F-Spondin Interaction with the Apolipoprotein E Receptor ApoEr2 Affects Processing of Amyloid Precursor Protein (original) (raw)
Abstract
A recent study showed that F-spondin, a protein associated with the extracellular matrix, interacted with amyloid precursor protein (APP) and inhibited β-secretase cleavage. F-spondin contains a thrombospondin domain that we hypothesized could interact with the family of receptors for apolipoprotein E (apoE). Through coimmunoprecipitation experiments, we demonstrated that F-spondin interacts with an apoE receptor (apoE receptor 2 [ApoEr2]) through the thrombospondin domain of F-spondin and the ligand binding domain of ApoEr2. Full-length F-spondin increased coimmunoprecipitation of ApoEr2 and APP in transfected cells and primary neurons and increased surface expression of APP and ApoEr2. Full-length F-spondin, but none of the individual F-spondin domains, increased cleavage of APP and ApoEr2, resulting in more secreted forms of APP and ApoEr2 and more C-terminal fragments (CTF) of these proteins. In addition, full-length F-spondin, but not the individual domains, decreased production of the β-CTF of APP and Aβ in transfected cells and primary neurons. The reduction in APP β-CTF was blocked by receptor-associated protein (RAP), an inhibitor of lipoprotein receptors, implicating ApoEr2 in the altered proteolysis of APP. ApoEr2 coprecipitated with APP α- and β-CTF, and F-spondin reduced the levels of APP intracellular domain signaling, suggesting that there are also intracellular interactions between APP and ApoEr2, perhaps involving adaptor proteins. These studies suggest that the extracellular matrix molecule F-spondin can cluster APP and ApoEr2 together on the cell surface and affect the processing of each, resulting in decreased production of Aβ.
Alzheimer's disease (AD) is characterized by the presence of β-amyloid plaques, composed predominantly of the Aβ peptide, a 40- or 42-amino-acid cleavage product of the amyloid precursor protein (APP). APP, a transmembrane protein, undergoes extracellular cleavage by one of two proteases, α- or β-secretase, resulting in the formation of a large N-terminal extracellular fragment (APPs) and smaller, membrane-bound C-terminal fragments (CTF). If the initial cleavage event occurs via β-secretase, then cleavage of the CTF by γ-secretase results in the formation of Aβ. Recent evidence suggests that F-spondin, a developmentally regulated neuronal protein associated with the extracellular matrix, may act as a regulator of APP processing (8). Specifically, F-spondin binds to the extracellular domain of APP and inhibits β-secretase cleavage (8). Thus, F-spondin may serve as a regulator of Aβ formation.
Despite the importance of APP in the formation of β- amyloid plaques, apolipoprotein E (apoE) remains the best defined genetic risk factor for late-onset AD (27). In AD brains, apoE is a component of most plaques and the APOE genotype correlates with the levels of Aβ deposition (23, 27). apoE may affect Aβ production, aggregation, or clearance and has been referred to as a pathological chaperone (5, 18, 34). Conversely, apoE may affect the development of AD indirectly through other processes, including cholesterol transport and synapse formation (16), neurite outgrowth (20), or destabilization of microtubules (19).
apoE binds to members of the low-density lipoprotein (LDL) receptor family, including the apoE receptor 2 (ApoEr2) and the LDL receptor-related protein (LRP); each of these receptors binds numerous ligands (26). At the molecular level, ligand interactions with members of this receptor family affect several intracellular signaling pathways, leading to the inhibition of the c-Jun N-terminal kinase 1/2 pathway and the activation of the extracellular signal-regulated kinase 1/2 pathway (9). Interestingly, apoE receptors and APP undergo similar proteolytic cleavage by γ-secretase, leading to the release of intracellular domains that interact with common adaptor proteins (10, 17). We hypothesize that extracellular ligands of ApoEr2 could affect the processing of APP, either directly or through the modulation of intracellular signaling pathways.
In this work, we demonstrate that F-spondin, in addition to interacting with APP and affecting its processing, also interacts with ApoEr2 and affects its processing. The effects of F-spondin on proteolysis of APP depends on the interaction of F-spondin with apoE receptors. We suggest that there could exist a tripartite complex of APP, F-spondin, and ApoEr2 on the cell surface, affecting processing of the membrane-bound molecules.
MATERIALS AND METHODS
Vector construction.
We produced several deletion constructs of ApoEr2 and F-spondin molecules. The following constructs of mouse ApoEr2 were generated with a signal peptide and a C-terminal hemagglutinin (HA) tag in the pcDNA flux3 expression vector: ApoEr2-1 (residues 1 to 89), ApoEr2-2 (residues 1 to 214), ApoEr2-3 (residues 1 to 401), ApoEr2-4 (residues 1 to 614), ApoEr2-5 (residues 615 to 670), and ApoEr2-6 (residues 671 to 842). Full-length ApoEr2 (ApoEr2-7 [residues 1 to 842]) lacked ligand binding repeats 4 to 6 and included the alternately spliced cytoplasmic insert (1). F-spondin constructs were produced in the PsecTag2/HygroB expression vector with a C-terminal c-myc tag: residues 1 to 225, reelin domain; residues 206 to 501, spondin domain; residues 502 to 807, thrombospondin domain. Three constructs of the thrombospondin domain were produced by PCR: repeats 1 and 2, repeats 3 and 4, and repeats 5 and 6. APP constructs were generated with a signal peptide in the PsecTag2/HygroB expression vector: residues 671 to 770, C99 fragment; and residues 687 to 770, C83 fragment. The recombinant DNA was confirmed by sequencing, and expression of correctly sized proteins was confirmed by Western blotting (data not shown).
Cell lines and culture conditions.
COS7 and HEK293 cells were maintained in Opti-MEM (Invitrogen) with 10% fetal bovine serum (FBS) (Life Technologies, Inc.) in a 10% CO2 incubator. Reelin-, spondin-, thrombospondin-, or full-length F-spondin-conditioned medium and control medium were prepared as described previously (4). Briefly, COS7 cells were transfected with the reelin, spondin, thrombospondin, or F-spondin plasmid or with a pSecTag2/HygroB vector. After 24 h, the cells were transferred to Opti-MEM serum-free medium (Invitrogen). Conditioned media were collected at 48 h posttransfection, concentrated 10- fold with a Centricon centrifugal filter with a 10,000-molecular-weight cutoff (Millipore), and stored at −80°C in small aliquots. Purified receptor-associated protein (RAP) was obtained from Dudley Strickland. Cells were preincubated in the presence of 1 μM RAP overnight and then exposed to either control medium or F-spondin-containing medium for 12 h.
Antibodies.
For transfected cells, we used anti-HA (Abcam) or anti-c-myc (Abcam) antibodies. For analysis of APP, we used 22C11 (identifying the extracellular domain of APP) (Chemicon), 6E10 (identifying secreted APPα and β-CTF) (Signet), and c1/6.1 and 369 (recognizing the C-terminal domain of APP). Anne Cataldo provided c1/6.1, and Sam Gandy provided 369. Antibody R2, against the thrombospondin domain of F-spondin, was provided by Avihu Klar. Antibody 5810 was raised in a rabbit against a recombinant protein of the first three ligand binding repeats of mouse ApoEr2 and does not cross-react with human ApoEr2 (data not shown). ApoEr2 antibody α-19, against its C terminus, was kindly provided by Johannes Nimpf.
Quantification of ApoEr2 and APP proteolytic fragments.
COS7 cells were transiently transfected with APP and indicated plasmids or ApoEr2 and indicated plasmids and then cultured in Dulbecco modified Eagle medium containing 10% FBS for 24 h. Cells were maintained for another 24 h in serum-free media with or without F-spondin-derived proteins. Secreted fragments were determined by Western blot analysis of the media (secreted APPα, 6E10 antibody; secreted ApoEr2, 5810 antibody). CTF were measured by Western blotting from cell lysates (APP αCTF, C1/6.1; APP β-CTF, 6E10; ApoEr2 CTF, HA antibodies). Aβ40 and Aβ42 levels in the conditioned media were determined byenzyme-linked immunosorbent assays, using 1A10 (anti-Aβ40) or 1C3 (anti-Aβ42) as a capture antibody and 12B2, which recognizes both mouse and human Aβ as a detection antibody (Immuno-Biological Laboratories) (11).
Primary neuronal cell culture.
Primary mouse embryonic cortical neuron cultures were prepared from embryonic day 16 Swiss-Webster mice as previously described (22). Brain cortices were chopped and trypsinized for 10 min at 37°C. After trypsinization, 0.4 μg/ml trypsin inhibitor, 0.025% DNase, and 12 mM MgSO4 were added and mixed until tissue was thoroughly homogenized. Cells were then transferred to neurobasal medium containing B27 serum supplement, 1 mM glutamine, gentamicin, and Ara-C. Neurons were seeded on 50 μg/ml poly-d-lysine-coated 12-well tissue culture plates at a density of 2 × 106 cells.
Coimmunoprecipitations.
Transfected COS7 cells were washed with phosphate-buffered saline (PBS) and lysed in buffer containing 50 mM Tris-HCl (pH 8.0), 0.15 M NaCl, 1% Nonidet P-40, phosphatase inhibitors (Sigma), and protease inhibitors (Roche). For immunoprecipitation, the lysates were incubated for 2 h at 4°C with the anti-HA antibody or an anti-6E10 antibody bound to protein G-Sepharose beads (Amersham Biosciences). The precipitates were then washed three times with lysis buffer and resuspended in sodium dodecyl sulfate (SDS) sample buffer. The samples were separated by SDS-polyacrylamide gel electrophoresis on 4 to 15% polyacrylamide gels, transferred electrophoretically to nitrocellulose membranes, and blocked with 5% nonfat dry milk. The blots were incubated with antibodies at room temperature for 1 h. Horseradish peroxidase-conjugated secondary antibodies were visualized by using an enhanced chemiluminescence detection system and exposed to film.
Biotin-labeled cell surface proteins.
COS7 cells were transiently transfected with APP and ApoEr2 in Fugene 6 (Roche) according to the manufacturer's protocol and cultured for 24 h in Dulbecco modified Eagle medium containing 10% FBS. After 24 h, medium was changed to serum-free medium, and then F-spondin-containing medium was added. After 24 h, cells were washed twice with PBS, and surface proteins were labeled with sulfo-NHS-SS-biotin [sulfosuccinimidyl 2-(biotinamido)-ethyl-1,3-dithiopropionate)] [500 μl] at 500 μg/ml PBS (Pierce) under gentle shaking at 4°C for 30 min. Fifty microliters of quenching solution was added to cells, which were washed twice with Tris-buffered saline. Cells were lysed in 500 μl lysis buffer, collected with a cell scraper, disrupted by sonication on ice, incubated for 30 min on ice, and clarified by centrifugation (10,000 × g, 2 min). To isolate biotin-labeled proteins, lysate was added to immobilized NeutrAvidin gel (50 μl) and incubated at room temperature for 1 h. Gels were washed five times with wash buffer and incubated 1 h with SDS-polyacrylamide gel electrophoresis sample buffer including 50 mM dithiothreitol. Eluants were analyzed by immunoblotting.
Fe65-dependent APP luciferase transactivation assay.
The Fe65-dependent APP luciferase transactivation assay was performed by the method of Cao and Sudhof (3, 15). HEK293 cells were cotransfected with the APP-Gal4 construct, Fe65-myc, and ApoEr2-HA, as well as the pG5E1B-luciferase reporter plasmid (to measure activation) and a β-galactosidase plasmid (to normalize transfection efficiency). Luciferase activity from cell lysates was determined in triplicate using the luciferase assay kit (Promega) by VICTOR2 (Perkin-Elmer). Results were normalized to β-galactosidase expression levels by using a β-galactosidase enzyme assay kit (Promega).
Surgical procedures.
For brain injections, adult male Sprague-Dawley rats (230 to 250 g; Taconic) were anesthetized with ketamine/xylazine and placed in a David Korf stereotaxic apparatus (David Korf Instruments, Tujunga, CA). Single-guide cannulae (33GA; Plastics One, Inc.) were implanted into the hippocampus (final coordinates relative to Bregma: anterior/posterior, −3.8 mm; medial/lateral, −2.2 mm; dorsal/ventral, −2.6 mm) by the method of Watson (32). Cannulae were secured to the skull with the aid of three stainless steel mounting screws and cranioplastic cement. After surgery, rats were allowed to recover for 6 days before the F-spondin treatment. F-spondin was administered at 4 μl daily for 7 days. Animals were sacrificed on day 8, and the hippocampus was collected. Tissue was homogenized in radioimmunoprecipitation assay buffer with phosphatase and protease inhibitors.
Statistical analyses.
Experiments were repeated a minimum of four times unless otherwise noted. All data were analyzed by analysis of variance with Graphpad Prism 4 software, using Tukey's multiple-comparison test for posthoc analyses with significance determined at a P of <0.05. Descriptive statistics were calculated with StatView 4.1 and displayed as an expressed mean ± standard error of the mean.
RESULTS
F-spondin interacts with ApoEr2.
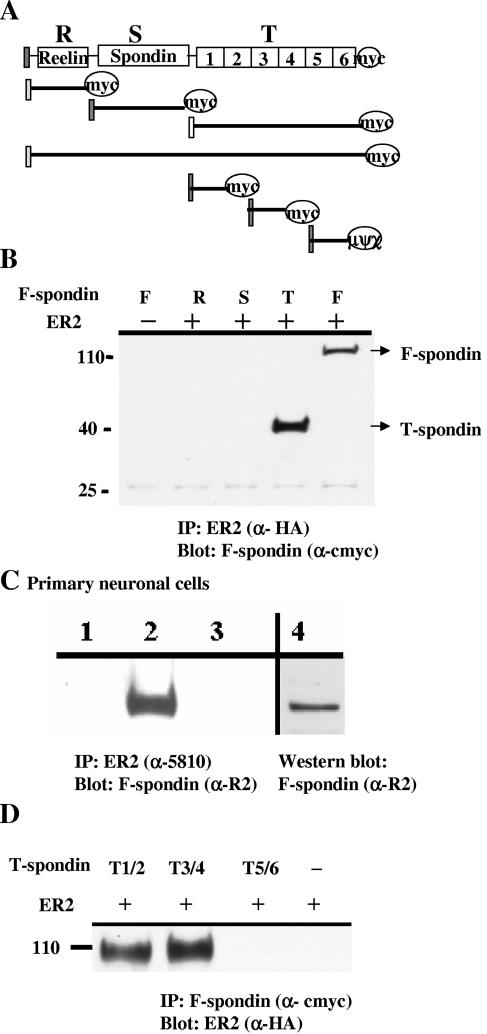
To test whether F-spondin interacted with apoE receptors, we produced several recombinant c-_myc_-tagged F-spondin constructs (Fig. 1A) and a construct of full-length ApoEr2 tagged at its C terminus with HA. We transfected COS7 cells with ApoEr2 and F-spondin constructs, immunoprecipitated ApoEr2 with anti-HA antibody, and probed with anti-c-myc antibody. Full-length F-spondin coprecipitated with ApoEr2 but did not precipitate in the absence of ApoEr2 (Fig. 1B). To determine which domain of F-spondin interacted with ApoEr2, we transfected COS7 cells with ApoEr2 and F-spondin constructs containing only the reelin, spondin, or thrombospondin domain. We immunoprecipitated ApoEr2 with the anti-HA antibody and probed with anti-c-myc antibody. The thrombospondin domain of F-spondin interacted with ApoEr2 (Fig. 1B), but the reeler and spondin domains did not. All F-spondin constructs were similarly expressed (data not shown). The specificity of the coimmunoprecipitations for only some F-spondin constructs with apoEr2 suggests that the interaction is not merely due to the fact that the two proteins were overexpressed in the same cells.
FIG. 1.
F-spondin interacts with ApoEr2. A. Several secreted deletion mutants of C-terminal myc-tagged F-spondin were produced: residues 1 to 225, reelin domain (R); residues 206 to 501, spondin domain (S); residues 502 to 807, thrombospondin domain (T). Three constructs of the thrombospondin domain were produced by PCR: repeats 1 and 2, repeats 3 and 4, and repeats 5 and 6. B. COS7 cells were transiently transfected with ApoEr2 (HA tagged) with constructs of F-spondin. ApoEr2 was immunoprecipitated (IP) with anti-HA antibody (α-HA), and precipitates were probed with anti-c-myc antibody (αcmyc). ApoEr2 precipitated full-length F-spondin (F) and the thrombospondin domain (T) of F-spondin, but not the reelin (R) or spondin (S) domain. C. Primary neuronal proteins were immunoprecipitated (IP) with anti-5810 (α5810) (recognizing ApoEr2) and probed with an anti-R2 (α-R2) (recognizing F-spondin) (lane 2). For negative controls, reactions were conducted without 5810 antibody (lane 3) or without cell lysates (lane 1). Lane 4 demonstrated F-spondin expression in the lysates. D. ApoEr2 was coexpressed with each of the three thrombospondin constructs. IP of constructs demonstrated interactions of ApoEr2 with repeats 1 and 2 and repeats 3 and 4 but not repeats 5 and 6.
To test the findings in transfected cells, we examined whether the native proteins could be coimmunoprecipitated from primary neuronal cultures of mouse embryonic neurons. We immunoprecipitated ApoEr2 with the anti-5810 antibody and probed with anti-R2 antibody (which recognizes F-spondin). Immunoprecipitation of ApoEr2 from neuronal lysates results in precipitation of F-spondin (Fig. 1C). No F-spondin was detected in control immunoprecipitation experiments performed with nonimmune serum or performed without ApoEr2 antibody.
We then tested which part of the thrombospondin domain interacted with ApoEr2. The thrombospondin domain of F-spondin consists of six repeats. We generated constructs containing repeats 1 and 2, repeats 3 and 4, or repeats 5 and 6. Each thrombospondin construct was expressed with ApoEr2, immunoprecipitated with anti-c-myc antibody, and probed with anti-HA antibody. We found that the N-terminal thrombospondin repeats (1-4) interacted with ApoEr2, but repeats 5 and 6 did not (Fig. 1D). Again, the lack of coprecipitation of ApoEr2 with thrombospondin repeats 5 and 6 supports the specificity of these findings.
The ligand binding domain of ApoEr2 interacts with F-spondin.
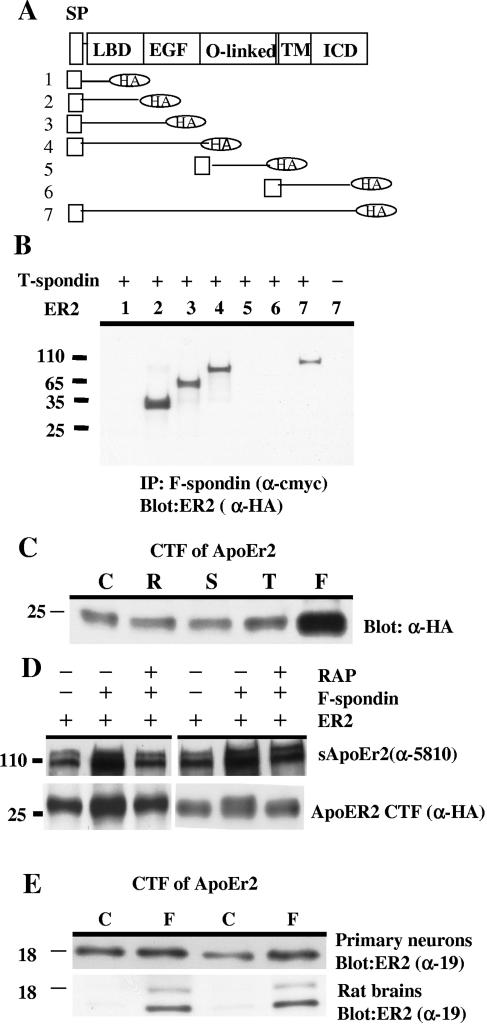
We examined which domains of ApoEr2 interacted with this thrombospondin fragment of F-spondin. We generated deletion constructs of ApoEr2 (Fig. 2A) altering expression of the ligand binding domain, the epidermal growth factor repeats (A and B), and the O-linked glycosylation domain. We cotransfected COS7 cells with the thrombospondin domain of F-spondin and these ApoEr2 deletion mutants. We found that constructs containing the entire ApoEr2 ligand binding domain immunoprecipitated with F-spondin (Fig. 2B) but that constructs lacking the ligand binding domain did not. We conclude that the ligand binding domain of ApoEr2, containing ligand binding domain repeats 3, 7, and 8, is required for the interaction of the thrombospondin domain of F-spondin (Fig. 2B).
FIG. 2.
F-spondin interacts with the ligand binding domain of ApoEr2 and promotes proteolysis. A. Several constructs of ApoEr2 with an N-terminal signal peptide and a C-terminal HA tag were produced: construct 1, residues 1 to 89; construct 2, residues 1 to 214; construct 3, residues 1 to 401; construct 4, residues 1 to 614; construct 5, residues 615 to 670; construct 6, residues 671 to 842; and construct 7, residues 1 to 842. SP, signal peptide; LBD, ligand binding domain; EGF, epidermal growth factor; TM, transmembrane; ICD, intracellular domain. B. COS7 cells were transfected with ApoEr2 constructs and the F-spondin thrombospondin domain (possessing the c-myc tag). Cell lysates were immunoprecipitated with anti-c-myc antibody (αcmyc) and probed with anti-HA antibody (α-HA). Only ApoEr2 constructs containing residues 90 to 214 immunoprecipitated with the thrombospondin domain of F-spondin. C. COS7 cells transfected with full-length ApoEr2 were treated with control medium (C) or conditioned medium containing the reelin (R), spondin (S), or thrombospondin (T) domain or full-length F-spondin (F). Cell lysates were analyzed for CTF of ApoEr2 with anti-HA antibody (α-HA), demonstrating increased ApoEr2 CTF only after treatment with full-length F-spondin. D. COS7 cells were transfected with ApoEr2, preincubated with 1 μM RAP, and then treated with the F-spondin-containing medium or control medium. Secreted ApoEr2 was measured in conditioned media with antibody 5810 (α-5810), and ApoEr2 CTF were detected from cell lysates with anti-HA antibody (α-HA). F-spondin treatment increased secreted ApoEr2 and ApoEr2 CTF; these increases were blocked by RAP. E. Primary neurons were treated with F-spondin medium, and endogenous ApoEr2 CTF was measured in cell lysates using α-19 (top blot). F-spondin-conditioned medium (F) was injected into rat hippocampus, and hippocampal proteins were analyzed similarly. Both primary neurons and brain tissue showed increased ApoEr2 CTF after F-spondin treatment. C, control medium.
We then asked whether F-spondin affected ApoEr2 processing by examining production of ApoEr2 CTF after exposing the cells to F-spondin. For these experiments we transfected COS7 cells with full-length ApoEr2 and treated them with conditioned media containing full-length F-spondin or one of the three F-spondin domains. We found that treatment with full-length F-spondin, but not any of the single F-spondin domains, increased C-terminal fragments of ApoEr2 (Fig. 2C). Of note, although the thrombospondin domain interacted with ApoEr2 (Fig. 1), it did not affect ApoEr2 cleavage.
RAP inhibited ApoEr2 processing by F-spondin.
To test whether the effects of F-spondin on ApoEr2 processing were due to binding of F-spondin to ApoEr2, we used an antagonist of the LDL receptor family, RAP (33). COS7 cells were transfected with ApoEr2, preincubated with 1 μM RAP, and treated with F-spondin-containing medium or control medium. As described above, we found that F-spondin treatment increased secreted ApoEr2 and ApoEr2 CTF. However, preincubation with RAP blocked F-spondin induction of secreted ApoEr2 and ApoEr2 CTF (Fig. 2D). These data suggest that the effects of F-spondin were mediated by interacting with ApoEr2.
We then asked whether F-spondin also affected endogenous ApoEr2 in neurons, using an antibody against the C-terminal fragment of ApoEr2. Primary neurons were treated with F-spondin-containing medium, and ApoEr2 CTF was measured in the cell lysates. We found that F-spondin treatment caused an increase in ApoEr2 CTF (Fig. 2E, top blot). We also injected F-spondin-containing medium into rat hippocampus to examine the effects of F-spondin in vivo. Again, we observed an increase in two ApoEr2 CTF (Fig. 2E, bottom blot).
APP interacts with the reelin and spondin domains of F-spondin.
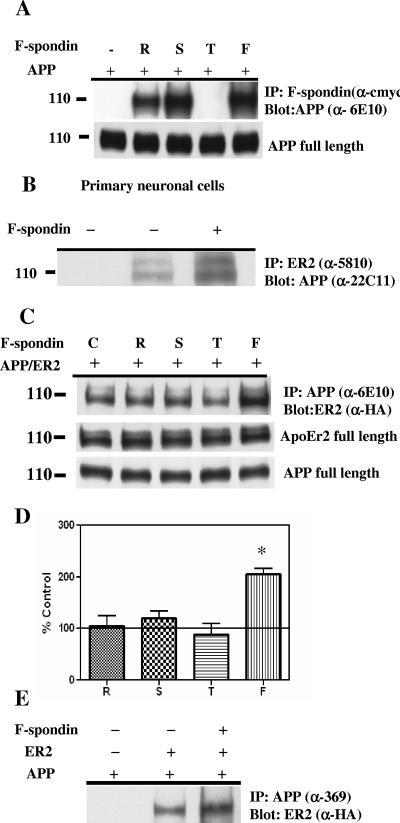
A recent study showed that F-spondin interacted with APP and inhibited β-secretase cleavage (8), thus providing some insight into the mechanism of APP metabolism. We confirmed that APP interacts specifically with F-spondin, via the reeler and spondin domains, but not the thrombospondin domain (Fig. 3A). Thus, F-spondin interacted with both APP and ApoEr2 but through different domains. These data suggested that F-spondin could provide an extracellular link between APP and ApoEr2. We were concerned that transient cotransfection of APP with F-spondin in COS7 cells may have affected APP expression, but we found that the levels of APP were consistent (Fig. 3A, bottom blot).
FIG. 3.
F-spondin increases coimmunoprecipitation of APP and ApoEr2. A. COS7 cells were transfected with full-length APP and constructs of F-spondin domains (reelin [R], spondin [S], and thrombospondin [T]) or full-length F-spondin (F). Cell lysates were immunoprecipitated (IP) with anti-c-myc (α-cmyc) and probed with 6E10 antibody (α-6E10). APP coprecipitated with reelin and spondin domains of F-spondin, but not with the thrombospondin domain. Expression of full-length APP in cell extracts was not altered after treatment. B. Primary neuronal proteins were immunoprecipitated with anti-5810 (α-5810) (recognizing ApoEr2) and probed with anti-22C11 (α-22C11) (recognizing APP) after control (middle lane) or F-spondin-containing medium treatment (rightmost lane). For a negative control, no precipitating antibody was used (leftmost lane). C. COS7 cells expressing full-length APP and ApoEr2 were treated with control medium (C) or medium containing the reelin (R), spondin (S), or thrombospondin (T) domain or full-length F-spondin (F). Cell lysates were immunoprecipitated with 6E10 antibody and probed with HA. Increased coprecipitation of APP and ApoEr2 was observed in the presence of full-length F-spondin. The levels of APP and ApoEr2 were not altered after treatment. D. Levels of ApoEr2 after coprecipitation with APP were quantified and compared to control conditions (defined as 100%). Full-length F-spondin treatment increased APP/apoEr2 coprecipitation, by 105% (P < 0.05). E. APP and ApoEr2 were coexpressed in COS7 cells, and cellular proteins were immunoprecipitated with the 369 antibody and probed with anti-HA antibody. Full-length APP was coprecipitated with ApoEr2.
These data suggested that APP and ApoEr2 might coimmunoprecipitate. Thus, we examined whether the native proteins could be coprecipitated from primary neuronal cultures of mouse embryonic neurons. We immunoprecipitated ApoEr2 with the anti-5810 antibody and probed with 22C11 antibody (recognizing APP). We found that immunoprecipitation of ApoEr2 from neuronal lysates coprecipitated APP, whereas no APP was detected in control immunoprecipitation experiments performed with nonimmune serum or without ApoEr2 antibody (Fig. 3B).
We then asked whether this interaction was increased by F-spondin. We coexpressed full-length APP with ApoEr2 and treated cells with media containing the single F-spondin domains (reelin, spondin, and thrombospondin) or media containing full-length F-spondin overnight. Interestingly, when cells were incubated with full-length F-spondin-containing medium, we observed increased coimmunoprecipitation of APP and ApoEr2 (Fig. 3C), but not when cells were incubated with any single F-spondin domain. Again, we were concerned that coexpression of APP and ApoEr2 in COS7 cells may affect their expression, so we determined the levels of APP and ApoEr2 in these cells. The levels of APP and ApoEr2 did not vary across conditions (Fig. 3C). Quantification of Western blots demonstrated that APP/ApoEr2 coprecipitation was increased by 105% after full-length F-spondin treatment (Fig. 3D), but no significant changes were observed after treatment with media containing the F-spondin fragments.
To test whether the interaction between APP and apoEr2 required full-length APP or only secreted APP, we coexpressed full-length APP with ApoEr2 and treated cells with media containing full-length F-spondin overnight. We immunoprecipitated APP with the C-terminal 369 antibody and probed with anti-HA antibody (for ApoEr2). We observed increased coimmunoprecipitation of APP and ApoEr2 after F-spondin treatment (Fig. 3E), demonstrating that full-length APP interacted with apoEr2.
F-spondin promotes APP and ApoEr2 cell surface levels and processing.
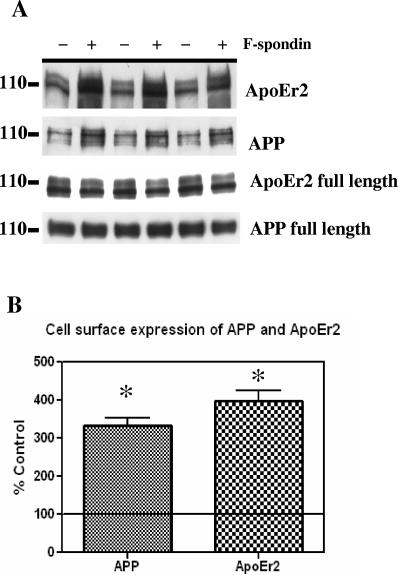
We hypothesized that F-spondin bound the extracellular domains of APP and ApoEr2, clustering these molecules together and potentially affecting their presence on the cell surface. To measure cell surface expression of APP and ApoEr2, we coexpressed these molecules in COS7 cells and treated cells with control or F-spondin-containing medium for 12 h. Cell surface proteins were biotin labeled, isolated with avidin beads, and immunoblotted for ApoEr2 or APP. We observed that F-spondin treatment promoted cell surface levels of both APP and ApoEr2 (Fig. 4A). Expression of total cell-associated levels of ApoEr2 and APP were not altered after F-spondin treatment (Fig. 4A, bottom two blots).
FIG. 4.
F-spondin increases cell surface levels of ApoEr2 and APP. A. COS7 cells expressing full-length APP and ApoEr2 were treated with control medium (−) or F-spondin-containing medium (+) for 12 h. Cell surface proteins were labeled with biotin, isolated with avidin beads, and immunoblotted with 5810 for ApoEr2 or with C1/6.1 for APP. Increased surface levels of APP and ApoEr2 were observed after F-spondin treatment. The levels of cellular APP were not altered after F-spondin treatment; the levels of ApoEr2 showed a slight decrease after F-spondin treatment. B. The increases in cell surface APP and ApoEr2 after F-spondin treatment were quantified from the Western blots. Surface levels were increased between 200 and 300% (asterisks indicate statistical significance [P < 0.01]).
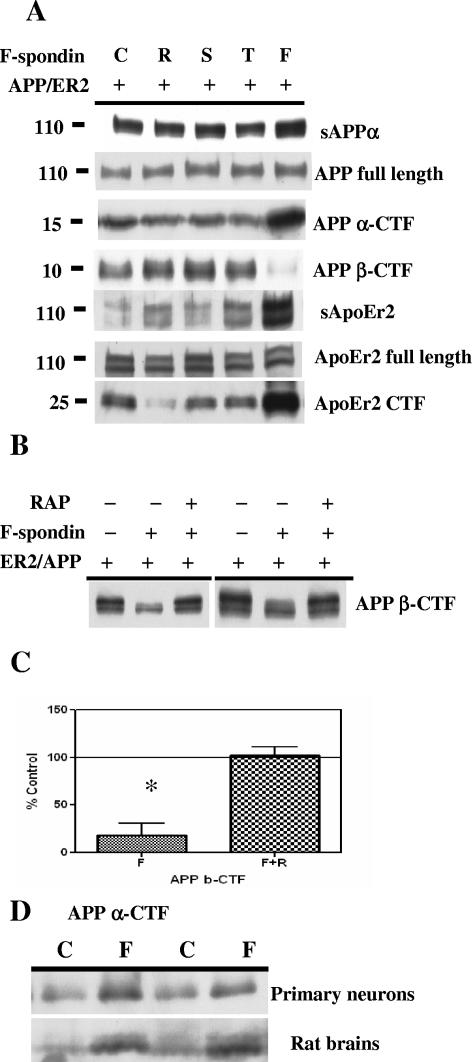
To examine whether F-spondin affected APP and ApoEr2 processing, we coexpressed APP and ApoEr2 and treated cells with control medium or medium containing reelin, spondin, thrombospondin, or full-length F-spondin. We measured secreted APP, full-length APP, CTF of APP, and β-CTF. The F-spondin-containing media were probed for the presence of secreted forms of APP and ApoEr2, but none were observed (data not shown). We observed increased levels of secreted APP and APP CTF after treatment with full-length F-spondin but not after treatment with any of the single F-spondin domains (Fig. 5A). We also found that F-spondin treatment decreased β-CTF (Fig. 5A). The levels of total cellular APP were not altered. We also measured secreted ApoEr2, full-length ApoEr2, and ApoEr2 CTF. We found that F-spondin treatment increased secreted ApoEr2 and ApoEr2 CTF but did not affect cellular ApoEr2 (Fig. 5A). Together, these data suggest that F-spondin promotes an interaction between cell surface APP and ApoEr2, inhibiting β-secretase cleavage.
FIG. 5.
F-spondin alters APP processing. A. COS7 cells expressing APP and ApoEr2 were treated with control medium (C) or with media containing reelin (R), spondin (S), or thrombospondin (T) or full-length F-spondin (F). Conditioned media were analyzed for secreted forms of APP (sAPPα) (6E10) and ApoEr2 (5810); cell lysates were analyzed for full-length APP (c1/6.1), α-CTF of APP (c1/6.1), β-CTF of APP (6E10), full-length ApoEr2 (5810), or CTF of ApoEr2 (anti-HA). Full-length F-spondin, but not the individual domains, increased secreted forms and CTF of APP and ApoEr2 but decreased β-CTF of APP. The levels of full-length APP and ApoEr2 were not altered. B. COS7 cells were transfected with APP or ApoEr2, preincubated with 1 μM RAP, and then treated with the F-spondin-containing medium or control medium. Cell lysates were harvested, and β-CTF of APP were detected with 6E10. The decreased level of β-CTF of APP was inhibited by RAP. C. The decrease in the level of β-CTF was quantified from Western blots, and the level significantly decreased over 80% after full-length F-spondin treatment (P < 0.05). D. Primary neurons were treated with control medium (C) or medium containing F-spondin (F), and the level of APP CTF was measured in cell lysates with antibody C1/6.1 (top blot). F-spondin was injected into rat hippocampus, and hippocampal proteins were similarly analyzed for APP CTF (bottom blot). Both primary neurons and brain tissue showed increased APP CTF after F-spondin treatment.
To test whether the effects of F-spondin on APP β-CTF were due to ApoEr2, COS7 cells were cotransfected with ApoEr2 and APP, preincubated with 1 μM RAP, and treated with the F-spondin-containing medium or control medium. As described above, we found that F-spondin treatment decreased APP β-CTF, but preincubation with RAP blocked this effect (Fig. 5B and C). These data suggest that the effects of F-spondin on APP processing require interactions with apoE receptors.
We then asked whether F-spondin also affected endogenous APP processing, using an antibody against the C terminus of APP. Primary neurons were treated with F-spondin-containing medium, and the levels of APP CTF in the cell lysates were measured. We found that F-spondin treatment increased in APP CTF (Fig. 5D, top blot), consistent with the increase in APP α-CTF in transfected cells (Fig. 5A). We also injected control or F-spondin-containing media into rat hippocampus to examine the effects of F-spondin in vivo. We again observed an increase in APP CTF in this system (Fig. 5D, bottom blot).
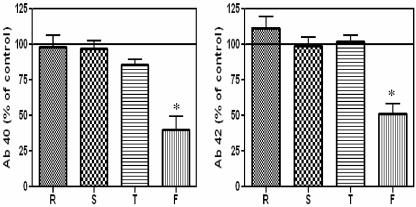
Since F-spondin affected APP processing, we examined whether it affected Aβ production. We found that F-spondin treatment decreased secreted Aβ40 levels (by 70%) and Aβ42 levels (by 50%) in COS7 cells (Fig. 6). We also tested whether F-spondin decreased Aβ40 or Aβ42 in primary neurons, as we had observed for the transfected COS7 cells. Exposure of cultured neurons to medium containing full-length F-spondin decreased the level of Aβ by 70% compared to the level in cells treated with control medium (data not shown). These data are consistent with F-spondin reducing the level of APP β-CTF.
FIG. 6.
F-spondin decreases Aβ40 (Ab 40) and Aβ42 (Ab 42). COS7 cells expressing APP and ApoEr2 were treated with control medium (set to 100%) or medium containing reelin (R), spondin (S), or thrombospondin (T) or full-length F-spondin (F). Aβ40 and Aβ42 levels in the conditioned media were determined by enzyme-linked immunosorbent assay. Full-length F-spondin, but not individual domains, decreased secreted Aβ40 levels (by 70%, P < 0.05) and Aβ42 (by 50%, P < 0.05).
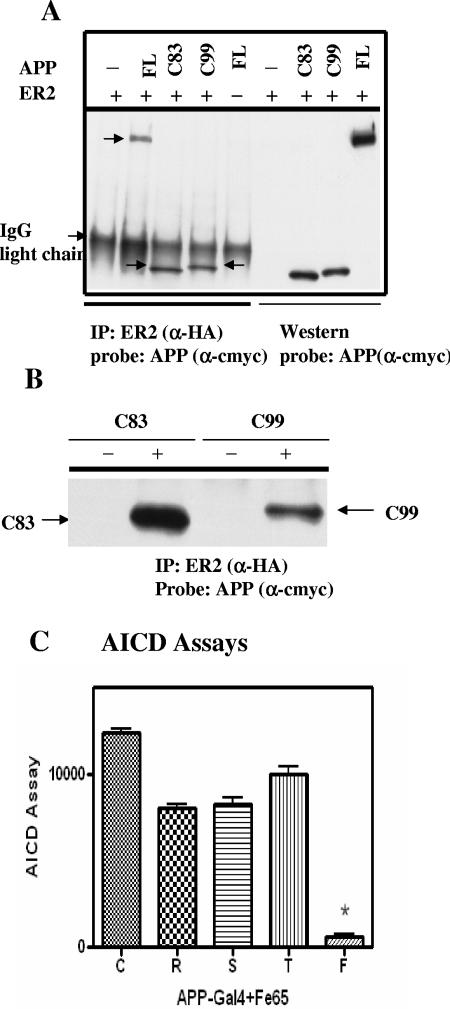
To examine whether interactions between APP and ApoEr2 could affect other APP fragments, we expressed constructs of APP α- and β-CTF (C83 and C99), with ApoEr2. Surprisingly, we observed that these APP CTF also coprecipitated with ApoEr2 (Fig. 7). We were concerned that the interactions between ApoEr2 and APP CTF were not biologically relevant. For a negative control, we expressed APP and ApoEr2 in separate cells and, after their expression, mixed the cell lysates. ApoEr2 was immunoprecipitated with the anti-HA antibody, and the precipitate was probed with anti-c-myc antibody (recognizing APP). APP and ApoEr2 coimmunoprecipitated when they were coexpressed in the same cells, but not in separate cells (Fig. 7B).
FIG. 7.
APP CTF interact with ApoEr2. A. COS7 cells were transfected with full-length ApoEr2 (HA tagged) and full-length APP (FL), C83, C99 (myc tagged), or vector alone (−). Cell lysates were immunoprecipitated (IP) with anti-HA (α-HA) and probed with anti-c-myc (α-cmyc). Western blotting with the anti-c-myc antibody (α-cmyc) demonstrated cell expression of APP constructs. All three APP constructs coprecipitated with ApoEr2. The position of immunoglobulin G (IgG) light chain is shown to the left of the gel. B. APP constructs and ApoEr2 were expressed in separate COS7 cell cultures, and mixed lysates (−) or APP constructs and ApoEr2 were expressed in the same cells (+). Cell lysates were immunoprecipitated (IP) with anti-HA and probed with anti-myc. APP constructs and ApoEr2 did not associate if not expressed in the same cells. C. HEK293 cells were cotransfected with APP-Gal4, Fe65, ApoEr2, and luciferase reporter constructs. Cells were treated with control medium (C) or medium containing reelin (R), spondin (S), thrombospondin (T), or full-length F-spondin (F). Only full-length F-spondin significantly decreased Fe65-dependent APP luciferase transactivation (P < 0.01).
Finally, we asked whether F-spondin treatment affected the cytoplasmic product of γ-secretase cleavage of APP CTF, the APP intracellular domain (AICD). We used an Fe65-dependent APP luciferase transactivation assay to assess AICD production in the presence of each domain of F-spondin or full-length F-spondin. HEK293 cells were cotransfected with APP-Gal4, Fe65, and ApoEr2 (3). Cells without Fe65 showed no response, as expected (3) (data not shown). As observed in the previous report (8), F-spondin resulted in 90% reduction of transactivation (Fig. 7C). Again, single domains of F-spondin did not affect production of APP fragments.
DISCUSSION
In this work, we demonstrate that F-spondin coimmunoprecipitates with the apoE receptor ApoEr2 both in transfected cells and in primary neurons (Fig. 1). This interaction occurs via the ligand binding domain of ApoEr2 (specifically domain 3, 7, or 8) (Fig. 2B), and the thrombospondin domain of F-spondin (specifically, the first four thrombospondin repeats) (Fig. 1D). The interaction of thrombospondin repeats with an apoE receptor is consistent with the interaction of thrombospondin 1 with another apoE receptor, LRP (6). Interestingly, F-spondin binds the extracellular matrix via the last two thrombospondin repeats (30), which do not bind ApoEr2. These repeats are cleaved from F-spondin by plasmin (30), which would disrupt interactions between cell surface ApoEr2 and the extracellular matrix. The interaction between F-spondin, extracellular matrix, and cell surface receptors may be important for the functions of F-spondin in axonal migration (29) or neurite outgrowth after nerve injury (2).
Full-length F-spondin increases cleavage of ApoEr2, resulting in increased secreted ApoEr2 and increased ApoEr2 CTF (Fig.2C and D). These findings are consistent with our earlier studies that binding of apoE to the ligand binding domain of ApoEr2 led to increased extracellular metalloproteinase cleavage and intramembranous γ-secretase cleavage of ApoEr2 (10). These effects on cleavage may be related to increased transfer of intracellular pools of ApoEr2 to the cell surface after treatment with F-spondin (Fig. 4). Interestingly, the effect of F-spondin on ApoEr2 proteolysis depends on the presence of full-length protein and does not occur with only the thrombospondin domain that interacts with ApoEr2 (Fig. 2C). Thus, the effects of F-spondin on cleavage of apoE receptors may require the binding of F-spondin to other surface molecules, such as APP. This hypothesis is consistent with the effects of apoE receptor clustering for signal transduction in neurons (25).
In this work, we confirmed the report that F-spondin interacted with APP (8). The earlier study showed interaction via the spondin domain, although we found interactions with both the reelin and spondin domains (Fig. 3). Whichever domain interacts with APP in vivo, the current work suggests that a single F-spondin molecule could interact with both APP (via its N-terminal domains) and ApoEr2 (via its C-terminal domain). We immunoprecipitated ApoEr2 with APP both in transfected cells and in primary neurons, and this immunoprecipitation was increased by the presence of full-length (but not truncated) F-spondin. This experiment provides evidence that there could exist a tripartite complex of APP, F-spondin, and ApoEr2.
The interaction between F-spondin and APP in COS7 cells decreased β-cleavage of APP (8). We confirmed these findings (Fig. 5A and B), using media containing F-spondin as opposed to coexpressing F-spondin in cells (8). The β-cleavage of APP may be affected by the increased levels of APP on the cell surface after exposure to F-spondin (Fig. 4). We further demonstrated that this effect depended on full-length (not truncated) F-spondin and demonstrated that full-length F-spondin decreased secreted Aβ levels (Fig. 6). Furthermore, we demonstrated that these effects on APP proteolysis depend on interactions with apoE receptors, since the inhibitor of the apoE receptor family, RAP, blocked the effects of F-spondin on APP proteolysis (Fig. 5B). This model is consistent with the results of several other studies that showed that APP physically interacted with another apoE receptor, LRP (14, 24), and that this interaction affected APP processing (13, 31).
In contrast to its effects on β-cleavage of APP, F-spondin treatment increased the levels of α-CTF of APP and CTF of ApoEr2 in transfected cells, in primary neurons, and in brain (Fig. 2, 3, and 5). These observations are in accordance with the effects of ligands binding to ApoEr2 (10). However, the dramatic increase in APP α-CTF is in contrast to the reduction in AICD signaling after F-spondin treatment (8) (Fig. 7C), since AICD can be produced from α-CTF. We hypothesize that the change in AICD signaling may not be due to a change in AICD levels, but in binding of Fe65 to AICD or a subsequent step in AICD signaling. Since F-spondin changes trafficking of APP (as evidenced by the increase of surface APP), it is possible that F-spondin could also affect the interaction of APP with cytoplasmic adaptor proteins.
We were surprised to find that in addition to the extracellular interaction between APP and ApoEr2 observed (which is increased in the presence of F-spondin), there is an intracellular interaction. Membrane-bound APP CTF (C99 and C83) with few extracellular amino acids still immunoprecipitated with ApoEr2 (Fig. 7A and B). Again, there is an analogous situation with APP and LRP, with the C-terminal domains of both proteins binding to different domains of the adaptor protein Fe65 (12, 21, 28), affecting processing of both LRP (7) and APP (21). It is possible that adaptor proteins, such as Fe65, could form an intracellular link between ApoEr2 and APP.
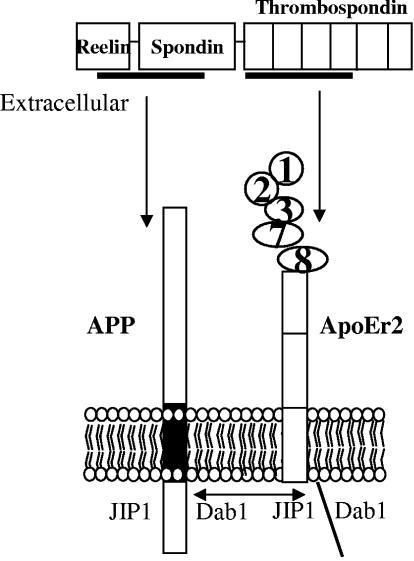
From these various experiments, we have developed a model of interaction of APP and ApoEr2 (Fig. 8). In this model, binding of APP and ApoEr2 to different domains of F-spondin clusters the transmembrane molecules together, allowing increased interaction of their C-terminal domains. These domains share adaptor proteins, such as JIP and disabled-1, potentially affecting signaling cascades mediated by these proteins. The potential clustering of APP and ApoEr2 leads to altered cleavage of each, both via extracellular metalloproteinases and intramembranous γ-secretase cleavage. The shared fates and functions of APP and apoE receptors suggest that apoE and other ligands of lipoprotein receptors could affect APP processing and thus influence Aβ levels.
FIG. 8.
Model of APP, ApoEr2, and F-spondin interactions. The reelin and spondin domains of F-spondin interact with an extracellular domain of APP, and the thrombospondin domain interacts with the ligand binding domain of ApoEr2 (containing repeats 1, 2, 3, 7, and 8). Full-length F-spondin can form a tripartite complex with APP and ApoEr2. APP and ApoEr2 also interact via intracellular domains, perhaps involving adaptor proteins Dab1 (disabled-1) and JIP (JNK-interacting protein).
Acknowledgments
We thank T. C. Sudhof for providing full-length F-spondin plasmid DNA; Avihu Klar for antibodies and advice; and Anne Cataldo, Sam Gandy, and Johannes Nimpf for antibodies. We also thank Oksana Berezovska and Dudley Strickland for useful reagents and materials. We thank Ana Pocivavsek for excellent technical assistance.
This work is supported by NIH grant AG14473.
REFERENCES
- 1.Brandes, C., L. Kahr, W. Stockinger, T. Hiesberger, W. J. Schneider, and J. Nimpf. 2001. Alternative splicing in the ligand binding domain of mouse ApoE receptor-2 produces receptor variants binding reelin but not alpha 2-macroglobulin. J. Biol. Chem. 276**:**22160-22169. [DOI] [PubMed] [Google Scholar]
- 2.Burstyn-Cohen, T., A. Frumkin, Y. T. Xu, S. S. Scherer, and A. Klar. 1998. Accumulation of F-spondin in injured peripheral nerve promotes the outgrowth of sensory axons. J. Neurosci. 18**:**8875-8885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao, X., and T. C. Sudhof. 2001. A transcriptively [sic] active complex of APP with Fe65 and histone acetyltransferase Tip60. Science 293**:**115-120. [DOI] [PubMed] [Google Scholar]
- 4.Chen, K., P. G. Ochalski, T. S. Tran, N. Sahir, M. Schubert, A. Pramatarova, and B. W. Howell. 2004. Interaction between Dab1 and CrkII is promoted by Reelin signaling. J. Cell Sci. 117**:**4527-4536. [DOI] [PubMed] [Google Scholar]
- 5.Cho, H. S., B. T. Hyman, S. M. Greenberg, and G. W. Rebeck. 2001. Quantitation of apoE domains in Alzheimer disease brain suggests a role for apoE in Aβ aggregation. J. Neuropathol. Exp. Neurol. 60**:**342-349. [DOI] [PubMed] [Google Scholar]
- 6.Godyna, S., G. Liau, I. Popa, S. Stefansson, and W. S. Argraves. 1995. Identification of the low density lipoprotein receptor-related protein (LRP) as an endocytic receptor for thrombospondin-1. J. Cell Biol. 129**:**1403-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guenette, S. Y., Y. Chang, B. T. Hyman, R. E. Tanzi, and G. W. Rebeck. 2002. Low-density lipoprotein receptor-related protein levels and endocytic function are reduced by overexpression of the FE65 adaptor protein, FE65L1. J. Neurochem. 82**:**755-762. [DOI] [PubMed] [Google Scholar]
- 8.Ho, A., and T. C. Sudhof. 2004. Binding of F-spondin to amyloid-beta precursor protein: a candidate amyloid-beta precursor protein ligand that modulates amyloid-beta precursor protein cleavage. Proc. Natl. Acad. Sci. USA 101**:**2548-2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoe, H. S., D. C. Harris, and G. W. Rebeck. 2005. Multiple pathways of apolipoprotein E signaling in primary neurons. J. Neurochem. 93**:**145-155. [DOI] [PubMed] [Google Scholar]
- 10.Hoe, H. S., and G. W. Rebeck. 2005. Regulation of ApoE receptor proteolysis by ligand binding. Mol. Brain Res. 137**:**31-39. [DOI] [PubMed] [Google Scholar]
- 11.Horikoshi, Y., G. Sakaguchi, A. G. Becker, A. J. Gray, K. Duff, P. S. Aisen, H. Yamaguchi, M. Maeda, N. Kinoshita, and Y. Matsuoka. 2004. Development of Aβ terminal end-specific antibodies and sensitive ELISA for Aβ variant. Biochem. Biophys. Res. Commun. 319**:**733-737. [DOI] [PubMed] [Google Scholar]
- 12.Kinoshita, A., C. M. Whelan, C. J. Smith, I. Mikhailenko, G. W. Rebeck, D. K. Strickland, and B. T. Hyman. 2001. Demonstration by fluorescence resonance energy transfer of two sites of interaction between the low-density lipoprotein receptor-related protein and the amyloid precursor protein: role of the intracellular adapter protein Fe65. J. Neurosci. 21**:**8354-8361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knauer, M. F., R. A. Orlando, and C. G. Glabe. 1996. Cell surface APP751 forms complexes with protease nexin 2 ligands and is internalized via the low density lipoprotein receptor-related protein (LRP). Brain Res. 740**:**6-14. [DOI] [PubMed] [Google Scholar]
- 14.Kounnas, M. Z., R. D. Moir, G. W. Rebeck, A. I. Bush, W. S. Argraves, R. E. Tanzi, B. T. Hyman, and D. K. Strickland. 1995. LDL receptor-related protein, a multifunctional ApoE receptor, binds secreted beta-amyloid precursor protein and mediates its degradation. Cell 82**:**331-340. [DOI] [PubMed] [Google Scholar]
- 15.Lleo, A., O. Berezovska, P. Ramdya, H. Fukumoto, S. Raju, T. Shah, and B. T. Hyman. 2003. Notch1 competes with the amyloid precursor protein for gamma-secretase and down-regulates presenilin-1 gene expression. J. Biol. Chem. 278**:**47370-47375. [DOI] [PubMed] [Google Scholar]
- 16.Mauch, D. H., K. Nagler, S. Schumacher, C. Goritz, E. C. Muller, A. Otto, and F. W. Pfrieger. 2001. CNS synaptogenesis promoted by glia-derived cholesterol. Science 294**:**1354-1357. [DOI] [PubMed] [Google Scholar]
- 17.May, P., Y. K. Reddy, and J. Herz. 2002. Proteolytic processing of low density lipoprotein receptor-related protein mediates regulated release of its intracellular domain. J. Biol. Chem. 277**:**18736-18743. [DOI] [PubMed] [Google Scholar]
- 18.Namba, Y., M. Tomonaga, H. Kawasaki, E. Otomo, and K. Ikeda. 1991. Apolipoprotein E immunoreactivity in cerebral amyloid deposits and neurofibrillary tangles in Alzheimer's disease and kuru plaque amyloid in Creutzfeldt-Jakob disease. Brain Res. 541**:**163-166. [DOI] [PubMed] [Google Scholar]
- 19.Nathan, B. P., K. C. Chang, S. Bellosta, E. Brisch, N. Ge, R. W. Mahley, and R. E. Pitas. 1995. The inhibitory effect of apolipoprotein E4 on neurite outgrowth is associated with microtubule depolymerization. J. Biol. Chem. 270**:**19791-19799. [DOI] [PubMed] [Google Scholar]
- 20.Nathan, B. P., Y. Jiang, G. K. Wong, F. Shen, G. J. Brewer, and R. G. Struble. 2002. Apolipoprotein E4 inhibits, and apolipoprotein E3 promotes neurite outgrowth in cultured adult mouse cortical neurons through the low-density lipoprotein receptor-related protein. Brain Res. 928**:**96-105. [DOI] [PubMed] [Google Scholar]
- 21.Pietrzik, C. U., I. S. Yoon, S. Jaeger, T. Busse, S. Weggen, and E. H. Koo. 2004. FE65 constitutes the functional link between the low-density lipoprotein receptor-related protein and the amyloid precursor protein. J. Neurosci. 24**:**4259-4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qiu, Z., D. K. Strickland, B. T. Hyman, and G. W. Rebeck. 2002. α2-Macroglobulin exposure reduces calcium responses to N-methyl-D-aspartate via low density lipoprotein receptor-related protein in cultured hippocampal neurons. J. Biol. Chem. 277**:**14458-14466. [DOI] [PubMed] [Google Scholar]
- 23.Rebeck, G. W., J. S. Reiter, D. K. Strickland, and B. T. Hyman. 1993. Apolipoprotein E in sporadic Alzheimer's disease: allelic variation and receptor interactions. Neuron 11**:**575-580. [DOI] [PubMed] [Google Scholar]
- 24.Rebeck, G. W., R. D. Moir, S. Mui, D. K. Strickland, R. E. Tanzi, and B. T. Hyman. 2001. Association of membrane-bound amyloid precursor protein APP with the apolipoprotein E receptor LRP. Brain Res. Mol. Brain Res. 87**:**238-245. [DOI] [PubMed] [Google Scholar]
- 25.Strasser, V., D. Fasching, C. Hauser, H. Mayer, H. H. Bock, T. Hiesberger, J. Herz, E. J. Weeber, J. D. Sweatt, A. Pramatarova, B. Howell, W. J. Schneider, and J. Nimpf. 2004. Receptor clustering is involved in Reelin signaling. Mol. Cell. Biol. 24**:**1378-1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strickland, D. K., M. Z. Kounnas, and W. S. Argraves. 1995. LDL receptor-related protein: a multiligand receptor for lipoprotein and proteinase catabolism. FASEB J. 9**:**890-898. [DOI] [PubMed] [Google Scholar]
- 27.Strittmatter, W. J., A. M. Saunders, D. Schmechel, M. Pericak-Vance, J. Enghild, G. S. Salvesen, and A. D. Roses. 1993. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc. Natl. Acad. Sci. USA 90**:** 1977-1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trommsdorff, M., J. P. Borg, B. Margolis, and J. Herz. 1998. Interaction of cytosolic adaptor proteins with neuronal apolipoprotein E receptors and the amyloid precursor protein. J. Biol. Chem. 273**:**33556-33560. [DOI] [PubMed] [Google Scholar]
- 29.Tzarfati-Majar, V., T. Burstyn-Cohen, and A. Klar. 2001. F-spondin is a contact-repellent molecule for embryonic motor neurons. Proc. Natl. Acad. Sci. USA 98**:**4722-4727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tzarfaty-Majar, V., R. Lopez-Alemany, Y. Feinstein, L. Gombau, O. Goldshmidt, E. Soriano, P. Munoz-Canoves, and A. Klar. 2001. Plasmin-mediated release of the guidance molecule F-spondin from the extracellular matrix. J. Biol. Chem. 276**:**28233-28241. [DOI] [PubMed] [Google Scholar]
- 31.Ulery, P. G., J. Beers, I. Mikhailenko, R. E. Tanzi, G. W. Rebeck, B. T. Hyman, and D. K. Strickland. 2000. Modulation of beta-amyloid precursor protein processing by the low density lipoprotein receptor-related protein (LRP). Evidence that LRP contributes to the pathogenesis of Alzheimer's disease. J. Biol. Chem. 275**:**7410-7415. [DOI] [PubMed] [Google Scholar]
- 32.Watson, P. 1998. The rat brain in stereotaxic coordinates. Academic Press, San Diego, Calif.
- 33.Williams, S. E., J. D. Ashcom, W. S. Argraves, and D. K. Strickland. 1992. A novel mechanism for controlling the activity of alpha 2-macroglobulin receptor/low density lipoprotein receptor-related protein. Multiple regulatory sites for 39-kDa receptor-associated protein. J. Biol. Chem. 267**:**9035-9040. [PubMed] [Google Scholar]
- 34.Wisniewski, T., and B. Frangione. 1992. Apolipoprotein E: a pathological chaperone protein in patients with cerebral and systemic amyloid. Neurosci Lett. 135**:**235-238. [DOI] [PubMed] [Google Scholar]