BANK regulates BCR-induced calcium mobilization by promoting tyrosine phosphorylation of IP3 receptor (original) (raw)
Abstract
B-cell activation mediated through the antigen receptor is dependent on activation of protein tyrosine kinases (PTKs) such as Lyn and Syk and subsequent phosphorylation of various signaling proteins. Here we report on the identification and characterization of the B-cell scaffold protein with ankyrin repeats (BANK), a novel substrate of tyrosine kinases. BANK is expressed in B cells and is tyrosine phosphorylated upon B-cell antigen receptor (BCR) stimulation, which is mediated predominantly by Syk. Overexpres sion of BANK in B cells leads to enhancement of BCR-induced calcium mobilization. We found that both Lyn and inositol 1,4,5-trisphosphate receptor (IP3R) associate with the distinct regions of BANK and that BANK promotes Lyn-mediated tyrosine phosphorylation of IP3R. Given that IP3R channel activity is up-regulated by its tyrosine phosphorylation, BANK appears to be a novel scaffold protein regulating BCR-induced calcium mobilization by connecting PTKs to IP3R. Because BANK expression is confined to functional BCR-expressing B cells, BANK-mediated calcium mobilization may be specific to foreign antigen-induced immune responses rather than to signaling required for B-cell development.
Keywords: B-cell antigen receptor/calcium mobilization/inositol 1,4,5-trisphosphate receptor/scaffold protein/tyrosine phosphorylation
Introduction
B-cell antigen receptor (BCR) engagement triggers a cascade of signal transduction events; cytoplasmic protein tyrosine kinase (PTK) activation is the earliest of the known events. This PTK activation results in the tyrosine phosphorylation of many proteins, including BCR Ig-α and Ig-β chains, phosphatidylinositol 3-kinase (PI3K), Vav, Cbl and phospholipase C-γ2 (PLC-γ2; Kurosaki, 1999). Several proteins which are tyrosine phosphorylated remain to be identified for further establishment of the importance of PTKs in B-cell activation.
Among the substrates, PLC-γ2 is a key enzyme for IP3 production. The PLC-γ2–calcium pathway is one of the most extensively studied biochemical cascades with respect to BCR signaling. Namely, BCR-induced tyrosine phosphorylation of PLC-γ2 is responsible for an increase in its activity, which allows conversion of phosphatidylinositol 4,5-bisphosphate (PIP2) to the second messengers inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). DAG activates protein kinase C (PKC). IP3 binds IP3 receptor (IP3R), which is localized primarily on the endoplasmic reticulum (ER) and stimulates the release of calcium from intracellular stores. PLC-γ2 activity is also regulated by cellular localization. Recently, an essential role of the adaptor protein BLNK (also called SLP-65 or BASH) in PLC-γ2 membrane localization and tyrosine phosphorylation was deduced from studies of BLNK-deficient DT40 chicken B-cell lines (Ishiai et al., 1999; Kurosaki and Tsukada, 2000). Upon BCR stimulation, BLNK is phosphorylated by Syk, which recruits Btk and PLC-γ2 to BLNK via their respective SH2 domains. The BLNK complex is targeted to the plasma membrane by an unidentified mechanism, in which Btk is thought to phosphorylate and activate PLC-γ2, leading to IP3 production and calcium mobilization.
IP3R, a critical component of BCR signaling (Sugawara et al., 1997), is a calcium channel. Its channel activity is modulated by phosphorylation. For example, IP3R is phosphorylated on a serine residue by protein kinase A (PKA) and the activity of isolated IP3R increases after incubation with the catalytic subunit of PKA (Nakade et al., 1994). IP3R is also phosphorylated by protein kinases C and G, and by Ca2+-calmodulin-dependent kinase II (CaMKII) in vitro (Komalavilas and Lincoln, 1994; Cameron et al., 1995). Moreover, IP3R is suggested as a substrate of Src family PTKs in antigen receptor signaling. T-cell antigen receptor (TCR)-induced tyrosine phosphorylation of IP3R is reduced in Fyn-deficient mice, and IP3R channel activity is increased by addition of Src family PTKs in vitro (Jayaraman et al., 1996). Therefore, calcium mobilization from intracellular stores, which is a function of IP3R (Sugawara et al., 1997), is regulated both by IP3 production and phosphorylation of IP3R.
In contrast to the mechanism of PLC-γ2 activation, little is known about direct regulation of IP3R activity by PTKs. By analogy with the mechanism of PLC-γ2 activation, it is logical to assume that a yet unidentified scaffold protein brings ER-associated IP3Rs into close proximity with BCR-associated PTKs. This idea is supported by the previous finding that, in T cells, IP3Rs are localized below TCR cap structures after TCR stimulation (Khan et al., 1992). In this paper, we report on the identification and characterization of a novel B cell-restricted scaffold protein that appears to regulate direct coupling between IP3R and BCR-associated PTKs. This protein possesses ankyrin repeats and we refer to it as BANK.
Results
Identification of BANK, a novel B-cell scaffold protein with ankyrin repeats
BCR engagement triggers a cascade of signal transduction events; Lyn PTK activation is the earliest of the known events. To identify new substrates for Lyn, we performed solid-phase phosphorylation screening (Fukunaga and Hunter, 1997; Lock et al., 1998). One of the potential substrates isolated during this screening contained a portion of the coding sequence for a novel ankyrin repeat-containing protein. This sequence was nearly identical to that of KAIA1273, one of the sequences deposited in DDBJ/EMBL/GenBank from a full-length cDNA project (Suzuki et al., 1997). On the basis of sequence information for KAIA1273, the entire coding sequence of the substrate was obtained by RT–PCR of RNA isolated from Daudi B cells. Nucleotide sequencing of the BANK cDNA obtained by RT–PCR revealed that KAIA1273 has some mutations, including nucleotide deletions. The BANK cDNA encodes a protein of 755 amino acids with a calculated mol. wt of 85 500 Da, although transient expresson of BANK in HEK293T cells yielded proteins estimated to be ∼100 kDa by SDS–PAGE (see below). The discrepancy may be due to the acidic nature of the protein, which has a calculated pI of 5.3.
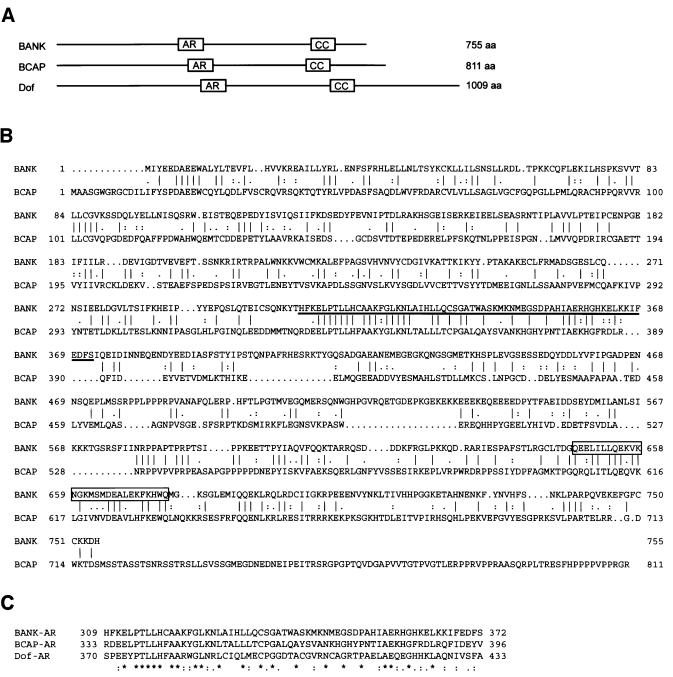
Comparison of the deduced primary amino acid sequence of BANK with the NCBI protein database revealed weak homology to mouse and chicken BCAP proteins (Okada et al., 2000) and Drosophila Dof protein (Figure 1; Vincent et al., 1998). These proteins have ankyrin repeat-like sequences and short sequences redicted to form coiled-coil structures. There is high similarity between the ankyrin repeat-like sequences of BANK, BCAP and Dof proteins (Figure 1C). BANK contains 23 tyrosine residues that may be phosphorylated. Among these tyrosines, Y454DDL motif, YExL motif (Y94ELL) and two Y[I/V]x[V/I] motifs (Y116ISV and Y458VFI) may serve as potential binding sites for the SH2 domains of Src family PTKs, Shc and SHP-2, respectively (Songyang et al., 1993, 1994).
Fig. 1. Identification of a novel protein BANK. (A) Schematic representation of predicted motifs and structures of human BANK, mouse BCAP and Drosophila Dof proteins. The ankyrin repeat-like motifs (amino acids 309–372 of BANK) and the presumptive coiled coils (647–675) are indicated by boxes AR and CC, respectively. (B) Alignment of the amino acid sequences of human BANK and mouse BCAP proteins was performed with the GAP program. The vertical bars indicate identical amino acids, while colons and dots indicate similar amino acids. The ankyrin repeat-like motif is underlined and the predicted coiled coil is boxed. (C) The sequences of the ankyrin repeat-like motifs of human BANK, mouse BCAP and Drosophila Dof proteins were aligned by the Clustal program.
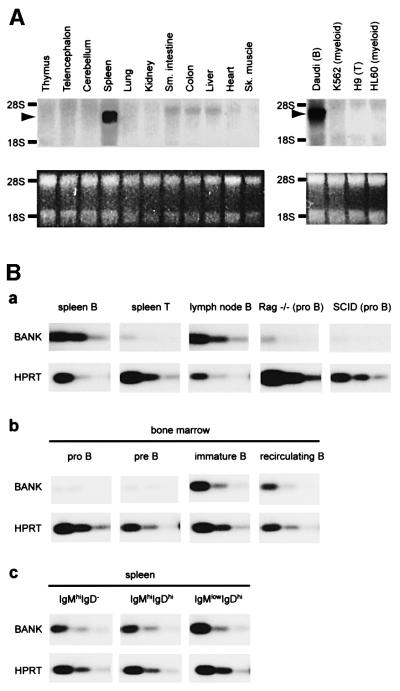
The tissue distribution of BANK mRNA was investigated by northern blot analysis of various mouse tissues and human cell lines (Figure 2A). We detected a prominent transcript of 3.2 kb, predominantly in spleen. BANK transcript was detected in a B-cell line (Daudi) but not in a T-cell line (H9) or myeloid cell lines (K562 and HL60). On the basis of these data, we assumed that BANK was expressed predominantly in B cells. Expression of BANK in B cells was further examined by semi-quantitative RT–PCR (Figure 2B). As expected from northern blot analysis of various cell lines, the expression levels of BANK in splenic B cells and lymph node B cells were very high, whereas splenic T cells showed very low levels of BANK expression. RT–PCR analysis of BANK expression in Rag-deficient mice and SCID mice, in which B-cell development is blocked in the pro B-cell stage, revealed that the expression of BANK in pro B cells was very low (Figure 2B, a). RT–PCR analysis of isolated bone marrow lymphoid cells showed that BANK was expressed at high levels in immature B cells and recirculating B cells, and at low levels in pro B cells and pre B cells (Figure 2B, b). We also sorted splenic B cells into immature IgMhiIgD–, IgMhiIgDhi and the most mature IgMlowIgDhi populations. BANK expression maintained at the same level in these subpopulations (Figure 2B, c). Because BANK expression is confined to functional BCR-expressing B cells, BANK may be specifically utilized in the B-cell response to foreign antigens.
Fig. 2. Cell type-specific expression of BANK mRNA. (A) Northern blot analysis of BANK expression in mouse tissues and human cell lines (upper parts). As a loading control, images of the ethidium bromide staining of RNA are shown in the lower parts. (B) Semi-quantitative RT–PCR analysis of BANK expression in B- and T-lineage cells. The RNA of described cells was isolated and reverse transcribed to cDNA. RT–PCR reactions were performed using three different template concentrations by 3.5-fold dilution. The amount of cDNA in the first dilution corresponds to 300 cells. (a) Cells indicated were isolated by MACS as described in Materials and methods. (b and c) Cells indicated were isolated by FACS as described in Materials and methods.
A search of the NCBI human genome database revealed that the human BANK gene is included in a contig that maps to human chromosome 4q22–24. In the NCBI OMIM database, there is no information suggesting that this locus is involved in any hereditary B-cell disorder.
BCR-induced tyrosine phosphorylation of BANK
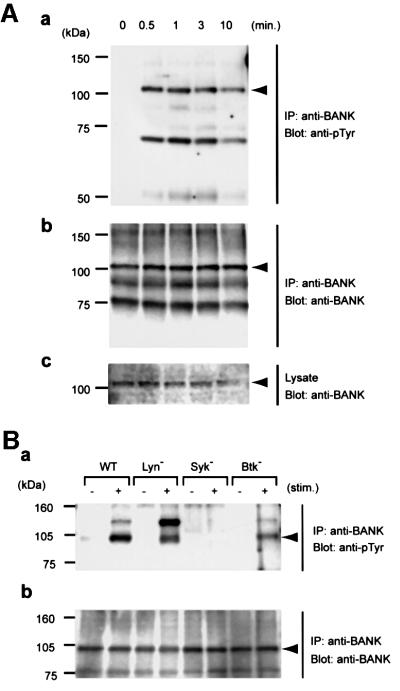
To determine whether BANK functions as a PTK substrate in vivo, we examined tyrosine phosphorylation of BANK in BCR-activated Daudi B cells. Immunoprecipitation analysis of BANK from resting and BCR-activated Daudi B cells revealed that BANK underwent receptor-dependent tyrosine phosphorylation, which peaked at 1 min after BCR cross-linking (Figure 3A).
Fig. 3. BCR-induced tyrosine phosphorylation of BANK. (A) Tyrosine phosphorylation of BANK in Daudi cells. At the time points indicated after BCR stimulation (10 µg/ml anti-human IgM), whole-cell lysates and anti-BANK immunoprecipitates were analyzed by immunoblotting with anti-phosphotyrosine (anti-pTyr, a) or anti-BANK (b and c; immunoprecipitates and whole-cell lysates, respectively). The positions of BANK are indicated by arrowheads. (B) BANK tyrosine phos phorylation in DT40 cells deficient in Lyn, Syk or Btk, unstimulated (minus symbol) or stimulated with 4 µg/ml anti-chicken IgM (M4) for 2 min (plus symbol). All experiments were performed at least three times.
We then analyzed BANK tyrosine phosphorylation in chicken DT40 B-cell lines lacking Lyn, Syk or Btk (Figure 3B; Takata et al., 1994; Takata and Kurosaki, 1996). Tyrosine phosphorylation of BANK was almost completely abolished in Syk-deficient DT40 cells, indicating that tyrosine phosphorylation of BANK upon BCR cross-linking was Syk dependent. However, originally we cloned BANK as a substrate for Lyn. The discrepancy might be due to the difference in species: BANK is phosphorylated by Syk in chicken DT40 B cells and BANK identified as a Lyn substrate is of human origin. Alternatively, the fidelity of the in vitro phosphorylation screening assay might be low. This could be the case because a synthetic peptide that is effectively phosphorylated by Lyn is also a good substrate for Syk (Schmitz et al., 1996).
Proteins with mol. wts of 120, 70 and 50 kDa (p120, p70 and p50, respectively), which were tyrosine phosphorylated upon BCR stimulation, were present in anti-BANK immunoprecipitates from DT40 (Figure 3B) and Daudi (Figure 3A) B cells, respectively. The nature of these proteins is unclear, but their presence in a species-specific manner is intriguing. Interestingly, tyrosine phosphorylation of p120 was markedly increased in Lyn-deficient DT40 cells. Future studies addressing the function of p120 would help establish the molecular basis of BANK function.
BANK is involved in BCR-induced calcium mobilization from intracellular calcium stores
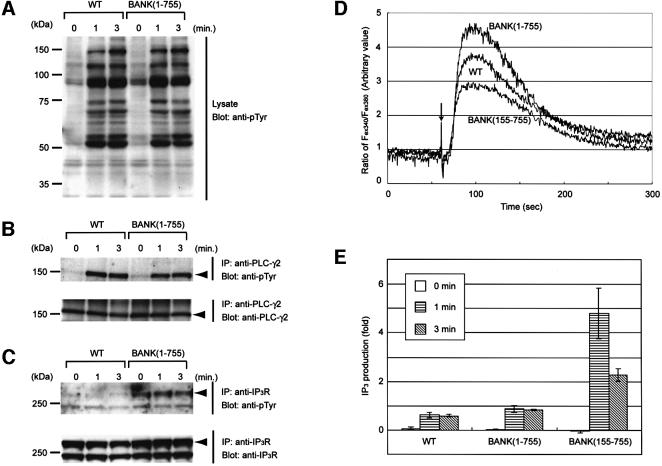
To address the physiological function of BANK, we generated DT40 cell lines that overexpress full-length BANK (BANK 1–755). Expression of BANK by these cell lines was confirmed by immunoblot analysis (data not shown). The level of BCR expression on the surface of BANK (1–755) DT40 cells was essentially the same as that of parental DT40 cells (data not shown). No change in BCR-induced overall tyrosine phosphorylation was detected between the wild-type and BANK (1–755) DT40 cells (Figure 4A), suggesting that BCR-associated PTKs such as Lyn and Syk were activated normally. Unaltered tyrosine kinase activity of Lyn was further confirmed by the apparently same tyrosine phosphorylation level of Cbl, which is a major substrate of Lyn in B cells (Tezuka et al., 1996; Yasuda et al., 2000), after BCR cross-linking (data not shown).
Fig. 4. Regulation of BCR-induced calcium mobilization by BANK. (A) BCR-induced tyrosine phosphorylation in wild-type (WT) and BANK-overexpressing (BANK 1–755) DT40 cells. (B) Tyrosine phosphorylation of PLC-γ2. (C) Tyrosine phosphorylation of type 2 IP3R. At the time points indicated after BCR stimulation (4 µg/ml M4), whole-cell lysates (A) and anti-PLC-γ2 (B) and anti-type 2 IP3R (C) immunoprecipitates were analyzed by immunoblotting with anti-pTyr. (D) Intracellular free calcium levels in Fura-2-loaded cells were monitored by spectrofluorometry after stimulation with M4 (4 µg/ml). The ratios of the fluorescence intensities at excitation wavelengths of 340 nm (Fex340) and 380 nm (Fex380) are shown. An arrow indicates the time point of M4 antibody addition. These data are representative of five independent experiments with two independent BANK-overexpressing DT40 clones (BANK 1–755), mutant BANK-overexpressing DT40 clones (BANK 155–755) and parental DT40 cells (WT). (E) BCR-induced IP3 production in BANK(1–755), BANK(155–755) and wild-type DT40 cells. Results are shown as the mean from three independent experiments. Error bars represent the SD from the mean.
Because one of the hallmarks of BCR-induced signaling is calcium mobilization, we examined the possible role of BANK in calcium signaling. Fura-2-loaded wild-type and BANK (1–755) DT40 cells were stimulated with anti-BCR antibody, and the increase in intracellular free calcium concentrations, [Ca2+]i, was measured in the presence of EGTA, which chelates the extracellular calcium. As shown in Figure 4D, the peak concentration of [Ca2+]i after BCR stimulation was markedly increased. This result suggests that BANK is involved in BCR-induced calcium mobilization from intracellular calcium stores. The increase in the peak concentration of [Ca2+]i after BCR stimulation was still observed in the non-chelating condition, namely in the presence of Ca2+, but not EGTA, in extracellular solutions (data not shown).
The enhancement of calcium mobilization in BANK (1–755) cells may be due to the stimulation of PLC-γ2 activity. However, levels of tyrosine phosphorylation of PLC-γ2 were virtually identical between wild-type and BANK (1–755) cells (Figure 4B), suggesting that PLC-γ2 is activated similarly in these cells (Nishibe et al., 1990; Weiss et al., 1991; Rebecchi and Pentyala, 2000). Indeed, BCR-induced IP3 generation was not significantly changed between wild-type and BANK (1–755) cells (Figure 4E; p = 0.12 in _t_-test). Interestingly, levels of tyrosine phosphorylation of type 2 IP3R were significantly enhanced (Figure 4C; see below).
To determine the functional domain of BANK, we generated DT40 cell lines that overexpressed BANK lacking the 154 N-terminal amino acids (BANK 155–755), an originally isolated portion of BANK in phosphorylation screening. Surprisingly, BCR-induced calcium mobilization in BANK (155–755) cells was not increased, but rather decreased (Figure 4D), although BCR-induced IP3 generation and BCR-induced tyrosine phosphorylation of PLC-γ2 were remarkably increased (Figure 4E; p = 0.03 in _t_-test and data not shown). The mechanism of the upregulation of PLC-γ2 activity is unknown, although this result strongly suggests that BANK regulates calcium mobilization through the IP3 production-independent pathways.
BANK–Lyn–IP3R trimolecular complex formation in vivo
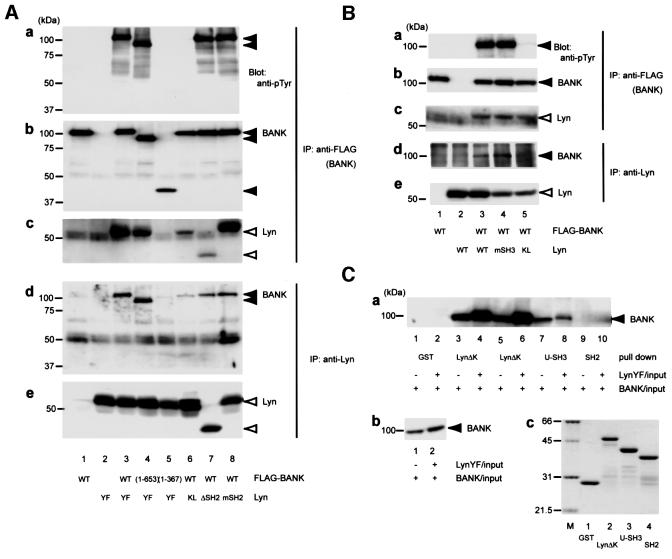
BANK was originally isolated as a substrate for Lyn. To examine the possible interactions between BANK and Lyn, we carried out co-immunoprecipitation analysis. Lyn was co-precipitated with BANK only when both FLAG-tagged BANK and active Lyn (LynY508F, YF) were expressed in HEK293T cells (Figure 5A, c, lane 3). Under such conditions, BANK was tyrosine phosphorylated (Figure 5A, a, lane 3). Conversely, BANK was present in Lyn immunoprecipitates (Figure 5A, d, lane 3). When the kinase-dead mutant Lyn (LynK275L, KL) was expressed instead of active Lyn, the interaction between BANK and Lyn was reduced remarkably (Figure 5A, lane 6). These results strongly suggest that the interaction between BANK and Lyn requires tyrosine phosphorylation of BANK.
Fig. 5. Association between BANK and Lyn. (A and B) HEK293T cells were transiently transfected with combinations of plasmids expressing various FLAG-tagged mutant BANK and Lyn mutants. Anti-FLAG (BANK) and anti-Lyn immunoprecipitates were subjected to immunoblotting with anti-pTyr, anti-BANK and anti-Lyn antibodies. (C) HEK293T cells were transiently transfected with plasmids expressing FLAG-tagged BANK, with (+) or without (–) expressing LynY508F. The lysates were incubated with 1 µg of the GST fusion proteins indicated immobilized on glutathione– Sepharose. The precipitates were washed and immunoblotted with anti-BANK antibody (a). The amount of BANK in HEK293T cell lysates (b). The amount and purity of GST fusion proteins were verified by Coomassie Blue staining (c; M, molecular weight standards). Abbreviations are explained in the text.
To study the mechanism of BANK–Lyn interaction, we generated various mutant BANK and mutant Lyn, and interactions in HEK293T cells were tested. As shown in Figure 5A (lanes 4 and 5), BANK (1–653) associated with Lyn, whereas BANK (1–367) did not. Furthermore, BANK (1–653) was tyrosine phosphorylated, whereas BANK (1–367) was not. This result suggests that residues 367–653 in BANK were required for association with Lyn and tyrosine phosphorylation of BANK. This region has less similarity with BCAP and does not contain the ankyrin repeats nor the coiled coil. There are eight tyrosine residues in BANK (367–653), among which Tyr454 is the closest to a favored binding site for the SH2 domain of Src family PTKs. However, the BANK mutant in which Tyr454 was mutated to Phe could associate with Lyn and was tyrosine phosphorylated with levels comparable to wild-type BANK in HEK293T cells (data not shown). Hence, other tyrosine residues are likely to be involved in BANK–Lyn association.
As the association of LynKL with BANK was much weaker than that of LynYF, the interaction between BANK and Lyn seems to be largely dependent on tyrosine phosphorylation of BANK. This suggests involvement of the SH2 domain of Lyn in the interaction. To test this possibility, we mutated or deleted the SH2 domain of LynYF to generate mSH2 or ΔSH2, respectively (mSH2, Lyn having R156ES to D156EF and Y508F mutations; ΔSH2, Lyn lacking residue 129–226 with Y508F mutation). Residue Arg156 in the Lyn SH2 domain is a critical residue that recognizes the phosphorylated tyrosine residue. As shown in Figure 5A (lanes 7 and 8), both mSH2 and ΔSH2 associated with BANK. The level of association of mSH2 with BANK was similar to that of LynYF (lane 3), whereas that of ΔSH2 was reduced to the same level as LynKL (lane 6). These results suggest that to some extent (levels seen in BANK and LynKL, Figure 5A, lane 6) BANK can associate with Lyn in a manner irrelevant to BANK phosphorylation, which does not require the Lyn SH2 domain. The full interaction of BANK and Lyn requires the Lyn SH2 domain in a mode different from the canonical SH2 domain–phospho tyrosine interaction. As for the former association, the SH3 domain–proline-rich sequence-based interaction does not appear to be involved because the mutation P114L (mSH3) in Lyn did not affect BANK–Lyn interaction in HEK293T cells (Figure 5B). As for the latter association, it is possible that BANK interacts with activated and autophosphorylated Lyn through the unidentified tyrosine kinase binding motif in BANK. However, this possibility was excluded by the following experimental data. BANK and LynYF were transiently transfected to cells in separate dishes and the cell lysates were mixed. From the mixed cell lysates, Lyn was not detectably immunoprecipitated with BANK (data not shown). This result strongly argues that BANK–Lyn interaction is enhanced by tyrosine phosphorylation of BANK itself.
Next, we carried out glutathione _S_-transferase (GST) pull-down experiments. HEK293T cells were transiently transfected with plasmids expressing FLAG-tagged BANK together with or without LynYF plasmids. The lysates were pulled down with GST fusion proteins containing various regions of Lyn (Figure 5C). The data showed that phosphorylated BANK interacted more tightly with LynΔK (residues 1–226) than unphosphorylated BANK (lanes 3–6). The unique region and the SH3 domain of Lyn (U-SH3, residues 1–118) interacted with unphosphorylated BANK. The level of this interaction was similar to that of LynΔK–unphosphorylated BANK association (lanes 3 and 7). Slightly stronger interaction of LynΔK with unphosphorylated BANK than that of Lyn U-SH3 might be explained by the residual tyrosine phosphorylation of BANK by endogenously expressed Lyn in HEK293T cells (see below). The level of the interaction between BANK and U-SH3 was not affected by tyrosine phosphorylation of BANK (lanes 7 and 8). Unexpectedly, the SH2 domain of Lyn alone (residues 129–226) did not interact with BANK significantly (lanes 9 and 10).
Taken together, these data suggest that there are two modes of interaction between BANK and Lyn. One is phosphorylation independent and is mediated through the unique and the SH3 domain of Lyn. The other is BANK phosphorylation dependent and is possibly mediated through the SH2 domain and the surrounding linker sequences of Lyn. These two modes are irrelevant to the canonical SH3 domain–proline-rich sequence-based interactions and the canonical SH2 domain–phosphotyrosine interactions. Tyrosine phosphorylation of BANK would affect BANK–Lyn interaction by changing the conformation of BANK.
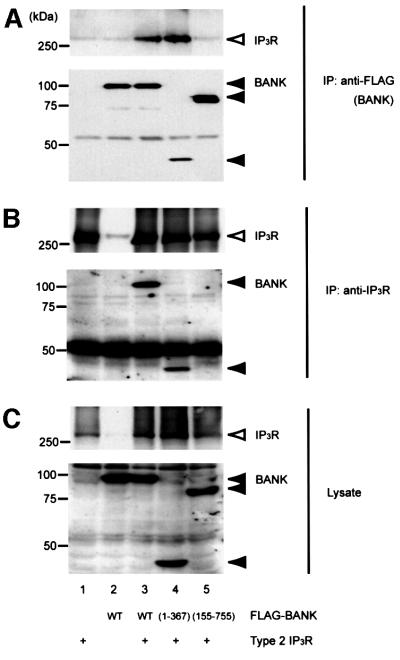
BANK is involved in BCR-induced calcium mobilization from intracellular calcium stores, but PLC-γ2 seems to be activated normally upon BCR stimulation in BANK-overexpressing cells (Figure 4). This raises a possibility that BANK may directly regulate IP3R activity. To examine this, the possible interaction between BANK and IP3R in vivo was analyzed by co-immunoprecipitation experiments. Among the three IP3R subunit subtypes, type 2 IP3R, which has the highest sensitivity to IP3 and is required for the long-lasting, regular calcium oscillations that occur upon BCR activation (Miyakawa et al., 1999), appears to be the most important subunit in BCR signaling. Type 2 IP3R was co-precipitated with BANK only when both FLAG-tagged BANK and type 2 IP3R were expressed in HEK293T cells (Figure 6A). Conversely, BANK was present in type 2 IP3R immunoprecipitates (Figure 6B). We observed that BANK (155–755), which could not enhance calcium mobilization in DT40 B cells (Figure 4D), did not associate with IP3R (Figure 6, lane 5). In contrast, BANK lacking the 388 C-terminal amino acids (BANK 1–367) associated with IP3R (Figure 6, lane 4). These results suggest that IP3R associates with BANK amino acids 1–154, a distinct region from that involved in the interaction with Lyn (BANK 367–653). In vitro studies showed that ankyrin is associated with calcium-release channels including IP3R and ryanodine receptor (Bourguignon et al., 1993, 1995). However, the ankyrin repeat of BANK is unlikely to be involved in its association with IP3R because BANK (155–755) containing the ankyrin repeat did not associate with IP3R. Full-length BANK and BANK lacking 309–372 (ΔANK) associated comparably with IP3R in a consistent manner (data not shown). An association of type 1 IP3R with BANK was also observed in similar experiments (data not shown).
Fig. 6. In vivo association between BANK and IP3R. HEK293T cells were transiently transfected with combinations of plasmids expressing various FLAG-tagged mutant BANK and type 2 IP3R. Anti-FLAG (BANK, A) and anti-IP3R (B) immunoprecipitates and whole-cell lysates (C) were subjected to immunoblotting with anti-IP3R (top) or anti-FLAG (bottom) antibodies. These data are representative of four independent experiments with similar results.
BANK promotes Lyn-mediated tyrosine phosphorylation of IP3R
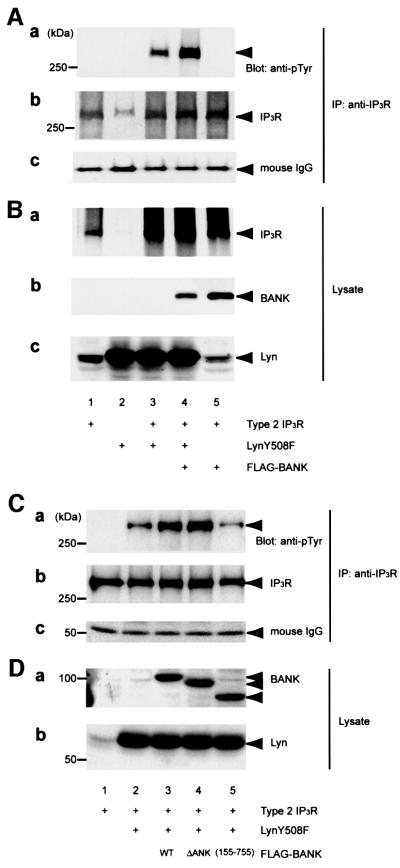
Because IP3R is a substrate of Src family PTKs in T cells (Jayaraman et al., 1996) and tyrosine phosphorylation of IP3R was significantly increased in BANK-overexpressing DT40 B cells (Figure 4C), we examined the effect of BANK on tyrosine phosphorylation of IP3R by Lyn. HEK293T cells were transiently transfected with various combinations of plasmids expressing type 2 IP3R, active Lyn and FLAG-tagged BANK. Immunoblotting of IP3R immunoprecipitates with anti-phosphotyrosine (anti-pTyr) antibody revealed that tyrosine phosphorylation of IP3R was significantly increased in the presence of BANK (Figure 7A, a, lanes 3 and 4). This result is consistent with that observed in BANK-overexpressing DT40 B cells (Figure 4C). The amounts of IP3R in these immunoprecipitates and cell lysates were similar (Figure 7A, b and B, a). The amounts of Lyn in cell lysates were identical (Figure 7B, c). Similarly, enhancement of Lyn-mediated tyrosine phosphorylation of type 1 IP3R in the presence of BANK was observed (data not shown). No such enhancements were observed when other ER-associated substrates for Lyn identified during the phosphorylation screening were tested in place of IP3Rs (data not shown). Finally, we examined the effect of BANK mutations on Lyn-mediated tyrosine phosphorylation of IP3R. BANK (155–755), which associates with Lyn (data not shown) but not with IP3R (Figure 6), failed to promote tyrosine phosphorylation of IP3R, whereas BANK ΔANK still promoted tyrosine phosphorylation of IP3R (Figure 7C and D). These observations strongly suggest the importance of the interaction between BANK and IP3R in promotion of tyrosine phosphorylation of IP3R.
Fig. 7. Promotion of Lyn-mediated tyrosine phosphorylation of IP3R by BANK. HEK293T cells were transiently transfected with various combinations of plasmids expressing FLAG-tagged BANK, LynY508F and type 2 IP3R. (A) IP3R was immunoprecipitated and subjected to immunoblotting with anti-pTyr antibody (a). The filter used in (a) was stripped and reprobed with anti-IP3R (b). The amounts of anti-IP3R antibody used in immunoprecipitation were equal (c). Expression of BANK, IP3R and Lyn in whole-cell lysates was confirmed by immunoblotting (B). The effects of BANK mutations were examined in similar experiments (C and D). All experiments were performed at least four times with similar results.
Discussion
In the present study, we isolated a novel B-cell-restricted scaffold protein, BANK, which has a domain organization characteristic of an emerging family of proteins, currently comprising Dof and BCAP. Dof is a downstream regulator that is specific for the fibroblast growth factor receptor (FGFR)-mediated signal transduction pathway in Drosophila (Vincent et al., 1998). BCAP is a tyrosine kinase substrate that connects BCR to PI3K activation through its four YxxM motifs, which are favored binding sites for the SH2 domains of the PI3K p85 subunit (Okada et al., 2000). Despite the similar structural organization with ankyrin repeats and coiled-coil domains, the overall homology between BANK and BCAP is only 33%. BANK has no YxxM motif and is not associated with p85 in BCR-activated Daudi B cells (data not shown). Instead, we have observed an interaction between BANK and Lyn in a tyrosine phosphorylation-dependent manner (Figure 5). In B cells, Syk mediates tyrosine phosphorylation of BANK (Figure 3), which may promote association between BANK and Lyn.
In addition to the role of a kinase-binding protein, BANK also associates with IP3R in vivo (Figure 6). Initially, we assumed that this association might involve the ankyrin repeats of BANK, since in vitro studies have shown that ankyrins are associated with IP3R and ryanodine receptor (Bourguignon et al., 1993, 1995). However, further analysis has revealed that the interaction is mediated by the N-terminal region (amino acids 1–154) of BANK and that, unexpectedly, the most conserved region of the ankyrin repeat, amino acids 309–372 of BANK, is not involved. Detailed characterization of the interaction site of BANK with IP3R will help to identify novel regulators of IP3R function in B cells and other cells.
The physiological importance of BANK in B cells can be deduced from the enhancement of BCR-induced calcium mobilization from intracellular calcium stores in BANK-overexpressing DT40 B-cell lines (Figure 4). Previous studies have shown that IP3R is tyrosine phosphorylated and up-regulated by Src family PTKs (Jayaraman et al., 1996). In DT40 chicken B cells, Lyn deficiency results in a considerable delay in the [Ca2+]i increase without impairment of IP3 production. In contrast, deficiency in Syk or Btk results in almost complete impairment of IP3 production and consequent calcium release (Takata et al., 1994). These differences suggest that Lyn may regulate IP3R functions independently of the PLC-γ2 pathway in B cells, possibly through tyrosine phosphorylation of IP3Rs. We speculate that the up-regulated calcium release in BANK-overexpressing B cells could be caused by the BANK-dependent enhancement of tyrosine phosphorylation of IP3R (Figures 4C and 7). A previous study demonstrated that delayed calcium mobilization of Lyn-deficient DT40 cells can be restored by back-transfection of Fyn with the SH2 domain mutation (R176ES to D176EC) as well as wild-type Fyn (Takata and Kurosaki, 1995). This observation is consistent with and supports our results that the BANK–Lyn interaction was not affected by the point mutation of the SH2 domain of Lyn (Figure 5A, lane 8). It is also possible that increased calcium mobilization is caused by altered production of IP3. However, this would be unlikely, since levels of tyrosine phosphorylation of PLC-γ2 were virtually equal and IP3 production was not changed significantly between wild-type and BANK-overexpressing cells (Figure 4B).
In T cells, IP3Rs are localized below TCR cap structures after TCR stimulation (Khan et al., 1992). BANK may have a role in targeting IP3R within the antigen receptor capped structures in B cells. Thereby, IP3Rs might be located in close proximity not only to BCR-associated PTKs, but also to PLC-γ2, which is also near to BCR. This may allow effective conversion of PLC-γ2-generated IP3 signals to calcium signals.
Data are accumulating that proteins involved in signaling may be physically coupled, emphasizing the biological importance of kinase-anchoring proteins. One good example in B cells is BLNK. BLNK is phosphorylated by Syk upon BCR stimulation and provides binding sites for Btk and PLC-γ2. Btk then phosphorylates and activates PLC-γ2. In other cell types, PSD-95 binds directly to both Fyn and NMDA receptor subunits and promotes Fyn-mediated tyrosine phosphorylation of NMDA receptor subunits (Tezuka et al., 1999). In the present study, we found that BANK promotes Lyn-mediated tyrosine phosphorylation of IP3R, possibly by arranging IP3R and Lyn kinase domain into a favorable configuration.
BANK has potential SH2 binding sites for Shc (Y94ELL) and SHP-2 (Y116ISV and Y458VFI). It may have important roles in the regulation of Shc and SHP-2 functions. In addition, BANK has a putative coiled-coil structure that could be available for protein–protein interactions. Recently, IP3R-associated cGMP kinase substrate (IRAG) has been suggested to interact with cGMP kinase Iβ (cGKIβ) through the coiled-coil domain of IRAG (Schlossmann et al., 2000). cGKIβ also phosphorylates IP3R and modulates its channel activity (Komalavilas and Lincoln, 1994). BANK might associate with other kinases, such as cGKIβ, through its coiled-coil domain and further regulate IP3R activity.
Expression of BANK is confined to B cells (Figure 2), whereas IP3Rs are expressed in a greater variety of cells. IRAG is expressed strongly in the smooth muscle cells of the trachea, aorta and uterus. IRAG attenuates IP3-evoked calcium release in the presence of cGKIβ and cGMP, suggesting the importance of IRAG in the NO–cGMP– cGKI pathway, which leads to a reduction in intracellular calcium and relaxation of vascular tone (Schlossmann et al., 2000). In neurons, the product of the immediate early gene Homer associates with both metabotropic glutamate receptors (mGluRs) and IP3Rs, and ectopic expression of Homer in Purkinje neurons alters mGluR-induced calcium release from intracellular stores (Tu et al., 1998). These three examples suggest that cells utilize different scaffold proteins, which regulate IP3R functions according to different external stimuli and their specific receptor systems, highlighting the importance of these scaffold proteins. The physiological importance of BANK and its association with IP3Rs would be further established by loss-of-function analysis.
Materials and methods
Solid-phase phosphorylation screening and isolation of BANK cDNA
Solid-phase phosphorylation screening was carried out essentially as described previously (Lock et al., 1998, 1999) with a purified GST–Lyn fusion protein and anti-pTyr antibody PY69 (BD Transduction Laboratories). The full-length BANK cDNA was obtained by RT–PCR of RNA isolated from Daudi B cells on the basis of sequence information from the full-length cDNA project (Suzuki et al., 1997). A fragment of the mouse BANK cDNA (corresponding to amino acids 448–694 of human BANK) was obtained by RT–PCR of RNA isolated from mouse spleen.
Sequences were analyzed with a sequence analysis package from the Genetics Computer Group (Institute of Medical Science, University of Tokyo, Tokyo, Japan).
Northern blot analysis
Northern blot analysis was performed as described previously (Kim et al., 1999). Briefly, total RNAs were isolated from various tissues of adult mice and cell pellets of different cell lines with Isogen (Nippon Gene, Japan). Ten micrograms of total RNA were separated on 1% agarose– formaldehyde gels, transferred to nylon membranes and probed with 32P-labeled mouse or human BANK cDNAs (corresponding to amino acids 448–694 and 1–248 of human BANK, respectively).
Semi-quantitative RT–PCR analysis
Bone marrow cells, splenocytes and lymph node cells were isolated from wild-type 129 sv, Rag-deficient and SCID mice. Splenic B cells and lymph node B cells were purified by CD43 depletion; splenic T cells were purified by depletion of non-T (B220, TER119, Mac-1 and Gr-1 positive) cells by magnetic cell sorting (MACS; Miltenyi Biotec). Pro-B (IgM–, B220+, CD43+) and pre-B (IgM–, B220+, CD43–) cells were separated by fluorescence-activated cell sorting (FACS) with a FACStar (Becton Dickinson). The purity of the isolated population was confirmed by FACS analysis. Purified cells for further experiments were >98% pure.
Total RNA was isolated from 1 × 105 purified lymphocytes with TRIzol reagent (Gibco-BRL). To remove any contaminating DNA, RNA was treated with DNaseI (Gibco-BRL) before cDNA synthesis. RNA was reverse transcribed in a 25 µl reaction mixture with the First Strand cDNA synthesis kit (Gibco-BRL). RT–PCR was carried out in a 30 µl reaction mixture that contained 1 µl of cDNA template, 10 pmol of specific oligonucleotide primer pair and 5 U of Taq polymerase (Sigma). Cycle parameters for amplification of BANK were 28 cycles of 1 min at 94°C, 1 min at 60°C and 1 min at 72°C. Cycle parameters for amplification of HPRT were 25 cycles of 1 min at 94°C, 1 min at 65°C and 1 min at 72°C. The amplified products were identified and visualized by Southern blotting with specific oligonucleotide probes.
For mouse BANK, the oligonucleotide primers were 5′-CAGTAAGAGTGTCAAGAA-3′ (5′ primer) and 5′-CAATAATGTTGTCTCGTA-3′ (3′ primer). The Southern blot probe was 5′-GAGCTTCAT CCACAGACA-3′. For mouse HPRT, the 5′ primer was 5′-GCTGGT GAAAAGGACCTCT-3′, the 3′ primer was 5′-CACAGGACTAGAACACCTGC-3′ and the Southern blot probe was 5′-TGGATACAGGCCAGACTTTGT-3′.
Antibodies and expression vectors
Rabbit polyclonal antibodies were raised against a GST fusion protein comprising amino acids 1–248 of human BANK and affinity purified. Other antibodies used were anti-pTyr antibody 4G10 (Upstate Biotechnology), anti-FLAG antibody M2 (Sigma), anti-chicken PLC-γ2 antibody (Yasuda et al., 2000), anti-Lyn polyclonal antibody (Yamanashi et al., 1989), anti-Lyn monoclonal antibody LYN8 (Wako, Japan) and anti-type 2 IP3R antibody KM1083 (Sugiyama et al., 1994).
FLAG-tagged BANK expression plasmid was constructed in pME18S vector (the SRα-promoter). Various mutant BANK expression plasmids were generated by endonuclease digestion (BANK 1–367, BANK 1–653), PCR (BANK lacking amino acids 309–372, ΔANK) or originally isolated in phosphorylation screening (BANK 155–755). Lyn expression plasmids were in pME18S vectors. Various mutant Lyn plasmids were generated by PCR. The constructs were verified by DNA sequencing. Type 2 IP3R was expressed under the control of the cytomegalovirus (CMV) promoter. For stable transfection, FLAG-tagged BANK cDNA was subcloned into pApuro vector (Yasuda et al., 2000).
Cell culture, transfection and cell stimulation
HEK293T cells were cultured as described previously (Nakazawa et al., 2001). Daudi B cells were maintained in RPMI 1640 supplemented with 10% fetal calf serum (FCS) and 50 µM 2-mercaptoethanol. Wild-type and various mutant chicken DT40 B cells were maintained in RPMI 1640 supplemented with 10% FCS, 1% chicken serum and 50 µM 2-mercaptoethanol. Stable transfection of DT40 cells was performed as described previously (Yasuda et al., 2000). Transient transfection of HEK293T cells using calcium phosphate precipitation was performed as described previously (Nakazawa et al., 2001). BCR stimulation was performed essentially as described previously (Yasuda et al., 2000).
Immunoprecipitation and immunoblotting
Cells were solubilized in NP-40 lysis buffer [1% NP-40, 50 mM Tris pH 7.4, 100 mM NaCl, 5 mM EDTA, 50 U/ml trasylol (Bayer), 1 mM Na3VO4]. Pre-cleared lysates were incubated sequentially with the appropriate antibodies and protein G–Sepharose (Amersham Pharmacia Biotech). The immunoprecipitates were washed at least four times with lysis buffer. Western blot analysis was performed as described previously (Tezuka et al., 1999).
Flow cytometry analysis
BCR expression on the surfaces of wild-type and mutant DT40 cells was determined as described previously (Yasuda et al., 2000) with a FACSCalibur (Becton Dickinson).
Calcium measurements
Measurements of cytosolic free calcium concentrations were performed with an acetoxymethyl ester of fura-2 (fura-2/AM; Molecular Probes). Cells (2.5 × 106) were washed once and suspended in Hanks’ balanced salt solution (HBSS) containing 10 mM HEPES pH 7.2, 0.025% bovine serum albumin (BSA) and 1 mM CaCl2, and then loaded with 3 µM fura-2/AM at 30°C for 30 min. Cells were washed twice with HBSS containing 10 mM HEPES pH 7.2, 0.025% BSA and 1.8 mM EGTA, and diluted to 106 cells/ml. Continuous monitoring of fluorescence from the cell suspension was performed with a CAF-110 spectrofluorometer (JASCO) at excitation wavelengths of 340 and 380 nm and an emission wavelength of 500 nm. The fluorescence intensities were normalized by subtracting background levels measured in the presence of EGTA and Triton X-100.
IP3 production analysis
Cells (2.0 × 106) suspended in 50 µl of culture medium were stimulated with M4 (2 µg/50 µl) at 37°C for the times indicated. Kinetic analysis of IP3 production from cells (2.0 × 106) was performed with the Biotrak IP3 assay system (Amersham Pharmacia Biotech) according to the manufacturer’s protocol. Results are shown as the mean from three independent experiments. Error bars represent the SD from the mean.
DDBJ/EMBL/GenBank accession number
The human BANK cDNA sequence data has been submitted to the DDBJ/EMBL/GenBank nucleotide database under accession No. AB063170.
Acknowledgments
Acknowledgements
We thank Dr T.Kurosaki for various DT40 mutant lines, Dr S.Sugano for KAIA1273 cDNA, Dr M.Hattori and Dr K.Uchida for valuable discussion, and Dr D.O’Carroll for critical reading of the manuscript. This work was supported by grants in aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- Bourguignon L.Y., Jin,H., Iida,N., Brandt,N.R. and Zhang,S.H. (1993) The involvement of ankyrin in the regulation of inositol 1,4,5-trisphosphate receptor-mediated internal Ca2+ release from Ca2+ storage vesicles in mouse T-lymphoma cells. J. Biol. Chem., 268, 7290–7297. [PubMed] [Google Scholar]
- Bourguignon L.Y., Chu,A., Jin,H. and Brandt,N.R. (1995) Ryanodine receptor–ankyrin interaction regulates internal Ca2+ release in mouse T-lymphoma cells. J. Biol. Chem., 270, 17917–17922. [DOI] [PubMed] [Google Scholar]
- Cameron A.M., Steiner,J.P., Roskams,A.J., Ali,S.M., Ronnett,G.V. and Snyder,S.H. (1995) Calcineurin associated with the inositol 1,4,5-trisphosphate receptor–FKBP12 complex modulates Ca2+ flux. Cell, 83, 463–472. [DOI] [PubMed] [Google Scholar]
- Fukunaga R. and Hunter,T. (1997) MNK1, a new MAP kinase-activated protein kinase, isolated by a novel expression screening method for identifying protein kinase substrates. EMBO J., 16, 1921–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiai M. et al. (1999) BLNK required for coupling Syk to PLCγ2 and Rac1-JNK in B cells. Immunity, 10, 117–125. [DOI] [PubMed] [Google Scholar]
- Jayaraman T., Ondrias,K., Ondriasova,E. and Marks,A.R. (1996) Regulation of the inositol 1,4,5-trisphosphate receptor by tyrosine phosphorylation. Science, 272, 1492–1494. [DOI] [PubMed] [Google Scholar]
- Khan A.A., Steiner,J.P., Klein,M.G., Schneider,M.F. and Snyder,S.H. (1992) IP3 receptor: localization to plasma membrane of T cells and co-capping with the T cell receptor. Science, 257, 815–818. [DOI] [PubMed] [Google Scholar]
- Kim M., Tezuka,T., Suziki,Y., Sugano,S., Hirai,M. and Yamamoto,T. (1999) Molecular cloning and characterization of a novel cbl-family gene, cbl-c. Gene, 239, 145–154. [DOI] [PubMed] [Google Scholar]
- Komalavilas P. and Lincoln,T.M. (1994) Phosphorylation of the inositol 1,4,5-trisphosphate receptor by cyclic GMP-dependent protein kinase. J. Biol. Chem., 269, 8701–8707. [PubMed] [Google Scholar]
- Kurosaki T. (1999) Genetic analysis of B cell antigen receptor signaling. Annu. Rev. Immunol., 17, 555–592. [DOI] [PubMed] [Google Scholar]
- Kurosaki T. and Tsukada,S. (2000) BLNK: connecting Syk and Btk to calcium signals. Immunity, 12, 1–5. [DOI] [PubMed] [Google Scholar]
- Lock P., Abram,C.L., Gibson,T. and Courtneidge,S.A. (1998) A new method for isolating tyrosine kinase substrates used to identify fish, an SH3 and PX domain-containing protein and Src substrate. EMBO J., 17, 4346–4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock P., Casagranda,F. and Dunn,A.R. (1999) Independent SH2-binding sites mediate interaction of Dok-related protein with RasGTPase-activating protein and Nck. J. Biol. Chem., 274, 22775–22784. [DOI] [PubMed] [Google Scholar]
- Miyakawa T., Maeda,A., Yamazawa,T., Hirose,K., Kurosaki,T. and Iino,M. (1999) Encoding of Ca2+ signals by differential expression of IP3 receptor subtypes. EMBO J., 18, 1303–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakade S., Rhee,S.K., Hamanaka,H. and Mikoshiba,K. (1994) Cyclic AMP-dependent phosphorylation of an immunoaffinity-purified homotetrameric inositol 1,4,5-trisphosphate receptor (type I) increases Ca2+ flux in reconstituted lipid vesicles. J. Biol. Chem., 269, 6735–6742. [PubMed] [Google Scholar]
- Nakazawa T., Komai,S., Tezuka,T., Hisatsune,C., Umemori,H., Semba,K., Mishina,M., Manabe,T. and Yamamoto,T. (2001) Characterization of Fyn-mediated tyrosine phosphorylation sites on GluRε2 (NR2B) subunit of the _N-_methyl-d-aspartate receptor. J. Biol. Chem., 276, 693–699. [DOI] [PubMed] [Google Scholar]
- Nishibe S., Wahl,M.I., Hernandez-Sotomayor,S.M., Tonks,N.K., Rhee,S.G. and Carpenter,G. (1990) Increase of the catalytic activity of phospholipase C-γ1 by tyrosine phosphorylation. Science, 250, 1253–1256. [DOI] [PubMed] [Google Scholar]
- Okada T., Maeda,A., Iwamatsu,A., Gotoh,K. and Kurosaki,T. (2000) BCAP: the tyrosine kinase substrate that connects B cell receptor to phosphoinositide 3-kinase activation. Immunity, 13, 817–827. [DOI] [PubMed] [Google Scholar]
- Rebecchi M.J. and Pentyala,S.N. (2000) Structure, function, and control of phosphoinositide-specific phospholipase C. Physiol. Rev., 80, 1291–1335. [DOI] [PubMed] [Google Scholar]
- Schlossmann J. et al. (2000) Regulation of intracellular calcium by a signalling complex of IRAG, IP3 receptor and cGMP kinase Iβ. Nature, 404, 197–201. [DOI] [PubMed] [Google Scholar]
- Schmitz R., Baumann,G. and Gram,H. (1996) Catalytic specificity of phosphotyrosine kinase Blk, Lyn, c-Src and Syk as assessed by phage display. J. Mol. Biol., 260, 664–677. [DOI] [PubMed] [Google Scholar]
- Songyang Z. et al. (1993) SH2 domains recognize specific phosphopeptide sequences. Cell, 72, 767–778. [DOI] [PubMed] [Google Scholar]
- Songyang Z. et al. (1994) Specific motifs recognized by the SH2 domains of Csk, 3BP2, fps/fes, GRB-2, HCP, SHC, Syk, and Vav. Mol. Cell. Biol., 14, 2777–2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara H., Kurosaki,M., Takata,M. and Kurosaki,T. (1997) Genetic evidence for involvement of type 1, type 2 and type 3 inositol 1,4,5-trisphosphate receptors in signal transduction through the B-cell antigen receptor. EMBO J., 16, 3078–3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama T. et al. (1994) Monoclonal antibodies distinctively recognizing the subtypes of inositol 1,4,5-trisphosphate receptor: application to the studies on inflammatory cells. FEBS Lett., 354, 149–154. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Yoshitomo-Nakagawa,K., Maruyama,K., Suyama,A. and Sugano,S. (1997) Construction and characterization of a full length-enriched and a 5′-end-enriched cDNA library. Gene, 200, 149–156. [DOI] [PubMed] [Google Scholar]
- Takata M. and Kurosaki,T. (1995) The catalytic activity of Src-family tyrosine kinase is required for B cell antigen receptor signaling. FEBS Lett., 374, 407–411. [DOI] [PubMed] [Google Scholar]
- Takata M. and Kurosaki,T. (1996) A role for Bruton’s tyrosine kinase in B cell antigen receptor-mediated activation of phospholipase C-γ2. J. Exp. Med., 184, 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata M., Sabe,H., Hata,A., Inazu,T., Homma,Y., Nukada,T., Yamamura,H. and Kurosaki,T. (1994) Tyrosine kinases Lyn and Syk regulate B cell receptor-coupled Ca2+ mobilization through distinct pathways. EMBO J., 13, 1341–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezuka T., Umemori,H., Fusaki,N., Yagi,T., Takata,M., Kurosaki,T. and Yamamoto,T. (1996) Physical and functional association of the cbl proto-oncogene product with an src-family protein tyrosine kinase, p53/56lyn, in the B cell antigen receptor-mediated signaling. J. Exp. Med., 183, 675–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezuka T., Umemori,H., Akiyama,T., Nakanishi,S. and Yamamoto,T. (1999) PSD-95 promotes Fyn-mediated tyrosine phosphorylation of the _N-_methyl-d-aspartate receptor subunit NR2A. Proc. Natl Acad. Sci. USA, 96, 435–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu J.C., Xiao,B., Yuan,J.P., Lanahan,A.A., Leoffert,K., Li,M., Linden,D.J. and Worley,P.F. (1998) Homer binds a novel proline-rich motif and links group 1 metabotropic glutamate receptors with IP3 receptors. Neuron, 21, 717–726. [DOI] [PubMed] [Google Scholar]
- Vincent S., Wilson,R., Coelho,C., Affolter,M. and Leptin,M. (1998) The Drosophila protein Dof is specifically required for FGF signaling. Mol. Cell, 2, 515–525. [DOI] [PubMed] [Google Scholar]
- Weiss A., Koretzky,G., Schatzman,R.C. and Kadlecek,T. (1991) Functional activation of the T-cell antigen receptor induces tyrosine phosphorylation of phospholipase C-γ1. Proc. Natl Acad. Sci. USA, 88, 5484–5488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanashi Y., Mori,S., Yoshida,M., Kishimoto,T., Inoue,K., Yamamoto,T. and Toyoshima,K. (1989) Selective expression of a protein-tyrosine kinase, p56lyn, in hematopoietic cells and association with production of human T-cell lymphotropic virus type I. Proc. Natl Acad. Sci. USA, 86, 6538–6542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda T., Maeda,A., Kurosaki,M., Tezuka,T., Hironaka,K., Yamamoto,T. and Kurosaki,T. (2000) Cbl suppresses B cell receptor-mediated phospholipase C (PLC)-γ2 activation by regulating B cell linker protein PLC-γ2 binding. J. Exp. Med., 191, 641–650. [DOI] [PMC free article] [PubMed] [Google Scholar]