T cells to inhibit γδ T cell homeostasis may be due simply to their greater cell number. Such reasoning could explain why depletion of NK cells, a smaller lymphocyte population, does not allow γδ T cell homeostatic expansion in TCRδ-/- mice. Additionally, although γδ T cells are capable of inhibiting their own homeostatic expansion, this effect was detectable only when the competing γδ T cells greatly outnumbered the population of interest. Nevertheless, greater cell number alone is not sufficient to explain the inhibitory effect of αβ T cells on γδ T cell homeostasis. Despite similar numbers at the time of harvest, purified CD8+ αβ T cells dramatically reduced γδ T cell reconstitution, whereas CD4+ αβ T cells had no significant effect.
Our studies suggest that IL-15 and, to a lesser extent, IL-7 contribute to γδ T cell homeostasis. Consistent with our results, a recent study using a different system also suggested that both IL-7 and IL-15 support γδ T cell homeostatic proliferation (2). The dual contribution of these cytokines to lymphoid γδ T cell homeostasis likely explains the observed persistence of Thy-1+ lymphoid γδ T cells in IL-15-/- mice (26). Competition for cytokine resources, primarily IL-15, is at least partly responsible for the interplay between αβ T cells and γδ T cells during homeostatic expansion. Similar to γδ T cells, both IL-7 and IL-15 have been implicated in maintenance of the CD8+ αβ T cell population (13, 46). More than 50% of CD8+ αβ T cells express high levels of both IL-7Rα and IL-2Rβ 5 days after adoptive transfer into TCRβ-/-/δ-/- hosts (data not shown). Furthermore, the inhibitory effect of αβ T cells on γδ T cell homeostatic expansion was eliminated in the presence of excess exogenous IL-15. Although we did not directly test the ability of IL-7 to rescue γδ T cell homeostatic expansion, it is likely that competition for IL-7 plays only a secondary role in our model.
If αβ T cells are capable of outcompeting γδ T cells for IL-15 stores, one might expect αβ T cells to express higher levels of the required cytokine receptors, compared with γδ T cells. However, the donor γδ T cells used in our studies express IL-2Rβ and IL-15Rα at a level that is comparable with or exceeds that of αβ T cells, both before and after adoptive transfer (data not shown). Thus, the relative disadvantage of γδ T cells, compared with αβ T cells, during homeostatic expansion (Fig. 3_B_) is not likely due to poor cytokine binding. Instead, they may have a decreased propensity for survival and/or a more limited inherent proliferative potential, compared with αβ T cells. In support of this hypothesis, the majority of lymphoid γδ T cells are thought to be short-lived, compared with αβ T cells (10, 51). Of note, whereas cytokines play an important role in αβ T cell homeostasis, several studies have revealed a role for T cell antigen receptor (TCR)-MHC interactions in maintaining the αβ T cell pool (52, 53). Such interactions might prolong αβ T cell survival, providing an early advantage over most γδ T cells. Additionally, αβ T cells may actively inhibit γδ T cell homeostasis by inducing γδ T cell apoptosis through apoptosis-mediating surface antigen Fas (CD95)-Fas ligand (CD95L) interactions, as has been suggested for memory T cell homeostasis (54). Given an initial advantage, cotransferred αβ T cells could quickly exceed the γδ T cell population and would then provide substantial competition for essential cytokine stores.
γδ T cells are known to have potent antitumor effects both in vitro and in murine tumor models (55-58). In human studies, in vivo γδ T cell expansions have been shown to correlate with temporary tumor regression and increased survival of leukemia and myeloma patients (59-61). In light of these findings, the possibility of using γδ T cells in cancer immunotherapy is now being explored, and such studies have already shown some promise. However, animal studies suggest that prolonged maintenance of donor γδ T cells may be essential to achieve complete tumor regression (62, 63). Defining the mechanisms responsible for regulating γδ T cell homeostasis may be critical to the success of such therapies.
Acknowledgments
We thank Dr. Philippa Marrack (National Jewish Medical and Research Center) for generously providing antibody-producing hybridomas used in our studies and Dr. Marrack and Dr. Laurent Gapin for critical reading of the manuscript. This work was supported by National Institutes of Health Grants 2R01AI44920 (to R.L.O.), 2T32AI000048 (to J.D.F.), and 1R01HL65410 and 2R01AI40611 (to W.K.B.). J.D.F. was also supported by a fellowship from the American Heart Association (05200005Z) and C.L.R. by an Arthritis Foundation Investigator Award.
Abbreviations: CFSE, carboxyfluorescein diacetate succinimidyl ester; NK, natural killer.
References
- 1.Born, W., Cady, C., Jones-Carson, J., Mukasa, A., Lahn, M. & O'Brien, R. (1999) Adv. Immunol. 71**,** 77-144. [PubMed] [Google Scholar]
- 2.Baccala, R., Witherden, D., Gonzalez-Quintial, R., Dummer, W., Surh, C. D., Havran, W. L. & Theofilopoulus, A. N. (2005) J. Immunol. 174**,** 4606-4612. [DOI] [PubMed] [Google Scholar]
- 3.Bell, E. B., Sparshott, S. M., Drayson, M. T. & Ford, W. L. (1987) J. Immunol. 139**,** 1379-1384. [PubMed] [Google Scholar]
- 4.Cabatingan, M. S., Schmidt, M. R., Sen, R. & Woodland, R. T. (2002) J. Immunol. 169**,** 6795-6805. [DOI] [PubMed] [Google Scholar]
- 5.Matsuda, J. L., Gapin, L., Sidobre, S., Kieper, W. C., Tan, J. T., Ceredig, R., Surh, C. D. & Kronenberg, M. (2002) Nat. Immunol. 3**,** 966-974. [DOI] [PubMed] [Google Scholar]
- 6.Miller, R. A. & Stutman, O. (1984) J. Immunol. 133**,** 2925-2932. [PubMed] [Google Scholar]
- 7.Prlic, M., Blazar, B. R., Farrar, M. A. & Jameson, S. C. (2003) J. Exp. Med. 197**,** 967-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ranson, T., Vosshenrich, C. A., Corcuff, E., Richard, O., Laloux, V., Lehuen, A. & Di Santo, J. P. (2003) Proc. Natl. Acad. Sci. USA 100**,** 2663-2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rocha, B., Dautigny, N. & Pereira, P. (1989) Eur. J. Immunol. 19**,** 905-911. [DOI] [PubMed] [Google Scholar]
- 10.Tough, D. F. & Sprent, J. (1998) J. Exp. Med. 187**,** 357-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boursalian, T. E. & Bottomly, K. (1999) J. Immunol. 162**,** 3795-3801. [PubMed] [Google Scholar]
- 12.Ferreira, C., Barthlott, T., Garcia, S., Zamoyska, R. & Stockinger, B. (2000) J. Immunol. 165**,** 3689-3694. [DOI] [PubMed] [Google Scholar]
- 13.Schluns, K. S., Kieper, W. C., Jameson, S. C. & Lefrancois, L. (2000) Nat. Immunol. 1**,** 426-432. [DOI] [PubMed] [Google Scholar]
- 14.Seddon, B. & Zamoyska, R. (2002) J. Immunol. 169**,** 3752-3759. [DOI] [PubMed] [Google Scholar]
- 15.Tan, J. T., Dudl, E., LeRoy, E., Murray, R., Sprent, J., Weinberg, K. I. & Surh, C. D. (2001) Proc. Natl. Acad. Sci. USA 98**,** 8732-8737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kennedy, M. K., Glaccum, M., Brown, S. N., Butz, E. A., Viney, J. L., Embers, M., Matsuki, N., Charrier, K., Sedger, L., Willis, C. R., et al. (2000) J. Exp. Med. 191**,** 771-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ku, C. C., Murakami, M., Sakamoto, A., Kappler, J. & Marrack, P. (2000) Science 288**,** 675-678. [DOI] [PubMed] [Google Scholar]
- 18.Lodolce, J. P., Boone, D. L., Chai, S., Swain, R. E., Dassopoulos, T., Trettin, S. & Ma, A. (1998) Immunity 9**,** 669-676. [DOI] [PubMed] [Google Scholar]
- 19.Cooper, M. A., Bush, J. E., Fehniger, T. A., VanDeusen, J. B., Waite, R. E., Liu, Y., Aguila, H. L. & Caligiuri, M. A. (2002) Blood 100**,** 3633-3638. [DOI] [PubMed] [Google Scholar]
- 20.Fehniger, T. A., Suzuki, K., Ponnappan, A., VanDeusen, J. B., Cooper, M. A., Florea, S. M., Freud, A. G., Robinson, M. L., Durbin, J. & Caligiuri, M. A. (2001) J. Exp. Med. 193**,** 219-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ranson, T., Vosshenrich, C. A., Corcuff, E., Richard, O., Muller, W. & Di Santo, J. P. (2003) Blood 101**,** 4887-4893. [DOI] [PubMed] [Google Scholar]
- 22.Maeurer, M. J. & Lotze, M. T. (1998) Int. Rev. Immunol. 16**,** 309-322. [DOI] [PubMed] [Google Scholar]
- 23.Schlissel, M. S., Durum, S. D. & Muegge, K. (2000) J. Exp. Med. 191**,** 1045-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laky, K., Lewis, J. M., Tigelaar, R. E. & Puddington, L. (2003) J. Immunol. 170**,** 4087-4094. [DOI] [PubMed] [Google Scholar]
- 25.De Creus, A., Van Beneden, K., Stevenaert, F., Debacker, V., Plum, J. & Leclercq, G. (2002) J. Immunol. 168**,** 6486-6493. [DOI] [PubMed] [Google Scholar]
- 26.Schluns, K. S., Nowak, E. C., Cabrera-Hernandez, A., Puddington, L., Lefrancois, L. & Aguila, H. L. (2004) Proc. Natl. Acad. Sci. USA 101**,** 5616-5621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kubo, R. T., Born, W., Kappler, J. W., Marrack, P. & Pigeon, M. (1989) J. Immunol. 142**,** 2736-2742. [PubMed] [Google Scholar]
- 28.Unkeless, J. C. (1979) J. Exp. Med. 150**,** 580-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grabstein, K. H., Waldschmidt, T. J., Finkelman, F. D., Hess, B. W., Alpert, A. R., Boiani, N. E., Namen, A. E. & Morrissey, P. J. (1993) J. Exp. Med. 178**,** 257-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sudo, T., Nishikawa, S., Ohno, N., Akiyama, N., Tamakoshi, M. & Yoshida, H. (1993) Proc. Natl. Acad. Sci. USA 90**,** 9125-9129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanaka, T., Tsudo, M., Karasuyama, H., Kitamura, F., Kono, T., Hatakeyama, M., Taniguchi, T. & Miyasaka, M. (1991) J. Immunol. 147**,** 2222-2228. [PubMed] [Google Scholar]
- 32.Zurawski, S. M., Mosmann, T. R., Benedik, M. & Zurawski, G. (1986) J. Immunol. 137**,** 3354-3360. [PubMed] [Google Scholar]
- 33.Tomonari, K. (1988) Immunogenetics 28**,** 455-458. [DOI] [PubMed] [Google Scholar]
- 34.Lyons, A. B. & Parish, C. R. (1994) J. Immunol. Methods 171**,** 131-137. [DOI] [PubMed] [Google Scholar]
- 35.Mombaerts, P., Clarke, A. R., Hooper, M. L. & Tonegawa, S. (1991) Proc. Natl. Acad. Sci. USA 88**,** 3083-3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Itohara, S., Mombaerts, P., Lafaille, J., Iacomini, J., Nelson, A., Clarke, A. R., Hooper, M. L., Farr, A. & Tonegawa, S. (1993) Cell 72**,** 337-348. [DOI] [PubMed] [Google Scholar]
- 37.Mombaerts, P., Clarke, A. R., Rudnicki, M. A., Iacomini, J., Itohara, S., Lafaille, J. J., Wang, L., Ichikawa, Y., Jaenisch, R., Hooper, M. L. & Tonegawa, S. (1992) Nature 360**,** 225-231. [DOI] [PubMed] [Google Scholar]
- 38.Pennington, D. J., Silva-Santos, B., Shires, J., Theodoridis, E., Pollitt, C., Wise, E. L., Tigelaar, R. E., Owen, M. J. & Hayday, A. C. (2003) Nat. Immunol. 4**,** 991-998. [DOI] [PubMed] [Google Scholar]
- 39.Bender, J., Mitchell, T., Kappler, J. & Marrack, P. (1999) J. Exp. Med. 190**,** 367-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ernst, B., Lee, D. S., Chang, J. M., Sprent, J. & Surh, C. D. (1999) Immunity 11**,** 173-181. [DOI] [PubMed] [Google Scholar]
- 41.Fung-Leung, W. P., Schilham, M. W., Rahemtulla, A., Kundig, T. M., Vollenweider, M., Potter, J., van Ewijk, W. & Mak, T. W. (1991) Cell 65**,** 443-449. [DOI] [PubMed] [Google Scholar]
- 42.Koller, B. H., Marrack, P., Kappler, J. W. & Smithies, O. (1990) Science 248**,** 1227-1230. [DOI] [PubMed] [Google Scholar]
- 43.Lahn, M., Kanehiro, A., Takeda, K., Terry, J., Hahn, Y. S., Aydintug, M. K., Konowal, A., Ikuta, K., O'Brien, R. L., Gelfand, E. W. & Born, W. K. (2002) Proc. Natl. Acad. Sci. USA 99**,** 8850-8855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nunez-Cruz, S., Aguado, E., Richelme, S., Chetaille, B., Mura, A. M., Richelme, M., Pouyet, L., Jouvin-Marche, E., Xerri, L., Malissen, B. & Malissen, M. (2003) Nat. Immunol. 4**,** 999-1008. [DOI] [PubMed] [Google Scholar]
- 45.Marrack, P., Bender, J., Hildeman, D., Jordan, M., Mitchell, T., Murakami, M., Sakamoto, A., Schaefer, B. C., Swanson, B. & Kappler, J. (2000) Nat. Immunol. 1**,** 107-111. [DOI] [PubMed] [Google Scholar]
- 46.Tan, J. T., Ernst, B., Kieper, W. C., LeRoy, E., Sprent, J. & Surh, C. D. (2002) J. Exp. Med. 195**,** 1523-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kitamura, D., Roes, J., Kuhn, R. & Rajewsky, K. (1991) Nature 350**,** 423-426. [DOI] [PubMed] [Google Scholar]
- 48.Marrack, P. & Kappler, J. (2004) Annu. Rev. Immunol. 22**,** 765-787. [DOI] [PubMed] [Google Scholar]
- 49.Kaufmann, S. H. E., Blum, C. & Yamamoto, S. (1993) Proc. Natl. Acad. Sci. USA 90**,** 9620-9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ladel, C. H., Blum, C. & Kaufmann, S. H. (1996) Infect. Immun. 64**,** 1744-1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tough, D. F. & Sprent, J. (1994) J. Exp. Med. 179**,** 1127-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kirberg, J., Berns, A. & von Boehmer, H. (1997) J. Exp. Med. 186**,** 1269-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tanchot, C., Lemonnier, F. A., Perarnau, B., Freitas, A. A. & Rocha, B. (1997) Science 276**,** 2057-2062. [DOI] [PubMed] [Google Scholar]
- 54.Callard, R. E., Stark, J. & Yates, A. J. (2003) Trends Immunol. 24**,** 370-375. [DOI] [PubMed] [Google Scholar]
- 55.Zocchi, M. R., Ferrarini, M. & Rugarli, C. (1990) Eur. J. Immunol. 20**,** 2685-2689. [DOI] [PubMed] [Google Scholar]
- 56.Kunzmann, V., Bauer, E., Feurle, J., Weissinger, F., Tony, H. P. & Wilhelm, M. (2000) Blood 96**,** 384-392. [PubMed] [Google Scholar]
- 57.Girardi, M., Oppenheim, D. E., Steele, C. R., Lewis, J. M., Glusac, E., Filler, R., Hobby, P., Sutton, B., Tigelaar, R. E. & Hayday, A. C. (2001) Science 294**,** 605-609. [DOI] [PubMed] [Google Scholar]
- 58.Penninger, J. M., Wen, T., Timms, E., Potter, J., Wallace, V. A., Matsuyama, T., Ferrick, D., Sydora, B., Kronenberg, M. & Mak, T. W. (1995) Nature 375**,** 241-244. [DOI] [PubMed] [Google Scholar]
- 59.Wilhelm, M., Kunzmann, V., Eckstein, S., Reimer, P., Weissinger, F., Ruediger, T. & Tony, H. P. (2003) Blood 102**,** 200-206. [DOI] [PubMed] [Google Scholar]
- 60.Lamb, L. S., Jr., Henslee-Downey, P. J., Parrish, R. S., Godder, K., Thompson, J., Lee, C. & Gee, A. P. (1996) J. Hematother. 5**,** 503-509. [DOI] [PubMed] [Google Scholar]
- 61.Dieli, F., Gebbia, N., Poccia, F., Caccamo, N., Montesano, C., Fulfaro, F., Arcara, C., Valerio, M. R., Meraviglia, S., Di Sano, et al. (2003) Blood 102**,** 2310-2311. [DOI] [PubMed] [Google Scholar]
- 62.Zheng, B. J., Chan, K. W., Im, S., Chua, D., Sham, J. S., Tin, P. C., He, Z. M. & Ng, M. H. (2001) Int. J. Cancer 92**,** 421-425. [DOI] [PubMed] [Google Scholar]
- 63.Chen, J., Niu, H., He, W. & Ba, D. (2001) Int. Arch. Allergy Immunol. 125**,** 256-263. [DOI] [PubMed] [Google Scholar]