NEW EMBO MEMBER’S REVIEW: Actin cytoskeleton regulation through modulation of PI(4,5)P2 rafts (original) (raw)
Abstract
The phosphoinositide lipid PI(4,5)P2 is now established as a key cofactor in signaling to the actin cytoskeleton and in vesicle trafficking. PI(4,5)P2 accumulates at membrane rafts and promotes local co-recruitment and activation of specific signaling components at the cell membrane. PI(4,5)P2 rafts may thus be platforms for local regulation of morphogenetic activity at the cell membrane. Raft PI(4,5)P2 is regulated by lipid kinases (PI5-kinases) and lipid phosphatases (e.g. synaptojanin). In addition, GAP43-like proteins have recently emerged as a group of PI(4,5)P2 raft-modulating proteins. These locally abundant proteins accumulate at inner leaflet plasmalemmal rafts where they bind to and co-distribute with PI(4,5)P2, and promote actin cytoskeleton accumulation and dynamics. In keeping with their proposed role as positive modulators of PI(4,5)P2 raft function, GAP43-like proteins confer competence for regulated morphogenetic activity on cells that express them. Their function has been investigated extensively in the nervous system, where their expression promotes neurite outgrowth, anatomical plasticity and nerve regeneration. Extrinsic signals and intrinsic factors may thus converge to modulate PI(4,5)P2 rafts, upstream of regulated activity at the cell surface.
Keywords: actin regulation/anatomical plasticity/nerve sprouting/PI(4,5)P2/rafts
Introduction
During development, nerve cells establish precise networks of processes that provide the basis for nervous system functioning. To a large extent the organization of such neural networks is predetermined genetically to produce adaptive species-specific behavior. As the young animal becomes autonomous, neural networks produce behavior through circuitry that is thought to be largely anatomically stable. The transition from nervous system construction to nervous system functioning is accompanied by a dramatic downregulation of anatomical growth and growth competence in nerve cells. Anatomical plasticity associated with experience can, however, still take place on a local scale in the adult, where persistent alterations in activity lead to rearrangements of synaptic connections. In addition, lesions of nerve tracts can induce regenerative growth and functional repair. Competence for process outgrowth is thus a regulatable property of neurons. Understanding molecular mechanisms of anatomical plasticity regulation in the nervous system will provide fundamental insights into how morphogenetic processes are regulated by intrinsic and extrinsic factors. In addition, such knowledge should provide mechanistic leads to develop strategies for repair in the nervous system.
Process outgrowth and synaptic rearrangements coincide with the expression of specific sets of genes in neurons (Skene, 1989). Among these, GAP43-like proteins mediate competence for anatomical plasticity (Skene, 1989; Benowitz and Routtenberg, 1997; Caroni, 1997). During development, these proteins are coexpressed at very high levels in most, and possibly all neurons. In contrast, expression levels in the adult are substantially lower, and restricted to partially overlapping subsets of neurons. Within cells, GAP43-like proteins accumulate at detergent-insoluble, cholesterol-dependent rafts at the inner surface of the cell membrane. At these rafts, they co-distribute with and modulate the lipid second messenger PI(4,5)P2 (Laux et al., 2000). As outlined below, recent studies have provided evidence that these proteins modulate the assembly of PI(4,5)P2-containing rafts to regulate actin dynamics at the cell surface (Frey et al., 2000; Laux et al., 2000). Separate studies have uncovered central roles for PI(4,5)P2 clusters and rafts in the recruiting and modulation of key components that regulate the actin cytoskeleton (Shibasaki et al., 1997; Ma et al., 1998; Higgs and Pollard, 2000; Raucher et al., 2000; Rohatgi et al., 2000; Rozelle et al., 2000) and the endocytotic machinery (Cremona et al., 1999; Gillooly and Stenmark, 2001). These combined findings have raised the possibility that one way of determining a cell’s intrinsic competence for morphogenetic activity and anatomical plasticity is by altering the molecular machinery that modulates PI(4,5)P2 rafts through changes in gene expression. Below, I focus on the possible roles and regulation of PI(4,5)P2 rafts, and the significance of this regulation for anatomical plasticity in the nervous system.
Broad range of functions for GAP43-like proteins
GAP43-like proteins are mechanistically and functionally related, and include GAP43, CAP23, MARCKS and MacMARCKS (Aderem, 1995; Wiederkehr et al., 1997). GAP43 and MARCKS are the two main PKC substrates in the brain. GAP43 (also known as B50, neuromodulin and F1) was discovered as a protein that is rapidly transported into growing axons, and is expressed by neurons when they grow processes (Skene, 1989). Mice lacking GAP43 exhibit specific defects in growth cone navigation and major deficits in the construction of topographic projections between sensory neurons and their target fields (see for example, Maier et al., 1999). Heterozygous mutants exhibit partial phenotypes, suggesting that the expression levels of this protein are critical for axon guidance. Gain-of-function experiments in mice have provided evidence that GAP43 is an intrinsic determinant of anatomical plasticity in neurons, where its expression levels define a cell’s competence to respond to local signals by anatomical rearrangements and growth (Aigner et al., 1995; Caroni et al., 1997). It has been a puzzling fact that in addition to its well established functions in nerve growth, GAP43 is also involved in stimulus-induced secretion, endocytosis and long-term potentiation of synaptic transmission (Benowitz and Routtenberg, 1997).
CAP23 (also known as NAP22 and BASP1) is a further intrinsic determinant of process outgrowth and anatomical plasticity in neurons (Caroni et al., 1997). Like GAP43, it is expressed at high levels during development and is re-expressed by those lesioned neurons that are competent to regenerate axons. Unlike GAP43, which accumulates selectively in axons, CAP23 decorates all compartments of the neuronal plasmamembrane. GAP43 and CAP23 have closely related functions in vivo (Frey et al., 2000). The two proteins synergize in promoting nerve sprouting (Caroni et al., 1997), and expression of both proteins appears to be required to confer competence for long-distance regenerative axon growth in the adult (Bomze et al., 2001).
Finally, the closely related proteins MARCKS (also known as 80K) and MacMARCKS (also known as MRP) (Aderem, 1995) are expressed at very high levels in the nervous system, where their absence leads to dramatic developmental deficits in morphogenesis and process outgrowth (see for example, Stumpo et al., 1995). In addition, and in contrast to GAP43 and CAP23, they are also expressed in a regulated manner by a wide range of non-neural cells. Their function has been studied most extensively in fibroblasts and circulating blood cells, where they promote cell adhesion and migration (Myat et al., 1997). In cultured fibroblasts, MARCKS expression is downregulated at confluency, concomitant with a loss of focal adhesions and actin stress fibers, and is rapidly reinduced by stimuli that overcome contact inhibition.
Taken together, these functional findings suggest that GAP43-like proteins share the property of conferring competence for morphogenetic processes to cells that express them. The wide variety of these processes suggests that the common target of these proteins may be a general mechanism affecting morphogenesis and motility.
Actin cytoskeleton modulation by GAP43-like proteins
GAP43-like proteins are highly hydrophilic, with characteristic biochemical, biophysical and functional properties in common, and no enzymatic activity. Shared properties include the presence of a basic domain that binds acidic phospholipids including PI(4,5)P2, calcium/calmodulin, actin filaments and PKC in a mutually exclusive manner (Hartwig et al., 1992; He et al., 1997; Wiederkehr et al., 1997; Denisow et al., 1998; Maekawa et al., 1999; Wang et al., 2001). GAP43-like proteins induce comparable dynamic actin-based structures such as filopodia and microspikes at the periphery of non-neuronal cells. A large body of experiments both in transfected cell lines and in vivo (see for example Wiederkehr et al., 1997; Laux et al., 2000) point to raft association and the properties of the basic domains as critical determinants of GAP43-like protein function. With respect to downstream targets of GAP43-like protein function, from phagocytosis to growth cone guidance, the unifying motive appears to be actin cytoskeleton regulation. The combined available evidence therefore suggests that GAP43-like proteins potentiate actin cytoskeleton assembly in a process that requires their accumulation at PI(4,5)P2-containing rafts and the presence of the basic domain.
PI(4,5)P2 rafts and actin cytoskeleton regulation
It has long been known that PI(4,5)P2 can sequester actin-binding proteins such as profilin, gelsolin, CapZ and cofilin in vitro, but its possible roles in actin cytoskeleton regulation in living cells remained controversial. This situation has changed recently, as several in vivo studies have assigned critical modulatory cofactor roles for PI(4,5)P2 in signaling pathways promoting actin cytoskeleton recruitment (for example the cdc42→WASP→ Arp2/3 pathway) and organization (for example the Rho→ ERM-protein pathway). In these processes, PI(4,5)P2 seems to act through its local concentration to lower pathway activation thresholds in a spatially defined manner. This is achieved by providing a local environment that recruits and activates cdc42, WASP and ERM proteins (Chong et al., 1994; Hirao et al., 1996; MacKay et al., 1997; Ma et al., 1998; Loisel et al., 1999; Macheski and Insall, 1999; Matsui et al., 1999; Rohatgi et al., 1999; Higgs and Pollard, 2000; Lamb et al., 2000; Mullins, 2000; Quang et al., 2000; Rohatgi et al., 2000; Tolias et al., 2000; Wear et al., 2000). Critical local recruitment roles for PI(4,5)P2 have also been assigned unequivocally to the assembly and budding of endosomes (Cremona et al., 1999; Gillooly and Stenmark, 2001). These findings have thus highlighted the significance of understanding how PI(4,5)P2 levels and pools are regulated locally (Hinchliffe, 2000; Gillooly and Stenmark, 2001). Although definitive answers to these questions will have to await imaging data from living cells, important potential clues are already available. Thus, PI(4,5)P2 is concentrated at membrane rafts (Pike and Miller, 1998), and Rho- and Arf-type GTPases can activate PI(4,5)P2-synthesizing PI5-kinases by inducing recruitment of the kinases at rafts (Chong et al., 1994; Godi et al., 1999; Honda et al., 1999).
What then are rafts, and in what ways may they relate to actin cytoskeleton regulation by PI(4,5)P2? In membranes rich in cholesterol, lipids with unsaturated fatty acid chains partition into microdomains that resist solubilization by non-ionic detergents. These microdomains have been designated DIGs (detergent-insoluble glycolipids), or rafts (Harder et al., 1998; Ledesma et al., 1999; Brown and London, 2000). Due to the specific distributions of cholesterol and the appropriate lipids in cellular membranes, rafts are predominantly found at the plasma membrane and at early endocytotic and late exocytotic compartments. The combination of highly unsaturated sphingolipids and cholesterol is particularly abundant at the outer leaflet of the plasma membrane, where rafts are best characterized. Inner leaflet plasma membrane rafts, where PI(4,5)P2 accumulates, are less well understood, and may assemble from phosphatidylethanolamine in the presence of cholesterol. The unique lipid environment at rafts drives the selective accumulation of specific classes of proteins, including GPI-linked, cell-surface proteins, double-acylated cytosolic proteins (e.g. src-family tyrosine kinases) and subgroups of transmembrane proteins (e.g. EphB receptors). These remain associated with small elementary raft units of ∼30 nm diameter for at least several minutes (Pralle et al., 2000).
Elementary rafts are thought to play important roles in signaling at the cell membrane and in membrane trafficking, by assembling into raft-based platforms in response to specific stimuli, such as receptor activation or cell adhesion. With respect to PI(4,5)P2 and actin cytoskeleton regulation, it has recently been shown that actin assembly can be initiated with remarkable efficiency at PI(4,5)P2 rafts, where it can drive membrane and vesicle movements (Harder and Simons, 1999; Merrifield et al., 1999; Taunton et al., 2000). In signaling, PI(4,5)P2 rafts may act as effective sites of local PI(4,5)P2 presentation due to selective recruitment of activated lipid kinases at the rafts. According to this view, PI(4,5)P2 raft regulation may affect the local synthesis of PI(4,5)P2, upstream of the role of this lipid second messenger in actin cytoskeleton regulation. In addition, raft assembly may also enhance more directly the activation of cdc42/WASP and ERM proteins by PI(4,5)P2. PI(4,5)P2 rafts thus appear to be attractive candidate targets of regulation in the production of local changes in actin dynamics and organization.
Modulation of PI(4,5)P2 rafts
Raft regulation has been investigated in the most detail in T cell activation. In this context, T-cell receptor engagement triggers raft recruitment through the co-activator CD28, followed by the assembly of a signaling platform of tyrosine kinases and adaptor proteins that greatly enhances signal strength (Lanzavecchia et al., 1999; Penninger and Crabtree, 1999; Viola et al., 1999; Rebres et al., 2001). That strength determines the onset and kinetics of T cell activation. The assembly of subplasmalemmal actin-based structures and platforms such as microspikes or focal adhesions also appears to depend on signal strength, as reflected by the recruitment and activation of e.g. WASP or ERM proteins. Therefore, by analogy to the role of rafts in T cell activation, it is tempting to speculate that modulation of WASP and ERM protein recruitment and activation by PI(4,5)P2 may involve regulated recruitment or assembly of PI(4,5)P2 rafts. This may involve two possible types of mechanism: regulation of PI(4,5)P2 contents at rafts, and regulation of PI(4,5)P2 raft effectiveness through raft assembly. By further analogy to T cell activation, these regulatory processes may involve both extrinsic signals and intrinsic competence components.
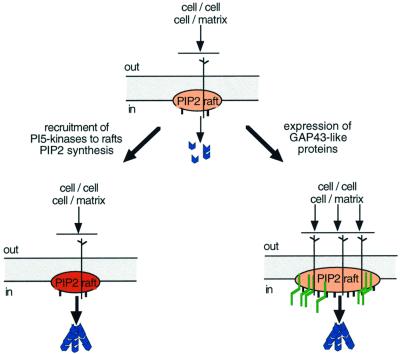
Recent findings on the mechanism of action of GAP43-like proteins suggest that these may indeed modulate the assembly of PI(4,5)P2 rafts. Thus, GAP43-like proteins promoted PI(4,5)P2 cluster assembly and partial resistance of the clusters against dispersion by the cholesterol-extracting drug cyclodextrin (Laux et al., 2000). Similarly to their effects in vivo, these effects of GAP43-like proteins had a marked dose-dependent component. GAP43-like protein constructs, lacking the basic domain (ΔBD-constructs) that binds PI(4,5)P2, still accumulated at PI(4,5)P2 rafts where they interfered with cluster assembly and PI(4,5)P2 accumulation at rafts (Laux et al., 2000). Thus, GAP43-like proteins may function as positive modulators of PI(4,5)P2 raft function, and the corresponding ΔBD-constructs may interfere with that function in a dominant-negative manner. Consistent with the notion that the effects on PI(4,5)P2 rafts reflect the function of GAP43-like proteins, wild-type and mutant constructs had opposite effects on actin recruitment and assembly at the cell membrane, and during cell adhesion, nerve sprouting and nerve regeneration in vivo (Laux et al., 2000; Frey et al., 2000). Based on these results, we would like to propose a positive cofactor role for GAP43-like proteins as intrinsic determinants of PI(4,5)P2 raft function, downstream of PI(4,5)P2 synthesis but upstream of actin cytoskeleton regulation, and possibly also in membrane trafficking (Figure 1).
Fig. 1. Pathways of PI(4,5)P2 raft modulation and actin cytoskeleton regulation at rafts. Top: PI(4,5)P2 (red) accumulates at subplasma lemmal rafts. Left: Signals through e.g. Rho-GTPases recruit activated PI5-kinases at rafts leading to enhanced contents of PI(4,5)P2 at rafts (darker red). Right: GAP43-like proteins (green) accumulate at PI(4,5)P2 rafts. By promoting raft assembly, GAP43-like proteins enhance signal strength at PI(4,5)P2 rafts. In both cases, elevated signaling by PI(4,5)P2 at rafts would enhance recruitment and activation of WASP proteins and ERM proteins, to promote actin polymerization and actin filament assembly (blue). Local interactions with extracellular matrix or cell surface components can modulate raft function, e.g. through tyrosine kinase activation, mediating spatial control of actin regulation, and promoting further signal amplification. See text for further details.
The model in Figure 1 emphasizes the potential roles of PI(4,5)P2 raft regulation in signaling to the actin cytoskeleton and provides a candidate mechanistic frame for understanding the roles of GAP43-like proteins as intrinsic determinants augmenting morphogenetic and motile activity in expressing cells. It also raises a number of questions for future investigation. These include: (i) If PI(4,5)P2 raft modulation should indeed be a major mechanism in the control of regulation by PI(4,5)P2, then what further molecular components are involved in this process? What is the composition of PI(4,5)P2 rafts and how are they regulated? Does raft modulation also affect PI(4,5)P2 synthesis at rafts? (ii) Actin cytoskeleton assembly and recruitment is frequently promoted by both PI(4,5)P2-modulated pathways and enhanced levels of protein tyrosine phosphorylation (Harder and Simons, 1999; Rebres et al., 2001)—is there crosstalk between PI(4,5)P2 raft modulation and the assembly of src-kinase accumulating rafts? (iii) What molecular mechanisms mediate spatial control of PI(4,5)P2 raft modulation? For GAP43-like proteins, the basic domains that bind PI(4,5)P2 are regulated by calcium/calmodulin and phosphorylation by PKC; this regulation may link local signaling through calcium and/or PKC to PI(4,5)P2 raft modulation. (iv) How do GAP43-like proteins accumulate at PI(4,5)P2 rafts? Does their basic domain predominantly regulate raft assembly, or does it also directly mask PI(4,5)P2, thereby regulating its availability? How do GAP43-like proteins synergize in promoting anatomical plasticity?
Conclusion
It has recently become clear that PI(4,5)P2 is an activating cofactor critical for signaling both to the actin cytoskeleton and in vesicle trafficking. In these pathways PI(4,5)P2 seems to act by providing a local environment at the membrane that promotes the co-recruitment and activation of signaling components. One therefore needs to know how PI(4,5)P2 pools are activated locally. Since PI(4,5)P2 is associated with lipid rafts, regulation likely involves mechanisms controlling raft recruitment. These may affect PI(4,5)P2 synthesis at rafts, and/or the effectiveness of raft PI(4,5)P2 in downstream signaling. It now turns out that a class of functionally related proteins that confers intrinsic competence for morphogenetic activity and regulated motility accumulates at and modulates PI(4,5)P2 rafts. GAP43-like proteins may thus be a first group of PI(4,5)P2 raft modulators. The fact that GAP43-like proteins can be regulated locally by calcium/calmodulin and PKC provides a possible link between extracellular signals and PI(4,5)P2 raft regulation that can be explored experimentally. Given the function of GAP43-like proteins in clinically relevant processes such as neural plasticity, nerve regeneration and cell migration, the novel findings potentially have significant clinical implications.
Acknowledgments
Acknowledgements
The Friedrich Miescher Institut is a branch of the Novartis Research Foundation.
References
- Aderem A. (1995) The MARCKS family of protein kinase-C substrates. Biochem. Soc. Trans., 23, 587–591. [DOI] [PubMed] [Google Scholar]
- Aigner L., Arber,S., Kapfhammer,J.P., Laux,T., Schneider,C., Botteri,F., Brenner,H.R. and Caroni,P. (1995) Overexpression of the neural growth-associated protein GAP-43 induces nerve sprouting in the adult nervous system of transgenic mice. Cell, 83, 269–278. [DOI] [PubMed] [Google Scholar]
- Benowitz L.I. and Routtenberg A. (1997) GAP-43: an intrinsic determinant of neuronal development and plasticity. Trends Neurosci., 20, 84–91. [DOI] [PubMed] [Google Scholar]
- Bomze H.M., Bulsara,K.R., Iskandar,B.J., Caroni,P. and Skene,P.J.H. (2001) Spinal axon regeneration evoked by replacing two growth cone proteins in adult neurons. Nature Neurosci., 4, 38–43. [DOI] [PubMed] [Google Scholar]
- Brown D.A. and London,E. (2000) Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J. Biol. Chem., 275, 17221–17224. [DOI] [PubMed] [Google Scholar]
- Caroni P. (1997) Intrinsic neuronal determinants that promote axonal sprouting and elongation. BioEssays, 19, 767–775. [DOI] [PubMed] [Google Scholar]
- Caroni P., Aigner,L. and Schneider,C. (1997) Intrinsic neuronal determinants locally regulate extrasynaptic and synaptic growth at the adult neuromuscular junction. J. Cell. Biol., 136, 679–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong L.D., Traynor-Kaplan,A., Bokoch,G.M. and Schwartz,M.A. (1994) The small GTP-binding protein Rho regulates a phosphatidyl inositol 4-phosphate 5-kinase in mammalian cells. Cell, 79, 507–513. [DOI] [PubMed] [Google Scholar]
- Cremona O. et al. (1999) Essential role of phosphoinositide metabolism in synaptic vesicle recycling. Cell, 99, 179–188. [DOI] [PubMed] [Google Scholar]
- Denisow G., Wanaski,S., Luan,P., Glaser,M. and McLaughlin,S. (1998) Binding of basic peptides to membranes produces lateral domains enriched in the acidic lipids phosphatidylserine and phosphatidyl inositol 4,5-bisphosphate: an electrostatic model and experimental results. Biophys. J., 74, 731–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey D., Laux,T., Xu,L., Schneider,C. and Caroni,P. (2000) Shared and unique roles of CAP23 and GAP43 in actin regulation, neurite outgrowth and anatomical plasticity. J. Cell. Biol., 149, 1443–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillooly D.J. and Stenmark,H. (2001) A lipid oils the endocytosis machine. Science, 291, 993–994. [DOI] [PubMed] [Google Scholar]
- Godi A., Pertile,P., Meyers,R., Marra,P., Di Tullio,G., Iurisci,C., Luini,A., Corda,D. and De Matteis,M.A. (1999) ARF mediates recruitment of PtdIns-4-OH kinase-β and stimulates synthesis of PtdIns(4,5)P2 on the Golgi complex. Nature Cell Biol., 1, 280–287. [DOI] [PubMed] [Google Scholar]
- Harder T. and Simons,K. (1999) Clusters of glycolipids and glycosylphosphatidylinositol-anchored proteins in lymphoid cells: accumulation of actin regulated by local tyrosine phosphorylation. Eur. J. Immunol., 29, 556–562. [DOI] [PubMed] [Google Scholar]
- Harder T., Scheiffele,P., Verkade,P. and Simons,K. (1998) Lipid domain structure of the plasma membrane revealed by patching of the membrane components. J. Cell Biol., 141, 929–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig J.H., Thelen,M., Rosen,A., Janmey,P.A., Nairn,A.C. and Aderem,A. (1992) MARCKS is an actin filament crosslinking protein regulated by protein kinase C and calcium-calmodulin. Nature, 356, 618–622. [DOI] [PubMed] [Google Scholar]
- He Q., Dent,E.W. and Meiri,K.F. (1997) Modulation of actin filament behavior by GAP-43 (neuromodulin) is dependent on the phosphorylation status of serine 41, the protein kinase C site. J. Neurosci., 17, 3515–3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgs H.N. and Pollard,T.D. (2000) Activation by Cdc42 and PIP(2) of Wiskott–Aldrich syndrome protein (WASp) stimulates actin nucleation by Arp2/3 complex. J. Cell Biol., 150, 1311–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinchliffe K. (2000) Intracellular signaling: is PIP2 a messenger too? Curr. Biol., 10, R104–R105. [DOI] [PubMed] [Google Scholar]
- Hirao M., Sato,N., Kondo,T., Yonemura,S., Monden,M., Sasaki,T., Takai,Y. and Tsukita,S. (1996) Regulation mechanism of ERM (Ezrin/Radixin/Moesin) protein/plasma membrane association: possible involvement of phosphatidylinositol turnover and rho-dependent signaling pathway. J. Cell Biol., 135, 37–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda A. et al. (1999) Phosphatidylinositol 4-phosphate-kinase a is a downstream effector of the small G protein ARF6 in membrane ruffle formation. Cell, 99, 521–532. [DOI] [PubMed] [Google Scholar]
- Lamb R.F., Roy,C., Diefenbach,T.J., Vinters,H.V., Johnson,M.W., Jay,D.G. and Hall,A. (2000) The TSC1 tumor suppressor hamartin regulates cell adhesion through ERM proteins and the GTPase Rho. Nature Cell Biol., 2, 281–287. [DOI] [PubMed] [Google Scholar]
- Lanzavecchia A., Iezzi,G. and Viola,A. (1999) From TCR engagement to T cell activation: a kinetic view of cell behavior. Cell, 96, 1–4. [DOI] [PubMed] [Google Scholar]
- Laux T., Fukami,K., Thelen,M., Golub,T., Frey,D. and Caroni,P. (2000) GAP43, MARCKS, and CAP23 modulate PI(4,5)P2 at plasmalemmal rafts, and regulate cell cortex actin dynamics through a common mechanism. J. Cell Biol., 149, 1455–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledesma M.D., Brugger,B., Bunning,C., Wieland,F.T. and Dotti,C.G. (1999) Maturation of the axonal plasma membrane requires upregulation of sphingomyelin synthesis and formation of protein–lipid complexes. EMBO J., 18, 1761–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loisel T.P., Boujemaa,R., Pantaloni,D. and Carlier,M.-F. (1999) Reconstitution of actin-based motility of Listeria and Shigella using pure proteins. Nature, 401, 613–616. [DOI] [PubMed] [Google Scholar]
- Ma L., Cantley,L.C., Janmey,P.A. and Kirschner,M.W. (1998) Corequirement of specific phosphoinositides and small GTP-binding protein Cdc42 in inducing actin assembly in Xenopus egg extracts. J. Cell Biol., 140, 1125–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macheski L.M. and Insall,R.H. (1999) Signaling to actin dynamics. J. Cell Biol., 146, 267–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKay D.J.G., Esch,F., Furttmayr,H. and Hall,A. (1997) Rho- and Rac-dependent assembly of focal adhesion complexes and actin filaments in permeabilized fibroblasts: an essential role for Ezrin/Radixin/Moesin proteins. J. Cell Biol., 138, 927–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa S., Sato,C., Kitajima,K., Funatsu,N., Kumanogoh,H. and Sokawa,Y. (1999) Cholesterol-dependent localization of NAP-22 on a neuronal membrane microdomain (raft). J. Biol. Chem., 274, 21369–21374. [DOI] [PubMed] [Google Scholar]
- Maier D.L., Mani,S., Donovan,S.L., Soppet,D., Tessarollo,L., McCasland,J.S. and Meiri,K.F. (1999) Disrupted cortical map and absence of cortical barrels in growth-associated protein (GAP)-43 knockout mice. Proc. Natl Acad. Sci. USA, 96, 9397–9402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T., Yonemura,S., Tsukita,Sh. and Tsukita, Sa. (1999) Activation of ERM proteins in vivo by Rho involves phosphatidylinositol 4-phosphate 5-kinase and not ROCK kinases. Curr. Biol., 9, 1259–1262. [DOI] [PubMed] [Google Scholar]
- Merrifield C.J., Moss,S.E., Ballestrem,C., Imhof,B.A., Giese,G., Wunderlich,I. and Almers,W. (1999) Endocytic vesicles move at the tips of actin tails in cultured mast cells. Nature Cell Biol., 1, 72–74. [DOI] [PubMed] [Google Scholar]
- Mullins R.D. (2000) How WASP-family proteins and the Arp2/3 complex convert intracellular signals into cytoskeletal structures. Curr. Opin. Cell Biol., 12, 91–96. [DOI] [PubMed] [Google Scholar]
- Myat M.M., Anderson,S., Allen,L.A. and Aderem,A. (1997) MARCKS regulates membrane ruffling and cell spreading. Curr. Biol., 7, 611–614. [DOI] [PubMed] [Google Scholar]
- Penninger J.M. and Crabtree,G.R. (1999) The actin cytoskeleton and lymphocyte activation. Cell, 96, 9–12. [DOI] [PubMed] [Google Scholar]
- Pike L.J. and Miller,J.M. (1998) Cholesterol depletion delocalizes phosphatidylinositol bisphosphate and inhibits hormone-stimulated phosphatidylinositol turnover. J. Biol. Chem., 273, 22298–22304. [DOI] [PubMed] [Google Scholar]
- Pralle A., Keller,P., Florin,E.L., Simons,K. and Horber,J.K. (2000) Sphingolipid-cholesterol rafts diffuse as small entities in the plasma membrane of mammalian cells. J. Cell Biol., 148, 997–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quang C.T., Gautreau,A., Arpin,M. and Treisman,R. (2000) Ezrin function is required for ROCK-mediated fibroblast transformation by the Net and Dbl oncogenes. EMBO J., 19, 4565–4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raucher D., Stauffer,T., Chen,W., Shen,K., Guo,S., York,J.D., Sheetz,M.P. and Meyer,T. (2000) Phosphatidylinositol 4,5-bisphosphate functions as a second messenger that regulates cytoskeleton-plasma membrane adhesion. Cell, 100, 221–228. [DOI] [PubMed] [Google Scholar]
- Rebres R.A., Green,J.M., Reinhold,M.I., Ticchioni,M. and Brown,E.J. (2001) Membrane raft association of CD47 is necessary for actin polymerization and protein kinase C Θ translocation in its synergistic activation of T cells. J. Biol. Chem., 276, 7672–7680. [DOI] [PubMed] [Google Scholar]
- Rohatgi R., Ma,L., Miki,H., Lopez,M., Kirchhausen,T., Takenawa,T. and Kirschner,M.W. (1999) The interaction between N-WASP and the Arp2/3 complex links Cdc42-dependent signals to actin assembly. Cell, 97, 221–231. [DOI] [PubMed] [Google Scholar]
- Rohatgi R., Ho,H.Y. and Kirschner,M.W. (2000) Mechanism of N-WASP activation by CDC42 and phosphatidylinositol 4,5-bisphosphate. J. Cell Biol., 150, 1299–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozelle A.L. et al. (2000) Phosphatidylinositol 4,5-bisphosphate induces actin-based movement of raft-enriched vesicles through WASP–Arp2/3. Curr. Biol., 10, 311–320. [DOI] [PubMed] [Google Scholar]
- Shibasaki Y., Ishihara,H., Kizuki,N., Asano,T., Oka,Y. and Yazaki,Y. (1997) Massive actin polymerization induced by phosphatidylinositol-4-phosphate 5-kinase in vivo. J. Biol. Chem., 272, 7578–7581. [DOI] [PubMed] [Google Scholar]
- Skene J.H. (1989) Axonal growth-associated proteins. Annu. Rev. Neurosci., 12, 127–156. [DOI] [PubMed] [Google Scholar]
- Stumpo D.J., Bock,C.B., Tuttle,J.S. and Blackshear,P.J. (1995) MARCKS deficiency in mice leads to abnormal brain development and perinatal death. Proc. Natl Acad. Sci. USA, 92, 944–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taunton J., Rowning,B.A., Coughlin,M.L., Wu,M., Moon,R.T., Mitchison,T.J. and Larabell,C.A. (2000) Actin-dependent propulsion of endosomes and lysosomes by recruitment of N-WASP. J. Cell Biol., 148, 519–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolias K.F., Hartwig,J.H., Ishihara,H., Shibasaki,Y., Cantley,L.C. and Carpenter,C.L. (2000) Type I α phosphatidylinositol-4-phosphate 5-kinase mediates Rac-dependent actin assembly. Curr. Biol., 10, 153–156. [DOI] [PubMed] [Google Scholar]
- Viola A., Schroeder,S., Sakakibara,Y. and Lanzavecchia,A. (1999) T lymphocyte costimulation mediated by reorganization of membrane microdomains. Science, 283, 680–682. [DOI] [PubMed] [Google Scholar]
- Wang J., Arbuzova,A., Hangyas-Mihalyne,G. and McLaughlin,S. (2001) The effector domain of myristoylated alanine-rich C kinase substrate (MARCKS) binds strongly to phosphatidylinositol 4,5-bisphosphate (PIP2). J. Biol. Chem., 276, 5012–5019. [DOI] [PubMed] [Google Scholar]
- Wear M.A., Schafer,D.A. and Cooper,J.A. (2000) Actin dynamics: assembly and disassembly of actin networks. Curr. Biol., 10, R891–R895. [DOI] [PubMed] [Google Scholar]
- Wiederkehr A., Staple,J. and Caroni,P. (1997) The motility-associated proteins GAP-43, MARCKS, and CAP-23 share unique targeting and surface activity-inducing properties. Exp. Cell. Res., 236, 103–116. [DOI] [PubMed] [Google Scholar]