The δ subunit of AP-3 is required for efficient transport of VSV-G from the trans-Golgi network to the cell surface (original) (raw)
Abstract
Vesicular stomatitis virus glycoprotein (VSV-G) is a transmembrane protein that functions as the surface coat of enveloped viral particles. We report the surprising result that VSV-G uses the tyrosine-based di-acidic motif (-YTDIE-) found in its cytoplasmic tail to recruit adaptor protein complex 3 for export from the trans-Golgi network. The same sorting code is used to recruit coat complex II to direct efficient transport from the endoplasmic reticulum to the Golgi apparatus. These results demonstrate that a single sorting sequence can interact with sequential coat machineries to direct transport through the secretory pathway. We propose that use of this compact sorting domain reflects a need for both efficient endoplasmic reticulum export and concentration of VSV-G into specialized post-trans-Golgi network secretory-lysosome type transport containers to facilitate formation of viral coats at the cell surface.
The mechanism(s) by which diverse types of cargo exit the sorting environment of the trans-Golgi network (TGN) to multiple destinations remains to be clarified. At least three major types of cargo exit the TGN: (i) those destined for the apical or basolateral cell surface through a constitutive pathway, (ii) those directed to the endosomal/lysosomal system, and (iii) those directed into regulated, post-Golgi secretory compartments (1–4). One set of pathways makes use of adaptor protein (AP) complexes to direct sorting. Four different APs (AP1–4) of distinct subunit composition are currently recognized (2, 5, 6). Interaction with APs can involve both tyrosine-based sorting motifs (YXXΦ; where X can be polar residues and Φ is a bulky hydrophobic residue) (7) as well as di-leucine/acidic residue-containing motifs found in the cytoplasmic tail of transmembrane cargo (2). AP-2 complexes mediate endocytosis through clathrin coats at the cell surface (2), and AP-1A adaptor complexes containing the μ1A subunit direct clathrin-dependent sorting of soluble enzymes from the TGN to the endosomal/lysosomal pathway (8). A novel AP-1 complex (AP-1B) containing the μ1B isoform restricted to polarized epithelial cells facilitates the sorting of the low density lipoprotein receptor from the TGN to the basolateral surface through the tyrosine-based sorting motif NPXY (9, 10). AP-4 has recently been suggested to promote the basolateral targeting of a restricted set of proteins (11). Finally, AP-3 complexes direct the transport of transmembrane cargo from the TGN to the vacuole in yeast (5). In mammalian cells, AP-3 directs cargo to lysosomes (12) and specialized secretory lysosome-type compartments that include melanosomes and platelets (13, 14).
Given the complexity of selective export of cargo from the TGN, we used a two-hybrid approach to identify sorting factors/coat complexes that interact with the tyrosine-based sorting motif (YTDIE) found in the cytoplasmic tail of vesicular stomatitis virus glycoprotein (VSV-G), a type 1 transmembrane protein. VSV-G is transported from the TGN to cell surface by using both vesicular and tubular containers (15–17). We now report that AP-3, but not AP-1 or AP-2 coat complexes, interact with the YTDIE export motif and that AP-3 is involved in transport of VSV-G to the cell surface in vivo. Binding to AP-3 involves a novel interaction with the δ subunit of AP-3 coats. Our results demonstrate that VSV-G contains a compact sorting domain that uses overlapping codes to recruit sequential cytosolic coat machineries that dictate movement from the endoplasmic reticulum (ER) to the cell surface.
Materials and Methods
Materials.
C57BL/6J and Mocha mice were obtained from The Jackson Laboratory. Rabbit antibodies against α-, γ-, and δ-adaptin were provided by M. S. Robinson (University of Cambridge, Cambridge, U.K.). VSV-G antibodies (T25I and 8G5) have been described (18, 19). The H4A3 mAb was obtained from the Developmental Studies Hybridoma Bank (University of Iowa, Iowa City).
DNA Constructs and Two-Hybrid Screening.
pAR VSV-G-YxDxE, -AxDxE, and -YxAxA have been described (20). Two-hybrid bait vectors were constructed by standard techniques using the 29-residue cytoplasmic tail of VSV-G sequences with the indicated mutations (21). The Human HeLa MATCHMAKER cDNA library was obtained from CLONTECH, and two-hybrid screens of a HeLa cell cDNA library in pGAD-GH were performed and evaluated according to the manufacturer's instructions.
Transfection and Transport.
HeLa cells were transfected with pAR vector encoding either VSV-G-YxDxE, AxDxE, or YxAxA, and cells were lysed and immunoprecipitated with a mouse antibody against VSV-G as described (22). Where indicated, cells were pulse-labeled with [35S]methionine (0.1 mCi/ml) at 37°C and chased for the indicated times at 20°C or 37°C and analyzed as described (22). To detect surface VSV-G, biotinylation was performed by incubating the cells with 0.5 mg/ml Sulfo-NHS-Biotin (Pierce) in PBS at 4°C (30 min) and quenched with 50 mM NH4Cl in PBS. Cells were lysed with 1% Triton X-100 in TBS-150 (50 mM Tris/150 mM NaCl, pH 7.5). Total VSV-G was immunoprecipitated (22, 23), washed and eluted with 0.1 ml of 1% SDS in TBS-150 at 90°C for 3 min, and diluted with 1 ml of 1% Triton X-100 in TBS-150. Biotinylated VSV-G was subsequently recovered with streptoavidin-agarose and surface VSV-G was eluted with 2× SDS/PAGE sample buffer.
Results
The Cytoplasmic Tail of VSV-G Binds the δ Subunit of AP-3.
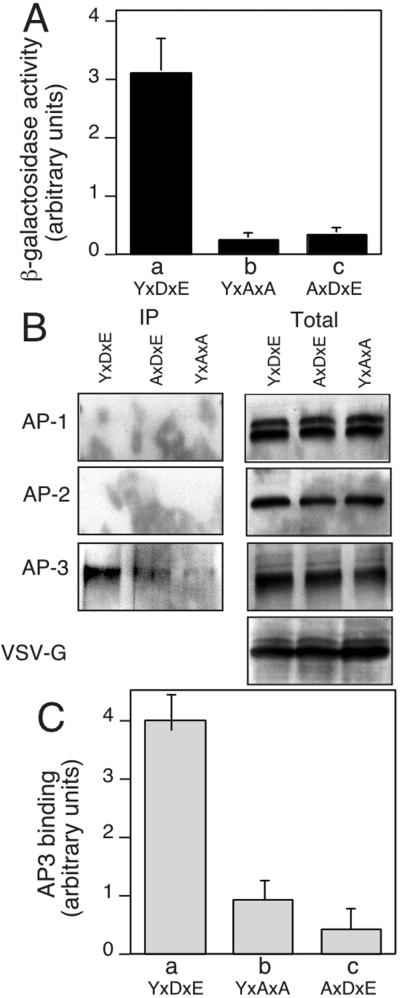
To identify the machinery involved in transport of VSV-G from the TGN to the cell surface, we screened a cDNA library by using a yeast two-hybrid selection for proteins that bind the wild-type sequence. Subsequently, we evaluated the ability of positive clones to interact with VSV-G tail mutants in which the YTDIE motif was mutated to either YxAxA (to remove the di-acidic code involved in ER export) (18, 20) or AxDxE (to interfere with the function of the critical Tyr residue involved in TGN sorting to the basolateral surface) (24). In this way, we could focus on prey clones that interact only with the complete YTDIE motif. Of 91 clones recovered in the first screen, five clones were recovered in the second screen, two of which encoded a fragment (residues 578–825) of δ-adaptin, a subunit of the AP-3 complex (Fig. 1A).
Figure 1.
Specific interaction of VSV-G and AP-3. (A) Two-hybrid analysis of the interaction of δ-adaptin (residues 578–825) with wild-type (YxDxE), AxDxE, and YxAxA sorting motifs found in the VSV-G cytoplasmic tail. β-Galactosidase activity of Y190 strains coexpressing the indicated VSV-G tail and δ-adaptin constructs was performed in triplicate. (B) Coimmunoprecipitation of VSV-G and APs. HeLa cells were transfected with either pAR wild-type VSV-G or the AxDxE or YxAxA mutants, and VSV-G was immunoprecipitated by using anti-VSV-G antibody. The VSV-G containing immunoprecipitates (Left) or 10% of total lysate used for immunoprecipitation (Right) were immunoblotted by using γ-, α-, and δ-adaptin antibodies. (C) Quantitation of recovery in B adjusted for total AP-3 in lysate.
To determine whether the interaction of δ-adaptin with the VSV-G cytoplasmic tail was important physiologically, we examined whether they could be coprecipitated in vivo. HeLa cells were transfected with VSV-G containing either wild-type VSV-G (YxDxE) or the YxAxA or AxDxE mutants, lysed, and immunoprecipitated by anti-VSV-G antibody. The resulting immunoprecipitates were examined by using quantitative immunoblotting. Whereas AP-3 was found to interact strongly with wild-type VSV-G (YxDxE) with ≈7% of the total AP3 pool recovered in the immunoprecipitate, the AxDxE mutant bound with nearly 4- to 5-fold less efficiency based on quantitative immunoblotting (Fig. 1 B and C). A similar level of reduced binding was detected with the YxAxA mutant lacking the acidic residues (Fig. 1 B and C). Although consistent with the two-hybrid results, the differences observed in retention of partial binding in vivo by the AxDxE and YxAxA mutants could reflect the contributions of other components of the AP3 complex, in particular, the μ subunit known to be involved in recognition of tyrosine-based motifs. The latter result is consistent with previous observations that acidic residues are critical for AP-3 binding to other cargo proteins (25–29). The interactions with AP-3 were specific, because neither AP-1A nor AP-2 could be detected in the VSV-G immunoprecipitate (Fig. 1B). Thus, our results show that the δ subunit of AP-3 specifically recognizes the YxDxE motif in nonpolarized cells.
AP-3 Facilitates Export of VSV-G from the TGN.
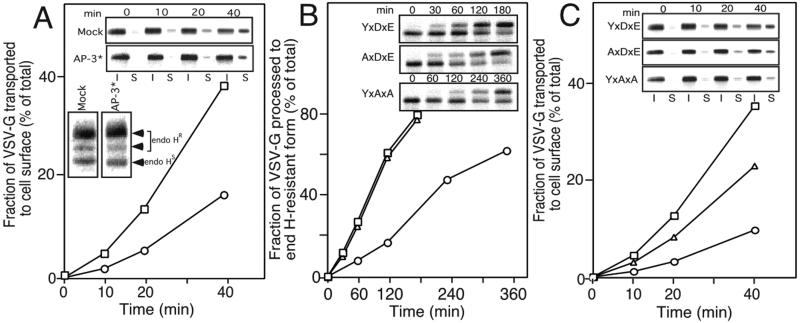
The above results suggest that the interaction of VSV-G with AP-3 requires both Tyr and di-acidic resides. As both have also been implicated in coat complex II recruitment (18, 20, 30), which step(s) in the secretory pathway use the AP-3 coat to promote cargo selection? Given that the AP-3 prey clone isolated in the two-hybrid screen encoded only a fragment (residues 578–825) of the full-length δ-adaptin (1,153 aa), this fragment must contain the VSV-G binding region and therefore may behave as a competitive inhibitor of normal AP-3 function in vivo (31). To test this possibility, the AP-3 fragment (AP-3*) and VSV-G were cotransfected into HeLa cells and its effect on the transport of VSV-G was determined. For this purpose, we used a variant form tsO45 VSV-G (VSV-Gts) that has a temperature-sensitive transport phenotype. When cells are transfected with VSV-Gts at 39.5°C (the restrictive temperature), the protein is retained in the ER because of a folding defect. Transfer of cells to the permissive temperature (32°C) rapidly reverses this defect, causing VSV-Gts to be synchronously exported from the ER (32, 33). Delivery to the Golgi was determined by measuring the extent of processing of VSV-Gts to endoglycosidase H (endo H)-resistant forms by Golgi-associated α-mannosidases and glycosyltransferases.
As shown in Fig. 2A Lower Inset, overexpression of the AP-3* did not have any affect on the extent of processing of VSV-Gts to endo H-resistant forms. Next, we examined whether the transport of VSV-Gts from the TGN to the plasma membrane (PM) was affected by overexpression of the AP-3* by using cell surface biotinylation (34). To only measure transport from the TGN to the cell surface, transfected cells were labeled with [35S]Met at 39.5°C and chased for 3 h at 20°C, a temperature that leads to the accumulation of VSV-G in the TGN (35). Subsequently, cells were incubated for the indicated time period at 37°C, transferred to ice, and biotinylated, and cell surface VSV-Gts was recovered on streptavidin beads. In contrast to the lack of effect of the AP-3* on ER to Golgi transport, the rate of TGN to PM transport (Fig. 2A Upper Inset) was significantly (30–40%) inhibited (Fig. 2A). These results are consistent with the localization of AP-3 to the TGN (36–38). We conclude that the transport step in which VSV-G uses AP-3 for delivery to the cell surface is initiated at the TGN.
Figure 2.
AP-3 can facilitate TGN-PM transport of VSV-G. (A) The AP-3 fragment inhibits TGN to PM transport of VSV-G. Baby hamster kidney (BHK) cells were transfected with the pAR wild-type VSV-G and either the pCR3.1 (□) (mock) or pCR3.1-AP-3 fragment (AP-3*) (○). Cell surface (S) and intracellular forms are indicated (I). (Inset) AP-3* does not inhibit ER to Golgi transport of VSV-G. BHK cells were transfected with wild-type VSV-G and either pCR3.1 (mock) or the AP-3*, labeled, and processed for endo H resistance. (B) ER to Golgi transport of VSV-G at 20°C. Appearance of endo H-resistant forms of wild-type (YxDxE) (□) or the AxDxE (▵) and YxAxA (○) mutants in BHK cells was measured for the indicated time at 20°C. (C) TGN to PM transport of VSV-G. Appearance of biotinylated forms of wild-type (YxDxE) (□) or the AxDxE (▵) and YxAxA (○) mutants in BHK cells was measured for the indicated time at 37°C after either 100 min (YxDxE and AxDxE) or 240 min (YxAxA) at 20°C.
Mutant Sorting Codes Show Reduced Kinetics of TGN to PM Transport.
If AP-3 is specifically required for transport of VSV-G from the TGN to the cell surface, then the YxAxA and AxDxE VSV-Gts mutants, which have reduced affinity for AP-3 (Fig. 1B), should exhibit reduced kinetics of TGN to PM transport compared with wild-type protein. Because the YxAxA mutant is transported from the ER to the Golgi at a much slower rate than the wild-type protein (20), we first measured the kinetics of ER to Golgi transport of VSV-Gts wild type (YxDxE) and the YxAxA and AxDxE mutants during incubation of cells at 20°C to determined the time point at which 50% of the protein acquired endo H resistance. These values were 100 min for the YxDxE and AxDxE mutants and 240 min for YxAxA (Fig. 2B), consistent with previous results (20). After accumulation of 50% of the total VSV-G in the TGN, we then determined the rate of TGN to PM transport of endo H-resistant forms by cell surface biotinylation. We analyzed only the initial 40-min time period when no more than 50% of the wild-type VSV-G had reached the cell surface to exclude any contribution of cell surface labeling from newly synthesized VSV-G exiting the ER. Both the YxAxA and AxDxE mutants showed significantly impaired rates of TGN to PM transport compared with wild-type VSV-Gts (Fig. 2C). Importantly, the efficiency of TGN to PM transport (YxDxE>AxDxE>YxAxA) was consistent with the relative affinity of VSV-G for AP-3 (YxDxE>AxDxE>YxAxA) (Figs. 1B and 2C). Taken together, these data reinforce the conclusion that AP-3 functions in TGN to PM transport of VSV-G.
Transport of VSV-G Is Markedly Reduced in Mocha Mice Fibroblasts Lacking AP-3.
Analyses of hereditary deficiencies have now clearly shown that loss of AP-3 function results in defective biosynthesis of the secretory lysosomes including melanosomes and platelet granules (5, 13, 31, 39–45). For example, mutations in both δ-adaptin (Mocha mouse) and β3A-adaptin (Hermansky Pudlak syndrome and Pearl mouse) cause defects in the biogenesis of the melanosome pigment melanin as a consequent of failure to efficiently sort enzymes required for melanin synthesis from the TGN to this regulated pathway (41, 42, 45).
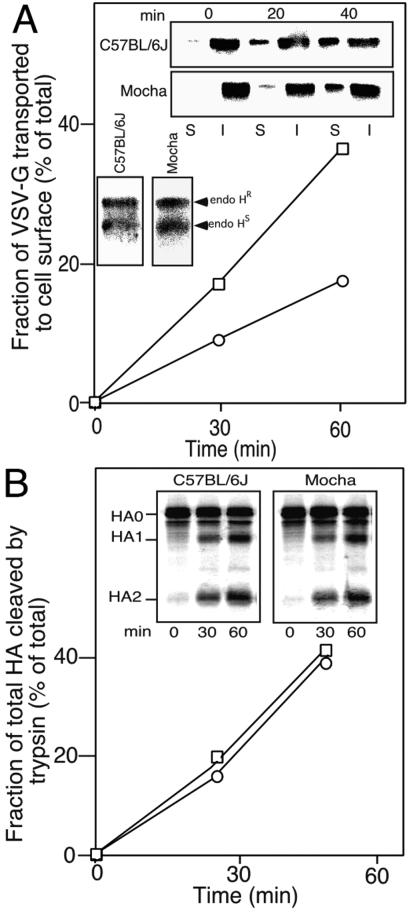
To further characterize the role of AP-3 in VSV-G transport, we established a primary skin fibroblast culture from both δ-adaptin deficient (Mocha) and control (C57BL/6J) mice (45). Mocha and control fibroblasts were transfected with wild-type VSV-Gts and transport from the TGN after accumulation at 20°C was measured with surface biotinylation. Consistent with the above results, TGN to PM transport of VSV-Gts in the Mocha fibroblast was significantly slower than control fibroblast (Fig. 3A). The reduced rate of transport in Mocha fibroblasts was specific for the TGN to PM step, as the rate of transport of VSV-G from the ER to the cis/medial Golgi compartments (based on the rate of processing of VSV-G to endo H-resistant forms) was identical to control fibroblasts (Fig. 3B Inset).
Figure 3.
VSV-G but not HA uses AP-3 for its transport to cell surface. (A) Appearance of biotinylated forms of VSV-G in C57BL/6J (□) and Mocha (○) fibroblasts was measured at the indicated time at 37°C after a 100-min preincubation at 20°C. (Inset) Appearance of endo H-resistant forms of VSV-G in C57BL/6J and Mocha fibroblasts was measured after a 100-min preincubation at 20°C. (B) C57BL/6J (□) and Mocha (○) fibroblasts were transfected with HA, labeled for 20 min with [35S]Met, chased with unlabeled Met for the indicated time at 37°C, digested with trypsin to cleave cell surface HA, and lysed as described (64). Total HA including uncleaved (HA0 representing intracellular HA) and cleaved (HA1 and HA2 representing cell surface HA) forms was immunoprecipitated with anti-HA antibody. The values for surface delivery of HA are expressed as a percent of HA1 + HA2/HA0 + HA1 + HA2.
To examine whether impaired TGN to PM transport in Mocha fibroblasts was specific for cargo directed to the basolateral surface, we also measured the rate of cell surface transport of the apical marker protein influenza virus hemaggulutinin (HA) (46–48). In contrast to VSV-G, whose transport to the PM was reduced in Mocha mice, HA transport to cell surface was unaffected (Fig. 3B). Thus, Mocha fibroblasts recapitulate the TGN sorting activities that separate basolateral and apical targeted cargo in polarized epithelial cells (47).
VSV-G Overexpression Interferes with Segregation of the AP-3-Dependent Cargo Protein LAMP1 into the Regulated Pathway.
If VSV-G is an authentic cargo for an AP-3-dependent transport carrier, it is likely that transport through this specific pathway is saturable. Thus, overexpression of VSV-G may compete with other cargo molecules that normally use AP-3 for export from the TGN. For example, the cell surface levels of the lysosomal proteins CD63/Lamp3, LAMP1 and Lamp2, but not M6PR or transferrin receptor, are increased in primary fibroblasts from Hermansky Pudlak syndrome patients defective in the (AP-3) β3A subunit (41). Moreover, disruption of AP-3 function results in the delivery of the lysosomal marker Lgp120 to the cell surface (12).
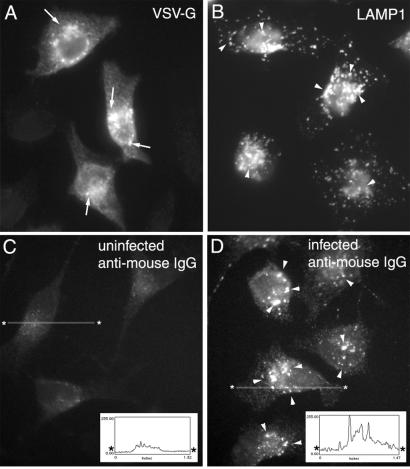
To address this possibility, we followed the effect of VSV-G expression on disrupting the normal transport of LAMP1 to the lysosome by infecting HeLa cells with wild-type VSV. Dislocation of LAMP1 to the cell surface can be detected by incubation of cells in the presence of a LAMP1-specific mouse monoclonal IgG. If transport to the cell surface occurs, binding of plasma membrane-localized LAMP1 leads to internalization and the accumulation of the antibody in the lysosome. Internalization can be quantitated by using a mouse-specific secondary antibody and indirect immunofluoresence of fixed, permeabilized cells. As shown in Fig. 4A, HeLa cells infected with VSV for 4 h express VSV-G in the secretory pathway including the Golgi and cell surface. HeLa cells have an abundant population of LAMP1-containing lysosomes as shown by antibody labeling of endogenous LAMP1 in fixed, permeabilized cells (Fig. 4B). In uninfected, control cells that do not express of VSV-G, incubation in the presence of the LAMP1-specific antibody in the medium for 45 min did not result in internalization and labeling of the endogenous lysosome population (Fig. 4C). In contrast, after incubation of infected cells for 45 min with anti-LAMP1 IgG, punctate structures containing the LAMP1-specific mouse IgG were readily detected (Fig. 4C). These results suggest that either biosynthetic LAMP1 was displaced to the cell surface from its normal AP3-dependent pathway during the time course of infection and/or that a recycling LAMP1 pool between endosome/lysosome compartments and the TGN was misdirected to the cell surface. Quantitation of these results indicate that whereas >95% of infected cells showed striking internalization of the LAMP1-specific IgG to the lysosome (300 cells counted), less than 2% of control cells showed comparable levels of LAMP1 antibody internalization. The efficiency of internalization of LAMP1-specific antibody in VSV-G-expressing cells is illustrated by the ratio of the pixel intensity of antibody-containing punctate structures to background cytoplasmic staining. This difference was generally 5- to 10-fold that observed in control cells (Fig. 4 C and D Insets). We conclude that VSV-G can usurp the endogenous TGN-derived AP-3 trafficking pathways to promote its efficient delivery to the cell surface.
Figure 4.
VSV-G can compete with LAMP1 for AP-3-mediated packaging in the TGN. Uninfected (B and C) or infected HeLa cells (A and D) with VSV at 32°C as described (33) were incubated in the presence of mouse anti-LAMP1 mAb (C and D) for 45 min at 32°C, fixed, permeabilized, and processed for indirect immunofluorescence microscopy with the indicated antibodies as described (33). Images typical of >300 cells were examined for each indicated condition. Arrows in A indicate VSV-G in Golgi elements, arrowheads in B and D indicate distribution of LAMP1 containing punctate structures. Asterisked line indicates location of density profile (pixels) shown in C and D Insets. (Magnifications: ×63.)
VSV-G Recruits AP-3 to the TGN.
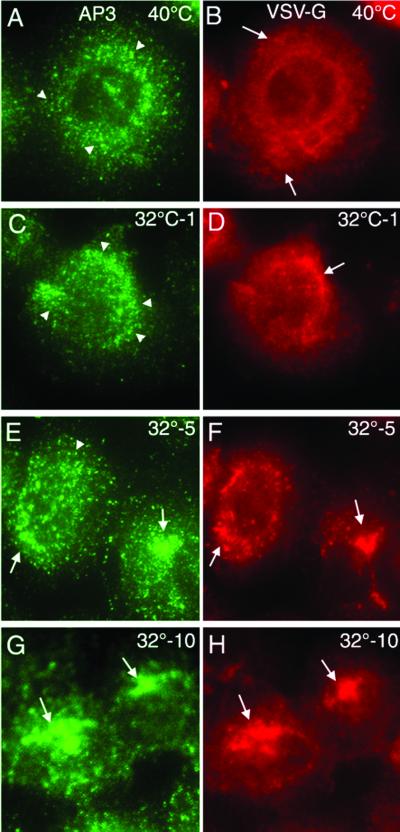
VSV infection of cells leads to reduction in host–protein synthesis. After 4 h of infection VSV-G is a major form of cargo transiting the secretory pathway. Indeed, we have previously demonstrated that retention of VSV-Gts in the ER during infection at 39.5°C not only prevents VSV-G export, but results in inhibition of coat complex II assembly and budding from the ER, reflecting lack of available cargo (49). Transfer to 32°C results in a synchronous burst of vesicles containing VSV-G budding from the ER, demonstrating that cargo can modulate coat complex II assembly (49). Similarly, the mannose-6-phosphate receptor can stimulate recruitment of AP-1 and GGA adaptors to the TGN for clathrin-mediated export (50–53), and lysosomal glycoproteins can promote recruitment of AP-3 coats to the TGN (12). If VSV-G indeed recruits AP-3 for transport to the cell surface, a specific prediction is that release of VSV-Gts from the ER in virus-infected cells should lead to localization of AP-3 to the TGN. This process should occur after a brief lag reflecting the time period (_t_1/2 ≈5–10 min) for VSV-G to reach terminal Golgi compartments (54, 55).
In infected BHK cells held at 39.5°C (Fig. 5A) or noninfected cells at either 32°C or 39.5°C (data not shown), AP-3 had a nearly random punctate distribution, reflecting association of AP-3 with endosomal compartments (37). This finding is in contrast to the diffuse distribution of VSV-G in the ER (Fig. 5B). After transfer to 32°C, there was a striking time-dependent recruitment of AP-3 to the perinculear Golgi region (Fig. 5 A, C, E, and G) corresponding to the typical kinetics of transport of VSV-G from the ER to the Golgi compartments (Fig. 5 B, D, F, and H). By 10 min of incubation where VSV-G populates the entire Golgi apparatus, 70–80% of infected cells show striking overlap with AP-3 in the Golgi region (Fig. 5 G and H). Thus, movement of a single type of cargo results in the sequential recruitment of the coat complex II (49) and AP-3 coat machineries to promote transport through the secretory pathway.
Figure 5.
VSV-G recruits AP-3 to the TGN. BHK cells were infected with the tsO45 VSV variant at 39.5°C for 4 h and subsequently transferred to ice (A and B) or incubated at 32°C for 1 (C and D), 5 (E and F) or 10 (G and H) min before transfer to ice. The distribution of VSV-G and AP-3 (δ-adaptin) were visualized by Texas red and Alexa 488, respectively. (Magnifications: ×63.)
Discussion
We have provided evidence that export of VSV-G from the TGN can use AP-3 adaptor complexes through an interaction with the δ-subunit: (i) we observed specific binding of the VSV-G tail to the δ subunit with two-hybrid analysis, (ii) VSV-G binding to AP-3 but not AP-1 or AP-2 could be readily detected in vivo, (iii) the AP-3 fragment inhibited TGN to cell surface, but not ER to Golgi transport, (iv) cell surface transport of VSV-G transport was reduced in Mocha mice fibroblasts lacking the AP-3 complex, (v) overexpression of VSV-G displaced the AP3 substrate LAMP1 to the cell surface, and (vi) synchronized release of VSV-G from the ER in viral-infected cells results in the recruitment of AP-3 to the TGN. Thus, our data suggests a new role for AP-3 and the δ subunit in transport of basolateral-sorted proteins containing an acidic tyrosine-based cytoplasmic sorting motif from the TGN.
Heterotetrameric APs (1–4) have a similar structure and are composed of two large chains (α/γ/δ/ɛ and β1–4), a medium chain (μ1–4), and a small light chain (σ1–4). The α/γ/δ/ɛ chains have been shown to bind accessory factors (56–58). Our data now suggest that the δ subunit has sorting function. It is intriguing that the 578–825 δ subunit fragment recovered in the two-hybrid screen spans a putative region of the δ subunit that includes the hinge and the ear, regions implicated in the interaction with Eps15, epsin, amphiphysin, and γ-synergin (56–58). Although the function of these accessory factors is unknown, we now suggest that VSV-G may also modulate AP-3 function through the δ subunit to promote rapid TGN export by AP-3.
The μ chains are now generally thought to recognize signals conforming to the YxxΦ motif found in the VSV-G cytoplasmic tail (2). Although we have not investigated directly the ability of the VSV-G YTDIE sorting motif to bind μ1–4 subunits, other studies have clearly demonstrated that closely related peptide sequences show strong interaction (2, 59). The structure of μ2 suggests that the Tyr and Φ residues fit into a hydrophobic pocket and that the identity of the Φ residue and residues flanking the critical Tyr play an important role in recognition of specific μ subunits. For example, μ1, μ2, μ3A, and μ4 prefer Leu, Leu, Ile, and Phe residues at the Φ position, and neutral, basic, acidic, or basic residues at the Tyr + 2 position, respectively (2). Interestingly, the YTDIE motif of VSV-G conforms best to the μ3A consensus recognition motif with an acidic residue in the Tyr + 2 position and Ile in the Φ position. Indeed, we found that mutation of these residues to Ala reduces the rate of TGN export. Acidic stretches associated with di-leucine motifs of the vam3 SNARE protein are also important in binding to μ3 (25). Thus, export of the homotrimeric VSV-G from the TGN may involve association with the AP-3 complex through both the δ and μ3 subunits. Such multivalent interactions with AP-3 subunits could mediate the assembly of the coat lattice to ensure highly efficient sorting and concentration of VSV-G for export from the TGN.
Why is VSV-G transported to the cell surface rather than being targeted to lysosomes or secretory lysosomes? One possibility is that AP-3 could direct the recruitment of all classes of AP-3 interacting proteins into a common vesicle/tubular carrier budding from the TGN, and that these different cargo molecules are segregated from one another in a post-TGN compartment such as a recycling endosome. However, the absence of VSV-G in endosomal compartments (16) argues against this possibility. A second possibility stems from the observation that cargo sequestered into regulated secretory pathways emerging from the TGN are diverted from the constitutive pathway by the specific removal of SNAp receptors that modulate constitutive fusion to the plasma membrane (60). Given the ability of VSV-G to usurp the function of the putative AP-3-regulated pathway, we suggest that it is both the type of cargo and the type of coat that dictates the targeting information recruited to or retained in carrier intermediates exiting the TGN. Indeed, two distinct classes of AP-3-coated structures, clathrin-associated and clathrin-independent AP-3s, have been reported (61), and a third may involve a novel form of clathrin (62). Thus, a combination of cargo, AP-3, and other coat components may favor coassembly with specific vesicle targeting proteins that direct the fate of the transport container to either the lysosome (yeast vacuole) (i.e., LAMP1), regulated secretory lysosome (i.e., tyrosinase), or constitutive pathways (VSV-G, other?). Although VSV-G can use AP-3 to promote export from the TGN, it is not the only pathway to the surface. We found only 50% inhibition of transport in the absence of AP-3 function in the Mocha mouse fibroblast lacking AP-3. Thus, VSV-G as well as other forms of cargo may be able to use other coat machineries in the absence of normal AP-3 function (9, 17). Given the recent report of the involvement of AP-4 in the basolateral sorting of tyrosine-motif containing proteins (11), AP-4 could serve as an alternative trafficking pathway, a possibility that remains to be explored.
Importantly, we have discovered that the di-acidic motif in the cytoplasmic tail of VSV-G participates in at least two sequential selection events in the secretory pathway—the first being efficient export from ER (18, 20) and the second, transport from the TGN to the cell surface by a AP-3-dependent mechanism. Thus, the YTDIE code appears to have the properties of a compact, multifunctional sorting domain that can be used to transport cargo through the entire secretory pathway. Because the Tyr residue, but not the acidic residues (DxE), have previously been shown to direct the fidelity of sorting of VSV-G from the TGN to the basolateral surface (63) it is now apparent that the DxE motif may supplement the specific role of Tyr in sorting by improving the efficiency of capture into AP-3-coated structures (18, 20). The use of a multifunctional sorting domain may reflect a need for both efficient ER export and prepackaging of VSV-G into specialized secretory lysosome-type transport containers to promote efficient formation of the dense VSV-G-containing viral coat at the cell surface.
Acknowledgments
N.N. was a Senior Postdoctoral Fellow of American Cancer Society-California Division. This work was supported by National Institutes of Health Grant GM42336 and National Cancer Institute Grant CA58689, Imaging Core C.
Abbreviations
TGN
trans-Golgi network
AP
adaptor protein
VSV-G
vesicular stomatitis virus glycoprotein
ER
endoplasmic reticulum
endo H
endoglycosidase H
PM
plasma membrane
HA
hemaggulutinin
BHK
baby hamster kidney
References
- 1.Hirst J, Robinson M S. Biochim Biophys Acta. 1998;1404:173–193. doi: 10.1016/s0167-4889(98)00056-1. [DOI] [PubMed] [Google Scholar]
- 2.Kirchhausen T. Annu Rev Cell Dev Biol. 1999;15:705–732. doi: 10.1146/annurev.cellbio.15.1.705. [DOI] [PubMed] [Google Scholar]
- 3.Lewin D A, Mellman I. Biochim Biophys Acta. 1998;1401:129–145. doi: 10.1016/s0167-4889(97)00130-4. [DOI] [PubMed] [Google Scholar]
- 4.Scales S J, Gomez M, Kreis T E. Int Rev Cytol. 2000;195:67–144. doi: 10.1016/s0074-7696(08)62704-7. [DOI] [PubMed] [Google Scholar]
- 5.Odorizzi G, Cowles C R, Emr S D. Trends Cell Biol. 1998;8:282–288. doi: 10.1016/s0962-8924(98)01295-1. [DOI] [PubMed] [Google Scholar]
- 6.Dell'Angelica E C, Mullins C, Bonifacino J S. J Biol Chem. 1999;274:7278–7285. doi: 10.1074/jbc.274.11.7278. [DOI] [PubMed] [Google Scholar]
- 7.Bonifacino J S, Dell'Angelica E C. J Cell Biol. 1999;145:923–926. doi: 10.1083/jcb.145.5.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le Borgne R, Hoflack B. Biochim Biophys Acta. 1998;1404:195–209. doi: 10.1016/s0167-4889(98)00057-3. [DOI] [PubMed] [Google Scholar]
- 9.Folsch H, Ohno H, Bonifacino J S, Mellman I. Cell. 1999;99:189–198. doi: 10.1016/s0092-8674(00)81650-5. [DOI] [PubMed] [Google Scholar]
- 10.Folsch H, Pypaert M, Schu P, Mellman I. J Cell Biol. 2001;152:595–606. doi: 10.1083/jcb.152.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simmen T, Honing S, Icking A, Tikkanen R, Hunziker W. Nat Cell Biol. 2002;4:154–159. doi: 10.1038/ncb745. [DOI] [PubMed] [Google Scholar]
- 12.Le Borgne R, Alconada A, Bauer U, Hoflack B. J Biol Chem. 1998;273:29451–29461. doi: 10.1074/jbc.273.45.29451. [DOI] [PubMed] [Google Scholar]
- 13.Rohn W M, Rouille Y, Waguri S, Hoflack B. J Cell Sci. 2000;113:2093–2101. doi: 10.1242/jcs.113.12.2093. [DOI] [PubMed] [Google Scholar]
- 14.Andrews N W. Trends Cell Biol. 2000;10:316–321. doi: 10.1016/s0962-8924(00)01794-3. [DOI] [PubMed] [Google Scholar]
- 15.Hirschberg K, Miller C M, Ellenberg J, Presley J F, Siggia E D, Phair R D, Lippincott-Schwartz J. J Cell Biol. 1998;143:1485–1503. doi: 10.1083/jcb.143.6.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keller P, Toomre D, Diaz E, White J, Simons K. Nat Cell Biol. 2001;3:140–149. doi: 10.1038/35055042. [DOI] [PubMed] [Google Scholar]
- 17.Simon J P, Ivanov I E, Adesnik M, Sabatini D D. Methods. 2000;20:437–454. doi: 10.1006/meth.2000.0957. [DOI] [PubMed] [Google Scholar]
- 18.Nishimura N, Bannykh S, Slabough S, Matteson J, Altschuler Y, Hahn K, Balch W E. J Biol Chem. 1999;274:15937–15946. doi: 10.1074/jbc.274.22.15937. [DOI] [PubMed] [Google Scholar]
- 19.Rowe T, Aridor M, McCaffery J M, Plutner H, Nuoffer C, Balch W E. J Cell Biol. 1996;135:895–911. doi: 10.1083/jcb.135.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishimura N, Balch W E. Science. 1997;277:556–558. doi: 10.1126/science.277.5325.556. [DOI] [PubMed] [Google Scholar]
- 21.Rose M D, Winston F, Hieter P. Methods in Yeast Genetics: A Laboratory Course Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1990. pp. 155–159. [Google Scholar]
- 22.Dascher C, VanSlyke J K, Thomas L, Balch W E, Thomas G. Methods Enzymol. 1995;257:174–188. doi: 10.1016/s0076-6879(95)57023-3. [DOI] [PubMed] [Google Scholar]
- 23.Sauve D M, Ho D T, Roberge M. Anal Biochem. 1995;226:382–383. doi: 10.1006/abio.1995.1242. [DOI] [PubMed] [Google Scholar]
- 24.Thomas D C, Brewer C B, Roth M G. J Biol Chem. 1993;268:3313–3320. [PubMed] [Google Scholar]
- 25.Darsow T, Burd C G, Emr S D. J Cell Biol. 1998;142:913–922. doi: 10.1083/jcb.142.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Honing S, Sandoval I V, von Figura K. EMBO J. 1998;17:1304–1314. doi: 10.1093/emboj/17.5.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohno H, Fournier M C, Poy G, Bonifacino J S. J Biol Chem. 1996;271:29009–29015. doi: 10.1074/jbc.271.46.29009. [DOI] [PubMed] [Google Scholar]
- 28.Stepp J D, Huang K, Lemmon S K. J Cell Biol. 1997;139:1761–1774. doi: 10.1083/jcb.139.7.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vowels J J, Payne G S. EMBO J. 1998;17:2482–2493. doi: 10.1093/emboj/17.9.2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sevier C S, Weisz O A, Davis M, Machamer C E. Mol Biol Cell. 2000;11:13–22. doi: 10.1091/mbc.11.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ooi C E, Moreira J E, Dell'Angelica E C, Poy G, Wassarman D A, Bonifacino J S. EMBO J. 1997;16:4508–4518. doi: 10.1093/emboj/16.15.4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lafay F. J Virol. 1974;14:1220–1228. doi: 10.1128/jvi.14.5.1220-1228.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Plutner H, Davidson H W, Saraste J, Balch W E. J Cell Biol. 1992;119:1097–1116. doi: 10.1083/jcb.119.5.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peranen J, Auvinen P, Virta H, Wepf R, Simons K. J Cell Biol. 1996;135:153–167. doi: 10.1083/jcb.135.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Griffiths G, Pfeiffer S, Simons K, Matlin K. J Cell Biol. 1985;101:949–964. doi: 10.1083/jcb.101.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cowles C R, Odorizzi G, Payne G S, Emr S D. Cell. 1997;91:109–118. doi: 10.1016/s0092-8674(01)80013-1. [DOI] [PubMed] [Google Scholar]
- 37.Simpson F, Peden A A, Christopoulou L, Robinson M S. J Cell Biol. 1997;137:835–845. doi: 10.1083/jcb.137.4.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dell'Angelica E C, Ohno H, Ooi C E, Rabinovich E, Roche K W, Bonifacino J S. EMBO J. 1997;16:917–928. doi: 10.1093/emboj/16.5.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dell'Angelica E C, Aguilar R C, Wolins N, Hazelwood S, Gahl W A, Bonifacino J S. J Biol Chem. 2000;275:1300–1306. doi: 10.1074/jbc.275.2.1300. [DOI] [PubMed] [Google Scholar]
- 40.Mullins C, Hartnell L M, Bonifacino J S. Mol Gen Genet. 2000;263:1003–1014. doi: 10.1007/pl00008688. [DOI] [PubMed] [Google Scholar]
- 41.Dell'Angelica E C, Shotelersuk V, Aguilar R C, Gahl W A, Bonifacino J S. Mol Cell. 1999;3:11–21. doi: 10.1016/s1097-2765(00)80170-7. [DOI] [PubMed] [Google Scholar]
- 42.Feng L, Seymour A B, Jiang S, To A, Peden A A, Novak E K, Zhen L, Rusiniak M E, Eicher E M, Robinson M S, et al. Hum Mol Genet. 1999;8:323–330. doi: 10.1093/hmg/8.2.323. [DOI] [PubMed] [Google Scholar]
- 43.Mullins C, Hartnell L M, Wassarman D A, Bonifacino J S. Mol Gen Genet. 1999;262:401–412. doi: 10.1007/s004380051099. [DOI] [PubMed] [Google Scholar]
- 44.Zhen L, Jiang S, Feng L, Bright N A, Peden A A, Seymour A B, Novak E K, Elliott R, Gorin M B, Robinson M S, Swank R T. Blood. 1999;94:146–155. [PubMed] [Google Scholar]
- 45.Kantheti P, Qiao X, Diaz M E, Peden A A, Meyer G E, Carskadon S L, Kapfhamer D, Sufalko D, Robinson M S, Noebels J L, Burmeister M. Neuron. 1998;21:111–122. doi: 10.1016/s0896-6273(00)80519-x. [DOI] [PubMed] [Google Scholar]
- 46.Brandli A W, Parton R G, Simons K. J Cell Biol. 1990;111:2909–2921. doi: 10.1083/jcb.111.6.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoshimori T, Keller P, Roth M G, Simons K. J Cell Biol. 1996;133:247–256. doi: 10.1083/jcb.133.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ikonen E, Simons K. Semin Cell Dev Biol. 1998;9:503–509. doi: 10.1006/scdb.1998.0258. [DOI] [PubMed] [Google Scholar]
- 49.Aridor M, Bannykh S I, Rowe T, Balch W E. J Biol Chem. 1999;274:4389–4399. doi: 10.1074/jbc.274.7.4389. [DOI] [PubMed] [Google Scholar]
- 50.Le Borgne R, Griffiths G, Hoflack B. J Biol Chem. 1996;271:2162–2170. doi: 10.1074/jbc.271.4.2162. [DOI] [PubMed] [Google Scholar]
- 51.Le Borgne R, Schmidt A, Mauxion F, Griffiths G, Hoflack B. J Biol Chem. 1993;268:22552–22556. [PubMed] [Google Scholar]
- 52.Puertollano R, Aguilar R C, Gorshkova I, Crouch R J, Bonifacino J S. Science. 2001;292:1712–1716. doi: 10.1126/science.1060750. [DOI] [PubMed] [Google Scholar]
- 53.Zhu Y, Drake M T, Kornfeld S. Methods Enzymol. 2001;329:379–387. doi: 10.1016/s0076-6879(01)29099-5. [DOI] [PubMed] [Google Scholar]
- 54.Bannykh S, Aridor M, Plutner H, Rowe T, Balch W E. Cold Spring Harbor Symp Quant Biol. 1995;60:127–137. doi: 10.1101/sqb.1995.060.01.016. [DOI] [PubMed] [Google Scholar]
- 55.Davidson H W, Balch W E. J Biol Chem. 1993;268:4216–4226. [PubMed] [Google Scholar]
- 56.Benmerah A, Begue B, Dautry-Varsat A, Cerf-Bensussan N. J Biol Chem. 1996;271:12111–12116. doi: 10.1074/jbc.271.20.12111. [DOI] [PubMed] [Google Scholar]
- 57.Page L J, Sowerby P J, Lui W W, Robinson M S. J Cell Biol. 1999;146:993–1004. doi: 10.1083/jcb.146.5.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ramjaun A R, McPherson P S. J Neurochem. 1998;70:2369–2376. doi: 10.1046/j.1471-4159.1998.70062369.x. [DOI] [PubMed] [Google Scholar]
- 59.Aguilar R C, Boehm M, Gorshkova I, Crouch R J, Tomita K, Saito T, Ohno H, Bonifacino J S. J Biol Chem. 2001;276:13145–13152. doi: 10.1074/jbc.M010591200. [DOI] [PubMed] [Google Scholar]
- 60.Eaton B A, Haugwitz M, Lau D, Moore H P. J Neurosci. 2000;20:7334–7344. doi: 10.1523/JNEUROSCI.20-19-07334.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dell'Angelica E C, Klumperman J, Stoorvogel W, Bonifacino J S. Science. 1998;280:431–434. doi: 10.1126/science.280.5362.431. [DOI] [PubMed] [Google Scholar]
- 62.Liu S H, Towler M C, Chen E, Chen C Y, Song W, Apodaca G, Brodsky F M. EMBO J. 2001;20:272–284. doi: 10.1093/emboj/20.1.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thomas D C, Roth M G. J Biol Chem. 1994;269:15732–15739. [PubMed] [Google Scholar]
- 64.Copeland C S, Doms R W, Bolzau E M, Webster R G, Helenius A. J Cell Biol. 1986;103:1179–1191. doi: 10.1083/jcb.103.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]