Balancing the activation state of the endothelium via two distinct TGF-β type I receptors (original) (raw)
Abstract
The generation of mice lacking specific components of the transforming growth factor-β (TGF-β) signal tranduction pathway shows that TGF-β is a key player in the development and physiology of the cardiovascular system. Both pro- and anti-angiogenic properties have been ascribed to TGF-β, for which the molecular mechanisms are unclear. Here we report that TGF-β can activate two distinct type I receptor/Smad signalling pathways with opposite effects. TGF-β induces phosphorylation of Smad1/5 and Smad2 in endothelial cells and these effects can be blocked upon selective inhibition of ALK1 or ALK5 expression, respectively. Whereas the TGF-β/ALK5 pathway leads to inhibition of cell migration and proliferation, the TGF-β/ALK1 pathway induces endothelial cell migration and proliferation. We identified genes that are induced specifically by TGF-β-mediated ALK1 or ALK5 activation. Id1 was found to mediate the TGF-β/ALK1-induced (and Smad-dependent) migration, while induction of plasminogen activator inhibitor-1 by activated ALK5 may contribute to the TGF-β-induced maturation of blood vessels. Our results suggest that TGF-β regulates the activation state of the endothelium via a fine balance between ALK5 and ALK1 signalling.
Keywords: ALK/angiogenesis/signal transduction/Smad/TGF-β
Introduction
Angiogenesis encompasses an activation and resolution phase. In the activation phase, increased vascular permeability and basement membrane degradation allows endothelial cells (ECs) to proliferate and migrate into the extracellular space and form new capillary sprouts. In the resolution phase, ECs cease proliferation and migration, reconstitute the basement membrane and promote vessel maturation. Mesenchymal cells are recruited and subsequently differentiate into pericytes and smooth muscle cells surrounding the newly formed vessel. The transition from the resolution to activation phase and vice versa is determined by an intricately regulated balance between inducers and inhibitors (Folkman and D’Amore, 1996; Risau et al., 1997; Carmeliet et al., 2000).
Transforming growth factor-β (TGF-β) is thought to play a pivotal role during vascular remodelling and the resolution phase of angiogenesis and is generally regarded as an inhibitor of angiogenesis (Pepper, 1997). Under most culture conditions, TGF-β inhibits the proliferation and migration of ECs, stimulates extracellular matrix (ECM) accumulation and stimulates the differentiation of mesenchymal cells into pericytes and smooth muscle cells (Sawdey et al., 1989; Madri et al., 1992; Hirschi et al., 1998). However, stimulatory effects of TGF-β have also been reported on angiogenesis in vivo (Roberts et al., 1986; Yang and Moses, 1990; Koh et al., 1995; Fajardo et al., 1996; Ananth et al., 1999) and ECs in vitro (Plouet and Gospodarowicz, 1989; Madri et al., 1992; Gajdusek et al., 1993; Iruela-Arispe and Sage, 1993; Pepper et al., 1993; Vernon and Sage, 1999). The mechanisms by which TGF-β acts as inhibitor or promoter for blood vessel formation are not understood.
TGF-β regulates cellular processes by binding to a heteromeric complex of type I and type II serine/threonine kinase receptors (Massagué, 1998). The type I receptor, also known as activin receptor-like kinase (ALK), acts downstream of the type II receptor and propagates the signal to the nucleus by phosphorylating specific members of the Smad family, receptor-regulated (R)-Smads, at their extreme C-terminal serine residues. Phosphorylated R-Smads form complexes with the common partner (Co)-Smad, i.e. Smad4, which accumulate in the nucleus where they participate in transcriptional regulation of target genes (Derynck et al., 1998).
In most cell types, TGF-β signals via TβRI, also known as ALK5. ECs also express ALK1, in addition to ALK5. Both type I receptors have been shown to bind TGF-β in transfected COS cells (Attisano et al., 1993; ten Dijke et al., 1994) and more recently in non-transfected ECs (Oh et al., 2000). Interestingly, while activated ALK5 induces the phosphorylation of Smad2 and Smad3, activated ALK1 has been shown to induce the phosphorylation of Smad1 and Smad5 in transfected COS cells (Chen and Massagué, 1999; Oh et al., 2000). Gene ablation studies in mice have revealed the importance of TβRII, ALK1, ALK5 and Smad5 in angiogenesis (Goumans and Mummery, 2000), and a human vascular disorder hereditary haemorrhagic telengiectasia (HHT), has been linked to mutations in ALK1 and endoglin, an endothelial accessory TGF-β receptor (McAllister et al., 1994; Johnson et al., 1996).
To gain more insight into the molecular mechanisms by which TGF-β regulates EC function, we investigated the contribution of ALK1 and ALK5 to TGF-β signalling in ECs. Our results reconcile previously published observations on inhibitory and stimulatory effects of TGF-β on blood vessel formation, and suggest that TGF-β regulates the activation/differentiation state of the endothelium via two oppositely acting TGF-β type I receptors, ALK1 and ALK5.
Results
TGF-β induces Smad2 and Smad5 phosphorylation in ECs
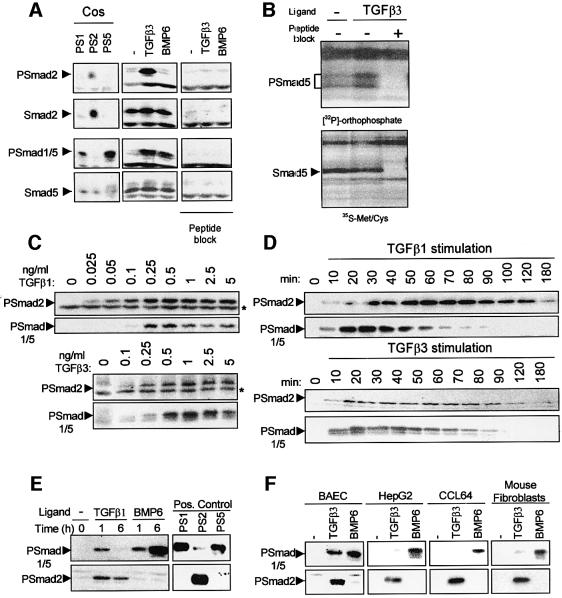
ALK1 and ALK5 have been shown to activate Smads differentially when overexpressed in COS cells (Oh et al., 2000). In order to examine the activation of endogenous Smads in non-transfected primary ECs, we developed a PSmad1 antibody that specifically recognizes phosphorylated Smad1 and/or Smad5 (Smad1/5) and a PSmad2 antibody that specifically detects phosphorylated Smad2 (Figure 1A, and Material and methods). Stimulation of mouse embryonic endothelial cells (MEECs) with TGF-β for 1 h resulted in phosphorylation of Smad2 and Smad5. Using specific Smad antibodies, we found that MEECs express Smad2 and Smad5, but Smad1 could not be detected (Figure 1A and data not shown). In all primary ECs studied [bovine aortic endothelial (BAE), bovine corneal endothelial (BCE), yolk sac (YS) and bovine microvascular endothelial (BME) cells], TGF-β was found to induce Smad1/5 and Smad2/3 phosphorylation (data not shown). As expected, stimulating ECs with bone morphogenetic protein 6 (BMP6) led to the phosphorylation of Smad5, but not Smad2 (Figure 1A and data not shown). To confirm that TGF-β stimulation resulted in Smad5 phosphorylation, we carried out orthophosphate labelling of MEECs in the absence or presence of TGF-β and immunoprecipitation of cell lysates with Smad5-specific antibody. Using this independent method, TGF-β was demonstrated to induce a significant increase in Smad5 phosphorylation in MEECs (Figure 1B). The specificity of the protein recognized by the Smad5 antibody was demonstrated by less of the phosphorylated band in the presence of the cognate peptide (Figure 1B). Thus, TGF-β induces Smad2 and Smad5 phosphorylation in ECs.
Fig. 1. TGF-β induces the phosphorylation of Smad2 and Smad5 in ECs. (A) TGF-β-induced Smad2 and Smad5 phosphorylation in ECs. MEECs were stimulated with 5 ng/ml TGF-β3 or 50 ng/ml BMP6 for 60 min at 37°C before lysis. Whole-cell extracts were fractionated by 6% SDS–PAGE and blotted. The filters were incubated with PSmad2, which specifically recognizes phosphorylated Smad2, PSmad1, which specifically recognizes phosphorylated Smad1/5, and Smad2 and Smad5 antibodies, in the absence or presence of blocking peptide to which the antibody was raised. (B) MEECs were labelled with [32P]orthophosphate and treated or not with 10 ng/ml TGF-β3. Cell lysates were immunoprecipitated with anti-Smad5 antibody and separated by SDS–PAGE followed by autoradiography. Cell lysates that had been labelled with [35S]Met/Cys show the presence of Smad5 protein after immunoprecipitation. (C) Dose response of TGF-β-induced Smad2 versus Smad1/5 phosphorylation. BAECs were stimulated with different concentrations of TGF-β1 or TGF-β3 for 1 h before lysis, and were fractionated and blotted. The filters were incubated with the PSmad2 and PSmad1/5 antibodies. The asterisk indicates an aspecific band recognized by the antibody showing equal loading. (D) Kinetics of TGF-β-induced Smad2 versus Smad1/5 phosphorylation. BAECs were stimulated with 5 ng/ml TGF-β1 or TGF-β3 for the indicated time before lysis. After fractionation and blotting, the filters were incubated with PSmad2 and PSmad1/5 antibodies. (E) Kinetics of TGF-β-induced Smad2 phosphorylation versus TGF-β-induced Smad1/5 phosphorylation, and TGF-β- versus BMP-induced Smad5 phosphorylation. MEECs were stimulated with 5 ng/ml TGF-β1 or 50 ng/ml BMP6 for 1 or 6 h before lysis, fractionated by 6% SDS–PAGE and blotted. The filters were incubated with the PSmad2 and PSmad1 antibodies. As a positive control, COS cell lysate transfected with either Smad1/caALK1 (PS1), Smad2/caALK5 (PS2) or Smad5/caALK1 (PS5) was used. (F) TGF-β3 efficiently induces Smad5 phosphorylation in endothelial cells, but not in other cell types. BAECs, HepG2 cells, CCL64 cells or MEECs were stimulated with 5 ng/ml TGF-β3 or 50 ng/ml BMP6 for 1 h before lysis, fractionated by 6% SDS–PAGE and blotted. The filters were incubated with the PSmad2 and PSmad1 antibodies.
Differential dose response and kinetics of TGF-β-induced Smad2 versus Smad5 phosphorylation
We examined the effect of different doses on TGF-β-induced Smad2 versus Smad5 phosphorylation (Figure 1C). TGF-β induced substantial Smad2 phosphorylation at 0.025 ng/ml and reached a maximum at 0.25 ng/ml, which remained at this level at higher doses. In contrast, TGF-β-induced Smad5 phosphorylation peaked at 0.25–0.5 ng/ml and decreased at higher doses. TGF-β1 and TGF-β3 induced similar dose–response patterns on Smad phosphorylation (Figure 1C). We examined the kinetics of TGF-β-induced Smad2 versus Smad5 phosphorylation (Figure 1D). Peak levels for TGF-β-induced Smad2 and Smad5 phosphorylation were reached after 1 h and 30 min of stimulation, respectively (Figure 1D). However, while TGF-β-induced Smad2 phosphorylation remained stable over time, Smad5 was no longer phosphorylated after 90 min of TGF-β treatment. TGF-β1 and TGF-β3 induced similar kinetic profiles (Figure 1D). Thus, TGF-β-induced Smad2 phosphorylation is much more stable than TGF-β-induced Smad5 phosphorylation in ECs. Interestingly, in contrast to TGF-β, BMP-induced Smad1/5 remained phosphorylated after 6 h of ligand stimulation (Figure 1E).
To evaluate whether the TGF-β-induced Smad5 phosphorylation is EC specific, we analysed which Smads become phosphorylated after TGF-β stimulation in non-ECs. TGF-β induced Smad2 phosphorylation in all cell lines examined. However, while BMP6 efficiently phosphorylates Smad1/5, only very little phosphorylated Smad1/5 protein was detectable in the non-ECs challenged with TGF-β. This is in contrast to BAECs in which TGF-β is as potent as BMP6 in stimulating phosphorylation of Smad1/5 (Figure 1F).
Differential Smad activation by ALK1 and ALK5 in ECs
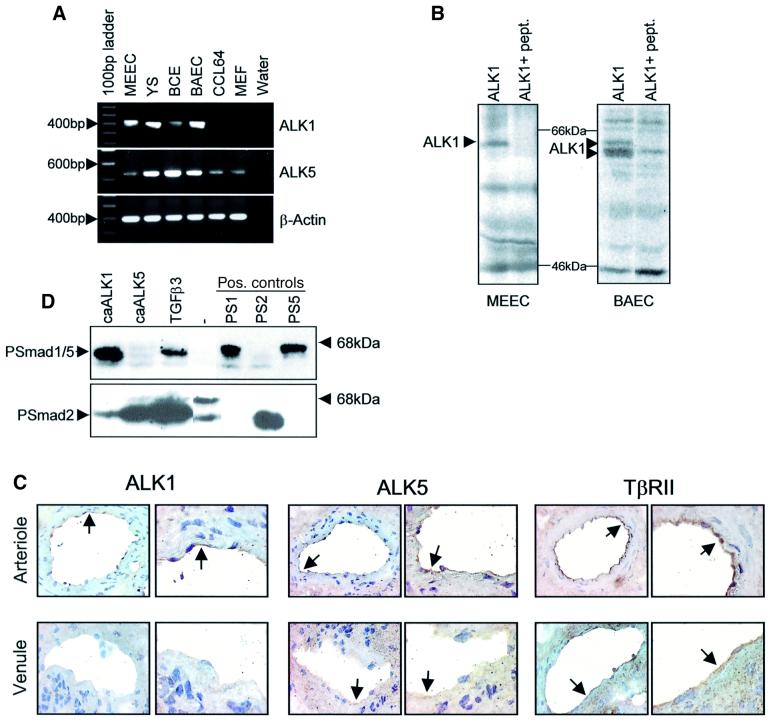
One striking difference between ECs and other cell types is the presence of the EC-specific TGF-β type I receptor ALK1, capable of binding TGF-β in non-transfected ECs (Oh et al., 2000). Indeed, ALK1 mRNA is only detected in ECs, whereas ALK5 mRNA is present in all cell types analysed (Figure 2A). In addition, both MEECs and BAECs express ALK1 protein (Figure 2B). Moreover, by performing immunohistochemistry, we detected expression of ALK1 and ALK5, as well as TβRII in blood vessels in submucosal areas of human colon (Figure 2C). Interestingly, expression of ALK1 in the ECs of arterioles is significantly higher than in venous ECs (Figure 2C, arrows). ALK5 expression in ECs of arterioles is moderately higher compared with veins, and the muscle and fibroblast layers in arterioles are weakly positive. TβRII is expressed in the ECs of arteries and veins as well as in the muscle and fibroblast layer (Figure 2C).
Fig. 2. Differential R-Smad activation by ALK1 and ALK5 in ECs. (A) The expression of ALK1 and ALK5 was analysed by RT–PCR. The PCR products were loaded on a 1% agarose gel and stained with ethidium bromide. Amplified products of ALK5, ALK1 and β-actin are indicated on the right of the figure. (B) To verify the expression of ALK1 protein, ECs were labelled with [35S]Met/Cys and immunoprecipitated for ALK1 using an ALK1-specific antibody, in the absence or presence of the cognate peptide to which the antibody was raised. (C) ALK1, ALK5 and TβRII are expressed in blood vessels in human colon. Immunohistochemistry was performed on cryosections from human colon using antibodies specifically recognizing ALK1, ALK5 or TβRII to determine the presence of these receptors in arteries and veins. As can be seen in the figure, all receptors are expressed in arterioles and veins albeit at different levels. (D) caALK1 and caALK5 phosphorylated different R-Smads. BAECs were infected with adenovirus expressing either caALK1 or caALK5 with a m.o.i. of 500. Sixteen hours after infection, the cells were washed. After 30 h, cells were lysed, fractionated by 6% SDS–PAGE and blotted. The filters were incubated with the PSmad2 and PSmad1 antibodies.
The difference in Smad phosphorylation observed between ECs and non-ECs might be caused by the expression of ALK1 in ECs, but not in other cell types. We therefore infected BAECs with adenovirus expressing either constitutively active (ca)ALK1 or caALK5 and analysed the effect on phosphorylation of Smad1/5 and Smad2. Whereas TGF-β stimulation leads to the phosphorylation of both types of R-Smad, caALK1 phosphorylates only Smad1/5, while caALK5 results in only Smad2 phosphorylation (Figure 2D). These data suggest that there are two signalling pathways downstream of TGF-β in ECs, one through ALK5 phosphorylating Smad2 and the other via ALK1 leading to Smad1/5 phosphorylation.
Direct implication of ALK1 and ALK5 in TGF-β signalling
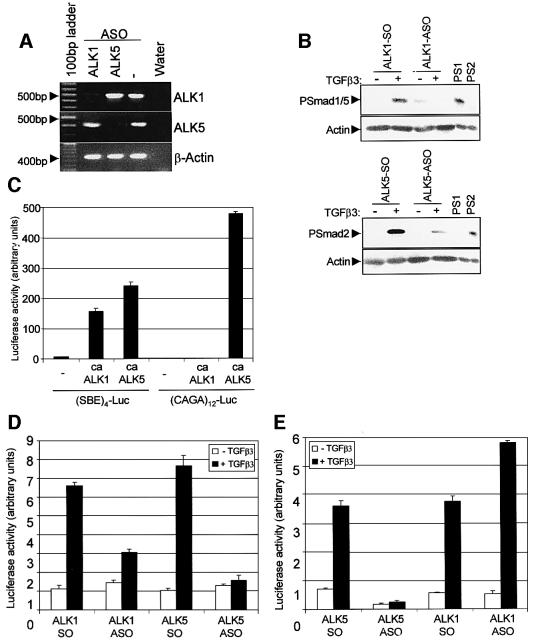
To test the direct involvement of ALK1 and ALK5 in TGF-β signalling in ECs, we designed antisense oligonucleotides (ASOs) to interfere selectively with the expression of either ALK1 or ALK5 and analysed their effect on TGF-β-induced responses in ECs. To show that indeed the ASOs target the degradation of ALK transcripts in a specific manner, we analysed the mRNA levels of ALK1 and ALK5 in MEECs treated with ASOs. Treatment of MEECs with ALK1 or ALK5 ASOs induced a strong down-regulation of expression levels for ALK1 or ALK5, respectively. Neither ALK5 mRNA nor ALK1 mRNA levels were affected by ASOs of the other receptor type, and the levels of β-actin mRNA were unaffected (Figure 3A). The ASO-induced decrease in ALK1 and ALK5 transcripts in MEECs resulted in a reduced capacity of TGF-β to phosphorylate Smad1/5 and Smad2 proteins, respectively (Figure 3B).
Fig. 3. Effect of antisense ALK1 or antisense ALK5 on TGF-β-induced Smad phosphorylation and transcriptional activity. (A) Antisense oligonucleotides (ASOs) target the degradation of ALK transcripts. ASOs or sense oligonucleotides (SOs) were introduced into MEECs by hypo-osmotic shock. To confirm the specificity of the antisense action, RNA was isolated from treated cells and RT–PCR was performed with primers specific for ALK1, ALK5 and β-actin. The amplified product is indicated on the right of the figure. (B) ALK1 and ALK5 ASOs reduce TGF-β-induced Smad phosphorylation. MEECs were loaded twice with oligonucleotides for 2 days. Subsequently, the cells were starved for 4 h and stimulated with 0.5 ng/ml TGF-β3. After 1 h, cells were lysed, fractionated by 6% SDS–PAGE and blotted. The filters were incubated with PSmad2, PSmad1 and actin antibodies as a loading control. (C) ALK1 and ALK5 can induce (SBE)4-luc, but only ALK5 can activate (CAGA)12-luc. MEECs were transfected either with (SBE)4-luc or (CAGA)12-luc in the absence or presence of caALK1 or caALK5, and luciferase activity was measured. A representative experiment using triplicate samples, corrected for transfection efficiency, is shown. (D and E) ALK1 or ALK5 ASOs affect TGF-β-induced transcriptional activity. MEECs loaded with oligonucleotides were transfected with either (SBE)4-luc (D) or (CAGA)12-luc (E), unstimulated or stimulated with 5 ng/ml TGF-β3, and luciferase activity was measured. A representative experiment using triplicate samples, corrected for transfection efficiency, is shown.
We next used these ASOs in a transcriptional response assay using TGF-β-inducible reporters, (SBE)4-luc (Jonk et al., 1998) and (CAGA)12-luc (Dennler et al., 1998). Whereas caALK1 and caALK5 activated (SBE)4-luc, (CAGA)12-luc was activated by caALK5, but not caALK1 (Figure 3C). Both reporters were activated by TGF-β in MEECs loaded with sense oligonucleotides (SOs). However, the stimulatory effect on (SBE)4-luc was greatly reduced when the cells were loaded with ALK1 ASO or abolished when the expression of ALK5 mRNA was blocked (Figure 3D). As expected, ALK5 ASO, but not ALK1 ASO, blocked TGF-β-induced (CAGA)12-luc activity (Figure 3E). These experiments indicate that both ALK1 and ALK5 are involved in TGF-β-induced transcriptional responses, and that ALK1 and ALK5 can both function as TGF-β type I receptors in ECs.
ALK1 and ALK5 have opposing effects on the activation state of the endothelium
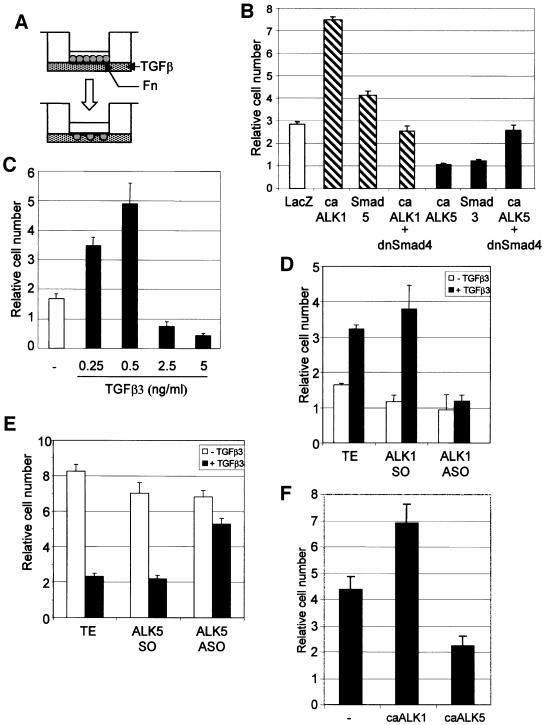
Having demonstrated the presence of two distinct TGF-β type I receptor signalling pathways in ECs, we wanted to elucidate the role of ALK1 and ALK5 in blood vessel formation. EC migration requires tight control during angiogenesis, and TGF-β is known to affect this response both positively and negatively (Muller et al., 1987; Sato and Rifkin, 1989; Yang and Moses, 1990; Basson et al., 1992; Pepper, 1997). We plated adenovirally infected cells expressing caALK1 or caALK5 on to the upper chamber of an 8 µm pore size transwell and measured the transmigration after 16 h (Figure 4A). MEECs expressing caALK1 exhibited increased migratory behaviour (2.5-fold), while the expression of caALK5 resulted in a 3-fold decrease in migration (Figure 4B). This difference in migratory behaviour was already visible after 6 h of migration, and addition of TGF-β to only the lower chamber or on both sides of the well did not change the outcome, suggesting that TGF-β affects the chemokinetic properties of the cells (data not shown). Similar effects were observed in infected BAECs in a scratch assay and analysed by time-lapse microscopy. LacZ-infected cells had a migration speed of 15 µm/h, caALK1 enhanced the migration to 21 µm/h, while ALK5 signal transduction greatly inhibited (1.7 µm/h) the capacity of the cells to close the wound (data not shown). To determine whether the effect TGF-β has on migration is dependent on the Smad pathway, we infected the MEECs with Smad5 or Smad3 adenovirus (Figure 4B). ECs infected with Smad5 or Smad3 could mimic the effects observed with caALK1 or caALK5, respectively. Expression of a dominant-negative form of Smad4, a common intracellular component necessary for Smad-dependent ALK1 and ALK5 signalling, blocked both the ALK1-induced increase and the ALK5-induced decrease of EC migration. Together, these results indicate that ALK1 and ALK5 mediate opposite effects on EC migration in a Smad-dependent manner.
Fig. 4. ALK1 and ALK5 have opposing effects on EC migration and proliferation. (A) Chemokinesis assay. Infected endothelial cells were seeded in the upper chamber of a fibronectin (Fn)-coated transwell. In the lower chamber, culture medium with or without ligand was added. After 6 or 16 h, the cells that had migrated to the other side of the filter were counted after staining with crystal violet. (B) Opposite effect of ALK1 and ALK5 on EC migration. MEECs infected with the adenovirus indicated were seeded into transwells. After 16 h, the number of transmigrated cells was assessed by staining with crystal violet. The mean and SD of three replicate wells of a representative experiment is shown. (C) Biphasic dose–response for TGF-β on migration. MEECs were incubated with different doses of TGF-β3 and used in a transwell migration assay. (D and E) ASOs blocking ALK1- induced and ALK5-inhibited migration. MEECs were loaded with ALK1 ASOs (D) or ALK5 ASOs (E) used in a transwell migration assay in the absence or presence of 0.25 (D) or 5 (E) ng/ml TGF-β3. (F) Opposite effect of ALK1 and ALK5 on EC proliferation. BAECs were infected with the adenovirus indicated 30 h before seeding. After 5 days, the cell number per well was counted. The mean and SD of three replicate wells of a representative experiment is shown.
When we examined the dose dependence of the TGF-β effect on EC migration, we noted a biphasic dose–response curve as previously reported (Gajdusek et al., 1993; Pepper et al., 1993) (Figure 4C). TGF-β was found to stimulate migration of YS ECs at concentrations from 0.25 to 0.5 ng/ml, while a higher dose of TGF-β inhibited EC migration (Figure 4C). The same biphasic response was observed in all ECs analysed. Distinct TGF-β responses at different thresholds or differential use of TGF-β receptors may explain this biphasic dose–response curve. To test whether ALK1 or ALK5 is involved in causing opposite responses at different TGF-β concentrations, we examined the effect of ALK1 ASO on migration in the ‘low dose’ response range, and the effect of ALK5 ASO on migration in the ‘high dose’ response range. Consistent with the notion that distinct TGF-β type I receptors are involved in generating the biphasic dose–response curve, we found that decreased ALK1 expression or ALK5 expression inhibited TGF-β-mediated stimulation or inhibition of migration, respectively (Figure 4D and E). ECs treated with ALK1 ASO remained inhibited in migration upon challenge with ‘high dose’ TGF-β (data not shown).
To examine the effect of caALK1 or caALK5 on EC proliferation, we infected BAECs with ALK-expressing adenoviruses and determined the change in cell number after 5 days by direct counting. caALK1 resulted in a reproducible increase in cell proliferation, while caALK5 resulted in a decrease in proliferation (Figure 4F). Thus, ALK1 promotes EC proliferation and migration, while ALK5 inhibits these responses.
Id1 is a specific downstream target gene of ALK1 in ECs
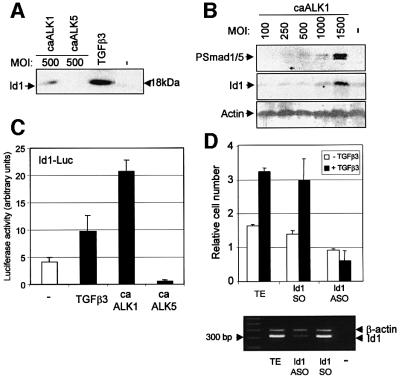
The identification of two opposing TGF-β signalling cascades in ECs led us to investigate the possible downstream targets of ALK1 and ALK5, which would clarify their opposing function in angiogenesis. BMPs that signal via the same Smad proteins as ALK1, are known to stimulate expression of Id proteins (inhibitors of differentiation or DNA binding) (Hollnagel et al., 1999; Korchynskyi and ten Dijke, 2002), which act as dominant inhibitors of basic helix–loop–helix (bHLH) transcription factors. Upon stimulation of BAECs with TGF-β, Id1 expression was up-regulated robustly (Figure 5A). Infect ing BAECs with adenovirus expressing caALK1 or caALK5 revealed that caALK1 selectively up-regulated Id1 protein. Furthermore, an increase in adenoviral caALK1 infection resulted in increased Smad1/5 phosphorylation and Id1 expression (Figure 5B). Consistent with these findings, TGF-β and caALK1 activated the Id1 promoter luciferase (Id1-luc) reporter, while caALK5 decreased Id1-luc activity compared with control levels (Figure 5C; Tournay and Benezra, 1996). Id1 has been reported to enhance migration of certain cells (Lin et al., 2000). We therefore tested the effect of Id1 ASO on TGF-β-mediated stimulation of migration. Treatment of cells with Id1 ASOs, but not Id1 sense oligonucleotides (SOs), inhibited TGF-β-mediated promotion of EC migration (Figure 5D). Id1 ASOs were shown specifically to inhibit Id1 mRNA expression (Figure 5D). This suggests that Id1 is an important downstream effector of ALK1 in ECs.
Fig. 5. Id1 is a specific downstream target gene of ALK1. (A) Effect of ALK1 and ALK5 on Id1 expression. BAECs were stimulated with TGF-β3 for 1 h prior to lysis or infected with adenovirus expressing either caALK1 or caALK5 with an m.o.i. of 500. After 30 h, cells were lysed, fractionated by 12.5% SDS–PAGE and blotted. The filter was incubated with an anti-Id1 antibody. (B) Increased ALK1 signalling results in increased Smad1/5 phosphorylation followed by increased Id1 expression. BEACs were infected with an increasing m.o.i. of caALK1. After 30 h, cells were lysed, fractionated and blotted. The filters were incubated with a PSmad1/5 and Id1 antibody, as well as an actin antibody to show equal loading. (C) Effect of ALK1 and ALK5 on an Id1 reporter. MEECs infected with adenoviruses expressing either caALK1 or caALK5 were used in a transcriptional response assay. The cells were transfected with Id1-luc and stimulated or not with 5 ng/ml TGF-β3. After 20 h, luciferase activity was measured and values are corrected for transfection efficiency as measured by β-galactosidase activity. A representative experiment using triplicate samples is shown. (D) Id1 ASOs inhibit TGF-β-induced EC migration. MEECs loaded with Id1 ASOs were used in a transwell migration assay in the absence or presence of 0.25 ng/ml TGF-β3. To confirm the specificity of the antisense action, RNA was isolated from treated cells and RT–PCR was performed with primers specific for Id1 and β-actin.
PAI-1 is a specific downstream target of ALK5 in ECs
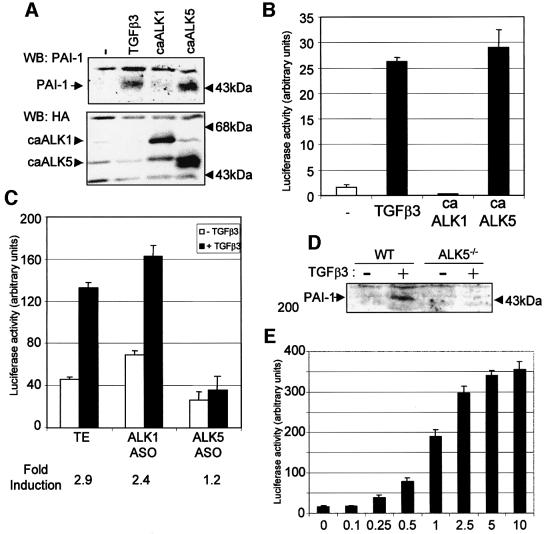
Our data suggest that ALK5 is involved in the resolution phase of angiogenesis. Proteinase inhibitors, such as plasminogen activator inhibitor (PAI)-1, have an important function during vessel maturation in preventing degradation of the provisional ECM around the nascent vessel. PAI-1 is induced by TGF-β in ECs (Figure 6A; Sawdey et al., 1989). We therefore analysed the effect of ALK1 and ALK5 on PAI-1 expression. Ectopic expression of caALK5, but not caALK1, resulted in an increase in the expression of PAI-1 protein in MEECs (Figure 6A). Using a PAI-1 promoter luciferase reporter, we also found that TGF-β and caALK5, but not caALK1, activated this reporter (Figure 6B). Inhibiting the expression of ALK5 by loading the ECs with ASOs (Figure 6C) abolished the TGF-β-induced activation of the PAI-1 promoter, showing that ALK5 expression is necessary for PAI-1 induction upon TGF-β stimulation. This observation was confirmed when we used ECs isolated from the ALK5 knockout mouse. While wild-type ECs show a clear induction of the PAI-1 protein upon TGF-β stimulation, no TGF-β-induced PAI-1 protein could be detected in the ALK5–/– ECs. The induction of PAI-1 by TGF-β in ECs did not follow a biphasic response curve. A plateau in the inducibility of the PAI-1 promoter reporter was reached when the concentration of TGF-β was increased to 10 ng/ml (Figure 6E).
Fig. 6. PAI-1 is a specific downstream target of ALK5. (A) Effect of ALK1 and ALK5 on PAI-1 expression. MEECs were stimulated with TGF-β3 for 16 h prior to lysis or infected with the adenovirus indicated with an m.o.i. of 500. After 30 h, cells were lysed, fractionated by 7.5% SDS–PAGE and blotted. The filter was incubated with an anti-PAI-1 antibody that specifically recognizes PAI-1 protein and an anti-HA antibody to show the expression of the HA-tagged type I receptors. (B) TGF-β and caALK5 induce the PAI-1 promoter. MEECs were transfected with p800-luc reporter and caALK1 or caALK5, and stimulated or not with 5 ng/ml TGF-β3. After 20 h, luciferase activity was measured and values are corrected for transfection efficiency as measured by β-galactosidase activity. A representative experiment using triplicate samples is shown. (C) ALK5 ASO inhibit TGF-β3-induced PAI-1 promoter activity. MEECs were transfected with p800-luc reporter, loaded with oligonucleotides and stimulated or not with 5 ng/ml TGF-β3. After 20 h, luciferase activity was measured and values are corrected for transfection efficiency as measured by β-galactosidase activity. A representative experiment using triplicate samples is shown. (D) ALK5 is necessary for PAI-1 induction upon TGF-β stimulation. Wild-type or ALK5-deficient MEECs were stimulated with 5 ng/ml TGF-β3 for 6 h. Cells were lysed, fractionated by 7.5% SDS–PAGE and blotted. The filter was incubated with an anti-PAI-1 antibody that specifically recognizes PAI-1 protein. (E) TGF-β dose response on PAI-1 promoter activation. MEECs were transfected with p800-luc reporter and stimulated with increasing doses of TGF-β3. After 20 h, luciferase activity was measured and values are corrected for transfection efficiency as measured by β-galactosidase activity. A representative experiment using triplicate samples is shown.
The data above suggest that TGF-β signalling via ALK5 results in deposition of a layer of ECM proteins around the primitive vessels leading to maturation of the endothelium.
Discussion
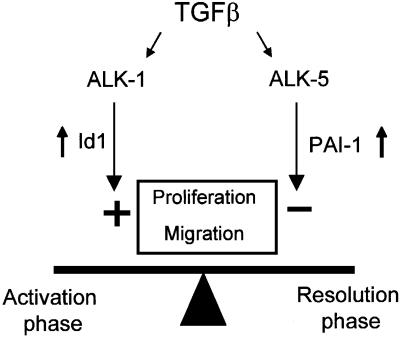
In the present study, we show that TGF-β in ECs can signal via two different type I receptors with opposite effects (Figure 7). Whereas activation of ALK5 by TGF-β results in inhibition of migration and proliferation, TGF-β-induced ALK1 activation results in increased migration and proliferation of ECs. The activation state of the endothelium may thus be dependent on the balance of ALK1 versus ALK5 activation that is induced by TGF-β. Our results provide a framework to understand previously conflicting reports in which pro- and anti-angiogenic properties were ascribed to TGF-β.
Fig. 7. Schematic figure of the functional role of ALK1 and ALK5 in ECs. TGF-β regulates the state of the endothelium via a fine balance between ALK5 and ALK1 signalling. Activation of ALK5 by TGF-β induces PAI-1 expression and inhibits migration and proliferation, whereas TGF-β-induced ALK1 activation induces Id1 expression and stimulates migration and proliferation. The ratio of TGF-β signals via ALK1 versus ALK5 will determine whether TGF-β will have a pro- or anti-angiogenic effect.
Both ALK1 and ALK5 are functional TGF-β type I receptors in endothelial cells. This statement is based upon our following observations: (i) whereas caALK1 induces Smad1/5 phosphorylation, caALK5 induces Smad2 phosphorylation in ECs; (ii) caALK1 cannot induce Smad2 phosphorylation, and caALK5 does not induce Smad1/5 phosphorylation, (iii) TGF-β induces phosphorylation of Smad1/5 and Smad2 in ECs, and these effects can be blocked upon selective inhibition of ALK1 or ALK5 expression, respectively; (iv) TGF-β only induces Smad1/5 phosphorylation in ECs as they express ALK1, but not in non-ECs that are ALK1 deficient; (v) the kinetics and dose response of TGF-β-induced Smad1/5 phosphorylation are distinct from TGF-β-induced Smad2 phosphorylation, suggesting that they may be occurring via distinct kinases; (vi) caALK1 or caALK5 induces or inhibits EC migration, respectively, and TGF-β-mediated induction or inhibition of EC migration is blocked by inhibiting ALK1 or ALK5 expression, respectively; and (vii) TGF-β activates the transcriptional reporters Id-luc and PAI-1-luc, which are selectively induced by caALK1 or caALK5 in ECs, respectively. Previous reports that TGF-β can bind to ALK1 and ALK5 in ECs (Oh et al., 2000) and that phenotypes of mice lacking ALK1 (Oh et al., 2000; Urness et al., 2000) or ALK5 (Larsson et al., 2001) and TβRII and Smad5-deficient mice are reminiscent of that of TGF-β1 null mice, which die in utero due to vascular defects (Goumans and Mummery, 2000) are fully consistent with our data.
Based upon the enhanced expression of angiogenic factors, such as vascular endothelial growth factor (VEGF), and their receptors, in ALK1 knockout mice, Oh et al. (2000) have proposed that ALK1 has an inhibitory effect on the state of the endothelium. This contrasts with our findings, which show that ALK1 stimulates migration and proliferation of ECs. The enhanced expression of angiogenic factors in ALK1-deficient mice may not be caused by a lack of ALK-1 signalling, but rather by secondary effects due to hypoxia caused by the altered vascular phenotype.
Previous observations have shown that low doses of TGF-β could promote EC migration, whereas high doses of TGF-β could inhibit EC migration (Pepper, 1997). Here we show that each phase of this biphasic dose–response curve of TGF-β on migration may be attributable to a distinct receptor, with ALK1 stimulating migration and ALK5 inhibiting migration. Consistent with this notion, we found that TGF-β-induced migration follows the same trend as TGF-β-induced Smad5 phosphorylation. How ever, we do not exclude the possibility that ALK5 activation at a ‘high’ dose of TGF-β affects ALK1 signalling downstream of Smad5 phosphorylation.
We show that the TGF-β/ALK1-induced migration occurs, in part, via up-regulation of Id1. Interference with ALK1 and Id1 expression abrogates TGF-β-induced migration of ECs. TGF-β/ALK1 activates the Id1 transcriptional reporter (Figure 6C), which is a Smad-dependent response (Korchynskyi and ten Dijke, 2002). This is consistent with the finding that the stimulatory effect of TGF-β/ALK1 on migration is Smad dependent (Figure 4B). Id proteins have been described as playing a central role in the control of mammalian cell growth, differentiation and tumorigenesis. Down-regulation of Id is necessary for terminal differentiation in many developmental processes including angiogenesis and tumour vascularization (Norton, 2000). Id1 has been reported to enhance proliferation, migration and invasiveness of mouse mammary ECs (Lin et al., 2000) and there is a strong correlation between Id1 expression and vascular invasion of breast cancer (Lin et al., 2000). More importantly, Id proteins are required for the proliferative and invasive phenotype of ECs during angiogenesis (Lyden et al., 1999). Besides mediating the stimulatory effect of TGF-β on migration, Id1 may also serve as an effector for the TGF-β/ALK1 pathway in mediating the stimulatory effect on proliferation.
PAI-1 is a potent inhibitor of vascular cell migration in vitro (Stefansson and Lawrence, 1996) and angiogenesis in the chick CAM in vivo (Stefansson et al., 2001). Since PAI-1 was identified as an ALK5-specific target, ALK5 signal transduction in ECs contributes to basement membrane formation, supporting a role for ALK5/Smad signalling in maturation of the blood vessel during the resolution phase.
It will be important to unravel how the expression and signalling activity of ALK1 and ALK5 is regulated since this, together with particular TGF-β concentrations, is important for determining whether endothelial cells become active or quiescent upon exposure to biologically active TGF-β. One possible candidate for this fine tuning is the accessory receptor endoglin. Just as we observed by interfering with the expression of ALK1, inhibition of endoglin expression was found to counteract the inhibitory effect of TGF-β on endothelial cell migration (Li et al., 2000). Ectopic expression of endoglin in cell lines has been reported to modulate their responses to TGF-β possibly by favouring signalling via the ALK1 pathway (Letamendia et al., 1998). Thus, both endoglin and ALK1 are positive signals for angiogenesis and negative regulators of ALK5 signalling. This, taken together with the similar knockout phenotypes in mice and clinical manifestations of HHT1 and HHT2, affecting loss of one allele of endoglin or ALK1, respectively, suggests that endoglin may potentiate the ALK1/Smad5 pathway. The importance of endoglin in pathogenic angiogenesis is illustrated by its strongly enhanced expression in ECs that undergo neovascularization (Bodey et al., 1998). We currently are analysing the expression of ALK1 in tumour endothelial cells and whether possible activation of its downstream pathway might be involved in TGF-β-induced tumour angiogenesis.
Materials and methods
Ligands and cells
Recombinant TGF-β1 and TGF-β3 were obtained from Drs N.Ferrara (Genetech, Inc.) and K.Iwata (OSI Pharmaceuticals), respectively. All assays were performed with both ligands with essentially the same results. Recombinant BMP6 was a gift from Dr K.Sampath (Curis, Inc.). MEECs and YS cells were cultured routinely as previously described (Larsson et al., 2001). COS-7 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal calf serum (FCS), l-glutamine and penicillin/streptomycin. Primary BAECs were cultured in low-glucose DMEM (Gibco-BRL) with 10% calf serum, l-glutamine and antibiotics, and grown in a 10% CO2-containing atmosphere.
RNA isolation and RT–PCR
Total RNA was isolated using RNeasy columns (Qiagen) according to the manufacturer’s instructions. RT–PCRs were performed as described by Goumans et al. (1999). The PCRs were performed using a PTC-200 Peltier thermal cycler (MJ Research). The PCR primers used have been described previously (Goumans et al., 1999; Rosendahl et al., 2001).
Metabolic labelling and [32P]orthophosphate labelling of ECs, and SDS–PAGE
ECs were metabolically labelled and immunoprecipitated, or [32P]orthophosphate labelled as described by Nakao et al. (1997). Cell extracts were subjected to immunoprecipitation with anti-ALK1 (ten Dijke et al., 1994) or anti-Smad5 antibody (Tamaki et al., 1998) and separated by SDS–PAGE. The gels were fixed, dried and autoradiography was performed.
Tissue collection and immunohistochemistry
Tissue collection and immunohistochemistry were performed as described by Rosendahl et al. (2001).
Adenoviral infection of ECs
ECs were infected with adenovirus expressing LacZ, caALK1 or caALK5 using a multiplicity of infection (m.o.i.) of 500. After 16 h, the cells were washed and allowed to recover for 24 h prior to use in the indicated assays.
Western blot analysis
Western blot analysis was performed as described by Larsson et al. (2001). PSmad1 and PSmad2 antibodies specifically recognize phosphorylated Smad1/5 or phosphorylated Smad2 in transfected COS cells (Persson et al., 1998) and non-transfected ECs (Figure 1A). Recognition of phosphorylated Smad2 by PSmad2 antibody can be blocked by pre-incubating this antibody with cognate phosphorylated Smad2 peptide, but not with equivalent non-phosphorylated Smad2 peptide or phosphorylated Smad1 peptide. Recognition of phosphorylated Smad1 by PSmad1 antibody could be blocked by cognate phosphorylated Smad1 peptide, but not with equivalent non-phosphorylated Smad1 peptide or phosphorylated Smad2 peptide (Figure 1 and data not shown). Primary antibodies against Smad1, Smad2 (Nakao et al., 1997), Smad5 (Tamaki et al., 1998), Id1 (Santa Cruz) and PAI-1 (Santa Cruz) have been described previously. Detection was performed by enhanced chemoluminescence (ECL).
Transient transfections and transcriptional reporter assays
MEECs were transfected with 0.5 µg of (CAGA)12-luc (Dennler et al., 1998), 0.5 µg of (SBE)4-luc (Jonk et al., 1998), 0.5 µg of Id1-luc (Tournay and Benezra, 1996) or 0.5 µg of p800-luc, in the absence or presence of an expression plasmid encoding caALK5 or caALK1. The assay was performed as described by Larsson et al. (2001).
EC proliferation assay
ECs were seeded in DMEM containing 1% serum at a density of 2 × 104 cells per 24-well plate. The next day, TGF-β was added at 2.5 ng/ml. After 5 days, cells were trypsinized and cell number per well was counted using a Casy-1 cell counter (Schäfer Systems).
EC migration assay
Migration (chemokinesis) was measured using a Boyden chamber. A Coster nucleopore filter (8 µm pore) was coated with fibronectin overnight at 4°C. The chamber was washed with phosphate-buffered saline (PBS) and the lower chamber was filled with DMEM with or without serum and with or without TGF-β3. Cells were trypsinized and suspended at a final concentration of 50 000 cells/ml in DMEM. A 150 µl aliquot of the cell suspension was added to the upper chamber and incubated at 37°C. After 16 h, the cells were washed and the upper surface was wiped to remove the non-migrating cells. The membranes were fixed in methanol, washed with water, stained and the numbers of cells present on the lower surface were counted.
Scratch assay
Adenoviral infected BAECs were grown to confluence, after which an ∼100 µm scratch was made. Closure of the wound was followed by time-lapse microscopy with a 15 min interval for 16 h.
EC loading of oligonucleotides
The following sequence-specific S-oligonucleotides were used: ALK1 SO, 5′- TGGGTAGAGGGAGTGAAG-3′; ALK1 ASO, 5′-CTTCACTCCCTCTACCCA-3′; ALK5 SO: 5′-GCGGCGGGACCATGGAGGCGG-3′; ALK5 ASO, 5′-CGCCGCCCTGGTACCTCCGCC-3′; Id1 SO, 5′-GTAGAGAAATGGGAACGC-3′; and Id1 ASO, 5′-GCGTTCCCATTTCTCTAC-3′. MEECs were loaded with oligonucleotides using Influx pinocytic cell-loading reagent according to the manufacturer’s procedures (Molecular Probes). After loading, the cells were allowed to recover for at least 10 min before being used.
Acknowledgments
Acknowledgements
We thank Martijn Brugman and Midory Thorikay for expert technical assistance, and Drs Carl-Henrik Heldin, Christine Mummery, Kohei Miyazono and Tatsuru Ohta for valuable discussions. We are grateful to Drs N.Ferrara, K.Iwata and K.Sampath for recombinant ligands, S.Karlsson, M.Pepper and J.Plouet for endothelial cells, A.Haimovitz-Friedman for BAECs, R.Benezra for the Id1 promoter construct, and Santa Cruz Biotechnology for Id1 antibody. This work was supported by grants from The Netherlands Heart Foundation (grant 99-046), Dutch Cancer Society (NKI 2000-22117), Dutch Organisation for Scientific Research (MW 902-16-295) and Ludwig Institute for Cancer Research to P.t.D.
References
- Ananth S., Knebelmann,B., Gruning,W., Dhanabal,M., Walz,G., Stillman,I.E. and Sukhatme,V.P. (1999) Transforming growth factor β1 is a target for the von Hippel–Lindau tumor suppressor and a critical growth factor for clear cell renal carcinoma. Cancer Res., 59, 2210–2216. [PubMed] [Google Scholar]
- Attisano L., Carcamo,J., Ventura,F., Weis,F.M., Massagué,J. and Wrana,J.L. (1993) Identification of human activin and TGFβ type I receptors that form heteromeric kinase complexes with type II receptors. Cell, 75, 671–680. [DOI] [PubMed] [Google Scholar]
- Basson C.T., Kocher,O., Basson,M.D., Asis,A. and Madri,J.A. (1992) Differential modulation of vascular cell integrin and extracellular matrix expression in vitro by TGF-β1 correlates with reciprocal effects on cell migration. J. Cell. Physiol., 153, 118–128. [DOI] [PubMed] [Google Scholar]
- Bodey B., Bodey,B.,Jr, Siegel,S.E. and Kaiser,H.E. (1998) Over-expression of endoglin (CD105): a marker of breast carcinoma-induced neo-vascularization. Anticancer Res., 18, 3621–3628. [PubMed] [Google Scholar]
- Carmeliet P. (2000) Mechanisms of angiogenesis and arteriogenesis. Nature Med., 6, 389–395. [DOI] [PubMed] [Google Scholar]
- Chen Y.G. and Massagué,J. (1999) Smad1 recognition and activation by the ALK1 group of transforming growth factor-β family receptors. J. Biol. Chem., 274, 3672–3677. [DOI] [PubMed] [Google Scholar]
- Dennler S., Itoh,S., Vivien,D., ten Dijke,P., Huet,S. and Gauthier,J.M. (1998) Direct binding of Smad3 and Smad4 to critical TGFβ-inducible elements in the promoter of human plasminogen activator inhibitor-type 1 gene. EMBO J., 17, 3091–3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck R., Zhang,Y. and Feng,X.H. (1998) Smads: transcriptional activators of TGF-β responses. Cell, 95, 737–740. [DOI] [PubMed] [Google Scholar]
- Fajardo L.F., Prionas,S.D., Kwan,H.H., Kowalski,J. and Allison,A.C. (1996) Transforming growth factor β1 induces angiogenesis in vivo with a threshold pattern. Lab. Invest., 74, 600–608. [PubMed] [Google Scholar]
- Folkman J. and D’Amore,P.A. (1996) Blood vessel formation: what is its molecular basis? Cell, 87, 1153–1155. [DOI] [PubMed] [Google Scholar]
- Gajdusek C.M., Luo,Z. and Mayberg,M.R. (1993) Basic fibroblast growth factor and transforming growth factor β-1: synergistic mediators of angiogenesis in vitro. J. Cell. Physiol., 157, 133–144. [DOI] [PubMed] [Google Scholar]
- Goumans M.-J. and Mummery,C. (2000) Functional analysis of the TGFβ receptor/Smad pathway through gene ablation in mice. Int. J. Dev. Biol., 44, 253–265. [PubMed] [Google Scholar]
- Goumans M.-J., Zwijsen,A., van Rooijen,M.A., Huylebroeck,D., Roelen,B.A. and Mummery,C.L. (1999) Transforming growth factor-β signalling in extraembryonic mesoderm is required for yolk sac vasculogenesis in mice. Development, 126, 3473–3483. [DOI] [PubMed] [Google Scholar]
- Hirschi K.K., Rohovsky,S.A. and D’Amore,P.A. (1998) PDGF, TGF-β and heterotypic cell–cell interactions mediate endothelial cell-induced recruitment of 10T1/2 cells and their differentiation to a smooth muscle fate. J. Cell Biol., 141, 805–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollnagel A., Oehlmann,V., Heymer,J., Ruther,U. and Nordheim,A. (1999) Id genes are direct targets of bone morphogenetic protein induction in embryonic stem cells. J. Biol. Chem., 274, 19838–19845. [DOI] [PubMed] [Google Scholar]
- Iruela-Arispe M.L. and Sage,E.H. (1993) Endothelial cells exhibiting angiogenesis in vitro proliferate in response to TGF-β1. J. Cell Biochem., 52, 414–430. [DOI] [PubMed] [Google Scholar]
- Johnson D.W. et al. (1996) Mutations in the activin receptor-like kinase 1 gene in hereditary haemorrhagic telangiectasia type 2. Nature Genet., 13, 189–195. [DOI] [PubMed] [Google Scholar]
- Jonk L.J., Itoh,S., Heldin,C.-H., ten Dijke,P. and Kruijer,W. (1998) Identification and functional characterization of a Smad binding element (SBE) in the JunB promoter that acts as a transforming growth factor-β, activin and bone morphogenetic protein-inducible enhancer. J. Biol. Chem., 273, 21145–21152. [DOI] [PubMed] [Google Scholar]
- Koh G.Y., Kim,S.J., Klug,M.G., Park,K., Soonpaa,M.H. and Field,L.J. (1995) Targeted expression of transforming growth factor-β1 in intracardiac grafts promotes vascular endothelial cell DNA synthesis. J. Clin. Invest., 95, 114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korchynskyi O. and ten Dijke,P. (2002) Identification and functional characterization of distinct critically important BMP-specific response elements in the Id1 promoter. J. Biol. Chem., 277, 4883–4891. [DOI] [PubMed] [Google Scholar]
- Larsson J. et al. (2001) Abnormal angiogenesis but intact hematopoietic potential in TGF-β type I receptor-deficient mice. EMBO J., 20, 1663–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letamendia A., Lastres,P., Botella,L.M., Raab,U., Langa,C., Velasco,B., Attisano,L. and Bernabeu,C. (1998) Role of endoglin in cellular responses to transforming growth factor-β. A comparative study with β-glycan. J. Biol. Chem., 273, 33011–33019. [DOI] [PubMed] [Google Scholar]
- Li C., Hampson,I.N., Hampson,L., Kumar,P., Bernabeu,C. and Kumar,S. (2000) CD105 antagonizes the inhibitory signaling of transforming growth factor β1 on human vascular endothelial cells. FASEB J., 14, 55–64. [DOI] [PubMed] [Google Scholar]
- Lin C.Q., Singh,J., Murata,K., Itahana,Y., Parrinello,S., Liang,S.H., Gillett,C.E., Campisi,J. and Desprez,P.Y. (2000) A role for Id-1 in the aggressive phenotype and steroid hormone response of human breast cancer cells. Cancer Res., 60, 1332–1340. [PubMed] [Google Scholar]
- Lyden D. et al. (1999). Id1 and Id3 are required for neurogenesis, angiogenesis and vascularization of tumour xenografts. Nature, 401, 670–677. [DOI] [PubMed] [Google Scholar]
- Madri J.A., Bell,L. and Merwin,J.R. (1992) Modulation of vascular cell behavior by transforming growth factors β. Mol. Reprod. Dev., 32, 121–126. [DOI] [PubMed] [Google Scholar]
- Massagué J. (1998) TGF-β signal transduction. Annu. Rev. Biochem., 67, 753–791. [DOI] [PubMed] [Google Scholar]
- McAllister K.A. et al. (1994) Endoglin, a TGF-β binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. Nature Genet., 8, 345–351. [DOI] [PubMed] [Google Scholar]
- Muller G., Behrens,J., Nussbaumer,U., Bohlen,P. and Birchmeier,W. (1987) Inhibitory action of transforming growth factor β on endothelial cells. Proc. Natl Acad. Sci. USA, 84, 5600–5604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao A. et al. (1997) TGF-β receptor-mediated signalling through Smad2, Smad3 and Smad4. EMBO J., 16, 5353–5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton J.D. (2000) ID helix–loop–helix proteins in cell growth, differentiation and tumorigenesis. J. Cell Sci., 113, 3897–3905. [DOI] [PubMed] [Google Scholar]
- Oh S.P. et al. (2000) Activin receptor-like kinase 1 modulates transforming growth factor-β1 signaling in the regulation of angiogenesis. Proc. Natl Acad. Sci. USA, 97, 2626–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper M.S. (1997) Transforming growth factor-β: vasculogenesis, angiogenesis and vessel wall integrity. Cytokine Growth Factor Rev., 8, 21–43. [DOI] [PubMed] [Google Scholar]
- Pepper M.S., Vassalli,J.D., Orci,L. and Montesano,R. (1993) Biphasic effect of transforming growth factor-β1 on in vitro angiogenesis. Exp. Cell Res., 204, 356–363. [DOI] [PubMed] [Google Scholar]
- Persson U., Izumi,H., Souchelnytskyi,S., Itoh,S., Grimsby,S., Engström,U., Heldin,C.-H., Funa,K. and ten Dijke,P. (1998) The L45 loop in type I receptors for TGF-β family members is a critical determinant in specifying Smad isoform activation. FEBS Lett., 434, 83–87. [DOI] [PubMed] [Google Scholar]
- Plouet J. and Gospodarowicz,D. (1989) Transforming growth factor β-1 positively modulates the bioactivity of fibroblast growth factor on corneal endothelial cells. J. Cell. Physiol., 141, 392–399. [DOI] [PubMed] [Google Scholar]
- Risau W. (1997) Mechanisms of angiogenesis. Nature, 386, 671–674. [DOI] [PubMed] [Google Scholar]
- Roberts A.B. et al. (1986) Transforming growth factor type β: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc. Natl Acad. Sci. USA, 83, 4167–4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosendahl A., Checchin,D., Fehniger,T.E., ten Dijke,P., Heldin,C.-H. and Sideras,P. (2001) Activation of the TGF-β/activin-Smad2 pathway during allergic airway inflammation. Am. J. Respir. Cell Mol. Biol., 25, 60–68. [DOI] [PubMed] [Google Scholar]
- Sato Y. and Rifkin,D.B. (1989) Inhibition of endothelial cell movement by pericytes and smooth muscle cells: activation of a latent transforming growth factor-β1-like molecule by plasmin during co-culture. J. Cell Biol., 109, 309–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawdey M., Podor,T.J. and Loskutoff,D.J. (1989) Regulation of type 1 plasminogen activator inhibitor gene expression in cultured bovine aortic endothelial cells. Induction by transforming growth factor-β, lipopolysaccharide and tumor necrosis factor-α. J. Biol. Chem., 264, 10396–10401. [PubMed] [Google Scholar]
- Stefansson S. and Lawrence,D.A. (1996) The serpin PAI-1 inhibits cell migration by blocking integrin αVβ3 binding to vitronectin. Nature, 383, 441–443. [DOI] [PubMed] [Google Scholar]
- Stefansson S., Petitclerc,E., Wong,M.K., McMahon,G.A., Brooks,P.C. and Lawrence,D.A. (2001) Inhibition of angiogenesis in vivo by plasminogen activator inhibitor-1. J. Biol. Chem., 276, 8135–8141. [DOI] [PubMed] [Google Scholar]
- Tamaki K., Souchelnytskyi,S., Itoh,S., Nakao,A., Sampath,K., Heldin,C.-H. and ten Dijke,P. (1998) Intracellular signaling of osteogenic protein-1 through Smad5 activation. J. Cell. Physiol., 177, 355–363. [DOI] [PubMed] [Google Scholar]
- ten Dijke P., Yamashita,H., Ichijo,H., Franzén,P., Laiho,M., Miyazono,K. and Heldin,C.-H. (1994) Characterization of type I receptors for transforming growth factor-β and activin. Science, 264, 101–104. [DOI] [PubMed] [Google Scholar]
- Tournay O. and Benezra,R. (1996) Transcription of the dominant-negative helix–loop–helix protein Id1 is regulated by a protein complex containing the immediate-early response gene Egr-1. Mol. Cell. Biol., 16, 2418–2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urness L.D., Sorensen,L.K. and Li,D.Y. (2000) Arteriovenous malformations in mice lacking activin receptor-like kinase-1. Nature Genet., 26, 328–331. [DOI] [PubMed] [Google Scholar]
- Vernon R.B. and Sage,E.H. (1999) A novel, quantitative model for study of endothelial cell migration and sprout formation within three-dimensional collagen matrices. Microvasc. Res., 57, 118–133. [DOI] [PubMed] [Google Scholar]
- Yang E.Y. and Moses,H.L. (1990) Transforming growth factor β1-induced changes in cell migration, proliferation and angiogenesis in the chicken chorioallantoic membrane. J. Cell Biol., 111, 731–741. [DOI] [PMC free article] [PubMed] [Google Scholar]