Identification of MEFV-Independent Modifying Genetic Factors for Familial Mediterranean Fever (original) (raw)
Abstract
Familial Mediterranean fever (FMF) is a recessively inherited disorder predisposing to renal amyloidosis and associated with mutations in MEFV, a gene encoding a protein of unknown function. Differences in clinical expression have been attributed to _MEFV_-allelic heterogeneity, with the M694V/M694V genotype associated with a high prevalence of renal amyloidosis. However, the variable risk for patients with identical MEFV mutations to develop this severe complication, prevented by lifelong administration of colchicine, strongly suggests a role for other genetic and/or environmental factors. To overcome the well-known difficulties in the identification of modifying genetic factors, we investigated a relatively homogeneous population sample consisting of 137 Armenian patients with FMF from 127 independent families living in Armenia. We selected the SAA1, SAA2, and APOE genes—encoding serum amyloid proteins and apolipoprotein E, respectively—as well as the patients' sex, as candidate modifiers for renal amyloidosis. A stepwise logistic-regression analysis showed that the _SAA1_α/α genotype was associated with a sevenfold increased risk for renal amyloidosis, compared with other SAA1 genotypes (odds ratio [OR] 6.9; 95% confidence interval [CI] 2.5–19.0). This association, which was present whatever the MEFV genotype, was extremely marked in patients homozygous for M694V (11/11). The risk for male patients of developing renal amyloidosis was fourfold higher than that for female patients (_OR_=4.0; 95% _CI_=1.5–10.8). This association, particularly marked in patients who were not homozygous for M694V (34.0% vs. 11.6%), was independent of _SAA1_-allelic variations. Polymorphisms in the SAA2 or APOE gene did not appear to influence susceptibility to renal amyloidosis. Overall, these data, which provide new insights into the pathophysiology of FMF, demonstrate that susceptibility to renal amyloidosis in this Mendelian disorder is influenced by at least two MEFV_-independent factors of genetic origin—_SAA1 and sex—that act independently of each other.
Introduction
Mutations in pyrin/marenostrin, a protein of unknown function, have been identified in patients with familial Mediterranean fever (FMF [MIM 249100]) (French FMF Consortium 1997; International FMF Consortium 1997; Bernot et al. 1998; Booth et al. 1998_b_; Cazeneuve et al. 1999), an autosomal recessive condition particularly common in several populations of Mediterranean extraction (Sohar et al. 1967), in which the prevalence reaches as high as 1/200 individuals. This disease is characterized by a marked variability in clinical expression between and within families (Sohar et al. 1967), a phenotypic feature that, combined with the absence of pathognomonic clinical and biological signs, makes the diagnosis of FMF difficult to establish. This diagnosis is indeed based on the occurrence of recurrent episodes of fever and serosal inflammation, usually revealed by sterile peritonitis, arthritis, and/or pleurisy, which may lead to unnecessary invasive investigations. However, the major concern in FMF is the risk of amyloidosis, mainly renal, which, in the absence of daily and lifelong administration of colchicine (Zemer et al. 1986), develops over years and progresses to renal failure.
The identification of the gene responsible for FMF (designated “_MEFV_”) (French FMF Consortium 1997; International FMF Consortium 1997) has opened up new ways to manage the disease—especially by providing the first objective test of diagnostic value (Cazeneuve et al. 1999)—and to decipher the pathophysiology of this disorder. The first genotype-phenotype correlation studies showed that the M694V homozygous genotype was associated with a more severe form of the disease, as judged by an earlier age at onset, a higher prevalence of pleurisy (Dewalle et al. 1998), a higher frequency of arthritis (Dewalle et al. 1998; Cazeneuve et al. 1999), and, most importantly, in patients who did not have access to a regular colchicine therapy, a higher prevalence of renal amyloidosis (Cazeneuve et al. 1999; Shohat et al. 1999). However, it should be emphasized that this latter observation was assessed at the population level, whereas, at the individual level, it is well established that not all patients with the M694V homozygous genotype develop renal amyloidosis (Cazeneuve et al. 1999; Shohat et al. 1999), and, conversely, not all the patients with FMF who have renal amyloidosis carry the M694V homozygous genotype (Yalcinkaya et al. 1998; Cazeneuve et al. 1999; Shohat et al. 1999). Taken together, these observations strongly suggest that the MEFV gene does not account for the total genetic contribution to renal amyloidosis susceptibility and that as-yet-unknown modifying factors may have a protective or deteriorating effect on renal function. The identification of these factors would thus be of critical importance in both improving the accuracy of renal amyloidosis–risk prediction in individual patients and extending our understanding of the pathophysiology of this major complication of the disease.
To investigate the mechanisms underlying such a variability in the risk for patients with FMF to develop renal amyloidosis, we have examined potential risk factors in a large cohort of independent Armenian patients living in Armenia; in this relatively homogeneous population sample, the complexity of genetic and environmental factors contributing to renal amyloidosis susceptibility is indeed expected to be minimal. We considered the genotype at the SAA1, SAA2, and APOE loci, as well as the patients' sex, as good candidate modifier factors for this severe complication. Indeed, the amyloid A1 (AA1) and A2 (AA2) proteins, which result from proteolytic cleavage of the serum amyloid A1 (SAA1) and A2 (SAA2) proteins, are major protein components of the amyloid A deposits found in secondary amyloidosis (Liepnieks et al. 1995); the apolipoprotein Eɛ4 (APOEɛ4) allele is a well-known risk factor for both the late-onset familial and the sporadic forms of Alzheimer disease, two conditions characterized by brain deposition of the β-amyloid protein (Saunders et al. 1993). We also examined the influence of sex on renal amyloidosis, given the unbalanced sex ratio documented in nearly all FMF population samples studied so far (Wolff 1994). Finally, to overcome the confounding effect of the M694V homozygous genotype on the possible association of each studied variable with renal amyloidosis, we also took into account the MEFV genotype.
Patients and Methods
Patients
We investigated 137 patients with FMF (age 3–99 years, mean 27.7 years; male:female ratio 1.40) from 127 unrelated families living in Armenia. Clinical features were recorded through a standardized form, and the diagnosis of FMF was made according to established clinical criteria (Livneh et al. 1997). The duration of the disease represents the delay between the onset of clinical signs and the time of this study; these data were available in 129/137 patients. Forty-seven individuals presented with renal amyloidosis, as diagnosed by persistent proteinuria during childhood or early adulthood of patients with FMF (Zemer et al. 1986), and/or nephrotic syndrome or chronic renal failure. Since the aim of this study was to evaluate the impact of several factors on the occurrence of renal amyloidosis, we oriented our recruitment toward patients presenting with this complication, thereby explaining the overall higher percentage (47/137 [34%]) of Armenian patients with FMF presenting with renal amyloidosis than has been reported elsewhere (Meyerhoff 1980; Cazeneuve et al. 1999). Because colchicine was not easily available in Armenia, patients could not have access to daily administration of colchicine until 1998 or 1999. Informed consent was obtained from all patients or their parents.
Molecular Genetic Analyses
MEFV Mutation Detection
Genomic DNA was isolated, using standard procedures, from peripheral leukocytes. The mutation analysis was performed according to Cazeneuve et al. (1999). In brief, we searched for the M694V and V726A mutations by means of restriction-enzyme analyses performed on PCR products spanning these sites. In patients carrying, at most, only one of these two mutations, we completed the MEFV gene analysis by screening all 10 coding exons and their flanking intronic sequences for mutations, using denaturing gradient gel electrophoresis. Samples displaying a shift in mobility were subsequently directly sequenced.
Analysis of Polymorphisms in Candidate Modifying Genes
The SAA1α, SAA1β, and SAA1γ isoforms are encoded by the V52-A57, A52-V57, and A52-A57 SAA1 alleles, respectively. The SAA2α and SAA2β isoforms are encoded by the H71 and R71 SAA2 alleles, respectively. We searched for the A/V52 and A/V57 polymorphisms of the SAA1 gene and the H/R71 polymorphism of the SAA2 gene by restriction-enzyme analysis of the PCR fragments spanning these sites, as described elsewhere (Moriguchi et al. 1999).
The ɛ2, ɛ3, and ɛ4 ApoE isoforms are encoded by the C112-C158, C112-R158, and R112-R158 APOE alleles, respectively. The R/C112 and R/C158 polymorphisms of the APOE gene were studied by restriction-enzyme analysis, as follows: the genotype at position 112 was determined by _Fsp_I restriction analysis of a 147-bp PCR product generated with primers Apo112-f (5′-GAAGGCCTACAAATCGGAAC-3′) and Apo112-r (5′-CCGCGGTACTGCACCAGGCGGCTG-3′ [this oligonucleotide is a mismatched primer designed to create an _Fsp_I restriction site in the presence of the R112 allele]); the genotype at position 158 was determined by _Hae_II restriction analysis of a 272-bp PCR product generated with primers Apo158-f (5′-CGGCGAGGTGCAGGCCATGC-3′) and Apo158-r (5′-AGGCCTGGGCCCGCTCCTGT-3′). Restriction fragments were separated on a 2% agarose gel.
Statistical Analyses
In univariate analyses, factors associated with the presence of renal amyloidosis were tested using the χ2 test (or Fisher's exact test when appropriate) for categorical data and one-way ANOVA test (or Mann-Whitney nonparametric test when appropriate) for quantitative data. To avoid the confounding effect of the M694V homozygous genotype, which is strongly related to renal amyloidosis, we subsequently tested the factors potentially related to renal amyloidosis after adjustment on the MEFV genotype, using the Mantel-Haenszel test and a two-way ANOVA test for categorical data and quantitative data, respectively. Finally, to point out factors independently associated with renal amyloidosis, we performed a multivariate analysis by means of a stepwise logistic regression analysis, allowing factors with _P_⩽.10 in the univariate analysis to enter the model. Factors with P<.05 in logistic regression were kept in the model. We also tested first-degree interaction between the patients' sex and the genotype at the SAA1 locus. The interaction between the genotype at the MEFV locus and the genotype at the SAA1 locus was not tested, since a cell equal to 0 made the estimation of the coefficient impossible.
Results
The study cohort, consisting of 137 patients with FMF, was divided into two groups, according to the presence (_n_=47) or absence (_n_=90) of renal amyloidosis at study entry. These two groups of patients (mean age 27.4±13.5 or 27.8±16.2 years, respectively; _P_=.806) were subsequently compared with each other with respect to the following variables: the genotype at the MEFV, SAA1, SAA2, and APOE loci, the patients' sex, the duration of the disease, and the age at onset.
Genotypes at the MEFV, SAA1, SAA2, and APOE Loci and Renal Amyloidosis
Table 1 summarizes the relationship between the genotypes at the MEFV and candidate modifier loci and the presence or absence of renal amyloidosis, as assessed by means of univariate analyses:
Table 1.
Genotypes of the Patients at the MEFV, SAA1, SAA2, and APOE Loci, According to the Presence or Absence of Renal Amyloidosis (Univariate Analysis)[Note]
| No. (%) of Patients with Genotype | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MEFV | SAA1 | SAA2 | APOE | |||||||||
| Patients | M694V/M694V | Othera | P | α/α | Otherb | P | α/α | Otherc | P | ɛ3/ɛ4 | Otherd | P |
| With RA | 24 (51.1) | 23 (48.9) | 22 (46.8) | 25 (53.2) | 35 (74.5) | 12 (25.5) | 5 (10.6) | 42 (89.4) | ||||
| Without RA | 17 (18.9) | 73 (81.1) | 15 (16.7) | 75 (83.3) | 56 (62.2) | 34 (37.8) | 13 (14.4) | 77 (85.6) | ||||
| Overall | .0001 | .0002 | .150 | .530 |
_MEFV._—The frequency of the M694V homozygous genotype in the group of patients with renal amyloidosis (51.1%) was significantly higher than that observed in the group of patients without renal amyloidosis (18.9%, _P_=.0001). In contrast, there was no significant difference in the frequency of the other most common MEFV genotypes (i.e., M694V/V726A, M694V/M680I, and M680I/V726A) between the two groups of patients (data not shown).
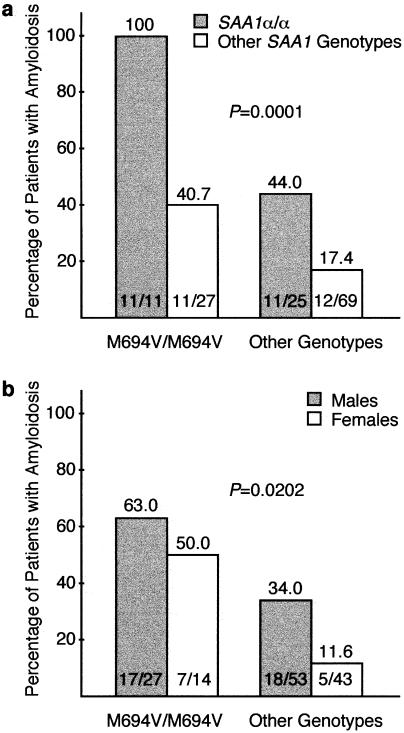
SAA1.—As shown in table 1, the frequency of renal amyloidosis in the group of patients carrying the _SAA1_α/α genotype was higher than that observed in patients carrying another SAA1 genotype (_P_=.0002). This difference persisted when adjusted on the MEFV genotype (fig. 1_a_; _P_=.0001) and was extremely marked in patients with the M694V homozygous genotype; it is indeed noteworthy that, in this latter group, all the patients who carried the _SAA1_α/α genotype (11/11) presented with renal amyloidosis. In contrast, whatever the genotype at the MEFV locus, the frequency of renal amyloidosis among patients carrying only one _SAA1_α allele and among those who did not carry any SAA1α allele did not differ statistically—that is, M694V homozygous genotype, 43.7% versus 42.9%; non-M694V homozygous genotypes, 18.9% versus 15.2% (data not shown).
Figure 1.
Influence of the genotype at the SAA1 locus (a) and of sex (b) on the risk of developing renal amyloidosis, according to MEFV genotypes (Mantel-Haenszel test).
_SAA2._—The frequency of renal amyloidosis was not significantly different in patients carrying the _SAA2_α/α genotype, compared with those with other SAA2 genotypes (_P_=.150; table 1). Similar results were obtained after adjustment on the MEFV genotype (M694V homozygous genotype: 66.7% vs. 42.9%; non-M694V homozygous genotypes: 26.7% vs. 18.7%; Mantel-Haenszel test: _P_=.178; data not shown).
_APOE._—There was no difference in the frequency of renal amyloidosis between the group of patients carrying at least one APOEɛ4 allele (i.e., ɛ3/ɛ4 genotype in our cohort of patients) and the groups of patients carrying another APOE genotype (_P_=.530; table 1). Similar results were obtained after adjustment on the MEFV genotype (M694V homozygous genotype: 57.1% vs. 58.8%; non-M694V homozygous genotypes: 10.0% vs. 25.9%; Mantel-Haenszel test: _P_=.493; data not shown), or when the group of patients carrying at least one ɛ2 allele was compared with that of patients who did not carry any ɛ2 allele (data not shown).
Sex-Related Prevalence of Renal Amyloidosis
The overall male:female ratio in our cohort of patients with FMF was 1.40 (80:57). The male:female ratio in patients with renal amyloidosis was higher than that of patients without renal amyloidosis (35:12 [2.92] vs. 45:45 [1.00], _P_=.006; table 2). This difference persisted when adjusted on the MEFV genotype (fig. 1b; _P_=.0202) and was particularly marked in patients who did not carry the M694V homozygous genotype; indeed, in this latter group, 34% (18/53) of male patients presented with renal amyloidosis, compared with only 11.6% (5/43) of female patients.
Table 2.
Clinical Characteristics of the Patients, According to the Presence or Absence of Renal Amyloidosis (Univariate Analysis)[Note]
| Sex (No. [%] of Patients) | |||||||
|---|---|---|---|---|---|---|---|
| Patients | Male | Female | P | Age at Onset (years, mean ± SD) | P | Duration of Disease (years, mean ± SD) | P |
| With RA | 35 (74.5) | 12 (25.5) | 9.1 ± 10.5 | 18.7 ± 10.3 | |||
| Without RA | 45 (50.0) | 45 (50.0) | 12.1 ± 10.5 | 16.3 ±12.8 | |||
| Overall | .006 | .022 | .142 |
Age at Onset, Duration of the Disease at Study Entry, and Renal Amyloidosis
The duration of the disease among patients with renal amyloidosis (_n_=44) was similar to that among patients without renal amyloidosis (_n_=85; 18.7±10.3 and 16.3±12.8 years, respectively; _P_=.142; table 2). However, the age at onset of the disease was significantly lower in patients with renal amyloidosis than in those without this complication (9.1±10.5 vs. 12.1±10.5; _P_=.022; table 2). This association actually reflects the unbalanced distribution of MEFV genotypes between the two groups, since the age at onset is lower in patients with the M694V homozygous genotype than in those who carried another MEFV genotype (5.8±5.3 and 7.9±9.7 vs. 13.4±11.0 and 10.8±11.6, in patients with and without renal amyloidosis, respectively; _P_=.016).
Multivariate Analysis
The odds ratios (ORs) presented in table 3 were estimated from the stepwise logistic model that takes into account the duration of the disease. In our population sample, the M694V homozygous genotype was associated with a sevenfold increased risk for renal amyloidosis, compared with other MEFV genotypes (_OR_=6.8; 95% _CI_=2.7–17.8). The results obtained with regard to SAA1 genotype showed that the patients who carried the _SAA1_α homozygous genotype had a sevenfold increased risk for renal amyloidosis, compared with those who carried another SAA1 genotype (_OR_=6.9; 95% _CI_=2.5–19.0). The risk for men with FMF to develop renal amyloidosis was found to be fourfold higher than that for women with FMF (_OR_=4.0; 95% _CI_=1.5–10.8). We observed no evidence of interaction between the patient's sex and the genotype at the SAA1 locus. This finding therefore suggests that genetic variations at the SAA1 locus predisposes to renal amyloidosis in a manner that is independent of the patient's sex.
Table 3.
Factors Associated With Renal Amyloidosis (Multivariate Logistic Regression Analysis)[Note]
| Variable | Percentage(No./Total)of Patients with RA | Adjusted ORsa(95% CI) | P |
|---|---|---|---|
| MEFV genotype: | |||
| M694V/M694V | 58.5 (24/41) | 6.8 (2.7–17.8) | .0001 |
| Otherb | 24.0 (23/96) | 1 | |
| Sex: | |||
| Male | 43.7 (35/80) | 4.0 (1.5–10.8) | .003 |
| Femaleb | 21.1 (12/57) | 1 | |
| SAA1 genotype: | |||
| α/α | 59.5 (22/37) | 6.9 (2.5–19.0) | .001 |
| Otherb | 25.0 (25/100) | 1 |
Discussion
Variability in clinical expression of the disease phenotype is a common feature of many genetic disorders. In theory, this phenomenon may result from allelic heterogeneity and/or from the influence of environmental or modifying genetic factors. Although identification of such modifier genes represents one of the major current challenges in human genetics, these complex studies are usually hampered by the potentially large number of independent genetic and environmental factors that may contribute to the disease phenotype, thereby making correlations between the phenotype and candidate modifier factors difficult to establish. In the case of FMF, the variable risk for patients carrying the same MEFV mutations to develop renal amyloidosis strongly suggests a role for other genetic and/or environmental factors. In the present study, we have selected and tested several candidate modifier factors and identified two _MEFV_-independent factors of genetic origin, SAA1 and sex, that indeed affect this risk by acting independently of each other.
In an attempt to overcome the well-known difficulties in identifying modifying genetic factors, we focused our efforts on the investigation of a large population sample of Armenian patients living in Armenia. Indeed, in this relatively homogeneous population, we expected the patients not only to share a common genetic background but also to have been exposed to similar environmental factors—two conditions that would facilitate the isolation of major modifying factors of genetic origin. The MEFV genotype was also taken into account in the interpretation of the data. Previous genotype-phenotype correlation studies indeed showed an association between the M694V homozygous genotype at the MEFV locus and the risk of occurrence of renal amyloidosis in patients with FMF (Cazeneuve et al. 1999; Shohat et al. 1999). As expected, a similar association was found in the present study. Therefore, to overcome the confounding effect of the M694V homozygous genotype on the interpretation of the data, the comparisons between patients with and without renal amyloidosis were adjusted on the MEFV genotype.
The mean duration of the disease in patients with renal amyloidosis was found to be similar to that of patients without renal amyloidosis, ruling out a bias related to follow-up duration. However, the age at onset of the disease in our cohort of patients was lower in individuals with the M694V homozygous genotype than in those with another MEFV genotype, an observation that is similar to that recorded in patients of Sephardic Jewish extraction who have FMF (Dewalle et al. 1998). The age at onset was also lower in patients with renal amyloidosis than in those without this complication. These last two observations are actually linked and reflect the unbalanced distribution of MEFV genotypes between the two groups.
Candidate modifiers of genetic origin in FMF-associated renal amyloidosis would include factors that participate in amyloid deposits. We therefore investigated the genes encoding the major acute-phase serum amyloid A1 (SAA1) and A2 (SAA2) proteins, which are precursors of the amyloid A (AA) proteins present in the amyloid fibrils found in AA secondary amyloidosis. The present study identified a significant and important relationship between the SAA1 genotype and the risk for patients with FMF to develop renal amyloidosis. The _SAA1_α/α genotype was indeed associated with a sevenfold increased risk for renal amyloidosis, compared with other SAA1 genotypes, thereby demonstrating the pivotal contribution of the SAA1 locus in the occurrence of this major complication. This result relates to the data recorded in patients with rheumatic arthritis or juvenile chronic arthritis, two non-Mendelian disorders of unknown etiology characterized by a chronic inflammatory syndrome: in these disorders, the risk of developing secondary amyloidosis was shown to be higher in patients with the _SAA1_α/α genotype than in those carrying another SAA1 genotype (Booth et al. 1998_a_). Our study also revealed that the presence of only one _SAA1_α allele did not confer a high susceptibility to renal amyloidosis, thereby indicating that, in patients with FMF, the predisposition to this particular complication linked to the _SAA1_α allele may be considered to be a recessive trait. In addition, it is striking to note that the association of the _SAA1_α/α genotype with renal amyloidosis, which was present whatever the MEFV genotype, was extremely marked in M694V-homozygous patients. Renal amyloidosis was indeed present in all the patients (_n_=11 [100%]) who were homozygous for both the _SAA1_α and M694V alleles. Because no patient without renal amyloidosis carried these genotypes at the SAA1 and MEFV loci, it was impossible to calculate the OR associated with the interaction between theses two factors, although this interaction is likely to be high. Altogether, these data raise the question of the mechanism(s) by which the _SAA1_α/α genotype predisposes to secondary amyloidosis. Although the relationship between this genotype and the occurrence of renal amyloidosis is not straightforward, at least three hypotheses can be advanced. First, the SAA plasma level might be higher in patients carrying the _SAA1_α/α genotype than in those carrying another SAA1 genotype. However, in the Japanese population, in which the risk for patients with rheumatic arthritis or juvenile chronic arthritis to develop secondary amyloidosis has been shown to be associated with the _SAA1_γ/γ genotype (Baba et al. 1995; Moriguchi et al. 1999), the SAA plasma level of healthy subjects carrying this genotype was lower than that of control individuals with another SAA1 genotype (Yamada et al. 1999). This observation therefore does not support the hypothesis that the risk for secondary amyloidosis is directly related to the plasma concentration of SAA. Second, because macrophages are thought to play a key role in the formation of amyloid A proteins and amyloid fibrils from the SAA protein (Kluve-Beckerman et al. 1999), it is tempting to speculate that the high susceptibility of the _SAA1_α/α genotype to secondary amyloidosis may result from a greater macrophage uptake of the SAA1α isoform, compared with that of β and γ isoforms. Consistent with this hypothesis, it has been shown that the amyloidogenic SAA isoform in mice, SAA1.1, displays an increased plasma clearance, compared with nonamyloidogenic SAA proteins (Meek et al. 1986; Kluve-Beckerman et al. 1997), and specifically binds RAGE—a multiligand immunoglobulin superfamily cell surface molecule expressed in macrophages—with nanomolar affinity (Kisilevsky 2000; Yan et al. 2000). One also cannot exclude the possibility that the human SAA1α isoform may undergo a macrophage processing different from that of β and γ isoforms. Last, the αAA1 isoform may also have a high intrinsic potential to promote fibrillogenesis, compared with that of β and γ AA1 isoforms. Further studies focused on the processing of each SAA1 isoform in macrophages and on the assessment of their respective amyloidogenic potentialities might be helpful in deciphering the molecular basis of the greater susceptibility for _SAA1_α homozygous patients to develop renal amyloidosis. Despite the structural and functional similarities between SAA1 and SAA2, the SAA2 locus did not significantly affect the risk of occurrence of renal amyloidosis in our population sample. This result may, however, be related to the minority representation of AA2 in amyloid deposits (Liepnieks et al. 1995).
We also considered the APOE genotype to be a good candidate modifier factor for renal amyloidosis in FMF. Indeed, although the mechanisms by which AA proteins accumulate in amyloidosis are not fully understood, several lines of evidence indicate the role of apolipoprotein E (ApoE) in this phenomenon: the ApoE protein has been identified in the FMF-associated amyloid deposits found in vivo (Wisniewski and Frangione 1992); furthermore, in vitro incubation of native AA peptides with the ApoE protein resulted in a higher degree of polymerization of amyloid A peptide, compared with control incubations performed without ApoE (Castaño et al. 1995). In addition, the APOEɛ4 allele has clearly been associated with an increased risk of developing Alzheimer disease (Saunders et al. 1993), a disorder characterized by brain deposition of the β-amyloid protein. The role of the APOEɛ4 allele in promoting fibrillogenesis of the amyloid Aβ peptide is also supported by in vitro studies that showed that ApoE, and especially the ApoEɛ4 isoform, accelerate the spontaneous fibrillogenesis of amyloid β peptide (Wisniewski et al. 1994). However, in this study, we did not find any evidence supporting the hypothesis that, in patients with FMF, allelic variations at the APOE locus modify the risk of occurrence of renal amyloidosis. Even though the number of our patients carrying the ɛ4 allele is relatively small, a major effect of this allele should have been identified, if it existed. This result, however, does not rule out the possibility that the different ApoE isoforms may have slight differential effects on the fibrillogenesis of AA proteins.
It is usually found that FMF is more frequent in men than in women (Wolff 1994). The present study showed that men and women were equally represented among patients without amyloidosis (45 men and 45 women), whereas the male:female ratio was clearly unbalanced in patients with amyloidosis (35 men and 12 women). Men with FMF had a fourfold increased risk for renal amyloidosis, compared with women with FMF, the difference being particularly marked in patients who did not carry the M694V homozygous genotype (34.0% vs. 11.6%). The mechanism by which the patient's sex can modify the risk of amyloidosis is unclear. Although sex steroid hormones are known to influence the concentration of several acute-phase proteins (Whicher 1983), whether the inflammatory syndrome in patients with FMF is more important in men than in women has not yet been documented.
In most Mendelian disorders that are inherited in a simple fashion, it is now well established that individuals with identical mutations in the disease-causing gene may differ clinically. These observations, which reflect the existence of additional environmental factors or genes that contribute to the disease phenotypes, support the concept that, for these Mendelian disorders, the phenotypes are, in fact, complex traits (reviewed by Romeo and McKusick 1994; Dipple and McCabe 2000). The modifier factors of genetic origin accounting for this phenotypic diversity can be classified into three categories, with allelic variations involving the disease gene itself, a locus genetically linked to the disease gene, or a disease-gene–independent locus (Feingold 2000). A relatively limited but increasing number of observations that fall into the two first categories have been reported in human populations. This is, for instance, the case for fatal familial insomnia and familial Creutzfeldt-Jakob disease, two neurological disorders linked to the same mutation of the prion-protein gene but associated with different apparently benign polymorphisms in the same gene (Goldfarb et al. 1992), whereas the disease severity in spinal muscular atrophy, a recessive disorder due to abnormalities in the SMN1 gene, is associated with deletions of the H4F5 gene, which maps a few kilobases upstream of SMN1 (Scharf et al. 1998). In contrast with these situations, the present study identifies modifier factors of genetic origin that are not linked to the disease-causing gene. To our knowledge, there are very few precedents for this situation in the human species, in which such modifiers cannot be easily identified, although their action is often suspected. One analogue is the familial hypertrophic cardiomyopathy due to mutations in the β-MHC gene, in which severity is associated with an insertion/deletion polymorphism in the ACE gene (Tesson et al. 1997). In this study, we have identified a key role for the SAA1 locus in conferring genetic susceptibility to renal amyloidosis, the most severe complication of FMF, which can be prevented by lifelong administration of colchicine. Male patients are also clearly more exposed to the risk of developing renal amyloidosis, compared with female patients with FMF. In light of these results, it would certainly be of particular interest to investigate other FMF population samples, following a similar candidate-gene approach. Taken together, the identification of these two _MEFV_-independent modifying genetic factors for FMF provides insights into the pathophysiology of the FMF-associated secondary amyloidosis A and raises the possibility that determination of the SAA1 genotype might be useful in assessing the patient's prognosis with respect to renal function.
Acknowledgments
We are grateful to the families for agreeing to participate in the study. This work was supported by grants from the Association Française contre les Myopathies and the Fondation Calouste Gulbenkian (Lisbon). H.A. and T.S. were the recipients of a grant from the Institut National de la Santé et de la Recherche Médicale.
Electronic-Database Information
The accession number and URL for data in this article are as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/omim/ (for FMF [MIM 249100])
References
- Baba S, Masago S, Takahashi T, Kasama T, Sugimura H, Tsugane S, Tsutsui Y, Shirasawa H (1995) A novel allelic variant of serum amyloid A, SAA1 γ: genomic evidence, evolution, frequency, and implication as a risk factor for reactive systemic AA-amyloidosis. Hum Mol Genet 4:1083–1087 [DOI] [PubMed] [Google Scholar]
- Bernot A, da Silva C, Petit J, Cruaud C, Caloustian C, Castet V, Ahmed-Arab M, Dross C, Dupont M, Cattan D, Smaoui N, Dode C, Pecheux C, Nedelec B, Medaxian J, Rozenbaum M, Rosner I, Delpech M, Grateau G, Demaille J, Weissenbach J, Touitou I (1998) Non-founder mutations in the MEFV gene establish this gene as the cause of familial Mediterranean fever. Hum Mol Genet 7:1317–1325 [DOI] [PubMed] [Google Scholar]
- Booth D, Booth S, Gillmore J, Hawkins P, Pepys M (1998_a_) SAA1 alleles as risk factors in reactive systemic AA amyloidosis. Amyloid 5:262–265 [DOI] [PubMed] [Google Scholar]
- Booth DR, Gillmore JD, Booth SE, Pepys MB, Hawkins PN (1998_b_) Pyrin/marenostrin mutations in familial Mediterranean fever. QJM 91:603–606 [DOI] [PubMed]
- Castaño EM, Prelli F, Pras M, Frangione B (1995) Apolipoprotein E carboxyl-terminal fragments are complexed to amyloids A and L. J Biol Chem 270:17610–17615 [DOI] [PubMed] [Google Scholar]
- Cazeneuve C, Sarkisian T, Pêcheux C, Dervichian M, Nédelec B, Reinert P, Ayvazyan A, Kouyoumdjian J-C, Ajrapetyan H, Delpech M, Goossens M, Dodé C, Grateau G, Amselem A (1999) MEFV-gene analysis in Armenian patients with familial Mediterranean fever: diagnostic value and unfavorable renal prognosis of the M694V homozygous genotype—genetic and therapeutic implications. Am J Hum Genet 65:88–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewalle M, Domingo C, Rozenbaum M, Ben-Chetrit E, Cattan D, Bernot A, Dross C, Dupont M, Notarnicola C, Levy M, Rosner I, Demaille J, Touitou I (1998) Phenotype-genotype correlation in Jewish patients suffering from familial Mediterranean fever (FMF). Eur J Hum Genet 6:95–97 [DOI] [PubMed] [Google Scholar]
- Dipple KM, McCabe ERB (2000) Phenotypes of patients with “simple” Mendelian disorders are complex traits; thresholds, modifiers, and systems dynamics. Am J Hum Genet 66:1729–1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feingold J (2000) Les gènes modificateurs dans les maladies héréditaires. Médecine/sciences 16:I–V [Google Scholar]
- Goldfarb LG, Petersen RB, Tabaton M, Brown P, LeBlanc AC, Montagna P, Cortelli P, Julien J, Vital C, Pendelbury WW, Haltia M, Wills PR, Hauw JJ, McKeever PE, Monari L, Schrank B, Swergold GD, Autilio-Gambetti L, Gajdusek DC, Lugaresi E, Gambetti P (1992) Fatal familial insomnia and familial Creutzfeldt-Jakob disease: disease phenotype determined by a DNA polymorphism. Science 258:806–808 [DOI] [PubMed] [Google Scholar]
- Kisilevsky R (2000) Amyloids: tombstones or triggers? Nat Med 6:633–634 [DOI] [PubMed] [Google Scholar]
- Kluve-Beckerman B, Liepnieks JJ, Wang L, Benson MD (1999) A cell culture system for the study of amyloid pathogenesis. Am J Pathol 155:123–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluve-Beckerman B, Yamada T, Hardwick J, Liepnieks JJ, Benson MD (1997) Differential plasma clearance of murine acute-phase serum amyloid A proteins SAA1 and SAA2. Biochem J 322:663–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liepnieks J, Kluve-Beckerman B, Benson M (1995) Characterization of amyloid A protein in human secondary amyloidosis: the predominant deposition of serum amyloid A1. Biochim Biophys Acta 1270:81–86 [DOI] [PubMed] [Google Scholar]
- Livneh A, Langevitz P, Zemer D, Zaks N, Kees S, Lidar T, Migdal A, Padeh S, Pras M (1997) Criteria for the diagnosis of familial Mediterranean fever. Arthritis Rheum 40:1879–1885 [DOI] [PubMed] [Google Scholar]
- Meek RL, Hoffman JS, Benditt EP (1986) Amyloidogenesis: one serum amyloid A isotype is selectively removed from the circulation. J Exp Med 163:499–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerhoff J (1980) Familial Mediterranean fever: report of a large family, review of the literature, and discussion of the frequency of amyloidosis. Medicine 59:66–77 [PubMed] [Google Scholar]
- Moriguchi M, Terai C, Koseki Y, Uesato M, Nakajima A, Inada S, Nishinarita M, Uchida S, Nakajima A, Yoon Kim S, Chen C-L, Kamatani N (1999) Influence of genotypes at SAA1 and SAA2 loci on the development and the length of latent period of secondary AA-amyloidosis in patients with rheumatoid arthritis. Hum Genet 105:360–366 [DOI] [PubMed] [Google Scholar]
- Romeo G, McKusick V (1994) Phenotypic diversity, allelic series and modifier genes. Nat Genet 7:451–453 [DOI] [PubMed] [Google Scholar]
- Saunders A, Strittmatter W, Schmechel D, George-Hyslop P, Pericak-Vance M, Joo S, Rosi B, Gusella J, Crapper-MacLachlan D, Alberts M, Hulette C, Crain B, Goldgaber D, Roses A (1993) Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer's disease. Neurology 43:1467–1472 [DOI] [PubMed] [Google Scholar]
- Scharf J, Endrizzi M, Wetter A, Huang S, Thompson T, Zerres K, Dietrich W, Wirth B, Kunkel L (1998) Identification of a candidate modifying gene for spinal muscular atrophy by comparative genomics. Nat Genet 20:83–86 [DOI] [PubMed] [Google Scholar]
- Shohat M, Magal N, Shohat T, Chen X, Dagan T, Mimouni A, Danon Y, Lotan R, Ogur G, Sirin A, Schlezinger M, Halpern G, Schwabe A, Kastner D, Rotter J, Fischel-Ghodsian N (1999) Phenotype-genotype correlation in familial Mediterranean fever: evidence for an association between Met694Val and amyloidosis. Eur J Hum Genet 7:287–292 [DOI] [PubMed] [Google Scholar]
- Sohar E, Gafni J, Pras M, Heller H (1967) Familial Mediterranean fever: a survey of 470 cases and review of the literature. Am J Med 43:227–253 [DOI] [PubMed] [Google Scholar]
- Tesson F, Dufour C, Moolman J, Carrier L, al-Mahdawi S, Chojnowska L, Dubourg O, Soubrier E, Brink P, Komajda M, Guicheney P, Schwartz K, Feingold J (1997) The influence of the angiotensin I converting enzyme genotype in familial hypertrophic cardiomyopathy varies with the disease gene mutation. J Mol Cell Cardiol 29:831–838 [DOI] [PubMed] [Google Scholar]
- The French FMF Consortium (1997) A candidate gene for familial Mediterranean fever. Nat Genet 17:25–31 [DOI] [PubMed] [Google Scholar]
- The International FMF Consortium (1997) Ancient missense mutations in a new member of the RoRet gene family are likely to cause familial Mediterranean fever. Cell 90:797–807 [DOI] [PubMed] [Google Scholar]
- Whicher JT (1983) Abnormalities of plasma proteins. In: Williams DL, Marks V (eds) Biochemistry in Clinical Practice. William Heinemann Medical Books Limited, London, pp 221–251 [Google Scholar]
- Wisniewski T, Castano E, Golabek A, Vogel T, Frangione B (1994) Acceleration of Alzheimer's fibril formation by apolipoprotein E in vitro. Am J Pathol 145:1030–1035 [PMC free article] [PubMed] [Google Scholar]
- Wisniewski T, Frangione B (1992) Apolipoprotein E: a pathological chaperone protein in patients with cerebral and systemic amyloidosis. Neurosci Lett 135:235–238 [DOI] [PubMed] [Google Scholar]
- Wolff SM (1994) Familial Mediterranean fever (familial paroxysmal polyserositis). In: Isselbacher KJ, Braunwald E, Wilson JD, Martin JB, Fauci AS, Kasper DL (eds) Harrison's principles of internal medicine, 13th ed. McGraw-Hill, Inc. Health Professions Division, New York, pp 1684–1686 [Google Scholar]
- Yalcinkaya F, Akar N, Misirlioglu M (1998) Familial Mediterranean fever—amyloidosis and the Val726Ala mutation. N Engl J Med 338:993 [DOI] [PubMed]
- Yamada T, Wada A, Itoh Y, Itoh K (1999) Serum amyloid A1 alleles and plasma concentrations of serum amyloid A. Amyloid 6:199–204 [DOI] [PubMed]
- Yan S-D, Zhu H, Zhu A, Golabek A, Du H, Roher A, Yu J, Soto C, Schmidt A-M, Stern D, Kindy M (2000) Receptor-dependent cell stress and amyloid accumulation in systemic amyloidosis. Nat Med 6:643–651 [DOI] [PubMed] [Google Scholar]
- Zemer D, Pras M, Sohar E, Modan M, Cabili S, Gafni J (1986) Colchicine in the prevention and treatment of the amyloidosis of familial Mediterranean fever. N Engl J Med 314:1001–1005 [DOI] [PubMed] [Google Scholar]