Concomitant loss of proapoptotic BH3-only Bcl-2 antagonists Bik and Bim arrests spermatogenesis (original) (raw)
Abstract
The BH3-only proteins of the Bcl-2 family initiate apoptosis through the activation of Bax-like relatives. Loss of individual BH3-only proteins can lead either to no phenotype, as in mice lacking Bik, or to marked cell excess, as in the hematopoietic compartment of animals lacking Bim. To investigate whether functional redundancy with Bim might obscure a significant role for Bik, we generated mice lacking both genes. The hematopoietic compartments of bik −/− bim −/− and bim −/− mice were indistinguishable. However, although testes develop normally in mice lacking either Bik or Bim, adult bik −/− bim −/− males were infertile, with reduced testicular cellularity and no spermatozoa. The testis of young bik −/− bim −/− males, like those lacking Bax, exhibited increased numbers of spermatogonia and spermatocytes, although loss of Bik plus Bim blocked spermatogenesis somewhat later than Bax deficiency. The initial excess of early germ cells suggests that spermatogenesis fails because supporting Sertoli cells are overwhelmed. Thus, Bik and Bim share, upstream of Bax, the role of eliminating supernumerary germ cells during the first wave of spermatogenesis, a process vital for normal testicular development.
Keywords: apoptosis, BH3-only protein, Bik, Bim, spermatogenesis
Introduction
Programmed cell death (apoptosis) is a genetically controlled mechanism of cell suicide that removes redundant, damaged or harmful cells during development and maintains tissue homeostasis in adults (Strasser et al, 2000; Adams, 2003; Danial and Korsmeyer, 2004). The Bcl-2 family consists of both death-preventing and -promoting members (Cory et al, 2003; Danial and Korsmeyer, 2004). Its prosurvival members share at least three of the four conserved regions, termed Bcl-2 homology regions 1–4 (BH1–4), and BH1, BH2 and BH3 are also present in the proapoptotic ‘Bax-like' proteins (Bax, Bak and Bok), which are structurally similar to Bcl-2. In contrast, members of the more divergent proapoptotic group, the ‘BH3-only' proteins (e.g. Bad, Bim, Bik and Puma), are related to Bcl-2 (and each other) only by the short BH3 region, which is essential for their proapoptotic function (Huang and Strasser, 2000). Combined loss of Bax and Bak renders many (perhaps all) cell types resistant to most physiological and experimentally applied apoptotic stimuli (Lindsten et al, 2000; Rathmell et al, 2002). Notably, overexpressed BH3-only proteins cannot kill such cells, establishing that Bax or Bak is required for their function (Cheng et al, 2001; Zong et al, 2001). It appears that the binding of BH3-only proteins to their prosurvival relatives promotes the activation of Bax and Bak (Willis et al, 2005), which are then thought to subvert outer mitochondrial membrane integrity, unleashing proteins such as cytochrome c that lead to activation of the caspases that demolish the cell (Huang and Strasser, 2000; Cory et al, 2003; Danial and Korsmeyer, 2004).
Gene disruption in mice has shown that each BH3-only protein is responsible for initiating apoptosis in response to only select stimuli and only within certain cell types (Bouillet et al, 1999; Putcha et al, 2001; Whitfield et al, 2001; Akiyama et al, 2003; Shibue et al, 2003; Villunger et al, 2003). Furthermore, the protective effect conveyed by the absence of a single BH3-only protein is usually not as marked as that conferred by overexpression of Bcl-2 or loss of both Bax and Bak (Deckwerth et al, 1996; Miller et al, 1997; Bouillet et al, 1999; Putcha et al, 2001; Whitfield et al, 2001; Villunger et al, 2003; Imaizumi et al, 2004). In view of the multiplicity of BH3-only proteins and overlaps in their expression patterns, a plausible explanation for such limited effects is redundancy and compensation by other BH3-only proteins.
In particular, we wondered whether the absence of an overt phenotype in mice lacking Bik (also known as Blk or Nbk) (Coultas et al, 2004) might be due to compensation by Bim. Loss of Bim impairs a range of cytotoxic responses in a spectrum of hematopoietic and other cell types (Bouillet et al, 1999, 2001, 2002; Putcha et al, 2001; Whitfield et al, 2001; Hildeman et al, 2002; Akiyama et al, 2003; Enders et al, 2003), but its loss rarely fully blocks the apoptotic response, indicating that at least one other cell death initiator must act in parallel. We previously reported that expression of Bik overlaps that of Bim in the hematopoietic system and is upregulated in response to certain apopototic stimuli partially dependent on Bim for apoptosis (Coultas et al, 2004). Their expression also overlaps in cardiomyocytes and epithelial tissues of the kidney and mammary gland (L Coultas and A Strasser, unpublished observations). Hence, Bik and Bim might act together in cell types both within and beyond the hematopoietic system.
To investigate the hypothesis that Bik and Bim share certain physiological roles in the initiation of apoptosis, we have characterized mice lacking both proteins. We report the unexpected finding that Bik and Bim share a critical role in spermatogenesis, a developmental process in which members of two other factions of the Bcl-2 family, namely the proapoptotic Bax (Knudson et al, 1995; Russell et al, 2002) and the antiapoptotic Bcl-w (Print et al, 1998; Ross et al, 1998), are known to have central roles. Our study thus identifies the essential BH3-only initiators of an apoptotic program required for normal sperm development.
Results
The hematopoietic phenotype of bim−/− mice is not augmented by concomitant loss of bik
Interbreeding of mice deficient for Bik or Bim produced animals lacking both genes in expected numbers and, except for male sterility (see below), they were overtly phenotypically normal. Since Bik and Bim are both expressed in lymphoid and myeloid cells (O'Reilly et al, 2000; Coultas et al, 2004), we explored whether the hematopoietic perturbation in bim knockout mice was exacerbated by concomitant absence of bik. We first examined the size and cellular composition of the hematopoietic organs. The spleen, enlarged in bim _−/_− mice due to excessive mature T and B lymphocytes, granulocytes and macrophages, was not further increased in size or cellularity by additional loss of bik (Figure 1A). Similarly, the bone marrow of bik −/− bim −/− mice showed the same reduction in pro/pre-B and immature B lymphoid populations and elevation in mature B and macrophage populations observed in bim −/− mice (Figure 1B). Finally, T-cell development in the thymus of bik −/− bim −/− animals was indistinguishable from bim −/− mice, with the same reduction in CD4+8+ (pre-T) cell numbers and elevation in CD4−8− (pro-T) and mature (CD4+8− and CD4−8+) T-cell populations (Figure 1C).
Figure 1.
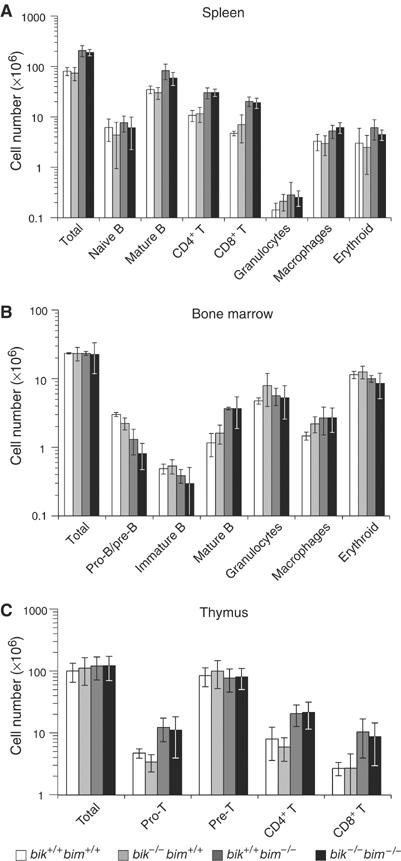
Cell type composition of hematopoietic organs from wt (bik +/+ bim +/+), bik −/− bim +/+, bik +/+ bim −/− and bik −/− bim −/− mice. (A) The total number of splenocytes from 8- to 13-week-old wt, bik −/− bim +/+, bik +/+ bim −/− and bik −/− bim −/− mice was determined and the number of naïve (sIgM+ sIgD−) and mature B cells (sIgMlo sIgDhi), mature T cells (CD4+ CD8− or CD4− CD8+), macrophages (Mac1+ Gr-l−), granulocytes (Mac1+ Gr-l+) and nucleated erythroid progenitors (Ter119+) was determined by FACS analysis. (B) The total number of bone marrow cells from 8- to 13-week-old wt, bik −/− bim +/+, bik +/+ bim −/− and bik −/− bim −/− mice was determined and the number of pro-B and pre-B cells (B220+ sIgM− sIgD−), immature (sIgM+ sIgD−) and mature (sIgMlo sIgDhi) B cells, macrophages (Mac1− Grl+) and granulocytes (Mac1+ Grl+) was determined by FACS analysis. (C) The total number of thymocytes from 8- to 13-week-old wt, bik −/− bim +/+, bik +/+ bim −/− and bik −/− bim −/− mice was determined and the number of pro-T (CD4−8−), pre-T (CD4+ 8+) and mature T (CD4+ 8− or CD4−8+) cells was determined by FACS analysis. Data shown represent means±s.d. of 3–5 mice of each genotype.
We next determined the sensitivity of bik −/− bim −/− lymphocytes to apoptotic stimuli. Thymocytes from bik −/− bim −/− mice proved to be no more resistant to spontaneous death in culture (cytokine deprivation) or treatment with the calcium ionophore ionomycin than bim _−/_− thymocytes (Figure 2A). Furthermore, concomitant loss of Bik did not augment the moderate resistance of _bim_−/− thymocytes to etoposide or dexamethasone, or provide any protection against phorbol myristate acetate (Figure 2A). Although B cells lacking Bim are resistant to crosslinking of their antigen receptor by anti-μ F(ab′)2 antibody fragments, they are not as refractory as _bcl_-2 transgenic B cells, implicating other BH3-only proteins (Enders et al, 2003). Since B cells upregulate Bik following antigen receptor stimulation (Jiang and Clark, 2001; Coultas et al, 2004), we tested the sensitivity of immature as well as mature B cells from bik −/− bim −/− mice to anti-μ F(ab′)2 antibody fragments. They were no more resistant than bim −/− lymphocytes (Figure 2B and data not shown). Furthermore, the resistance of Bim-deficient mature B and T lymphocytes to death in response to cytokine withdrawal or treatment with etoposide or dexamethasone was not enhanced by concurrent loss of Bik (Figure 2B and data not shown). In conclusion, we could find no evidence that Bik cooperates with Bim to regulate apoptosis within the hematopoietic system.
Figure 2.
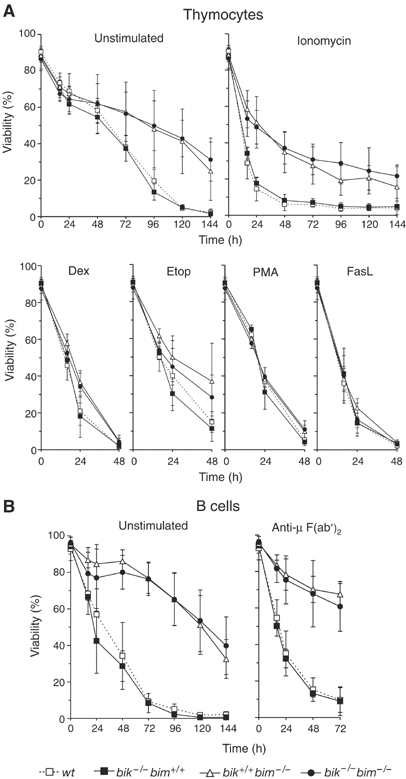
Susceptibility of bik −/− bim −/− lymphocytes to apoptotic stimuli. (A) Thymocytes from wt (bik +/+ bim +/+), bik −/− bim +/+, bik +/+ bim −/− and bik −/− bim −/− mice were harvested and cultured in the presence of dexamethasone (Dex 10−6 M), etoposide (Etop 1 μg/ml), PMA (2 ng/ml), ionomycin (1 μg/ml), crosslinked FasL (100 ng/ml) or in the absence of exogenous stimuli (unstimulated) for the indicated times. (B) Mature B cells were negatively sorted from lymph nodes of wt (bik +/+ bim +/+), bik −/− bim +/+, bik +/+ bim −/− and bik −/− bim −/− mice and cultured in the presence of anti-IgM crosslinking F(ab′) antibody fragments at 5 μg/ml or in the absence of exogenous stimuli (unstimulated) for the indicated times. Data shown represent means±s.d. of 4–6 mice of each genotype.
Reduced fertility and testicular weight in Bik/Bim double knockout males
Although the fertility of both male and female bik −/− or bim −/− mice is normal_, bik_ −/− bim −/− males failed to sire offspring when mated to C57BL/6 females. Their infertility was not due to defective sexual behaviour, because the mated females routinely exhibited vaginal plugs. In contrast to the males, bik −/− bim −/− females were fertile and produced normal numbers of offspring.
Whereas the testes of bik −/− and bim −/− males were of normal weight, those of bik −/− bim −/− littermates were much smaller, while the remainder of the male reproductive tract appeared normal (Figure 3A). In subsequent analyses, we compared the bik −/− bim −/− testicular defect with that well-characterized in Bax-deficient mice (Knudson et al, 1995; Russell et al, 2002). The reduction in testicular weight became apparent after postnatal (PN) day 21 and the weight drop in adults (∼60%) was comparable to that in the bax −/− testes (Figure 3B). The reduction was unlikely to be hormone related, as the weight of the androgen-dependent vesicular gland (seminal vesicle) was normal (data not shown), and preliminary results from six adult mutant animals suggest that serum concentrations of inhibin and follicle-stimulating hormone (mFSH) are also normal. For unknown reasons, around half of all bik −/− bim −/− and bax −/− males exhibited reduced body weight, but even the males with normal body weight had a low testicular weight.
Figure 3.
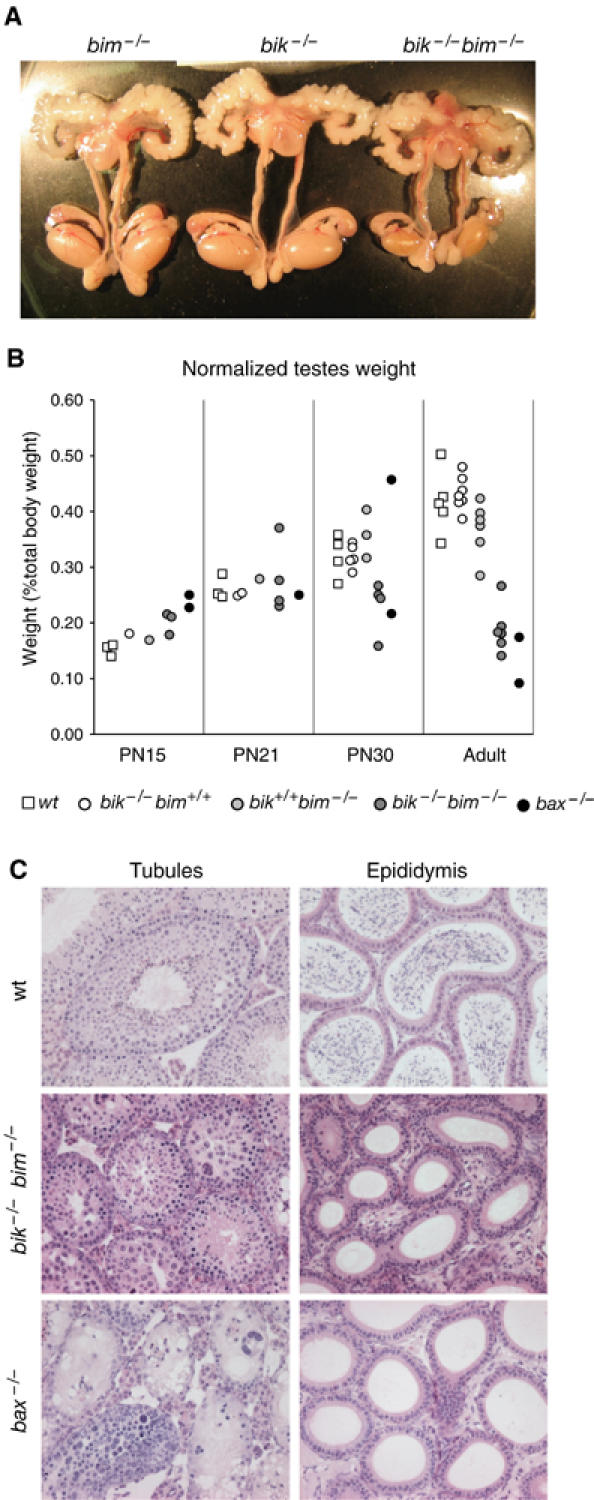
Reduced testicular size and absence of spermatozoa in bik −/− bim −/− males. (A) Dissected reproductive tracts from representative bik −/− bim +/+, bik +/+ bim −/− and bik −/− bim −/− males showing reduced testicular size, but otherwise normal appearance. Testes of bik −/− bim +/+, bik +/+ bim −/− mice were indistinguishable from those of wt males. (B) Weights of testes from wt, bik −/− bim +/+, bik +/+ bim −/−, bik −/− bim −/− and bax −/− males at PN day 15, 21, 30 and adult. Each data point represents the weight of one testis from a single male normalized to body weight. (C) Histological appearance of tubules and epididymis from testes of adult wt, bik −/− bim −/− and bax −/− males.
Histologic analysis of testes from adult bik −/− bim −/− males showed abnormal tubules. Abnormal features varied between tubules but included vacuolation, multi-nucleate syncytia, accumulation of spermatocytes and a qualitative reduction in tubule cellularity, diameter and lumen size (Figure 3C). The tubules differed markedly from those of _bax_−/− males, in which some tubules showed a general accumulation of early germ cell types while others appeared deficient in germ cells (Figure 3C). Another difference was that spermatids, never seen in bax −/− tubules, appeared in some bik −/− bim −/− tubules (although less frequently than in wild type (wt)). Like bax −/− males, however, mature sperm were never observed in the epididymis of bik −/− bim −/− males (Figure 3C).
Loss of Bim does not exacerbate the spermatogenesis defect in Bax-deficient mice
To investigate further the relationship between Bim and Bax in spermatogenesis, we examined male mice lacking both Bim and Bax (Hutcheson et al, 2005). The _bim_−/−_bax_−/− males were sterile and their spermatogenic defect was indistinguishable from that in _bax_−/− males. The seminiferous epithelium was grossly dysmorphic, the tubule diameter was abnormally decreased and no spermatids were present (Supplementary Figure 1). In contrast, males lacking both Bim and Bak (Hutcheson et al, 2005), like those lacking Bim or Bak alone, were fertile and their testes had a normal histological appearance and exhibited all major germ cell types, including elongated sperm (Supplementary Figure 1). These observations indicate that Bim exerts its function in spermatogenesis mostly through Bax and not Bak (see Discussion).
Bik and Bim are expressed in both germ cells and Sertoli cells of seminiferous tubules
We next investigated the expression pattern of bik and bim in the testis. As expected, Northern blot analysis confirmed bik gene expression in wt but not bik −/− or bik −/− bim −/− testes, while Western blotting revealed Bim in wt but not bim −/− or bik −/− bim −/− testes (Figure 4A and B). The level of bik expression was not elevated over wt in bim −/− testes, and Bim protein expression was normal in bik −/− testes (Figure 4A and B), demonstrating that neither gene was upregulated to compensate for the absence of the other. In situ hybridization analysis indicates that bim is expressed in essentially all germ cell types and Sertoli cells (Meehan et al, 2001), and immunohistochemistry has revealed Bim protein in elongating spermatids and Sertoli cells (O'Reilly et al, 2000). Our in situ hybridization analysis revealed bik mRNA in spermatogonia and spermatocytes (Figure 4C and D). The presence of a β-galactosidase reporter gene in the mutant bik locus (Coultas et al, 2004) allowed staining for the enzyme activity in bik heterozygous mice as a surrogate for Bik expression. The staining confirmed Bik expression in spermatogonia, albeit not spermatocytes, and revealed expression in Sertoli cells (Figure 4E). Collectively, these results show that Bik and Bim are each expressed in both germ cells and supporting Sertoli cells of the testis.
Figure 4.
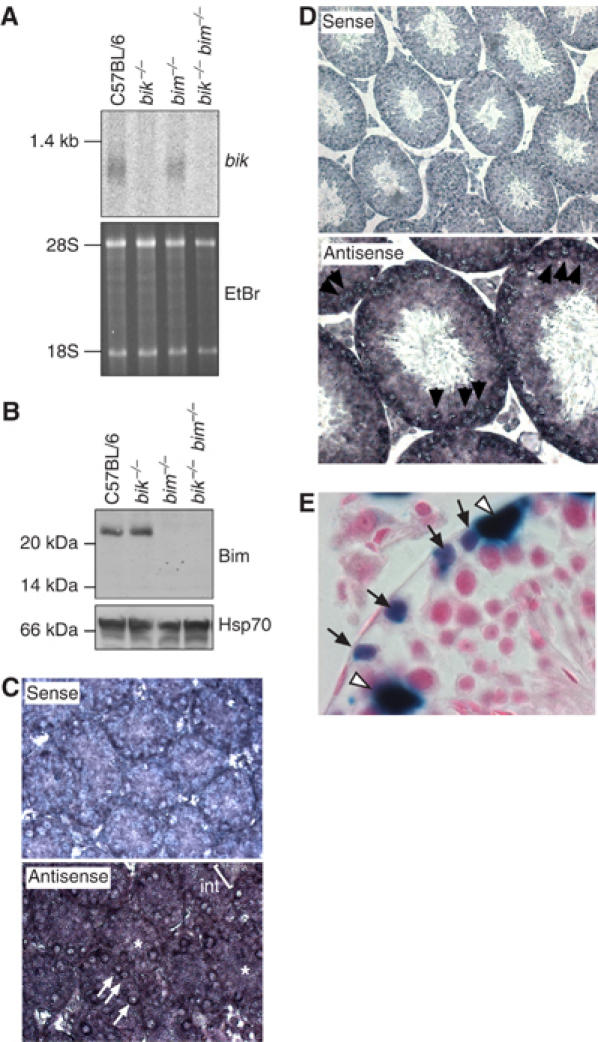
Expression of Bik and Bim in testes. (A) Northern blot analysis of poly(A)+ RNA extracted from the testes of adult wt C57BL/6, bik −/−, bim −/− and bik −/− bim −/− mice. The blot was probed with a cDNA probe containing the entire bik coding region. Ethidium bromide stain of the RNA is shown as a loading control; 28S and 18S rRNA species are indicated. (B) Western blot analysis of Bim protein expression in whole-cell lysates extracted from the testes of adult wt C57BL/6, bik −/−, bim −/− and bik −/− bim −/− mice. Probing with an anti-Hsp70 mAb was used as a loading control. (C) Bik gene expression assessed in PN5 testes by in situ hybridization, showing a strong signal in spermatogonia (arrows) and a less intense signal in the Sertoli cell cytoplasm (asterisk), while the interstitial signal was weak to absent (Int). Sense probe (top panel) is included as a negative control. (D) Bik gene expression in the adult testis was assessed by in situ hybridization, showing strongest signal with spermatocytes (arrows). Sense probe (top panel) is included as a negative control. (E) β-Galactosidase assay as a surrogate for bik expression in bik +/− adult testes. β-Galactosidase activity was evident in spermatogonia (arrows) and Sertoli cells (open arrow head).
Spermatogenesis arrests at a later developmental stage in bik−/−bim−/− than in bax−/− testes
We next sought to identify the stage at which spermatogenesis arrests in bik −/− bim −/− males, and compare this to the well-defined block in the bax −/− testes (Russell et al, 2002). We first examined the DNA content of germ cells from the adult testis by flow cytometry. DNA content analysis readily separates the postmeiotic haploid spermatids and spermatozoa (1N) from the 2N spermatogonia and 2° spermatocytes and the 4N spermatocytes (Figure 5A). Consistent with previous reports (Knudson et al, 1995; Russell et al, 2002), the Bax-deficient testes lacked haploid cells (Figure 5A), whereas the adult bik −/− bim −/− testes had a notable 1N peak, albeit far smaller than that in wt testes (Figure 5A). This result indicates that the bik −/− bim −/− males produce modest numbers of a postmeiotic cell type, whereas maturation in the Bax-deficient testes arrests before meiosis is complete, in the preleptotene spermatocyte phase (Russell et al, 2002).
Figure 5.
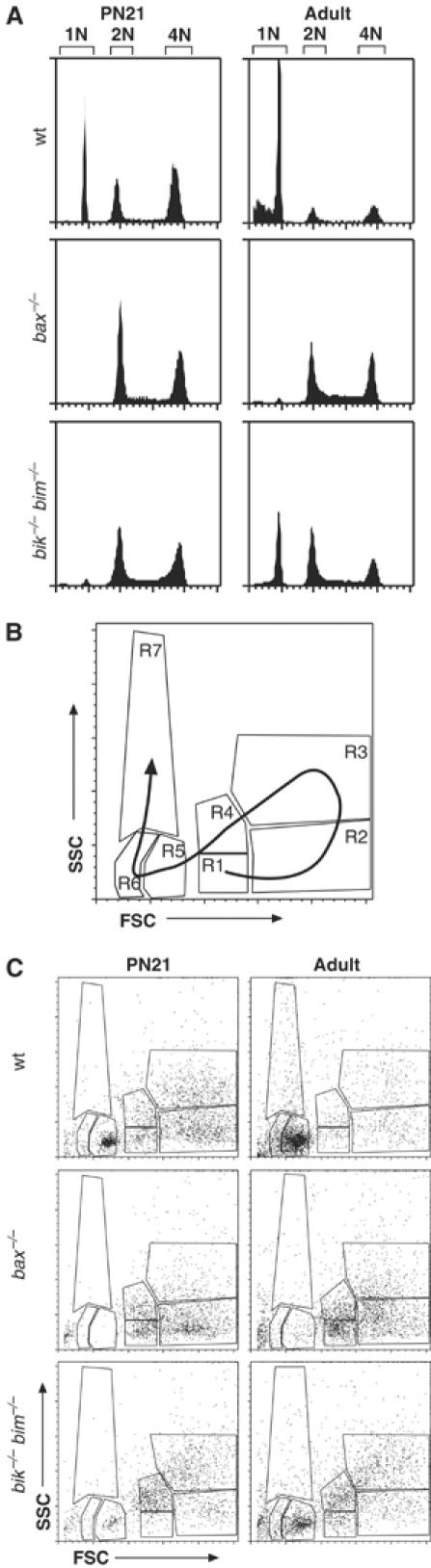
Profiling germ cell development in bik −/− bim −/− mice by FACS. (A) DNA content of bik −/− bim −/− male germ cells was assessed by PI staining and FACS analysis and compared to that in wt and bax −/− germ cells. Cells with sub-1N DNA content represent spermatozoa with condensed chromatin, such that PI cannot fully access the DNA. (B) Schematic diagram of germ cell analysis by FACS. Development of mature spermatozoa from spermatogonia (R1 and R4) follows the direction of the arrow on the FSC/SSC plot. The premeiotic 2N spermatogonia appear in regions R1 and R4. On replicating their DNA to become 4N primary spermatocytes, they shift to R2. When the spermatocytes condense their chromosomes, they shift into R3 as late-stage spermatocytes. The first meiotic division produces 2° spermatocytes, which transit back through R1 and R4 before entering R5 as round spermatids after the second meiotic division. As spermatids elongate and condense their DNA they occupy R5 and R6, and finally on becoming mature spermatozoa they reach R7 (although mature spermatozoa contribute to R5, R6 and R7) (Malkov et al, 1998). (C) FACS profiles of germ cell suspensions prepared from wt, bik −/− bim −/− and bax −/− testes at PN21 and adults, showing arrested germ cell development at the early primary spermatocyte stage in bax −/− mice and at the round spermatid stage in bik −/− bim −/− mice.
Germ cell populations can be delineated by two-parameter FACS analysis of the forward- and side-scatter properties of the nuclei (Malkov et al, 1998) (Figure 5B). At PN15, when only spermatogonia and primary spermatocytes are present, both the bik −/− bim −/− and the bax −/− testes appeared relatively normal by FSC/SSC and DNA content analysis (data not shown). By PN21, shortly after onset of meiosis and spermatid production, the bik −/− bim −/− testes showed a marked reduction, compared to wt, in the round spermatid population (R5) and an increase in the R4 population (Figure 5C), indicative of a disproportionate representation of spermatogonia and spermatocytes. In adult bik −/− bim −/− testes (Figure 5C), spermatogonia and spermatocytes (in R1, R3 and R4) were still over-represented, but there were some uncondensed spermatids, seen in R6, albeit fewer than in wt. In contrast, at all ages the composition of bik −/− or bim −/− testes was indistinguishable from wt (data not shown).
Although germ cell maturation in bik −/− bim −/− testes resembled bax −/− testes in the over-representation of early germ cell types and lack of mature spermatids, there were notable differences. Consistent with previous reports (Knudson et al, 1995; Russell et al, 2002), the light scatter profiles confirmed that the bax −/− testes accumulated only very early germ cell types (spermatogonia and primary spermatocytes), whereas bik −/− bim −/− testes contained some later-stage spermatocytes (Figure 5C). For example, the early R1 and R2 populations were much more prominent in the bax −/− than the bik −/− bim −/− testes at both PN21 and the adult. Moreover, whereas bax −/− males produce very few, if any, round spermatids (Knudson et al, 1995; Russell et al, 2002), a small number of round, uncondensed spermatids (R5) developed in the bik −/− bim −/− testes.
Histological assessment (Figure 6) confirmed that germ cell maturation was abnormal in bik −/− bim −/− testes, albeit distinct from that in bax −/− animals. At PN15 (Figure 6A), the spermatogonia layer in bik −/− bim −/− tubules, as in the bax −/− testes, was more pronounced than in controls (e.g. bim −/−), being up to three cells thick. Clusters of round cells appeared in the centre of some tubules from bik −/− bim −/− testes, but these clusters were more frequent in bax −/− tubules. By PN21 (Figure 6B), round spermatids were apparent in tubules of control testes, but few bik −/− bim −/− tubules contained round spermatids at this age, and their spermatogonia cell layer still contained more cells than controls. Furthermore, the bik −/− bim −/− tubules differed from bax −/− tubules in that the latter contained no spermatids at all and their spermatocytes appeared less mature than those in bik −/− bim −/− mice. At PN30 (Figure 6C), when elongating spermatids were present in control tubules (arrowheads), bik −/− bim −/− tubules were filled with spermatocytes and some contained round spermatids, but none exhibited elongating spermatids. In contrast, the bax mutant tubules contained primarily spermatogonia and pre-leptotene spermatocytes, and had changed little in appearance from PN15 and PN21. Collectively, these data indicate that germ cell maturation in the bik −/− bim −/− testes arrests at a later stage than in bax −/− testes.
Figure 6.
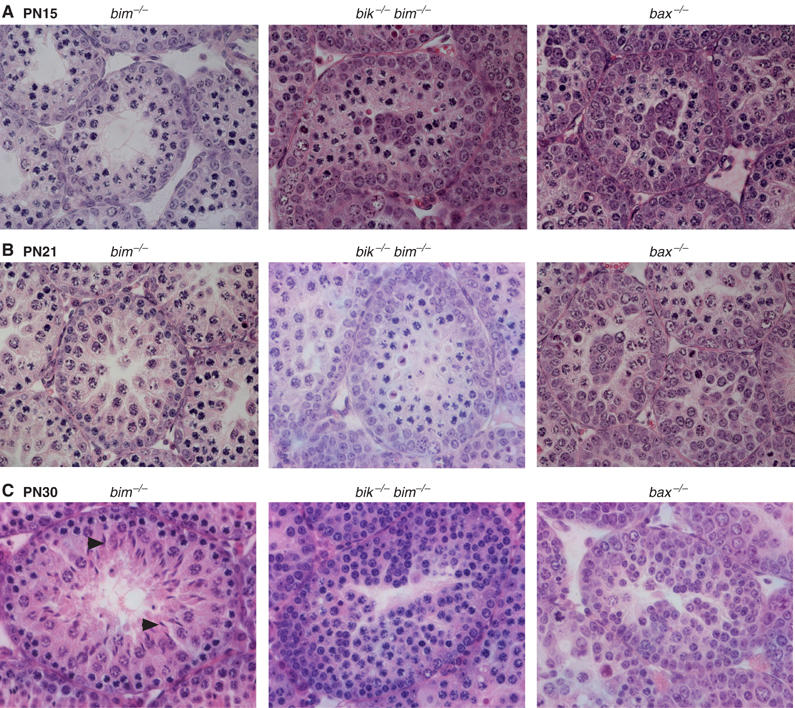
Histological appearance of developing bik −/− bim −/− testes. At all ages, the bim −/− testis (left panels) was indistinguishable from a wt or bik −/− testis (data not shown). (A) Sections of PN15 testes, stained with hematoxylin and eosin, showing increased thickness of the spermatogonial (outer) cell layer and cell clusters in the centre of bik −/− bim −/− and bax −/− tubules compared to controls. (B) Sections of PN21 testes, stained with hematoxylin and eosin, showing that bik −/− bim −/− testes have increased layers of spermatogonia, compared to bim −/− testes. (C) Sections of PN30 testes, stained with hematoxylin and eosin, showing accumulation of spermatocytes in bik −/− bim −/− testes. By comparison, bax −/− tubules show preferential accumulation of spermatogonia and some early spermatocytes. Some bik −/− bim −/− tubules contained round spermatids, but not the elongating spermatids (arrowheads) observed in bim −/−, bik −/− and wt controls.
Reduced cellularity in adult bik−/−bim−/− testes is preceded by overabundance of c-Kit+ germ cells
The developmental arrest in the Bax-deficient testes has been attributed to overcrowding of the supporting seminiferous epithelium (Sertoli cells) by the excess spermatogonia and primary spermatocytes in the young mice (Russell et al, 2002). We investigated whether the block in the bik −/− bim −/− testis has a similar basis. Consistent with the reduced testicular weight, germ cell populations in adult bik −/− bim −/− testis were also reduced by as much as 10-fold compared to bik −/− and bim −/− controls, which were indistinguishable from wt mice (Figure 7A). In striking contrast, germ cell number at PN15 in both bik −/− bim −/− and bax −/− testes exceeded that of controls (Figure 7A). Interpretation of the germ cell numbers at PN21 was complicated because several of the bik −/− bim −/− and bax −/− males analysed were runts, but the bik −/− bim −/− males of normal body weight still had a clear increase in testis cellularity over the normal controls.
Figure 7.
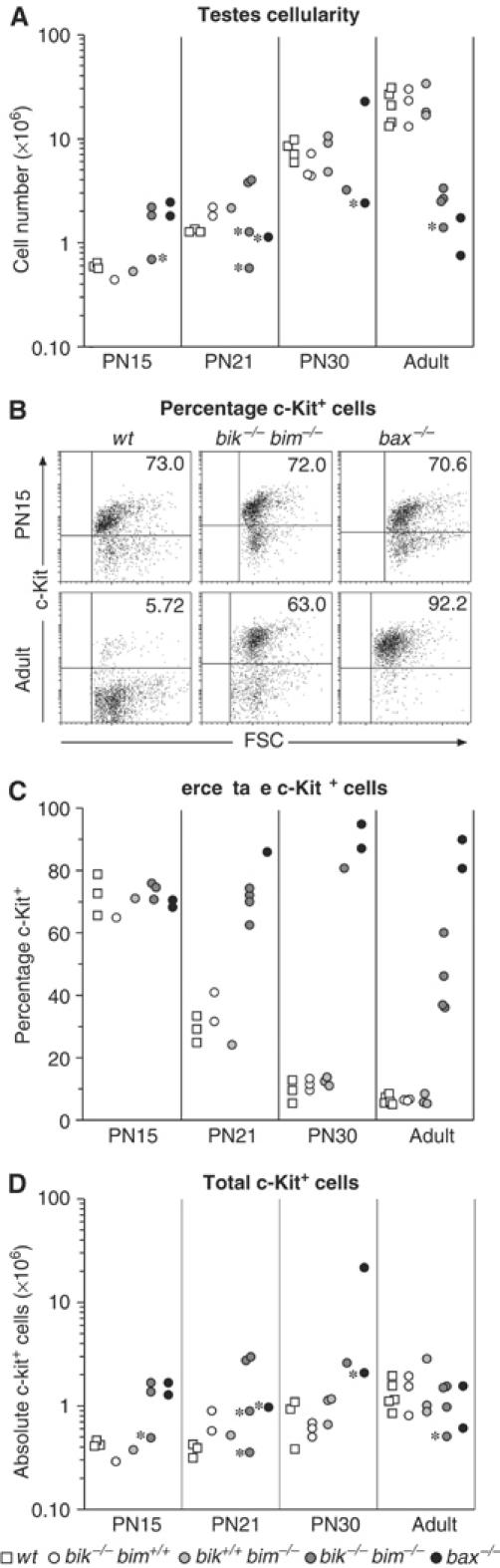
Accumulation of c-Kit+ germ cells in bik −/− bim −/− testes. (A) Total cellularity of wt, bik −/−, bim −/−, bik −/− bim −/− and bax −/− testes taken at PN15, PN21, PN30 and adult (8–15 weeks old). Each data point represents the number of germ cells isolated from a single testis of one animal. Asterisks mark testes taken from runts. (B) Representative FACS profiles of PN15 and adult (8–15 weeks) wt, bik −/− bim −/− and bax −/− testes stained for c-Kit expression. (C) The percentage of c-Kit+ cells in germ cell preparations from PN15, PN21, PN30 and adult (8–15 weeks) wt, bik −/−, bim −/−, bik −/− bim −/− and bax −/− testes. Each data point represents the percentage of c-Kit+ germ cells from a single testis of one animal. (D) Absolute numbers of c-Kit+ cells in the germ cell preparations of PN15, PN21, PN30 and adult testes from wt, bik −/−, bim −/−, bik −/− bim −/− and bax −/− testes. Each data point represents the number of germ cells isolated from a single testis of one animal. Asterisks mark testes taken from runts.
To investigate whether the excess cells in the young bik −/− bim −/− testes represented early germ cell stages, we stained single-cell suspensions for c-Kit, a marker of spermatogonia and some primary spermatocytes, and enumerated c-Kit+ germ cells by flow cytometry (Figure 7B and C). Except in some runts, the testes of young bik −/− bim −/− males did contain substantially more c-Kit+ germ cells than controls (Figure 7D). The excess in early germ cell numbers in bik −/− bim −/− and bax −/− testes was evident from at least PN15, prior to the spermiogenic phase, when round spermatids develop into mature spermatozoa, and persisted up to PN30 (Figure 7C). While the number of c-Kit+ germ cells in adult bik −/− bim −/− testes appeared to be normal (Figure 7D), their proportional representation within the germ cell population was up to 10-fold higher than in control testes, due to the dearth of mature (c-Kit−) germ cells in the mutant (Figure 7B). The proportion of c-Kit+ cells was lower in the bik −/− bim −/− than bax −/− testes (Figure 7B) because some c-Kit− germ cell types, such as late spermatocytes and spermatids, were produced in the bik/bim double knockout but not the bax −/− testis. These results re-enforce our conclusion that early germ cells dominate the testes of bik −/− bim −/− males, but that the block occurs at a later stage of spermatogenesis than in the bax −/− testis.
Discussion
Since mice lacking Bim have defects in apoptosis in response to multiple stimuli in cell types ranging from the hematopoietic to the nervous system (see Introduction), we hypothesized that concomitant loss of another BH3-only protein, such as Bik, might exacerbate the phenotype provoked by Bim loss, or even elicit a novel phenotype not seen in the absence of either alone.
Despite the coexpression of Bik and Bim in lymphoid and myeloid cells (Coultas et al, 2004), the hematopoietic phenotypes of bim −/− mice (Bouillet et al, 1999) were not enhanced by Bik loss. The composition of hematopoietic organs in bik −/− bim −/− mice and the response of their lymphocytes to a range of apoptotic stimuli in vitro were indistinguishable from those of mice lacking Bim alone. Thus, Bik is not a major initiator of physiological cell death in the developing or mature hematopoietic system, nor in the response to any of the cellular stresses tested. Evidence that the BH3-only protein Puma is needed for a number of cytotoxic responses in lymphocytes (Jeffers et al, 2003; Villunger et al, 2003) suggests that Bim and Puma are the dominant initiators of apoptosis in the hematopoietic compartment (Figure 8A). Furthermore, recent findings suggest that Bim and Puma are more potent initiators of apoptosis than Bik because they both can readily engage all the prosurvival family members, whereas Bik has only low affinity for Bcl-2 and Mcl-1 (Chen et al, 2005). Since both Bcl-2 and Mcl-1 play vital roles in the hematopoietic system (Veis et al, 1993; Bouillet et al, 2001; Opferman et al, 2003), it is explicable that loss of Bim rescued the hematopoietic deficit in Bcl-2 knockout mice (Bouillet et al, 2001), whereas loss of Bik did not (Coultas et al, 2004). Thus, Bik may have merely a supporting role in hematopoietic cells, because it cannot engage all the relevant prosurvival family members (Figure 8A) (Chen et al, 2005).
Figure 8.
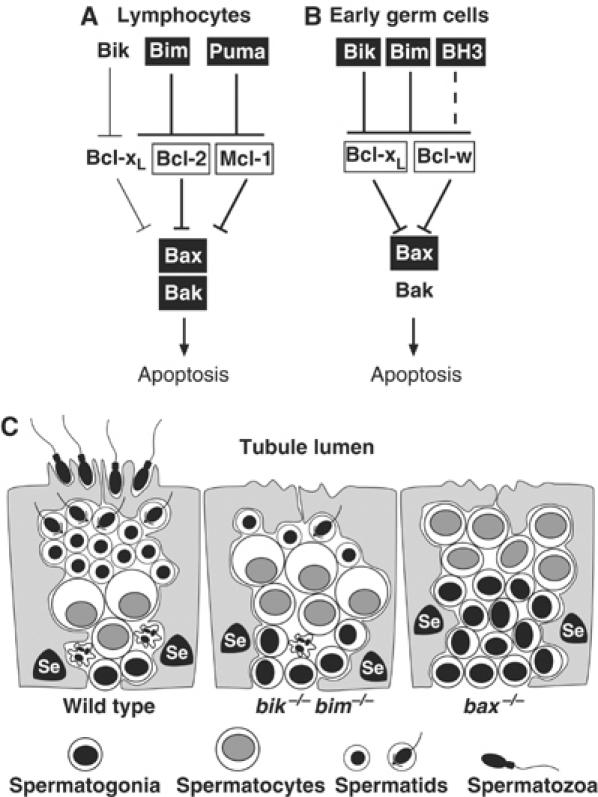
Model for the disrupted spermatogenesis in bik −/− bim −/− mice. The key regulators of apoptosis within lymphocytes (A) and early germ cells (B) are compared (see text). For each cell type, boxed genes indicate dominant Bcl-2 family regulators of apoptosis and thick lines denote dominant pathways. The BH3-only protein postulated to cooperate with Bim and Bik in germ cells is indicated as BH3. Bcl-xL is assumed to have a greater role in spermatogonia than Bcl-w because loss of a single allele of the former but not the latter impairs male germ cell apoptosis (Kasai et al, 2003). (C) During the first wave of normal spermatogenesis (wt), the excess of germ cells, particularly spermatogonia, is eliminated by apoptosis (crinkly cell) because the Sertoli cells (Se) cannot provide sufficient support. In the bik −/− bim −/− and bax −/− testes, the failure of apoptosis during the first wave produces an abnormal excess of spermatogonia and spermatocytes that is presumed to overwhelm the Sertoli cells, leading to aborted testicular development.
Our hypothesis that the double knockout mice might exhibit novel phenotypes not found in the Bim or Bik single knockout animals was confirmed when the bik −/− bim −/− males proved to lack mature sperm. Whereas testicular development appeared completely normal in bik −/− or bim −/− mice, and indeed even in bik −/− bim +/− or bik +/− bim −/− males, the testes of sexually mature bik −/− bim −/− animals were atrophied, had far fewer germ cells and produced no sperm. While unexpected, the phenotype was not altogether surprising because the Bcl-2 family has important roles in the control of apoptosis during spermatogenesis (Print and Loveland, 2000). Bax-deficient males or those expressing a _bcl_-xL or _bcl_-2 transgene specifically in the germ cells have a similar defect (Knudson et al, 1995; Motoyama et al, 1995; Rodriguez et al, 1997; Russell et al, 2002). These mutations impair spermatogenesis during its first wave and are believed to be germ cell autonomous. In contrast, in mice lacking the prosurvival Bcl-w, attrition of both germ and Sertoli cells begins later and extends over months (Print et al, 1998; Ross et al, 1998, 2001; Russell et al, 2001).
During PN germ cell development, when germ cells first undergo their complex program of differentiation to become mature spermatozoa, apoptosis is essential (Print and Loveland, 2000). The first wave of spermatogenesis, beginning by PN5 in mice, is accompanied by a wave of apoptosis that peaks 2–3 weeks after birth and is especially prominent in spermatogonia (Rodriguez et al, 1997; Wang et al, 1998). Only 25% of spermatogonial descendents are estimated to reach the preleptotene spermatocyte stage, the remainder being eliminated by apoptosis (Allan et al, 1987; Print and Loveland, 2000).
Successful germ cell maturation requires a precise balance between the numbers of germ cells and neighbouring Sertoli cells, which support their survival, proliferation and differentiation (Orth et al, 1988). The initial neonatal overproduction of spermatogonia is thought to be countered by apoptosis to establish this critical ratio (Allan et al, 1987) (Figure 8C). Young bax −/− males have an excess of early germ cell types, particularly spermatogonia and preleptotene spermatocytes. Spermatogenesis is presumed to abort in these mice because Sertoli cells cannot properly nurture the excess germ cells (Knudson et al, 1995; Russell et al, 2002). A similar mechanism seems to account for the sterility of bik −/− bim −/− males (Figure 8C). As in bax −/− mice, the testes of young bik −/− bim −/− males contained an abnormal excess of early germ cells, as assessed by c-Kit staining and histological examination, and germ cell maturation failed during the first wave of differentiation. Hence, overcrowding of the seminiferous epithelium by the copious early germ cells during that wave is the likely cause of defective spermatogenesis in both mutant strains (Figure 8C). This model implies a germ cell autonomous role for Bik and Bim, as proposed for Bax (Russell et al, 2002). Although Bik and Bim are also expressed in Sertoli cells, we have observed no obvious abnormalities in Sertoli cells, and it seems unlikely that an increase in their numbers would impair germ cell development. The bik −/− bim −/− testicular phenotype is very different from the delayed atrophy in Bcl-w-deficient mice, ascribed to attrition in both the germ and Sertoli cell populations (Print et al, 1998; Ross et al, 1998, 2001; Russell et al, 2001).
Since testicular development is normal in mice lacking Bak, it is very likely that the BH3-only proteins act through Bax and not Bak. To confirm this genetically, we analysed males lacking Bim and Bax, or Bim and Bak. Concomitant loss of Bim did not exacerbate the spermatogenesis defect seen in Bax-deficient mice, and the testes of animals lacking both Bim and Bak were completely normal. These findings support the view that Bik and Bim cooperate to initiate early germ cell apoptosis in a pathway that requires Bax and not Bak (Figure 8B).
Nevertheless, spermatogenesis did arrest later in bik −/− bim −/− than bax −/− males (Figure 8C). Whereas spermatogonia and preleptotene spermatocytes accumulated in bax −/− tubules, the bik −/− bim −/− tubules were dominated by more mature spermatocytes and contained some haploid spermatids. Our interpretation is that Bik and Bim act in the same germ cell types as Bax, but that some initial apoptosis persists in the absence of Bik and Bim but not in the absence of Bax. If so, spermatogenesis might proceed further without Bik and Bim, until sufficient germ cells accumulated to overwhelm the seminiferous epithelium. On this hypothesis, the Sertoli cells would then be unable to provide sufficient differentiation-promoting cues to nurture further germ cell development, explaining why the spermatids that form in the bik −/− bim −/− testes do not differentiate further. Why might bik −/− bim −/− but not bax −/− germ cells exhibit residual apoptosis? We speculate that a third BH3-only protein (‘BH3' in Figure 8B) helps to promote germ cell apoptosis in a Bax-dependent manner. For example, Bad is expressed in spermatogonia at the onset of spermatogenic differentiation (Meehan et al, 2001), where it might facilitate Bax-dependent apoptosis in conjunction with Bik and Bim, which are also expressed in spermatogonia at this time (Meehan et al, 2001).
Why might Bik and Bim appear to act in parallel in germ cells but not in hematopoietic cells? One potential explanation would be that Bik preferentially targets prosurvival family members whose prosurvival role is more important in germ cells than hematopoietic cells. Bcl-w is one such protein. Although Bcl-w is present in both germ and hematopoietic cells, male Bcl-w knockout mice become sterile due to abnormal germ cell apoptosis, yet have a normal hematopoietic system (Print et al, 1998; Ross et al, 1998; O'Reilly et al, 2001; Russell et al, 2001). Bcl-xL is also clearly required for male germ cell survival, since even a partial reduction in its level provokes Bax-dependent male germ cell apoptosis (Rucker et al, 2000). Significantly, Bik and Bim efficiently bind to both Bcl-w and Bcl-xL (Chen et al, 2005). Therefore, we propose that in the wt juvenile testis, Bim and Bik are activated in supernumerary spermatogonia that do not receive sufficient trophic support from Sertoli cells. They then engage and thereby inactivate both Bcl-w and Bcl-xL, unleashing Bax to initiate the apoptotic programme (Figure 8B). Presumably both Bik and Bim are needed to overwhelm both of these prosurvival proteins.
By generating mice lacking the BH3-only family members Bik and Bim, we have for the first time demonstrated a requirement for BH3-only proteins in the control of male germ cell differentiation in the PN gonad. The findings thus uncover the first critical in vivo role for Bik and identified a novel role for Bim. By directly comparing the phenotypes of bik −/− bim −/− and bax −/− mice, we have shown that Bik and Bim are likely to be the essential triggers for Bax-dependent apoptosis in spermatogonia, but that another BH3-only protein may also act in early germ cells. More generally, our finding that Bik and Bim share a previously unsuspected essential physiological role suggests that various combinations of the BH3-only proteins will prove to be responsible for mediating numerous other vital developmentally programmed cell death programs in different tissues. Indeed, it seems likely that most cytotoxic stimuli are funnelled through specific subsets of these death ligands (Huang and Strasser, 2000; Cory et al, 2003; Danial and Korsmeyer, 2004).
Materials and methods
Mice
All experiments with mice were performed according to the guidelines of the Melbourne Directorate Animal Ethics Committee. To generate mice lacking Bik and Bim, we first crossed bik −/− mice (Coultas et al, 2004) and bim −/− mice (Bouillet et al, 1999) and backcrossed their bik +/− bim +/− offspring to either bik −/− or bim −/− animals to produce progeny with one gene homozygous for the null allele and the other heterozygous for the null allele (bik −/− bim +/− or bik +/− bim −/−). These two lines were maintained separately and brother/sister mated to generate double knockout and control genotype animals. All were on a C57BL/6 background: the bik −/− mice were made from C57BL/6 ES cells and maintained on a C57BL/6 background (Coultas et al, 2004), while the bim −/− animals, generated from 129SV ES cells, have been backcrossed to C57BL/6 mice for eight or more generations (Bouillet et al, 2001). Mice were genotyped at the bik and bim loci by PCR as described previously (Bouillet et al, 1999, 2001; Coultas et al, 2004). The bax+/− mice, originally produced from 129Sv ES cells (Knudson et al, 1995), were purchased from Jackson Labs and had been backcrossed for 10 generations or more onto the C57BL/6 background. Mice lacking both Bim and Bax (_bim_−/−_bax_−/−) or Bim and Bak (_bim_−/−_bak_−/−) have been described previously (Hutcheson et al, 2005). Protocols for genotyping of mice will be provided upon request.
Northern blotting, Western blotting and RT–PCR
Northern blotting for bik gene expression was performed on polyA(+) enriched RNA isolated from adult testes as described previously (Coultas et al, 2004). Western blotting for Bim expression was performed on 150 μg total protein isolated from adult testes by homogenization in RIPA buffer as described, using the rat anti-Bim monoclonal antibody 3C5 (Alexis; a gift from L O'Reilly and D Huang). Probing with N6 anti-HSP70 monoclonal antibody (a gift from Drs W Welch and R Anderson) was used as a loading control.
Cell culture and viability assays
Isolated unsorted primary thymocytes and negatively sorted lymph node B cells (CD4−, CD8−) were cultured essentially as described (Coultas et al, 2004). To assess viability, cultured cells were harvested and incubated with 2 μg/ml of propidium iodide (PI) (Sigma) on ice for 5 min and analysed on a FACScan analyser (Becton-Dickinson) to determine the percentage of cells negative for PI.
Male germ cell isolation
Male germ cells were isolated from testes using a protocol adapted from Malkov et al (1998). Testes were dissected and decapsulated to release the tubules, which were then incubated in 0.25 mg/ml collagenase type 3 (Worthington) at 32°C for up to 30 min with agitation. Dispersed tubules were allowed to settle and washed twice to remove peritubular cells. Washed tubules were then incubated with 0.25 mg/ml trypsin (GibcoBRL) and 1 μg/ml DNaseI (Roche) at 32°C for 10 min with agitation. Trypsin digestion was terminated by adding an equal volume of DME 10% FCS containing 0.5 mg/ml soybean trypsin inhibitor (Sigma). The suspension was centrifuged at 400 g for 5 min and tubules resuspended in DME 10% FCS and disaggregated into a single-cell suspension by trituration using a flame-polished Pasteur pipette. Aggregates were removed by filtering the cell suspension through a 50 μm Nitex filter (Sefar, 03-50/31). Cells were then resuspended in a defined volume of DME 10% FCS, stained with trypan blue and counted in a hemocytometer.
Immunofluorescence staining, cell sorting and flow-cytometric analysis
Immunofluorescence staining of cells for FACS analysis and cell sorting was performed as described (Coultas et al, 2004). Mature B cells for cell survival assays were negatively sorted from lymph nodes as a CD4−, CD8− population and confirmed to be ⩾98% B220+, as described previously (Coultas et al, 2004).
Germ cell suspensions were stained either for DNA content and nuclear size analysis or for expression of the c-Kit cell surface marker. For DNA content and nuclear analysis, 2 × 105 germ cells were rinsed once with balanced salt solution (with Ca2+ and Mg2+), resuspended in 0.2 ml of cold PI staining solution (10 mM Tris, pH 8.0, 1 mM NaCl, 0.1% Nonidet P40, 50 μg/ml PI, 10 μg/ml RNaseA), vortexed for 2–3 s and incubated on ice for 10 min to lyse the plasma membrane and stain nuclear DNA. Nuclear size and complexity were determined on a FACScan analyser as described (Malkov et al, 1998). DNA content was assessed on a FACS analyser (Becton Dickinson) as described, with PI detected in the FL3 channel with amplification set to linear (Malkov et al, 1998). Surface staining of germ cells for c-Kit was performed using a standard FACS staining protocol (Coultas et al, 2004). Briefly, 2 × 105 germ cells were stained with a biotin-conjugated rat anti-mouse c-Kit monoclonal antibody (ACK-4; gift of Professor S Nishikawa), which was revealed using R-phycoerythrin-conjugated streptavidin (Caltag). Viable (PI−) c-Kit+ germ cells were then detected using a FACScan analyser.
Histology and in situ hybridization
Testes were dissected from mice and separated from the epididymis before fixing both for 6 h in Bouin's fixative and processing and embedding for paraffin. Fixed testes and epididymis were sectioned and stained with hematoxylin and eosin. In situ hybridization was performed essentially as described (Meinhardt et al, 1998). Briefly, Bouin's fixed sections were washed in 0.2 M HCl, digested with proteinase K and treated with 0.1 M triethanolamine with acetic anhydride before prehybridization. Sections were then hybridized to a digoxigenin-labelled antisense RNA probe specific for the coding sequence of mouse bik. Bik signal was revealed using alkaline phosphatase-conjugated anti-digoxigenin antibody Fab fragments (Roche) and BCIP/NBT substrate (Pierce, Rockford, IL, USA), and cells were then counterstained with hematoxylin. A corresponding sense control RNA probe was used on parallel sections in every experiment for all conditions tested.
Supplementary Material
Supplementary Figure 1
Acknowledgments
We thank Dr V Marsden for assistance with Western blotting in Figure 4B; A Szczepny for in situ hybridization; Dr F Battye, C Tarlinton, V Lapatis and C Clark for cell sorting; A Naughton, C Tilbrook, J Morrow, N Clark and K Birchall for animal husbandry; S Mihajlovic and E Tsui for histology; S Cory, D Huang, A Harris, D Vaux, H Puthalakath and L O'Reilly for insightful discussions. This work was supported by fellowships and grants from the NHMRC (Canberra) #143792 and #334011 to KLL, the Dr Josef Steiner Cancer Research Foundation (Bern), the Leukemia and Lymphoma Society of America, and the NIH (CA80188). LC was a recipient of a Cancer Council Victoria Postdoctoral Cancer Research Fellowship.
References
- Adams JM (2003) Ways of dying: multiple pathways to apoptosis. Genes Dev 17: 2481–2495 [DOI] [PubMed] [Google Scholar]
- Akiyama T, Bouillet P, Miyazaki T, Kadono Y, Chikuda H, Chung UI, Fukuda A, Hikita A, Seto H, Okada T, Inaba T, Sanjay A, Baron R, Kawaguchi H, Oda H, Nakamura K, Strasser A, Tanaka S (2003) Regulation of osteoclast apoptosis by ubiquitylation of proapoptotic BH3-only Bcl-2 family member Bim. EMBO J 22: 6653–6664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan DJ, Harmon BV, Kerr JFR (1987) Cell death in spermatogenesis. In Perspectives on Mammalian Cell Death, Potten CS (ed) pp 229–258. Oxford: Oxford University Press [Google Scholar]
- Bouillet P, Cory S, Zhang L-C, Strasser A, Adams JM (2001) Degenerative disorders caused by Bcl-2 deficiency are prevented by loss of its BH3-only antagonist Bim. Dev Cell 1: 645–653 [DOI] [PubMed] [Google Scholar]
- Bouillet P, Metcalf D, Huang DCS, Tarlinton DM, Kay TWH, Köntgen F, Adams JM, Strasser A (1999) Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science 286: 1735–1738 [DOI] [PubMed] [Google Scholar]
- Bouillet P, Purton JF, Godfrey DI, Zhang L-C, Coultas L, Puthalakath H, Pellegrini M, Cory S, Adams JM, Strasser A (2002) BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature 415: 922–926 [DOI] [PubMed] [Google Scholar]
- Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, Colman PM, Day CL, Adams JM, Huang DCS (2005) Differential targeting of pro-survival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell 17: 393–403 [DOI] [PubMed] [Google Scholar]
- Cheng EH, Wei MC, Weiler S, Flavell RA, Mak TW, Lindsten T, Korsmeyer SJ (2001) BCL-2, BCL-xL sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol Cell 8: 705–711 [DOI] [PubMed] [Google Scholar]
- Cory S, Huang DCS, Adams JM (2003) The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene 22: 8590–8607 [DOI] [PubMed] [Google Scholar]
- Coultas L, Bouillet P, Stanley EG, Brodnicki TC, Adams JM, Strasser A (2004) Proapoptotic BH3-only Bcl-2 family member Bik/Blk/Nbk is expressed in hemopoietic and endothelial cells but is redundant for their programmed death. Mol Cell Biol 24: 1570–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danial NN, Korsmeyer SJ (2004) Cell death: critical control points. Cell 116: 205–219 [DOI] [PubMed] [Google Scholar]
- Deckwerth TL, Elliott JL, Knudson CM, Johnson EM Jr, Snider WD, Korsmeyer SJ (1996) BAX is required for neuronal death after trophic factor deprivation and during development. Neuron 17: 401–411 [DOI] [PubMed] [Google Scholar]
- Enders A, Bouillet P, Puthalakath H, Xu Y, Tarlinton DM, Strasser A (2003) Loss of the pro-apoptotic BH3-only Bcl-2 family member Bim inhibits BCR stimulation-induced apoptosis and deletion of autoreactive B cells. J Exp Med 198: 1119–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildeman DA, Zhu Y, Mitchell TC, Bouillet P, Strasser A, Kappler J, Marrack P (2002) Activated T cell death in vivo mediated by pro-apoptotic Bcl-2 family member, Bim. Immunity 16: 759–767 [DOI] [PubMed] [Google Scholar]
- Huang DCS, Strasser A (2000) BH3-only proteins—essential initiators of apoptotic cell death. Cell 103: 839–842 [DOI] [PubMed] [Google Scholar]
- Hutcheson J, Scatizzi JC, Bickel E, Brown NJ, Bouillet P, Strasser A, Perlman H (2005) Combined loss of proapoptotic genes Bak or Bax with Bim synergizes to cause defects in hematopoiesis and in thymocyte apoptosis. J Exp Med 201: 1949–1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi K, Benito A, Kiryu-Seo S, Gonzalez V, Inohara N, Lieberman AP, Kiyama H, Nunez G, Leiberman AP (2004) Critical role for DP5/Harakiri, a Bcl-2 homology domain 3-only Bcl-2 family member, in axotomy-induced neuronal cell death. J Neurosci 24: 3721–3725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffers JR, Parganas E, Lee Y, Yang C, Wang J, Brennan J, MacLean KH, Han J, Chittenden T, Ihle JN, McKinnon PJ, Cleveland JL, Zambetti GP (2003) Puma is an essential mediator of p53-dependent and -independent apoptotic pathways. Cancer Cell 4: 321–328 [DOI] [PubMed] [Google Scholar]
- Jiang A, Clark EA (2001) Involvement of Bik, a proapoptotic member of the Bcl-2 family, in surface IgM-mediated B cell apoptosis. J Immunol 166: 6025–6033 [DOI] [PubMed] [Google Scholar]
- Kasai S, Chuma S, Motoyama N, Nakatsuji N (2003) Haploinsufficiency of Bcl-x leads to male-specific defects in fetal germ cells: differential regulation of germ cell apoptosis between the sexes. Dev Biol 264: 202–216 [DOI] [PubMed] [Google Scholar]
- Knudson CM, Tung KSK, Tourtellotte WG, Brown GAJ, Korsmeyer SJ (1995) Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science 270: 96–99 [DOI] [PubMed] [Google Scholar]
- Lindsten T, Ross AJ, King A, Zong W, Rathmell JC, Shiels HA, Ulrich E, Waymire KG, Mahar P, Frauwirth K, Chen Y, Wei M, Eng VM, Adelman DM, Simon MC, Ma A, Golden JA, Evan G, Korsmeyer SJ, MacGregor GR, Thompson CB (2000) The combined functions of proapoptotic Bcl-2 family members Bak and Bax are essential for normal development of multiple tissues. Mol Cell 6: 1389–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkov M, Fisher Y, Don J (1998) Developmental schedule of the postnatal rat testis determined by flow cytometry. Biol Reprod 59: 84–92 [DOI] [PubMed] [Google Scholar]
- Meehan T, Loveland KL, de Kretser D, Cory S, Print CG (2001) Developmental regulation of the bcl-2 family during spermatogenesis: insights into the sterility of bcl-w−/− male mice. Cell Death Differ 8: 225–233 [DOI] [PubMed] [Google Scholar]
- Meinhardt A, O'Bryan MK, McFarlane JR, Loveland KL, Mallidis C, Foulds LM, Phillips DJ, de Kretser DM (1998) Localization of follistatin in the rat testis. J Reprod Fertil 112: 233–241 [DOI] [PubMed] [Google Scholar]
- Miller TM, Moulder KL, Knudson CM, Creedon DJ, Deshmukh M, Korsmeyer SJ, Johnson EM (1997) Bax deletion further orders the cell death pathway in cerebellar granule cells and suggests a caspase-independent pathway to cell death. J Cell Biol 139: 205–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motoyama N, Wang FP, Roth KA, Sawa H, Nakayama K, Nakayama K, Negishi I, Senju S, Zhang Q, Fujii S, Loh DY (1995) Massive cell death of immature hematopoietic cells and neurons in Bcl-x deficient mice. Science 267: 1506–1510 [DOI] [PubMed] [Google Scholar]
- Opferman JT, Letai A, Beard C, Sorcinelli MD, Ong CC, Korsmeyer SJ (2003) Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature 426: 671–676 [DOI] [PubMed] [Google Scholar]
- O'Reilly LA, Cullen L, Visvader J, Lindeman G, Print C, Bath ML, Huang DCS, Strasser A (2000) The pro-apoptotic BH3-only protein Bim is expressed in hemopoietic, epithelial, neuronal and germ cells. Am J Pathol 157: 449–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly LA, Print C, Hausmann G, Moriishi K, Cory S, Huang DCS, Strasser A (2001) Tissue expression and subcellular localization of the pro-survival molecule Bcl-w. Cell Death Differ 8: 486–494 [DOI] [PubMed] [Google Scholar]
- Orth JM, Gunsalus GL, Lamperti AA (1988) Evidence from Sertoli Cell-depleted rats indicates that spermatid number in adults depends on numbers of Sertoli cells produced during perinatal development. Endocrinology 122: 787–794 [DOI] [PubMed] [Google Scholar]
- Print CG, Loveland KL (2000) Germ cell suicide: new insights into apoptosis during spermatogenesis. BioEssays 22: 423–430 [DOI] [PubMed] [Google Scholar]
- Print CG, Loveland KL, Gibson L, Meehan T, Stylianou A, Wreford N, de Kretser D, Metcalf D, Köntgen F, Adams JM, Cory S (1998) Apoptosis regulator Bcl-w is essential for spermatogenesis but appears otherwise redundant. Proc Natl Acad Sci USA 95: 12424–12431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putcha GV, Moulder KL, Golden JP, Bouillet P, Adams JM, Strasser A, Johnson EMJ (2001) Induction of Bim, a proapoptotic BH3-only Bcl-2 family member, is critical for neuronal apoptosis. Neuron 29: 615–628 [DOI] [PubMed] [Google Scholar]
- Rathmell JC, Lindsten T, Zong W-X, Cinalli RM, Thompson CB (2002) Deficiency in Bak and Bax perturbs thymic selection and lymphoid homeostasis. Nat Immunol 3: 932–939 [DOI] [PubMed] [Google Scholar]
- Rodriguez I, Ody C, Araki K, Garcia I, Vassalli P (1997) An early and massive wave of germinal cell apoptosis is required for the development of functional spermatogenesis. EMBO J 16: 2262–2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross AJ, Amy SP, Mahar PL, Lindsten T, Knudson CM, Thompson CB, Korsmeyer SJ, MacGregor GR (2001) BCLW mediates survival of postmitotic Sertoli cells by regulating BAX activity. Dev Biol 239: 295–308 [DOI] [PubMed] [Google Scholar]
- Ross AJ, Waymire KG, Moss JE, Parlow AF, Skinner MK, Russell LD, MacGregor GR (1998) Testicular degeneration in _Bclw_-deficient mice. Nat Genet 18: 251–256 [DOI] [PubMed] [Google Scholar]
- Rucker EB III, Dierisseau P, Wagner KU, Garrett L, Wynshaw-Boris A, Flaws JA, Hennighausen L (2000) Bcl-x and Bax regulate mouse primordial germ cell survival and apoptosis during embryogenesis. Mol Endocrinol 14: 1038–1052 [DOI] [PubMed] [Google Scholar]
- Russell LD, Chiarini-Garcia H, Korsmeyer SJ, Knudson CM (2002) Bax-dependent spermatogonia apoptosis is required for testicular development and spermatogenesis. Biol Reprod 66: 950–958 [DOI] [PubMed] [Google Scholar]
- Russell LD, Warren J, Debeljuk L, Richardson LL, Mahar PL, Waymire KG, Amy SP, Ross AJ, MacGregor GR (2001) Spermatogenesis in Bclw-deficient mice. Biol Reprod 65: 318–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibue T, Takeda K, Oda E, Tanaka H, Murasawa H, Takaoka A, Morishita Y, Akira S, Taniguchi T, Tanaka N (2003) Integral role of Noxa in p53-mediated apoptotic response. Genes Dev 17: 2233–2238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser A, O'Connor L, Dixit VM (2000) Apoptosis signaling. Ann Rev Biochem 69: 217–245 [DOI] [PubMed] [Google Scholar]
- Veis DJ, Sorenson CM, Shutter JR, Korsmeyer SJ (1993) Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell 75: 229–240 [DOI] [PubMed] [Google Scholar]
- Villunger A, Michalak EM, Coultas L, Müllauer F, Böck G, Ausserlechner MJ, Adams JM, Strasser A (2003) p53- and drug-induced apoptotic responses mediated by BH3-only proteins Puma and Noxa. Science 302: 1036–1038 [DOI] [PubMed] [Google Scholar]
- Wang RA, Nakane PK, Koji T (1998) Autonomous cell death of mouse male germ cells during fetal and postnatal period. Biol Reprod 58: 1250–1256 [DOI] [PubMed] [Google Scholar]
- Whitfield J, Neame SJ, Paquet L, Bernard O, Ham J (2001) Dominant-negative c-Jun promotes neuronal survival by reducing BIM expression and inhibiting mitochondrial cytochrome c release. Neuron 29: 629–643 [DOI] [PubMed] [Google Scholar]
- Willis SN, Chen L, Dewson G, Wei A, Naik E, Fletcher JI, Adams JM, Huang DC (2005) Pro-apoptotic Bak is sequestered by Mc1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev 19: 1294–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong WX, Lindsten T, Ross AJ, MacGregor GR, Thompson CB (2001) BH3-only proteins that bind pro-survival Bcl-2 family members fail to induce apoptosis in the absence of Bax and Bak. Genes Dev 15: 1481–1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1