Wide spectrum of tumors in knock-in mice carrying a Cdk4 protein insensitive to INK4 inhibitors (original) (raw)
Abstract
We have introduced a point mutation in the first coding exon of the locus encoding the cyclin-dependent kinase 4 (Cdk4) by homologous recombination in embryonic stem cells. This mutation (replacement of Arg24 by Cys) was first found in patients with hereditary melanoma and renders Cdk4 insensitive to INK4 inhibitors. Here, we report that primary embryonic fibroblasts expressing the mutant Cdk4R24C kinase are immortal and susceptible to transformation by Ras oncogenes. Moreover, homozygous _Cdk4_R24C/R24C mutant mice develop multiple tumors with almost complete penetrance. The most common neoplasia (endocrine tumors and hemangiosarcomas) are similar to those found in pRb+/– and p53–/– mice. This Cdk4 mutation cooperates with p53 and p27Kip1 deficiencies in decreasing tumor latency and favoring development of specific tumor types. These results provide experimental evidence for a central role of Cdk4 regulation in cancer and provide a valuable model for testing the potential anti-tumor effect of Cdk4 inhibitors in vivo.
Keywords: animal models/cell cycle regulation/cyclin-dependent kinase/INK4 inhibitors/tumor development
Introduction
The central role of cyclin/cyclin-dependent kinase (Cdk) complexes and their regulation in the modulation of cell cycle progression has been widely recognized (Morgan, 1995; Sherr, 2000). Cdk4 and Cdk6 are two cell cycle kinases involved in cell cycle initiation of resting cells as a response to mitogenic signals, as well as in the progression through the G1/S transition. These kinases associate with the growth factor-regulated D-type cyclins (D1, D2, D3) to form active complexes that phosphorylate and inactivate the retinoblastoma protein (pRb). The molecular mechanisms involved in Cdk regulation have been extensively studied (Morgan, 1995; Pavletich, 1999; Sherr and Roberts, 1999). For instance, Cdks can be regulated by activating and inactivating phosphorylations, binding of inhibitory proteins and interaction with chaperone molecules that provide a proper folding and transport to the nucleus.
Two families of Cdk inhibitors have been described based on functional and structural homologies. The INK4 family of inhibitors, p16_INK4a_, p15_INK4b_, p18_INK4c_ and p19_INK4d_, are structurally characterized by ankyrin repeats, and specifically bind to Cdk4 and Cdk6 preventing their interaction with D-cyclins. The second group of inhibitors, known as the Cip/Kip family, is composed of three members, p21_Cip1/Waf1/Sdi1_, p27_Kip1_ and p57_Kip2_. These proteins are primary inhibitors of Cdk2 activity by forming ternary complexes with cyclin E- and cyclin A-bound Cdk2 proteins. Interestingly, members of the Cip/Kip family can also bind and form ternary complexes with cyclin D–Cdk4/6 kinases. However, in this case the ternary complexes can retain kinase activity. These and other observations have led to the hypothesis that cyclin D–Cdk4/6 complexes may participate in the sequential activation of downstream cyclin E–Cdk2 complexes by removing circulating Cip/Kip molecules that may bind to cyclin E–Cdk2 complexes (reviewed by Sherr and Roberts, 1999).
Increasing evidence, obtained primarily from human tumors, indicates that regulators of the cell cycle including cyclins, Cdks and Cdk inhibitors are either themselves targets for genetic modification in cancer or are disrupted by other oncogenic events (Hirama and Koeffler, 1995; Sherr, 2000). Most of these mutations result in deregulation of the G1 kinases, Cdk4 and Cdk6 (Sherr, 2000). For instance, overexpression of cyclins or deletion of the INK4a–ARF locus are among the most frequent alterations in human malignancies (Peters, 1994; Ruas and Peters, 1998). Deletion of this locus results in loss of two different proteins, p16INK4a and p19ARF. p19ARF is a tumor suppressor involved in stabilization of p53 (Sharpless and DePinho, 1999; Sherr, 2000). The adjacent INK4b locus, which encodes p15INK4b, is also frequently co-deleted as well as inactivated by promoter hypermethylation (Ruas and Peters, 1998). Expression of Cdk4 or Cdk6 is also altered in human cancer by mechanisms involving gene amplification or chromosomal translocations (Khatib et al., 1993; Schmidt et al., 1994; Chilosi et al., 1998; Corcoran et al., 1999). Finally, miscoding mutations in the CDK4 locus have been observed in familiar and sporadic melanoma (Wolfel et al., 1995; Zuo et al., 1996). These mutations render a Cdk4 protein whose kinase activity becomes insensitive to INK4 inhibitors.
Recently, the role of some of these proteins in normal homeostasis as well as in tumor development has been studied in gene-targeted mice. Strains lacking each of the INK4 inhibitory proteins have already been described (for a review see Malumbres et al., 2000a). Whereas p16INK4a and p15INK4b are rather weak tumor suppressors, p18INK4c-deficient mice are highly susceptible to pituitary as well as to other types of tumors (Franklin et al., 1998; Latres et al., 2000; Krimpenfort et al., 2001; Sharpless et al., 2001). No tumors have been detected in p19INK4d knock-out mice (Zindy et al., 2000). The function of D1 and D2 cyclins has also been investigated. Mice lacking D1 cyclin have reduced size and display severe retinopathy due to impaired development of the retina and limited development of mammary acinar cells during pregnancy (Fantl et al., 1995; Sicinski et al., 1995). On the other hand, D2 cyclin deficiency results in female sterility due to a defect in granulosa cell proliferation and male hypoplastic testis (Sicinski et al., 1996). Finally, the role of Cdks in vivo has only been investigated in the case of Cdk4 (Rane et al., 1999; Tsutsui et al., 1999). Mice lacking Cdk4 are viable but have reduced size and severe proliferative defects in certain endocrine cells, primarily testicular Leydig cells and pancreatic β-cells (Rane et al., 1999; J.Martin, S.L.Hunt, P.Dubus, R.Sotillo, M.Malumbres, S.Ortega and M.Barbacid, in preparation).
To understand the role of Cdk4 in neoplastic development, we have engineered a single miscoding mutation in the first exon of the mouse Cdk4 locus by gene targeting (Rane et al., 1999). The resulting knock-in mice, Cdk4R24C/R24C, express an endogenous Cdk4 protein that carries the Arg to Cys substitution (Cdk4R24C), the same mutation found in melanoma patients (Wolfel et al., 1995; Zuo et al., 1996). Here, we report that primary embryonic fibroblasts expressing the endogenous Cdk4R24C allele are immortal and susceptible to oncogenic transformation. In addition, homozygous, as well as heterozygous, Cdk4R24C mice develop a wide spectrum of tumors from different cell lineages. This mutation cooperates with deficiencies in other tumor suppressor genes such as p53 or _p27_Kip1. These results highlight the central role of Cdk4 regulation in cell transformation and provide a valuable model for developing cancer therapy strategies based on Cdk4 inhibition.
Results
Growth properties of Cdk4R24C/R24C mouse embryo fibroblasts
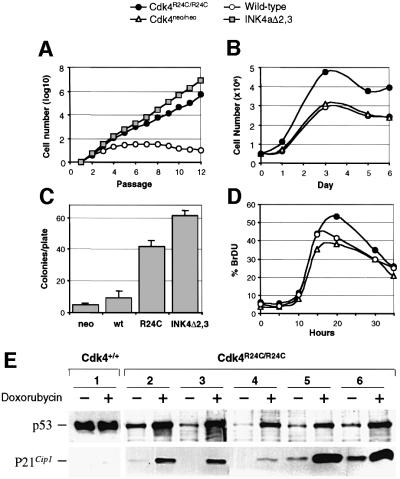
Mouse embryonic fibroblasts (MEFs) obtained from Cdk4R24C/R24C mice express normal levels of a Cdk4 protein that does not bind p16_INK4a_ and induces hyperphosphorylation of pRb (Rane et al., 1999). When maintained in a 3T3 passage scheme, these MEFs grow faster than wild-type MEFs (Figure 1). The population doubling time in the exponential phase of proliferation is ∼18–20 h for Cdk4R24C/R24C cells, 16–18 h for control INK4a–ARF-deficient cells (MEFs derived from INK4aΔ2,3 mice that lack p16_INK4a_ and p19_ARF_ proteins; Serrano et al., 1996) and 28–30 h for wild-type cells. Cdk4R24C/R24C MEFs show an almost undetectable crisis period, and do not enter into senescence after many passages (Figure 1A), a result reminiscent of that observed with INK4a–ARF mutant cells (Serrano et al., 1996). Cdk4R24C/R24C cells display other properties characteristic of a higher proliferative potential. Cdk4R24C/R24C monolayers achieve higher cellular densities (Figure 1B), show a higher efficiency in colony formation when seeded at low density (Figure 1C), and a higher number of cells enter simultaneously into S phase after starvation and re-stimulation with serum (Figure 1D).
Fig. 1. Growth properties of Cdk4R24C/R24C MEFs. MEFs derived from wild-type (open circles), Cdk4R24C/R24C (filled circles), Cdk4neo/neo (open triangles) and INK4aΔ2,3 (gray squares) mice were utilized. (A) Growth of MEFs according to a 3T3 protocol; (B) cell density; (C) platting efficiency; (D) DNA synthesis after serum re-stimulation; and (E) functional analysis of the p53 pathway in immortal clones derived from wild-type MEFs (clone 1) or Cdk4R24C/R24C MEFs (clones 2–6). Immortal wild-type MEFs show overexpression of a p53 protein that is not able to induce p21_Cip1_ after treatment with doxorubycin. All five Cdk4R24C/R24C clones display normal p53 and p21_Cip1_ induction after doxorubycin treatment. Results shown in (A–D) correspond to an average of three to five different experiments.
It has been proposed that replicative senescence in INK4a–ARF–/– MEFs results from the p53-dependent growth inhibitory effect of p19ARF (Kamijo et al., 1997; Sherr and DePinho, 2000), since spontaneous immortalization of MEFs is usually accompanied by either mutation of p53 or deletion of the INK4a–ARF locus. To examine the functional status of the p53 pathway, we have analyzed the p53-dependent response to DNA damage induced after doxorubycin insult in Cdk4R24C/R24C MEFs. As illustrated in Figure 1E, each (5/5) of the immortal Cdk4R24C/R24C MEF clones examined displayed a significant accumulation of p53 accompanied by p21Cip1 induction. In contrast, most clones (4/6) carrying the normal Cdk4 alleles lose a functional p53 pathway as determined by either absence of p53 protein, overexpression of p53 due to point mutations or lack of p21Cip1 induction (Figure 1E). These results indicate that immortal Cdk4R24C/R24C cells are resistant to p19ARF/p53-induced senescence.
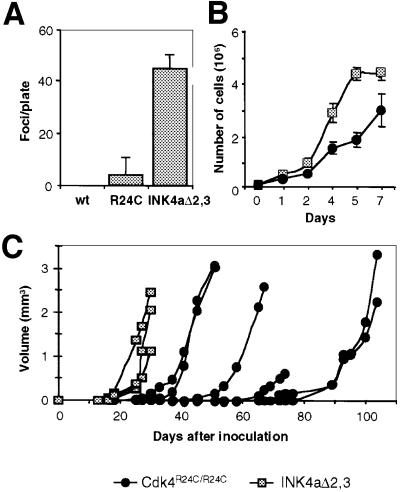
Cdk4R24C/R24C MEFs, although immortal, do not show a morphologically transformed phenotype and are not capable of growing under the skin of nude mice. However, these mutant MEFs are susceptible to transformation by Ras oncogenes (Figure 2A). The susceptibility of Cdk4R24C/R24C MEFs to Ras transformation is similar to that observed in p15_INK4b_–/– MEFs (Latres et al., 2000) and lower than in INK4a–ARF–/– MEFs (Figure 2A). In addition, Cdk4R24C/R24C clones grow slower than INK4a–ARF–/– cells even after being transformed by Ras oncogenes (Figure 2B). These observations suggest that defects in the p19_ARF_/p53 pathway contribute to Ras susceptibility as well as to Ras transforming activity.
Fig. 2. Susceptibility of primary MEFs to transformation by Ras oncogenes. (A) Number of transformed foci per plate in wild-type (wt), Cdk4R24C/R24C or INK4aΔ2,3 MEFs after transfection with oncogenic H-Ras. (B) Growth curves of Ras-transformed clones derived from Cdk4R24C/R24C MEFS (filled circles) and INK4aΔ2,3 MEFs (squares). Data represent the average and standard deviation of two experiments with two different clones. (C) Growth of tumors after subcutaneous injection of individual Ras-transformed clones in nude mice. No tumors were observed after injection of non-transfected Cdk4R24C/R24C MEFs.
Transformed Cdk4R24C/R24C MEFs induced tumors when injected into BALB/c nude mice, although with decreased incidence and longer latency than Ras-transformed INK4a–ARF–/– cells (Figure 2C). Whereas all four INK4a–ARF–/– clones produced tumors in 15–20 days after injection, only six out of 12 (50%) Cdk4R24C/R24C clones gave tumors and with longer latency (25–80 days). No tumors were observed after injection of non- transfected Cdk4R24C/R24C cells. To analyze whether neoplastic growth of Cdk4R24C/R24C MEFs requires subsequent mutations in the p53 pathway, we tested the cellular levels of p53, p19_ARF_ and MDM2 in six subcutaneous tumors derived from Cdk4R24C/R24C or INK4a–ARF–/– cells. We did not detect overexpression of MDM2 by immunohistochemical analysis. p19_ARF_ expression in Cdk4R24C/R24C cells was difficult to evaluate due to the low levels of ARF protein in tumors and in surrounding normal cells. p53 expression was very low (∼0.1% positive cells) in all tumors regardless of whether they were derived from Cdk4R24C/R24C or INK4a–ARF–/– cells. Therefore, it is likely that the Cdk4 R24C mutation can contribute to the tumorigenic properties of Ras-transformed MEFs without additional contribution of the p53 pathway.
Tumor susceptibility in Cdk4R24C/R24C mice
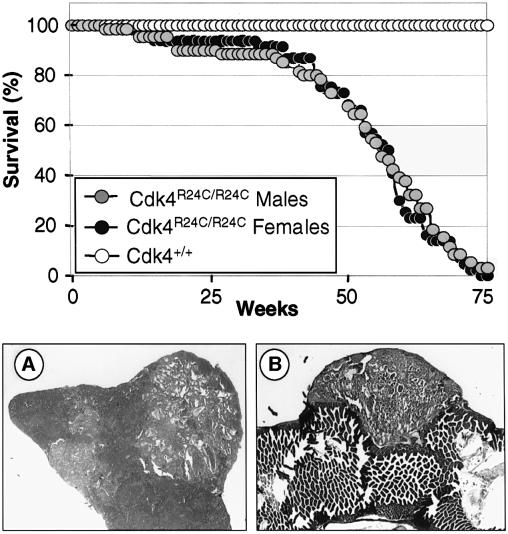
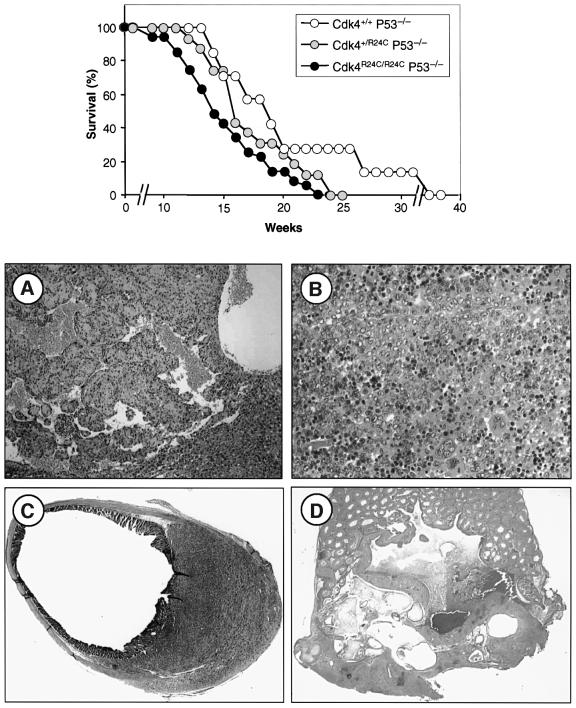
Cdk4R24C/R24C mice are born at the expected Mendelian ratio, are fertile and develop normally (Rane et al., 1999). However, these mice develop detectable tumors after 8 months and most of them are dead by 16 months of age (Figure 3). Necropsy analysis of a cohort of 105 Cdk4R24C/R24C dead mice showed a wide spectrum of tumors, including malignancies of mesenchymatous origin (67% incidence), epithelial endocrine (55%), epithelial non-endocrine (24%) and, to a lesser extent, hematopoietic malignancies (3%) (Table I). Many mice exhibited multiple tumors of independent origin with an average of almost two tumors per animal (Table II). An independent cohort of 50 mice sacrificed at 14–16 months of age, without external signs of disease, revealed a high incidence of tumors with a similar distribution pattern. However, these mice displayed a reduced incidence of sarcomas and pituitary tumors, suggesting that these malignancies are the major cause of death in Cdk4R24C/R24C mice (Table I). Heterozygous Cdk4+/R24C mice also developed the same type of malignancies, although with increased latency (data not shown) indicating the dominant nature of this mutation.
Fig. 3. (Top panel) Survival curve of Cdk4R24C/R24C male (gray circles) or female (black circles) mice compared with wild-type littermates (open circles). (A) Representative section of a hemangiosarcoma in the spleen (200×). (B) Metastasis of a hemangiosarcoma in the intestine epithelium (100×).
Table I. Tumor incidence in Cdk4R24C/R24C mice.
| Tumor type | Spontaneous death (n = 105) | Killed at 14–16 months (n = 50) | ||
|---|---|---|---|---|
| Mesenchymatous tumorsa | 71 | (67%) | 16 | (32%) |
| hemangiosarcomas | 59 | (56%) | 11 | (22%) |
| other sarcomasb | 6 | (6%) | 2 | (4%) |
| benign mesenchymatous tumorsc | 8 | (8%) | 3 | (6%) |
| Epithelial endocrine tumorsa | 58 | (55%) | 34 | (68%) |
| Leydig cell tumors (males onlyd) | 38 | (62%) | 21 | (70%) |
| pancreatic endocrine tumors | 32 | (31%) | 21 | (42%) |
| pituitary tumors | 23 | (22%) | 3 | (6%) |
| other endocrine tumorse | 2 | (2%) | 0 | (0%) |
| Epithelial non-endocrine tumorsa | 25 | (24%) | 21 | (42%) |
| lung adenomas/adenocarcinomas | 19 | (18%) | 16 | (32%) |
| hepatocellular tumorsf | 6 | (6%) | 6 | (12%) |
| other epithelial tumorsg | 3 | (3%) | 5 | (10%) |
| Lymphoid/myeloproliferative tumors | 3 | (3%) | 1 | (2%) |
| lymphoma | 2 | (2%) | 0 | (0%) |
| mast cell | 1 | (1%) | 1 | (2%) |
Table II. Number of different primary tumors per mouse at death.
| Tumors per mouse | Males (n = 61) | Females (n = 44) | Total (n = 105) | |||
|---|---|---|---|---|---|---|
| 0 | 3 | (5%) | 4 | (9%) | 7 | (7%) |
| 1 | 22 | (36%) | 14 | (30%) | 35 | (33%) |
| 2 | 21 | (34%) | 17 | (39%) | 38 | (36%) |
| 3 | 7 | (11%) | 7 | (16%) | 14 | (13%) |
| 4 | 7 | (11%) | 3 | (7%) | 10 | (10%) |
| 5 | 1 | (2%) | 0 | (0%) | 1 | (1%) |
| Average | 1.93 | 1.81 | 1.89 |
Soft tissue sarcomas developed in 62% of Cdk4R24C/R24C mice with hemangiosarcoma the most frequent subtype (56% incidence; Figure 3A), a result reminiscent of that observed with p15_INK4b_–/– mice (Latres et al., 2000). These tumors appeared at multiple sites (spleen, liver, subcutaneous or deep soft tissues) and were often accompanied by metastasis (54% of the cases; Figure 3B). Among endocrine tumors (Figure 4), those of Leydig cell origin were the most frequent (62% incidence), followed by those derived from pancreatic β-islet cells (31%) and pituitary cells (22%). Leydig cell tumors were preceded by hyperplasia in most of the mice analyzed. These tumors were often bilateral and multifocal but did not metastasize (except in one case) and did not affect the overall survival since male Cdk4R24C/R24C mice have similar survival rates as female mice (Figure 2).
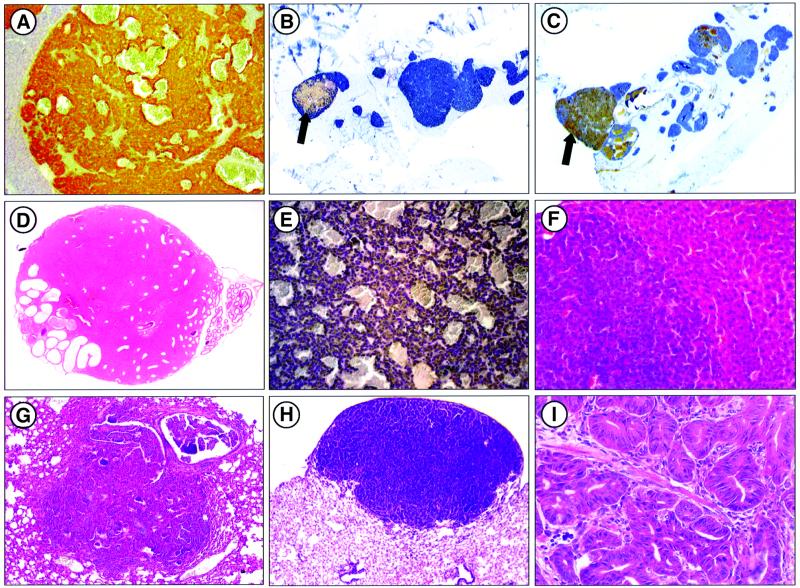
Fig. 4. Endocrine and epithelial non-endocrine tumors in Cdk4R24C/R24C mice. (A) Pancreatic endocrine tumor positive for insulin immunostaining (100×). (B–C) Adenomatous pancreatic islets showing cells positive for (B) insulin (blue) and glucagon (brown cells indicated by an arrow) or (C) insulin and PP (brown cells indicated by an arrow) (100×). (D) Leydig cell tumor with extension to the epididymis (200×). (E) Adenocarcinoma of the adenohypophysis immunoreactive for prolactin (200×). (F) Liver adenoma (200×). (G) Lung adenocarcinoma (100×). (H) Alveolar bronchiolar adenoma (400×). (I) Intestinal adenoma with in situ carcinoma (400×).
Pancreatic endocrine tumors were also preceded by severe endocrine hyperplasia. In a panel of 17 pancreas analyzed in detail, we identified 273 small adenomas (16 adenomas/pancreas) ranging from 0.5 to 1.5 mm in diameter. A high proportion of these adenomas (89%) consisted of mostly (>95%) insulin-producing β-cells. However, a few adenomatous islets were made almost exclusively of pancreatic polypeptide (PP)- and glucagon-producing cells (four and one islets, respectively). Moreover, a significant percentage of these pancreatic adenomas (∼10%) contained a mixture of endocrine cells including β, α and PP cells (Figure 4A–C). These results indicate that improper Cdk4 regulation can affect the proliferative properties of a variety of endocrine pancreatic cells. However, no hyperplastic somatostatin-producing delta cells have been identified in the pancreas of Cdk4R24C/R24C mice.
Most of the pituitary tumors observed in Cdk4R24C/R24C mice were adenomas with a variety of hormone expression patterns. However, ∼15% of these tumors appeared to be carcinomas, most of them positive for prolactine expression. Bronchio-alveolar adenomas and adenocarcinomas of the lung were the third most common tumor in Cdk4R24C/R24C mice, a result reminiscent of those found in p18_INK4c_–/–; p27_Kip1_–/– double mutant mice (Franklin et al., 2000). Finally, adenoma or hyperplasia of the mammary gland, hepatocytes, salivary gland, adrenal subcapsullar cells, prostatic and endometrial cells (1–7% incidence) were also observed.
Other common pathologies detected in Cdk4R24C/R24C mice included extramedular hematopoiesis in spleen and liver (43% incidence) associated with myeloid metaplasia (13%), lymphoid hyperplasia in the spleen (6%) and in the lymph nodes (13%), glomerular cysts (27%), chronic nephropathy (9%) and ductal ectasia of mammary glands (1%). Many of these pathologies have also been reported in mice deficient for p15_INK4b_ and/or p18_INK4c_ inhibitors (Latres et al., 2000). In addition, organomegaly was common in male genital annexed glands such as seminal vesicles, coagulating glands and prostate, without evidence of histological abnormalities.
Absence of INK4a, INK4b, ARF and p53 alterations in Cdk4R24C tumors
Alterations of the genes encoding p16INK4a, p15INK4b and p19ARF proteins are frequently found in rodent tumors (Ruas and Peters, 1998). Whereas these loci are commonly deleted or inactivated by promoter hypermethylation, point mutations are rare. To investigate whether these alterations also occur in Cdk4R24C/R24C tumors, we analyzed deletion of the individual _p16_INK4a, _p15_INK4b and _p19_ARF genes as well as the methylation status of their respective promoters. Southern analysis of DNA tumor samples did not show deletions in any of these three genes in a panel of 31 tumors (13 sarcomas, seven Leydig cell tumors, seven pituitary tumors and four hepatocellular tumors). Similarly, no methylation of their respective promoters was detected in any of these samples (data not shown).
The status of the p53 gene was also studied in Cdk4R24C/R24C tumors. Exons 5–9 of the murine p53 gene from 17 tumor samples, including representative epithelial tumors and sarcomas, were amplified by PCR using intronic primers. No point mutations were found in any of the samples after amplification and DNA sequencing (data not shown). To further analyze the integrity of the p53 pathway, we studied the protein levels of MDM2, p19ARF and p53 in these tumors. Western blot and immunohistochemical detection of these proteins was performed in a representative number of sarcomas (18 samples), Leydig cell tumors (11 samples), pancreatic endocrine adenomas (10 samples), and tumors of the liver (four samples), lung (four samples) and pituitary (five samples). Overexpression of MDM2 was only detected in two out of 11 (18%) pancreatic endocrine adenomas. The presence of p19ARF was difficult to evaluate due to the low level of expression in most organs. However, when the comparison was possible, p19ARF levels in tumor cells were equivalent to those of the corresponding normal cells. Similarly, no differences were detected in the levels of p53 in any of the tumors analyzed. These results suggest that loss of Cdk4 regulation by INK4 inhibitors is sufficient to induce a variety of tumors in vivo, not requiring cooperation by a mutated p19ARF/p53 pathway, at least in most cases.
Cdk4 and Cdk2 activity and p27Kip1 complexes in Cdk4R24C tissues
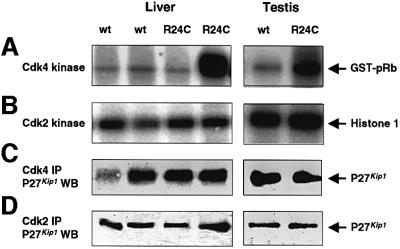
The high susceptibility to tumor development in homozygous as well as heterozygous Cdk4R24C mice suggests that the R24C mutation has a dominant effect in the fate of several cellular lineages. This mutation makes the Cdk4 kinase resistant to INK4 inhibition (Wolfel et al., 1995) and results in pRb hyperphosphorylation in cultured MEFs (Rane et al., 1999). We tested Cdk4 and Cdk2 kinase activity in tissues derived from wild-type and Cdk4R24C/R24C mice. Higher Cdk4 activity was observed in most, but not all (eight out of 12), tissues derived from Cdk4R24C/R24C mice (Figure 5). In contrast, no significant increases in Cdk2 activity were observed (Figure 5). Next, we assessed whether the Cdk4R24C mutation affected the redistribution of p27_Kip1_ between Cdk4 and Cdk2 complexes. As illustrated in Figure 5, we did not observe significant differences in the distribution of p27_Kip1_ in Cdk4R24C/R24C cells when compared with those derived from wild-type mice.
Fig. 5. (A) Cdk4 and (B) Cdk2 kinase activity in representative liver and testis samples from Cdk4R24C/R24C mice. Kinase activity was assayed after immunoprecipitation with Cdk4 or Cdk2 antibodies. R24C lanes depict examples of one out of four tissues in which we did not observe increased Cdk4 kinase activity and of two out of eight tissues in which the Cdk4 kinase activity was elevated. In none of the samples, we observed significantly increased Cdk2 activity. (C and D) Distribution of p27_Kip1_ in complexes formed with (C) Cdk4 or (D) Cdk2 in the above samples. IP, immunoprecipitation; WB, western blot. The migration of the Cdk4 and Cdk2 substrates, GST–pRB and Histone H1, respectively and of p27_Kip1_ is indicated by arrows.
Cooperation between Cdk4R24C and p53 deficiency
The low frequency of ARF/MDM2/p53 mutations in Cdk4R24C/R24C tumors does not preclude the possibility of cooperation between Cdk4 deregulation and p53 mutations. To examine this question, we crossed Cdk4R24C/R24C mutant mice with p53–/– knock-out animals. Double mutant Cdk4R24C/R24C;p53–/– or Cdk4+/R24C;p53–/– mice die before reaching 4 months of age, significantly earlier than their Cdk4+/+;p53–/– littermates (Figure 6; Table III). These mice display an increased number of sarcomas (mainly hemangiosarcomas and leiomyosarcomas) with shorter latency. The increased incidence of these tumors probably accounts for the lower incidence of diffuse large cell lymphomas in the Cdk4R24C/R24C;p53–/– and Cdk4+/R24C;p53–/– mice (Table III). Moreover, a significant fraction of these double mutant mice (10%) develop immature teratomas, a tumor not found in any of their single mutant siblings, although a low incidence of teratomas (<2%) has been described previously in p53–/– mice (Jacks et al., 1994). These results suggest that these key regulators exert growth control at different threshold levels in different cell types.
Fig. 6. Cooperation between the Cdk4R24C allele and p53 deficiency. (Top panel) Tumor-free survival curve of p53-deficient mice carrying one or two Cdk4R24C alleles. (A–D) Representative sections of tumors obtained from Cdk4R24C/R24C;p53–/– double mutant mice. (A) Hemangiosarcoma located in the liver (100×). (B) Typical diffuse large cell lymphoma in spleen (400×). (C) Leiomyosarcoma with intermediate differentiation spreading to the large intestine/rectum area (200×). (D) Teratoma in the testis formed by mature structures such as cysts lined by columnar or epidermoid epithelium (center), bone (right) and cartilage (left), and a large immature area (200×).
Table III. Tumors in Cdk4R24C/p53 double mutant mice.
| | Cdk4+/+;p53–/– (n = 11) | Cdk4+/R24C;p53–/– (n = 15) | Cdk4R24C/R24C;p53–/– (n = 41) | | | | | | ----------------------------- | ----------------------------- | -------------------------------- | ----------- | ----- | -- | ----- | | Average age at death | 4.7 monthsa | 3.9 months | 3.3 monthsa | | | | | Tumor type | | | | | | | | lymphoma | 9 | (82%) | 11 | (73%) | 21 | (51%) | | sarcoma | 2 | (18%) | 6 | (40%) | 20 | (49%) | | teratoma | 0 | (0%) | 0 | (0%) | 4 | (10%) |
Cooperation between the Cdk4R24C mutation and p27Kip1 deficiency
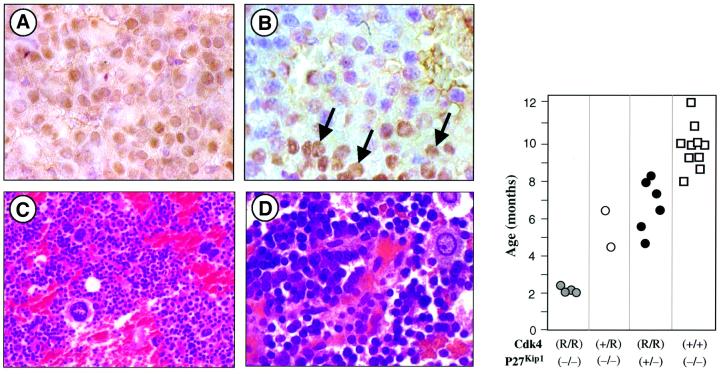
Cdk4R24C/R24C mice develop pituitary tumors with high penetrance, a phenotype also observed in mice targeted for other components of the INK4/Cdk4/Rb pathway, pRb+/– and p18INK4c–/–. Moreover, mutations in Cip/Kip inhibitors such as p27Kip1 also result in pituitary tumors (reviewed in Malumbres et al., 2000a). In fact, concomitant loss of p18INK4c–/– and p27Kip1 results in significant reduction of tumor latency, thus suggesting cooperation between INK4 and Cip/Kip inhibitors (Franklin et al., 1998). To determine whether p27Kip1 plays a role in the development of pituitary tumors in Cdk4R24C/R24C mice, we exam ined p27Kip1 expression by immunohistochemical staining. Wild-type pituitary tissue shows p27Kip1 immunoreactivity in most endocrine cells from the adenohypophysis and the pars intermedia. However, p27Kip1 staining is either lost or severely reduced in most (9/10) Cdk4R24C/R24C pituitary tumors analyzed (Figure 7).
Fig. 7. Cooperation between the Cdk4R24C mutation and loss of p27Kip1 in the development of pituitary tumors. (A) p27Kip1 expression in wild-type adenohypophysis. Most nuclei from endocrine cells are positive (1000×). (B) Cdk4R24C/R24C adenoma of the adenohypophysis showing areas of positive cells (arrows) and tumoral regions negative for p27Kip1 immunoreactivity (1000×). (C and D) Undifferentiated tumor of the pars intermedia in a Cdk4R24C/R24C;p27Kip1–/– mouse [hematoxylin and eosin staining; (C) 400×; (D)1000×]. (Right panel) Latency of pituitary tumors in animals derived from the crosses between Cdk4R24C/R24C and p27Kip1–/– mice. The _y_-axis represents the age of the mice at death in the different genotypes.
Next, to assess the extent of the cooperation between the Cdk4R24C mutation and loss of p27Kip1 in tumor development, we crossed Cdk4R24C/R24C mice with animals deficient in this Cip/Kip inhibitor. Pituitary tumors in Cdk4R24C/R24C mice were detectable at 14–16 months of age. Likewise, p27Kip1-deficient mice died of pituitary tumors with an average latency of 10 months (Figure 7). In contrast, Cdk4R24C/R24C;p27Kip1–/– double mutant mice died of pituitary tumors with an average latency of ∼2 months (Figure 7). These tumors, unlike those found in Cdk4R24C/R24C mice, were mainly undifferentiated neoplasias of the pars intermedia, similar to pituitary tumors arising in pRb+/–; p27Kip1–/– mutants (Park et al., 1999). Cdk4+/R24C;p27Kip1–/– and Cdk4R24C/R24C;p27Kip1+/– mice also developed pituitary tumors with complete penetrance. However, these strains displayed increased latency (5.6 months in Cdk4+/R24C;p27Kip1–/–; 6.7 months in Cdk4R24C/R24C;p27Kip1+/–). These results, in agreement with the biochemical data shown in Figure 5, suggest that tumor development induced by the Cdk4R24C mutation is not due to increased sequestration of p27Kip1, at least in pituitary cells.
Discussion
Available evidence has suggested that the p19_ARF_/p53 rather than the INK4/pRb pathway is primarily responsible for inducing senescence (Harvey and Levine, 1991; Kamijo et al., 1997). However, concomitant deletion of the three members of the Rb family of proteins also confers immortality to these cells since MEFs deficient for pRb, p107 and p130 do not sustain functional mutations in the p53 pathway even upon prolonged passage in culture (Dannenberg et al., 2000; Sage et al., 2000). Here, we show that expression of a mutant Cdk4 whose kinase activity cannot be down-regulated by INK4 inhibitors is also sufficient to confer immortality to MEFs. MEFs derived from Cdk4R24C/R24C embryos grow significantly faster than wild-type MEFs and have properties similar to those observed in MEFs lacking the three Rb family members. Moreover, none of these properties require mutations in the p19_ARF_/p53 pathway. Thus, Cdk4 activity, when resistant to INK4 inhibition, is sufficient to inactivate the entire Rb protein family, at least during immortalization of MEFs in culture. These results suggest that the INK4 proteins are the primary effectors responsible for maintaining the activity of pRb family members during senescence. Whether this activity requires all or a subset of INK4 inhibitors, remains to be determined. However, since concomitant deletion of p15_INK4b_ and p18_INK4c_ proteins does not result in MEF immortalization (Latres et al., 2000), we conclude that proper control of Cdk4 activity during senescence must require the coordinated action of at least three members of the INK4 protein family.
Cdk4R24C/R24C MEFs are also susceptible to transformation by Ras oncogenes, a property shared by MEFs defective in all Rb family members (Dannenberg et al., 2000; Sage et al., 2000). Interestingly, this property can be attributed to the specific action of single INK4 proteins since we have previously shown that p15_INK4b_-deficient MEFs can be transformed by Ras oncogenes (Latres et al., 2000; Malumbres et al., 2000b). In contrast, loss of either p18_INK4c_ or p19_INK4d_ proteins does not confer this property (Latres et al., 2000; Zindy et al., 2000). These results indicate that certain phenotypic consequences of the unregulated Cdk4 activity, such as susceptibility to Ras-induced transformation, can be independently controlled by specific INK4 inhibitors.
Deregulation of G1/S cell cycle kinases is one of the most frequent events in human cancer. Cdk4R24C/R24C mice develop a wide spectrum of tumors. Indeed, the intense tumor susceptibility of these mutant mice contrasts with the collective data showing that disabling single INK4 family members does not dramatically increase the rate of spontaneous tumor development (Roussel, 1999; Malumbres et al., 2000a). These observations suggest that INK4 proteins have significant overlapping properties in most cell types. This might explain not only the increased incidence of tumor formation in Cdk4R24C/R24C mice, but also the significantly wider spectrum of tumor types. Interestingly, many of the tumors observed in Cdk4R24C/R24C mice originate from endocrine cells of the pancreas, pituitary or testis, the very same cell types that have been previously shown to have limited proliferative properties in mice lacking Cdk4 expression (Rane et al., 1999; J.Martin, S.L.Hunt, P.Dubus, R.Sotillo, M.Malumbres, S.Ortega and M.Barbacid, in preparation). These results suggest that Cdk4 plays a central role in controlling the proliferation of these endocrine cells.
Some of the tumors found in Cdk4R24C/R24C mice, such as soft tissue sarcomas, reproduce findings with human tumors where oncogenic amplification and overexpression of Cdk4 has commonly been reported (Khatib et al., 1993; Wei et al., 1999; Wunder et al., 1999). In these tumors, Cdk4 is frequently co-amplified with other genes including MDM2 (Khatib et al., 1993; Wunder et al., 1999). Cdk4 overexpression has also been reported in other human tumors such as breast cancer, gliomas or meningiomas (He et al., 1994; Schmidt et al., 1994; Weber et al., 1997; An et al., 1999). Surprisingly, Cdk4R24C mutant mice do not develop melanomas, the tumor type observed in humans with the same genotype. Recent studies in which these mutant mice have been exposed to various skin carcinogenesis protocols have resulted in the frequent appearance of invasive melanomas (Sotillo et al., 2001). These results indicate that human melanoma may result from a combination of cell cycle mutations followed by additional genotoxic events induced by environmental carcinogens.
The most frequent tumor types observed in Cdk4R24C/R24C mice match those previously found in mice deficient in INK4 inhibitors, albeit with lower incidence (Franklin et al., 1998; Latres et al., 2000; Krimpenfort et al., 2001; Sharpless et al., 2001). For instance, hemangiosarcoma is the predominant tumor type in p15INK4b-deficient mice and pituitary tumors are predominant in p18INK4c null mice. Hematopoietic malignancies and other minor pathologies such as renal cysts, mammary gland ectasia or pancreatic β-cell hyperplasia were also described in the p15INK4b–p18INK4c double knockout or in the single mutants (Latres et al., 2000). Of particular interest are pituitary tumors which in addition to p18INK4c null mice have been found in other cell cycle mutant mice such as pRb+/– and p27Kip1–/– (Jacks et al., 1992; Hu et al., 1994; Fero et al., 1996; Kiyokawa et al., 1996; Nakayama et al., 1996; Franklin et al., 1998; Latres et al., 2000). These tumors, however, arise mostly from the pars intermedia [75% in the case of the pRb mutant mice (Nikitin et al., 1999) and 98% in the case of p18INK4c–/– mice (Latres et al., 2000)], whereas those expressing a mutated Cdk4R24C protein are mostly of adenohypophysis origin (80%). However, when pRb is conditionally deleted in specific cells (IRBP-Cre;Rb/floxed mice), all the pituitary tumors develop from the adenohypophysis (Vooijs and Berns, 1999). Since Cdk4 acts downstream of p18INK4c and upstream of pRb, these results illustrate the delicate balance that these proteins interplay in regulating cell proliferation in cells of common ectodermic origin.
Cdk4R24C tumors are quite similar to those found in mice deficient for p53 (hemangiosarcomas and lung adenocarcinomas) and in mice heterozygous for pRb and p53 (pituitary adenomas, pancreatic islet cell adenocarcinomas, hemangiosarcoma and lymphomas) (Donehower et al., 1992; Jacks et al., 1994; Williams et al., 1994; Harvey et al., 1995). We have not found mutations in the p53 tumor suppressor gene in a wide panel of representative Cdk4R24C/R24C tumors, indicating that they arise without cooperating p53 mutations. However, when we expressed the Cdk4R24C mutant protein in a p53–/– background, the incidence of sarcomas and teratomas increased significantly, illustrating the different requirements of distinct cell types to undergo neoplastic transformation.
Previous studies have illustrated the cooperation between the INK4/pRb and the p27_Kip1_ pathways in inducing pituitary tumors (Franklin et al., 1998, 2000; Park et al., 1999). Interestingly, loss of p27_Kip1_ reverts some of the defects caused by loss of Cdk4 (Tsutui et al., 1999) and cyclin D1 (Geng et al., 2001; Tong and Pollard, 2001). These observations are consistent with the hypothesis that Cdk4–cyclin D complexes may play a role in sequestering p27_Kip1_ during G1/S transition to activate the Cdk2 kinase (Sherr and Roberts, 1999). According to this hypothesis, lack of INK4-mediated inhibition of Cdk4 in Cdk4R24C/R24C mice should increase the availability of Cdk4 molecules to form Cdk4–Cyclin D–p27_Kip1_–ternary complexes. However, our results are not consistent with this hypothesis. First of all, we have not observed significant p27_Kip1_ redistribution into Cdk4–Cyclin D complexes in tissues expressing the Cdk4 R24C kinase. Moreover, pituitary tumors of Cdk4R24C/R24C mice invariably lose p27_Kip1_ expression. Finally, and perhaps most compelling, is the dramatic decrease in tumor latency observed in Cdk4R24C/R24C;p27_Kip1_ double mutant mice. Therefore, although our results do not preclude a role of Cdk4 in sequestering p27_Kip1_, they suggest that loss of INK4 inhibition by the Cdk4 R24C mutation is more likely to increase Cdk4-dependent pRb phosphorylation than sequestration of p27_Kip1_.
Proliferative disorders such as cancer are recognized as diseases of the cell cycle. G1/S-phase control mechanisms are aberrant in most human tumors and INK4/Cdk4/Rb regulation seems to be a central step in this control (Sherr, 2000). These results have validated the utilization of the cell cycle as a target for drug discovery (Brooks and La Tangue, 1999), underscoring the urgent need to develop specific inhibitors that downregulate this pathway in tumor cells. An ideal target for pharmacological intervention would be essential for growth or survival of tumors but dispensable in normal tissues. Cdk4 fulfils these requirements since Cdk4 is not essential for mouse development (Rane et al., 1999; Tsutsui et al., 1999). Yet, loss of just one of its multiple regulatory mechanisms due to lack of INK4 binding is sufficient to elicit a wide variety of tumors in many cell types. If disabling INK4 inhibitors is a hallmark of many cancers (Ruas and Peters, 1998; Sherr, 2000), then the most efficacious treatment would be to restore their effect by therapeutic inhibition of Cdk4 activity, an approach more readily applicable than restoring INK4 function. Cdk4R24C/R24C mice should provide an invaluable tool to test the anti-tumor properties of inhibitors of Cdk4 and possibly Cdk2 kinase activity, as well as future inhibitors of Cdk4–Cyclin D complexes, and thus help to speed up the process of translational research.
Materials and methods
Mice
Cdk4R24C/R24C mice expressing an endogenous Cdk4R24C protein were obtained by gene-targeting techniques as described previously (Rane et al., 1999). Cdk4-deficient mice (Cdk4neo/neo) were also reported (Rane et al., 1999). Cdk4R24C/R24C and Cdk4neo/neo mice were maintained in a mixed 129/Sv × CD-1 background. Mice deficient for the p53 or p27_Kip1_ tumor suppressor genes have been published previously (Jacks et al., 1994; Fero et al., 1996). Athymic BALB/c nu/nu mice were purchased from Charles River Laboratories. All mice were maintained according to institutional guidelines.
MEF assays
MEFs were isolated by standard techniques and maintained in a 3T3 scheme with Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum. Immortalization and proliferation assays were performed as described (Latres et al., 2000). The status of the p53 pathway was assessed essentially as described by Pantoja and Serrano (1999). Focus assays were performed by calcium phosphate-mediated transfection of MEFs with pAL8, an expression plasmid carrying an oncogenic H-ras allele under the control of its own promoter (Latres et al., 2000). Transformed clones (foci) were isolated, expanded and injected into nude mice (5 × 105 cells per injection). Tumor volume (V) was calculated using the formula V = (_d_2 × D)/2 where d is the minor diameter and D the major diameter of the tumor.
Necropsy and pathological analysis
Mice were sacrificed at any sign of disease and normal or pathological tissue samples were recovered for histological and molecular analysis. A group of 50 apparently healthy Cdk4R24C/R24C mice was sacrificed at the age of 16 months. Formalin-fixed pituitary samples were stained with antibodies against Adrenocorticotropic hormone, follicle-stimulating hormone, growth hormone, prolactin, luteinizing hormone and thyroid-stimulating hormone (kindly provided by A.F.Parlow, NHPP, NIDDK). Endocrine tumors of the pancreas were stained with antibodies against insulin, glucagon, somatostatin (Dako Corp.) and PP (Monosan). Deletion or hypermethylation of the _p16_INK4a, _p15_INK4b and _p19_ARF genes was studied as described (Malumbres et al., 1997; Meléndez et al., 2000). To analyze the presence of mutations in p53, we previously obtained the sequence of the mouse introns 4–8. These sequence data have been submitted to the DDBJ/EMBL/GenBank databases under accession No. AF367373. We then designed specific primers to amplify exons 5–9 of p53 and the presence of mutations in the tumor samples was scored by direct sequencing of amplified products. Sequence (5′–3′) of primers is as follows: mp53-5F, 5′-TACTCTCCTCCCCCTCAATAAG-3′; mp53-I6R, 5′-CGGGTTGCTAGAAAGTCAACA-3′; mp53-I6F, 5′-TGCCGAACAGGT GGAATATC-3′; mp53-I7R, 5′-TGGAACAGAAACAGGCAGAA-3′; mp53-I8F, 5′-TCTCGGGGTTCCTGTAACTG-3′; mp53–9R, 5′-GGATCTTGAGGGTGAAATACTCTCC-3′. Expression of p27Kip1 (clone DCS-72.F6, Neomarkers), p53 (NCL-p53-CM5p, Novocastra), p19ARF (SC-7403 from Santa Cruz Biotechnology, or R562 from Abcam) and MDM2 (SC-965, Santa Cruz Biotechnology) was analyzed by immunohistochemistry in paraffin sections of the tumors using the indicated antibodies.
Immunoprecipitation, kinase and western blot analysis
Tissue samples or cultured cells were lysed in a solution containing 50 mM Tris–HCl pH 7.4, 150 mM NaCl, 0.5% nonidet P-40 and protease inhibitors. For immunoprecipitation, cell lysates were incubated with 5 µg of anti-Cdk4 or anti-Cdk2 antibody (Santa Cruz Biotechnology) and 200 µl of protein A beads on a rotating wheel at 4°C for 2 h. Cdk4 kinase assays were performed with 100 ng of glutathione _S_-transferase (GST)–Rb (Santa Cruz Biotechnology) as a substrate. Histone H1 (1 µg per reaction; Roche Biochemicals) was used as a Cdk2 substrate. Kinase assays were performed in a solution containing 20 mM Tris–HCl pH 8, 1 mM EGTA, 10 mM MgCl2, 1 mM dithiothreitol, 25 µM cold ATP and 10 µCi of [γ-32P]ATP. Mixtures were incubated at 30°C for 30 min, and the reactions were stopped by the addition of SDS loading buffer. Reaction mixtures were run on a 12% SDS–polyacrylamide gel that was stained with Coomassie Blue. Quantification of phosphorylated proteins was performed on a PhosphorImager (Molecular Dynamics). Gels stained with Coomassie Blue were also scanned by densitometry to quantify the exact amount of protein present on the gel by NIH image software. For western blots, whole cell extracts or immunoprecipitates, prepared as above, were separated on a 12% SDS–PAGE gel. Following electrophoresis, proteins were transferred to nitrocellulose before incubation with the following antibodies: Cdk4 (C-22), Cdk2 (M2) and p21Cip1 (C-19, all from Santa Cruz Biotechnology), p53 (CM-5, Novocastra) and p27Kip1 (DCS-72.F6, Neomarkers). The antibodies were detected using anti-mouse or anti-rabbit Ig horseradish peroxidase linked whole antibody and visualized by the enhanced chemiluminiscent detection system (Amersham).
Acknowledgments
Acknowledgements
We thank Carmen Gómez, Henry Gomez, Maribel Muñoz and Michelle Turmo for excellent technical assistance, M.Serrano for the INK4aΔ2,3 MEFs and A.F.Parlow for antibodies against pituitary hormones. This work was supported by grants from Association pour la Recherche contre le Cancer (to P.D.), Comunidad Autónoma de Madrid, Spain (to M.M.), Pfizer S.A., (to M.B.) and V Framework Program of the EU (to M.B.).
References
- An H.X., Beckmann,M.W., Reifenberger,G., Bender,H.G. and Niederacher,D. (1999) Gene amplification and overexpression of CDK4 in sporadic breast carcinomas is associated with high tumor cell proliferation. Am. J. Pathol., 154, 113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks G. and La Thangue,N.B. (1999) The cell cycle and drug discovery: the promise and the hope. Drug Discov. Today, 5, 455–464. [DOI] [PubMed] [Google Scholar]
- Chilosi M. et al. (1998) Differential expression of cyclin-dependent kinase 6 in cortical thymocytes and T-cell lymphoblastic lymphoma/leukemia. Am. J. Pathol., 152, 209–217. [PMC free article] [PubMed] [Google Scholar]
- Corcoran M.M. et al. (1999) Disregulation of cyclin dependent kinase 6 expression in splenic marginal zone lymphoma through chromosome 7q translocations. Oncogene, 18, 6271–6277. [DOI] [PubMed] [Google Scholar]
- Dannenberg J.-H., van Rossum,A., Schuijff,L. and te Riele,H. (2000) Ablation of the retinoblastoma gene family deregulates G1 control causing immortalization and increased cell turnover under growth-restricting conditions. Genes Dev., 14, 3051–3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donehower L.A., Harvey,M., Slagle,B.L., McArthur,M.J., Montgomery,C.A., Butel,J.S. and Bradley,A. (1992) Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature, 356, 215–221. [DOI] [PubMed] [Google Scholar]
- Fantl V., Stamp,G., Andrews,A., Rosewell,I. and Dickson,C. (1995) Mice lacking cyclin D1 are small and show defects in eye and mammary gland development. Genes Dev., 9, 2364–2372. [DOI] [PubMed] [Google Scholar]
- Fero M.L. et al. (1996) A syndrome of multi-organ hyperplasia with features of gigantism, tumorigenesis and female sterility in p27Kip1-deficient mice. Cell, 85, 733–744. [DOI] [PubMed] [Google Scholar]
- Franklin D.S., Godfrey,V.L., Lee,H., Kovalev,G.I., Schoonhoven,R., Chen-Kiang,S., Su,L. and Xiong,Y. (1998) CDK inhibitors p18INK4c and p27Kip1 mediate two separate pathways to collaboratively suppress pituitary tumorigenesis. Genes Dev., 12, 2899–2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin D.S., Godfrey,V.L., O’Brien,D.A., Deng,C. and Xiong,Y. (2000) Functional collaboration between different cyclin-dependent kinase inhibitors suppresses tumor growth with distinct tissue specifity. Mol. Cell. Biol., 20, 6147–6158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng Y., Yu,Q., Sicinska,E., Das,M., Bronson,R.T. and Sicinski,P. (2001) Deletion of the p27Kip1 gene restores normal development in cyclin D1-deficient mice. Proc. Natl Acad. Sci. USA, 98, 194–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey D.M. and Levine,A.J. (1991) p53 alteration is a common event in the spontaneous immortalization of primary BALB/c murine embryo fibroblasts. Genes Dev., 5, 2375–2385. [DOI] [PubMed] [Google Scholar]
- Harvey M., Vogel,H., Lee,Y.-H.P., Bradley,A. and Donehower,A. (1995) Mice deficient in both p53 and Rb develop tumors primarily of endocrine origin. Cancer Res., 55, 1146–1151. [PubMed] [Google Scholar]
- He J., Allen,J.R., Collins,V.P., Allalunis-Turner,M.J., Godbout,R., Day,R.S. and James,C.D. (1994) CDK4 amplification is an alternative mechanism to p16 homozygous deletion in glioma cell lines. Cancer Res., 54, 5804–5807. [PubMed] [Google Scholar]
- Hirama T. and Koeffler,H.P. (1995) Role of the cyclin-dependent kinase inhibitors in the development of cancer. Blood, 86, 841–854. [PubMed] [Google Scholar]
- Hu N., Gutsmann,A., Herbert,D.C., Bradley,A., Lee,W.H. and Lee,E.Y. (1994) Heterozygous Rb-1 delta 20/+ mice are predisposed to tumors of the pituitary gland with nearly complete penetrance. Oncogene, 9, 1021–1027. [PubMed] [Google Scholar]
- Jacks T., Fazeli,A., Schmitt,E.M., Bronson,R.T., Goodell,M.A. and Weinberg,R.A. (1992) Effects of an Rb mutation in the mouse. Nature, 359, 295–300. [DOI] [PubMed] [Google Scholar]
- Jacks T., Remington,L., Williams,B.O., Schmitt,E.M., Halachmi,S., Bronson,R.T. and Weinberg,R.A. (1994) Tumor spectrum analysis in p53-mutant mice. Curr. Biol., 4, 1–7. [DOI] [PubMed] [Google Scholar]
- Kamijo T., Zindy,F., Roussel,M.F., Quelle,D.E., Downing,J.R., Ashmun,R.A., Grosveld,G. and Sherr,C.J. (1997) Tumor suppression at the mouse INK4a locus mediated by the alternative reading frame product p19ARF. Cell, 91, 649–659. [DOI] [PubMed] [Google Scholar]
- Khatib Z.A., Matsushime,H., Valentine,M., Shapiro,D.N., Sherr,C.J. and Look,A.T. (1993) Coamplification of the CDK4 gene with MDM2 and GLI in human sarcomas. Cancer Res., 53, 5535–5541. [PubMed] [Google Scholar]
- Kiyokawa H. et al. (1996) Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27Kip1. Cell, 85, 721–732. [DOI] [PubMed] [Google Scholar]
- Krimpenfort P., Quon,K.C., Mooi,W.J., Loonstra,A. and Berns,A. (2001) Loss of p16Ink4a confers susceptibility to metastatic melanoma in mice. Nature, 413, 83–86. [DOI] [PubMed] [Google Scholar]
- Latres E., Malumbres,M., Sotillo,R., Martín,J., Ortega,S., Martín-Caballero,J., Flores,J.M., Cordón-Cardo,C. and Barbacid,M. (2000) Limited overlapping roles of p15ink4b and p18INK4c cell cycle inhibitors in proliferation and tumorigenesis. EMBO J., 19, 3496–3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malumbres M., Pérez de Castro,I., Santos,J., Meléndez,B., Mangues,R., Serrano,M., Pellicer,A. and Fernéndez-Piqueras,J. (1997) Inactivation of the cyclin-dependent kinase inhibitor p15INK4b by deletion and de novo methylation with independence of p16INK4a alterations in murine primary T-cell lymphomas. Oncogene, 14, 1361–1370. [DOI] [PubMed] [Google Scholar]
- Malumbres M., Ortega,S. and Barbacid,M. (2000a) Genetic analysis of mammalian cyclin-dependent kinases and their inhibitors. Biol. Chem., 381, 827–838. [DOI] [PubMed] [Google Scholar]
- Malumbres M., Pérez de Castro,I., Hernández,M.I., Jiménez,M., Corral,T. and Pellicer,A. (2000b) Cellular response to oncogenic Ras involves induction of the Cdk4 and Cdk6 inhibitor p15INK4a. Mol. Cell. Biol., 20, 2915–2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meléndez B., Malumbres,M., Pérez de Castro,I., Santos,J., Pellicer,A. and Fernández-Piqueras,J. (2000) Characterization of the murine p19ARF promoter CpG island and its methylation pattern in primary lymphomas. Carcinogenesis, 21, 817–821. [DOI] [PubMed] [Google Scholar]
- Morgan D.O. (1995) Principles of Cdk regulation. Nature, 374, 131–134. [DOI] [PubMed] [Google Scholar]
- Nakayama K., Ishida,N., Shirane,M., Inomata,A., Inoue,T., Shishido,N., Horii,I. and Loh,D.Y. (1996) Mice lacking p27Kip1 display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell, 85, 707–720. [DOI] [PubMed] [Google Scholar]
- Nikitin A.Y., Juarez-Perez,M.I., Li,S., Guang,L. and Lee,W.H. (1999) RB-mediated suppression of spontaneous multiple neuroendocrine neoplasia and lung metastases in Rb+/– mice. Proc. Natl Acad. Sci. USA, 96, 3916–3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantoja C. and Serrano,M. (1999) Murine fibroblasts lacking p21 undergo senescence and are resistant to transformation by oncogenic Ras. Oncogene, 18, 4974–4982. [DOI] [PubMed] [Google Scholar]
- Park M.S., Rosai,J., Nguyen,H.T., Capodieci,P., Cordon-Cardo,C. and Koff,A. (1999) p27 and pRb are on overlapping pathways suppressing tumorigenesis in mice. Proc. Natl Acad. Sci. USA, 96, 6382–6387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavletich N.P. (1999) Mechanisms of cyclin-dependent kinase regulation: structures of cdks, their cyclin activators, and Cip and INK4 inhibitors. J. Mol. Biol., 287, 821–828. [DOI] [PubMed] [Google Scholar]
- Peters G. (1994) The D-type cyclins and their role in tumorigenesis. J. Cell Sci., S18, 89–96. [DOI] [PubMed] [Google Scholar]
- Rane S.G., Dubus,P., Mettus,R.V., Galbreath,E.J., Boden,G., Reddy,E.P. and Barbacid,M. (1999) Loss of Cdk4 expression causes insulin-deficient diabetes and Cdk4 activation results in β-islet cell hyperplasia. Nature Genet., 22, 44–52. [DOI] [PubMed] [Google Scholar]
- Roussel M.F. (1999) The INK4 family of cell cycle inhibitors in cancer. Oncogene, 18, 5311–5317. [DOI] [PubMed] [Google Scholar]
- Ruas M. and Peters,G. (1998) The p16INK4a/CDKN2A tumor suppressor and its relatives. Biochim. Biophys. Acta, 1378, F115–F177. [DOI] [PubMed] [Google Scholar]
- Sage J., Mulligan,G.J., Attardi,L.D., Miller,A., Chen,S., Williams,B., Theodorou,E. and Jacks,T. (2000) Targeted disruption of the three Rb-related genes leads to loss of G1 control and immortalization. Genes Dev., 14, 3037–3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt E.E., Ichimura,K., Reifenberger,G. and Collins,V.P. (1994) CDKN2 (p16/MTS1) gene deletion or Cdk4 amplification occurs in the majority of glioblastomas. Cancer Res., 54, 6321–6324. [PubMed] [Google Scholar]
- Serrano M., Lee,H.-W., Chin,L., Cordon-Cardo,C., Beach,D. and DePinho,R.A. (1996) Role of the INK4a locus in tumor suppression. Cell, 85, 27–37. [DOI] [PubMed] [Google Scholar]
- Sharpless N.E. and DePinho,R.A. (1999) The INK4A/ARF locus and its two gene products. Curr. Opin. Genet. Dev., 9, 22–30. [DOI] [PubMed] [Google Scholar]
- Sharpless N.E., Bardeesy,N., Lee,K.H., Carrasco,D., Castrillon,D.H., Aguirre,A.J., Wu,E.A., Horner,J.W. and DePinho,R.A. (2001) Loss of p16Ink4a with retention of p19Arf predisposes mice to tumorigenesis. Nature, 413, 86–91. [DOI] [PubMed] [Google Scholar]
- Sherr C.J. (2000) Cancer cell cycles revisited. Cancer Res., 60, 3689–3695. [PubMed] [Google Scholar]
- Sherr C.J. and Roberts,J.M. (1999) CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev., 13, 1501–1512. [DOI] [PubMed] [Google Scholar]
- Sherr C.J. and DePinho,R.A. (2000) Cellular senescence: mitotic clock or culture shock. Cell, 102, 407–410. [DOI] [PubMed] [Google Scholar]
- Sicinski P. et al. (1995) Cyclin D1 provides a link between development and oncogenesis in the retina and breast. Cell, 82, 621–630. [DOI] [PubMed] [Google Scholar]
- Sicinski P. et al. (1996) Cyclin D2 is an FSH-responsive gene involved in gonadal cell proliferation and oncogenesis. Nature, 384, 470–474. [DOI] [PubMed] [Google Scholar]
- Sotillo R., Garcia,J.F., Ortega,S., Martin,J., Dubus,P., Barbacid,M. and Malumbres,M. (2001) Invasive melanoma in Cdk4-targeted mice. Proc. Natl Acad. Sci. USA, 98, 13312–13317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong W. and Pollard,J.W. (2001) Genetic evidence for the interactions of cyclin D1 and p27Kip1 in mice. Mol. Cell. Biol., 21, 1319–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui T., Hesabi,B., Moons,D.S., Pandolfi,P.P., Hansel,K.S., Koff,A. and Kiyokawa,H. (1999) Targeted disruption of CDK4 delays cell cycle entry with enhanced p27Kip1 activity. Mol. Cell. Biol., 19, 7011–7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vooijs M. and Berns,A. (1999) Developmental defects and tumor predisposition in Rb mutant mice. Oncogene, 18, 5293–5303. [DOI] [PubMed] [Google Scholar]
- Weber R.G., Boström,J., Wolter,M., Baudis,M., Collins,V.P., Reifenberger,G. and Lichter,P. (1997) Analysis of genomic alterations in benign, atypical, and anaplastic meningiomas: toward a genetic model of meningioma progression. Proc. Natl Acad. Sci. USA, 94, 14719–14724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei G., Lonardo,F., Ueda,T., Kim,T., Huvos,A.G., Healey,J.H. and Ladanyi,M. (1999) CDK4 gene amplification in osteosarcoma: reciprocal relationship with INK4A gene alterations and mapping of 12q13 amplicons. Int. J. Cancer, 80, 199–204. [DOI] [PubMed] [Google Scholar]
- Williams B.O., Remington,L., Albert,D.M., Mukai,S., Bronson,R.T. and Jacks,T. (1994) Cooperative tumorigenic effects of germline mutations in Rb and p53. Nature Genet., 7, 480–484. [DOI] [PubMed] [Google Scholar]
- Wolfel T. et al. (1995) A p16INK4a-insensitive Cdk4 mutant targeted by cytilytic lymphocytes in a human melanoma. Science, 269, 1281–1284. [DOI] [PubMed] [Google Scholar]
- Wunder J.S., Eppert,K., Burrow,S.R., Gokgoz,N., Bell,R.S., Andrulis,I.L. and Gogkoz,N. (1999) Co-amplification and overexpression of CDK4, SAS and MDM2 occurs frequently in human parosteal osteosarcomas. Oncogene, 18, 783–788. [DOI] [PubMed] [Google Scholar]
- Zindy F., van Deursen,J., Grosveld,G., Sherr,C.J. and Roussel,M.F. (2000) INK4d-deficient mice are fertile despite testicular atrophy. Mol. Cell. Biol., 20, 372–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo L., Weger,J., Yang,Q., Goldstein,A.M., Tucker,M.A., Walker,G.J., Hayward,N. and Dracopoli,N.C. (1996) Germline mutations in the p16INK4a binding domain of Cdk4 in familial melanoma. Nature Genet., 12, 97–99. [DOI] [PubMed] [Google Scholar]