Mutational and Haplotype Analyses of Families with Familial Partial Lipodystrophy (Dunnigan Variety) Reveal Recurrent Missense Mutations in the Globular C-Terminal Domain of Lamin A/C (original) (raw)
Abstract
Familial partial lipodystrophy (FPLD), Dunnigan variety, is an autosomal dominant disorder characterized by marked loss of subcutaneous adipose tissue from the extremities and trunk but by excess fat deposition in the head and neck. The disease is frequently associated with profound insulin resistance, dyslipidemia, and diabetes. We have localized a gene for FPLD to chromosome 1q21-q23, and it has recently been proposed that nuclear lamin A/C is altered in FPLD, on the basis of a novel missense mutation (R482Q) in five Canadian probands. This gene had previously been shown to be altered in autosomal dominant Emery-Dreifuss muscular dystrophy (EDMD-AD) and in dilated cardiomyopathy and conduction-system disease. We examined 15 families with FPLD for mutations in lamin A/C. Five families harbored the R482Q alteration that segregated with the disease phenotype. Seven families harbored an R482W alteration, and one family harbored a G465D alteration. All these mutations lie within exon 8 of the lamin A/C gene—an exon that has also been shown to harbor different missense mutations that are responsible for EDMD-AD. Mutations could not be detected in lamin A/C in one FPLD family in which there was linkage to chromosome 1q21-q23. One family with atypical FPLD harbored an R582H alteration in exon 11 of lamin A. This exon does not comprise part of the lamin C coding region. All mutations in FPLD affect the globular C-terminal domain of the lamin A/C protein. In contrast, mutations responsible for dilated cardiomyopathy and conduction-system disease are observed in the rod domain of the protein. The FPLD mutations R482Q and R482W occurred on different haplotypes, indicating that they are likely to have arisen more than once.
Introduction
Familial partial lipodystrophy (FPLD), Dunnigan variety (MIM 151660), is an autosomal dominant disorder characterized by marked loss of subcutaneous adipose tissue from the extremities and trunk at puberty and by predisposition to insulin resistance and its complications. Affected patients may also have excess fat deposition in the head and neck areas. We have previously localized FPLD to human chromosome 1q21-q23, with use of a cohort of five multiply affected families of European origin (Peters et al. 1998). Two groups (Jackson et al. 1998; Anderson et al. 1999) have confirmed this localization in families from the United Kingdom and the United States.
Recently, a candidate gene for FPLD was described (Cao and Hegele 2000). This gene, lamin A/C, encodes a component of the nuclear lamina, a polymeric structure intercalated between chromatin and the inner membrane of the nuclear envelope (Gerace and Blobel 1982). The lamin A/C (LMNA) gene undergoes alternative splicing to produce two nuclear laminar proteins: lamin A and lamin C (Lin and Worman 1993). Lamins A and C form dimers through their rod domain (Fisher et al. 1986; McKeon et al. 1986), and they bind and assemble on the surface of mitotic chromosomes at specific sites on the rod (Glass and Gerace 1990; Glass et al. 1993). Lamins A and C also associate with integral proteins of the nuclear envelope, such as lamin-associated polypeptides 1A and 1B (Foisner and Gerace 1993), lamin B receptor (Worman et al. 1990), and emerin (Squarzoni 1998). The rod domain is flanked by hydrophobic N- and C-terminal domains (Fisher et al. 1986; McKeon et al. 1986).
Mutations in LMNA have been described in autosomal dominant Emery-Dreifuss muscular dystrophy (EDMD-AD; Bonne et al. 1999), a disease characterized by regional and progressive skeletal muscle wasting and weakness and by cardiac abnormalities. Missense mutations in the rod domain of LMNA have also been shown to cause dilated cardiomyopathy and conduction-system disease (Fatkin et al. 1999).
Because we were interested in evaluating the role of the lamin A/C gene in FPLD, we performed a mutational analysis of affected family members in a cohort of families with the disease. We detected four independent mutations in members of 14 families. One mutation has been described elsewhere (Cao and Hegele 2000). The other three have not previously been reported. Two mutations affected the same codon and were seen on a variety of different haplotypes, indicating that they are likely to be recurrent mutations. All the alterations occur in the globular C-terminal portion of the protein, and none of the alterations occur in the rod domain of lamin A/C, unlike the dilated cardiomyopathy and conduction-system-disease defects that are clustered in this region. The region harboring FPLD mutations also harbors different missense alterations that are responsible for EDMD-AD. One FPLD alteration occurs in exon 11 and affects lamin A only.
Families and Methods
Sample Collection
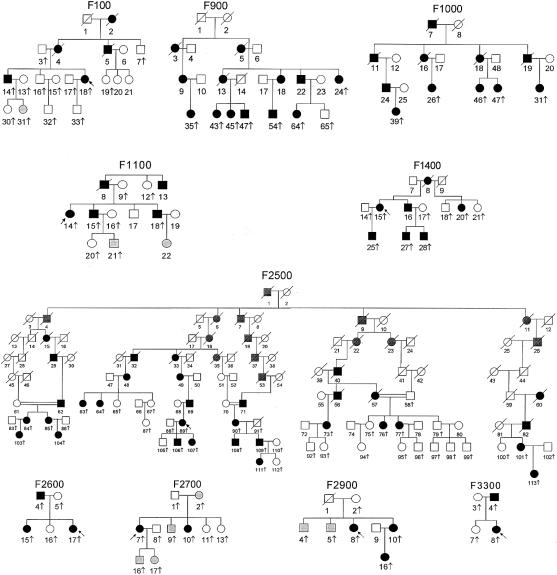
Five families with FPLD have been described elsewhere (Peters et al. 1998). Pedigrees of the additional 10 families are shown in figure 1. All families were recruited through the referral of an affected female as an index case. All families were of European origin, except for family F2600, which was of Asian Indian origin (table 1). The protocol was approved by the appropriate institutional review boards, and all subjects gave informed consent. The phenotype was classified as “affected,” “unaffected,” or “uncertain,” on the basis of history, physical examination, review of the medical records, responses to a written questionnaire, and inspection of photographs (when available). Lack of subcutaneous fat and extreme muscularity in all extremities, commencing at puberty, was considered the essential criterion for diagnosis. Another important diagnostic criterion was excess fat accumulation in the face and neck, which resulted in a cushingoid appearance. Additional supportive criteria included (1) the presence of acanthosis nigricans or hirsutism and (2) laboratory data confirming the presence of diabetes mellitus, hypertriglyceridemia, or low serum levels of HDL cholesterol. In some patients, characteristic body-fat distribution, as seen on whole-body magnetic-resonance images, provided confirmation of the diagnosis (Garg et al. 1999). Determination of the phenotype was easily accomplished in women, but, in some men and prepubertal children, it was classified as “uncertain.” Lymphoblastoid cell lines were established by Epstein-Barr–virus transformation of peripheral blood lymphocytes, and genomic DNA was isolated either from these cells or buffy coat, by use of routine methods.
Figure 1.
Pedigrees of families with FPLD, Dunnigan variety. The pedigrees and each family member are numbered for identification. Unblackened squares denote unaffected males; blackened squares, affected males; unblackened circles, unaffected females; blackened circles, affected females; circles and squares with a diagonal slash, deceased subjects; gray-shaded circles and squares, phenotype uncertain. Vertical arrows denote subjects for which DNA was available, whereas slanting arrows indicate probands from each family.
Table 1.
Lamin A/C Mutations and Haplotypes Segregating with Disease in Families with FPLD[Note]
| Haplotypes Corresponding to Loci Analyzed | ||||||||
|---|---|---|---|---|---|---|---|---|
| Family | D1S305 | D1S303 | D1S1595 | D1S2140 | D1S2777 | D1S2624 | D1S1600 | Mutation Detected |
| F100 | 2 | 4 | 3 | 4 | 7 | 2 | 4 | NDa |
| F200 | 5 | 1 | 5 | 6 | 1 | 4 | 4 | R482Q |
| F500 | 1 | 1 | 4 | 6 | 1 | 2 | 6 | R482Q |
| F1000 | 5 | 1 | 5 | 6 | 5 | 2 | 3 | R482Q |
| F2500 | 5 | 1 | 7 | 6 | 1 | 4 | 3 | R482Q |
| F3300 | 1 | 1 | 5 | 6 | 1 | 1 | 3 | R482Q |
| F300 | 6 | 1 | 11 | 2 | 1 | 5 | 4 | R482W |
| F600 | 1 | 1 | 5 | 6 | 5 | 2 | 4 | R482W |
| F700 | 2 | 1 | 5 | 6 | 1 | 1 | 4 | R482W |
| F900 | 1 | 4 | 11 | 2 | 9 | 2 | 4 | R482W |
| F1400 | 6 | 4 | 5 | 6 | 2 | 4 | 3 | R482W |
| F2600 | 5 | 1 | 7 | 7 | 5 | 1 | 3 | R482W |
| F2900 | 8 | 1 | 3 | 4 | 1 | 1 or 2 | 5 | R482W |
| F2700 | 2 | 1 | 5 | 6 | 1 | 1 | 4 | R582H |
| F1100 | 2 | 1 | 5 | 6 | 1 | 1 | 3 | G465D |
Clinical Features
In all families, affected subjects—particularly, affected women—had an increased frequency of hypertriglyceridemia, low levels of serum HDL cholesterol, and diabetes mellitus (with age at onset primarily at >20 years). Some patients had eruptive xanthomas and recurrent episodes of acute pancreatitis as a result of extreme hypertriglyceridemia. Other manifestations in affected subjects included acanthosis nigricans, hypertension, and atherosclerotic vascular disease, including coronary heart disease. Some affected women had irregular menstrual periods, hirsutism, and polycystic ovarian syndrome. The proband from family F100 complained of myopathy, but the clinical features were not similar to those seen in patients with EDMD-AD.
Genotyping and Haplotype Reconstruction
DNA from family members was subjected to semiautomated genotyping of polymorphic microsatellites from the FPLD interval. Genotyping methods are described elsewhere (Bennett et al. 2000). Haplotypes were reconstructed by means of the computer software CYRILLIC and were verified by eye.
Mutational Analysis of Lamin A/C
Primers that would amplify each exon of the lamin A/C gene from genomic DNA templates were designed from published sequence information (Lin and Worman 1993; GenBank accession numbers L12399, L12400 and L12401). PCR products for each exon from each individual were subjected to analysis by means of denaturing high-performance liquid chromatography (DHPLC) (Underhill et al. 1997). DHPLC was performed with use of the “Wave” (Transgenomic). Products were eluted with a linear acetonitrile (J. T. Baker) gradient at a flow rate of 0.9 ml/min. The beginning and end points of the gradient were adjusted according to the size of the PCR products. Generally, analysis took <10 min, including column regeneration and reequilibration of the starting conditions. Samples that exhibited DHPLC variants were DNA sequenced with use of the Big Dye Terminator Cycle Sequencing Ready Reaction sequencing kit (PE Biosystems), and reactions were analyzed with the use of an ABI PRISM 377 DNA sequencer.
Results
Haplotype Reconstruction
Haplotypes segregating with disease in each family are listed in table 1; they correspond to alleles from the following polymorphic satellite–containing loci: D1S305, D1S303, D1S1595, D1S2140, D1S2777, D1S2624, and D1S1600. Most haplotypes were very different, although some harbored the same mutation in lamin A/C. Families F2500 and F200 may harbor a core “6, 1, 4” haplotype at D1S2140, D1S2777, and D1S2624.
Mutational Analysis
Table 1 describes the lamin A/C mutations detected in the families studied. In families F200, F500, F1000, F2500, and F3300, the previously described R482Q (CGG→CAG) alteration in exon 8 (Cao and Hegele 2000) was detected. Seven families—F300, F600, F700, F900, F1400, F2600, and 2900—harbored a different alteration R482W (CGG→TGG) in the same codon. One family (F1100) harbored a G465D (GGC→GAC) alteration. In the affected members of all families, the variant allele segregated with disease. We were unable to detect mutations in lamin A/C in affected members of family F100, in which there was linkage. None of these variants were seen in more than 140 normal chromosomes from CEPH.
One family with atypical FPLD (F2700) harbored a novel R582H (CGC→CAC) alteration within exon 11 of the lamin A/C gene. Sequences encoded by this exon are found in the lamin A mRNA only.
We detected two polymorphisms that resulted in silent changes: one in exon 5 (GCT→GCC; codon 297) and one in exon 7 (GAT→GAC; codon 446). These were also seen in several expressed sequence tag clones. Chromosomes from one unaffected CEPH individual (individual 1701) harbored an R633C (CGC→TGC) alteration in exon 11 of lamin A. This change was not seen in 139 other normal chromosomes from CEPH and is likely to be a rare variant that is unrelated to the diseases described in the present study.
Discussion
Fifteen families with FPLD were examined for molecular alterations in the lamin A/C gene on human chromosome 1q21. Four alterations were detected—three within exon 8 and one within exon 11. One mutation, R482Q, was seen in five of the families in the present study and was first described in five probands from Canada (Cao and Hegele 2000). One of the families in the present study is also of Canadian origin and could be related to one of the kindreds described elsewhere (Cao and Hegele 2000); however, it could reflect an earlier founder effect. The other three mutations have not previously been described. Two alterations—R482Q and R482W—disrupted the same codon and were seen on a variety of different haplotypes, indicating that they are likely to be recurrent mutations. To date, all families with FPLD described in the literature have been of European origin (Garg 2000). However, one of the families (family F2600) in the present study is of Asian Indian origin. This family harbored the most frequently observed mutation, R482W, on a novel haplotype.
Both the R482Q and the R482W mutations occur within a stretch of CCGG nucleotides and result in a CTGG change. Of the mammalian DNA, 2%–7% is converted to 5-methylcytosine (Vanyushin et al. 1970), and >90% of the 5-methylcytosine residues in the DNA of higher eukaryotes are bordered by a G residue on the 3′ side (Doskocil and Sorm 1962; Grippo et al. 1968). Ehrlich and Wang (1981) propose that 5-methylcytosine can be mutated to thymidine via deamination and mismatch repair. The observation that both the R482Q and R482W mutations occur on several different haplotypes suggests that they have arisen more than once, possibly by means of the mechanism described above.
The third alteration found within exon 8 is a G465D change. The fourth alteration, R582H, occurred within exon 11 of the lamin A/C gene and can affect the lamin A protein only. In FPLD, all missense mutations identified in exons 8 and 11 change amino acids conserved through different species and in different lamins (fig. 2).
Figure 2.
Amino-acid-sequence alignment of lamins A, B, and C from various species. A, Conservation of Gly465 and Arg482 of lamin A/C is shown in boldface type. B, Conservation of Arg582 of lamin A is shown in boldface type. Xenopus = Xenopus laevis.
DHPLC and sequencing analysis failed to reveal a mutation within the lamin A/C coding sequence in members of family F100, in which linkage to chromosome 1q21 was found. Mutational analyses of the regulatory elements of this gene—as well as Southern blotting to detect large rearrangements—are required to rule out alterations in other regions.
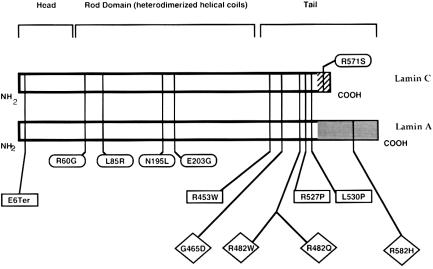
The distribution, within lamin A/C, of mutations that are responsible for three different diseases—EDMD-AD, dilated cardiomyopathy and conduction-system disease, and FPLD—are illustrated in figure 3. The lamin A/C gene is encoded by 12 exons, and alternative splicing within exon 10 gives rise to the two isoforms (Lin and Worman 1993). The first 566 amino acids of lamins A and C are identical. Lamin C contains 6 additional C-terminal amino acids, and lamin A contains 128 additional C-terminal amino acids. Pre–lamin A peptide undergoes farnesylation at the peptide sequence CAAX within the C-terminal and is cleaved to lamin A (Sinensky et al. 1994).
Figure 3.
Locations of mutations in lamin A/C in EDMD-AD (squares), dilated cardiomyopathy with conduction-system-disease defects (ovals), and FPLD (diamonds). Organization of the proteins is also indicated, and locations of the hydrophobic head and carboxy-terminal regions, with respect to the rod domain, are shown. The regions of lamin A and C that diverge from each other are indicated at the carboxyl-termini.
All of the alterations in FPLD occur in the globular C-terminal portion of the lamin A/C protein, and none of the alterations occur in the α-helical rod domain, unlike the dilated cardiomyopathy and conduction-system-disease defects that are clustered in this region (Fatkin et al. 1999). Only one mutation causing dilated cardiomyopathy and conduction-system disease occurs in the lamin C tail domain. In contrast, missense mutations in the hydrophobic C-terminal region of lamin A/C result in EDMD-AD (Bonne et al. 1999). One case of EDMD-AD is the result of a nonsense mutation within codon 6 of the protein. However, this may result in loss of the protein entirely, because of nonsense-mediated RNA degradation, or it may result in a nonfunctional, highly truncated protein. It is interesting that three FPLD mutations cluster in exon 8, a region that also harbors EDMD-AD changes. The overlap between FPLD and EDMD-AD changes is interesting, since FPLD and EDMD-AD appear to affect different developmental processes. FPLD appears to affect adipocyte apoptosis or differentiation, and EDMD-AD changes result in regional and progressive skeletal muscle wasting and cardiac abnormalities.
The observation that one FPLD mutation occurs within the C-terminal region of lamin A (R582H; exon 11) contrasts with a single missense mutation within the C-terminal of lamin C in dilated cardiomyopathy and conduction-system disease. In two affected sisters that underwent clinical evaluation and that were from the family that harbors this mutation, reduction in subcutaneous fat from both the gluteal region and the medial aspects of the thighs was less pronounced, compared with that in other women with FPLD. One of these sisters had diabetes mellitus, borderline hypertriglyceridemia, and irregular menstrual periods, but the other had normal glucose tolerance, normal serum lipids, and regular menstrual periods. Neither sister had acanthosis nigricans. However, this type of clinical heterogeneity is seen in other families with FPLD; ∼50% of affected women have diabetes mellitus, and only one-third have irregular periods and acanthosis nigricans. The R582H alteration may disrupt some interactions of lamin A with chromatin, since it has been shown that carboxy-terminal sequence elements of lamin A are required for the observed lamin-chromatin interaction (Hoger et al. 1991).
The results of the present study confirm and extend findings from a previous report (Cao and Hegele 2000) indicating that missense mutations in the lamin A/C gene cosegregate with FPLD. However, it is not clear how the alterations described in the present study lead to adipocyte apoptosis or initiate loss of fat at puberty. Additional studies are also required to determine why different alterations within lamin A/C are responsible for three clinically distinct diseases.
Acknowledgments
We thank the members of the families studied, for their invaluable contribution to this project; Drs. David C. Robbins, Robert A. Kreisberg, Andrea Dunaif, Richard Legro, Mark D. Shepherd, Irene Sills, Margo Denke, Steven Aronoff, Noralane M. Lindor, Tu T. Nguyen, and Evelyn Cintron, for referring family members for investigation; Angela Osborn and Rebecca Cochran, for technical help; and the nursing and dietetic services of the General Clinical Research Center, for patient care support. This work was supported by National Institutes of Health grants R01-DK54387 and M01-RR00633, the Southwestern Medical Foundation, and the Washington University Medical Center Division of Human Genetics.
_Note added in proof.—_Recently, Shackleton et al. (2000) also confirmed the findings of Cao and Hegele (2000) and reported missense mutations limited to exon 8 in ten families and three individuals with FPLD (Dunnigan variety). They also observed the R482Q and R482W mutations and report novel R482L and K486N mutations.
Electronic-Database Information
Accession numbers and URLs for data in this article are as follows:
- CEPH, http://www.cephb.fr/
- GenBank, http://www.ncbi.nlm.nih.gov/Genbank/index.html (for human nuclear lamin A and nuclear lamin C gene, exon 1 [accession number L12399]; human nuclear lamin A and nuclear lamin C gene, exon 2 [accession number L12400]; and human nuclear lamin A and nuclear lamin C gene, exons 3–12, and complete alternative mRNAs [accession number L12401])
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for FPLD [MIM 151660])
References
- Anderson JL, Khan M, David WS, Mahdavi Z, Nuttall FQ, Krech E, West SG, et al (1999) Confirmation of linkage of hereditary partial lipodystrophy to chromosome 1q21-22. Am J Med Genet 82:161–165 [PubMed]
- Bennett LB, Roach SE, Bowcock AM (2000) A locus for paroxysmal kinesigenic dyskinesia maps to human chromosome 16. Neurology 54:125–130 [DOI] [PubMed]
- Bonne G, Di Barletta MR, Varnous S, Becane HM, Hammouda EH, Merlini L, Muntoni F, et al (1999) Mutations in the gene encoding lamin A/C cause autosomal dominant Emery-Dreifuss muscular dystrophy. Nat Genet 21:285–288 [DOI] [PubMed]
- Cao H, Hegele RA (2000) Nuclear lamin A/C R482Q mutation in Canadian kindreds with Dunnigan-type familial partial lipodystrophy. Hum Mol Genet 9:109–112 [DOI] [PubMed]
- Doskocil J, Sorm F (1962) Distribution of 5-methylcytosine in pyrimidine sequences of deoxyribonucleic acids. Biochim Biophys Acta 55:953–959 [DOI] [PubMed] [Google Scholar]
- Ehrlich M, Wang RYH (1981) 5-Methylcytosine in eukaryotic DNA. Science 212:1350–1357 [DOI] [PubMed]
- Fatkin D, MacRae C, Sasaki T, Wolff MR, Porcu M, Frenneaux M, Atherton J, et al (1999) Missense mutations in the rod domain of the lamin A/C gene as causes of dilated cardiomyopathy and conduction-system disease. N Engl J Med 341:1715–1724 [DOI] [PubMed]
- Fisher DZ, Chaudhary N, Blobel G (1986) cDNA sequencing of nuclear lamins A and C reveals primary and secondary structural homology to intermediate filament proteins. Proc Natl Acad Sci USA 83:6450–6454 [DOI] [PMC free article] [PubMed]
- Foisner R, Gerace L (1993) Integral membrane proteins of the nuclear envelope interact with lamins and chromosomes, and binding is modulated by mitotic phosphorylation. Cell 73:1267–1279 [DOI] [PubMed]
- Garg A (2000) Lipodystrophies. Am J Med 108:143–152 [DOI] [PubMed] [Google Scholar]
- Garg A, Peshock RM, Fleckenstein JL (1999) Adipose tissue distribution pattern in patients with familial partial lipodystrophy (Dunnigan variety). J Clin Endocrinol Metab 84:170–174 [DOI] [PubMed]
- Gerace L, Blobel G (1982) Nuclear lamina and the structural organization of the nuclear envelope. Cold Spring Harb Symp Quant Biol 46:967–978 [DOI] [PubMed]
- Glass JR, Gerace L (1990) Lamins A and C bind and assemble at the surface of mitotic chromosomes. J Cell Biol 111:1047–1057 [DOI] [PMC free article] [PubMed]
- Glass CA, Glass JR, Taniura H, Hasel KW, Blevitt JM, Gerace L (1993) The α-helical rod domain of human lamins A and C contains a chromatin binding site. EMBO J 12:4413–4424 [DOI] [PMC free article] [PubMed]
- Grippo P, Laccarino M, Paris E, Scarono E (1968) Methylation of DNA in developing sea urchin embryos. J Mol Biol 36:195–208 [DOI] [PubMed]
- Hoger TH, Krohne G, Kleinschmidt JA (1991) Interaction of Xenopus lamins A and LII with chromatin in vitro mediated by a sequence element in the carboxyterminal domain. Exp Cell Res 197:280–289 [DOI] [PubMed]
- Jackson SNJ, Pinkney J, Bargiotta A, Veal CD, Howlett TA, McNally PG, Corral R, et al (1998) A defect in the regional deposition of adipose tissue (partial lipodystrophy) is encoded by a gene at chromosome 1q. Am J Hum Genet 63:534–540 [DOI] [PMC free article] [PubMed]
- Lin F, Worman HJ (1993) Structural organization of the human gene encoding nuclear lamin A and nuclear lamin C. J Biol Chem 268:16321–16326 [PubMed]
- McKeon FD, Kirschner MW, Caput D (1986) Homologies in both primary and secondary structure between nuclear envelope and intermediate filament proteins. Nature 319:463–469 [DOI] [PubMed]
- Peters JM, Barnes R, Bennett L, Gitomer WM, Bowcock AM, Garg A (1998) Localization of the gene for familial partial lipodystrophy (Dunnigan variety) to chromosome 1q21-22. Nat Genet 18:292–295 [DOI] [PubMed]
- Shackleton S, Lloyd DJ, Jackson SN, Evans R, Niermeijer MF, Singh BM, Schmidt H, et al (2000) LMNA, encoding lamin A/C, is mutated in partial lipodystrophy. Nature Genet 24:153–156 [DOI] [PubMed]
- Sinensky M, Fantle K, Trujillo M, McLain T, Kupfer A, Dalton M (1994) The processing pathway of prelamin A. J Cell Sci 107:61–67 [DOI] [PubMed]
- Squarzoni S (1998) Immunocytochemical detection of emerin within the nuclear matrix. Neuromuscul Disord 8:338–344 [DOI] [PubMed]
- Underhill PA, Jin L, Lin AA, Mehdi SQ, Jenkins T, Vollrath D, Davis RW, et al (1997) Detection of numerous Y chromosome biallelic polymorphisms by denaturing high-performance liquid chromatography. Genome Res 7:996–1005 [DOI] [PMC free article] [PubMed]
- Vanyushin BF, Tkacheva SG, Belozersky AN (1970) Rare bases in animal DNA. Nature 225:948–949 [DOI] [PubMed]
- Worman HJ, Evans CD, Blobel G (1990) The lamin B receptor of the nuclear envelope inner membrane: a polytopic protein with eight potential transmembrane domains. J Cell Biol 111:1535–1542 [DOI] [PMC free article] [PubMed]