mda-7 (IL-24) mediates selective apoptosis in human melanoma cells by inducing the coordinated overexpression of the GADD family of genes by means of p38 MAPK (original) (raw)
Abstract
Subtraction hybridization identified melanoma differentiation-associated gene-7 (_mda_-7) as a gene induced during terminal differentiation in human melanoma cells. On the basis of structure, chromosomal localization and cytokine-like properties, _mda_-7 is classified as IL-24. Administration of _mda_-7/IL-24 by means of a replication-incompetent adenovirus (Ad._mda_-7) induces apoptosis selectively in diverse human cancer cells without inducing harmful effects in normal fibroblast or epithelial cells. The present studies investigated the mechanism underlying this differential apoptotic effect. Infection of melanoma cells, but not normal immortal melanocytes, with Ad._mda_-7 induced a time- and dose-dependent increase in expression, mRNA and protein, of a family of growth arrest and DNA damage (GADD)-inducible genes, which correlated with induction of apoptosis. Among the members of the GADD family of genes, GADD153, GADD45α, and GADD34 displayed marked, and GADD45γ showed minimal induction. Treatment of melanoma cells with SB203580, a selective inhibitor of the p38 mitogen-activated protein kinase (MAPK) pathway, effectively inhibited Ad._mda_-7-induced apoptosis. Additional support for an involvement of the p38 MAPK pathway in Ad._mda_-7-mediated apoptosis was documented by using an adenovirus expressing a dominant negative mutant of p38 MAPK. Infection with Ad._mda_-7 increased the phosphorylation of p38 MAPK and heat shock protein 27 in melanoma cells but not in normal immortal melanocytes. In addition, SB203580 effectively inhibited Ad._mda_-7-mediated induction of the GADD family of genes in a time- and dose-dependent manner, and it effectively blocked Ad._mda_-7-mediated down-regulation of the antiapoptotic protein BCL-2. Inhibition of GADD genes by an antisense approach either alone or in combination also effectively blocked Ad._mda_-7-induced apoptosis in melanoma cells. These results support the hypothesis that Ad._mda_-7 mediates induction of the GADD family of genes by means of the p38 MAPK pathway, thereby resulting in the selective induction of apoptosis in human melanoma cells.
Keywords: melanoma differentiation-associated gene-7‖growth arrest DNA damage-inducible gene family‖programmed cell death
Abnormalities in differentiation represent a distinctive characteristic and frequent event in many histologically dissimilar cancers (1–3). Exploiting these defects in tumor cells represents a potentially less toxic form of therapy, “differentiation therapy” (4, 5). Treatment of human melanoma cells with a combination of fibroblast IFN-β and the protein kinase C activator mezerein results in irreversible growth arrest, terminal differentiation, and eventually programmed cell death (apoptosis) (2, 6). To define the gene expression changes associated with and potentially causative of these profound changes in melanoma cell physiology, we have used various subtraction hybridization strategies (7–11). This approach resulted in the identification of _mda-_7 as a unique gene selectively up-regulated during the process of terminal differentiation and irreversible growth arrest in melanoma cells (11). Current data suggest that _mda-_7 is a member of the IL-10 subfamily, which now includes IL-19, IL-TIF, AK-155, and IL-20 (12–16). On the basis of the presence of an IL-10 signature sequence, a 49-aa N-terminal signal peptide, physical location in the human genome on chromosome locus 1q32 in an apparent cytokine cluster, including IL-10, IL-19, and IL-20, and its ability to signal through the IL-20R complex, _mda-_7 has now been classified as IL-24 (16–18).
An intriguing property of _mda-_7/IL-24 is its ability, when expressed by means of a replication-incompetent adenovirus, Ad._mda-_7, to induce apoptosis in many human cancer cell contexts, but sparing normal human cells from toxicity (19). Ad._mda-_7 infection of melanoma, breast carcinoma, colon carcinoma, prostate carcinoma, small cell lung carcinoma, and pancreatic carcinoma (when used in combination with antisense-K-ras oligonucleotide) culminates in apoptosis (19–23). However, normal melanocytes, endothelial cells, mammary and prostate epithelial cells, and skin fibroblasts are refractive to Ad._mda-_7-induced killing (19, 20, 22, 23). Although the apoptosis-inducing effect of _mda-_7 is well established, the pathway(s) responsible for this cancer-specific apoptosis remains to be elucidated. Moreover, on the basis of selective cancer-specific killing by Ad._mda-_7, this gene may prove effective in the gene-based therapy of cancer (19, 20, 23, 24).
Growth-arrest and DNA damage-inducible genes were originally isolated from UV radiation-treated cells and subsequently grouped according to their coordinate regulation by growth arrest and DNA damage (25). Later these genes were found to be stress response genes that were induced by UV radiation, chemical carcinogens, starvation, oxidative stress, and apoptosis-inducing agents such as tumor necrosis factor-α, C-2 ceramide, dimethyl sphingosine, anti-Fas antibody, and staurosporine (26–29). The five members of this family of growth arrest and DNA damage (GADD)-inducible genes are GADD34, GADD45α, GADD45β, GADD45γ, and GADD153, which encode highly acidic nuclear proteins with similar and unusual charge characteristics (30, 31). GADD34 is a 73-kDa protein that interacts with a diverse array of proteins within the cell (32–38). Some of these interactions facilitate growth suppression/apoptosis, whereas others indicate its involvement in translation initiation, DNA recombination or repair, mRNA transport, and transcriptional regulation. GADD45, a p53-regulated gene, codes for a 21-kDa protein that interacts with the products of two other p53-regulated genes, p21WAF1/CIP1/MDA-6 and proliferating cell nuclear antigen, and has been implicated in specific aspects of nucleotide excision repair (39, 40). GADD45 also interacts with MTK1 MAPKKK and thus mediates activation of p38 and JNK MAP kinases in response to environmental stress (30). GADD153, also known as CHOP10 (C/EBP-homologous protein), is a transcription factor containing the basic region-leucine zipper domain that heterodimerizes with members of the C/EBP family of transcription factors and interferes with C/EBP-mediated transcription (41). In addition, GADD153 itself can enhance gene transcription by binding to a DNA element or by interactions with other transcription factors like AP-1 (42, 43). Overexpression of each GADD gene causes growth inhibition and/or apoptosis, and combined overexpression of the GADD genes leads to synergistic or cooperative antiproliferative effects (31).
Subtractive hybridization, which identified _mda-_7 (7, 11), also identified up-regulation of GADD34 during the process of terminal differentiation and growth arrest in human melanoma cells (9). In the present study we document a direct relationship between ectopic expression of _mda-_7 in melanoma cells and induction of the GADD family of genes. We further demonstrate that the induction of the GADDs is regulated by p38 mitogen-activated protein kinase (MAPK) specifically in melanoma cells, and modifying this signaling pathway by pharmacological means or by using a replication incompetent adenovirus expressing a p38 MAPK dominant negative mutant [AdCMV-Flagp38(AGF)] protects melanoma cells from _mda-_7-induced apoptosis. These experiments establish that _mda-_7 induces the GADD family of genes by means of the p38 MAPK pathway, and this induction plays a central role in _mda-_7-mediated apoptosis of melanoma cells.
Materials and Methods
Cell Lines, Reagents, and Cell Growth Assays.
Normal immortal human melanocyte (FM516-SV; FM516); WM35 early radial growth phase primary human melanoma; and HO-1, FO-1, and MeWo metastatic melanoma cell lines were cultured as described (44–47). SB203580 was purchased from Sigma. Cell growth and viable cell numbers were monitored by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) staining as described (48).
Virus Construction and Infection Protocol.
The construction and purification of _mda-_7 expressing replication-defective Ad._mda-_7 have been described (22). The empty adenoviral vector (Ad.vec) was used as a control. A dominant negative kinase-deficient mutant p38 MAPK expressing virus [Flag-p38(AGF)] was prepared by cloning the kinase-inactive p38α (AGF) cDNA (49, 50) into an adenovirus transfer plasmid and made into a replication-incompetent Ad-5 (delE1, E3) virus as described (51, 52). The virus expresses p38α (T180GY-AGF) with a Flag-tag at its amino terminus. Viral infections were performed as described (22).
Plasmid Construction and Transfection Assays.
GADD153, GADD34, GADD45α, and GADD45γ coding sequences were amplified by reverse transcription–PCR. The sequences were verified and ligated into pcDNA3.1(+) (Invitrogen) vector in an antisense manner. Plasmids were purified by Qiagen Plasmid Purification Maxi Kit (Qiagen, Hilden, Germany). The day before transfection, 2 × 104 cells were plated into each well of a 96-well plate. Transient transfection was performed by using Maxfect transfection reagent (Molecular Probes) according to the manufacturer's instructions with 500 ng of DNA per well. The total DNA concentration was kept constant by the addition of empty vector.
RNA Isolation and Northern Blot Analysis.
Total RNA was extracted from the cells by using Qiagen RNeasy mini kit according to the manufacturer's protocol, and Northern blotting was performed as described (53). The cDNA probes used were full-length human GADD153, full-length human GADD45-α, β, and γ, a 500-bp fragment from human GADD34, and full-length human GAPDH.
Western Blot Analysis.
Western blotting was performed as described (22). The primary antibodies included GADD153 (1:500; rabbit polyclonal; Santa Cruz Biotechnology), GADD34 (1:200; rabbit polyclonal; Santa Cruz), GADD45 (1:200; mouse monoclonal; Santa Cruz), bcl-2 (1:1000; rabbit polyclonal; kindly provided by J. Reed), and EF1α (1:1000; mouse monoclonal; Upstate Biotechnology, Waltham, MA).
Phosphorylation of p38-MAPK and Heat Shock Protein (HSP) 27.
Cells were harvested in radioimmunoprecipitation assay buffer containing protease inhibitor mixture, 1 mM Na3VO4, and 50 mM NaF, and centrifuged at 12,000 rpm for 10 min at 4°C. The supernatant was used as total cell lysate. p38-MAPK was immunoprecipitated from 500 μg of the total cell lysate overnight at 4°C with 2 μl of rabbit polyclonal anti-p38-MAPK antibody (Calbiochem). After addition of protein A-agarose, incubation was continued for 2 h. Immunocomplexes were washed four times in radioimmunoprecipitation assay buffer, resuspended in 1× SDS-polyacrylamide gel lysis buffer, and transferred to a nitrocellulose membrane. The expressions of phospho-p38-MAPK and total p38-MAPK were detected by Western blot analysis with a rabbit polyclonal anti-phospho-p38-MAPK antibody (New England Biolabs), and anti-p38-MAPK antibody (Calbiochem), respectively. The expressions of phospho-HSP27 and total HSP27 in total cell lysate were detected by Western blot analysis by using a rabbit polyclonal anti-phospho-HSP27 antibody and a mouse monoclonal anti-HSP27 antibody, respectively (New England Biolabs).
DNA Fragmentation Assay.
Adherent and floating cells from a 10-cm dish were used for DNA fragmentation assays which were performed as described (22).
Cell Cycle Analysis.
Cells were harvested, washed in PBS, and fixed overnight at −20°C in 70% ethanol. The cells were treated with RNase A (1 mg/ml) at 37°C for 30 min and then with propidium iodide (50 μg/ml). Cell cycle was analyzed by using a FACScan flow cytometer and data were analyzed with CELLQUEST software (Becton Dickinson).
Statistical Analysis.
Statistical analysis was performed by using one-way ANOVA, followed by Fisher's protected least significant difference analysis.
Results
Infection of Human Melanoma Cells, but Not Normal Immortal Melanocytes, with Ad._mda_-7 Induces the GADD Family of Genes.
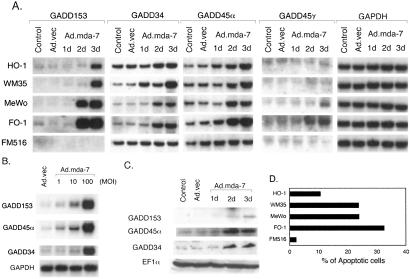
Melanoma cell lines were infected with either Ad._mda-_7 or Ad.vec, total RNA was extracted, and the expression pattern of mRNAs of the GADD family of genes was analyzed by Northern blot analysis. As shown in Fig. 1A, the expression of mRNAs for GADD153, GADD34, GADD45α, and GADD45γ was either undetectable or low in the control and Ad._vec_-infected melanoma cells. At day 1 after infection with Ad._mda-_7, when no effect occurs on the growth of these melanoma cells (22), no change in GADD gene expression is apparent. However, at days 2 and 3 after infection, a marked induction of GADD153, GADD34, GADD45α, and GADD45γ genes occurs in melanoma cells but not in FM516-SV (FM516) cells, which are immortalized normal human melanocytes (47). This induction correlates with previous studies indicating that melanoma cells begin dying 2 days after infection, and infection with Ad._mda-_7 causes selective apoptosis of melanoma cells but not of normal melanocytes (Fig. 1D) (22). Among the melanoma cell lines, HO-1 was the most refractory to Ad._mda-_7 induction of apoptosis (as measured by increases in the percentage of Ao cells), which correlated with a smaller induction of the GADD family of genes. The other three cell lines, FO-1, MeWo, and WM35, were readily killed by Ad._mda-_7 (Fig. 1D) (22) and displayed significant induction of the GADD family of genes. Among these three melanoma cell lines FO-1 was the most sensitive to Ad._mda-_7 and showed the maximum induction of the GADD family of genes. The expression level of GADD45γ mRNA was minimal in all of the cells and the induction level was also not as significant as it was for the other three members of this family. For GADD45γ autoradiography was performed for one week, whereas for the other mRNAs it was performed overnight. No expression of GADD45β could be detected by Northern blot analysis in any of the control or Ad._mda-_7-infected cells. However, reverse transcription–PCR analysis detected GADD45β mRNA, but its level did not change after Ad._mda-_7 infection (data not shown). The expression level of the housekeeping gene GAPDH did not change in any of the cell lines after Ad._mda-_7 infection.
Figure 1.
Infection with Ad._mda-_7 induces the GADD family of genes in melanoma cells but not in normal immortal melanocytes in a time- and dose-dependent manner. (A) Melanoma cells (HO-1, WM35, MeWo, and FO-1) and immortalized human melanocytes (FM516) were infected with either Ad.vec or with Ad._mda-_7 at a multiplicity of infection (MOI) of 100 pfu per cell for 3 days. Total RNA was extracted and Northern analysis was performed by using the indicated cDNA probes as described in Materials and Methods. (B) FO-1 cells were infected with either Ad.vec (100 pfu per cell) or Ad._mda-_7 (1, 10, and 100 pfu per cell) and Northern blot analysis was performed as indicated. (C) FO-1 cells were infected with either Ad.vec or Ad._mda-_7 (100 pfu per cell) for 3 days. Cell lysates were prepared and Western blot analysis was performed by using the indicated antibodies as described in Materials and Methods. (D) Percentage of cells displaying hypodiploidy (Ao), a measure of apoptosis, by fluorescence-activated cell sorter analysis 3 days after infection with a multiplicity of infection of 100 pfu per cell of Ad._mda-_7.
Studies were conducted with the FO-1 cell line to investigate further the relationship between Ad._mda-_7 infection and induction of the GADD family of genes. Ad._mda-_7 infection of FO-1 cells for 3 days resulted in a dose-dependent induction of the GADD family of genes (Fig. 1B). The level of induction of the GADD family mRNA gradually increased when cells were infected with a multiplicity of infection of 1, 10, or 100 plaque-forming units (pfu) per cell. Infection of FO-1 with 100 pfu per cell of Ad._mda-_7 also resulted in an increase in the protein levels of the respective GADD family of genes (Fig. 1C). Because no antibody-targeting GADD45γ is currently available, protein expression of this GADD family member was not determined. The level of the housekeeping protein EF1α was unchanged after Ad._mda-_7 infection documenting the specificity of the induction of the GADD family gene members.
Induction of the GADD Family of Genes by Ad._mda_-7 Proceeds Through the p38 MAPK Pathway.
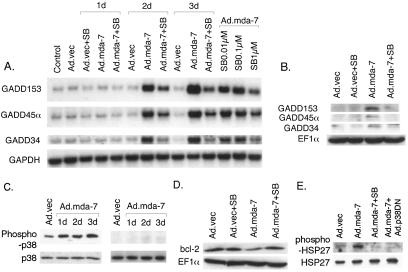
Experiments were performed to define the signaling pathway(s) involved in GADD family gene expression after infection with Ad._mda-_7. The GADD family of genes is induced by diverse stimuli, and one major pathway that is involved in this induction is by way of the p38 MAPK pathway (29, 54, 55). Because activation of the p38 MAPK pathway results in apoptosis in various cell types, the involvement of this pathway in Ad._mda-_7-mediated induction of GADD family of genes was examined. FO-1 cells were infected with Ad._mda-_7 and then the cells were treated with SB203580, a selective p38 MAPK inhibitor, to determine effects on induction of the GADD family of genes. SB203580 was also chosen for these studies because this inhibitor selectively blocks Ad._mda-_7-mediated cell death in several cancer cell types, besides melanoma cells (data not shown). Dose-response studies indicated that the most effective SB203580 concentration, without inducing toxicity, in FO-1 cells was 1 μM. Treatment with SB203580 alone did not affect the basal expression of any of the GADD family of genes (Fig. 2A). However, SB203580 significantly inhibited Ad._mda-_7-mediated induction of these genes at days 2 and 3. The inhibitory effect of SB203580 was dose-dependent with minimum inhibition at 0.01 μM and maximum inhibition at 1 μM (Fig. 2A). Western blotting also revealed that SB203580 could inhibit Ad._mda-_7-mediated induction of GADD153, GADD34, and GADD45α proteins (Fig. 2B).
Figure 2.
Treatment with SB203580 inhibits Ad._mda-_7-mediated induction of the GADD family of genes, p38 MAPK phosphorylation, and BCL-2 protein down-regulation. (A) FO-1 cells were infected with either Ad.vec or with Ad._mda-_7 (100 pfu per cell) and were treated with either 1 μM SB203580 for 3 days or with different concentrations of SB203580 for 2 days. Total RNA was extracted and Northern blot analysis was performed. (B) FO-1 cells were infected with either Ad.vec or Ad._mda-_7 (100 pfu per cell) and treated with 1 μM SB203580 for 3 days. Cell lysates were prepared and Western blot analysis was performed. (C) FO-1 (Left) and FM516 (Right) cells were infected with either Ad.vec or Ad._mda-_7 (100 pfu per cell) for 3 days. Cell lysates were prepared and Western blot analysis was performed with anti-phospho-p38 and anti-p38 MAPK antibodies as described in Materials and Methods. (D) FO-1 cells were infected with either Ad.vec or Ad._mda-_7 (100 pfu per cell) and were treated with 1 μM SB203580 for 3 days. Cell lysates were prepared and Western blot analysis was performed by using the indicated antibodies. (E) FO-1 cells were infected with either Ad.vec or Ad._mda-_7 (100 pfu per cell) and were either treated with 1 μM SB203580 or infected with dominant negative p38 MAPK (Ad.p38DN; 100 pfu per cell) for 3 days. Cell lysates were prepared and Western blot analysis was performed by using the indicated antibodies.
The effect of Ad._mda-_7 infection on p38 MAPK phosphorylation was tested (Fig. 2C). In FO-1 cells, the level of phospho-p38 MAPK increased significantly from day 1 to 3 after Ad._mda-_7 infection (Fig. 2C). In contrast, Ad._mda-_7 infection of FM516 cells did not induce p38 MAPK phosphorylation. In FO-1 cells and normal immortal melanocytes, equal amounts of total p38 MAPK was detected in Ad.vec and Ad._mda-_7-infected cells. The activation of p38 MAPK pathway results in phosphorylation of HSP27 by MAPKAP kinase 2 (56). To check whether phosphorylation of p38 MAPK by Ad._mda_-7 also results in the phosphorylation of this downstream target, the expression levels of phospho-HSP27 and total HSP27 in FO-1 cells were determined after Ad._mda_-7 infection. Ad._mda_-7 infection resulted in phosphorylation of HSP27 (Fig. 2E), and this phosphorylation could be blocked by treatment with SB203580 or by infection with a replication-incompetent adenovirus expressing a dominant negative p38 MAPK (49, 50), AdCMV-Flagp38(AGF). Equal amounts of total HSP27 could be detected in Ad.vec and Ad._mda_-7-infected FO-1 cells. These results further support the involvement of the p38 MAPK pathway in Ad._mda-_7-mediated killing of FO-1 cells.
Blocking p38 MAPK Protects Human Melanoma Cells from _mda_-7-Induced Apoptosis.
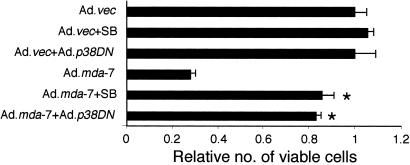
Experiments were next performed to determine the involvement of the p38 MAPK pathway in regulating cell viability after Ad._mda-_7 infection, which was achieved by blocking p38 MAPK pathway pharmacologically with SB203580 or by using AdCMV-Flagp38(AGF). FO-1 cells were infected with Ad._mda-_7, alone or with AdCMV-Flagp38(AGF), or they were treated with SB203580 and cell viability was determined by using the MTT assay 4 days later (Fig. 3). Infection with Ad._mda-_7 significantly reduced cell viability (≈75%) 4 days post infection (Fig. 3), while infection with AdCMV-Flagp38(AGF) or treatment after SB203580 reversed the _mda-_7 inhibitory effect.
Figure 3.
Inhibition of the p38 MAPK pathway protects FO-1 melanoma cells from Ad._mda-_7-mediated cell death. FO-1 cells were infected with either Ad.vec or Ad._mda-_7 (100 pfu per cell) and treated with 1 μM SB203580 or infected with Ad.p38DN (100 pfu per cell). Cell viability was measured by MTT assay after 4 days. Cell viability of Ad._vec_-treated cells was regarded as 1. *, significant differences from Ad.mda-7 (P < 0.0001).
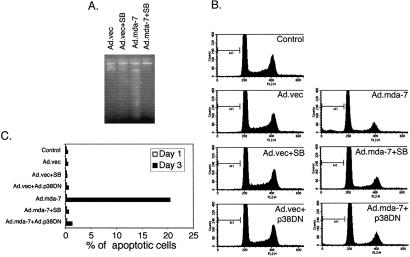
A series of experiments were also performed to determine if SB203580 or AdCMV-Flagp38(AGF) could inhibit Ad._mda-_7-mediated apoptosis, as monitored by DNA fragmentation, in FO-1 cells. Three days after infection of FO-1 cells with Ad._mda-_7 resulted in significant DNA fragmentation as compared with control and SB203580-treated cells (Fig. 4A). However, treatment with SB203580 effectively inhibited Ad._mda-_7-mediated DNA fragmentation in FO-1 cells. These results were confirmed by cell cycle analysis (Fig. 4B). One day after infection with Ad._mda-_7 no induction of apoptosis was apparent (Fig. 4C). However, at day 3, a significant percentage (≈20%) of cells were apoptotic after Ad._mda-_7 infection. Treatment with SB203580 or simultaneous infection with AdCMV-Flagp38(AGF) prevented the induction of apoptosis by Ad._mda-_7 (Fig. 4 B and C). These results suggest that Ad._mda-_7 induces the GADD family of genes by way of the p38 MAPK pathway, which then induce apoptosis.
Figure 4.
Inhibition of the p38 MAPK pathway protects cells from Ad._mda-_7-mediated apoptosis. (A) FO-1 cells were infected with either Ad.vec or Ad._mda-_7 (100 pfu per cell) and treated with 1 μM SB203580 for 3 days. DNA was isolated from the cells and fragmentation was analyzed as described in Materials and Methods. (B) FO-1 cells were infected with either Ad.vec or with Ad._mda-_7 (100 pfu per cell) and treated with 1 μM SB203580 or infected with Ad.p38DN (100 pfu per cell). Cell cycle was analyzed as described in Materials and Methods. (C) FO-1 cells were infected with either Ad.vec or with Ad._mda-7 (100 pfu per cell) and treated with 1 μM SB203580 or infected with Ad.p_38DN (100 pfu per cell). Percentage of apoptotic cells at days 1 and 3 after infection in each group were plotted.
Inhibition of p38 MAPK Prevents Ad._mda_-7 Down-Regulation of the Antiapoptotic Protein BCL-2.
Previous studies document that Ad._mda-_7 infection results in down-regulation of the antiapoptotic protein BCL-2 in FO-1 cells (22). We therefore determined whether SB203580 treatment could counteract this effect of Ad._mda-_7 on BCL-2 protein levels. Indeed, as shown in Fig. 2D, treatment with SB203580 prevented Ad._mda-_7-mediated down-regulation of the BCL-2 protein. This effect of SB203580 was specific for BCL-2 because it did not have any effect on Ad._mda-_7-mediated modulation of other pro- or antiapoptotic proteins (data not shown).
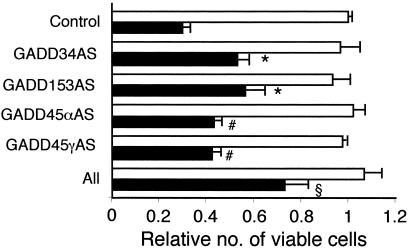
Blocking the GADD Family of Genes Inhibits Ad._mda_-7-Induced Apoptosis.
Involvement of the GADD family of genes in Ad._mda-_7-mediated apoptosis was also confirmed by an antisense approach. The different antisense constructs were transfected either alone or in combination into FO-1 cells and then the cells were infected with Ad.vec or Ad._mda-_7. Cell growth was analyzed after 4 days by MTT assay. As shown in Fig. 5, infection with Ad._mda-_7 reduced the viable cell number by ≈75% as compared with infection with Ad.vec. Transfection of the antisense constructs alone provided small but significant protection against Ad._mda-_7-mediated cell death. In contrast, the combination of all of the constructs provided significantly higher protection against Ad._mda-_7-mediated cell death, the cell number being reduced by only ≈25% as compared with the Ad._vec_-infected cells (Fig. 5). These results indicate that the coordinated overexpression of the GADD family of genes plays an important role in Ad._mda-_7-mediated apoptosis.
Figure 5.
Inhibition of the GADD family of genes protects FO-1 melanoma cells from Ad._mda-_7-mediated cell death. FO-1 cells were transfected with the indicated antisense construct either alone or in combination. After 24 h the cells were infected with either Ad.vec (white bars) or Ad._mda-_7 (black bars) (100 pfu per cell) for 3 days. Cell viability was measured by MTT assay. Cell viability of Ad._vec_-treated cells was regarded as 1. Significant differences from Control, Ad.mda-7 #, P < 0.05; *, P < 0.005; §, P < 0.0001.
Discussion
Mda-7/IL-24 holds significant promise for the gene-based therapy of cancers because of its unique capacity to kill cancer cells while sparing normal cells (19–24, 57). In this context, this gene is currently undergoing Phase I clinical trials and has been found in an initial study to be safe with only mild toxicities observed and to induce apoptosis in a large percentage of tumor volume when injected intratumorally (58). However, despite entry into the clinical arena, the molecular mechanism of _mda-_7 action still remains to be elucidated. The present study depicts a specific signaling pathway responsible for the apoptosis-inducing effect of Ad._mda-_7 in the framework of human melanoma cells from which _mda-_7/IL-24 was first identified and cloned (11).
Two recent reports demonstrate that _mda-_7/IL-24 can bind and signal through the IL20R1/IL22R2 and IL20R1/IL20R2 receptor complexes and this interaction causes phosphorylation of STAT 3 (18, 59). However, the biological relevance of STAT 3 phosphorylation is not known. The present study provides direct evidence indicating that the apoptotic effect of Ad._mda-_7 in melanoma cells is mediated by the p38 MAPK pathway. In numerous instances, activation of the p38 MAPK pathway has correlated with induction of apoptosis (60–66). These include the concomitant activation of p38 MAPK and apoptosis induced by diverse agents and experimental conditions, including nerve growth factor withdrawal, Fas ligation, and exposure to tumor necrosis factor-α, ceramides, sodium salicylates, peroxynitrite, and UV radiation (29, 60–67). In addition, the selective p38 MAPK inhibitor, SB203580, can block sodium salicylate-induced FS-4 fibroblast apoptosis, glutamate-induced cerebellar granule cell apoptosis, serum depletion-induced Rat-1 cell death, nerve growth factor withdrawal-induced PC12 cell apoptosis, tumor necrosis factor-α-mediated rat fetal brown adipocyte apoptosis, and TL1-induced bovine pulmonary artery endothelial cell apoptosis (60, 63–65). Activation of the GADD family of genes by p38 MAPK might represent a mechanism by which some of these protocols induce apoptosis (29, 54, 55). Fas- or ceramide-induced apoptosis is mediated by p38 MAPK and GADD153 (55). Oxidative stress by peroxynitrite induces GADD34, GADD45, and GADD153 by means of the p38 MAPK pathway and induces apoptosis in neuroblastoma cells (29).
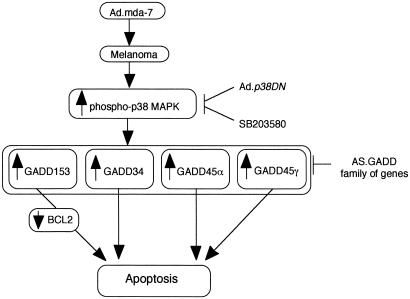
In the present study, the GADD family of genes was up-regulated both at mRNA and protein levels by Ad._mda-_7 in human melanoma, but not in normal immortal melanocytes. A similar up-regulation of the GADD family of genes (which correlates with apoptosis induction) is observed in glioblastoma multiforme and breast and prostate carcinoma cells, but not in their normal cellular equivalents, after infection with Ad._mda-_7 (unpublished data). This up-regulation in melanoma cells was coupled with the induction of apoptosis and was blocked by SB203580. GADD153 induces its apoptotic effect by down-regulating the activity of the _bcl-_2 promoter (70). It was previously observed that Ad._mda-_7 infection results in down-regulation of BCL-2 protein in many different cancer cell types, including melanoma (19, 21, 22). On the basis of these observations it was reasoned that if _bcl-_2 down-regulation was caused by Ad._mda-_7-mediated induction of GADD153, then treatment with SB203580 should restore the BCL-2 protein to its basal level. Indeed, as shown in Fig. 2D, SB203580 inhibited Ad._mda-_7-mediated BCL_-_2 down-regulation, indicating a signaling pathway involving Ad._mda-_7, p38 MAPK, GADD153, and _bcl-_2 (Fig. 6). A role of the GADD family of genes in Ad._mda-_7-mediated apoptosis in human melanoma cells is given further credence by the observation that inhibition of the GADD genes, either alone or in combination, counteracted Ad._mda-_7-mediated apoptosis. These results indicate that apoptosis induction in human melanoma cells by Ad._mda-_7 is mediated by the GADD family of genes, rather than up-regulation of the GADD family of genes occurring simply as a consequence of growth arrest and apoptosis.
Figure 6.
A hypothetical model of the involvement of the p38 MAPK pathway and the GADD family members of genes in mediating apoptosis in human melanoma cells by Ad._mda-_7.
A very significant question is why Ad._mda-_7 fails to elicit any detrimental effects in normal cells. Previous studies document that Ad._mda-_7 efficiently infects normal melanocytes and immortal FM516 melanocytes resulting in the production of MDA-7 protein, which is secreted into the medium (22). Apparently, the FM516 cells are not defective in the GADD-induction pathway, because the DNA-damaging agent methyl methanesulfonate induces the GADD family of genes in these cells (unpublished data). Infection of FM516 cells with Ad._mda-_7 does not result in phosphorylation of p38 MAPK, whereas this effect is observed in FO-1 cells that are induced to undergo apoptosis by _mda-_7. Although this finding may partially explain the resistance of FM516 cells to Ad._mda-_7, the reason why p38 MAPK is not phosphorylated in these cells is not understood. A recent report has documented that the p38 MAPK pathway is augmented in oncogenically transformed cells, in comparison with normal cells, making these tumor cells sensitive to genotoxic stress (69). This phenomenon might provide an explanation for the resistance of FM516 cells to Ad._mda-_7. The basal level of GADD34 mRNA, but not GADD153, expression in FM516 cells was higher than that in the melanoma cell lines (Fig. 1A). GADD34 functions as a negative regulator for the expression of GADD153 during unfolded protein response (70) providing a possible explanation for the differences in the levels of these two GADD family member genes in FM516 cells. Although both GADD34 and GADD153 are capable of inducing apoptosis either alone or in combination, in certain contexts they might be part of a check-and-balance loop for viability within the cell. It is possible that the high basal level of GADD34 in FM516 cells might give them some inherent resistance to _mda-_7-mediated killing. Future studies directed to explore this question further and to determine the generality of these relationships between changes in GADD gene family members and sensitivity and resistance to Ad._mda-_7-mediated apoptosis should prove very informative.
Acknowledgments
This research was supported by Department of Defense Grant DAMD17–98-1–8053, National Institutes of Health Grants CA35675 and CA74468, the Samuel Waxman Cancer Research Foundation, and the Chernow Endowment. P.B.F. is the Michael and Stella Chernow Urological Cancer Research Scientist.
Abbreviations
GADD
growth arrest and DNA damage
MAPK
mitogen-activated protein kinase
MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
pfu
plaque-forming unit
HSP
heat shock protein
References
- 1.Sachs L. Nature (London) 1978;274:535–539. doi: 10.1038/274535a0. [DOI] [PubMed] [Google Scholar]
- 2.Fisher P B, Grant S. Pharmacol Ther. 1985;27:143–166. doi: 10.1016/0163-7258(85)90067-1. [DOI] [PubMed] [Google Scholar]
- 3.Jiang H, Lin J, Fisher P B. Mol Cell Differ. 1994;2:221–239. [Google Scholar]
- 4.Leszczyniecka M, Roberts T, Dent P, Grant S, Fisher P B. Pharmacol Ther. 2001;90:105–156. doi: 10.1016/s0163-7258(01)00132-2. [DOI] [PubMed] [Google Scholar]
- 5.Waxman S. Differentiation Therapy. Rome: Ares-Serono Symposia Publishers; 1996. pp. 1–531. [Google Scholar]
- 6.Jiang H, Su Z Z, Boyd J, Fisher P B. Mol Cell Differ. 1993;1:41–66. [Google Scholar]
- 7.Jiang H, Fisher P B. Mol Cell Differ. 1993;1:285–299. [Google Scholar]
- 8.Kang D C, LaFrance R, Su Z Z, Fisher P B. Proc Natl Acad Sci USA. 1998;95:13788–13793. doi: 10.1073/pnas.95.23.13788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang H, Kang D C, Alexandre D, Fisher P B. Proc Natl Acad Sci USA. 2000;97:12684–12689. doi: 10.1073/pnas.220431297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang F, Adelman J, Jiang H, Goldstein N I, Fisher P B. Gene. 1999;236:125–131. doi: 10.1016/s0378-1119(99)00244-9. [DOI] [PubMed] [Google Scholar]
- 11.Jiang H, Lin J J, Su Z Z, Goldstein N I, Fisher P B. Oncogene. 1995;11:2477–2486. [PubMed] [Google Scholar]
- 12.Gallagher G, Dichensheets H, Eskdale J, Izotova L S, Mirochnitchenko O V, Peat J D, Vazquez N, Pestka S, Donnelly R P, Kotenko S V. Genes Immun. 2000;1:442–450. doi: 10.1038/sj.gene.6363714. [DOI] [PubMed] [Google Scholar]
- 13.Zhang R, Tan Z, Liang P. J Biol Chem. 2000;275:24436–24443. doi: 10.1074/jbc.M001958200. [DOI] [PubMed] [Google Scholar]
- 14.Xie M H, Aggarwal S, Ho W H, Foster J, Zhang Z, Stinson J, Wood W I, Goddard A D, Gurney A L. J Biol Chem. 2000;275:31335–31339. doi: 10.1074/jbc.M005304200. [DOI] [PubMed] [Google Scholar]
- 15.Kotenko S V, Izotova L S, Mirochnitchenko O V, Esterova E, Dickensheets H, Donnelly R P, Pestka S. J Biol Chem. 2001;276:2725–2732. doi: 10.1074/jbc.M007837200. [DOI] [PubMed] [Google Scholar]
- 16.Pestka S, Kotenko S V, Fisher P B. In: Encyclopedia of Hormones. Henry H L, Norman A W, editors. New York: Academic; 2002. , in press. [Google Scholar]
- 17.Huang E Y, Madireddi M T, Gopalkrishnan R V, Leszczyniecka M, Su Z Z, Lebedeva I V, Kang D, Jiang H, Lin J J, Alexandre D, et al. Oncogene. 2001;20:7051–7063. doi: 10.1038/sj.onc.1204897. [DOI] [PubMed] [Google Scholar]
- 18.Wang M, Tan Z, Zhang R, Kotenko S V, Liang P. J Biol Chem. 2002;277:7341–7347. doi: 10.1074/jbc.M106043200. [DOI] [PubMed] [Google Scholar]
- 19.Su Z Z, Madireddi M T, Lin J J, Young C S, Kitada S, Reed J C, Goldstein N I, Fisher P B. Proc Natl Acad Sci USA. 1998;95:14400–14405. doi: 10.1073/pnas.95.24.14400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saeki T, Mhashilkar A, Chada S, Branch C, Roth J A, Ramesh R. Gene Ther. 2000;7:2051–2057. doi: 10.1038/sj.gt.3301330. [DOI] [PubMed] [Google Scholar]
- 21.Su Z, Lebedeva I V, Gopalkrishnan R V, Goldstein N I, Stein C A, Reed J C, Dent P, Fisher P B. Proc Natl Acad Sci USA. 2001;98:10332–10337. doi: 10.1073/pnas.171315198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lebedeva I V, Su Z Z, Chang Y, Kitada S, Reed J C, Fisher P B. Oncogene. 2002;21:708–718. doi: 10.1038/sj.onc.1205116. [DOI] [PubMed] [Google Scholar]
- 23.Madireddi M T, Su Z Z, Young C S, Goldstein N I, Fisher P B. Adv Exp Med Biol. 2000;465:239–261. doi: 10.1007/0-306-46817-4_22. [DOI] [PubMed] [Google Scholar]
- 24.Mhashilkar A M, Schrock R D, Hindi M, Liao J, Sieger K, Kourouma F, Zou-Yang X H, Onishi E, Takh O, Vedvick T S, et al. Mol Med. 2001;7:271–282. [PMC free article] [PubMed] [Google Scholar]
- 25.Fornace A J, Neibert D W, Hollander M C, Luethy J D, Papathanasiou M, Fragoli J, Holbrook N J. Mol Cell Biol. 1989;9:4196–4203. doi: 10.1128/mcb.9.10.4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hollander M C, Sheikh M S, Yu K, Zhan Q, Iglesias M, Woodworth C, Fornace A J. Int J Cancer. 2001;96:22–31. doi: 10.1002/1097-0215(20010220)96:1<22::aid-ijc3>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 27.Price B, Calderwood S. Cancer Res. 1992;52:3814–3817. [PubMed] [Google Scholar]
- 28.Marten N W, Burke E J, Hayden J M, Straus D S. FASEB J. 1994;8:538–544. doi: 10.1096/fasebj.8.8.8181673. [DOI] [PubMed] [Google Scholar]
- 29.Oh-hashi K, Maruyama W, Isobe K. Free Radical Biol Med. 2001;30:213–221. doi: 10.1016/s0891-5849(00)00461-5. [DOI] [PubMed] [Google Scholar]
- 30.Takekawa M, Saito H. Cell. 1998;95:521–530. doi: 10.1016/s0092-8674(00)81619-0. [DOI] [PubMed] [Google Scholar]
- 31.Zhan Q, Lord K A, Alamo I, Jr, Hollander M C, Carrier F, Ron D, Kohn K W, Hoffman B, Liebermann D A, Fornace A J., Jr Mol Cell Biol. 1994;14:2361–2371. doi: 10.1128/mcb.14.4.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hollander M C, Zhan Q, Bae I, Fornace A J. J Biol Chem. 1997;272:13731–13737. doi: 10.1074/jbc.272.21.13731. [DOI] [PubMed] [Google Scholar]
- 33.Connor J H, Weiser D C, Li S, Hallenbeck J H, Shenolikar S. Mol Cell Biol. 2001;21:6841–6850. doi: 10.1128/MCB.21.20.6841-6850.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hasegawa T, Yagi A, Isobe K. Biochem Biophys Res Commun. 2000;267:593–596. doi: 10.1006/bbrc.1999.1991. [DOI] [PubMed] [Google Scholar]
- 35.Hasegawa T, Isobe K. Biochim Biophys Acta. 1999;1428:161–168. doi: 10.1016/s0304-4165(99)00060-4. [DOI] [PubMed] [Google Scholar]
- 36.Hasegawa T, Xiao H, Isobe K. Biochem Biophys Res Commun. 1999;256:249–254. doi: 10.1006/bbrc.1999.0275. [DOI] [PubMed] [Google Scholar]
- 37.Grishin A V, Azhipa O, Semenov I, Corey S J. Proc Natl Acad Sci USA. 2001;98:10172–10177. doi: 10.1073/pnas.191130798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adler H T, Chinery R, Wu D Y, Kussick S J, Payne J M, Fornace A J, Tkachuk D C. Mol Cell Biol. 1999;19:7050–7060. doi: 10.1128/mcb.19.10.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith M L, Chen I T, Zhan Q, Bae I, Chen C Y, Gilmer T M, Kastan M B, O'Connor P M, Fornace A J., Jr Science. 1994;266:1376–1380. doi: 10.1126/science.7973727. [DOI] [PubMed] [Google Scholar]
- 40.Vairapandi M, Balliet A G, Fornace A J, Jr, Hoffman B, Liebermann D A. Oncogene. 1996;12:2579–2594. [PubMed] [Google Scholar]
- 41.Ron D, Habener J F. Genes Dev. 1992;6:439–453. doi: 10.1101/gad.6.3.439. [DOI] [PubMed] [Google Scholar]
- 42.Ubeda M, Vallejo M, Habener J F. Mol Cell Biol. 1999;19:7589–7599. doi: 10.1128/mcb.19.11.7589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ubeda M, Wang X, Zinszner H, Wu I, Habener J F, Ron D. Mol Cell Biol. 1996;16:1479–1489. doi: 10.1128/mcb.16.4.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kerbel R S, Man M S, Dexter D. J Natl Cancer Inst. 1984;72:93–108. doi: 10.1093/jnci/72.1.93. [DOI] [PubMed] [Google Scholar]
- 45.Fisher P B, Prignoli D R, Hermo H, Weinstein I B, Pestka S. J Interferon Res. 1985;5:11–22. doi: 10.1089/jir.1985.5.11. [DOI] [PubMed] [Google Scholar]
- 46.Herlyn M. Cancer Metastasis Rev. 1990;9:101–112. doi: 10.1007/BF00046337. [DOI] [PubMed] [Google Scholar]
- 47.Melber K, Zhu G, Diamond L. Cancer Res. 1989;49:3650–3655. [PubMed] [Google Scholar]
- 48.Lebedeva I V, Rando R, Ojwang J, Cossum P, Stein C A. Cancer Res. 2000;60:6052–6060. [PubMed] [Google Scholar]
- 49.Raingeaud J, Gupta S, Rogers J S, Dickens M, Han J, Ulevitch R J, Davis R J. J Biol Chem. 1995;270:7420–7426. doi: 10.1074/jbc.270.13.7420. [DOI] [PubMed] [Google Scholar]
- 50.Taher M M, Baumgardner T, Dent P, Valerie K. Biochemistry. 1999;38:13055–13062. doi: 10.1021/bi9902900. [DOI] [PubMed] [Google Scholar]
- 51.Valerie K. In: Biopharmaceutical Drug Design and Development. Wu-Pong S, Rojanasakul Y, editors. Clifton, NJ: Humana; 1999. pp. 69–142. [Google Scholar]
- 52.Rosenberg E, Taher M M, Kuemmerle N B, Farnsworth J, Valerie K. Cancer Res. 2001;61:764–770. [PubMed] [Google Scholar]
- 53.Su Z Z, Shi Y, Fisher P B. Proc Natl Acad Sci USA. 1997;94:9125–9130. doi: 10.1073/pnas.94.17.9125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kultz D, Madhany S, Burg M B. J Biol Chem. 1998;273:13645–13651. doi: 10.1074/jbc.273.22.13645. [DOI] [PubMed] [Google Scholar]
- 55.Brenner B, Koppenhoefer U, Weinstock C, Linderkamp O, Lang F, Gulbins E. J Biol Chem. 1997;272:22173–22181. doi: 10.1074/jbc.272.35.22173. [DOI] [PubMed] [Google Scholar]
- 56.Rouse J, Cohen P, Trigon S, Morange M, Alonso-Llamazares A, Zamanillo D, Hunt T, Nebreda A. Cell. 1994;78:1027–1037. doi: 10.1016/0092-8674(94)90277-1. [DOI] [PubMed] [Google Scholar]
- 57.Jiang H, Su Z Z, Lin J J, Goldstein N I, Young C S, Fisher P B. Proc Natl Acad Sci USA. 1996;93:9160–9165. doi: 10.1073/pnas.93.17.9160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chada S, Nemunaitis J, Tong A, Zhang Y, Su D, Mhashilkar A, Yang H, Parker K, Wilson D, Merritt J, Coffee K. Cancer Gene Ther. 2001;8:S3. [Google Scholar]
- 59.Dumoutier L, Leemans C, Lejeune D, Kotenko S V, Renauld J C. J Immunol. 2001;167:3545–3549. doi: 10.4049/jimmunol.167.7.3545. [DOI] [PubMed] [Google Scholar]
- 60.Kummer J L, Rao P K, Heidenreich K A. J Biol Chem. 1997;272:20490–20494. doi: 10.1074/jbc.272.33.20490. [DOI] [PubMed] [Google Scholar]
- 61.Xia Z, Dickens M, Raingeaud J, Davis R J, Greenberg M E. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 62.Juo P, Kuo C J, Reynolds S E, Konz R F, Raingeaud J, Davis R J, Biemann H P, Blenis J. Mol Cell Biol. 1997;17:24–35. doi: 10.1128/mcb.17.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schwenger P, Bellosta P, Vietor I, Basilico C, Skolnik E Y, Vilcek J. Proc Natl Acad Sci USA. 1997;94:2869–2873. doi: 10.1073/pnas.94.7.2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kawasaki H, Morooka T, Shimohama S, Kimura J, Hirano T, Gotoh Y, Nishida E. J Biol Chem. 1997;272:18518–18521. doi: 10.1074/jbc.272.30.18518. [DOI] [PubMed] [Google Scholar]
- 65.Yue T L, Ni J, Romanic A M, Gu J L, Keller P, Wang C, Kumar S, Yu G L, Hart T, Wang X, et al. J Biol Chem. 1999;274:1479–1486. doi: 10.1074/jbc.274.3.1479. [DOI] [PubMed] [Google Scholar]
- 66.Valladares A, Alvarez A M, Ventura J J, Roncero C, Benito M, Porras A. Endocrinology. 2000;141:4383–4395. doi: 10.1210/endo.141.12.7843. [DOI] [PubMed] [Google Scholar]
- 67.Galibert M D, Carreira S, Goding C R. EMBO J. 2001;20:5022–5031. doi: 10.1093/emboj/20.17.5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McCullough K D, Martindale J L, Klotz L O, Aw T Y, Holbrook N J. Mol Cell Biol. 2001;21:1249–1259. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Benhar M, Dalyot I, Engelberg D, Levitzki A. Mol Cell Biol. 2001;21:6913–6926. doi: 10.1128/MCB.21.20.6913-6926.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Novoa I, Zeng H, Harding H P, Ron D. J Cell Biol. 2001;153:1011–1021. doi: 10.1083/jcb.153.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]