β-Catenin stabilization dysregulates mesenchymal cell proliferation, motility, and invasiveness and causes aggressive fibromatosis and hyperplastic cutaneous wounds (original) (raw)
Abstract
Fibroproliferative processes are a group of disorders in which there is excessive proliferation of spindle (mesenchymal fibroblast-like) cells. They range from hypertrophic scars to neoplasms such as aggressive fibromatosis. Cells from these disorders share cytologic similarity with fibroblasts present during the proliferative phase of wound healing, suggesting that they represent a prolonged wounding response. A critical role for β-catenin in mesenchymal cells in fibroproliferative processes is suggested by its high rate of somatic mutation in aggressive fibromatosis. Using a Tcf-reporter mouse we found that β-catenin protein level and Tcf-transcriptional activity are elevated in fibroblasts during the proliferative phase of healing. We generated a transgenic mouse in which stabilized β-catenin is expressed in mesenchymal cells under control of a tetracycline-regulated promoter. Fibroblasts from the transgenic mice exhibited increased proliferation, motility, and invasiveness when expressing stabilized β-catenin and induced tumors after induction of the transgene when grafted into nude mice. Mice developed aggressive fibromatoses and hyperplastic gastrointestinal polyps after 3 months of transgene induction and healed with hyperplastic cutaneous wounds compared with control mice, which demonstrates an important function for β-catenin in mesenchymal cells and shows a central role for β-catenin in wound healing and fibroproliferative disorders.
An important role in embryonic development and neoplasia is played by β-catenin, which is a mediator in the canonical Wnt (wingless) signaling pathway (1). When Wnt signaling is quiescent, a multiprotein complex including adenomatous polyposis coli (APC), β-catenin, axin, and glycogen synthase kinase-3β induces phosphorylation of serine and threonine residues in the amino terminus of β-catenin. This phosphorylation targets β-catenin for ubiquitinylation and subsequent degradation. Wnt signaling inhibits the ability of this multiprotein complex to phosphorylate β-catenin. Consequently, amino-terminal serine and threonine sites are not phosphorylated, leading to stabilization of β-catenin. Stabilized cytosolic β-catenin translocates into the nucleus where it binds to Tcf-Lef family proteins to form a transcriptional activation complex. In neoplasia, the β-catenin protein level can become elevated and transcriptionally active because of mutations in members of this multiprotein complex or in β-catenin itself (1–3).
Fibroproliferative disorders are a group of pathological processes in which there is excessive proliferation of spindle (mesenchymal fibroblast-like) cells. They range from hypertrophic scars to neoplasms such as the locally invasive tumor aggressive fibromatosis (4). During the proliferative phase of wound healing, mesenchymal (fibroblast-like) cells migrate into the healing wound, proliferate, and produce a disorganized matrix, providing the initial tensile strength (5). Fibroproliferative processes such as aggressive fibromatosis are composed of cells that share cytological similarity to the fibroblasts in the proliferative phase of wound healing, leading to the speculation that they represent an unchecked healing response (4). Thus, it is possible that molecular events implicated in the pathogenesis of aggressive fibromatosis also play a role in wound healing. A critical role for β-catenin in mesenchymal cell function is suggested by the high rate of somatic mutations in β-catenin in aggressive fibromatosis, in which a third of cases harbor somatic mutations in either β-catenin or APC, resulting in an elevated β-catenin protein level (6–8).
We report here that β-catenin protein is elevated and Tcf-dependent transcription is activated in fibroblasts during the proliferative phase of wound healing. To determine the role of β-catenin stabilization in mesenchymal (fibroblast) cell function, we generated a transgenic mouse model in which stabilized β-catenin is expressed in mesenchymal cells under the control of a tetracycline-regulated promoter. This mouse developed aggressive fibromatoses and hyperplastic cutaneous wounds with induction of the transgene, suggesting a common role for β-catenin in fibroproliferative processes. Furthermore, these data suggest that unchecked activation of a process important in normal wound healing causes neoplasia.
Materials and Methods
Generation of Transgenic Mice.
A Tcf-reporter construct was generated containing the lacZ gene downstream of a c-Fos minimal promoter and three consensus Tcf-binding motifs (9). Binding Tcf of a β-catenin–Tcf complex activates the expression of lacZ. The transgene was microinjected into murine embryos to produce transgenic mice expressing the reporter construct. A mouse line was generated that harbors an inducible, stabilized β-catenin, the expression of which was controlled by a tetracycline-regulated promoter. Phosphorylation of serine and threonine sites at the amino terminus of β-catenin regulate its ubiquitin-mediated degradation (9, 10). In vitro mutagenesis was used to change these sites at codons 33, 37, 41, and 45 to alanine in human β-catenin, which has nearly complete homology to murine β-catenin (11). An amino-terminal Myc-tag was added, and the construct was subcloned into the bidirectional pd2EGFP-TRE vector (CLONTECH; ref. 12). NIH 3T3 “tet-off” cells (CLONTECH) were used to verify that tetracycline regulated expression of the transgene, and that the stabilized version of β-catenin was transcriptionally active (transfected with the tcf-reporter construct, pTOPFLASH, or the control pFOPFLASH control reporter construct containing mutated tcf consensus binding sites). The transgene was microinjected into B6/SJL embryos, which were implanted into pseudopregnant female mice. Genotyping with Southern blot identified founders, which were mated with mice that expressed the reverse tetracycline transactivator (rtTA) under the regulation of the cytomegalovirus promoter (The Jackson Laboratory). Double-positive animals were identified by using Southern analysis and PCR.
Regulation of Stabilized β-Catenin Transgene Expression.
Double-transgenic progeny from each founder were evaluated for regulation of transgene expression. A slow-release doxycycline pellet (0.7 mg/day, Innovative Research of America) was implanted in the skin of half of the double-transgenic mice. After 1 week, ear biopsies from both the induced and uninduced mice from each founder were observed under low-power fluorescence microscopy to determine expression of the green fluorescent protein. Immunohistochemistry also was performed by using an anti-Myc-epitope antibody (CLONTECH). Double-transgenic progeny from three founders were found to demonstrate strict regulation of transgene expression. Additional double-transgenic mice were bred from these lines and analyzed for regulation of transgene expression in various tissues (skin, skeletal muscle, gastrointestinal tract, liver, and kidneys) by using Northern analysis, and Western analysis with the anti-Myc-epitope antibody was performed on immunoprecipitates obtained by using an anti-β-catenin antibody (Transduction Laboratories, Lexington, KY).
Transgene Induction and Phenotypic Analysis.
Six-week-old mice were used in all experimental groups. Transgene expression was induced in double-positive animals by using a 90-day slow-release doxycycline pellet (0.7 mg/day). Control mice consisted of double-transgenic mice that were not treated with doxycycline and nontransgenic littermates that were treated by using the slow-release pellet. Eight double-transgenic animals from each of the three founders treated with doxycycline (24 in total), 12 nontransgenic littermates treated with doxycycline, and 12 double-transgenic animals not treated with doxycycline were studied. The mice were killed at 5 months of age and observed for the presence of aggressive fibromatoses as reported previously (13). Gross examinations of the gastrointestinal tract, liver, kidneys, spleen, lung, and heart were conducted for palpable or visible tumors. Observed lesions were formalin-fixed, paraffin-embedded, and prepared for histologic evaluation with hematoxylin and eosin staining.
Primary Cell Cultures and Their Analysis.
Primary dermal fibroblast cultures were established from 6-week-old double-positive transgenic animals as reported (14). Doxycycline (1 μg/ml) was added to the medium to activate expression of the transgene. Transgene induction was verified by observation of green fluorescent protein under fluorescence microscopy and by using the anti-Myc-epitope antibody (Western analysis and immunohistochemistry). Control cells consisted of uninduced dermal fibroblast cells from double-transgenic animals and skin fibroblasts from nontransgenic littermates treated with doxycycline. Each experiment was performed 10 times except as noted. Cell proliferation was measured by using bromodeoxyuridine (BrdUrd) incorporation (15). BrdUrd (10 μM, Sigma) was added to the medium for 8 h. Cells were fixed in paraformaldehyde, and immunohistochemistry was performed by using an anti-BrdUrd-peroxidase antibody (Roche, Gipf-Oberfrick, Switzerland). The number of positively and negatively stained nuclei were counted over 10 high-power fields and averaged to determine the percentage of staining. Apoptosis was measured by using DNA laddering (Apoptotic DNA ladder, Roche Molecular Biochemicals; ref. 18). Cell motility was measured by using a modified Boyden chamber (16) and observing cell motion under time-lapse photomicroscopy (17). Equal numbers of fibroblasts (1 × 105) from the various animals were plated onto the top chamber of a modified Boyden chamber (10-mm tissue-culture inserts with 8-μm polycarbonate membranes, Becton Dickinson). The cells were allowed to migrate across the membrane for 24 h. The transgene was induced by treating the cells with doxycycline starting 24 h before being plated onto the modified Boyden chamber. The number of cells crossing the membrane was averaged over 10 high-powered fields counted. Time-lapse photomicroscopy was performed by using a Zeiss Axiovert microscope with a heated stage. Cells (50,000) were plated onto 25-ml culture dishes and photographed at 5-min intervals over 5 h. The rate of random-direction locomotion of 10 cells chosen at random in each dish was calculated with the aid of NORTHERN ECLIPSE IMAGE ANALYSIS software (Empix, Missisauga, ON) and expressed as μm traveled per hour. Five dishes of each cell type were examined, and the rate of motion was averaged over the cells measured. Invasion chambers were prepared by coating 10-mm tissue-culture inserts with 8-μm polycarbonate membranes with 85 μg/cm2 Matrigel (Becton Dickinson; ref. 18). All other techniques and parameters for the study were identical to those used for the studies with the modified Boyden chamber.
Grafting Cell Cultures into Nude Mice.
Primary cell cultures from double-transgenic mice were grafted into nude mice. Because the addition of extracellular matrix will stimulate tumor formation by some cell lines (19), Matrigel was mixed to the cells in half of the grafts, and 107 cells were mixed with either 150 μl of Matrigel or PBS and injected s.c. into the back of nude mice, half of which were treated with doxycycline. Tumor size was observed at weekly intervals, and the mice were killed at 4 weeks of age. The size of the excised tumors was measured with a caliper.
Wound Healing.
Four full-thickness 4-mm diameter skin wounds were created on the dorsum of the Tcf-reporter mice with a dermal biopsy punch (Miltex Instrument Company, Bethpage, NY). Mice were killed at 0, 2, 8, 14, and 24 days after wounding. Two of the wounds were processed for histology, and the remaining wounds were harvested independently of normal skin for protein extraction. Four animals were examined at each time point. β-Catenin protein level in the wounds was determined by using Western analysis. Membranes were stripped and reprobed with an anti-actin antibody (Oncogene Research Products, Cambridge, MA) as a loading control. For immunostaining, the sections were dewaxed, put in citrate buffer (pH 6), and microwaved for 15 min at 900 W. The antibodies used were against Ki-67 (Immunotech, Luminy, France), a nuclear marker of proliferating cells (20), and β-catenin (Transduction Laboratories). Routine hematoxylin and eosin, and Masson trichrome staining also was carried out on paraffin sections. LacZ expression was detected by incubating wound samples in 5-bromo-4-chloro-3-indolyl β-D-galactoside staining solution according to a protocol obtained from Specialty Media (Lavellette, NJ).
Wounding of β-catenin transgenic mice or controls was performed in a similar fashion, but wounds were harvested at 8, 14, and 24 days after wounding and processed for histology. Twelve mice were examined at each time point: four transgenic mice in which the transgene was induced, four transgenic mice without induction of the transgene, and four nontransgenic littermates treated with doxycycline. Wound size was determined by using histologic sections cut at a right angle to the skin surface across the wound. Serial sections were observed, and the section at the center of the wound, with the largest wound diameter, was chosen to measure wound size. A grid was used to measure the size of the epidermal and mesenchymal (or dermal) component of each wound.
Results
Regulation of Expression of the Mutant β-Catenin.
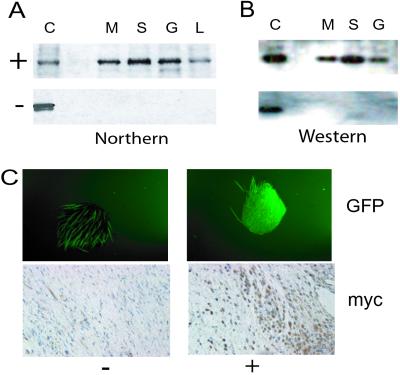
Double-positive transgenic mice from three founders were tested for regulation of transgene expression. Northern and Western analysis of β-catenin immunoprecipitates demonstrated tight regulation of transgene expression in the variety of tissue types examined (Fig. 1). Green fluorescent protein expression was regulated by doxycycline administration in a similar manner as detected by observation of specimens under low-power fluorescent microscopy (Fig. 1C).
Figure 1.
Regulation of the transgene by doxycycline in double-transgenic mice. (A) Northern analysis using a transgene-specific probe. +, mice treated with doxycycline; −, mice not treated; C, positive control; M, muscle; S, skin; G, gastrointestinal tract; L, liver. (B) Western analysis using an anti-Myc-epitope antibody on immunoprecipitates performed with an anti-β-catenin antibody on protein extracts. (C) Regulation of transgene expression as demonstrated by green fluorescent protein production in a full-thickness punch sample from a mouse ear (Upper, autofluorescence of hair is noted in the untreated sample) and by immunohistochemistry using an anti-Myc-epitope antibody in dermal tissue (Lower). Strict regulation of expression of the transgene by doxycycline administration is demonstrated.
Primary Cell Cultures Demonstrate Tcf-Dependent Transcriptional Activation.
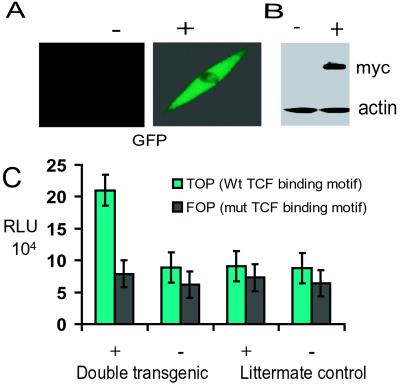
Regulation of transgene expression by doxycycline in primary fibroblast cultures was confirmed by using immunohistochemistry and Western analysis as well as by observation of green fluorescent protein expression under fluorescent microscopy. Fibroblast cell cultures were transfected with the Tcf-reporter construct, pTOPFLASH, or the pFOPFLASH control reporter construct containing mutated Tcf consensus binding sites. With doxycycline administration there was an 8-fold increase in pTOPFLASH luminescence. Without doxycycline administration, pTOPFLASH activity was approximately the same as that in fibroblasts derived from nontransgenic littermates. There was no difference in pFOPFLASH activity between any of the cell cultures (Fig. 2).
Figure 2.
Tcf-dependent transcriptional activation in primary fibroblast cell cultures. +, treated with doxycycline; −, not treated. The regulation of transgene expression in primary cell cultures is shown. A shows green fluorescent protein production, and B shows Western analysis using an anti-Myc-epitope antibody with actin as a loading control. (C) Tcf-transcriptional activation (pTOPFLASH) is increased significantly with induction on the transgene, compared with activation of a control reporter construct (pFOPFLASH) or control cell cultures. RLU, relative luciferase units; Wt, wild type; mut, mutant.
β-Catenin Stabilization Is Sufficient to Cause Aggressive Fibromatosis and Hyperplastic Gastrointestinal Polyps.
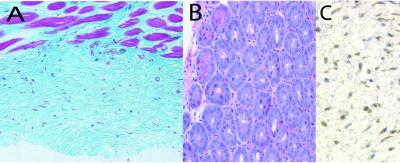
After 3 months of transgene induction, autopsy revealed aggressive fibromatoses in 18 of 24 mice and hyperplastic gastrointestinal polyps in all the mice. No tumors were detected in nontransgenic littermates treated with doxycycline, nor were they observed in double-transgenic mice not treated with doxycycline. All three founders exhibited both tumor types. There was a median of five fibromatoses and four gastrointestinal tumors in each mouse. The fibromatoses had a mean diameter of 2.0 mm and were located in both muscle and skin. Histologic observation showed that the fibromatoses were composed of spindle-shaped cells that infiltrated into surrounding normal structures, similar to that observed in human aggressive fibromatoses (Fig. 3A). The hyperplastic gastrointestinal polyps were similar in histology to those that develop in a mouse in which the expression of a mutant β-catenin is targeted to the gastrointestinal cells (Fig. 3B). We did not detect other tumor types in mice in which the transgene was induced. Myc immunohistochemistry (Fig. 3C) confirmed expression of the transgene in the fibromatoses.
Figure 3.
Histology of tumors formed in the mice. (A) An aggressive fibromatosis with spindle cells infiltrating into the muscle tissue. (B) A hyperplastic gastrointestinal polyp. (C) Immunohistochemistry using an anti-Myc-epitope antibody in an aggressive fibromatosis, illustrating expression of the transgene in the tumor.
Primary Fibroblasts Demonstrate Increased Proliferation, Cell Motility, and Invasiveness.
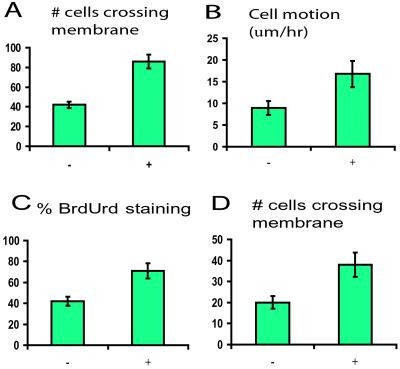
BrdUrd incorporation assays showed a significantly increased proliferation rate with induction of stabilized β-catenin in primary fibroblast cultures (Fig. 4). The rate of cell motility increased with transgene induction as measured by using a modified Boyden chamber and time-lapse photomicroscopy. Cell invasiveness was increased as determined by the number of cells passing through Matrigel after transgene induction (Fig. 4). All these differences were significant as determined by using the Student's t test (P < 0.005). Although no change in apoptosis rate as detected by using DNA laddering (data not shown) was observed with transgene induction, the apoptosis rate was quite low in all the cultures.
Figure 4.
Result of transgene stabilization on behavior of primary fibroblast cultures. +, treated with doxycycline; −, not treated. (A) Motility as measured by the number of cells per high-powered field crossing the membrane using a modified Boyden chamber. (B) Cell motion as measured by using time-lapse photomicroscopy. (C) Proliferation as measured by using the percentage of cells incorporating BrdUrd. (D) Cell invasiveness as measured by the number of cells per high-power field crossing Matrigel. In all cases, activation of the transgene resulted in a significant increase.
Primary Fibroblast Cell Cultures Form Tumors in Nude Mice When Expressing Stabilized β-Catenin.
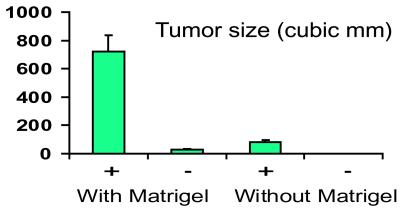
Primary fibroblast cell cultures from the double-transgenic mice were grown as grafts in nude mice with or without the addition of Matrigel to the cultures. With induction of the transgene, tumors derived from cells in PBS averaged 84 mm3 at 4 weeks, whereas no tumors formed in cases without transgene induction. When Matrigel was added to the cells, tumors in the mice treated with doxycycline averaged 720 mm3, whereas tumors in the mice not treated averaged 27 mm3 (Fig. 5). The differences in tumor size with and without induction of the transgene was significant in both cases at P < 0.05.
Figure 5.
Size of tumors formed with cells grafted into nude mice. +, treated with doxycycline; −, not treated. A significantly increased size is noted with induction of the transgene, with or without the use of Matrigel.
β-Catenin Protein Level Is Elevated and Tcf-Dependent Transcription Is Activated in Fibroblasts During the Proliferative Phase of Wound Healing.
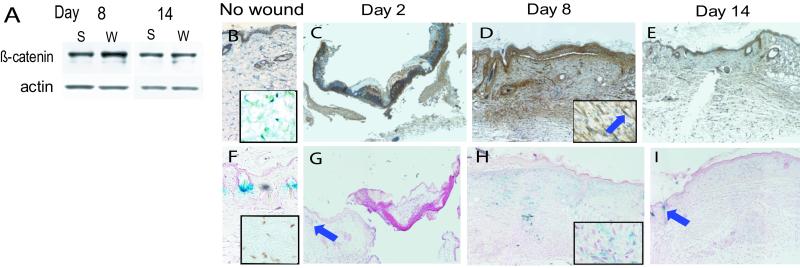
Two days after wounding, β-catenin levels were low in scar samples, likely because the isolated wound is essentially a blood clot with little tissue. By day 8, when fibroblasts are active during the proliferative phase of wound healing, β-catenin was elevated in the wound samples to double the level in unwounded tissues. After 2 weeks, when fibroblast activation is waning, β-catenin protein levels were only slightly higher in scar tissue, reflecting the change to the remodeling phase of wound healing (Fig. 6A). Immunohistochemisty for β-catenin showed intense and diffuse staining in fibroblasts (identified through their characteristic elongated nuclei and presence in the dermis) of the wound on day 8 (Fig. 6D). To assess whether Tcf-dependent transcription is activated during wound healing, we performed 5-bromo-4-chloro-3-indolyl β-D-galactoside staining of wounds from Tcf-galactosidase mice. As shown in Fig. 6H, staining on day 2 was visible only in hair follicles, where β-catenin is known to be activated during the hair cycle (21, 22). Eight days postwounding, prominent staining in the entire fibroblast-dense wound area was seen, indicating higher levels of β-catenin-mediated Tcf-dependent transcriptional activation. By day 14, staining again was limited to the follicular regions.
Figure 6.
β-Catenin-mediated tcf-dependent transcription is activated in the proliferative phase of wound healing. (A) Western analysis from wounds 8 and 14 days after wounding, showing an increase in β-catenin protein in the wound at day 8 (actin staining as a loading control). W, wound tissue; S, normal tissue. (B_–_E) β-Catenin immunohistochemistry, showing increased staining in the mesenchymal tissues (magnified view in box with arrow pointing to a fibroblast expressing β-catenin) at day 8 (D). (F_–_I) Tcf-activation in the reporter mouse. Blue staining indicates tcf activation. The arrows point to hair follicles, where tcf is known to be activated, as a positive control. The 8-day sample (H) shows tcf activation in the fibroblasts, better illustrated in the magnified view in the box. β-Catenin-mediated tcf-dependent activation in hair follicles, illustrated in the unwounded skin shown in F and by the arrow in G, may result in underestimation of the actual difference in β-catenin protein level when comparing with unwounded skin, because hair follicles are not present in wound tissue. The insets in B and F show a lack of β-catenin-mediated tcf-dependent activation in dermal fibroblasts in unwounded skin.
β-Catenin Protein Stabilization Results in Excessive Fibroblast Proliferation in Wound Healing.
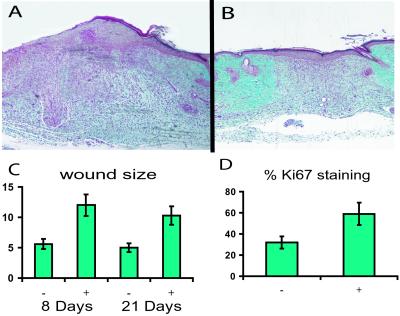
To determine how the β-catenin protein level regulates the proliferative phase of wound healing, we analyzed wound size and appearance 8, 14, and 24 days after wounding the β-catenin transgenic mice. Measurement of wound areas revealed that transgenic mouse wounds were more than two times the size of control wounds (Fig. 7). Histology sections showed that in contrast to control wounds, stabilized β-catenin-overexpressing mouse wounds were hypercellular and contained excessive collagen compared with control mice, a histologic appearance reminiscent of hypertrophic wounds or keloids. Ki-67 staining was present in a higher percentage of cells in β-catenin-overexpressing mouse wounds, indicating an increased rate of proliferation. Treatment of nontransgenic mice with doxycycline alone did not alter normal wound formation significantly. We found no significant difference in percentage of Ki-67 staining in cells from unwounded areas of the skin between mice in which the transgene was induced and controls. However, in the unwounded skin, the percentage of cells exhibiting Ki-67 staining was quite low (less than 4% of cells), making detection of a difference difficult.
Figure 7.
Wounds are larger in animals expressing the stabilized β-catenin. A histologic section taken through the middle of the wound 8 days after wounding. A larger size to the mesenchymal component of the wound in the mouse treated with doxycycline (A) is illustrated compared with a transgenic mouse not treated with doxycycline (B). In this particular sample, a thicker epithelial component is present also; however, there was not a significant difference in size of the epithelial component when taking all the samples into account. (C) Graphic representation of the difference in wound area between treated (+) and untreated (−) animals at 8 and 21 days after wounding. (D) Difference in Ki-67 staining (by percentage of cells) between wounds in mice receiving doxycycline (+) and those not receiving doxycycline (−) at 8 days after wounding.
Discussion
We generated a transgenic mouse model in which expression of a stabilized form of β-catenin is controlled tightly by a tetracycline-regulated promoter. Three months of transgene induction resulted in the development of aggressive fibromatoses and gastrointestinal tumors, demonstrating that β-catenin stabilization is sufficient to cause aggressive fibromatosis. β-Catenin protein level is elevated in wound fibroblasts during the proliferative phase of wound repair and is transcriptionally active. Furthermore, overexpression of stabilized β-catenin led to excessive scar formation, providing further evidence of the importance of β-catenin in the regulation of mesenchymal cells and in wound repair.
Expression of stabilized β-catenin starting at 6 weeks of age is sufficient to cause aggressive fibromatosis and gastrointestinal tumors in our mouse model. This situation is analogous to adult-onset tumors, in which a sporadic mutation later in life is causative, as opposed to a genetic neoplastic syndrome, in which a germ-line mutation is present. Most mouse models of neoplasia, including other models in which expression of a stabilized form of β-catenin is driven by a tissue-specific promoter (23), are more analogous to a genetic condition.
Select mice harboring APC mutations also develop aggressive fibromatosis. Interestingly, the Min mouse infrequently develops aggressive fibromatosis (24), whereas the _Apc_1638N mouse develops aggressive fibromatosis with regularity (13). One explanation for the difference in development of aggressive fibromatosis in these mice is that the site of Apc mutation results in a different protein level of β-catenin. Previous work shows that the fibromatoses formed in the _Apc_1638N mice have high protein levels of β-catenin (25, 26). Alternatively, the difference may be related to an alternative function of APC such as its role in maintaining chromosomal stability (27). Our cell-graft data suggest that β-catenin stabilization on its own is sufficient to cause fibroblasts to adopt a tumor phenotype, because normal fibroblast cell cultures on their own are incapable of producing tumors in nude mice (28). These data strongly suggest that β-catenin protein elevation is the fundamental cause of aggressive fibromatosis and implicates the β-catenin regulatory function of APC as responsible for the development of this tumor type in cases of APC germline mutants.
Expression of the transgene in fibroblasts derived from double-transgenic animals resulted in increased cell proliferation, motility, and invasiveness. Previous cell culture studies give conflicting data regarding the role of stabilized β-catenin expression in fibroblasts, with one demonstrating an increase in proliferation (29) and another demonstrating increased apoptosis (30). It is possible that these differences are caused by the level of expression of stabilized β-catenin, because there is evidence for a Tcf-independent mechanism by which β-catenin regulates apoptosis (30), which may become activated at higher levels of expression.
An increase in β-catenin level in wound tissue relative to normal skin was observed during the proliferative phase of repair. Although it is possible that the increase in β-catenin levels is the result of an increase in cell number during the proliferative phase, we compared the level of staining between normal skin fibroblasts and fibroblasts in the wound bed and noted that the latter cells had much more intense staining for β-catenin. β-Catenin was observed also in epithelial cells of hair follicles independent of the stage of healing. Because hair follicles do not regenerate after wounding, the β-catenin in hair follicles may have reduced the difference in β-catenin protein levels detected by Western analysis between normal and wounded skin. β-Catenin was observed also in the cell membrane region of epidermal cells in the healing wound. This result was not surprising, because β-catenin is a component of the adherens junction (31), which may be important during reepithelialization, because epidermal cells migrate and proliferate to cover the surface of the tissue defect and reestablish cadherin-catenin-mediated adherens junctions with adjacent cells (5). 5-Bromo-4-chloro-3-indolyl β-D-galactoside staining was prominent in fibroblasts of proliferative phase wounds from Tcf-galactosidase mice, indicating that Tcf-dependent transcription was activated. As expected, Tcf-mediated transcription was active also in follicular epithelial cells where β-catenin-mediated signaling is important in hair follicle organogenesis and in the hair cycle (21, 22). 5-Bromo-4-chloro-3-indolyl β-D-galactoside staining was not seen in epidermal epithelial cells, which was not surprising, because β-catenin is localized to the cell membrane in these cells, suggesting that Tcf-dependent transcription is not activated in these cells during reepithelialization.
There are a variety of mechanisms that might cause β-catenin elevation in fibroblasts during the proliferative phase of wound repair. One possibility is that growth factors present during the inflammatory phase stimulate β-catenin protein stabilization either by regulating Wnt expression (Wnt 4 is known to be expressed in early wound healing; ref. 32) or more directly regulating β-catenin protein stability. Because β-catenin-mediated Tcf-dependent transcription is activated in fibroblasts during the proliferative phase of wound healing, it is likely that it regulates the expression of genes, which may be important in the remodeling phase of wound healing. As such, β-catenin likely plays a central role in wound healing, mediating how factors liberated during the initial stages of wounding regulate factors important in mesenchymal tissue remodeling.
We observed the healing of wounds in stabilized β-catenin-overexpressing mice and found that the wounds were larger compared with controls, exhibiting increased amounts of collagen-rich tissue as well as a higher percentage of Ki-67-positive fibroblasts. The abnormal healing of skin wounds in these mice indicates that β-catenin is an important factor in fibroblast proliferation after injury, because β-catenin overexpression resulted in a heightened proliferative phase of wound healing, giving a wound with a histologic appearance quite reminiscent of hyperplastic wounds or keloids.
Taken together, our data suggest an important role for β-catenin in the regulation of mesenchymal cell function. The data demonstrate that stabilization of β-catenin alone is sufficient to cause aggressive fibromatosis and that β-catenin plays an important role in normal cutaneous wound healing, and it provides a model for adult-onset neoplasia. The phenotype of the wounds in the mice expressing stabilized β-catenin is reminiscent of hyperplastic wounds or keloids, suggesting a role for β-catenin in these conditions of pathologic wound healing.
Acknowledgments
This research was supported by grants from the Canadian Institutes of Health Research, the National Cancer Institute of Canada, funds from the Terry Fox Run, the Research Training Centre of the Hospital for Sick Children, and the Canadian Research Chairs Program.
Abbreviation
APC
adenomatous polyposis coli
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Polakis P. Genes Dev. 2000;14:1837–1851. [PubMed] [Google Scholar]
- 2.Taipale J, Beachy PA. Nature (London) 2001;411:349–354. doi: 10.1038/35077219. [DOI] [PubMed] [Google Scholar]
- 3.Korswagen HC, Clevers HC. Cold Spring Harbor Symp Quant Biol. 1999;64:141–147. doi: 10.1101/sqb.1999.64.141. [DOI] [PubMed] [Google Scholar]
- 4.Lattes R. In: Tumors of the Soft Tissues. Lattes R, editor. Washington, DC: Armed Forces Institute of Pathology; 1980. pp. 1–30. [Google Scholar]
- 5.Epstein F H. N Engl J Med. 1999;341:738–746. [Google Scholar]
- 6.Tejpar S, Nollet F, Li C, Wunder J S, Michils G, dal Cin P, Van Cutsem E, Bapat B, van Roy F, Cassiman J J, Alman B A. Oncogene. 1999;18:6615–6620. doi: 10.1038/sj.onc.1203041. [DOI] [PubMed] [Google Scholar]
- 7.Miyoshi Y, Iwao K, Nawa G, Yoshikawa H, Ochi T, Nakamura Y. Oncol Res. 1998;10:591–594. [PubMed] [Google Scholar]
- 8.Alman B A, Li C, Pajerski M E, Diaz-Cano S, Wolfe H J. Am J Pathol. 1997;151:329–334. [PMC free article] [PubMed] [Google Scholar]
- 9.Morin P J, Sparks A B, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler K W. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 10.Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. EMBO J. 1997;16:3797–3804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nollet F, Berx G, Molemans F, van Roy F. Genomics. 1996;32:413–424. doi: 10.1006/geno.1996.0136. [DOI] [PubMed] [Google Scholar]
- 12.Furth P A, St. Onge L, Boger H, Gruss P, Gossen M, Kistner A, Bujard H, Hennighausen L. Proc Natl Acad Sci USA. 1994;91:9302–9306. doi: 10.1073/pnas.91.20.9302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smits R, van der Houven van Oordt W, Luz A, Zurcher C, Jagmohan-Changur S, Breukel C, Khan P M, Fodde R. Gastroenterology. 1998;114:275–283. doi: 10.1016/s0016-5085(98)70478-0. [DOI] [PubMed] [Google Scholar]
- 14.Li C, Bapat B, Alman B A. Am J Pathol. 1998;153:709–714. doi: 10.1016/s0002-9440(10)65614-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alman B A, Greel D A, Ruby L K, Goldberg M J, Wolfe H J. J Orthop Res. 1996;14:722–728. doi: 10.1002/jor.1100140507. [DOI] [PubMed] [Google Scholar]
- 16.Li C, Nguyen Q, Cole W G, Alman B A. J Pediatr Orthop. 2001;21:372–377. [PubMed] [Google Scholar]
- 17.Taylor W R, Greenberg A H, Turley E A, Wright J A. Exp Cell Res. 1993;204:295–301. doi: 10.1006/excr.1993.1036. [DOI] [PubMed] [Google Scholar]
- 18.Nam S W, Clair T, Campo C K, Lee H Y, Liotta L A, Stracke M L. Oncogene. 2000;19:241–247. doi: 10.1038/sj.onc.1203263. [DOI] [PubMed] [Google Scholar]
- 19.Fridman R, Kibbey M C, Royce L S, Zain M, Sweeney M, Jicha D L, Yannelli J R, Martin G R, Kleinman H K. J Natl Cancer Inst. 1991;83:769–774. doi: 10.1093/jnci/83.11.769. [DOI] [PubMed] [Google Scholar]
- 20.Scholzen T, Gerdes J. J Cell Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 21.DasGupta R, Fuchs E. Development (Cambridge, UK) 1999;126:4557–4568. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- 22.Chan E F, Gat U, McNiff J M, Fuchs E. Nat Genet. 1999;21:410–413. doi: 10.1038/7747. [DOI] [PubMed] [Google Scholar]
- 23.Harada N, Tamai Y, Ishikawa T, Sauer B, Takaku K, Oshima M, Taketo M M. EMBO J. 1999;18:5931–5942. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Halberg R B, Katzung D S, Hoff P D, Moser A R, Cole C E, Lubet R A, Donehower L A, Jacoby R F, Dove W F. Proc Natl Acad Sci USA. 2000;97:3461–3466. doi: 10.1073/pnas.050585597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poon R, Smits R, Li C, Jagmohan-Changur S, Kong M, Cheon S, Yu C, Fodde R, Alman B A. Oncogene. 2001;20:451–460. doi: 10.1038/sj.onc.1204107. [DOI] [PubMed] [Google Scholar]
- 26.Smits R, Kielman M F, Breukel C, Zurcher C, Neufeld K, Jagmohan-Changur S, Hofland N, van Dijk J, White R, Edelmann W, et al. Genes Dev. 1999;13:1309–1321. doi: 10.1101/gad.13.10.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fodde R, Kuipers J, Rosenberg C, Smits R, Kielman M, Gaspar C, van Es J H, Breukel C, Wiegant J, Giles R H, Clevers H. Nat Cell Biol. 2001;3:433–438. doi: 10.1038/35070129. [DOI] [PubMed] [Google Scholar]
- 28.Ali I U, Rasheed S. Cancer Invest. 1987;5:17–24. doi: 10.3109/07357908709020302. [DOI] [PubMed] [Google Scholar]
- 29.Soler C, Grangeasse C, Baggetto L G, Damour O. FEBS Lett. 1999;442:178–182. doi: 10.1016/s0014-5793(98)01648-2. [DOI] [PubMed] [Google Scholar]
- 30.Kim K, Pang K M, Evans M, Hay E D. Mol Biol Cell. 2000;11:3509–3523. doi: 10.1091/mbc.11.10.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ko K, Arora P, Lee W, McCulloch C. Am J Physiol. 2000;279:C147–C157. doi: 10.1152/ajpcell.2000.279.1.C147. [DOI] [PubMed] [Google Scholar]
- 32.Labus M B, Stirk C M, Thompson W D, Melvin W T. Wound Repair Regen. 1988;6:58–64. doi: 10.1046/j.1524-475x.1998.60109.x. [DOI] [PubMed] [Google Scholar]