Hairless-Mediated Repression of Notch Target Genes Requires the Combined Activity of Groucho and CtBP Corepressors (original) (raw)
Abstract
Notch signal transduction centers on a conserved DNA-binding protein called Suppressor of Hairless [Su(H)] in Drosophila species. In the absence of Notch activation, target genes are repressed by Su(H) acting in conjunction with a partner, Hairless, which contains binding motifs for two global corepressors, CtBP and Groucho (Gro). Usually these corepressors are thought to act via different mechanisms; complexed with other transcriptional regulators, they function independently and/or redundantly. Here we have investigated the requirement for Gro and CtBP in Hairless-mediated repression. Unexpectedly, we find that mutations inactivating one or the other binding motif can have detrimental effects on Hairless similar to those of mutations that inactivate both motifs. These results argue that recruitment of one or the other corepressor is not sufficient to confer repression in the context of the Hairless-Su(H) complex; Gro and CtBP need to function in combination. In addition, we demonstrate that Hairless has a second mode of repression that antagonizes Notch intracellular domain and is independent of Gro or CtBP binding.
The Notch signaling pathway regulates many different cell fate decisions during the development of all metazoans. Upon ligand binding, the activated Notch receptor is cleaved and an intracellular fragment, NICD, is released. The active fragment NICD is translocated to the nucleus and functions as a transcriptional coactivator interacting with a DNA-binding protein, known as Suppressor of Hairless [Su(H)], in Drosophila species (CBF1 or RBP-Jk in mammals) (reviewed in references 2 and 32). Recently it was proposed that many nuclear effectors of developmental pathways act as a “molecular switch”; in the uninduced state they repress their target genes, through the recruitment of corepressors, and only after the signaling event are they transformed into activators (3, 7, 41). Indeed, the mammalian homolog of Su(H) acts as a transcriptional repressor in the absence of NICD by the indirect recruitment of histone deacetylases (overview in reference 32).
Repression of Notch target genes in Drosophila species is mediated by Hairless (H), which binds to DNA-bound Su(H) (4, 30). Combined overexpression of Su(H) and Hairless results in a strong inhibition of Notch-dependent transcription, indicating that binding of Hairless transforms Su(H) into a repressor (17, 30). Hairless in turn is reported to recruit two global corepressors, CtBP (30) and Groucho (Gro) (4). Both corepressors appear to contribute to repression during bristle shaft development, as mutations in either Gro or CtBP enhance the bristle loss caused by reducing Hairless function (4). However, deletion of the CtBP-binding domain from Hairless was sufficient to abrogate its repressive activity upon coexpression with Su(H) (30), even though the protein would have retained the ability to interact with Gro, suggesting nonredundant roles for the two corepressors. Thus, the relative roles of Gro and CtBP in Hairless-mediated repression remain to be clarified.
Gro and CtBP are implicated as corepressors for many different transcription factors; they mostly interact directly with the DNA-binding protein itself rather than requiring an additional intermediary such as Hairless. Gro itself was first identified as a corepressor for the E(spl) basic helix-loop-helix proteins encoded by the immediate downstream targets of Notch (23, 38). It has subsequently been shown to participate in many different repressor complexes, and tethering of Gro-binding domains to a DNA-binding protein is sufficient to convert it into a transcriptional repressor (16). Thus, Gro alone has the capability of repressing gene transcription. Likewise, CtBP is also sufficient to confer repressive function, and in the closely related proteins Snail, Escargot, and Worniu, it is indeed the CtBP-binding domain that confers distinct and essential repressive characteristics on Snail (20). Recently it has emerged that two transcription factors, Hairy and Brinker, have the ability to recruit both CtBP and Gro. Complexed with Brinker, the two proteins seem to contribute to repression of an overlapping set of targets; one set responds more strongly to Gro, a second more strongly to CtBP, and a third to both equally (13, 19, 46). In addition, Brinker has a further type of repressor activity which is independent of either corepressor and involves competition with other DNA-binding proteins. On Hairy target genes, Gro and CtBP appear to mediate separate pathways of repression, and CtBP is reported to interfere with Gro-mediated repression (39, 47). Furthermore, chromatin profiling indicates that cofactor recruitment is context dependent but suggests that repression of the majority of Hairy target genes involves CtBP acting in combination with dSir2 rather than Gro (5).
Here we have set out to investigate the contributions of Gro and CtBP to Hairless-mediated repression in Drosophila species. We have tested the residual activity of mutant forms of Hairless that are unable to recruit one or the other of the corepressors both in direct transcriptional assays in cell culture and in overexpression assays during wing development. We find that Hairless requires interaction with both cofactors for full repression; removal of one or the other interaction renders it as inactive as removing both in most assays. Thus, the two corepressors appear to act in combination. Furthermore, even when both motifs are deleted Hairless retains significant repressive activity for Notch target promoters, arguing that it has an additional mechanism of repression, most likely by interfering with the recruitment of the activating complex containing NICD.
MATERIALS AND METHODS
Analysis of protein-protein interactions.
Coimmunoprecipitations were performed as described earlier (34) using protein extracts of approximately 500 wild-type embryos. For immunoprecipitation, we used rabbit anti-Hairless A antibodies at 1:250 dilution; for detection we used rat anti-Hairless A at 1:500 (28, 29), mouse anti-Gro antibodies at 1:50 (14), and rat anti-CtBP antibodies at 1:100. In vitro transcription-translation was performed with a TNT T7/T3-coupled reticulocyte lysate system (Promega) using Hairless, Gro, and CtBP cDNAs cloned in BT vector (Stratagene). Immunoprecipitation was performed with rabbit anti-Gro antibodies (1:250). Precipitates and 16% of the input were probed using Western blot analysis with antibodies as outlined above. Proteins were sized with a prestained protein ladder (Fermentas) (∼10 to 180 kDa). Secondary AP-coupled antibodies (Jackson Lab) were used at 1:200. Antibodies directed against CtBP were raised against a glutathione _S_-transferase fusion protein in rats (Pineda ABservice, Berlin, Germany); the respective pGEX construct was kindly provided by S. Parkhurst.
Yeast two-hybrid protein interaction assays were performed as previously described (34) using VP16-dCtBP (40) or VP16-Gro and pEG-Gro (1). For bait constructs, pEG-HFL, -HΔS (= HC2), -HC3, -HCX, and -HΔC (= HC6) were used (see reference 27). pEG-GBDcontains amino acids 565 to 712; potential eh1-related Gro-binding motifs were mutated by changing aromatic amino acid F582 (GBD-1) or Y663 (GBD-2) into alanine. HΔG deletes amino acids 490 to 712. Counts begin with the first methionine (29). In the H*C mutation, the CtBP binding site was altered by in vitro mutagenesis from PLNLSKH to VIQITKR; in H*G, the wild-type GBD was replaced by GBD-2. Changes were confirmed by sequence analysis.
Analysis of target gene activation.
S2 cells were transfected with 1 μg of luciferase reporters containing Notch-responsive Su(H) sites (NME) or mutated sites (NRE) (8) and 0.2 μg of a control Renilla-expressing plasmid (tk-Renilla; Promega). Reporters also contained binding sites for Grainyhead (Grh), as described for the equivalent in vivo reporters [Gbe plus Su(H)-LacZ] (17). Expression from the two plasmids was measured by a dual luciferase assay (Promega). To analyze effects of Hairless on NICD, cells were cotransfected with 1 μg of pMT-ICN (15) and 0.5 μg of the relevant pMT-H construct (cloned by shuttling the respective coding sequences into pRmHa-3 [10]). For titration experiments, the amount of H constructs was adjusted as indicated. To analyze effects on Grh, cells were cotransfected with 1 μg of pMT-Grh and 0.5 μg of the relevant pMT-H construct. In all cases the total amount of transfected DNA was normalized to 3 μg by use of pMT-A (Invitrogen). Expression of NICD, Hairless, and/or Grh was induced by adding 0.6 mM Cu2SO4 6 h after transfection. Cells were harvested 18 h after addition of Cu2SO4. Integrity and the amounts of expressed H protein variants were assayed on Western blots.
Manipulation of gene activity in vivo.
Tissue-specific expression of respective transgenes was induced with a Gal4 upstream activation sequence (UAS) system (6) using omb-Gal4 and the indicated UAS lines or UAS-redStinger as a control (http://www.flybase.net/). UAS-Su(H) and UAS-H lines were as described previously (28, 34). Cloning details for UAS-HΔC (deletes amino acids 1062 to 1076), UAS-HΔG (deletes amino acids 490 to 712), and UAS-HΔGC (double deletion of amino acids 490 to 712 and 1062 to 1076) and UAS-Su(H)WRPW (WRPW codons added in frame at C terminus) are available upon request. Several transgenic fly lines were generated for each construct and tested for expressivity in vivo. Representative lines that have similar expression levels as determined by Western blot analysis and immunostaining of imaginal disks were used for further experiments. For repression assays the respective lines were crossed with the vg BE -LacZ line (24), and for coexpression they were recombined with UAS-Su(H).
Tissue-specific RNA interference (RNAi) was induced by misexpression of internal repeat constructs as outlined for pUdsGFPgro in reference 33; segments from the corresponding genes were PCR amplified, cloned into pHIBS, and shuttled into pUdsGFP or pUAST. pUdsGFP-SuH contains 445 nucleotides of the Su(H) cDNA in a double-headed orientation (coding sequence, nucleotides 808 to 1253); pUAST-dsH contains 285 nucleotides of Hairless (coding sequence, nucleotides 582 to 867); and pUAST-dsCtBP contains 296 nucleotides of CtBP (coding sequence, nucleotides 1 to 296). Several transgenic lines were established that behaved identically as determined on the basis of adult phenotypes and of the ability to knock down cognate mRNA-protein levels (as analyzed in situ and on Western blots).
Immunohistochemistry.
Staining of imaginal disks and Western blot analysis were performed as described before (34) using the following antisera: anti-Gro and anti-beta-galactosidase (developed by C. Delidakis and J. Sanes, respectively; obtained from Developmental Studies Hybridoma Bank, Department of Biological Sciences, University of Iowa, Iowa City, IA 52242); anti-H-A and anti-NTH(28, 29); and anti-CtBP and anti-Su(H) (18). Secondary antibodies coupled to alkaline phosphatase, fluorescein, Cy3, or Cy5 were purchased from Jackson Laboratory. Samples were embedded in Vectashield (Vector Lab) and analyzed on a Zeiss Axiophot linked to a Bio-Rad MRC1024 confocal microscope.
RESULTS
Binding and function of Hairless corepressors in vivo.
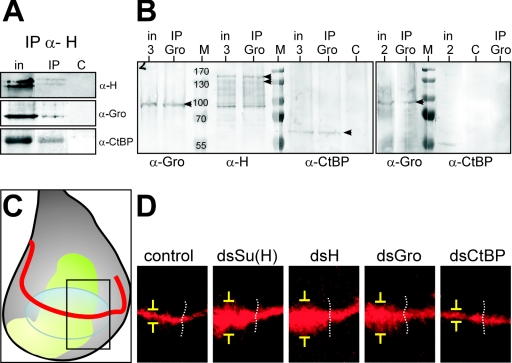
Hairless contains motifs that are able to interact directly with CtBP and Gro in vitro, and phenotypic analysis indicates that both factors may contribute to Hairless function in vivo (4). In order to further investigate the roles of the two corepressors we first established that they are interacting with Hairless in vivo. Hairless antibody was used to immunoprecipitate Hairless protein from embryonic extracts, and the presence of Gro or CtBP was analyzed. Both corepressor proteins were detected in the immunoprecipitates, confirming that they each can form complexes with Hairless in vivo (Fig. 1A). To address whether or not both proteins are present in the same complex, we asked whether CtBP could be coimmunoprecipitated with Gro. The proteins were translated in vitro; immunoprecipitations were carried out using Gro antibodies and a mixture of all three proteins or a mixture of Gro and CtBP only. The precipitates were subsequently probed for the presence of each protein. Robust levels of CtBP were detected in precipitates from the mixture containing all three proteins but not in those from the mixture containing Gro and dCtBP only (pairwise protein interaction between Gro and CtBP was also not detectable in a yeast two-hybrid assay; data not shown). These data show that dCtBP is present in the same complexes as Gro and Hairless and suggest that Hairless can recruit the two corepressors simultaneously (Fig. 1B). The converse immunoprecipitation using CtBP antibodies to precipitate complexes from a mixture of the three proteins likewise revealed Gro in the precipitate (not shown).
FIG. 1.
Relationship between Hairless and the corepressors CtBP and Gro. (A) Hairless binds to Gro and CtBP in vivo. Proteins immunoprecipitated (IP) from embryonic extracts by anti-Hairless antibodies were probed to detect Gro or CtBP as indicated. The input lane (in) contained 30% of the extract used for IP. C, control precipitate with unrelated antibody. Typically, Hairless is detected as two protein bands, 120 kDa and 150 kDa. (B) Hairless can recruit CtBP and Gro at the same time. Anti-Gro antiserum was used to immunoprecipitate in vitro-translated proteins from a mixture containing Hairless, Gro, and CtBP (input 3) or just Gro and CtBP (input 2). The presence of Gro, Hairless, and CtBP was detected in the immunoprecipitates (arrowheads) in comparison to input (in) (16%). CtBP was only detected when the mix contained H also but was not detected in the double mixture.Control, precipitate with unrelated antiserum, probed with anti-CtBP; M, protein ladder (sizes in kilodaltons). (C and D) Negative regulation of the vg BE -LacZ by Hairless, Su(H), and Gro. (C) Schematic drawing of a wing disk; the dorsoventral boundary (red), omb expression domain (green), and prospective wing blade (light blue) are indicated. The inset depicts the sector shown in panel D. (D) Effects on vg BE -LacZ (red) of expressing double-stranded (ds) RNAi constructs, as indicated, in the omb domain (white dotted lines indicate the right boundary of the domain). Note that double-stranded RNAi against Su(H), Hairless, and Gro but not CtBP causes derepression of vg BE -LacZ (yellow brackets). UAS-redStinger was overexpressed in the control.
To investigate the involvement of Gro and CtBP in Su(H)-Hairless-mediated gene repression in vivo we tested their effects on expression of the well-defined Notch target gene vestigial (vg). Expression of vg along the dorsoventral border is mediated by Su(H), which binds to the boundary enhancer element of vg (vg BE) (24). Expression driven by vg BE is restricted to a small strip of few cells (Fig. 1C and D), a situation reminiscent of single minded expression in the mesectoderm. There, the restriction of single minded expression to a single row of cells requires a combination of specific activation by Su(H) plus NICD in mesectodermal cells and specific repression in adjacent cells through an Su(H)-Hairless complex (30). In the complete absence of Su(H), single minded is expressed at intermediate levels in a broad domain (30), presumably due to the activity of other DNA-bound regulators that are no longer repressed by Su(H)-Hairless. We hypothesized that vg might likewise be repressed by Hairless outside of the domain of highest Notch signal and that this might allow us to investigate the requirement for corepressor CtBP and/or Gro. If this repression does take place, lowering the amount of either component of the presumptive repressor complex should result in a derepression of vg in the neighboring cells.
To test this assumption, we used the Gal4 system to locally reduce gene activity by RNAi (11, 33) and monitored Su(H)-dependent regulation of vg by use of a vg BE -LacZ reporter line (24). The omb-Gal4 driver was used to express the RNAi constructs in a limited domain at a late stage of development, after expression of vg BE -LacZ has been established (Fig. 1C and D). This approach confirmed our prediction (Fig. 1D): knock down of either Su(H) or Hairless activity in the central domain of the wing resulted in an upregulation of vg on either side of the boundary. Our results differ from previous work that showed a reduction of _vg_-encoded protein in clones of cells homozygous for a hypomorphic allele of Su(H) (24). Two aspects of our experiments could account for our ability to detect derepression when Su(H) is knocked down. First, Su(H) activity is removed at late stages, after vg BE -LacZ has already been activated. Therefore, the enhancer is already in a primed state in which the composition of the Su(H) complexes is likely to be critical to whether or not the enhancer is repressed [e.g., other activators are bound; Su(H)-Hairless is the break that keeps the enhancer off unless NICD is present]. Second, the RNAi could be more effective than the mutant clones at eliminating Su(H). Su(H) mutants frequently retain significant Su(H) activity due to perdurance of the protein-mRNA (26). The residual low level of Su(H) may be sufficient to keep target genes repressed, because loss of repression was seen in the embryo only when Su(H) function was eliminated using a deletion allele (31).
Reducing Gro activity resulted in a similar broadening of the vg expression domain, suggesting that Gro is part of the repression complex. Interference with CtBP activity did not affect expression of the vg reporter construct at all. Under these conditions, therefore, it appears that Gro is an essential corepressor but that CtBP is not. Although the RNAi caused a conspicuous reduction of CtBP protein amounts and defects in the adult wing (data not shown), we cannot rule out the possibility that there was insufficient knockdown of CtBP to elicit an effect on vg expression. Nevertheless, these data suggest that there might be differential requirements for the two corepressors at different enhancers or contexts. We therefore set out to investigate the contribution of Gro and CtBP to Hairless-mediated repression in more detail.
Contribution of CtBP and Gro to Hairless-mediated repression of Notch target gene activation.
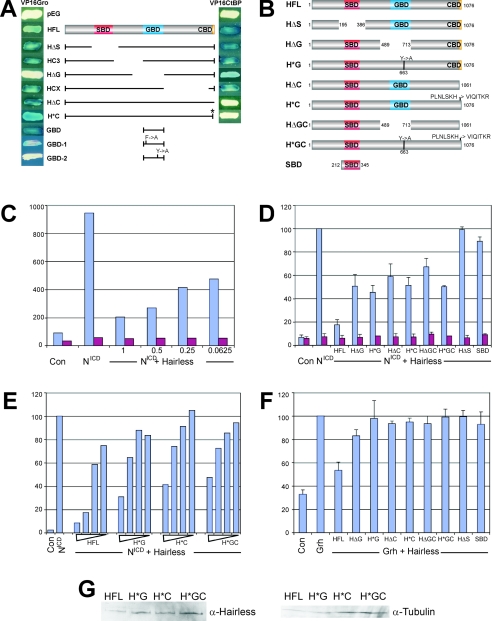
In order to dissect the roles of Gro and CtBP in mediating Hairless-dependent repression we generated mutant forms of Hairless that lacked the ability to bind either one or both of these cofactors. Previous experiments showed that a C-terminal motif is both necessary and sufficient for recruiting CtBP (4, 30). An isolated region encompassing one eh1-like motif (amino acids 616 and 723) is capable of binding to Gro but not when this motif is mutated (4). We first confirmed that deletion of the Gro- and CtBP-binding domains in the context of the full-length protein was sufficient to abrogate binding to the cognate corepressor by use of a yeast two-hybrid assay to measure interactions (Fig. 2A). This confirmed that a single domain is necessary for binding to Gro; deletion of this domain (ΔG; Fig. 2A) eliminates Gro binding. To avoid potential complications arising from deletion of the entire Gro-binding domain, we also tested the effects of mutating a single amino acid in the eh1-like motif and found that this was sufficient to eliminate binding to the isolated Gro-binding domain (GBD-2 Y→A; Fig. 2A). On the basis of this interaction analysis we constructed mutant forms of Hairless that lacked binding to one or both corepressors for expression in vivo (Fig. 2B). All of these mutated proteins were still able to interact with Su(H) (not shown). A mutant form of Hairless that lacked Su(H) binding (HΔS) was also constructed, along with a severely truncated polypeptide that contained only the Su(H) interaction domain [Su(H)-binding domain (SBD); Fig. 2B].
FIG. 2.
Both CtBP and Gro are necessary for Hairless-mediated repression in S2 cells. (A) Mapping Gro and CtBP interaction domains. Interactions of Hairless with CtBP or Gro were investigated by yeast two-hybrid assay: positive interactions appear blue on X-Gal indicator plates, negatives and control (pEG, empty vector) remain white. A central domain of about 150 amino acids was necessary (HΔG) and sufficient (GBD) for binding to Gro. This domain contains two eh1-related binding motifs (site 1, EFEKCSLED; site 2, SYSIHSLLG). Mutations within site 1 (F→A; GBD-1) retained Gro binding, whereas mutations within site 2 (Y→A, GBD-2) completely destroyed it. The CtBP interaction domain CBD was confined to a 15-amino-acid interval (HΔC). Conservative substitution within this motif (PLNLSKH to VIQITKR) resulted in a complete loss of CtBP binding (H*C). The Su(H) binding SBD domain is depicted in red. (B) Constructs for assay of residual Hairless activity in cell culture; remaining codons are numbered. (C to F) Effects of Hairless proteins on transcription of Notch reporters (NRE) in the presence of NICD (1 μg) (C to E) or Grh (1 μg) (F). S2 cells were transfected with the constructs indicated; control was empty vector (Con). The level of expression from the NRE (blue) (C to E) and NME [mutated Su(H) sites] (red) (C and D) was measured in comparison to cotransfected renilla plasmid; _y_-axis numbers indicate this relative luciferase activity from the NRE or NME reporters. Values for NRE in the presence of NICD or Grh clone were normalized to 100% (D to F). In panel C, differing amounts of each construct are indicated in micrograms. In panel E, the concentrations used were 1.5 μg, 0.5 μg, 0.125 μg, and 0.003 μg. (G) Hairless mutant proteins are expressed at similar levels. Cells transfected with 0.5 μg of the Hairless plasmids indicated (along with the other plasmids described for panel E) were analyzed using Western blots probed with anti-Hairless to detect the expression of the Hairless variants, and anti-tubulin, to detect overall protein levels.
The repressive contribution of the different corepressors was investigated using a cell culture assay with a Notch-dependent reporter that contains four Su(H) binding sites (NRE) (8). Expression of activated Notch (NICD) resulted in a 14-fold stimulation from the NRE, but this result was not seen when a reporter with mutated sites (NME) was used. Addition of Hairless repressed this stimulation by about 80% over a range of concentrations (Fig. 2C and E). No effect was seen with HΔS, confirming that the interaction with Su(H) is necessary for repression (Fig. 2D). Deletion and mutation of either the CtBP-binding domain (HΔC, H*C) or the Gro-binding domain (HΔG, H*G) had similar effects on repression. All four mutant forms of Hairless had severely compromised repressive activity but were still able to reduce the levels of activity in the presence of NICD by 50% (Fig. 2D). The relative effects of the different mutant forms were consistent over a wide range of concentrations (Fig. 2E and data not shown). In all cases the amount of repression increased when higher concentrations of protein were added, but the mutated constructs always showed compromised activity compared to the wild type. These data suggest, therefore, that CtBP and Gro are equally important for the repressive function of Hairless.
Since loss of one or the other corepressor domain led to a significant loss of repression we anticipated that mutating both domains together would have an additive effect and would eliminate all repressive activity of Hairless. Surprisingly, however, the double-mutant forms of the protein (HΔGC, H*GC) still retained repressive activity, reducing expression to 50 to 67% of the level seen with NICD alone, even though they could bind neither Gro nor CtBP (Fig. 2B, D, and E). Thus, lack of binding to both corepressors resulted in a reduction of Hairless activity similar to that seen in the absence of a single corepressor site, suggesting that the corepressors act in combination. The residual activity in the double mutant (H*GC) might be due to competition between H and NICD for binding to Su(H). If this were the case we would expect the effects to be sensitive to the amount of Hairless proteins added, which is what was observed (Fig. 2E). However, expression of the Hairless SBD alone was not sufficient to mediate repression, as might be expected for a simple competition model, which is consistent with data arguing that NICD and Hairless bind to nonoverlapping regions in Su(H) (9, 21, 35).
To test the repressive effects of Hairless in the absence of NICD, we investigated its ability to inhibit transcription in the presence of Grh. The NRE reporter also contains binding sites for the transcriptional activator Grh which stimulated transcription fourfold in the absence of NICD (Fig. 2F) and increased the stimulation seen in the presence of NICD (data not shown). Addition of full-length Hairless inhibited these effects, reducing transcription in the presence of Grh alone by 50%. Furthermore, this inhibitory effect was dependent on Su(H), as indicated by a lack of repression of HΔS, and required both CtBP and Gro, since Hairless proteins with either interaction domain mutated (HΔC, H*C, HΔG, H*G; Fig. 2B) had lost most of their repressive activity (Fig. 2F). Again, the levels of activity with the single mutants were similar to the levels seen with the double-mutant forms of the protein (HΔGC, H*GC) and all resulted in >90% of the expression seen with Grh (Fig. 2F). These experiments suggest that Hairless has two modes of repression, one that operates by repressing the transcriptional machinery through its recruitment of global corepressors and a second that operates by directly antagonizing NICD.
These data confirm therefore that both Gro and CtBP can function as corepressors with Hairless, and indeed both factors are necessary for full repression by Hairless on the NRE; preventing the interaction with one or the other factor severely compromises Hairless activity. This is in apparent contrast to the effects on vg BE -LacZ, for which only Gro appeared essential. Furthermore, the two cofactors appear to act together, since Hairless proteins lacking both interaction motifs retained a level of repression that was comparable to the results seen upon removing either alone.
Both Gro and CtBP recruitment motifs are required for repressive function of Hairless in vivo.
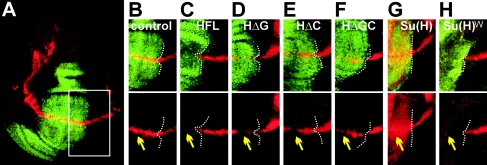
Overexpression of Hairless interferes with Notch signaling, as manifested by Notch loss-of-function phenotypes in many tissues with which it has been tested (for examples, see reference 27). In the wing, Hairless overexpression severely inhibits growth and results in wing margin defects, thickening of the veins, and aberrant bristles (Fig. 3B). This assay was used to monitor residual activity of Hairless mutant proteins in vivo. All three HΔ proteins exhibit nuclear localization in disks similar to that seen with the full-length Hairless construct (HFL) (see Fig. 4C to F) and are expressed at similar levels in the lines analyzed (data not shown). Overexpression of HΔC, HΔG, or H*G within the central domain of the wing by use of the omb-Gal4 driver caused phenotypes of wing margin loss and notching (Fig. 3C to E). These phenotypes are weaker than those seen with overexpression of HFL (Fig. 3B) and are consistent with the two mutant forms having reduced repressive function. HΔGC, which has completely lost its ability to interact with either corepressor, still caused thickened veins and notching, but the effects were weaker than those seen with the other constructs (Fig. 3F). Similar results were obtained when we monitored effects on the vg BE enhancer. vg BE -LacZ expression was strongly inhibited by overexpression of Hairless and was inhibited to a lesser extent when either or both of the interaction domains were missing (Fig. 4C to F).
FIG. 3.
Both Gro and CtBP binding motifs are required for Hairless-mediated repression in the wing. (A) In the wild-type (WT) wing, the five longitudinal veins (L1 to L5) are easily discernible. (B) Hairless overexpression (HFL) results in a small wing with thickened veins and clumps of bristles at the tip (see arrow and arrowhead in enlargement at right). (C to F) Effects of deletion constructs (HΔC, HΔG, H*G, HΔGC) are similar and less severe than that of Hairless; wings have some vein thickening (arrow) and disrupted margin (arrowhead). (G) Co-overexpression of HΔGC with Su(H) results in a dramatically enhanced phenotype with ectopic bristles and thickened wing veins and margin defects (arrow and arrowhead, respectively; see enlargement). (H) Su(H) overexpression. Wings lack veins (open arrow) and have balloon-like overgrowth; wings often cannot be inflated well after eclosion, as seen here. (I) Overexpression of Su(H)WRPW causes more-severe phenotypes than Hairless overexpression, with deep incisions (arrow). In all cases proteins were overexpressed using the omb-Gal4 driver. Wings are shown at same magnification; enlargements are shown in the right panels.
FIG. 4.
Requirement for Gro and CtBP for the repression of the Notch target gene vg. (A) Control disk expressing UAS-redStinger (green). The boxed area depicts a region shown at higher magnification in panels B and C to H. (C to H) Use of full-length Hairless (C) or Su(H)WRPW (H) results in complete repression of vg BE -LacZ (red), whereas HΔG (D), HΔC (E), and HΔGC (F) cause only partial repression (yellow arrows). The indicated proteins were overexpressed using the omb-Gal4 driver and detected by anti-Hairless or anti-Su(H) staining (green). The white dotted line indicates the right boundary of expression. (G) Overexpression of Su(H) induces vg BE -LacZ expression (yellow arrow) and causes increased growth of the wing disk.
We have shown before that combined overexpression of Su(H) and H leads to strong inhibition of Notch signaling, presumably by assembly of a repressor complex (17, 30). We therefore tested the effects of expressing the Hairless mutant proteins in combination with Su(H). Expression of full-length Hairless, HΔC, or HΔG with Su(H) resulted in larval or early pupal lethality, with severely distorted disks and extensive apoptosis. HΔGC and Su(H) combinations produced viable offspring, albeit with strongly enhanced phenotypes compared with that seen with HΔGC overexpression alone, that include supernumerary bristles along thickened veins (Fig. 3G). These phenotypes are qualitatively similar to those observed with full-length Hairless alone (Fig. 3B).
In other repressor complexes it appears that recruitment of either Gro or CtBP directly by the transcriptional regulator is sufficient to mediate repression. Why should Su(H) require a complex consisting of Hairless plus two further cofactors in some contexts? To investigate whether this is a characteristic of Su(H) we tested the effect of fusing a Gro binding motif (WRPW) to Su(H) directly. Su(H)WRPW proved itself to be a potent repressor in the wing, resulting in extreme wing nicking and inhibition of vg BE -LacZ expression (Fig. 3I and 4H). Thus, addition of a Gro recruitment domain to Su(H) converts it into a powerful repressor.
DISCUSSION
The Su(H) DNA-binding protein and its homologues in other species are pivotal in the transcriptional switch required for Notch pathway activation. In the absence of Notch, Su(H) recruits corepressors to the promoters of target genes, thus keeping them inactive until they are replaced by the activating complex containing Notch and Mastermind (reviewed in reference 41). Hairless is a key member of the Su(H) repressor complex, and previous Drosophila studies have shown that Hairless can interact with two different corepressors, Groucho and CtBP (4, 30). Here we have investigated the relative contributions of these two factors to Hairless-mediated repression. Our results indicate that Gro and CtBP act in combination within the Hairless complex, at least in certain contexts.
Both Gro and CtBP are well-characterized corepressors; they are directly recruited by transcriptional regulators and, in general, either protein alone is sufficient to mediate repression (reviewed in reference 13). In fact we have shown that conferring Gro-binding ability to Su(H) turns it in a potent repressor (Fig. 3 and 4). We had anticipated that this might also be the case for Hairless, with one or the other protein being sufficient to confer corepression function at specific enhancers. In cell culture, however, deletion of the Gro interaction domain or the CtBP interaction domain renders the protein as inactive as a deletion that removes both domains together. The results in flies are slightly different, with the double-domain deletion giving weaker phenotypes than deletion of either domain alone, although the defects are qualitatively similar. Together, the data suggest that in the absence of one corepressor the other functions poorly or not at all and, thus, that the two act together in many contexts.
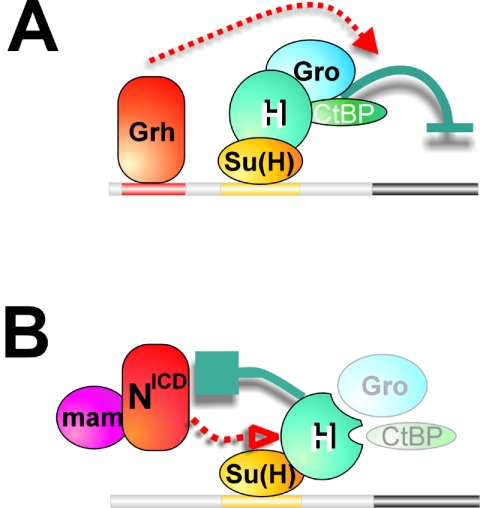
In addition we also discovered that Hairless has repressive activity in the absence of either Gro or CtBP binding. This activity is only observed in the presence of NICD, suggesting that Hairless has an additional mode of inhibition that involves direct antagonism of NICD. This accounts for approximately half of the repressive activity of Hairless in cell culture assays but does not appear to involve a simple competition for binding to Su(H), since the Su(H)-binding domain of Hairless, SBD, is insufficient for this activity. However, the full-length Hairless protein may contain an additional domain that can mask essential interfaces on Su(H) or on other essential factors (e.g., SKIP) (48) that are required for productive Su(H)-NICD complex formation. Thus, our data argue that Hairless displays two modes of inhibition, one via the combined function of both Gro and CtBP and another that targets NICD independently of either corepressor (Fig. 5).
FIG. 5.
Two modes of Hairless repression. (A) Active repression requires the combined activity of Gro and CtBP and is independent of Notch. In this example, the activator of transcription is Grh. (B) Competition with NICD: specific antagonism of NICD can occur independently of corepressors (as shown here) and is dose dependent on Hairless.
Two other transcriptional repressors, Hairy and Brinker, are reported to bind both Gro and CtBP. At certain targets Brinker only requires one or the other corepressor for its repression, whereas at others it requires both for full activity (13, 19, 46). Similarly, the basic helix-loop-helix repressor Hairy might require Gro or CtBP at different targets (5, 39, 47). Furthermore, it appears that CtBP attenuates the repressive action of Gro when bound to Hairy at the same time, so the two corepressors have been argued to act in different and mutually exclusive repression pathways. We found no evidence for such an antagonistic relationship in Hairless-mediated repression, but we note that the CtBP-binding domain in Hairless is a “suboptimal” interface like that in Hairy (47), so it remains possible that there is a more complex interplay between the two corepressors depending on the target enhancer.
Although our cell culture and in vivo assays reveal that Hairless differs from Brinker and Hairy in needing the combined actions of Gro and CtBP for repression, there may be circumstances in which one or the other is sufficient. For example, knockdown experiments using RNAi in the wing showed a requirement for Gro but not for CtBP in vg BE -LacZ repression, although it is possible that CtBP was not sufficiently compromised by our RNAi treatments. In addition, overexpression of Hairless proteins that retain the ability to bind one or the other corepressor produced more-severe phenotypes than HΔGC. Furthermore, in previous studies, phenotypes resulting from a mutation that removes the CtBP-binding domain from Hairless (H 22) were found to be weaker than the phenotypes seen with Hairless null mutations and could still be enhanced even by decreased amounts of Gro, arguing that H22 protein retained some Gro-dependent repressive activity (4). However, this observation is complicated by the fact that Gro functions with other DNA-binding proteins in the process of bristle development that was examined in the assay.
Previous studies of CtBP and Gro have argued that they mediate repression in qualitatively different ways, although both are thought to recruit histone deacetylases (12, 13, 42, 44, 47). Gro has predominantly been associated with so-called long-range repression, as it operates to dominantly silence modular enhancers. In contrast, CtBP appears to act in a local way to inhibit activators that are bound nearby (13, 36). However, these models do not appear compatible with a combined requirement for Gro and CtBP in Hairless-mediated repression. Furthermore, direct fusion of a Gro interaction domain to the Su(H) protein was sufficient to convert it into a potent repressor, as described for other transcriptional regulators (22). Why should Gro and CtBP therefore be interdependent in the context of Hairless recruitment? One simple explanation would be that one or the other corepressor is needed to specifically counteract NICD activation. For example, CtBP interferes with recruitment of p300 (25), a histone acetyltransferase that is reported to interact with mammalian NICD (37, 45). However, our data suggest that CtBP and Gro are both needed to repress Grh even in the absence of NICD, arguing that each corepressor can only perform a subset of its functions in the context of Hairless. Maybe the two corepressors recruit different enzymatic activities that are needed together to promote repression. If the Hairless complex were incompatible with oligomerization of Gro, which is reported to be important for stable repression (43), Gro might be able to recruit histone deacetylases but not to promote spreading of the repression complex. And if CtBP, which in mammals has been found complexed with methyl transferases as well as deacetylases (42), could recruit only histone methyl transferases, the corepressors would each confer a critical component on the Hairless complex. A more complete understanding of the molecular functions of Gro and CtBP in the context of chromatin dynamics and transcription complexes will be needed to determine why Hairless requires their coordinate activities in many developmental scenarios, as we have shown here.
Acknowledgments
We are indebted to members of the laboratories, especially I. Wech, I. Beck, C. Seitz, W. Staiber, and T. Stöβer, for invaluable technical support. We thank C. Delidakis, S. Parkhurst, S. Schreiber, and F. Schweisguth for constructs, antibodies, fly lines, and helpful discussions.
This work was supported by grants from the German Science Foundation to A.P. and D.M. (SFB 495), from the Medical Research Council to S.B. (G0200457) and from CONACYT to A.B.-P. A.K. is supported by an EMBO long-term Fellowship.
REFERENCES
- 1.Alifragis, P., G. Poortinga, S. M. Parkhurst, and C. Delidakis. 1997. A network of interacting transcriptional regulators involved in Drosophila neural fate specification revealed by the yeast two-hybrid system. Proc. Natl. Acad. Sci. USA 94**:**13099-13104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Artavanis-Tsakonas, S., M. D. Rand, and R. J. Lake. 1999. Notch signaling: cell fate control and signal integration in development. Science 284**:**770-776. [DOI] [PubMed] [Google Scholar]
- 3.Barolo, S., and J. W. Posakony. 2002. Three habits of highly effective signaling pathways: principles of transcriptional control by developmental cell signaling. Genes Dev. 16**:**1167-1181. [DOI] [PubMed] [Google Scholar]
- 4.Barolo, S., T. Stone, A. G. Bang, and J. W. Posakony. 2002. Default repression and Notch signaling: Hairless acts as an adaptor to recruit the corepressors Groucho and dCtBP to Suppressor of Hairless. Genes Dev. 16**:**1964-1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bianchi-Frias, D., A. Orian, J. J. Delrow, J. Vazquez, A. E. Rosales-Nieves, and S. M. Parkhurst. 13. July 2004, posting date. Hairy transcriptional repression targets and cofactor recruitment in Drosophila. PLoS Biol. 2**:**E178. (Online.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brand, A. H., and N. Perrimon. 1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118**:**401-415. [DOI] [PubMed] [Google Scholar]
- 7.Bray, S., and M. Furriols. 2001. Notch pathway: making sense of Suppressor of Hairless. Curr. Biol. 11**:**R217-R221. [DOI] [PubMed] [Google Scholar]
- 8.Bray, S., H. Musisi, and M. Bienz. 2005. Bre1 is required for Notch signaling and histone modification. Dev. Cell 8**:**279-286. [DOI] [PubMed] [Google Scholar]
- 9.Brou, C., F. Logeat, M. Lecourtois, J. Vandekerckhove, P. Kourilsky, F. Schweisguth, and A. Israel. 1994. Inhibition of the DNA-binding activity of Drosophila Suppressor of Hairless and of its human homolog, KBF2/RBP-J. kappa, by direct protein-protein interaction with Drosophila Hairless. Genes Dev. 8**:**2491-2503. [DOI] [PubMed] [Google Scholar]
- 10.Bunch, T. A., Y. Grinblat, and L. S. Goldstein. 1988. Characterization and use of the Drosophila metallothionein promoter in cultured Drosophila melanogaster cells. Nucleic Acids Res. 16**:**1043-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carthew, R. W. 2001. Gene silencing by double-stranded RNA. Curr. Opin. Cell Biol. 13**:**244-248. [DOI] [PubMed] [Google Scholar]
- 12.Chen, G., J. Fernandez, S. Mische, and A. J. Courey. 1999. A functional interaction between the histone deacetylase Rpd3 and the corepressor groucho in Drosophila development. Genes Dev. 13**:**2218-2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Courey, A. J., and S. Jia. 2001. Transcriptional repression: the long and the short of it. Genes Dev. 15**:**2786-2796. [DOI] [PubMed] [Google Scholar]
- 14.Delidakis, C., A. Preiss, D. A. Hartley, and S. Artavanis-Tsakonas. 1991. Two genetically and molecularly distinct functions involved in early neurogenesis reside within the Enhancer of split locus of Drosophila melanogaster. Genetics 129**:**803-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fehon, R. G., P. J. Kooh, I. Rebay, C. L. Regan, T. Xu, M. A. Muskavitch, and S. Artavanis-Tsakonas. 1990. Molecular interactions between the protein products of the neurogenic loci Notch and Delta, two EGF-homologous genes in Drosophila. Cell 61**:**523-534. [DOI] [PubMed] [Google Scholar]
- 16.Fisher, A. L., S. Ohsako, and M. Caudy. 1996. The WRPW motif of the hairy-related basic helix-loop-helix repressor proteins acts as a 4-amino-acid transcription repression and protein-protein interaction domain. Mol. Cell. Biol. 16**:**2670-2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Furriols, M., and S. Bray. 2000. Dissecting the mechanisms of Suppressor of Hairless function. Dev. Biol. 227**:**520-532. [DOI] [PubMed] [Google Scholar]
- 18.Gho, M., M. Lecourtois, G. Geraud, J. W. Posakony, and F. Schweisguth. 1996. Subcellular localization of Suppressor of Hairless in Drosophila sense organ cells during Notch signalling. Development 122**:**1673-1682. [DOI] [PubMed] [Google Scholar]
- 19.Hasson, P., B. Muller, K. Basler, and Z. Paroush. 2001. Brinker requires two corepressors for maximal and versatile repression in Dpp signalling. EMBO J. 20**:**5725-5736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hemavathy, K., X. Hu, S. I. Ashraf, S. J. Small, and Y. T. Ip. 2004. The repressor function of snail is required for Drosophila gastrulation and is not replaceable by Escargot or Worniu. Dev. Biol. 269**:**411-420. [DOI] [PubMed] [Google Scholar]
- 21.Hsieh, J. J., T. Henkel, P. Salmon, E. Robey, M. G. Peterson, and S. D. Hayward. 1996. Truncated mammalian Notch1 activates CBF1/RBPJk-repressed genes by a mechanism resembling that of Epstein-Barr virus EBNA2. Mol. Cell. Biol. 16**:**952-959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inbal, A., N. Halachmi, C. Dibner, D. Frank, and A. Salzberg. 2001. Genetic evidence for the transcriptional-activating function of Homothorax during adult fly development. Development 128**:**3405-3413. [DOI] [PubMed] [Google Scholar]
- 23.Jennings, B., A. Preiss, C. Delidakis, and S. Bray. 1994. The Notch signalling pathway is required for Enhancer of split bHLH protein expression during neurogenesis in the Drosophila embryo. Development 120**:**3537-3548. [DOI] [PubMed] [Google Scholar]
- 24.Kim, J., A. Sebring, J. J. Esch, M. E. Kraus, K. Vorwerk, J. Magee, and S. B. Carroll. 1996. Integration of positional signals and regulation of wing formation and identity by Drosophila vestigial gene. Nature 382**:**133-138. [DOI] [PubMed] [Google Scholar]
- 25.Kim, J. H., E. J. Cho, S. T. Kim, and H. D. Youn. 2005. CtBP represses p300-mediated transcriptional activation by direct association with its bromodomain. Nat. Struct. Mol. Biol. 12**:**423-428. [DOI] [PubMed] [Google Scholar]
- 26.Koelzer, S., and T. Klein. 2003. A Notch-independent function of Suppressor of Hairless during the development of the bristle sensory organ precursor cell of Drosophila. Development 130**:**1973-1988. [DOI] [PubMed] [Google Scholar]
- 27.Maier, D., J. Marquart, A. Thompson-Fontaine, I. Beck, E. Wurmbach, and A. Preiss. 1997. In vivo structure-function analysis of Drosophila Hairless. Mech. Dev. 67**:**97-106. [DOI] [PubMed] [Google Scholar]
- 28.Maier, D., A. C. Nagel, B. Johannes, and A. Preiss. 1999. Subcellular localization of Hairless protein shows a major focus of activity within the nucleus. Mech. Dev. 89**:**195-199. [DOI] [PubMed] [Google Scholar]
- 29.Maier, D., A. C. Nagel, and A. Preiss. 2002. Two isoforms of the Notch antagonist Hairless are produced by differential translation initiation. Proc. Natl. Acad. Sci. USA 99**:**15480-15485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morel, V., M. Lecourtois, O. Massiani, D. Maier, A. Preiss, and F. Schweisguth. 2001. Transcriptional repression by Suppressor of Hairless involves the binding of a Hairless-dCtBP complex in Drosophila. Curr. Biol. 11**:**789-792. [DOI] [PubMed] [Google Scholar]
- 31.Morel, V., and F. Schweisguth. 2000. Repression by Suppressor of Hairless and activation by Notch are required to define a single row of single-minded expressing cells in the Drosophila embryo. Genes Dev. 14**:**377-388. [PMC free article] [PubMed] [Google Scholar]
- 32.Mumm, J. S., and R. Kopan. 2000. Notch signaling: from the outside in. Dev. Biol. 228**:**151-165. [DOI] [PubMed] [Google Scholar]
- 33.Nagel, A. C., D. Maier, and A. Preiss. 2002. Green fluorescent protein as a convenient and versatile marker for studies on functional genomics in Drosophila. Dev. Genes Evol. 212**:**93-98. [DOI] [PubMed] [Google Scholar]
- 34.Nagel, A. C., I. Wech, and A. Preiss. 2001. Scalloped and strawberry notch are target genes of Notch signaling in the context of wing margin formation in Drosophila. Mech. Dev. 109**:**241-251. [DOI] [PubMed] [Google Scholar]
- 35.Nam, Y., A. P. Weng, J. C. Aster, and S. C. Blacklow. 2003. Structural requirements for assembly of the CSL.intracellular Notch1.Mastermind-like 1 transcriptional activation complex. J. Biol. Chem. 278**:**21232-21239. [DOI] [PubMed] [Google Scholar]
- 36.Nibu, Y., H. Zhang, and M. Levine. 2001. Local action of long-range repressors in the Drosophila embryo. EMBO J. 20**:**2246-2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oswald, F., B. Tauber, T. Dobner, S. Bourteele, U. Kostezka, G. Adler, S. Liptay, and R. M. Schmid. 2001. p300 acts as a transcriptional coactivator for mammalian Notch-1. Mol. Cell. Biol. 21**:**7761-7774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paroush, Z., R. L. Finley, Jr., T. Kidd, S. M. Wainwright, P. W. Ingham, R. Brent, and D. Ish-Horowicz. 1994. Groucho is required for Drosophila neurogenesis, segmentation, and sex determination and interacts directly with hairy-related bHLH proteins. Cell 79**:**805-815. [DOI] [PubMed] [Google Scholar]
- 39.Phippen, T. M., A. L. Sweigart, M. Moniwa, A. Krumm, J. R. Davie, and S. M. Parkhurst. 2000. Drosophila C-terminal binding protein functions as a context-dependent transcriptional co-factor and interferes with both mad and groucho transcriptional repression. J. Biol. Chem. 275**:**37628-37637. [DOI] [PubMed] [Google Scholar]
- 40.Poortinga, G., M. Watanabe, and S. M. Parkhurst. 1998. Drosophila CtBP: a Hairy-interacting protein required for embryonic segmentation and hairy-mediated transcriptional repression. EMBO J. 17**:**2067-2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schweisguth, F. 2004. Notch signaling activity. Curr. Biol. 14**:**R129-R138. [PubMed] [Google Scholar]
- 42.Shi, Y., J. Sawada, G. Sui, el B. Affar, J. R. Whetstine, F. Lan, H. Ogawa, M. P. Luke, and Y. Nakatani. 2003. Coordinated histone modifications mediated by a CtBP co-repressor complex. Nature 422**:**735-738. [DOI] [PubMed] [Google Scholar]
- 43.Song, H., P. Hasson, Z. Paroush, and A. J. Courey. 2004. Groucho oligomerization is required for repression in vivo. Mol. Cell. Biol. 24**:**4341-4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Subramanian, T., and G. Chinnadurai. 2003. Association of class I histone deacetylases with transcriptional corepressor CtBP. FEBS Lett. 540**:**255-258. [DOI] [PubMed] [Google Scholar]
- 45.Wallberg, A. E., K. Pedersen, U. Lendahl, and R. G. Roeder. 2002. p300 and PCAF act cooperatively to mediate transcriptional activation from chromatin templates by Notch intracellular domains in vitro. Mol. Cell. Biol. 22**:**7812-7819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Winter, S. E., and G. Campbell. 2004. Repression of Dpp targets in the Drosophila wing by Brinker. Development 131**:**6071-6081. [DOI] [PubMed] [Google Scholar]
- 47.Zhang, H., and M. Levine. 1999. Groucho and dCtBP mediate separate pathways of transcriptional repression in the Drosophila embryo. Proc. Natl. Acad. Sci. USA 96**:**535-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou, S., M. Fujimuro, J. J.-D. Hsieh, L. Chen, A. Miyamoto, G. Weinmaster, and S. D. Hayward. 2000. SKIP, a CBF1-associated protein, interacts with the ankyrin repeat domain of NotchIC to facilitate NotchIC function. Mol. Cell. Biol. 20**:**2400-2410. [DOI] [PMC free article] [PubMed] [Google Scholar]