A complex of the IL-1 homologue IL-1F7b and IL-18-binding protein reduces IL-18 activity (original) (raw)
Abstract
IL-1F7 was discovered in expressed sequence tag databases as a member of the increasing family of proteins sharing sequence homology to IL-1α/β, IL-1Ra, and IL-18. In the present study using immunohistochemical staining, IL-1F7 was localized in human peripheral monocytic cells, suggesting its role in immune regulation. Recombinant human IL-1F7b was shown to bind to the IL-18Rα but without IL-18 agonistic or antagonistic function. Using chemical cross-linking, we observed that, unlike IL-18, IL-1F7b fails to recruit the IL-18Rβ chain to form a functionally active, ternary complex with the IL-18Rα chain. IL-1F7b shares two conserved amino acids with IL-18 (Glu-35 and Lys-124), which participate in the interaction of IL-18 with the IL-18Rα chain as well as the IL-18-binding protein (IL-18BP), a secreted protein that neutralizes IL-18 activity. In testing whether IL-1F7b interacts with IL-18BP, we unexpectedly observed that IL-1F7b enhanced the ability of IL-18BP to inhibit IL-18-induced IFNγ by 25–30% in a human natural killer cell line. This effect was observed primarily at limiting concentrations of IL-18BP (3.12–12.5 ng/ml) and at a 50- to 100-fold molar excess of IL-1F7b. Similar results were obtained by using isolated human peripheral blood mononuclear cells. To study the molecular basis of this effect we performed binding studies of IL-1F7b and IL-18BP. After cross-linking, a high molecular weight complex consisting of IL-1F7b and IL-18BP was observed on SDS/PAGE. We propose that after binding to IL-18BP, IL-1F7b forms a complex with IL-18Rβ, depriving the β-chain of forming a functional receptor complex with IL-18Rα and thus inhibiting IL-18 activity.
Cytokines of the IL-1 family, including IL-18, possess a variety of inflammatory and immunoregulatory properties during first-line and secondary responses to infection (1, 2). Six members of the IL-1 gene family have been discovered from expressed sequence tag database searches (3–10). These proteins share a common β-barrel pattern consisting of 12 β-strands and significant amino acid homology with the IL-1 receptor antagonist (IL-1Ra), IL-1β, and IL-18. The new members of the IL-1 family are derived from a common ancestor, as are IL-1 and IL-18 (11, 12). Except for IL-18, each maps to the same region on human chromosome 2 (4, 11–13). On the basis of their structure these IL-1 family members potentially can act as agonistic or antagonistic ligands for members of the IL-1 receptor family; however, their biological function is presently unknown.
The IL-1 homologue IL-1F7 has five different splice variants (IL-1F7a–e) (4, 6, 9, 10, 12). The first isoform described, IL-1F7a, has a unique N terminus consisting of exon 3 of the IL-1F7 gene which is not present in the other splice variants of the gene. The short isoforms IL-1F7c, IL-1F7d, and IL-1F7e lack exon 4, 2, or both, respectively. Only IL-1F7b and -c containing exons 1 and 2 express an N-terminal prodomain that includes a potential caspase-1 cleavage site (14). In addition to these splice variants, amino acid polymorphisms (V31G and A42T) exist in IL-1F7b based on two base pair mutations in exon 2 (6, 9). Despite extensive database searches and sequencing of the _IL-1_-gene locus, no murine homologue of IL-1F7 has yet been found.
IL-1F7b shares significant sequence homology with IL-18. The hallmark for IL-18 activity is its ability to induce IFNγ in T cells or natural killer (NK) cells in the presence of IL-2, IL-12, or IL-15 as costimulants. The activity of IL-18 is mediated by the IL-18 receptor (IL-18R) complex consisting of the ligand-binding chain termed IL-18Rα (15) and a signaling chain termed IL-18Rβ (16, 17). On binding to the IL-18Rα chain and formation of the heterodimeric complex with the IL-18Rβ chain, IL-18 induces activation of IL-1 receptor-associated kinase and tumor necrosis factor (TNF) receptor-associated factor 6 (TRAF-6). These activated kinases eventually result in the translocation of nuclear factor κB (NF-κB) (18, 19). IL-1F7b has been reported to bind to the IL-18Rα by using a receptor pulldown assay (9) or surface plasmon resonance by using BiaCore techniques (14). A significant, but low-affinity binding of _K_d = 130 nM was observed primarily for the mature form of IL-1F7b without the propeptide, suggesting biological relevance to IL-1F7b processing by caspase-1 (14). Despite the binding to the IL-18Rα, no IL-18-like or antagonistic activity of either pro- or mature IL-1F7b was demonstrated (9, 14).
IL-18-binding protein (IL-18BP) is a naturally occurring, constitutively secreted inhibitor of IL-18. IL-18 binds to IL-18BP with a high affinity (_K_d = 400 pM) and neutralizes its activity (20, 21). In a previous report, we demonstrated that two charged amino acids in the sequence of IL-18 (Glu-42 and Lys-89) are crucial for the interaction of IL-18 both with the IL-18BP and with the IL-18Rα (22, 23).
In the present study we report that unlike IL-18, IL-1F7b fails to recruit the IL-18Rβ to form a functionally active, ternary complex with the IL-18Rα chain. Sequence alignment revealed that Glu-42 and Lys-89, which are critical for the interaction of IL-18 with IL-18Rα and IL-18BP, are conserved in IL-1F7b. Therefore, we tested whether IL-1F7b affects the ability of IL-18BP to neutralize IL-18 activity and studied binding of IL-1F7b to the IL-18BP by using chemical cross-linking.
Materials and Methods
Reagents and Cytokines.
All reagents were purchased from Sigma unless otherwise indicated. IL-2 was purchased from R&D Systems. Recombinant human IL-12, IL-15, and TNF-α were gifts provided by PeproTech (Rocky Hill, NJ). Recombinant IL-18 and IL-18BP were provided by Serono Pharmaceutical Research Institute (SPRI, Geneva, Switzerland). Recombinant IL-18Rα:ECD without six histidines (His6 tag) was a gift from R&D Systems. The third extracellular domain (ECD) of IL-18Rα (IL-18Rα:D3) used for cross-linking was expressed with an N-terminal His6 tag in Escherichia coli, and IL-18Rβ:ECD with a C-terminal His6 tag was expressed in COS7 cells as described (T.A., D. Novick, P.B., L.L.R., D. Y. Yoon, M. Rubinstein, C.A.D., and S.-H.K., unpublished work). The mAb against human IL-18BP (mAb 582) was a gift from D. Novick (Weizmann Institute of Science, Rehovot, Israel). The mAb against human IL-18Rα was purchased from R&D Systems. All restriction enzymes and Taq DNA polymerase were purchased from Invitrogen.
Cells and Cell Culture.
All cells and the human NK cell line used in this study were cultured as described (20). The KG-1 cell line was obtained from the American Type Culture Collection. For bioassays, NK or KG-1 cells were suspended at 0.5 × 106 cells per ml in culture medium (0.2 ml) in 96-flat-well plates in the presence of 0.5 ng/ml IL-12 or 10 ng/ml TNF-α, respectively. Different concentrations of IL-18, IL-18BP, and IL-1F7b were then added, and after 16–20 h at 37°C in humidified air with 5% CO2, the culture supernatants were collected for cytokine measurements.
Isolation of Peripheral Blood Mononuclear Cells (PBMC).
These studies were approved by the Combined Colorado Investigational Review Board, and all subjects gave informed consent. PBMC were purified either from platelet-depleted residual leukocytes or from heparinized blood of healthy donors (24). The isolated PBMC were kept on ice until the assay was started.
Protein Expression and Purification.
The following oligonucleotide primers were used to clone the IL-1F7b cDNA from a human spleen library (CLONTECH HL0011B, BD Biosciences CLONTECH): sense primer 5′-GTTGAGTAATAAACTCAACG, reverse primer 5′-GTTCAATGGGGCAGTTTC [specific for clone AF200496 (GenBank) (6)]. The IL-1F7b cDNA was reamplified by using a second pair of primers introducing cleavage sites for _Eco_RI at the 5′ and _Xba_I at the 3′ end (sense primer 5′-ATATGAATTCATGTCCTTTGTGGGGGAG; reverse primer 5′-TATATCTAGAAGTTTCCTAATCGCTGACC). By using TA-cloning the IL-1F7b cDNA was transferred into pGEM-T Easy (Promega) and the correct sequence was verified. The IL-1F7b cDNA was then ligated into pPROEX HTa (Invitrogen) for bacterial expression by using the _Eco_RI and _Xba_I site. The pPROEX HTa vector contains an N-terminal His6 tag for affinity purification of the expressed protein. His6-tagged IL-1F7b protein was expressed by following a recently described protocol (23) and recovered from inclusion bodies by treatment with 8 M urea. After affinity chromatography with Talon affinity resin (CLONTECH), purified IL-1F7b was used to immunize rabbits.
Alternatively, IL-1F7b cDNA was cloned into pMAL-p2X vector (New England Biolabs) by using _Eco_RI and _Xba_I cloning sites and expressed as fusion protein with maltose-binding protein (MBP). IL-1F7b/MBP fusion protein was expressed in competent E. coli as described above, recovered as soluble protein after sonication, and purified by affinity chromatography with amylose-coupled resin (New England Biolabs). The IL-1F7b/MBP fusion protein (total, 3 mg) was coupled to 1.5 ml of activated Sepharose (Affi-Gel Hz Immunoaffinity Kit, Bio-Rad) and used for affinity purification of IL-1F7b-specific IgG from rabbit serum.
Full-length (pro) and mature IL-1F7b (N terminus E21) used in bioassays and for cross-linking studies were produced in E. coli as described (14).
Immunization of Rabbits and Purification of IL-1F7b-Specific IgG.
IL-1F7b produced in E. coli by using pPROEX HTa expression plasmid was separated on a preparative SDS/polyacrylamide gel. The gel was stained with Coomassie blue (Bio-Rad), and the band containing IL-1F7b was excised. The IL-1F7b-containing gel was used to generate polyclonal sera in rabbits according to standard protocols (Rockland, Gilbertsville, PA).
Total IgG from rabbit IL-1F7b antiserum was precipitated by using ammonium sulfate. The IgG precipitate was dissolved in PBS and extensively dialyzed against PBS. The dialyzed IgG preparation was applied to IL-1F7b/MBP-coupled Affi-Gel. Bound IL-1F7b-specific IgG was eluted with sodium citrate buffer (50 mM citric acid/100 mM NaCl, pH 2.5), dialyzed against PBS, and concentrated by using a Centricon centrifugal filter device (Millipore).
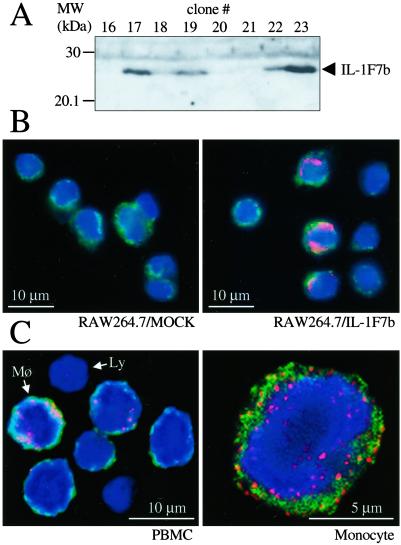
Specificity of the rabbit anti-IL-1F7b serum and affinity-purified IgG was tested by using RAW264.7 murine macrophage cells stable-transfected with full-length IL-1F7b in pTarget expression plasmid (Promega). Individual clones after stable transfection were lysed (5 × 106 cells) in PBS/1% Triton X-100. After centrifugation the lysate was separated by SDS/PAGE and blotted on nitrocellulose (Hybond ECL, Amersham Pharmacia). The blot was stained with rabbit IL-1F7b antiserum at a dilution of 1:500. For detection a peroxidase-labeled donkey anti-rabbit antibody was used (Jackson ImmunoResearch) and the Western blot was developed with enhanced chemiluminescence (NEN Life Science) (Fig. 7A). Alternatively, IL-1F7b-transfected RAW264.7 cells (clone 23) or Mock control was stained by using IL-1F7b affinity-purified rabbit IgG (Fig. 7B).
Figure 7.
Expression of IL-1F7b in transfected RAW264.7 cells and human PBMC. (A) After stable transfection lysates of individual clones (5 × 106 cells) were separated by SDS/PAGE and tested for IL-1F7b expression by using Western blot analysis. The rabbit anti-IL-1F7b serum (1:500 dilution) specifically stained IL-1F7b-positive clones. (B) Stable transfectants of RAW264.7 cells (Mock or IL-1F7b clone 23) were stained with affinity-purified rabbit anti- IL-1F7b IgG and visualized with confocal digital microscopy. (C) Freshly isolated human PBMC were stained against IL-1F7b by using affinity-purified polyclonal rabbit anti-IL-1F7b-IgG. Mø, monocyte; Ly, lymphocyte. Red dye, anti-IL-1F7b; green dye, membranes; blue dye, nuclear stain.
Cross-Linking Experiments.
Each purified protein (1.5 μg) was diluted in a final volume of 30 μl of PBS and incubated for 2 h on ice. Then bis(sulfosuccinimidyl) suberate (BS3; Pierce) was added to a final concentration of 1 mM and the mixture was incubated for 1 h at room temperature. The reaction was quenched by the addition of Tris⋅Cl, pH 7.4 (20 mM final concentration). After boiling for 5 min, the proteins were separated by using an SDS/10% polyacrylamide gel under reducing conditions (50 mM DTT) and blotted on nitrocellulose. The cross-linked proteins were detected by using indicated antibodies. For cross-linking studies of IL-1F7b and IL-18 to IL-18BP and subsequent detection by using antisera against IL-1F7b or IL-18, 10 μg of each protein was used in a total volume of 200 μl. The complexes were precipitated by using a mAb against IL-18BP at 10 μg/ml and protein G Sepharose (Amersham Pharmacia). After washing with cold PBS/0.05% Tween 20 the immunocomplexes were recovered from protein G beads by boiling in sample buffer and separated by reducing SDS/PAGE.
Measurement of Cytokines.
The liquid-phase electrochemiluminescence (ECL) method was used to measure IFNγ in cell culture supernatants and whole blood (25). The amount of ECL was determined by using an Origen Analyzer (Igen, Gaithersburg, MD). The limit of detection for IFNγ was 62 pg/ml.
Immunohistochemistry and Confocal Microscopy.
Freshly isolated human PBMC or RAW264.6 transfectants were washed in PBS and resuspended in 4% paraformaldehyde in PBS. After fixation for 15 min at room temperature the cells were spread on charged glass slides (Superfrost Plus, Fisher Scientific). Staining was performed by using affinity-purified rabbit-anti IL-1F7b IgG at 5 μg/ml in PBS containing 1% BSA or 5 μg/ml nonimmune rabbit IgG as negative control. A goat anti-rabbit antibody conjugated to Cy3 (Jackson ImmunoResearch) was used for detection. Nuclei were stained blue with 1 μg/100 ml bisbenzimide (Sigma). Glycoproteins were stained with Alexa488 conjugate WGA (Molecular Probes). Digital confocal imaging was performed by using a Leica DM RXA microscope equipped with SLIDEBOOK Software for Macintosh (Intelligent Imaging Innovations, Denver).
Statistical Analysis.
Data are expressed as the mean ± SEM. Differences between treated and nontreated groups were compared by using a paired Student's t test. Statistical significance was accepted within 95% confidence limits. Statistical analyses were performed with the statistical package STATVIEW 512+ (BrainPower, Calabasas, CA).
Results
IL-1F7b Lacks IL-18-Like Agonistic Activity.
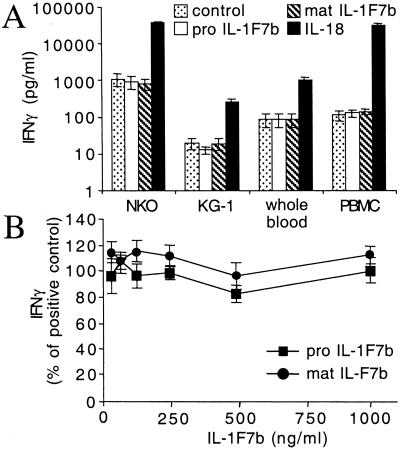
IL-1F7b has been shown to bind to the IL-18Rα chain (9, 14) suggesting that it might possess IL-18-like bioactivity. Therefore, we first evaluated whether IL-1F7b stimulates IFNγ production by using two different IL-18-sensitive human assays, human whole blood and PBMC. IL-1F7b was used as the full-length molecule (pro IL-1F7b) or expressed as mature molecule (mature IL-1F7b) with E21 as N terminus at the predicted caspase-1-cleavage site. As expected, IL-18 markedly stimulated IFNγ production (Fig. 1A). Neither pro nor mature IL-1F7b stimulated IFNγ production, suggesting that binding of IL-1F7b to the IL-18Rα chain does not progress to recruit the IL-18Rβ chain and form a functionally active ternary complex (Fig. 1A). The lack of an as-yet-unknown additional receptor chain necessary for IL-1F7b activity seemed unlikely, because consistent negative results were obtained for both primary human cells (whole blood, PBMC) and the cell lines NK and KG-1.
Figure 1.
IL-1F7b neither stimulates nor inhibits IFNγ production induced by IL-18. (A) Human NKO cells, cultures of whole human blood, PBMC [costimulated with IL-12 (1 ng/ml)], and KG-1 cells [costimulated with TNFα (10 ng/ml)] were treated with 100 ng/ml recombinant IL-1F7b (pro or mature form) or IL-18. After 18 h (48 h for KG-1) IFNγ was measured in the supernatant. (B) Induction of NK cells by IL-18 (20 ng/ml) in the presence of IL-12 (1 ng/ml) and increasing concentrations of pro or mature IL-1F7b. The data represent mean ± SEM of three independent experiments.
Additional experiments were performed to test whether IL-1F7b functions as a classic receptor antagonist by occupying IL-18-binding sites of the IL-18Rα chain and thus inhibiting its biological activity. When the human NK cell line was used, no inhibition of IL-18-induced IFNγ by pro or mature IL-1F7b occurred at concentrations of up to 40-fold molar excess of IL-1F7b over IL-18 (Fig. 1B). Low-affinity binding of IL-1F7b to the IL-18Rα might favor IL-18 binding, but even prolonged preincubation (maximal 6 h) of IL-1F7b with the cells before the addition of IL-18 did not affect IFNγ production. Similar results were obtained for human PBMC (data not shown).
IL-1F7b Does Not Modulate IL-18-Independent IFNγ Production.
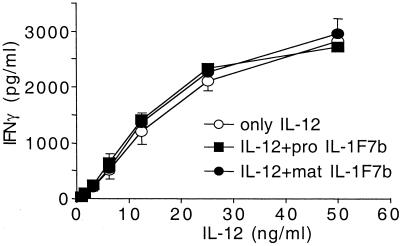
IL-1F7b was then tested for whether it alters IL-18-independent IFNγ production induced by a high concentration of IL-12. Both pro and mature IL-1F7b at a constant concentration of 250 ng/ml did not modulate the IL-12-induced IFNγ production in NK cells (Fig. 2). Taken together these results demonstrate that IL-1F7b does not stimulate or inhibit IFNγ secretion.
Figure 2.
IL-1F7b does not alter IL-12-induced IFNγ production. NKO cells were induced by IL-12 with or without IL-1F7b at a constant concentration of 250 ng/ml. After 18 h IFNγ was measured in the supernatant. Results are shown as mean ± SEM of three independent experiments.
IL-1F7b Binds to the Third ECD of the IL-18Rα but Fails to Recruit the IL-18Rβ to Form a Ternary Receptor Complex.
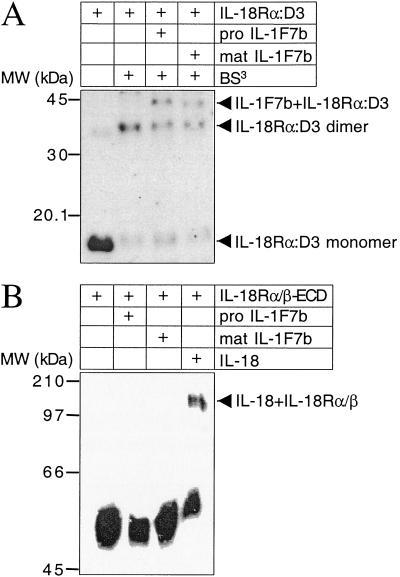
IL-18 binds to the IL-18Rα by the third ECD (IL-18Rα:D3) (T.A., D. Novick, P.B., L.L.R., D. Y. Yoon, M. Rubinstein, C.A.D., and S.-H.K., unpublished work). To characterize IL-1F7b binding to the IL-18Rα, the third ECD (D3) of the IL-18Rα was separately expressed in E. coli as His6-tagged protein and purified by Talon affinity chromatography (T.A., D. Novick, P.B., L.L.R., D. Y. Yoon, M. Rubinstein, C.A.D., and S.-H.K., unpublished work). Then, IL-1F7b was chemically cross-linked to the isolated IL-18Rα:D3. As shown in Fig. 3A, SDS/PAGE and Western blotting revealed a complex of 43 kDa corresponding to cross-linked IL-1F7b and the IL-18Rα:D3. Positive cross-linking was observed for both pro and mature IL-1F7b.
Figure 3.
Cross-linking of IL-1F7b and IL-18Rα-ECD 3. (A) Reducing SDS/PAGE of IL-1F7b cross-linked to IL-18Rα:D3. After blotting on nitrocellulose the cross-linked proteins were visualized by a mAb against the IL-18Rα. BS3, bis(sulfosuccinimidyl) suberate. (B) Formation of a ternary complex of the IL-18Rα- and -β-ECD in the presence of IL-18 but not IL-1F7b after chemical cross-linking. After Western blotting the complexes were visualized by an anti-His6 tag mAb against the His6-tagged IL-18Rβ.
These findings suggested that similar to IL-18 the IL-18Rα:D3 is crucial for IL-1F7b binding. On the basis of this observation, the ability of IL-1F7b to form a ternary receptor complex with the IL-18Rα and IL-18Rβ was studied. The extracellular domains of both the IL-18Rα and IL-18Rβ were produced in eukaryotic cells to ensure mammalian posttranslational modifications such as glycosylation (T.A., D. Novick, P.B., L.L.R., D. Y. Yoon, M. Rubinstein, C.A.D., and S.-H.K., unpublished work). Not unexpectedly, after chemical cross-linking with IL-18, a high molecular weight complex consisting of IL-18Rα, IL-18Rβ, and IL-18 was observed (Fig. 3B). But unlike IL-18, pro and mature IL-1F7b failed to recruit the IL-18Rβ chain to form a ternary complex with the IL-18Rα chain (Fig. 3B).
IL-1F7b Enhances the Ability of IL-18BP to Neutralize IL-18-Induced IFNγ in NK Cells.
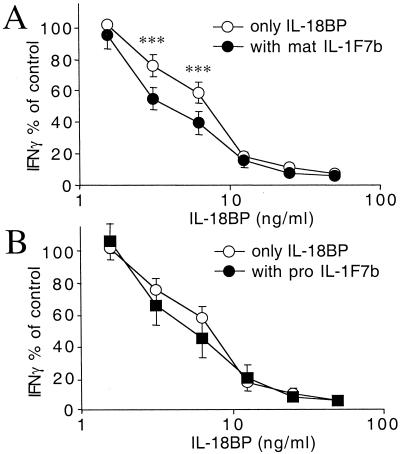
As shown in Fig. 4, IL-1F7b shares two conserved amino acids with IL-18 (E42 and K89). Mutations of either amino acid in IL-18 are critical for the activity of IL-18 as well as for the interaction of IL-18 with the IL-18BP (23). IL-1F7b contains E35 and K124, which are likely similar to E42 and K89 in IL-18. On the basis of the sequence similarity with IL-18, IL-1F7b might also interact with IL-18BP. Therefore, we next investigated whether IL-1F7b affects the ability of IL-18BP to neutralize IL-18. The human NK cell line was stimulated with a constant amount of IL-18 (25 ng/ml) and increasing concentrations of IL-18BP (1.56–50 ng/ml). IL-1F7b was added at a 10-fold molar excess to IL-18. As shown in Fig. 5A, at low concentrations of the IL-18BP, the presence of IL-1F7b increased the ability of IL-18BP to neutralize IL-18-induced IFNγ. At 6.25 ng/ml of IL-18BP, the activity of IL-18 was reduced from 76 to 55% by the presence of IL-1F7b (21% further decrease in activity). At 3.12 ng/ml of IL-18BP and in the presence of mature IL-1F7b, the activity of IL-18 was reduced from 59% to 40% (19% further decrease in activity). Pro IL-1F7b was less active than mature IL-1F7b (Fig. 5B). This effect of IL-1F7b was highly reproducible but observed only at a low concentration of the IL-18BP. Similar results were obtained with PBMC (data not shown).
Figure 4.
Sequence similarity of human IL-18 and IL-1F7b. Human IL-18 (GenBank accession no. D49950) and human IL-1F7b (accession no. AF200496) are shown. Alignment was generated by using Expert Protein Analysis System (ExPasy) with additional manual adjustment. The amino acid identity of IL-18 with IL-1F7b is 28% and the similarity 55%. The underlined amino acids represent the caspase-1-cleavage site in IL-18 and the predicted cleavage site in IL-1F7b.
Figure 5.
IL-1F7b enhances the ability of IL-18BP to inhibit the IL-18-induced IFNγ release by NKO cells. Mature IL-1F7b at 250 ng/ml (A, n = 9) or pro IL-1F7b at 250 ng/ml (B, n = 8_)_, IL-18 (25 ng/ml) and a dilution of IL-18BP in RPMI/10% FCS were incubated in 96-well microtiter plates for 1 h before the addition of NKO cells (0.5 × 106 per ml) and IL-12 (1 ng/ml). After 16 h the supernatant was collected and IFNγ was measured by ECL. Values are expressed as the percent change of IFNγ produced by NKO cells stimulated with IL-18 (25 ng/ml) plus IL-12 (1 ng/ml) in the absence of IL-1F7b or IL-18BP. Statistical analysis was performed by using Student's paired t test (***, P < 0.001).
IL-1F7b Binds to the IL-18BP.
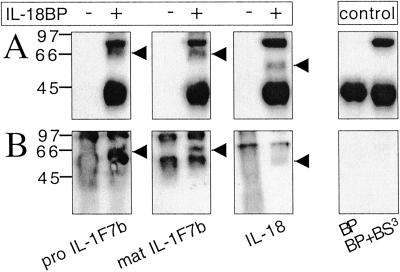
Because IL-1F7b inhibited IL-18-induced IFNγ production, but only in the presence of IL-18BP, we hypothesized that physical interaction of both proteins may occur. After chemical cross-linking, separation by SDS/PAGE, and blotting on nitrocellulose, an additional band with a molecular mass of 64–66 kDa was consistently observed on Western blots with anti-IL-18BP (Fig. 6A) and anti-IL-1F7b sera (Fig. 6B). This cross-linked band represents a complex of mature IL-1F7b/IL-18BP and pro IL-1F7b/IL-18BP, respectively, and reveals the interaction of IL-1F7b with IL-18BP in the fluid phase.
Figure 6.
Cross-linking of IL-1F7b and IL-18BP. (A) Detection of cross-linked proteins (1.5 μg each) on a Western blot by using a rabbit anti-IL-18BP serum. (B) Immunoprecipitation of cross-linked proteins (10 μg each) with a mAb against IL-18BP. Cross-linked IL-1F7b/IL-18BP and the control lanes (IL-18BP with or without BS3) were stained with a rabbit anti-IL-1F7b serum. IL-18/ IL-18BP complex was detected with a rabbit anti-IL-18 serum. BS3, bis(sulfosuccinimidyl) suberate.
Expression of IL-1F7b in Human Peripheral Blood Monocytes.
Anti-IL-1F7b-specific IgG was obtained by affinity purification from a polyclonal rabbit anti-IL-1F7b serum and used to study expression of IL-1F7 in human PBMC. The specificity of the rabbit anti-IL-1F7b serum and IgG preparation was tested by two different methods using murine RAW264.7 macrophage cells transfected with IL-1F7b cDNA. First, IL-1F7b antiserum specifically recognized IL-1F7b in the lysate of IL-1F7b-transfected RAW264.7 cells (Fig. 7A). Second, when confocal digital microscopy was used, affinity-purified anti-IL-1F7b IgG recognized IL-1F7b expression in transfected RAW264.7 but not Mock control cells (Fig. 7B).
Human PBMC were freshly isolated and stained by using the affinity-purified anti-IL-1F7b IgG. As shown in Fig. 7C Left, the expression of IL-1F7 in PBMC is restricted to the monocytic cell population. Absent or limited staining was observed for lymphocytes. IL-1F7 is expressed mainly in the cytoplasm localized to the inner surface of the plasma membrane as well as surrounding the nuclear membrane. The pattern of staining appears granular and is partly associated with the outer cell membrane, suggesting membrane translocation by way of secretory vesicles.
Discussion
Search of expressed sequence tag databases by using known members of the IL-1 family identified IL-1F7b as a member of the IL-1 family (4, 6, 9, 10). IL-1F7b shares two conserved amino acids with IL-18, which are critical for the interaction of IL-18 with the IL-18Rα as well as with the IL-18BP. Here, we show that the fluid-phase interaction of IL-1F7b with IL-18BP is sufficient for binding and cross-linking as well as resulting in a greater reduction in IL-18 activity. In accordance with previous reports, we demonstrated that IL-1F7b possess no IL-18-like agonistic or antagonistic properties. The expression of IL-1F7 in the monocytic cell population of PBMC raises the importance of IL-1F7b as a naturally expressed modulator of IL-18 activity in vivo.
Initially, binding of IL-1F7 to known members of the IL-1 receptor family was studied. Two research groups independently reported that IL-1F7 did not bind to any known member of the IL-1 receptor family or to the orphan receptors IL-1R4 (T1/ST2) and IL-1R6 (IL-1Rrp2) (4, 10). Furthermore, IL-1F7 did not possess IL-18-like agonistic or IL-18-antagonistic activity in NF-κB reporter assays (4). However, IL-1F7 does bind to the IL-18Rα as reported in two studies (9, 14). The use of different splice variants of IL-1F7 complicates these studies and may explain the contrary results. The variants of IL-1F7 used in the first studies have a different N terminus (IL-1F7a) (10) or lack a 40-amino acid segment in the N-terminal region of the protein [IL-1F7c (4)]. Thus, the integrity of the N terminus seems important for binding of IL-1F7 to the IL-18Rα. Like IL-18, IL-1F7b has a prodomain, which might be cleaved by caspase-1. Indeed, N-terminal processing of IL-1F7b by caspase-1 was reported and only mature IL-1F7b showed significant affinity to an IL-18Rα:Fc fusion protein (14).
In the present study, we used chemical cross-linking and showed that, like IL-18 (T.A., D. Novick, P.B., L.L.R., D. Y. Yoon, M. Rubinstein, C.A.D., and S.-H.K., unpublished work), pro and mature IL-1F7b bind to the third ECD of the IL-18Rα. The reported binding affinity of mature IL-1F7b to IL-18Rα is low (_K_d = 130 nM) compared with IL-18 (_K_d = 2.3 nM) (14), which may explain why IL-1F7b does not act as a classic receptor antagonist. In addition, we and others (9, 14) could not demonstrate IL-18-like agonistic activity of IL-1F7b by using two different IL-18-sensitive assays, human PBMC or cultured whole blood. The lack of agonistic activity is supported by our observation that, unlike IL-18, IL-1F7b fails to recruit the IL-18Rβ chain to form a functionally active, ternary complex with the IL-18Rα chain. The existence of an additional receptor chain necessary for IL-1F7b function is unlikely, because similar results were obtained with various cell lines and primary human cells. We also observed that IL-1F7b does not modulate IL-18-independent IFNγ production induced by IL-12.
The present data suggest that even when present at a 40-fold molar excess to IL-18, IL-1F7b does not act as a classic receptor antagonist. Furthermore, at high concentrations IL-1F7b does not show IL-18-like activity and does not trigger a negative signal to inhibit IL-18-independent IFNγ production. Because IL-1F7b shares two conserved amino acids (E35 and K124) with IL-18, both being critical for the interaction of IL-18 with the IL-18Rα as well as the IL-18BP, we tested whether IL-1F7b affects the ability of Il-18BP to neutralize IL-18 activity. We consistently observed that the addition of IL-1F7b enhanced the ability of IL-18BP to neutralize IL-18 activity by an additional 25–30% in a human NK cell line. This finding was unexpected, because we assumed that IL-1F7b bound to IL-18BP would ordinarily occupy binding sites for IL-18, thus decreasing its neutralizing activity. Moreover, we expected a reduced capacity of low concentrations of IL-18BP to neutralize IL-18. In fact, the enhanced neutralizing effect by IL-1F7b was observed only at molar ratio of IL-18BP to IL-18 of <0.4 and at a 10-fold molar excess of IL-1F7b to IL-18. These concentrations of the IL-18BP used to reveal inhibition of IL-18 activity are indeed those found in the circulation of healthy humans (26). Because IL-18BP has a high affinity to IL-18 (_K_d = 400 pM) (20), the neutralizing effect of Il-18BP is >90% at equimolar concentrations of IL-18BP and IL-18, and no additional effect of IL-1F7b can be observed.
To study the molecular basis of the enhanced reduction of IL-18 activity by IL-1F7b, we performed binding studies of IL-1F7b and IL-18BP. After cross-linking, a high molecular mass complex of 64–66 kDa was observed reflecting a complex of monomeric IL-1F7b and IL-18BP. Both the full-length and the mature forms of IL-1F7b bound to IL-18BP. Compared with IL-18, the binding of mature IL-1F7b to IL-18Rα is weak (_K_d = 130 nM) (14). Therefore, the binding affinity between IL-1F7b and IL-18BP is also likely to be relatively weak. Consistent with this hypothesis, we did not find specific binding of IL-1F7b to IL-18BP:Fc by using BiaCore techniques, in which one of the components is immobilized (14). In addition, bacterially expressed IL-1F7b may lack posttranslational modifications which might account for a low calculation of the affinity between the two proteins.
We propose that IL-1F7b binds IL-18BP at the same engagement sites for IL-18 by the conserved amino acids E35 and K124. After binding to IL-18BP, we further propose that IL-1F7b forms a complex with cell-bound IL-18Rβ and the resulting ternary complex deprives the β-chain to form a functional receptor complex with IL-18Rα. As a result of the formation of the IL-18BP/IL-1F7b/IL-18Rβ complex, the activity of IL-18 is reduced further than that due to neutralization of IL-18 by IL-18BP alone. Others have shown that the soluble IL-1RII binds to IL-1β and forms a complex with the cell-bound IL-1RAcP, thus preventing the IL-1RAcP from participation in IL-1 signal transduction (27). However, when we used the IL-18Rβ:ECD, we were unable to observe a complex with IL-18BP and IL-1F7b by cross-linking (data not shown). It is possible that membrane anchoring of the IL-18Rβ is critical for the formation of the heterotrimeric complex with IL-18BP and IL-1F7b and might account for the failure to detect a ternary complex by using the soluble IL-18Rβ:ECD.
Which role does IL-1F7b have in health and disease? Transcripts of IL-1F7 were detected by real-time PCR in several tissues but were most abundant in testis, thymus, and uterus (9). Protein expression for IL-1F7 was reported in a variety of normal and carcinoma tissues with strong staining in plasma cells (14). Here we show the immunohistochemical localization of IL-1F7 in the monocytic cell population of human PBMC, suggesting the role of IL-1F7b as a naturally expressed modulator of IL-18 activity in vivo.
Acknowledgments
This study was supported by National Institutes of Health Grant AI-15614 (to C.A.D.). P.B. is supported by the Deutsche Forschungsgemeinschaft (BU-1222/2-1).
Abbreviations
ECL
electrochemiluminescence
ECD
extracellular domain
His6 tag
six histidines
IL-18BP
IL-18-binding protein
IL-18R
IL-18 receptor
PBMC
peripheral blood mononuclear cells
TNF
tumor necrosis factor
MBP
maltose-binding protein
References
- 1.Dinarello C A. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- 2.Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Annu Rev Immunol. 2001;19:423–474. doi: 10.1146/annurev.immunol.19.1.423. [DOI] [PubMed] [Google Scholar]
- 3.Barton J L, Herbst R, Bosisio D, Higgins L, Nicklin M J. Eur J Immunol. 2000;30:3299–3308. doi: 10.1002/1521-4141(200011)30:11<3299::AID-IMMU3299>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 4.Busfield S J, Comrack C A, Yu G, Chickering T W, Smutko J S, Zhou H, Leiby K R, Holmgren L M, Gearing D P, Pan Y. Genomics. 2000;66:213–216. doi: 10.1006/geno.2000.6184. [DOI] [PubMed] [Google Scholar]
- 5.Debets R, Timans J C, Homey B, Zurawski S, Sana T R, Lo S, Wagner J, Edwards G, Clifford T, Menon S, et al. J Immunol. 2001;167:1440–1446. doi: 10.4049/jimmunol.167.3.1440. [DOI] [PubMed] [Google Scholar]
- 6.Kumar S, McDonnell P C, Lehr R, Tierney L, Tzimas M N, Griswold D E, Capper E A, Tal-Singer R, Wells G I, Doyle M L, Young P R. J Biol Chem. 2000;275:10308–10314. doi: 10.1074/jbc.275.14.10308. [DOI] [PubMed] [Google Scholar]
- 7.Lin H, Ho A S, Haley-Vicente D, Zhang J, Bernal-Fussell J, Pace A M, Hansen D, Schweighofer K, Mize N K, Ford J E. J Biol Chem. 2001;276:20597–20602. doi: 10.1074/jbc.M010095200. [DOI] [PubMed] [Google Scholar]
- 8.Mulero J J, Pace A M, Nelken S T, Loeb D B, Correa T R, Drmanac R, Ford J E. Biochem Biophys Res Commun. 1999;263:702–706. doi: 10.1006/bbrc.1999.1440. [DOI] [PubMed] [Google Scholar]
- 9.Pan G, Risser P, Mao W, Baldwin D T, Zhong A W, Filvaroff E, Yansura D, Lewis L, Eigenbrot C, Henzel W J, Vandlen R. Cytokine. 2001;13:1–7. doi: 10.1006/cyto.2000.0799. [DOI] [PubMed] [Google Scholar]
- 10.Smith D E, Renshaw B R, Ketchem R R, Kubin M, Garka K E, Sims J E. J Biol Chem. 2000;275:1169–1175. doi: 10.1074/jbc.275.2.1169. [DOI] [PubMed] [Google Scholar]
- 11.Nicklin M J, Barton J L, Nguyen M, FitzGerald M G, Duff G W, Kornman K. Genomics. 2002;79:718–725. doi: 10.1006/geno.2002.6751. [DOI] [PubMed] [Google Scholar]
- 12.Taylor S L, Renshaw B R, Garka K E, Smith D E, Sims J E. Genomics. 2002;79:726–733. doi: 10.1006/geno.2002.6752. [DOI] [PubMed] [Google Scholar]
- 13.Mulero J J, Nelken S T, Ford J E. Immunogenetics. 2000;51:425–428. doi: 10.1007/s002510050640. [DOI] [PubMed] [Google Scholar]
- 14.Kumar S, Hanning C R, Brigham-Burke M R, Rieman D J, Lehr R, Khandekar S, Kirkpatrick R B, Scott G F, Lee J C, Lynch F J, et al. Cytokine. 2002;18:61–71. doi: 10.1006/cyto.2002.0873. [DOI] [PubMed] [Google Scholar]
- 15.Torigoe K, Ushio S, Okura T, Kobayashi S, Taniai M, Kunikata T, Murakami T, Sanou O, Kojima H, Fujii M, et al. J Biol Chem. 1997;272:25737–25742. doi: 10.1074/jbc.272.41.25737. [DOI] [PubMed] [Google Scholar]
- 16.Born T L, Thomassen E, Bird T A, Sims J E. J Biol Chem. 1998;273:29445–29450. doi: 10.1074/jbc.273.45.29445. [DOI] [PubMed] [Google Scholar]
- 17.Kim S H, Reznikov L L, Stuyt R J, Selzman C H, Fantuzzi G, Hoshino T, Young H A, Dinarello C A. J Immunol. 2001;166:148–154. doi: 10.4049/jimmunol.166.1.148. [DOI] [PubMed] [Google Scholar]
- 18.Matsumoto S, Tsuji-Takayama K, Aizawa Y, Koide K, Takeuchi M, Ohta T, Kurimoto M. Biochem Biophys Res Commun. 1997;234:454–457. doi: 10.1006/bbrc.1997.6665. [DOI] [PubMed] [Google Scholar]
- 19.Robinson D, Shibuya K, Mui A, Zonin F, Murphy E, Sana T, Hartley S B, Menon S, Kastelein R, Bazan F, O'Garra A. Immunity. 1997;7:571–581. doi: 10.1016/s1074-7613(00)80378-7. [DOI] [PubMed] [Google Scholar]
- 20.Kim S H, Eisenstein M, Reznikov L, Fantuzzi G, Novick D, Rubinstein M, Dinarello C A. Proc Natl Acad Sci USA. 2000;97:1190–1195. doi: 10.1073/pnas.97.3.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Novick D, Kim S H, Fantuzzi G, Reznikov L L, Dinarello C A, Rubinstein M. Immunity. 1999;10:127–136. doi: 10.1016/s1074-7613(00)80013-8. [DOI] [PubMed] [Google Scholar]
- 22.Kim S H, Azam T, Yoon D Y, Reznikov L L, Novick D, Rubinstein M, Dinarello C A. Proc Natl Acad Sci USA. 2001;98:3304–3309. doi: 10.1073/pnas.051634098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim S H, Azam T, Novick D, Yoon D Y, Reznikov L L, Bufler P, Rubinstein M, Dinarello C A. J Biol Chem. 2002;277:10998–11003. doi: 10.1074/jbc.M108311200. [DOI] [PubMed] [Google Scholar]
- 24.Puren A J, Fantuzzi G, Gu Y, Su M S, Dinarello C A. J Clin Invest. 1998;101:711–721. doi: 10.1172/JCI1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Puren A J, Razeghi P, Fantuzzi G, Dinarello C A. J Infect Dis. 1998;178:1830–1834. doi: 10.1086/314481. [DOI] [PubMed] [Google Scholar]
- 26.Novick D, Schwartsburd B, Pinkus R, Suissa D, Belzer I, Sthoeger Z, Keane W F, Chvatchko Y, Kim S H, Fantuzzi G, et al. Cytokine. 2001;14:334–342. doi: 10.1006/cyto.2001.0914. [DOI] [PubMed] [Google Scholar]
- 27.Lang D, Knop J, Wesche H, Raffetseder U, Kurrle R, Boraschi D, Martin M U. J Immunol. 1998;161:6871–6877. [PubMed] [Google Scholar]