Long-Chain Base Kinase Lcb4 Is Anchored to the Membrane through Its Palmitoylation by Akr1 (original) (raw)
Abstract
Sphingoid long-chain base kinase Lcb4 catalyzes the production of the bioactive lipid molecules the long-chain base 1-phosphates. Although Lcb4 has no apparent transmembrane-spanning domain, it is tightly associated with the membrane. Here, we demonstrate that Lcb4 is modified by palmitoylation. This modification was greatly reduced in mutants for AKR1, which was recently identified as encoding a protein acyltransferase. In vitro experiments revealed that Akr1 indeed acts as a protein acyltransferase for Lcb4. Studies using site-directed mutagenesis indicated that Cys-43 and Cys-46 are palmitoylated. The loss of palmitoylation on Lcb4 caused several effects, including mislocalization of the protein to the cytosol, reduced phosphorylation, and loss of downregulation during the stationary phase. Although Akr2 is highly homologous to Akr1, the deletion of AKR2 did not result in any remarkable phenotypes. However, overproduction of Akr2 resulted in reduced amounts of Lcb4. We demonstrated that Akr2 is an unstable protein and is degraded in the vacuole. Akr2 exhibits high affinity for Lcb4, and in Akr2-overproducing cells this interaction caused unusual delivery of Lcb4 to the vacuole and degradation.
Many proteins are posttranslationally modified by the addition of lipids such as fatty acids, isoprenoids, and glycosylphosphatidylinositol. These lipid modifications have been shown to regulate protein stability, intracellular localization, and activities, as well as protein-protein interactions and protein-lipid interactions (4, 7, 35). Protein modification by fatty acids is mediated through amide (N acylation) linkages on N-terminal glycine residues or through thioester linkages (S acylation) on cysteine residues. Palmitoylation is the most commonly found S acylation, and many signaling proteins are thus modified (7, 35). There are generally no exact motifs for palmitoylation, although these proteins are often categorized based on the site of modification (36). Palmitoylation can regulate localization of the protein within the plasma membrane, often in lipid microdomains (7, 35).
The DHHC cysteine-rich domain (CRD) is a highly conserved region found in multiple proteins in most organisms (34). Yeast genetic approaches have recently identified two DHHC CRD-containing proteins, Akr1 and Erf2, as protein acyltransferases (PATs) (25, 26, 38). Akr1 is an 86-kDa integral membrane protein with six transmembrane segments (32) and six ankyrin motifs in its large hydrophilic N-terminal domain (19). The DHHC CRD domain is located in the cytoplasmic loop between the fifth and sixth transmembrane domains (32); since the Cys residue of this motif has been suggested to form an acyl-intermediate, this domain would appear to constitute an active site (26, 38). Akr1 is responsible for the palmitoylation of the C-terminal two Cys residues of the type I casein kinase Yck2, and in the Δ_akr1_ mutant the plasma membrane localization of Yck2 is abolished (2, 38). In addition, deletion of the AKR1 gene causes pleiotropic effects in yeast (8, 11, 19, 33). There is also increasing evidence in mammals that DHHC CRD proteins act as PATs (6, 9, 14, 21). For instance, the mammalian homolog of Akr1, HIP14/AKRL1, reportedly possesses PAT activity for H-/N-RAS and for several neuronal proteins (6, 14).
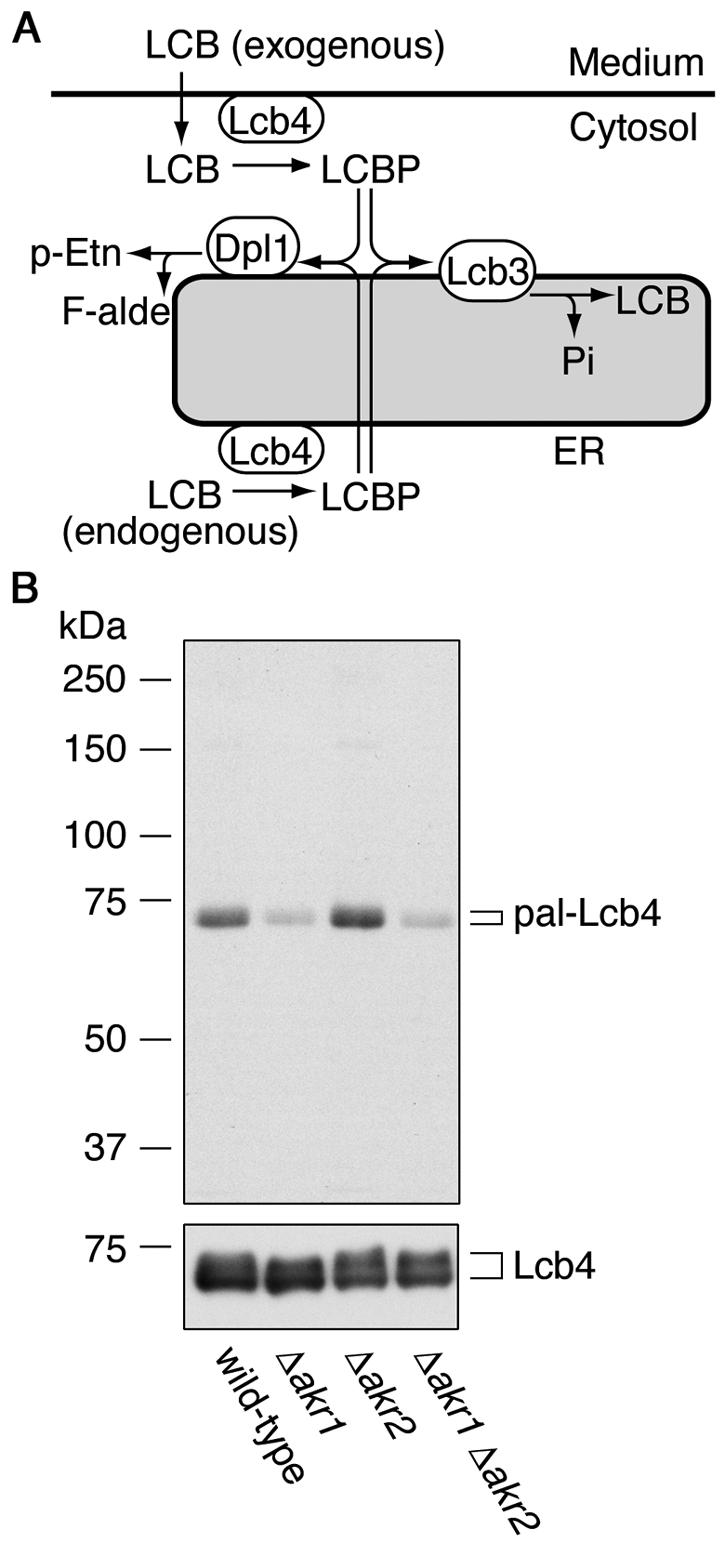
Sphingoid long-chain base 1-phosphates (LCBPs) are bioactive lipid molecules found in most organisms. The yeast Saccharomyces cerevisiae contains two LCBPs, dihydrosphingosine 1-phosphate and phytosphingosine (PHS) 1-phosphate, which have known roles in heat stress resistance, diauxic shift, and Ca2+ mobilization (3, 12, 27, 28, 41). Formation of LCBPs is catalyzed by the long-chain base (LCB) kinases Lcb4 (primarily) and Lcb5 (30). LCBPs are degraded either to fatty aldehydes and phosphoethanolamine by LCBP lyase (Dpl1) (39) or to LCBs by LCBP phosphatase (Lcb3) (29) (Fig. 1A). Studies found that a Δ_dpl1_ Δ_lcb3_ Δ_lcb4_ triple deletion mutant grew normally, yet a Δ_dpl1_ Δ_lcb3_ double mutant did not, indicating that an overaccumulation of LCBPs is cytotoxic (24, 43). Currently, the molecular mechanism of this toxicity and the target molecules of LCBPs are unknown.
FIG. 1.
Lcb4 is palmitoylated and Akr1 is involved. (A) Pathways for LCB and LCBP metabolism. Exogenous LCB is imported into the cells and then converted to LCBP by the kinase Lcb4. LCBP is then degraded in the ER via one of two pathways: conversion to long-chain fatty aldehyde (F-alde) and phosphoethanolamine (p-Etn) by the lyase Dpl1 or conversion to LCB by the phosphatase Lcb3. (B) KA311A (wild type), KHY689 (Δ_akr1_), KHY672 (Δ_akr2_), and KHY690 (Δ_akr1_ Δ_akr2_) cells, each harboring pAK514 (GAL1 promoter-LCB4), were grown at 30°C in SC medium lacking uracil. Lcb4 expression was induced by using YPGal medium and, after 1 h, cells were labeled with 0.3 mCi of [3H]palmitic acid for 2 h at 30°C. Lcb4 was immunoprecipitated from total cell lysates (equal in radioactivity) with anti-Lcb4 antibodies and separated by SDS-PAGE, followed by autoradiography (upper panel) or by immunoblotting with anti-Lcb4 antibodies (lower panel). pal-Lcb4, palmitoylated Lcb4.
In the present study, we found that Lcb4 is palmitoylated at Cys-43 and Cys-46 and that this palmitoylation plays an important function in the localization and downregulation of the protein. We further identified Akr1 as being responsible for the palmitoylation of Lcb4. In addition, we determined that another DHHC CRD-containing protein, Akr2, exhibits high affinity for Lcb4 and delivers it to the vacuole for degradation.
MATERIALS AND METHODS
Yeast strains and media.
S. cerevisiae strains used are listed in Table 1. The Δ_dpl1_::TRP1, Δ_lcb4_::LEU2, and Δ_pep4_::LEU2 constructions were described previously (1, 22, 23). The Δ_akr1_::URA3 and Δ_akr1_::LEU2 cells were constructed by replacing the 1.2-kb MscI-HindIII region in the AKR1 gene with the URA3 and LEU2 markers, respectively. The Δ_pep4_::KanMX4, Δ_akr2_::KanMX4, and Δ_lcb4_::KanMX4 cells were constructed by replacing each of their entire open reading frames (ORFs) with the KanMX4 marker. For construction of the Δ_lcb4_::TRP1 cells, the 1.1-kb HpaI-KpnI region in the LCB4 gene was replaced with the TRP1 marker. The cells were grown either in YPD medium (1% yeast extract, 2% peptone, and 2% glucose), YPGal medium (1% yeast extract, 2% peptone, and 2% galactose), or synthetic complete (SC) medium (0.67% yeast nitrogen base and 2% glucose) containing nutritional supplements.
TABLE 1.
Strains used in this study
| Strain | Genotype | Source or reference |
|---|---|---|
| SEY6210 | _MAT_α leu2-3,112 ura3-52 his3_-Δ_200 trp1_-Δ_901 lys2-801 suc2_-Δ_9 | 37 |
| KHY21 | SEY6210, Δ_dpl1_::TRP1 Δ_lcb3_::LEU2 sup | 22 |
| KHY92 | SEY6210, Δ_dpl1_::TRP1 Δ_lcb3_::LEU2 Δ_rsb1_::HIS3 sup | This study |
| KHY195 | SEY6210, Δ_pep4_::KanMX4 | 42 |
| KHY197 | SEY6210, Δ_akr2_::KanMX4 | This study |
| KHY199 | SEY6210, Δ_pep4_::LEU2 Δ_akr2_::KanMX4 | This study |
| KHY211 | SEY6210, Δ_akr1_::URA3 | This study |
| KHY212 | SEY6210, Δ_pep4_::KanMX4 Δ_akr1_::URA3 | This study |
| KHY543 | SEY6210, Δ_pep4_::LEU2 Δ_akr1_::URA3 Δ_akr2_::KanMX4 | This study |
| KHY544 | SEY6210, Δ_dpl1_::TRP1 Δ_akr1_::URA3 Δ_akr2_::KanMX4 | This study |
| SIY03 | SEY6210, Δ_lcb4_::TRP1 | This study |
| TVY1 | SEY6210, Δ_pep4_::LEU2 | 1 |
| TS11 | SEY6210, Δ_pep4_::LEU2 Δ_lcb4_::TRP1 | This study |
| KA311A | MATaura3 leu2 his3 trp1 | 15 |
| KHY672 | KA311A, Δ_akr2_::KanMX4 | This study |
| KHY689 | KA311A, Δ_akr1_::LEU2 | This study |
| KHY690 | KA311A, Δ_akr1_::LEU2 Δ_akr2_::KanMX4 | This study |
| FKY146 | KA311A, Δ_pep4_::LEU2 Δ_lcb4_::KanMX4 | This study |
| PJ69-4A | MATa_trp1-901 leu2-3,112 ura3-52 his3-200 gal4_Δ _gal80_Δ LYS2::GAL1-HIS3 GAL2-ADE2 met2::GAL7-lacZ | 18 |
Isolation of PHS-resistant multicopy suppressors.
Genomic DNA was prepared from KHY92 cells and partially digested with Sau3AI. The 4- to 10-kb fragments were ligated into the BamHI site of pRS426 (URA3 marker) (5). The yeast genomic library obtained was introduced into KHY21 cells, and transformants were selected at 30°C on SC medium lacking uracil. About 60,000 obtained colonies were pooled and diluted. Approximately 300,000 cells per plate were added to YPD plates containing 6 μM PHS (BIOMOL, Plymouth Meeting, PA) and 0.0015% Nonidet P-40 as a dispersant, followed by incubation at 30°C for 60 h. Plasmids were prepared from 41 PHS-resistant clones. These then were reintroduced into KHY21 cells, and the PHS resistance of the resulting transformants was examined. Eight plasmids again conferred PHS resistance on KHY21 cells. Sequence analysis revealed that seven plasmids contained the RSB2 (YKR088c) gene and one contained the AKR2 (YOR034c) gene. In the course of the subcloning studies, pAK222 (RSB2) and pAK234 (AKR2) plasmids were constructed by cloning a 2.3-kb DraI-DraI fragment of the RSB2 region and a 4.2-kb HpaI-EcoRI fragment of the AKR2 region, respectively, into pRS426.
Plasmid construction.
Yeast centromere-based (CEN) low-copy-number plasmids pRS313 (HIS3 marker), pRS314 (TRP1), and pRS315 (LEU2) (40) and 2μm-based high-copy-number plasmids pRS423 (HIS3) and pRS426 (URA3) (5) were used as vectors. The pWK79 (LCB4 2μm HIS3 marker) and pWK78 (LCB4, CEN, HIS3 marker) plasmids were described previously (17).
The pFK18 (LCB4-C1), pFK19 (LCB4-C2), pFK20 (LCB4-C3), pFK21 (LCB4-C4), pFK22 (LCB4-C5), pFK23 (LCB4-C6), pFK24 (LCB4-C7), pFK25 (LCB4-C8), pFK26 (LCB4-C9), pFK27 (LCB4-C10), pFK28 (LCB4-C11), and pFK29 (LCB4-C12) plasmids were constructed by site-directed mutagenesis using a QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA) and appropriate primers from pWK78. The pFK33 (LCB4-C1C2) plasmid was likewise constructed using the pFK18 plasmid as a template.
The pAK243 (AKR1) plasmid was constructed by cloning the AKR1 gene, amplified from SEY6210 genomic DNA, into pGEM-T Easy (Promega, Madison, WI). The 3.3-kb NotI-NotI fragment of the resulting plasmid (pAK239) was then cloned into the NotI site of pRS426, generating pAK243.
The pAK439 plasmid, a derivative of pRS423, was constructed to produce a C-terminally triple FLAG (3xFLAG)-tagged fusion protein. For the pAK456 plasmid (AKR1-3xFLAG), the AKR1 gene was amplified by using genomic DNA prepared from SEY6210 cells. The amplified fragment was cloned into pGEM-T Easy to generate pAK240. The pAK456 plasmid was produced by cloning the 3.2-kb SpeI-SpeI fragment of pAK240 into the SpeI site of pAK439. The pAK783 (AKR1-C500S-3xFLAG) plasmid was constructed by site-directed mutagenesis using a QuikChange site-directed mutagenesis kit from pAK456. To prepare the pAK457 plasmid (AKR2-3xFLAG), the AKR2 gene was amplified with genomic DNA as a template and then cloned into pGEM-T Easy. The resulting pAK242 plasmid was digested with SpeI, and the fragment was cloned into the SpeI site of pAK439 to create pAK457.
The pYES2 plasmid (Invitrogen) is a 2μm-based cloning vector (URA3 marker) under the control of the GAL1 promoter. The pAK514 (LCB4), pFK72 (LCB4-C1), pFK73 (LCB4-C2), and pFK74 (LCB4-C1C2) plasmids are derivatives of pYES2 and were constructed by amplifying the corresponding genes from the pWK78, pFK18, pFK19, and pFK33 plasmids. The resulting fragments were digested and cloned into the HindIII site of pYES2.
The pAK533 (3xFLAG-LCB4) and pFK53 (3xFLAG-LCB4-C1C2) plasmids were constructed by using the pAKNF314 plasmid, a _CEN-_based cloning vector (TRP1 marker) designed for N-terminal 3xFLAG-tagged protein expression under the control of the TDH3 promoter. The LCB4 gene was amplified by using the pWK78 plasmid as a template, and the resulting fragment was then cloned into the HindIII-BamHI site of pAKNF314, generating pAK533. pFK53 was constructed by cloning the 1.0-kb NdeI-MscI fragment of pFK33 into the NdeI-MscI site of pAK533.
For two-hybrid analysis, the pGAD-C1 (LEU2 marker) (18) and pGBDU-C1 (URA3 marker) (18) plasmids, which encode the GAL4 activation domain (AD) and GAL4 DNA-binding domain (BD), respectively, were used. The pWK05 (BD-LCB4), pAK318 (AD-AKR1), pAK312 (AD-AKR2), pAK320 (_AD-AKR1-Δ_C), pAK322 (_AD-AKR2-Δ_C), pAK779 (_AD-AKR1-Δ_N), pAK782 (_AD-AKR2-Δ_N), pAK780 (AD-AKR1-C500S), and pAK781 (AD-AKR2-C476S) plasmids were constructed by cloning the respective genes into the pGAD-C1 or pGBDU-C1 vector. The AKR1_-Δ_N, AKR1_-Δ_C, AKR2_-Δ_N, and AKR2_-Δ_C constructs were designed to lack amino acids 3 to 278, 324 to 765, 3 to 254, and 300 to 749, respectively.
In vivo [3H]palmitic acid labeling.
Cells were grown to confluence at 30°C in SC medium lacking uracil. Cells (ca. 6 × 107) were collected by centrifugation, washed and suspended in YPGal medium (3 ml), and incubated at 30°C for 30 min. Cells were then incubated with 3 μg of cerulenin/ml at 30°C for 30 min to inhibit fatty acid synthesis. After labeling with 0.3 mCi of [3H]palmitic acid (60 Ci/mmol; American Radiolabeled Chemical, St. Louis, MO) at 30°C for 2 h, cells were chilled on ice. Cell lysates were then prepared as described previously (22), and Lcb4 was immunoprecipitated from the lysates with equal levels of radioactivity using anti-Lcb4 antiserum (17) and protein A-Sepharose (Amersham Biosciences, Piscataway, NJ). Immunoprecipitates eluted with sodium dodecyl sulfate (SDS) sample buffer containing 10 mM 2-mercaptoethanol was separated by SDS-polyacrylamide gel electrophoresis (PAGE) and visualized by autoradiography using the fluorographic reagent EN3HANCE (Perkin-Elmer Life Sciences, Ontario, Canada).
Immunoblotting.
Immunoblotting was performed as described previously (22). Affinity-purified anti-Lcb4 (1:1,000 dilution) (17), anti-FLAG M2 (1 μg/ml; Stratagene), anti-Pgk1 (0.25 μg/ml; Molecular Probes, Eugene, OR), and anti-Dpm1 (2.5 μg/ml; Molecular Probes) antibodies were used as primary antibodies. Peroxidase-conjugated donkey anti-rabbit immunoglobulin G (IgG) F(ab′)2 fragment (1:7,500 dilution) and sheep anti-mouse IgG F(ab′)2 fragment (1:7,500 dilution) were used as secondary antibodies. Labeling was detected with an ECL detection kit (Amersham Biosciences).
As needed, electrophoresis was performed over a long period of time to more clearly separate bands of hyperphosphorylated and hypophosphorylated Lcb4. Protein dephosphorylation was performed with alkaline phosphatase as described previously (17).
Immunofluorescence microscopy.
Microscopic immunofluorescence analysis was done as described previously (22) with affinity-purified anti-Lcb4 antibodies (1:100) and Alexa Fluor 488 goat anti-rabbit IgG(H+L)-conjugated antibody (5 μg/ml; Molecular Probes). The stained cells were analyzed by fluorescence microscopy (Axioskop 2 PLUS; Carl Zeiss, Oberkochen, Germany).
Cytosol and membrane preparations.
To convert cells to spheroplasts, cells (ca. 2 × 108) were harvested, suspended in 1 ml of 100 mM Tris-HCl (pH 9.4) containing 40 mM 2-mercaptoethanol, and incubated for 10 min at room temperature. After centrifugation, cells were suspended in 2 ml of 50 mM Tris-HCl (pH 7.5) containing 1.2 M sorbitol and incubated with 50 μg of zymolyase 100T (Seikagaku Kogyo, Tokyo, Japan)/ml for 30 min at 30°C. The resulting spheroplasts were collected by centrifugation, suspended in buffer A (20 mM HEPES-NaOH [pH 7.5], 0.1 M sorbitol, 150 mM potassium acetate, 2 mM EDTA, 1 mM dithiothreitol, 1× protease inhibitor cocktail [Complete; Roche Diagnostics, Indianapolis, IN], and 1 mM phenylmethylsulfonyl fluoride), and sonicated. Unbroken cells were removed by centrifugation at 1,200 × g for 3 min at 4°C. The resulting supernatant was used as the total cell lysate or separated into membrane and soluble fractions by centrifugation at 100,000 × g for 1 h at 4°C.
Immunoprecipitation.
Spheroplasts were prepared as described above, lysed, and treated with 1% Triton X-100. Proteins were immunoprecipitated by using an M2 affinity gel (Sigma), separated by SDS-PAGE, and subjected to immunoblotting as described above.
Purification of 3xFLAG-tagged proteins.
Akr1-3xFLAG, Akr1-C500S-3xFLAG, 3xFLAG-Lcb4, and 3xFLAG-Lcb4-C1C2 were purified from KHY212 (Δ_akr1_ Δ_pep4_)/pAK456 (AKR1-3xFLAG), KHY212/pAK783 (AKR1-C500S-3xFLAG), KHY543 (Δ_akr1_ Δ_akr2_ Δ_pep4_)/pAK533 (3xFLAG-LCB4), and KHY543/pFK53 (3xFLAG-LCB4-C1C2) cells, respectively. Purification of Akr1-3xFLAG and Akr1-C500S-3xFLAG was performed essentially as described previously (38) with membrane fractions solubilized with 1% _n_-octyl-β-d-glucoside and anti-FLAG M2 agarose. 3xFLAG-Lcb4 and 3xFLAG-Lcb4-C1C2 were similarly purified by using soluble fractions and anti-FLAG M2 agarose.
In vitro palmitoylation.
In vitro palmitoylation was performed as described previously (38) with 7 μCi of [3H]palmitoyl-coenzyme A (CoA) (60 Ci/mmol; American Radiolabeled Chemical) and Akr1-3xFLAG and/or 3xFLAG-Lcb4. The reactions were terminated by adding a 10-fold volume of cold acetone. Proteins were suspended in SDS sample buffer containing 10 mM 2-mercaptoethanol, separated by SDS-PAGE, and visualized by autoradiography using the fluorographic reagent EN3HANCE.
RESULTS
Identification of the AKR2 gene.
Exogenously added LCBs are readily imported to yeast cells and converted to LCBPs by Lcb4 (Fig. 1A). Using the cytotoxic effects of accumulated LCBPs, we previously screened for a multicopy suppressor gene that would rescue the LCB-sensitive phenotype of the Δ_dpl1_ mutant. We identified the RSB1 gene and determined that Rsb1 functions as a putative LCB transporter/translocase (22). To discover additional genes, we repeated the screening with two modifications. First, instead of KHY13 (Δ_dpl1_) cells we used the more PHS-sensitive KHY21 (Δ_dpl1_ Δ_lcb3_) cells, which enabled us to use PHS at low concentrations (6 μM). In addition, we used a yeast genomic library constructed with DNA prepared from KHY92 cells, which carry the Δ_dpl1_ Δ_lcb3_ Δ_rsb1_ mutations, to avoid isolating these previously established genes.
We obtained several PHS-resistant clones, and subsequent sequencing analysis and subcloning experiments revealed that two genes, termed RSB2 and RSB3, were responsible for the phenotype. These were identical to ORFs YKR088c and YOR034c, respectively. RSB3 had previously been named AKR2 (11), so we use this nomenclature hereafter. As shown in Table 2, Akr2 overproduction affected PHS sensitivity more than Rsb2 overproduction, so we used AKR2 for further analysis.
TABLE 2.
PHS sensitivity
| Strain | Sensitivitya at PHS concn (μM): | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 5 | 7.5 | 10 | 12.5 | 15 | 20 | |
| KHY21/pRS426 | + | ± | − | − | − | − | − |
| KHY21/pAK222 (RSB2) | + | + | ± | − | − | − | − |
| KHY21/pAK234 (AKR2) | ++ | + | + | ± | − | − | − |
| KHY21/pAK243 (AKR1) | + | ± | − | − | − | − | − |
Akr2 is a polypeptide of 749 amino acids with a deduced molecular mass of 85.2 kDa. The PAT Akr1 shares high homology with Akr2 (37.9% identity and 64.2% similarity), yet the AKR1 gene could not function as a multicopy suppressor (Table 2). In addition, whereas deletion of the AKR1 gene results in pleiotropic effects (8, 11, 19, 33), the Δ_akr2_ cells grew normally and displayed no remarkable phenotypes, including PHS sensitivity (data not shown).
Akr1 is involved in the palmitoylation of Lcb4.
One possible mechanism behind the Akr2 rescue of the PHS-sensitive KHY21 cells would be a decrease in the activity of Lcb4. Like its homolog, the PAT Akr1, Akr2 contains a DHHC CRD, so we examined the possibility that Lcb4 might be regulated by palmitoylation. Wild-type, Δ_akr1_, Δ_akr2_, and Δ_akr1_ Δ_akr2_ cells overproducing Lcb4 from the GAL1 promoter were labeled with [3H]palmitic acid. [3H]Palmitoylated Lcb4 was apparent in the wild-type cells but far less so in the Δ_akr1_ cells (Fig. 1B, upper panel), although the protein levels were similar (lower panel). In all of the immunoblots, Lcb4 was detected as a doublet, with the upper and lower bands corresponding to hyper- and hypophosphorylated Lcb4, respectively (Fig. 5B), as reported (17). In contrast to the Δ_akr1_ cells, Lcb4 was normally palmitoylated in the Δ_akr2_ cells (Fig. 1B). Moreover, the palmitoylation in the Δ_akr1_ Δ_akr2_ cells was indistinguishable from that in the Δ_akr1_ cells.
FIG. 5.
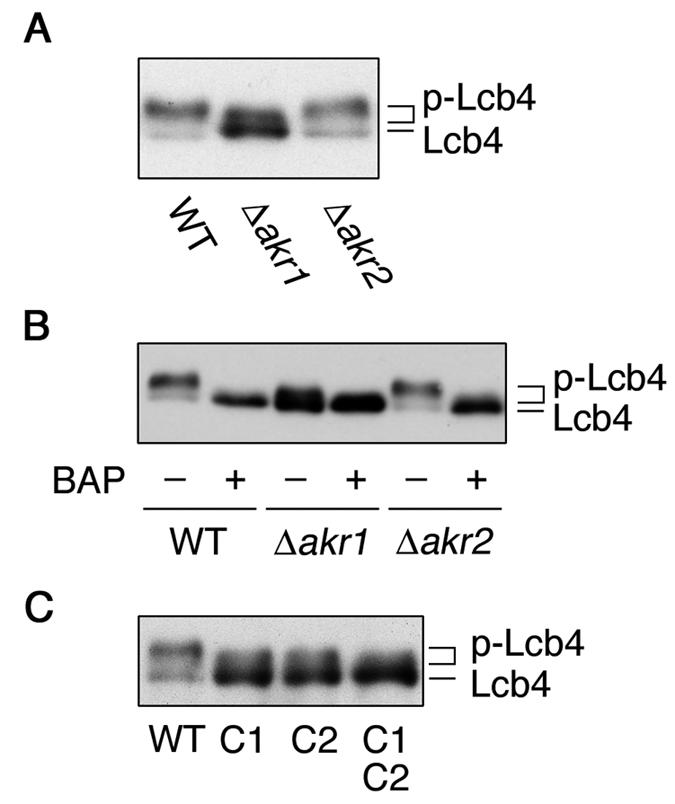
Palmitoylation is required for the phosphorylation of Lcb4. (A) Total lysates were prepared from SEY6210 (wild type), KHY211 (Δ_akr1_), and KHY197 (Δ_akr2_) cells. Proteins were separated by SDS-PAGE and detected by immunoblotting with anti-Lcb4 antibodies. (B) Total lysates prepared as in panel A were treated with or without bacterial alkaline phosphatase (BAP) and separated by SDS-PAGE, followed by immunoblotting with anti-Lcb4 antibodies. (C) Total lysates prepared from TS11 (Δ_lcb4_ Δ_pep4_) cells harboring pWK78 (LCB4), pFK18 (LCB4-C1), pFK19 (LCB4-C2), or pFK33 (LCB4-C1C2) were separated by SDS-PAGE, followed by immunoblotting with anti-Lcb4 antibodies. WT, wild type, p-Lcb4, hyperphosphorylated Lcb4.
Palmitoylation is required for the membrane association of Lcb4.
Lcb4 is tightly associated with the membrane (10, 13), although it has no apparent transmembrane-spanning domain. Therefore, we investigated whether palmitoylation is required for this association, using a fractionation experiment. In the wild-type and Δ_akr2_ cells most of the Lcb4 was associated with the membranes, whereas in the Δ_akr1_ cells ca. 80% was detected in the soluble fraction (Fig. 2A). The decrease in membrane association corresponded to the reduced palmitoylation of Lcb4 observed in the Δ_akr1_ cells (Fig. 1B). In addition, when examined by indirect immunofluorescence microscopy, Δ_akr1_ cells exhibited a diffuse intracellular fluorescence pattern for Lcb4 indicative of cytosolic localization (Fig. 2B), providing further evidence that palmitoylation is required for its membrane localization.
FIG. 2.
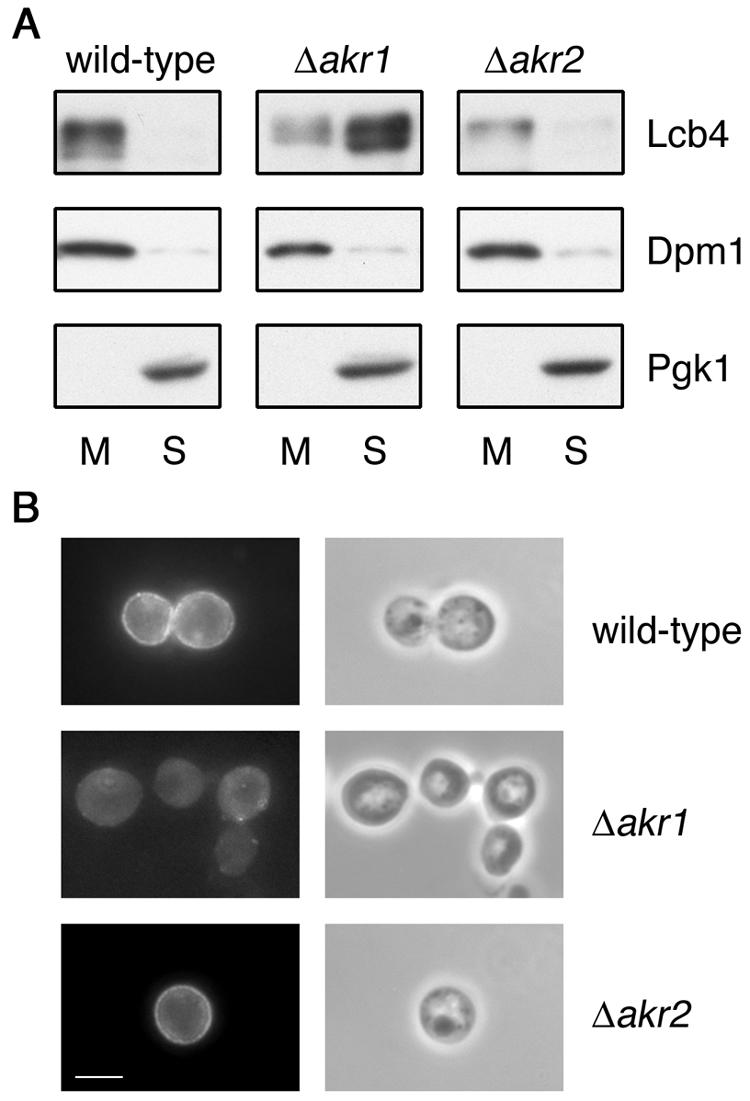
Akr1 is required for the membrane association of Lcb4. (A) Total lysates were prepared from KHY195 (wild-type), KHY212 (Δ_akr1_), and KHY199 (Δ_akr2_) cells. Membrane (M) and soluble (S) proteins were separated by centrifugation at 100,000 × g for 1 h and then by SDS-PAGE. Lcb4, Dpm1 (a membrane protein marker), and Pgk1 (a soluble protein marker) were then detected by immunoblotting. (B) SEY6210 (wild-type), KHY211 (Δ_akr1_), and KHY197 (Δ_akr2_) cells, each bearing pWK79 (LCB4), were fixed by formaldehyde, converted to spheroplasts, permeabilized with 0.1% Triton X-100, and stained with anti-Lcb4 antibodies (left panels). Phase-contrast images are also shown (right panels). Bar, 5 μm.
In contrast, in the wild-type and Δ_akr2_ cells Lcb4 staining was apparent on the cell perimeter. This localization pattern differs from prior accounts which localized Lcb4 to various endomembrane compartments, i.e., the endoplasmic reticulum (ER), Golgi, and endosomes (10, 13). These prior reports made use of an epitope-tagging strategy, whereas our analysis relies on the detection of wild-type Lcb4 using Lcb4-specific antibodies. Hemagglutinin (HA) tags attached to the Lcb4 C terminus, which were used in their studies, may have perturbed the Lcb4 localization.
Lcb4 is palmitoylated at Cys-43 and Cys-46 residues.
Palmitoylation occurs at Cys residues through thioester linkages, although no obvious motifs exist. For each of the 12 Cys residues (N-terminal C1 to C12) in Lcb4, we created a Cys-to-Ser mutant and performed fractionation experiments. Wild-type Lcb4 and the C3 to C12 mutant forms of Lcb4 were recovered mainly in the membrane fractions (Fig. 3A). However, Lcb4-C1 (Cys-43 mutant) and Lcb4-C2 (Cys-46 mutant), as well as the Lcb4-C1C2 double mutant, were predominantly detected in the soluble fractions (Fig. 3A and 3B). In addition, [3H]palmitoylation of Lcb4-C1 and Lcb4-C2 mutants was greatly reduced compared to that of wild-type Lcb4, and no [3H]palmitoylation was apparent in Lcb4-C1C2 (Fig. 3C). Together with the fact that palmitoylation often occurs at Cys residues arranged in clusters, these results indicate that two Cys residues (Cys-43 and Cys-46) of Lcb4 are palmitoylated, although why substitution of one of the Cys residues would affect the palmitoylation of the other is unknown.
FIG. 3.
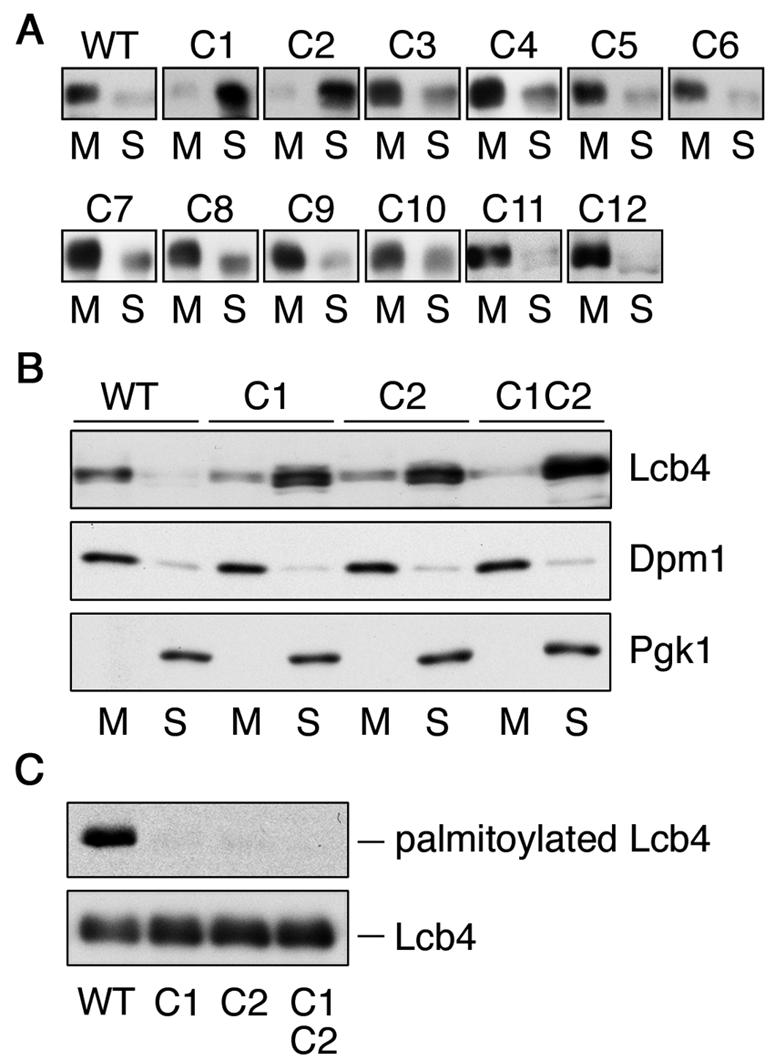
Cys-43 and Cys-46 residues of Lcb4 are palmitoylated. (A) Total lysates prepared from SIY03 (Δ_lcb4_) cells bearing pWK78 (LCB4), pFK18 (LCB4-C1), pFK19 (LCB4-C2), pFK20 (LCB4-C3), pFK21 (LCB4-C4), pFK22 (LCB4-C5), pFK23 (LCB4-C6), pFK24 (LCB4-C7), pFK25 (LCB4-C8), pFK26 (LCB4-C9), pFK27 (LCB4-C10), pFK28 (LCB4-C11), or pFK29 (LCB4-C12) were centrifuged at 100,000 × g for 1 h. Proteins in the resulting pellet (membrane fraction; M) and supernatant (soluble fraction; S) were separated by SDS-PAGE, followed by immunoblotting with anti-Lcb4. (B) Proteins were separated as in panel A by using total lysates prepared from TS11 (Δ_lcb4_ Δ_pep4_) cells bearing pWK78, pFK18, pFK19, or pFK33 (LCB4-C1C2). Lcb4, Dpm1, and Pgk1 were detected by immunoblotting. (C) FKY146 (Δ_lcb4_ Δ_pep4_) cells harboring pAK514 (GAL1 promoter-LCB4), pFK72 (LCB4-C1), pFK73 (LCB4-C2), or pFK74 (LCB4-C1C2) were labeled with 0.3 mCi of [3H]palmitic acid for 2 h at 30°C. Lcb4 was immunoprecipitated from total cell lysates (equal in radioactivity) by using anti-Lcb4 antibodies and separated by SDS-PAGE, followed by autoradiography (upper panel) or immunoblotting with anti-Lcb4 antibodies (lower panel). WT, wild type.
Akr1 palmitoylates Lcb4 in vitro.
We next investigated whether Akr1 is directly involved in the palmitoylation of Lcb4. When purified 3xFLAG-Lcb4 and Akr1-3xFLAG (Fig. 4A) were incubated in the presence of [3H]palmitoyl-CoA, palmitoylation of the 3xFLAG-Lcb4 was detected (Fig. 4B). In contrast, when purified 3xFLAG-Lcb4-C1C2 (Fig. 4A) was used, almost no palmitoylation was observed (Fig. 4C).
FIG. 4.
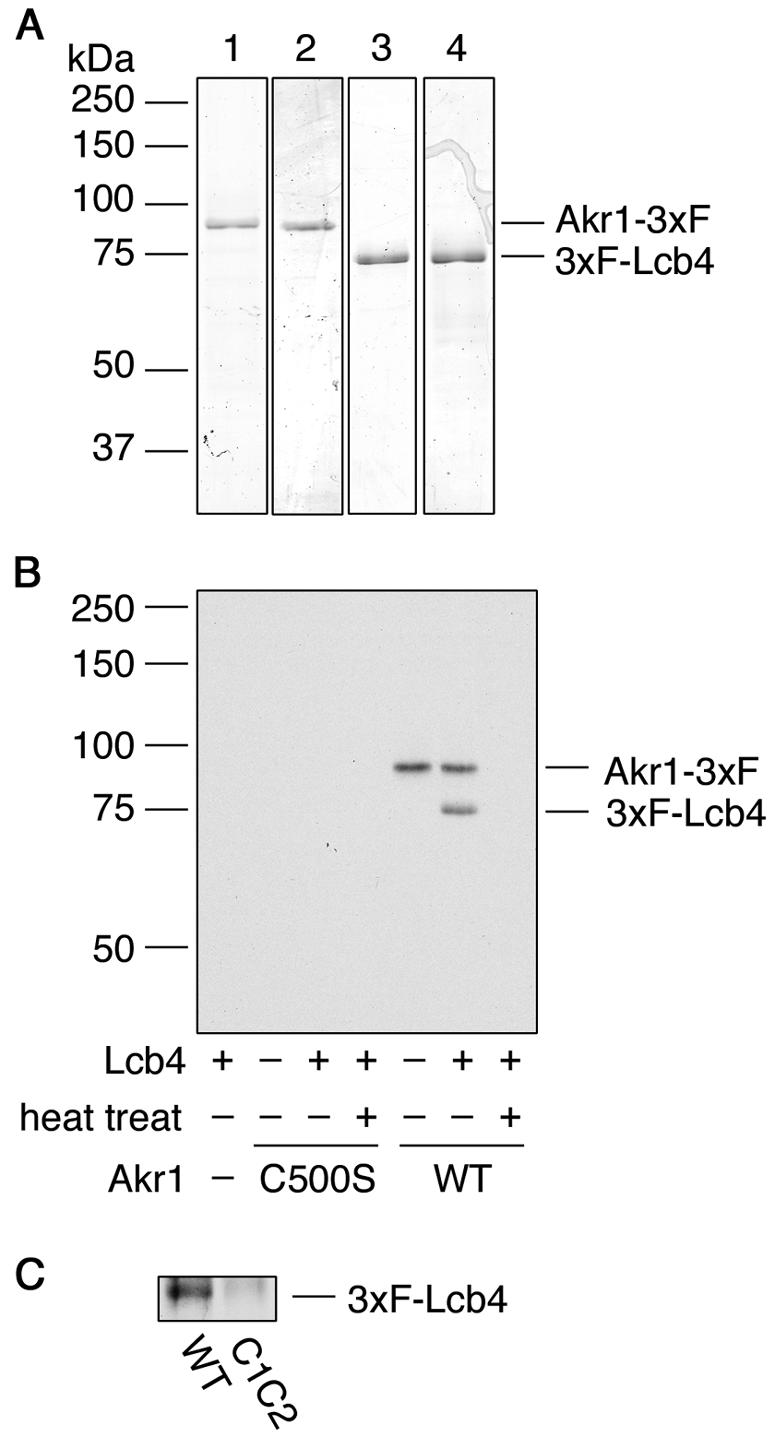
Akr1 palmitoylates Lcb4 in vitro. (A) Akr1-3xFLAG (lane 1), Akr1-C500S-3xFLAG (lane 2), 3xFLAG-Lcb4 (lane 3), and 3xFLAG-Lcb4-C1C2 (lane 4) were purified by using anti-FLAG M2 agarose. Purified proteins (0.5 μg) were separated by SDS-PAGE and stained with Coomassie brilliant blue. (B) Reaction mixtures containing [3H]palmitoyl-CoA, and purified proteins (0.1 μg of Akr1-3xFLAG and/or 0.24 μg of 3xFLAG-Lcb4) were incubated at 30°C for 1 h. As indicated, Akr1-3xFLAG protein was heat inactivated at 100°C for 15 min. (C) Akr1-3xFLAG (0.5 μg) was incubated with 3xFLAG-Lcb4 (1 μg) or 3xFLAG-Lcb4-C1C2 (1 μg) in the presence of [3H]palmitoyl-CoA at 37°C for 1 h. Proteins were separated by SDS-PAGE, followed by autoradiography. 3xF, 3xFLAG; WT, wild type.
Autopalmitoylation of Akr1-3xFLAG was also detected both in the presence or absence of Lcb4, a finding consistent with reports suggesting that autopalmitoylation represents the acyl-intermediate (26, 38). Palmitoylation of 3xFLAG-Lcb4 was not achieved with heat-inactivated Akr1-3xFLAG. Moreover, neither palmitoylation of 3xFLAG-Lcb4 nor autopalmitoylation of Akr1-3xFLAG was observed when Akr1-C500S-3xFLAG, a mutant for the active site, was used (Fig. 4B).
Palmitoylation is required for the phosphorylation of Lcb4.
We recently reported that Lcb4 is phosphorylated by Pho85 cyclin-dependent kinase and that the phosphorylation is important in the rapid downregulation of Lcb4 that occurs during the stationary growth phase of yeast (17). Therefore, we examined the relationship between phosphorylation and palmitoylation. In immunoblots of wild-type and Δ_akr2_ cells two bands of Lcb4 were detected (Fig. 5A). The major 73-kDa band represented a hyperphosphorylated form, since it shifted to the position of the 72-kDa band upon treatment with alkaline phosphatase (Fig. 5B). The gel mobility of the lower band exhibited little change after this treatment, suggesting that this band represents a hypophosphorylated (most likely nonphosphorylated) Lcb4. In immunoblots of the Δ_akr1_ cells, however, the hypophosphorylated 72-kDa Lcb4 was predominant (Fig. 5A); moreover, a minor band with an intermediate gel mobility was observed, suggesting a partially phosphorylated Lcb4. The phosphorylation patterns for the Lcb4-C1, Lcb4-C2, and Lcb4-C1C2 mutants were similar to that observed for the Lcb4 from the Δ_akr1_ cells (Fig. 5C). These results indicate that palmitoylation is required for the phosphorylation of Lcb4.
Consistent with our previous report (17), little Lcb4 was detected in wild-type cells 10 h after reaching stationary phase (Fig. 6A). On the other hand, the amounts of Lcb4 were nearly unchanged in Δ_akr1_ cells (Fig. 6A). In Δ_pho85_ cells the stabilization of Lcb4 was only partial (17), so palmitoylation apparently has a much stronger influence on the stability of the protein than phosphorylation does. Similar stabilization effects to those observed in the Δ_akr1_ cells were also found in the nonpalmitoylated mutants of Lcb4 (Fig. 6B).
FIG. 6.
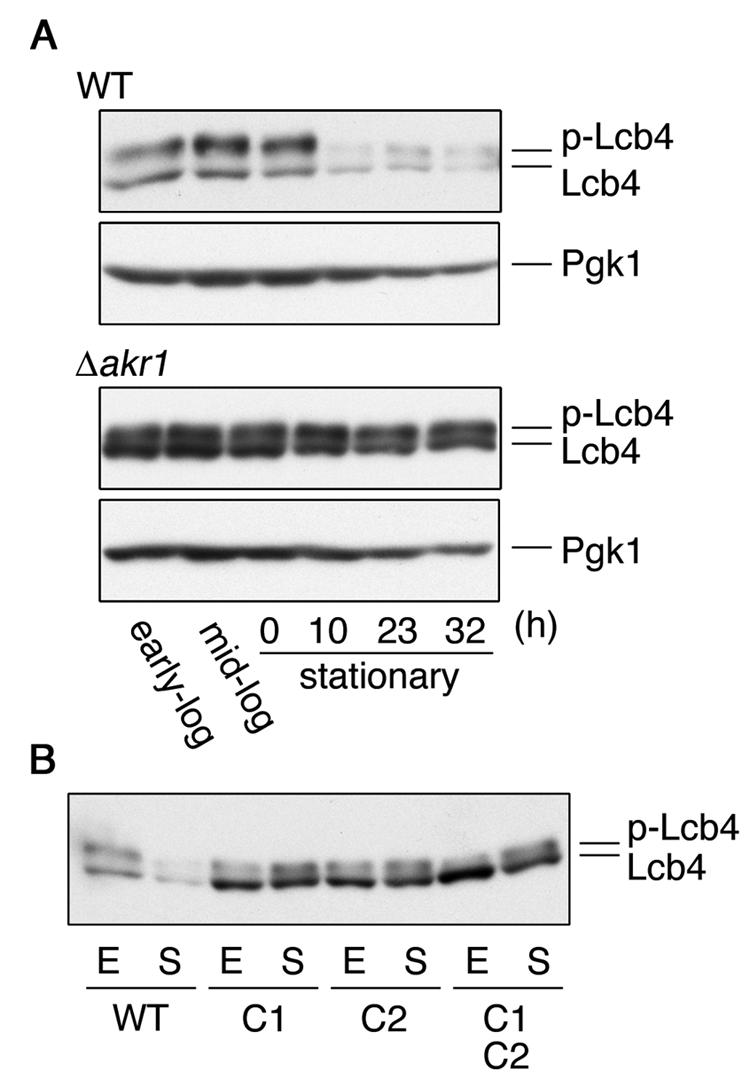
Palmitoylation is essential for the downregulation of Lcb4 during stationary phase. (A) SEY6210 (wild-type) and KHY211 (Δ_akr1_) cells grown overnight were diluted into fresh YPD medium at a density of A_600 = 0.3 and incubated at 30°C. Cells were harvested in early log phase, mid-log phase, and 0, 10, 23, or 32 h after reaching stationary phase. Total lysates were prepared from cells (ca. 2 × 107), and proteins (12 μg) were separated by SDS-PAGE and subjected to immunoblotting with anti-Lcb4 or anti-Pgk1 antibodies. (B) SIY03 (Δ_lcb4) cells bearing pWK78 (LCB4), pFK18 (LCB4-C1), pFK19 (LCB4-C2), or pFK33 (LCB4-C1C2) were harvested in early log phase (E) or 10 h after reaching stationary phase (S). Total cell lysates were prepared, and proteins (15 μg) were separated by SDS-PAGE, followed by immunoblotting with anti-Lcb4 antibodies. WT, wild type; p-Lcb4, hyperphosphorylated Lcb4.
Akr2 interacts with Lcb4.
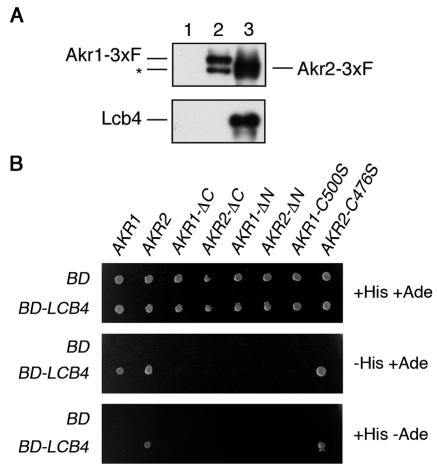
A comprehensive two-hybrid analysis previously suggested that Akr2 interacts with Lcb4 (16). We investigated this interaction by coimmunoprecipitation. Akr1-3xFLAG and Akr2-3xFLAG were efficiently immunoprecipitated from lysates of transfected cells, using anti-FLAG antibodies (Fig. 7A). Lcb4 was also detected in the Akr2-3xFLAG immunoprecipitate but not in the Akr1-3xFLAG immunoprecipitate (Fig. 7A). Thus, Lcb4 exhibits a stable interaction only with Akr2.
FIG. 7.
Akr2 interacts with Lcb4. (A) Total cell lysates prepared from KHY195 (Δ_pep4_)/pWK62 (LCB4)/pRS423 (vector) (lane 1), KHY195/pWK62/pAK456 (AKR1-3xFLAG) (lane 2), and KHY195/pWK62/pAK457 (AKR2-3xFLAG) (lane 3) cells were solubilized with 1% Triton X-100. Proteins were immunoprecipitated by using anti-FLAG M2 affinity gels and separated by SDS-PAGE, followed by immunoblotting with anti-FLAG (M2) or anti-Lcb4 antibodies. The asterisk indicates the degradation product of Akr1-3xFLAG. (B) Plasmids containing GLA4 AD fused with the indicated genes were introduced into PJ69-4A cells harboring pGBDU-C1 (GAL4 BD) or pWK05 (GAL4 BD-LCB4). Cells (ca. 3 × 105) were plated on leucine-free SC medium lacking uracil (top panel), uracil and histidine (middle panel), or uracil and adenine (bottom panel), followed by incubation at 30°C. 3xF, 3xFLAG.
To investigate the domain of Akr2 responsible for binding to Lcb4, we performed two-hybrid analyses, fusing the full-length AKR2 gene to the _GAL4_-AD and the full-length LCB4 gene to the _GAL4_-BD. We also prepared AD-AKR2_-Δ_N and AD-AKR2_-Δ_C, which are AD-AKR2 constructs lacking the domains encompassing the N-terminal ankyrin repeats and the C-terminal transmembrane region, respectively. Each AKR2 construct was introduced into PJ69-4A cells, together with BD-LCB4, and the resulting cells were plated on medium lacking histidine or adenine to monitor the expression of the reporter genes HIS3 and the more stringent ADE2. PJ69-4A cells expressing both AD-AKR2 and BD-LCB4 were able to grow on both plates (Fig. 7B), confirming the previous two-hybrid interaction (16). However, neither AD-AKR2_-Δ_N nor AD-AKR2_-Δ_C exhibited a two-hybrid interaction, suggesting that both domains are important for binding. On the other hand, AD-AKR2-C476S, in which the putative active site Cys residue was replaced with Ser, was as effective as AD-AKR2.
In contrast, PJ69-4A cells harboring AD-AKR1 and BD-LCB4 constructs grew in the absence of histidine but not in the absence of adenine (Fig. 7B), suggesting that the Akr1-Lcb4 interaction is weaker than the Akr2-Lcb4 interaction. Both N-terminal and C-terminal domains were, again, important; however, the active site mutant AKR1-C500S did not exhibit a two-hybrid interaction (Fig. 7B). Thus, Lcb4 interacts with both Akr1 and Akr2 but in different ways. The Akr1-Lcb4 interaction may be transient and reflect an enzyme-substrate relationship; a two-hybrid interaction between Akr1 and its substrate Yck2 has also been reported (16). On the other hand, the Akr2-Lcb4 interaction is stable and independent of the active site Cys residue.
Overproduction of Akr2 causes the rapid degradation of Lcb4.
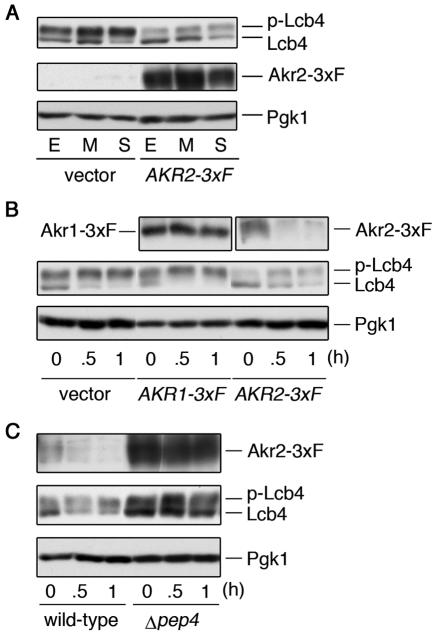
Deletion of AKR2 had no effect on palmitoylation or phosphorylation of Lcb4, whether the mutation was introduced into wild-type cells or Δ_akr1_ cells. However, since AKR2 was isolated as a multicopy suppressor, it is conceivable that its effects would become apparent when overproduced but not when deleted.
Throughout the growth phase, phosphorylated Lcb4 levels were markedly reduced by the overproduction of Akr2-3xFLAG, yet the hypophosphorylated form appeared to be unaffected (Fig. 8A). Thus, the total steady-state level of Lcb4 is reduced by the overproduction of Akr2. To test the possibility that this reduction is caused by the destabilization of Lcb4, we blocked any subsequent protein synthesis with cycloheximide and monitored the Lcb4 protein levels. In cells harboring the vector plasmid, the hypophosphorylated form of Lcb4 rapidly disappeared (Fig. 8B); however, since the hyper-phosphorylated form slightly increased within the first 30 min, the hypophosphorylated form was likely just converted to the hyperphosphorylated form. Overproduction of Akr1-3xFLAG had no effect on the stability of Lcb4, and Akr1-3xFLAG itself remained stable throughout the incubation (Fig. 8B). In contrast, in cells overproducing Akr2-3xFLAG, the amount of hyperphosphorylated Lcb4 at 0 h was already less than in control cells (Fig. 8B). The hypophosphorylated Lcb4 in cells overexpressing Akr2-3xFLAG again disappeared rapidly. However, in this case, this seemed to be due not to phosphorylation but to degradation, since the total Lcb4 amount decreased to ca. 50% (compared to levels at 0 h) even after a 1-h incubation period, at which time almost no degradation was observed in the control cells. Moreover, we found a rapid disappearance of Akr2-3xFLAG, indicating that it is highly unstable. The degradation of both Lcb4 and Akr2-3xFLAG apparently occurs in the vacuole, since the levels and half-life of each were significantly increased in a deletion mutant of PEP4, which encodes a vacuolar protease (Fig. 8C). These results indicate that Lcb4 is degraded in the vacuole, together with Akr2, when Akr2 is overproduced.
FIG. 8.
Overproduction of Akr2 causes destabilization of Lcb4. (A) SEY6210 cells bearing pRS423 (vector) or pAK457 (AKR2-3xFLAG) were harvested in early log phase (E), in mid-log phase (M), or at the beginning of stationary phase (S). Total lysates were prepared from cells (ca. 2 × 107), and proteins (9 μg) were separated by SDS-PAGE and immunoblotted with anti-Lcb4, anti-FLAG M2, and anti-Pgk1 antibodies. (B) SEY6210 cells bearing pRS423, pAK456 (AKR1-3xFLAG), or pAK457, grown to early log phase, were treated with cycloheximide (10 μg/ml). Cells were collected at the indicated time point after treatment, and total cell lysates were prepared. Proteins (10 μg) were separated by SDS-PAGE, followed by immunoblotting with anti-Lcb4, anti-FLAG M2, and anti-Pgk1 antibodies. (C) SEY6210 (wild-type) cells bearing pAK457 and TVY1 (Δ_pep4_) cells bearing pAK457 were treated with cycloheximide and processed as in panel B. 3xF, 3xFLAG; p-Lcb4, hyperphosphorylated Lcb4.
DISCUSSION
To date, there have been limited examples of palmitoylation proven to be true enzymatic reactions, since few PATs have been identified and those only very recently (9, 14, 26, 38). Indeed, Lcb4, as reported here, is only the second identified substrate of Akr1, after Yck2. Lcb4 palmitoylation was observed in wild-type cells, but only at greatly reduced levels in Δ_akr1_ cells (Fig. 1B). The efficiency of protein labeling by [3H]palmitic acid is generally low, since most of the added palmitic acid is consumed for lipid synthesis. For this reason, we could not detect endogenous Lcb4 by [3H]palmitic acid labeling. However, palmitoylation of endogenous Lcb4 is highly likely, since the mislocalization of endogenous Lcb4 in the soluble fraction of the Δ_akr1_ cells (Fig. 2) corresponded to the decreased palmitoylation (Fig. 1B). In addition, although the labeling of Lcb4 was largely reduced, it was not completely abolished in the Δ_akr1_ or Δ_akr1_ Δ_akr2_ cells. Yeast have five DHHC CRD-containing proteins, in addition to Akr1 and Akr2, and it is most likely that one of them is involved in the palmitoylation of Lcb4 in the absence of Akr1.
Although Akr1 and Akr2 share high homology, they seem not to function redundantly. Overproduction of Akr2 did not suppress the slow-growth observed in the Δ_akr1_ cells (data not shown). Deletion of the AKR2 gene also had no effect on the palmitoylation of Lcb4 when introduced into wild-type or Δ_akr1_ cells (Fig. 1B). Thus, at present there is no evidence that Akr2 functions as a PAT.
Although palmitoylation has no effect on the in vitro sphingosine kinase activity of Lcb4 (data not shown), it has several functions. Palmitoylation is essential for the membrane association (Fig. 2) and the phosphorylation of Lcb4 (Fig. 5). Phosphorylation, however, is not required for palmitoylation, since Lcb4 was recovered in the membrane fractions of Δ_pho85_ cells (data not shown). Palmitoylation is also necessary for the degradation of Lcb4. Since palmitoylation of Lcb4 is essential for its complete phosphorylation, which, in turn, is important for the ubiquitination that leads to degradation, one might suppose that loss of degradation would be a secondary effect of reduced phosphorylation. However, the degree of stabilization of Lcb4 in the Δ_akr1_ cells was much more significant compared to that in Δ_pho85_ cells (17).
Lcb4 is delivered to the lytic organelle vacuole, which is equivalent to a mammalian lysosome, for degradation. First, like most proteins, Lcb4 must be transported to the multivesicular body (MVB) compartment (17), in which invagination into vesicles and subsequent membrane fusion with the vacuole occurs (20). Vacuolar lipases and proteases then hydrolyze the MVB vesicle and its contents. Since cytosolic proteins cannot traverse the vacuolar membrane, invagination at the MVB is essential. The complete stabilization of Lcb4 in the Δ_akr1_ cells, then, would indicate that palmitoylation is required for the sorting of Lcb4 to the MVB/vacuole pathway.
We found here that Akr2 is highly unstable and is also degraded in the vacuole. Like other membrane proteins, Akr2 must be synthesized on the ER membrane; it might then be delivered to the Golgi apparatus and sorted to the vacuole. On the other hand, Akr1 is stable and localized in the Golgi apparatus (2, 38). When Akr1-3xFLAG and Akr2-3xFLAG were expressed from their own promoters, the intensity of the Akr2-3xFLAG band was much weaker (data not shown), suggesting that under normal conditions Akr1 exists in much greater amounts than Akr2.
Our current model for the fate of Lcb4 is as follows. Since Lcb4 has no signal sequence or transmembrane domain, it must be synthesized in the cytosol and is palmitoylated at the Golgi apparatus by Akr1. Normally, Lcb4 is then delivered to the plasma membrane via the Sec pathway, it may then be transported to the ER via an unknown pathway. Lcb4 is degraded constitutively but slowly during log phase by being transported to the vacuole via the MVB, but its degradation is accelerated at the stationary phase by an unknown mechanism (17). In contrast to this normal fate, once Akr2 surpasses Akr1 in quantity, as by forced expression from a plasmid, Lcb4 would tend to associate with Akr2, since the Akr2-Lcb4 interaction is stronger than the Akr1-Lcb4 interaction (Fig. 7). In this situation, Lcb4 is directly delivered to the vacuole together with Akr2, without being transported to the plasma membrane, even during log phase. This may be the reason that Akr2 was isolated as a multicopy suppressor, since reduced Lcb4 results in a decrease in cytotoxic LCBPs. Phosphorylation of Lcb4 may occur after it reaches the plasma membrane, since forced delivery to the vacuole by overproduction of Akr2 causes a decrease in the phosphorylation of Lcb4 (Fig. 8).
The two Cys residues (Cys-43 and Cys-46) of Lcb4 are conserved in another yeast LCB kinase, Lcb5 (Cys-91 and Cys94), and about one-third of Lcb5 associates with the membrane (30). We created an Lcb5 mutant in which the conserved Cys residues were mutated to Ser and found that its membrane localization was similar to that of the wild type. Moreover, the Δ_akr1_ mutation did not affect the localization of Lcb5 (F. Kurotsu, A. Kihara, and Y. Igarashi, unpublished results). These results suggest that Lcb5 is anchored to the membrane through another mechanism or that Cys residues other than Cys-91 and Cys-94 are palmitoylated, possibly by PATs other than Akr1.
The present study, together with our previous report (17), demonstrates that Lcb4 is regulated by several posttranslational modifications, including phosphorylation, ubiquitination, and palmitoylation. Phosphorylation of the mammalian sphingosine kinase SPHK1 has also been reported (31). In that case, phosphorylation induces the membrane translocation of SPHK1, although the precise molecular mechanism is unknown. Future studies are required to investigate whether regulation of LCB/sphingosine kinase by palmitoylation is conserved beyond species.
Acknowledgments
This study was supported by a Grant-In-Aid for Scientific Research on Priority Areas (B, 12140201) and a Grant-in-Aid for Young Scientists (B, 15770078) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
REFERENCES
- 1.Abeliovich, H., T. Darsow, and S. D. Emr. 1999. Cytoplasm to vacuole trafficking of aminopeptidase I requires a t-SNARE-Sec1p complex composed of Tlg2p and Vps45p. EMBO J. 18**:**6005-6016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babu, P., R. J. Deschenes, and L. C. Robinson. 2004. Akr1p-dependent palmitoylation of Yck2p yeast casein kinase 1 is necessary and sufficient for plasma membrane targeting. J. Biol. Chem. 279**:**27138-27147. [DOI] [PubMed] [Google Scholar]
- 3.Birchwood, C. J., J. D. Saba, R. C. Dickson, and K. W. Cunningham. 2001. Calcium influx and signaling in yeast stimulated by intracellular sphingosine 1-phosphate accumulation. J. Biol. Chem. 276**:**11712-11718. [DOI] [PubMed] [Google Scholar]
- 4.Casey, P. J. 1995. Protein lipidation in cell signaling. Science 268**:**221-225. [DOI] [PubMed] [Google Scholar]
- 5.Christianson, T. W., R. S. Sikorski, M. Dante, and P. Hieter. 1992. Multifunctional yeast high-copy-number shuttle vectors. Gene 110**:**119-122. [DOI] [PubMed] [Google Scholar]
- 6.Ducker, C. E., E. M. Stettler, K. J. French, J. J. Upson, and C. D. Smith. 2004. Huntingtin interacting protein 14 is an oncogenic human protein: palmitoyl acyltransferase. Oncogene 23**:**9230-9237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunphy, J. T., and M. E. Linder. 1998. Signalling functions of protein palmitoylation. Biochim. Biophys. Acta 1436**:**245-261. [DOI] [PubMed] [Google Scholar]
- 8.Feng, Y., and N. G. Davis. 2000. Akr1p and the type I casein kinases act prior to the ubiquitination step of yeast endocytosis: Akr1p is required for kinase localization to the plasma membrane. Mol. Cell. Biol. 20**:**5350-5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fukata, M., Y. Fukata, H. Adesnik, R. A. Nicoll, and D. S. Bredt. 2004. Identification of PSD-95 palmitoylating enzymes. Neuron 44**:**987-996. [DOI] [PubMed] [Google Scholar]
- 10.Funato, K., R. Lombardi, B. Vallee, and H. Riezman. 2003. Lcb4p is a key regulator of ceramide synthesis from exogenous long chain sphingoid base in Saccharomyces cerevisiae. J. Biol. Chem. 278**:**7325-7334. [DOI] [PubMed] [Google Scholar]
- 11.Givan, S. A., and G. F. Sprague, Jr. 1997. The ankyrin repeat-containing protein Akr1p is required for the endocytosis of yeast pheromone receptors. Mol. Biol. Cell 8**:**1317-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gottlieb, D., W. Heideman, and J. D. Saba. 1999. The DPL1 gene is involved in mediating the response to nutrient deprivation in Saccharomyces cerevisiae. Mol. Cell. Biol. Res. Commun. 1**:**66-71. [DOI] [PubMed] [Google Scholar]
- 13.Hait, N. C., K. Fujita, R. L. Lester, and R. C. Dickson. 2002. Lcb4p sphingoid base kinase localizes to the Golgi and late endosomes. FEBS Lett. 532**:** 97-102. [DOI] [PubMed] [Google Scholar]
- 14.Huang, K., A. Yanai, R. Kang, P. Arstikaitis, R. R. Singaraja, M. Metzler, A. Mullard, B. Haigh, C. Gauthier-Campbell, C. A. Gutekunst, M. R. Hayden, and A. El-Husseini. 2004. Huntingtin-interacting protein HIP14 is a palmitoyl transferase involved in palmitoylation and trafficking of multiple neuronal proteins. Neuron 44**:**977-986. [DOI] [PubMed] [Google Scholar]
- 15.Irie, K., M. Takase, K. S. Lee, D. E. Levin, H. Araki, K. Matsumoto, and Y. Oshima. 1993. MKK1 and MKK2, which encode Saccharomyces cerevisiae mitogen-activated protein kinase-kinase homologs, function in the pathway mediated by protein kinase C. Mol. Cell. Biol. 13**:**3076-3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ito, T., T. Chiba, R. Ozawa, M. Yoshida, M. Hattori, and Y. Sakaki. 2001. A comprehensive two-hybrid analysis to explore the yeast protein interactome. Proc. Natl. Acad. Sci. USA 98**:**4569-4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iwaki, S., A. Kihara, T. Sano, and Y. Igarashi. 2005. Phosphorylation by Pho85 cyclin-dependent kinase acts as a signal for the down-regulation of the yeast sphingoid long-chain base kinase Lcb4 during the stationary phase. J. Biol. Chem. 280**:**6520-6527. [DOI] [PubMed] [Google Scholar]
- 18.James, P., J. Halladay, and E. A. Craig. 1996. Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics 144**:**1425-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kao, L. R., J. Peterson, R. Ji, L. Bender, and A. Bender. 1996. Interactions between the ankyrin repeat-containing protein Akr1p and the pheromone response pathway in Saccharomyces cerevisiae. Mol. Cell. Biol. 16**:**168-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katzmann, D. J., G. Odorizzi, and S. D. Emr. 2002. Receptor downregulation and multivesicular-body sorting. Nat. Rev. Mol. Cell. Biol. 3**:**893-905. [DOI] [PubMed] [Google Scholar]
- 21.Keller, C. A., X. Yuan, P. Panzanelli, M. L. Martin, M. Alldred, M. Sassoe-Pognetto, and B. Luscher. 2004. The γ2 subunit of GABAA receptors is a substrate for palmitoylation by GODZ. J. Neurosci. Res. 24**:**5881-5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kihara, A., and Y. Igarashi. 2002. Identification and characterization of a Saccharomyces cerevisiae gene, RSB1, involved in sphingoid long-chain base release. J. Biol. Chem. 277**:**30048-30054. [DOI] [PubMed] [Google Scholar]
- 23.Kihara, A., T. Sano, S. Iwaki, and Y. Igarashi. 2003. Transmembrane topology of sphingoid long-chain base-1-phosphate phosphatase, Lcb3p. Genes Cells. 8**:**525-535. [DOI] [PubMed] [Google Scholar]
- 24.Kim, S., H. Fyrst, and J. Saba. 2000. Accumulation of phosphorylated sphingoid long chain bases results in cell growth inhibition in Saccharomyces cerevisiae. Genetics 156**:**1519-1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Linder, M. E., and R. J. Deschenes. 2004. Model organisms lead the way to protein palmitoyltransferases. J. Cell Sci. 117**:**521-526. [DOI] [PubMed] [Google Scholar]
- 26.Lobo, S., W. K. Greentree, M. E. Linder, and R. J. Deschenes. 2002. Identification of a Ras palmitoyltransferase in Saccharomyces cerevisiae. J. Biol. Chem. 277**:**41268-41273. [DOI] [PubMed] [Google Scholar]
- 27.Mandala, S. M., R. Thornton, Z. Tu, M. B. Kurtz, J. Nickels, J. Broach, R. Menzeleev, and S. Spiegel. 1998. Sphingoid base 1-phosphate phosphatase: a key regulator of sphingolipid metabolism and stress response. Proc. Natl. Acad. Sci. USA 95**:**150-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mao, C., J. D. Saba, and L. M. Obeid. 1999. The dihydrosphingosine-1-phosphate phosphatases of Saccharomyces cerevisiae are important regulators of cell proliferation and heat stress responses. Biochem. J. 342**:**667-675. [PMC free article] [PubMed] [Google Scholar]
- 29.Mao, C., M. Wadleigh, G. M. Jenkins, Y. A. Hannun, and L. M. Obeid. 1997. Identification and characterization of Saccharomyces cerevisiae dihydrosphingosine-1-phosphate phosphatase. J. Biol. Chem. 272**:**28690-28694. [DOI] [PubMed] [Google Scholar]
- 30.Nagiec, M. M., M. Skrzypek, E. E. Nagiec, R. L. Lester, and R. C. Dickson. 1998. The LCB4 (YOR171c) and LCB5 (YLR260w) genes of Saccharomyces encode sphingoid long chain base kinases. J. Biol. Chem. 273**:**19437-19442. [DOI] [PubMed] [Google Scholar]
- 31.Pitson, S. M., P. A. Moretti, J. R. Zebol, H. E. Lynn, P. Xia, M. A. Vadas, and B. W. Wattenberg. 2003. Activation of sphingosine kinase 1 by ERK1/2-mediated phosphorylation. EMBO J. 22**:**5491-5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Politis, E. G., A. F. Roth, and N. G. Davis. 2005. Transmembrane topology of the protein palmitoyl transferase Akr1. J. Biol. Chem. 280**:**10156-10163. [DOI] [PubMed] [Google Scholar]
- 33.Pryciak, P. M., and L. H. Hartwell. 1996. AKR1 encodes a candidate effector of the Gβγ complex in the Saccharomyces cerevisiae pheromone response pathway and contributes to control of both cell shape and signal transduction. Mol. Cell. Biol. 16**:**2614-2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Putilina, T., P. Wong, and S. Gentleman. 1999. The DHHC domain: a new highly conserved cysteine-rich motif. Mol. Cell Biochem. 195**:**219-226. [DOI] [PubMed] [Google Scholar]
- 35.Resh, M. D. 1999. Fatty acylation of proteins: new insights into membrane targeting of myristoylated and palmitoylated proteins. Biochim. Biophys. Acta 1451**:**1-16. [DOI] [PubMed] [Google Scholar]
- 36.Resh, M. D. 1996. Regulation of cellular signalling by fatty acid acylation and prenylation of signal transduction proteins. Cell. Signal. 8**:**403-412. [DOI] [PubMed] [Google Scholar]
- 37.Robinson, J. S., D. J. Klionsky, L. M. Banta, and S. D. Emr. 1988. Protein sorting in Saccharomyces cerevisiae: isolation of mutants defective in the delivery and processing of multiple vacuolar hydrolases. Mol. Cell. Biol. 8**:**4936-4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roth, A. F., Y. Feng, L. Chen, and N. G. Davis. 2002. The yeast DHHC cysteine-rich domain protein Akr1p is a palmitoyl transferase. J. Cell Biol. 159**:**23-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saba, J. D., F. Nara, A. Bielawska, S. Garrett, and Y. A. Hannun. 1997. The BST1 gene of Saccharomyces cerevisiae is the sphingosine-1-phosphate lyase. J. Biol. Chem. 272**:**26087-26090. [DOI] [PubMed] [Google Scholar]
- 40.Sikorski, R. S., and P. Hieter. 1989. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122**:**19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Skrzypek, M. S., M. M. Nagiec, R. L. Lester, and R. C. Dickson. 1999. Analysis of phosphorylated sphingolipid long-chain bases reveals potential roles in heat stress and growth control in Saccharomyces. J. Bacteriol. 181**:**1134-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uemura, S., A. Kihara, J. Inokuchi, and Y. Igarashi. 2003. Csg1p and newly identified Csh1p function in mannosylinositol phosphorylceramide synthesis by interacting with Csg2p. J. Biol. Chem. 278**:**45049-45055. [DOI] [PubMed] [Google Scholar]
- 43.Zhang, X., M. S. Skrzypek, R. L. Lester, and R. C. Dickson. 2001. Elevation of endogenous sphingolipid long-chain base phosphates kills Saccharomyces cerevisiae cells. Curr. Genet. 40**:**221-233. [DOI] [PubMed] [Google Scholar]