Mechanism of Entry Determines the Ability of Toxoplasma gondii To Inhibit Macrophage Proinflammatory Cytokine Production (original) (raw)
Abstract
Macrophages (Mφ) infected with tachyzoites of the opportunistic protozoan Toxoplasma gondii are blocked in production of the proinflammatory cytokines tumor necrosis factor alpha (TNF-α) and interleukin-12 (IL-12) in response to lipopolysaccharide (LPS) triggering, and this is associated with parasite-induced inhibition of NFκB translocation. Here, we demonstrate a requirement for active invasion in the ability of the parasite to mediate suppression. Neither soluble tachyzoite antigen nor secreted products were suppressive, and heat-inactivated, antibody-coated tachyzoites, which efficiently entered the cell through receptor-mediated uptake, failed to inhibit LPS responses. Cytochalasin D, a drug blocking tachyzoite invasion of, but not adherence to, Mφ, severely curtailed _Toxoplasma_-induced suppression. In addition, parasite-induced nonresponsiveness, as measured by TNF-α production, was reversed by treating infected cells with the toxoplasmastatic drugs pyrimethamine and 6-thioxanthine prior to LPS stimulation. A divergence in IL-12 and TNF-α responses was found during extended incubation of tachyzoites and Mφ in that 24 h of incubation of infected Mφ resulted in IL-12, but not TNF-α, secretion, and production of the latter cytokine remained suppressed when these cells were subjected to LPS triggering. Our results demonstrate that active invasion and survival of the parasite within the parasitophorous vacuole are required to induce and maintain Mφ cytokine-specific nonresponsiveness to LPS. They also show that the effects of Toxoplasma on IL-12 and TNF-α production are nonidentical, with the parasite exerting a longer-lasting suppression of the latter.
Microbial pathogens that parasitize macrophages (Mφ) must survive and replicate within the potentially hostile environment of the host cell. Mφ functions such as phagocytosis and phagolysosomal degradation, nitric oxide intermediate production, and respiratory burst activity are highly effective microbicidal effector mechanisms (1, 36, 55). Mφ may also play a role in triggering acquired immunity through release of cytokines such as interleukin-12 (IL-12) and presentation of peptide antigen (Ag) to T cells within the context of major histocompatibility complex (MHC) and costimulatory molecules (26, 29, 33). Bacterial and protozoan pathogens residing within Mφ have adopted unique strategies for intracellular survival. Protozoan parasites such as Leishmania major, while generally thought to reside within an acidified phagolysosome, have been suggested to actively block phagosome-endosome fusion (15). Trypanosoma cruzi trypomastigotes, which enter cells by a process of induced phagocytosis, escape into the host cell cytoplasm through a mechanism involving the pore-forming toxin Tc-TOX (4, 9).
A different strategy of intracellular survival is employed by Toxoplasma gondii and other apicomplexan parasites. For T. gondii, tachyzoite invasion is an active process involving parasite actin-based motility coupled with discharge of the contents of rhoptry and dense-granule apical organelles (13, 16, 18, 23). In this manner, T. gondii avoids entry into the phagolysosomal pathway, instead establishing and replicating within a specialized parasitophorous vacuole composed of host cell lipid and parasite-derived, as well as host-derived, proteins (39, 52). Indeed, the intracellular fate of T. gondii within the Mφ is determined by the route through which the parasite enters the host cell. Thus, tachyzoites entering the cell through Fc receptor-mediated phagocytosis find themselves within vacuoles that undergo normal acidification and lysosomal fusion, resulting in nonproductive infection (27, 41).
Many bacterial and protozoan pathogens are capable of subverting pathways leading to inflammatory cytokine responses. For example, L. major prevents IL-12 synthesis in Mφ by a mechanism that may involve intracellular pathways triggered by interaction with Mφ Fc receptors (12, 38, 53). In contrast, Salmonella spp_._ and Yersinia enterocolitica can block inflammatory responses by directly disabling the NFκB activation pathway (44, 49). Inasmuch as in vivo Toxoplasma infection is associated with robust proinflammatory cytokine production, the parasite has not generally been regarded as a pathogen that subverts early immunity. Nevertheless, we recently demonstrated a powerful immunosuppressive effect on the ability of infected Mφ to produce tumor necrosis factor alpha (TNF-α) and IL-12 in response to lipopolysaccharide (LPS) stimulation in vitro (11). Although the inhibitor molecule IκBα underwent infection-induced phosphorylation and degradation, NFκB p50-p65 heterodimers did not translocate to the nucleus and subsequent LPS triggering was unable to promote translocation (11). Similar results were recently reported by Shapira et al. (50). Highly relevant to these data is the fact that T. gondii infection has also been shown to result in down-regulation of Mφ MHC class II gene expression as a result of interference with the STAT-1α activation pathway (34, 35).
In this study, we further examined the ability of T. gondii to inhibit Mφ cytokine responses. We examined the role of activation stimulus, route of entry, and tachyzoite-parasitophorous vacuole persistence in the suppressive properties of the parasite. Our results demonstrate that TNF-α and IL-12 production induced by several microbial stimuli, in addition to LPS, are blocked by the parasite. It is of particular interest that heat-killed-antibody-opsonized tachyzoites entering the cell through Fc receptor-mediated endocytosis were unable to suppress Mφ cytokine production. Cytochalasin (CytD), a drug blocking the parasite's ability to invade Mφ but not its ability to adhere to Mφ, severely curtailed tachyzoite-mediated suppression. In addition, in cells in which active infection was halted by pyrimethamine and 6-thioxanthine treatment, the ability to respond to LPS triggering was restored. Finally, Mφ infected for 24 h produced IL-12 but TNF-α production remained potently suppressed. The latter cells were sensitive to LPS-triggered NFκB translocation, unlike cells infected for 2 to 3 h. Together, these results establish that active invasion and the presence of live parasites are required to initiate pathways leading to inhibition of cytokine production. The results also demonstrate a divergence in parasite-triggered IL-12 and TNF-α responses.
MATERIALS AND METHODS
Mice.
Female C57BL/6 animals (6 to 8 weeks of age) were obtained from The Jackson Laboratory (Bar Harbor, Maine). The mice were housed under specific-pathogen-free conditions in the Cornell University College of Veterinary Medicine animal facility, which is accredited by the Association for Assessment and Accreditation of Laboratory Care.
Parasites and Ag.
Tachyzoites of the virulent RH strain were maintained by biweekly passage on human foreskin fibroblasts in complete medium (CM) composed of Dulbecco modified Eagle medium (Life Technologies, Gaithersburg, Md.) supplemented with 1% fetal calf serum (HyClone, Logan, Utah), penicillin (100 U/ml; Life Technologies), and streptomycin (100 μg/ml; Life Technologies). Tachyzoites were harvested from freshly lysed cultures, washed once in endotoxin-free phosphate-buffered saline (PBS; Sigma Chemical Co., St Louis, Mo.), and employed for in vitro assays. In some cases, tachyzoites were liberated from heavily infected fibroblasts by forced passage of parasite-bearing cells through an 18-gauge needle. Tachyzoite opsonization was accomplished by heat inactivation of parasites (56°C, 25 min), followed by incubation (4°C, 25 min) with mouse anti-Toxoplasma serum obtained from animals chronically infected with T. gondii strain ME49. Unbound antibody was removed by washing prior to the use of tachyzoites in assays. Soluble tachyzoite Ag (STAg) was prepared by parasite sonication in the presence of a protease inhibitor cocktail, followed by high-speed centrifugation and supernatant collection, as described elsewhere (8).
Reagents.
LPS (Escherichia coli strain O26:B6), staphylococcal enterotoxin A (SEA), pyrimethamine, and 6-thioxanthine were obtained from Sigma. Murine recombinant gamma interferon (IFN-γ) was purchased from R & D Systems, Inc. (Minneapolis, Minn.). Heat-killed bacillus Calmette-Guérin (BCG) was kindly provided by D. Russell (Cornell University).
Mφ preparation.
Bone marrow-derived Mφ were prepared as described in detail elsewhere (24). Briefly, bone marrow was collected from tibia and femur bones of 6- to 8-week-old C57BL/6 mice. Cells were washed and resuspended in bone marrow culture medium, consisting of Dulbecco modified Eagle medium (Life Technologies), 10% fetal calf serum (HyClone), 5% horse serum (Life Technologies), 2 mM glutamine, penicillin (100 U/ml; Life Technologies), and streptomycin (100 μg/ml; Life Technologies). The medium was supplemented with 30% supernatant from confluent cultures of L929 cells (American Type Culture Collection, Manassas, Va.) as a source of Mφ colony-stimulating factor. After 4 days of culture, nonadherent cells were washed away and cells were replated in CM and subjected to infection and/or stimulation 18 h later.
Inhibition assays.
Mφ (2 × 105 per well in 96-well tissue culture plates) were infected by adding RH tachyzoites and briefly centrifuging plates (500 × g, 3 min) to initiate parasite-cell contact. After 2 h (37°C, 5% CO2), Mφ activation stimuli were added and plates were incubated for 30 min (37°C, 5% CO2). The cells were subsequently gently washed with 37°C CM, fresh medium was added, and the cells were returned to the incubator for 6 to 36 h, depending upon the experiment.
To examine the ability of CytD to block suppression, tachyzoites were pretreated (10 min, 0°C) with 1 μM drug (Sigma) and then infections were carried out in the continued presence of 1 μM CytD. Cells were subjected to LPS triggering, and supernatant was collected 6 h later as described above. For tachyzoite inactivation, pyrimethamine (final concentration, 10 μM) and 6-thioxanthine (final concentration, 40 μg/ml) were added 2 h after infection, cells were subjected to LPS stimulation 24 h later, and supernatants were collected 6 h after LPS treatment.
Transwell experiments were conducted by plating 106 bone marrow-derived Mφ on a 24-well plate containing a transwell insert (0.1 μM polycarbonate membrane; Costar, Cambridge, Mass.). Tachyzoites were placed in transwells or directly on cells, and 2 h later, Mφ were subjected to LPS triggering for cytokine analysis as described above.
Cytokine ELISA.
IL-12(p40) was measured by two-site enzyme-linked immunosorbent assay (ELISA) as previously described (8), and TNF-α was measured with a commercially available kit (B-D Pharmingen, San Diego, Calif.).
Fluorescence microscopy.
Visualization of NFκB p65 (RelA) nuclear translocation was achieved by confocal fluorescence microscopy as described in detail elsewhere (11). Briefly, Mφ were plated onto 12-mm glass coverslips and subjected to infection and LPS stimulation as described above. Cells were fixed in 3% formaldehyde, washed, and incubated with goat anti-p65 serum (Santa Cruz Biotechnology, Santa Cruz, Calif.) and rabbit anti-Toxoplasma serum diluted in PBS with 0.075% saponin (Sigma). After washing, cells were incubated with fluorescein isothiocyanate-conjugated anti-goat and Texas Red-conjugated anti-rabbit antibody (both reagents from Jackson Immunoresearch, West Grove, Pa.) diluted in PBS with 0.075% saponin and 5% normal mouse serum. Cells were washed and mounted with Pro-Long Antifade (Molecular Probes, Eugene, Oreg.). Fluorescence images were acquired with a Zeiss Axioskop equipped with an Axiocam and analyzed with Axiovision software (Carl Zeiss, Inc. Thornwood, N.Y.).
RESULTS
T. gondii inhibits Mφ cytokine production driven by multiple stimuli.
Our previous results demonstrated that tachyzoite infection blocked LPS-triggered Mφ IL-12 and TNF-α production. To determine whether suppressed cytokine production was restricted to Mφ activated with LPS, we infected cells and then, 2 h later, stimulated them with BCG and SEA, in addition to LPS. While LPS signals Mφ exclusively through Toll-like receptor 4 (TLR-4), BCG likely signals through multiple receptors, including TLR-2 and TLR-4 (25, 54). In contrast, SEA, a bacterial superantigen, activates Mφ by binding to MHC class II molecules (17). Six hours after stimulation, supernatants were collected for ELISA. As shown in Fig. 1, LPS was a strikingly more potent stimulus for Mφ inflammatory cytokine production than was either BCG or SEA. Nevertheless, parasite infection clearly inhibited cytokine production in response to both of these microbial stimuli, in addition to LPS.
FIG. 1.
Multiple NFκB activation stimuli are blocked by tachyzoite (TZ) preinfection. Bone marrow-derived Mφ (Ø) were infected with T. gondii (4:1 ratio of parasites to cells) and then, 24 h later, subjected to stimulation with LPS (100 ng/ml), heat-killed BCG (5 × 108/ml), SEA (50 μg/ml), or medium. After 30 min, stimuli were removed and fresh medium was added. A cytokine ELISA was performed on culture supernatants 6 h after addition of microbial activation stimuli. This experiment was repeated twice with essentially identical results.
Live infection, but not STAg, inhibits LPS-driven Mφ TNF-α and IL-12 production.
We previously showed that tachyzoite infection results in suppressed proinflammatory cytokine production in infected, but not bystander noninfected, Mφ (11). This result suggested that infection per se, and not release of soluble factors by extracellular parasites or infected cells, is required to mediate suppression. This issue was further addressed by comparing the abilities of live parasites and STAg, a soluble fraction from total parasite sonicate, to mediate cytokine suppression in Mφ. As shown in Fig 2, while tachyzoites blocked LPS-induced Mφ IL-12(p40) and TNF-α production, at no STAg dose tested was suppression evident. Importantly, we estimate that the largest dose examined was approximately equivalent to 107 tachyzoites, while the highest multiplicity of infection tested corresponded to 1.6 × 106 parasites.
FIG. 2.
Live parasites, but not STAg, block LPS-triggered Mφ IL-12(p40) and TNF-α production. Bone marrow-derived Mφ were infected with RH strain tachyzoites (TZ) or incubated with STAg. Two hours later, extracellular parasites and STAg were removed and LPS (100 ng/ml) was added. After 30 min, LPS was washed away, fresh medium was added, and 6 h later, supernatants were collected for cytokine ELISA. Symbols: •, cells incubated in the presence of LPS; ○, cells incubated in the absence of LPS. This experiment was repeated three times with equivalent results.
Tachyzoite release of soluble factors fails to account for parasite-mediated inhibition.
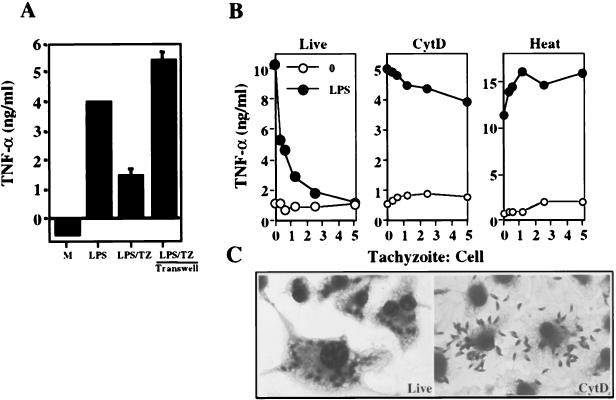
Although STAg failed to suppress Mφ responses, it was possible that live tachyzoites release a subset of inhibitory molecules not represented in our STAg preparation. Alternatively, it was possible that sonication during the course of STAg preparation results in loss of the biological activity of suppressive molecules. To address these issues, a transwell experiment was performed in which tachyzoites were separated from Mφ by a membrane that is impermeable to parasites but nonetheless allows free diffusion of macromolecules. As shown in Fig. 3A, when parasite-host cell contact was prevented, Mφ remained fully responsive to subsequent LPS triggering, unlike the situation in which parasites were placed directly into contact with host cells.
FIG. 3.
Direct contact between the parasite and host cell is necessary, but not sufficient, to mediate suppression of LPS-triggered TNF-α production. (A) Mφ were stimulated with LPS (100 ng/ml), preinfected for 2 h with a 1:1 ratio of parasites to host cells and then stimulated with LPS, or incubated for 2 h with the same number of tachyzoites (TZ) in a transwell and then stimulated with LPS. Cell supernatants were collected 6 h later for cytokine assay. M, medium. (B) Live, CytD (1 μM)- and heat-treated tachyzoites were cultured for 2 h with Mφ, LPS (100 ng/ml) was added, and supernatants were collected 6 h later for cytokine ELISA. For CytD, Mφ were maintained in the drug for the duration of the experiment. Cultures with live and heat-killed parasites were carried out in an a percentage of the solvent dimethyl sulfoxide equivalent to that used for the CytD experiment. (C) Diff-Quik staining of Mφ infected with live and CytD-treated tachyzoites. Cells were fixed and stained 6 h after parasite exposure. The proportions of cells containing internalized parasites were determined to be 99 and 5% in live and CytD experiments, respectively. This experiment was repeated three times with the same result.
These results suggested that the parasite exerted its effects from within the host cell. To examine this further, infection and subsequent LPS triggering were performed in the presence of CytD. The latter drug blocks both gliding motility and entry into host cells by disrupting the parasite actin cytoskeleton while leaving intact the parasite's ability to adhere to the cell surface membrane (16) (Fig. 3C). Treatment with CytD almost completely blocked suppression of Mφ cytokine production by T. gondii (Fig. 3B). Nevertheless, a small amount of suppression was evident relative to Mφ incubated with heat-inactivated tachyzoites, and this became more pronounced at higher tachyzoite/cell ratios (data not shown). While it is possible that the latter phenomenon results from rhoptry discharge and formation of empty vacuoles (evacuoles) (23), we cannot exclude the possibility of low-level host cell invasion in the presence of the drug.
Heat-inactivated tachyzoites internalized by receptor-mediated endocytosis fail to block LPS-triggered cytokine production.
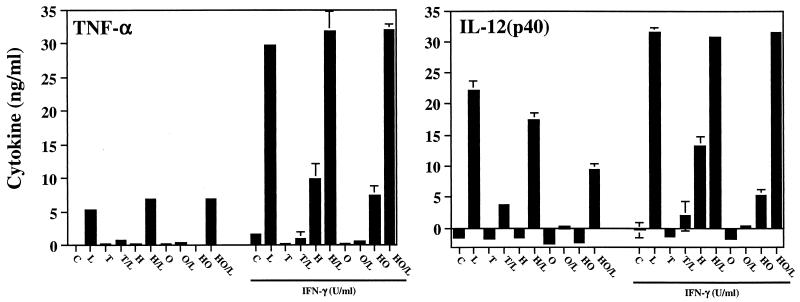
We then asked whether Mφ that had internalized antibody-coated tachyzoites by receptor-mediated endocytosis showed suppressed responses to LPS, as was the case for cells harboring live parasites. These experiments were complicated by the fact that live tachyzoites coated with antibodies may enter Mφ by both receptor-mediated endocytosis and active invasion. Therefore, we examined suppression mediated by live, heat-inactivated, opsonized live, and opsonized and heat-inactivated tachyzoites. In this set of experiments, we also examined whether Mφ preactivated with IFN-γ are subjected to parasite-induced suppression.
As shown in Fig. 4, tachyzoites subjected to either heat inactivation or heat inactivation followed by opsonization were unable to suppress LPS-triggered TNF-α production (H/L and HO/L) relative to live parasites (T/L) in Mφ that were not subjected to overnight IFN-γ activation. Live, antibody-coated tachyzoites maintained the ability to prevent LPS-driven Mφ TNF-α (O/L), most likely as a result of continued active invasion by the parasite. Similar results were obtained when IL-12 production in Mφ not preactivated with IFN-γ was examined. Nevertheless, in this case, we found that opsonized, heat-treated parasites displayed a partial (approximately 50%) ability to suppress IL-12 production (HO/L). In addition, live opsonized parasites were more effective at inhibiting LPS-induced IL-12 than were live parasites (O/L and T/L, respectively). The basis for this effect may be the reported ability of Fc receptor cross-linking to specifically suppress IL-12 production in Mφ (22).
FIG. 4.
Effects of heat inactivation and antibody coating on the ability of tachyzoites to suppress Mφ proinflammatory cytokine production. Cells were cultured with live, opsonized, heat-inactivated, or heat-inactivated and opsonized tachyzoites (3:1 ratio of parasites to cells) in the presence or absence of recombinant IFN-γ. Six hours after LPS stimulation, supernatants were collected for cytokine assay. Cells were cultured with medium alone (C), 100 ng of LPS per ml (L), live tachyzoites (T), live tachyzoites followed by LPS (T/L), heat-killed tachyzoites (H), heat-killed parasites followed by LPS (H/L), opsonized parasites (O), opsonized parasites followed by LPS (O/L), heat-killed and opsonized tachyzoites (HO), and heat-killed and opsonized tachyzoites followed by LPS (HO/L). In this experiment, the percentages of cells with internalized tachyzoites were 61% ± 15% (live), 25% ± 3% (heat killed), 75% ± 2% (opsonized), and 68% ± 14% (heat killed, opsonized). The data shown are representative of three experiments.
IFN-γ-preactivated Mφ were also sensitive to live and opsonized tachyzoite-induced blockade of LPS-induced cytokine responses (T/L and O/L in Fig. 4). Interestingly, while live tachyzoites (T in Fig. 4) alone failed to induce cytokine production by IFN-γ-activated Mφ, parasites subjected to heat inactivation (H in Fig. 4) became capable of inducing both IL-12 and TNF-α production by Mφ.
Drug-induced tachyzoite inactivation following infection reverses inhibition of LPS-triggered TNF-α production.
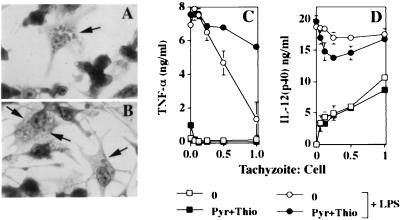
To determine whether live infection and parasite replication are required to maintain Mφ LPS nonresponsiveness, cells were infected and then, 2 h later, pyrimethamine and 6-thioxanthine were added to the cultures. The latter drugs interfere with nucleotide metabolic pathways required for parasite replication and long-term survival within the parasitophorous vacuole (45, 48). Treatment of infected cells with pyrimethamine results in parasite stasis and dissolution of the parasitophorous vacuole (27). As shown in Fig. 5A, in the absence of drug inhibitors, replicating parasites are clearly visible within a distinct parasitophorous vacuole. After 24 h in the presence of pyrimethamine and 6-thioxanthine, intracellular parasites were difficult to distinguish. In those cells where they could be identified, tachyzoites displayed a rounded-up morphology and, in most cases, a distinct parasitophorous vacuole was inapparent (Fig. 5B). At this time point, cells were pulsed for 30 min with LPS and supernatants were collected 6 h later for cytokine ELISA.
FIG. 5.
Inactivation of intracellular parasites with pyrimethamine (Pyr) and 6-thioxanthine (Thio) fails to reverse LPS-triggered TNF-α production. Bone marrow-derived Mφ were infected with live RH strain tachyzoites. Two hours later, a combination of pyrimethamine (10 μM) and 6-thioxanthine (40 μg/ml) or an equivalent dilution of carrier solvent alone was added. After 24 h, control and drug-treated cells (panels A and B; Diff-Quik staining) were pulsed for 30 min with LPS, fresh medium was added, and 6 h later, supernatants were collected for TNF-α and IL-12 ELISA (panels C and D, respectively). At the time of LPS addition, parallel cultures on glass coverslips were fixed and stained with Diff-Quik and examined by light microscopy (original magnification, ×100). The arrows in panels A and B point to parasites. The experiment was repeated three times with similar results.
Interestingly, we found that the TNF-α and IL-12 responses diverged at this late time point following infection. As shown in Fig. 5C, cells in which tachyzoites were subjected to 24-h drug inactivation exhibited a reversal in TNF-α suppression. In contrast, cells infected for 24 h showed dose-dependent IL-12(p40) production in the absence of further LPS stimulation (Fig. 5D), unlike the TNF-α response at this time point (Fig. 5C) and unlike IL-12 responses 6 h after infection (Fig. 2 and 4). Indirect immunofluorescence of IL-12 production confirmed the ELISA results and further showed that both infected and noninfected cells produced the cytokine (data not shown).
IL-12 production during late infection is accompanied by release of inhibition of NFκB activation.
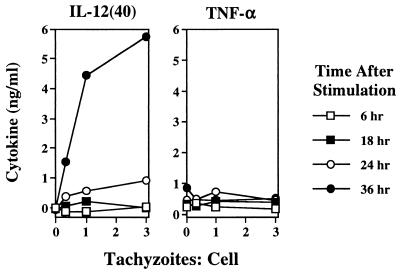
We further examined the kinetics of IL-12 production during infection with T. gondii relative to stimulation with LPS. Figure 6 shows that Mφ IL-12 production induced by tachyzoites was first detectable 24 h postinfection and that the response was maximal 36 h after initiation of infection. This differed from the response to LPS, which was strong at 6 h and peaked at 8 h after stimulation. At no time point did live parasites induce TNF-α production (Fig. 6). It is notable that in 30-h cultures, no TNF-α was produced and, indeed, LPS-induced TNF-α production remained strongly suppressed (Fig. 5C).
FIG. 6.
Kinetics of Mφ IL-12 production during T. gondii infection or LPS stimulation. Cultures with increasing amounts of tachyzoites or LPS were incubated for the indicated times, and supernatants were collected for cytokine ELISA. This experiment was repeated twice with essentially the same results.
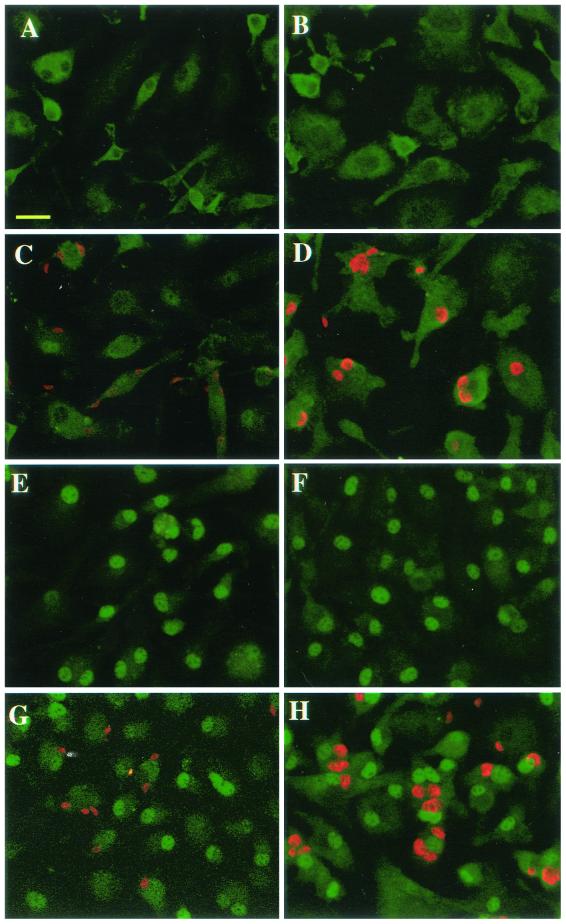
Previous results from our laboratory and others demonstrated inhibition of NFκB nuclear translocation during Toxoplasma infection of Mφ (11, 50). The observation that infected Mφ initiate IL-12 production after a delay of approximately 24 h led us to ask whether such cells maintain a block in NFκB translocation. To answer this question, Mφ were infected with parasites, triggered with LPS either 2 or 24 h later, and then examined for RelA nuclear translocation 60 min after endotoxin addition. As shown in Fig. 7, cells cultured in medium alone for 2 (panel A) or 24 (panel B) h displayed cytoplasmic NFκB localization, shown by green fluorescence. Similarly, Mφ infected with tachyzoites (shown in red) for 2 (panel C) or 24 (panel D) h displayed little evidence of RelA nuclear translocation. Triggering with LPS resulted in NFκB relocalization to the nucleus (panels E and F). As previously demonstrated, cells preinfected for 2 h displayed a block in NFκB nuclear translocation during LPS stimulation, unlike surrounding noninfected cells, in which NFκB was translocated to the nucleus (panel G). A different pattern was apparent in the case of Mφ infected 24 h prior to LPS triggering. Here, cells harboring tachyzoites were capable of NFκB translocation in response to LPS, as shown by strong green fluorescence in the nuclei of infected cells (Fig. 7H).
FIG. 7.
LPS-triggered RelA translocation is blocked 2 h post-Toxoplasma invasion but restored 24 h after infection. Mφ were infected (1:1 ratio of tachyzoites to cells), and then, either 2 (panels A, C, E, and G) or 24 (panels B, D, F, and H) h later, cells were triggered with LPS (100 ng/ml). Sixty minutes later, cells were fixed, permeabilized, and stained with antibodies to RelA (p65) (shown in green) and Toxoplasma (shown in red). Panels: A and B, medium; C and D, Toxoplasma; E and F, LPS; G and H, Toxoplasma preinfection plus LPS. Scale bar = 20 μm. This experiment was repeated twice with similar results.
DISCUSSION
T. gondii may enter Mφ through either active invasion or receptor-mediated uptake. Active invasion, completed within approximately 15 to 20 s (42), is a complex, parasite-initiated process involving sequential discharge of microneme, rhoptry, and dense-granule proteins. Microneme protein discharge is associated with initial attachment of the parasite to a host cell (10, 19). Rhoptry proteins are released concurrent with formation of a moving junction during cell penetration (23). Dense-granule exocytosis occurs in the nascent vacuole and is likely involved in modification of the intracellular compartment occupied by the tachyzoite (13). Phagocytosis and receptor-mediated endocytosis are host cell-mediated processes that may involve clathrin-based mechanisms (1). Phagocytosis typically leads to vacuole acidification and fusion of the vacuole with lysosomes. The latter processes are evaded during T. gondii invasion (27, 40, 51).
Our results suggest that active invasion and survival of the parasite within the parasitophorous vacuole are required to induce and maintain Mφ cytokine-specific nonresponsiveness to LPS. STAg and molecules secreted by live parasites failed to affect LPS-driven cytokine production, arguing against a receptor-mediated event at the cell surface. Additionally, in the presence of CytD, which allows parasite attachment but not invasion, tachyzoites were almost completely ineffective at suppressing responses to LPS. Nevertheless, a small amount of suppression was apparent in the presence of the drug. Indeed, at high parasite/cell ratios (approximately 20-fold relative to those of control cultures in the absence of CytD), levels of cytokine suppression were similar. Inasmuch as rhoptry discharge and evacuole formation may occur in the absence of tachyzoite invasion (23), it is possible that evacuole formation in the presence of CytD accounts for Mφ nonresponsiveness when high numbers of tachyzoites are present.
Heat-killed parasites entering host cells through phagocytosis or receptor-mediated endocytosis following opsonization failed to prevent LPS-triggered cytokine production. In addition, parasite-induced nonresponsiveness, as measured by TNF-α production, was reversed by drug inactivation of the parasites. Combined with our previous study showing that LPS nonresponsiveness was established within 30 min of infection (11), these results reveal that tachyzoite-mediated suppression occurs rapidly after infection and that its continued maintenance requires active parasites and/or an intact parasitophorous vacuole. A hypothesis we suggest is that Mφ nonresponsiveness is initiated during parasite entry, possibly involving discharge of apical organelles. Several tachyzoite proteins, including rhoptry-derived ROP-2 and dense-granule-derived GRA5, undergo transmembrane insertion into the parasitophorous vacuole (5, 30). It is possible that such proteins interfere with intracellular Mφ functions, leading to a block in NFκB activation.
Mφ nonresponsiveness to tachyzoite infection is not restricted to cells activated by LPS. Thus, BCG-driven TNF-α and IL-12(p40) production was also inhibited by preinfection. Components of BCG signal through both TLR-4 and TLR-2 (54). Since both receptors lie upstream of the IκB kinase activation pathway required for NFκB translocation (28), it is not surprising that the BCG response is blocked. Similarly, superantigen-induced MHC class II multimerization leads to IL-12(p40) production through an NFκB-dependent pathway (17) and, as predicted, tachyzoite infection also blocked this response.
The results presented here suggest that Toxoplasma possesses at least two mechanisms that result in suppressed proinflammatory cytokine production. Early during infection, production of IL-12 and TNF-α triggered by LPS is prevented, and this is associated with a blockade in NFκB nuclear import. However, after 24 h of infection, tachyzoites themselves elicit Mφ IL-12, yet the cells remain actively suppressed in the ability to produce TNF-α. Importantly, at this late time point, LPS-driven RelA translocation is no longer prevented in infected cells. These results suggest nonidentical control of IL-12 and TNF-α expression. While control of IL-12(p40) and TNF-α gene promoters is not fully understood, both contain binding sites for AP-1 and CCAAT enhancer-binding protein, in addition to NFκB (32, 43). Nevertheless, functional promoter analysis suggests that these genes are unlikely to be regulated identically (32, 46). Determination of how T. gondii targets different aspects of Mφ proinflammatory signaling cascades is an area of ongoing research.
Our experiments show that at 24 h post-Toxoplasma infection, when IL-12 is being produced, there is no evidence of NFκB translocation in infected cells. In this regard, it is of interest that production of IL-12 during T. gondii infection also occurs in the absence of NFκB family member c-Rel (37). Together, these results suggest that parasite-driven IL-12 production may occur independently of NFκB signaling pathways. Nevertheless, with regard to the present results, it is possible that parasite-driven nuclear translocation and re-export of RelA to the cytoplasm were events that preceded the 24-h time point at which cells were examined, a possibility that we are now examining.
Our findings and similar reports by others (50) suggesting that Toxoplasma disables the NFκB activation pathway parallel other studies demonstrating parasite interference with the Janus kinase/signal transducer and activator of transcription signaling pathway in Mφ. Thus, the parasite also down-regulates Mφ expression of IFN-γ-induced MHC class II molecules, a process dependent upon signaling by the Janus kinase/signal transducer and activator of transcription (34). The suppressive effect results from parasite interference with nuclear translocation of STAT1α, despite normal phosphorylation of the latter transcription factor (35). It is tempting to speculate that the inhibitory effects of Toxoplasma on NFκB and STAT1α stem from a common mechanism of action. A hypothesis consistent with these data is that T. gondii infection results in a nuclear protein import blockade.
Toxoplasma infection is well known to result in robust proinflammatory mediator production in vivo (2, 14, 56). Therefore, the parasite's suppressive effects on Mφ cannot be globally applicable to all cell types. Indeed, the present study shows that Mφ themselves produce IL-12(p40), but not TNF-α, during extended periods (i.e., >24 h) of contact with T. gondii. Both dendritic cells and neutrophils serve as early cytokine sources during infection (3, 6-8, 47). We are currently examining the role of NFκB activation during infection of these cell types with T. gondii. The present results showing Mφ IL-12 production during extended culture also reconcile our results with previous studies showing Mφ IL-12 production in response to T. gondii (20).
It is not clear why Toxoplasma possesses mechanisms to down-regulate Mφ proinflammatory responses. Control of infection depends upon balanced production of type 1 cytokines such as IL-12 and IFN-γ (2, 14, 21, 31, 56). Therefore, it is possible that the suppressive activity of the parasite represents an immune evasion strategy or a means by which to avoid overinduction of proinflammatory cytokines. Molecular identification of the parasite-inhibitory factor is required to understand its role in the host-parasite interaction and may lead to development of anti-inflammatory agents of broad clinical utility.
Acknowledgments
We thank Leesun Kim and David Sibley for advice and discussion during the course of this study.
This work was supported by National Institutes of Health grant AI47888.
REFERENCES
- 1.Aderem, A., and D. M. Underhill. 1999. Mechanisms of phagocytosis in macrophages. Annu. Rev. Immunol. 17**:**593-623. [DOI] [PubMed] [Google Scholar]
- 2.Alexander, J., and C. A. Hunter. 1998. Immunoregulation during toxoplasmosis. Chem. Immunol. 70**:**81-102. [DOI] [PubMed] [Google Scholar]
- 3.Aliberti, J., C. Reis e Sousa, M. Schito, S. Hieny, T. Wells, G. B. Huffnage, and A. Sher. 2000. CCR5 provides a signal for microbial induced production of IL-12 by CD8α+ dendritic cells. Nat. Immunol. 1**:**83-87. [DOI] [PubMed] [Google Scholar]
- 4.Andrews, N. W. 1994. From lysosomes to the cytosol: the intracellular pathway of Trypanosoma cruzi. Braz. J. Med. Biol. Res. 27**:**471-475. [PubMed] [Google Scholar]
- 5.Beckers, C. J. M., J.-F. Dubremetz, O. Mercereau-Puijalon, and K. A. Joiner. 1994. The Toxoplasma gondii rhoptry protein ROP 2 is inserted into the parasitophorous vacuole membrane, surrounding the intracellular parasite, and is exposed to the host cell cytoplasm. J. Cell Biol. 127**:**947-961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bliss, S. K., B. A. Butcher, and E. Y. Denkers. 2000. Rapid recruitment of neutrophils with prestored IL-12 during microbial infection. J. Immunol. 165**:**4515-4521. [DOI] [PubMed] [Google Scholar]
- 7.Bliss, S. K., A. J. Marshall, Y. Zhang, and E. Y. Denkers. 1999. Human polymorphonuclear leukocytes produce IL-12, TNF-α, and the chemokines macrophage-inflammatory protein-1α and -1β in response to Toxoplasma gondii antigens. J. Immunol. 162**:**7369-7375. [PubMed] [Google Scholar]
- 8.Bliss, S. K., Y. Zhang, and E. Y. Denkers. 1999. Murine neutrophil stimulation by Toxoplasma gondii antigen drives high level production of IFN-γ-independent IL-12. J. Immunol. 163**:**2081-2088. [PubMed] [Google Scholar]
- 9.Bogdan, C., and M. Rollinghoff. 1999. How do protozoan parasites survive inside macrophages? Parasitol. Today 15**:**22-28. [DOI] [PubMed] [Google Scholar]
- 10.Brecht, S., V. B. Carruthers, D. J. Ferguson, O. K. Giddings, G. Wang, U. Jaekle, J. M. Harper, L. D. Sibley, and D. Soldati. 2001. The Toxoplasma micronemal protein MIC4 is an adhesin composed of six conserved apple domains. J. Biol. Chem. 276**:**4119-4127. [DOI] [PubMed] [Google Scholar]
- 11.Butcher, B. A., L. Kim, P. F. Johnson, and E. Y. Denkers. 2001. Toxoplasma gondii tachyzoites inhibit proinflammatory cytokine induction in infected macrophages by preventing nuclear translocation of the transcription factor NFκB. J. Immunol. 167**:**2193-2201. [DOI] [PubMed] [Google Scholar]
- 12.Carrera, L., R. T. Gazzinelli, R. Badolato, S. Hieny, W. Muller, R. Kuhn, and D. L. Sacks. 1996. Leishmania promastigotes selectively inhibit interleukin 12 induction in bone marrow-derived macrophages from susceptible and resistant mice. J. Exp. Med. 183**:**515-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carruthers, V. B., and L. D. Sibley. 1997. Sequential protein secretion from three distinct organelles of Toxoplasma gondii accompanies invasion of human fibroblasts. Eur. J. Cell Biol. 73**:**114-123. [PubMed] [Google Scholar]
- 14.Denkers, E. Y., and R. T. Gazzinelli. 1998. Regulation and function of T-cell-mediated immunity during Toxoplasma gondii infection. Clin. Microbiol. Rev. 11**:**569-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Desjardins, M., and A. Descoteaux. 1997. Inhibition of phagolysosomal biogenesis by the Leishmania lipophosphoglycan. J. Exp. Med. 185**:**2061-2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dobrowski, J. M., and L. D. Sibley. 1996. Toxoplasma invasion of mammalian cells is powered by the actin cytoskeleton of the parasite. Cell 84**:**933-939. [DOI] [PubMed] [Google Scholar]
- 17.Du, C., and S. Sriram. 2000. Induction of interleukin-12/p40 by superantigens in macrophages is mediated by activation of nuclear factor-κB. Cell. Immunol. 199**:**50-57. [DOI] [PubMed] [Google Scholar]
- 18.Dubremetz, J. F. 1998. Host cell invasion by Toxoplasma gondii. Trends Microbiol. 6**:**27-30. [DOI] [PubMed] [Google Scholar]
- 19.Garcia-Reguet, N., M. Lebrun, M. N. Fourmaux, O. Mercereau-Puijalon, T. Mann, C. J. Beckers, B. Samyn, J. Van Beeumen, D. Bout, and J. F. Dubremetz. 2000. The microneme protein MIC3 of Toxoplasma gondii is a secretory adhesin that binds to both the surface of the host cells and the surface of the parasite. Cell. Microbiol. 2**:**353-364. [DOI] [PubMed] [Google Scholar]
- 20.Gazzinelli, R. T., S. Hieny, T. Wynn, S. Wolf, and A. Sher. 1993. IL-12 is required for the T-cell independent induction of IFN-γ by an intracellular parasite and induces resistance in T-cell-deficient hosts. Proc. Natl. Acad. Sci. USA 90**:**6115-6119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gazzinelli, R. T., M. Wysocka, S. Hieny, T. Scharton-Kersten, A. Cheever, R. Kuhn, W. Muller, G. Trinchieri, and A. Sher. 1996. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent upon CD4+ T cells and accompanied by overproduction of IL-12, IFN-γ, and TNF-α. J. Immunol. 157**:**798-805. [PubMed] [Google Scholar]
- 22.Grazia Cappiello, M., F. S. Sutterwala, G. Trinchieri, D. M. Mosser, and X. Ma. 2001. Suppression of Il-12 transcription in macrophages following Fc gamma receptor ligation. J. Immunol. 166**:**4498-4506. [DOI] [PubMed] [Google Scholar]
- 23.Hakansson, S., A. J. Charron, and L. D. Sibley. 2001. Toxoplasma evacuoles: a two-step process of secretion and fusion forms the parasitophorous vacuole. EMBO J. 20**:**3132-3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hakim, F. T., R. T. Gazzinelli, E. Y. Denkers, S. Hieny, G. M. Shearer, and A. Sher. 1991. CD8+ T cells from mice vaccinated against Toxoplasma gondii are cytotoxic for parasite-infected or antigen pulsed host cells. J. Immunol. 147**:**2310-2316. [PubMed] [Google Scholar]
- 25.Hoshino, K., O. Takeuchi, T. Kawai, H. Sanjo, T. Ogawa, Y. Takeda, K. Takeda, and S. Akira. 1999. Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J. Immunol. 162**:**3749-3752. [PubMed] [Google Scholar]
- 26.Hsieh, C. S., S. E. Macatonia, C. S. Tripp, S. F. Wolf, A. O'Garra, and K. M. Murphy. 1993. Development of TH1 CD4+ T cells through IL-12 produced by _Listeria_-induced macrophages. Science 260**:**547-549. [DOI] [PubMed] [Google Scholar]
- 27.Joiner, K. A., S. A. Fuhrman, H. M. Miettinen, L. H. Kasper, and I. Mellman. 1990. Toxoplasma gondii: fusion competence of parasitophorous vacuoles in Fc receptor-transfected fibroblasts. Science 249**:**641-646. [DOI] [PubMed] [Google Scholar]
- 28.Karin, M., and Y. Ben-Neriah. 2000. Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu. Rev. Immunol. 18**:**621-663. [DOI] [PubMed] [Google Scholar]
- 29.Ladel, C. H., C. Blum, A. Drehar, K. Reifenberg, and S. H. E. Kaufmann. 1995. Protective role of γ/δ T cells and α/β T cells in tuberculosis. Eur. J. Immunol. 25**:**2877-2881. [DOI] [PubMed] [Google Scholar]
- 30.Lecordier, L., C. Mercier, L. D. Sibley and M.-F. Cesbron-Delauw. 1999. Transmembrane insertion of the Toxoplasma gondii GRA5 protein occurs after soluble secretion into the host cell. Mol. Biol. Cell 10**:**1277-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liesenfeld, O., H. Kang, D. Park, T. A. Nguyen, C. V. Parkhe, H. Watanabe, T. Abo, A. Sher, J. S. Remington, and Y. Suzuki. 1999. TNF-α, nitric oxide and IFN-γ are all critical for development of necrosis in the small intestine and early mortality in genetically susceptible mice infected perorally with Toxoplasma gondii. Parasite Immunol. 21**:**365-376. [DOI] [PubMed] [Google Scholar]
- 32.Liu, H., P. Sidiropoulos, G. Song, L. J. Pagliari, M. J. Birrer, B. Stein, J. Anrather, and R. M. Pope. 2000. TNF-α gene expression in macrophages: regulation by NF-κB is independent of c-Jun or C/EBPβ. J. Immunol. 164**:**4277-4285. [DOI] [PubMed] [Google Scholar]
- 33.Locksley, R. M. 1993. Interleukin 12 in host defense against microbial pathogens. Proc. Natl. Acad. Sci. USA 90**:**5879-5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luder, C. G. K., T. Lang, B. Beurle, and U. Gross. 1998. Down-regulation of MHC class II molecules and inability to up-regulate class I molecules in murine macrophages after infection with Toxoplasma gondii. Clin. Exp. Immunol. 112**:**308-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luder, C. G. K., W. Walter, B. Beuerle, M. J. Maeurer, and U. Gross. 2001. Toxoplasma gondii down-regulates MHC class II gene expression and antigen presentation by murine macrophages via interference with nuclear translocation of STAT1α. Eur. J. Immunol. 31**:**1475-1484. [DOI] [PubMed] [Google Scholar]
- 36.MacMicking, J., Q. Xie, and C. Nathan. 1997. Nitric oxide and macrophage function. Annu. Rev. Immunol. 15**:**323-350. [DOI] [PubMed] [Google Scholar]
- 37.Mason, N., J. Aliberti, J. C. Caamano, H. C. Liou, and C. A. Hunter. 2002. Identification of c-Rel-dependent and -independent pathways of Il-12 production during infectious and inflammatory stimuli. J. Immunol. 168**:**2590-2594. [DOI] [PubMed] [Google Scholar]
- 38.McDowell, M. A., and D. L. Sacks. 1999. Inhibition of host cell signal transduction by Leishmania: observations relevant to the selective impairment of IL-12 responses. Curr. Opin. Microbiol. 2**:**438-443. [DOI] [PubMed] [Google Scholar]
- 39.Mordue, D. G., N. Dessai, M. Dustin, and L. D. Sibley. 1999. Invasion by Toxoplasma gondii establishes a moving junction that selectively excludes host cell plasma membrane proteins on the basis of their membrane anchoring. J. Exp. Med. 190**:**1783-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mordue, D. G., S. Hakansson, I. Niesman, and L. D. Sibley. 1999. Toxoplasma gondii resides in a vacuole that avoids fusion with host cell endocytic and exocytic vesicular trafficking pathways. Exp. Parasitol. 92**:**87-99. [DOI] [PubMed] [Google Scholar]
- 41.Mordue, D. G., and L. D. Sibley. 1997. Intracellular fate of vacuoles containing Toxoplasma gondii is determined at the time of formation and depends upon the mechanism of entry. J. Immunol. 159**:**4452-4459. [PubMed] [Google Scholar]
- 42.Morisaki, J. H., J. E. Heuser, and L. D. Sibley. 1995. Invasion of Toxoplasma gondii occurs by active penetration of the host cell. J. Cell Sci. 108**:**2457-2464. [DOI] [PubMed] [Google Scholar]
- 43.Murphy, T., M. Cleveland, P. Kulezka, J. Magram, and K. Murphy. 1995. Regulation of interleukin 12 p40 expression through an NF-κB half-site. Mol. Cell. Biol. 15**:**5258-5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neish, A. S., A. T. Gewirtz, H. Zeng, A. N. Young, M. E. Hobert, V. Karmali, A. S. Rao, and J. L. Madara. 2000. Prokaryotic regulation of epithelial responses by inhibition of IκB-α ubiquitination. Science 289**:**1560-1563. [DOI] [PubMed] [Google Scholar]
- 45.Pfefferkorn, E. R., and S. E. Borotz. 1994. Toxoplasma gondii: characterization of a mutant resistant to 6-thioxanthine. Exp. Parasitol. 79**:**374-382. [DOI] [PubMed] [Google Scholar]
- 46.Plevy, S. E., J. H. M. Gemberling, S. Hsu, A. J. Dorner, and S. T. Smale. 1997. Multiple control elements mediate activation of the murine and human interleukin 12 p40 promoters: evidence of functional synergy between C/EBP and Rel proteins. Mol. Cell. Biol. 17**:**4572-4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reis e Sousa, C., S. Hieny, T. Scharton-Kersten, D. Jankovic, H. Charest, R. N. Germain, and A. Sher. 1997. In vivo microbial stimulation induces rapid CD40L-independent production of IL-12 by dendritic cells and their re-distribution to T cell areas. J. Exp. Med. 186**:**1819-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reynolds, M. G., and D. S. Roos. 1998. A biochemical and genetic model for parasite resistance to antifolates: Toxoplasma gondii provides insights into pyrimethamine and cycloguanil resistance in Plasmodium falciparum. J. Biol. Chem. 273**:**3461-3469. [DOI] [PubMed] [Google Scholar]
- 49.Ruckdeschel, K., O. Mannel, K. Richter, C. A. Jacobi, K. Trulzsch, B. Rouot, and J. Heesemann. 2001. Yersinia outer protein P of Yersinia enterocolitica simultaneously blocks the nuclear factor-κB pathway and exploits lipopolysaccharide signaling to trigger apoptosis. J. Immunol. 166**:**1823-1831. [DOI] [PubMed] [Google Scholar]
- 50.Shapira, S. S., K. Speirs, A. Gerstein, J. Caamano, and C. A. Hunter. 2002. Suppression of NF-κB activation by infection with Toxoplasma gondii. J. Infect. Dis. 185**:**S66-S72. [DOI] [PubMed] [Google Scholar]
- 51.Sibley, L. D., R. Lawson, and J. L. Krahenbuhl. 1985. Phagosome acidification blocked by intracellular Toxoplasma gondii. Nature 315**:**416-419. [DOI] [PubMed] [Google Scholar]
- 52.Suss-Toby, E., E. J. Zimmerberg, and G. E. Ward. 1996. Toxoplasma invasion: the parasitophorous vacuole is formed from host cell plasma membrane and pinches off via a fusion pore. Proc. Natl. Acad. Sci. USA 93**:**8413-8418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sutterwala, F. S., G. J. Noel, R. Clynes, and D. M. Mosser. 1997. Selective suppression of interleukin-12 induction after macrophage receptor ligation. J. Exp. Med. 185**:**1977-1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tsuji, S., M. Matsumoto, O. Takeuchi, S. Akira, I. Azuma, A. Hayashi, K. Toyoshima, and T. Seya. 2000. Maturation of human dendritic cells by cell wall skeleton of Mycobacterium bovis bacillus Calmette-Guerin: involvement of toll-like receptors. Infect. Immun. 68**:**6883-6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vazquez-Torres, A., and F. C. Fang. 2001. Oxygen-dependent anti-salmonella activity of macrophages. Trends Microbiol. 9**:**29-33. [DOI] [PubMed] [Google Scholar]
- 56.Yap, G. S., and A. Sher. 1999. Cell-mediated immunity to Toxoplasma gondii: initiation, regulation and effector function. Immunobiology 201**:**240-247. [DOI] [PubMed] [Google Scholar]