Sphingolipid Partitioning into Ordered Domains in Cholesterol-Free and Cholesterol-Containing Lipid Bilayers (original) (raw)
Abstract
We have used fluorescence-quenching measurements to characterize the partitioning of a variety of indolyl-labeled phospho- and sphingolipids between gel or liquid-ordered and liquid-disordered lipid domains in several types of lipid bilayers where such domains coexist. In both cholesterol-free and cholesterol-containing lipid mixtures, sphingolipids with diverse polar headgroups (ranging from sphingomyelin and monoglycosylceramides to ganglioside GM1) show a net preference for partitioning into ordered domains, which varies modestly in magnitude with varying headgroup structure. The affinities of different sphingolipids for ordered lipid domains do not vary in a consistent manner with the size or other simple structural properties of the polar headgroup, such that for example ganglioside GM1 partitions between ordered and disordered lipid domains in a manner very similar to sphingomyelin. Ceramide exhibits a dramatically higher affinity for ordered lipid domains in both cholesterol-free and cholesterol-containing bilayers than do other sphingolipids. Our findings suggest that sphingolipids with a variety of headgroup structures will be enriched by substantial factors in liquid-ordered versus liquid-disordered regions of membranes, in a manner that is only modestly dependent on the nature of the polar headgroup. Ceramide is predicted to show a very strong enrichment in such domains, supporting previous suggestions that ceramide-mediated signaling may be compartmentalized to liquid-ordered (raft and raft-related) domains in the plasma membrane.
INTRODUCTION
Sphingolipids, together with cholesterol, constitute major lipid components of lipid rafts and related membrane microdomains in animal cell membranes (Thompson and Tillack, 1985; Rietveld and Simons, 1998; Brown, 1998; Masserini and Ravasi, 2001). Naturally occurring membrane sphingolipids present a considerable range of headgroup structures, ranging from ceramide itself to glycosphingolipids bearing diverse, and in some cases rather complex, oligosaccharide residues. The partitioning of sphingolipids into ordered lipid domains is promoted at least in part by their ceramide moieties, which are typically enriched in molecular species bearing long (C16- to C26-) saturated N-acyl chains. However, less is known about the effects of varying headgroup structure on the affinities of different sphingolipids for ordered lipid domains, and particularly for the liquid-ordered domains that appear to constitute the core element of lipid rafts in biological membranes (Simons and Ikonen, 1997; Brown and London, 1998a,b). This question is germane to understanding the relative abilities of different sphingolipids both to partition into existing raft domains and, potentially, to favor the formation of new raft structures during processes such as membrane biogenesis and remodeling. Some recent reports have moreover suggested that different populations of rafts and raft-related domains may be differentially enriched in particular sphingolipid components (Iwabuchi et al., 1998; Chigorno et al., 2000; Gómez-Mouton et al., 2001), raising the possibility that such heterogeneity could arise from differential interactions among raft sphingolipid components.
We have previously shown by a fluorescence-quenching method that sphingomyelin and monoglycosylceramides differ significantly in their partitioning between liquid-ordered and liquid-disordered domains in sphingolipid/phospholipid/cholesterol bilayers where the two types of domains coexist (Wang and Silvius, 2000). In this study we have used this general approach to examine the partitioning of a variety of simple and complex sphingolipids between ordered and disordered lipid domains in several types of cholesterol-free and cholesterol-containing lipid bilayers. Our results reveal that in general, sphingolipids bearing different polar headgroups exhibit only modest (albeit significant) differences in their affinities for ordered lipid domains, and that these affinities do not vary in a simple manner with the size of the oligosaccharide headgroup. As well, the relative affinities of different sphingolipids for ordered (gel or liquid-ordered) lipid domains appear to vary only modestly with variations in the composition of these domains. Finally, we find that ceramide itself exhibits a striking and uniquely high affinity for both gel and liquid-ordered lipid domains, which may be important to the role of this lipid in cellular signaling.
MATERIALS AND METHODS
Materials
Synthetic phosphatidylcholines and 1-acyl-lysophosphatidylcholines were purchased from Avanti Polar Lipids (Alabaster, AL) or prepared from the appropriate diacylphosphatidylcholines as described previously (Mason et al., 1981). Sulfogalactopsychosine (lysosulfatide) was prepared from bovine brain sulfatides (Sigma/Aldrich) as described by Koshy and Boggs (1983). 16-Hydroxyhexadecanoic acid and all reagents used to synthesize indolyl-labeled and brominated lipids were obtained from Aldrich Chemicals. Solvents were of reagent grade or better.
18-Hydroxyoctadecanoic acid was prepared from 16-hydroxyhexadecanoic acid as follows. 16-Hydroxyhexadecanoic acid (3 g, 11.0 mmol) was first converted successively to methyl 16-hydroxyhexadecanoate (with 0.3% methanolic H2SO4, 65°C, 30 min) and to methyl 16-(tetrahydropyranyl-2-oxy)hexadecanoate (Miyashita et al., 1977), and the doubly protected fatty acid was purified by flash chromatography on a column of silica gel 60, eluting with a gradient of 0–2% methanol in methylene chloride. The product was reduced with LiAlH4 (0.4 g, in 16 ml dry ether) to yield 16-(tetrahydropyranyl-2-oxy)hexadecan-1-ol. The latter product (3.02 g, 8.8 mmol) was converted successively to the mesylate (with 1.2 eq. methanesulfonyl chloride and 1.5 eq. triethylamine in 30 dry methylene chloride, 0°C, 1.5 h), to the nitrile (with 448 mg NaCN in dry dimethylsulfoxide, refluxing for 3 h under argon), to 17-(tetrahydropyranyl-2-oxy)heptadecanoic acid (with 5 ml 10 M aq. NaOH, 10 ml methoxyethanol, 95°C, 2.5 h), and to 17-(tetrahydropyranyl-2-oxy)heptadecan-1-ol (with LiAlH4 as described above). The latter compound was purified by flash chromatography on a column of silica gel 60, eluting with a gradient of 2–8% acetone in CH2Cl2. The purified product (1.95 g, 5.5 mmol) was again successively converted to the mesylate and to the nitrile, then hydrolyzed to 18-(tetrahydropyranyl-2-oxy)octadecanoic acid as described above. After flash chromatography on a column of silica gel 60, eluting with a gradient of 0–6% acetone in CH2Cl2, the latter compound was reacted first with ethanolic pyridinium tosylate to remove the dihydropyranyl residue (Miyashita et al., 1977), then with 1 M KOH in 10% aqueous methanol (37°C, 1 h) to remove a small amount of ethyl ester formed in the previous reaction. The crude products from the latter reaction were finally recrystallized from acetone to yield 18-hydroxyoctadecanoic acid (1.05 g = 32% overall yield based on 16-hydroxyhexadecanoic acid), which gave a single spot (comigrating with 16-hydroxyhexadecanoic acid) by thin-layer chromatography in 70:30:2 hexane/ethyl acetate/acetic acid.
Portions of 18-hydroxyoctadecanoic acid were converted to 18-bromooctadecanoic acid and to 18-(N-indolyl)octadecanoic acid (hereafter referred to as 18-bromostearic acid and 18-indolylstearic acid, respectively) as described previously (Silvius, 1992). 1-Acyl-2-(18-indolylstearoyl)- and 1-acyl-2-(18-bromostearoyl)-phosphatidylcholines were synthesized from the appropriate 1-acyl-lysophosphatidylcholines and fatty acid anhydrides as described previously (Mason et al., 1981). N-(18-indolylstearoyl)- and N-(18-bromostearoyl)-sphingomyelin were prepared by coupling the appropriate fatty acids to D-erythro-sphingosinephosphorylcholine (Matreya, Inc., Pleasant Gap, PA) in 90:10 (v/v) CH2Cl2/methanol in the presence of 10 eq. 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide and 5 eq. diisopropylethylamine (37°C, 5 h), then purified by preparative thin-layer chromatography. N-(18-bromostearoyl)-galactosylceramide, -glucosylceramide and -lactosylceramide were prepared similarly from 18-bromostearic acid and the appropriate glycosylsphingosines (Avanti Polar Lipids, Alabaster, AL). N-(18-indolylstearoyl)-galactosylceramide, -glucosylceramide, -lactosylceramide and –sulfatide were synthesized by coupling 18-indolylstearic acid to the appropriate glycosylsphingosines, either using the method of Kishimoto (1975) or as described by Koshy and Boggs (1983) but using succinimidyl 18-indolylstearate as the acylating agent. N-(18-indolylstearoyl)-ceramide trihexoside (globoside Gb3) and -ganglioside GM1 were synthesized by coupling succinimidyl 18-indolylstearate to lyso-ceramide trihexoside or lyso-GM1 (Matreya) using the procedure described by Schwarzmann and Sandhoff (1987).
Preparation of lipid samples
Lipid mixtures (normally containing 25–50 nmol lipid and incorporating 2 mol% fluorescent lipid) were mixed in 2:1 CH2Cl2/methanol and dried under a stream of nitrogen while warming to 50–55°C. After further drying under high vacuum to remove traces of solvents, samples were rehydrated in 0.44 ml of 25 mM NaCl, 10 mM 3-(N-morpholino)propanesulfonic acid, 1 mM ethylenediaminetetraacetic acid, pH 7.0 with warming under argon to a temperature above the phase transition temperature of the lipids (55°C [5 min] for samples not containing glycosphingolipids or 75°C [3 min] for glycosphingolipid-containing samples). The samples were then vortexed, cooled at <0.5°C/min from 55°C (65°C for glycosphingolipid-containing samples) to the final experimental temperature (20°C where not otherwise indicated) and incubated at the latter temperature for 48–96 h. In all cases it was confirmed that the final results were unchanged when the samples were initially hydrated at a temperature 10°C higher than those indicated above, or when the final incubation at the lower temperature was extended to twice the normal duration. Except where otherwise indicated, experimental quenching curves were determined at 20°C, a temperature where the lipid domain structures of many of the lipid mixtures examined has been relatively well-defined (Dietrich et al., 2001; Samsonov et al., 2001) and that gave good resolution of the quenching curves (relative to the inherent data scatter) for diverse fluorescent lipids in the various systems examined.
For fluorescence determination replicate aliquots (0.2 ml) of each incubated sample were withdrawn at the experimental temperature and mixed with 2.8 ml of either sample buffer or methanol. The fluorescence of the samples was determined in a Perkin-Elmer LS-50 spectrofluorometer with a thermostatted sample chamber (excitation/emission wavelengths 281 nm/321 nm, excitation/emission slitwidths 5 nm), and the ratio of the (blank-corrected) fluorescence readings in buffer and in methanol (_F_N = normalized fluorescence) was calculated. Fluorescence data are presented in the scaled form (F/_F_o)c suggested by London and co-workers (Chattopadhyay and London, 1987; Ahmed et al., 1997):
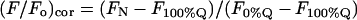 |
(1) |
|---|
where _F_N, _F_100%Q and _F_0%Q are the normalized fluorescence values measured for the fluorescent molecule in vesicles containing a given molar percentage (%Q), 100 mol% or 0 mol%, respectively, of quencher lipid in the nonsterol (= phospho- plus sphingolipid) fraction. Normalized fluorescence values measured for probes in bilayers where all of the phospholipid was brominated were typically 12–17% of those measured for the same probes in quencher-free bilayers.
Partition coefficients describing the relative affinities of fluorescent-labeled lipids for gel versus liquid-crystalline phases in bilayers combining dioleoyl PC with either 1-palmitoyl-2-(16-bromopalmitoyl)-PC or N-(18-bromostearoyl)-sphingomyelin were determined as described previously (London and Feigenson, 1981; Huang et al., 1988; Wang and Silvius, 2001), by fitting experimental quenching curves, over the range of bilayer compositions giving phase separation, to the equation
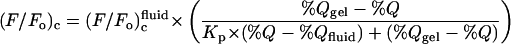 |
(2) |
|---|
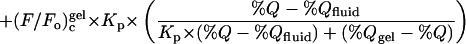
where %Q is the molar percentage of brominated (quencher) lipid in the nonsterol (= phospho- plus sphingolipid) fraction, %_Q_gel and %_Q_fluid define the limiting compositions for the region of phase separation, (F/_F_o)c, (F/_F_o)cgel and (F/_F_o)cfluid are the scaled fluorescence values measured for molar percentages of quencher equal to %Q, %_Q_gel and %_Q_fluid, respectively, and _K_p is the gel/fluid phase partition coefficient.
Quenching curves determined for fluorescent lipids in cholesterol-containing systems were fit (over the range of compositions giving domain segregation) to Eq. 2, in this case as a purely empirical fitting function, and were analyzed in two manners. First, the percentage of each fluorescent lipid X present in liquid-ordered domains in bilayers composed of 1:1:1 (molar proportions) dioleoyl PC, cholesterol and brominated lipid was estimated using the equation
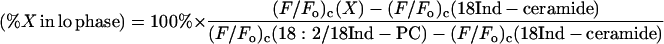 |
(3) |
|---|
where the relevant values of (F/_F_o)c at this composition were calculated from the fitted quenching curves for the indicated species, and where as discussed in the Results section the fluorescence values measured for N-(18-indolylstearoyl)-ceramide (18Ind-ceramide) and for 1-lineoleoyl-2-(18-indolylstearoyl)-PC (18:2/18Ind-PC) were used to estimate the scaled fluorescence values for labeled lipid molecules present in liquid-ordered and liquid-disordered domains, respectively. Application of this approach to calculate the percentages of different fluorescent lipids in cholesterol-free 1:1 BrDPPC/DOPC bilayers gave values that differed by <4% from the values calculated directly (and rigorously) using the measured partition coefficients for the lipid probes in this system. A second analysis, also based on fitting experimental quenching curves to Eq. 2, was applied to the data obtained for fluorescent lipids in bilayers combining cholesterol and DOPC with either 1-stearoyl-2-(18-bromostearoyl)-PC or N-(18-bromostearoyl)-sphingomyelin plus N-(18-bromostearoyl)-lactosylceramide. In this case the calculated slopes of the quenching curves at the limits of the region of domain segregation were used to estimate the relative affinities of different fluorescent lipids for liquid-ordered domains, as described previously (Wang and Silvius, 2000; Wang et al., 2000, 2001). Standard errors of quantities estimated by curve fitting were estimated using standard propagation-of-error analyses.
RESULTS
Gel/fluid phase partitioning of fluorescent phospho- and sphingolipids
The general structures of the indolyl-labeled (fluorescent) and brominated (quencher) phospholipids used in this study are shown in Fig. 1. As we have shown previously, the phase behavior of saturated brominated lipids like those employed here closely parallels that of the corresponding unbrominated species (Silvius et al., 1996). We have also shown that a saturated brominated galactosylceramide mixes with unsaturated phospholipids in a manner very similar to natural (bovine brain) galactosylceramide in bilayers combining these components with or without cholesterol (Curatolo, 1986; Silvius, 1992). For the experiments described later, it was important to utilize fluorescent probes whose labeled acyl chains would perturb as little as possible any potential differences in the partitioning of species with different headgroups between ordered and disordered lipid domains. For this purpose we examined N-indolyl lipids like those shown in Fig. 1, which like the related carbazole-labeled lipids (Abrams and London, 1992) position the fluorescent group deep within the bilayer interior.
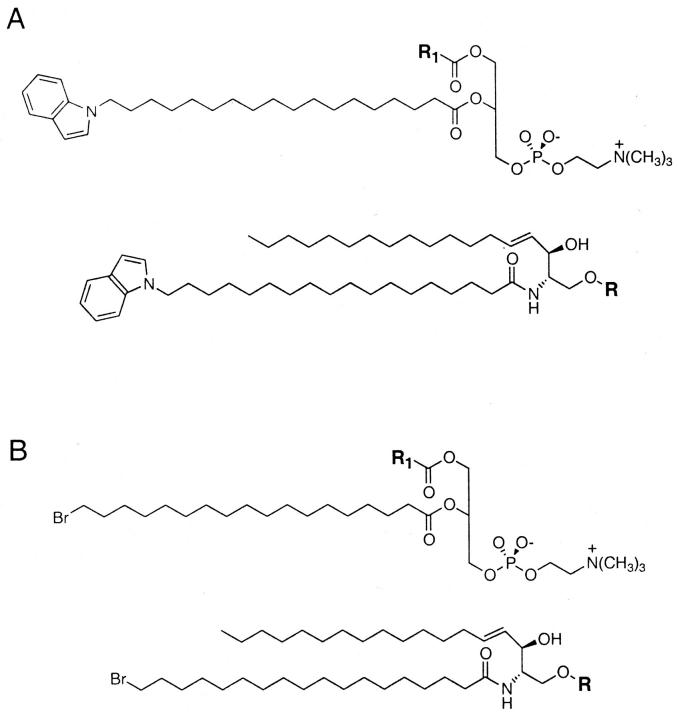
FIGURE 1.
General structures of (A) the fluorescent 18-indolylstearoyl-substituted and (B) the brominated phospho- and sphingolipids employed in this study. For the more complex glycosphingolipids utilized the polar headgroups R were as follows: lactosylceramide, R = Gal_β_1-4Glc-; ceramide trihexoside (globotriosylceramide, globoside Gb3), R = Gal_β_1-4Gal_β_1-4Glc-; ganglioside GM1, R = Gal_β_1-3GalNac_β_1-4(NeuAc_β_1-3)Gal_β_1-4Glc-. The acyl groups R1 present at the 1-position of the fluorescent phospholipids employed were as indicated in the text.
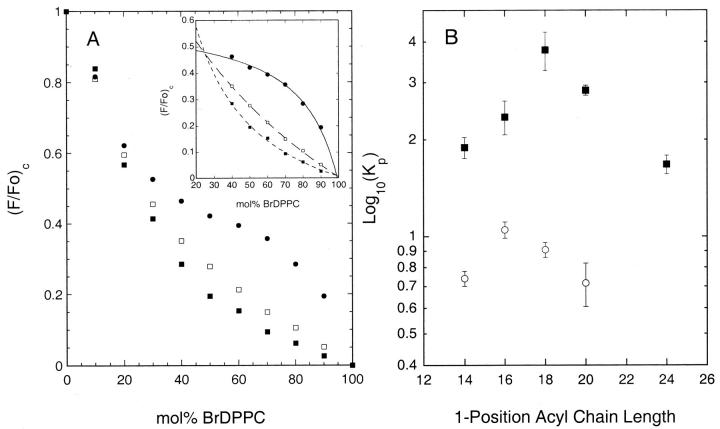
In preliminary experiments we compared the gel/liquid-crystalline phase partitioning of several saturated diacyl phosphatidylcholine analogs, labeled with an indolyl group on the 2-position acyl chain, in bilayers composed of varying proportions of 1-palmitoyl-2-(16-bromopalmitoyl)-PC (BrDPPC) and dioleoyl PC (DOPC) at 20°C. As illustrated in Fig. 2 A, the quenching curves (plots of scaled fluorescence versus the proportion of the brominated quencher lipid) determined for different indolyl-labeled species diverge markedly over a broad range of compositions. This behavior reflects the occurrence of gel/liquid-crystalline phase separation in this system and the different partitioning of the various labeled lipids between the coexisting phases (Wang and Silvius, 2001). As described previously (Huang et al., 1988; Ahmed et al., 1997; Wang and Silvius, 2001), and as illustrated in the inset to Fig. 2 A, by appropriate curve-fitting data like those shown in Fig. 2 A can be analyzed to determine the gel/fluid partition coefficient for each fluorescent lipid species. The results of these analyses are plotted in Fig. 2 B. For a series of 1-acyl-2-(18-indolylstearoyl)-PC's, the partition coefficient does not increase monotonically with the length of the 1-position acyl chain but rather peaks when this chain is 18 carbons in length and falls off as the length of this chain increases further. For a series of 1-acyl-2-(16-indolyl)-PC's a qualitatively similar pattern of results is observed, but the partition coefficient is now maximal when the 1-position acyl chain is 16 carbons long. These results indicate that these fluorescent lipids are best accommodated in ordered lipid domains when the indolyl-labeled acyl chain at the 2-position is at least as long as the unlabeled acyl chain at the 1-position. This arrangement presumably minimizes steric conflict between the 1-position acyl chain and the N-indolyl group, allowing the labeled lipid to occupy a smaller optimal area, and hence to partition better into ordered lipid domains. In light of this result, or the remainder of the studies described here we utilized fluorescent phospho- and sphingolipids incorporating an 18-indolylstearoyl chain, which in the case of sphingolipids should exhibit minimal steric conflict with either a C18- or a C20-sphingosine backbone.
FIGURE 2.
A) Quenching curves determined in BrDPPC/DOPC/(33 mol% cholesterol) bilayers at 20°C for (•) 1-linoleoyl-2-(18-indolylstearoyl)-PC, (□) 1-myristoyl-2-(18-indolylstearoyl) PC and (▪) 1-stearoyl-2-(18-indolylstearoyl)-PC. A inset) Illustration of fits of quenching curves to Eq. 2 over the region of phase separation, used to determine the (gel/fluid) partition coefficients of the fluorescent lipids. B) (Gel/fluid) phase partition coefficients determined in BrDPPC/DOPC bilayers at 20°C for (▪) 1-acyl-2-(18-indolylstearoyl)- or (○) 1-acyl-2-(16-indolylpalmitoyl)-phosphatidylcholines carrying saturated acyl chains of the indicated lengths at the 1-position. Samples were prepared and experimental quenching curves determined and analyzed as outlined in the text. Data shown in Figure 2 B represent the average (mean ± SE) of three independent experiments.
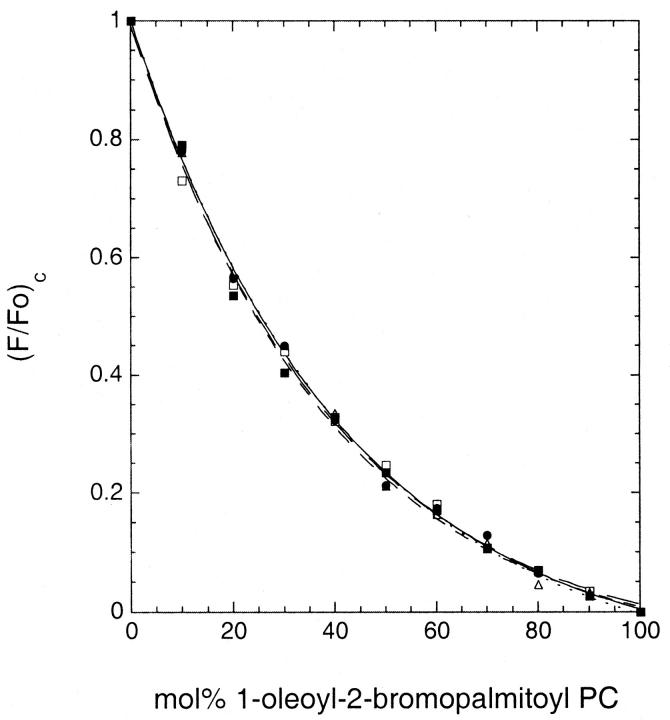
As illustrated in Fig. 3, in homogeneous fluid-phase mixtures of 1-oleoyl-2-(18-bromostearoyl)-PC and DOPC, the normalized fluorescence intensities for different (18-indolylstearoyl)-labeled phospho- and sphingolipids vary in an essentially identical manner with the proportion of the brominated quencher species. The experimental curves are well described by an exponential expression of the form used previously to describe quenching of the fluorescence of molecules incorporated into homogenous fluid bilayers (Chattopadhyay and London, 1987). These results demonstrate that in a homogeneous lipid mixture the different probes show very similar intrinsic susceptibilities to quenching by the brominated lipid species. This was true even for the more complex glycolipids examined (ceramide trihexoside and ganglioside GM1).
FIGURE 3.
Representative quenching curves determined for fluorescent phospho- and sphingolipids in homogeneous fluid-phase DOPC/1-oleoyl-2-(18-bromostearoyl)-PC bilayers at 20°C. (•) 1-linoleoyl-2-(18-indolylstearoyl)-PC, (▵) 1-myristoyl-2-(18-indolylstearoyl)-PC, (▪) N-(18-indolylstearoyl)-sphingomyelin, (□) N-(18-indolylstearoyl)-ceramide trihexoside, and (○) N-(18-indolylstearoyl)-ganglioside GM1. Quenching curves were determined as described in the text. The fitted curves represent the best fit of each data set to an equation of the form (F/Fo)c = A + B × exp(−C × (%brominated lipid)) .
In contrast to the behavior shown in Fig. 3, the quenching curves observed for different indolyl-labeled phospho- and sphingolipids in phase-separated BrDPPC/DOPC bilayers at 20°C diverged markedly, similar to the behavior illustrated in Fig. 2 A and again reflecting differential partitioning of the various fluorescent species between the gel and the liquid-crystalline phases. In Table I we summarize the gel/liquid-crystalline phase partition coefficients determined for the different fluorescent lipids by curve fitting analysis as described above. As expected the polyunsaturated 1-linoleoyl-2-(18-indolylstearoyl)-PC shows very low affinity for ordered lipid domains. By contrast, all of the fluorescent saturated phosphatidylcholines and sphingolipids examined show a preference for the gel over the liquid-crystalline phase. As detailed in the Discussion, comparison of the present results to previously reported data suggests that an 18-indolylstearoyl chain is roughly equivalent to a palmitoyl chain in its contribution to the partitioning of phospho- and sphingolipids into ordered lipid domains.
TABLE 1.
Gel/Fluid phase partition coefficients for the distribution of 18-indolylstearoyl- phospho- and sphingolipid probes in BrDPPC/DOPC bilayers at 20°C
| Kp(gel/fluid) in: | ||
|---|---|---|
| Fluorescent Lipid | BrDPPC/DOPC | BrSphM/DOPC |
| 18:2/(18Indolyl18:0)-PC | 0.195 ± 0.012 | |
| 18:1/(18Indolyl18:0)-PC | 0.206 ± 0.013 | |
| 14:0/(18Indolyl18:0)-PC | 1.89 ± 0.14 | 1.91 ± 0.14 |
| 18:0/(18Indolyl18:0)-PC | 3.78 ± 0.51 | |
| N-(18Indolyl18:0)-ceramide | 30.7 ± 8.0 | 48.4 ± 4.1 |
| N-(18Indolyl18:0)-sphingomyelin | 2.11 ± 0.12 | 3.75 ± 0.68 |
| N-(18Indolyl18:0)-sulfatide | 2.20 ± 2.28 | |
| N-(18Indolyl18:0)-galactocerebroside | 4.81 ± 0.55 | |
| N-(18Indolyl18:0)-glucocerebroside | 5.84 ± 0.74 | 9.31 ± 0.60 |
| N-(18Indolyl18:0)-lactocerebroside | 3.22 ± 0.38 | 5.76 ± 0.45 |
| N-(18Indolyl18:0)-ceramide trihexoside | 4.59 ± 0.66 | |
| N-(18Indolyl18:0)-ganglioside GM1 | 2.49 ± 0.20 |
The results presented in Table 1 reveal that the affinities of different sphingolipids for the gel phase in BrDPPC/DOPC bilayers vary significantly with the structure of the polar headgroup. The gel/liquid-crystalline phase partition coefficient measured for sphingomyelin in this system (2.11 ± 0.12) only slightly exceeds that determined for 14:0/(18-indolylstearoyl)-PC (1.89 ± 0.14), the phospholipid species with the most closely matching hydrocarbon chain lengths (Brown, 1998). The partition coefficients measured for the various fluorescent sphingolipids (excepting ceramide) vary over a roughly threefold range. Among the glycosphingolipids examined, affinity for the gel phase is not a simple function of headgroup size, being almost as high for ceramide trihexoside (globoside Gb3) as for the monoglycosylceramides, yet considerably lower for lactosyl ceramide and ganglioside GM1. Negatively charged substituents may decrease somewhat the affinity of glycosphingolipids for ordered domains (compare for example the partitioning of galactosylceramide and of its 3′-sulfated derivative sulfatide in the BrDPPC/DOPC system). Most strikingly, however, ceramide exhibits a much higher affinity for the BrDPPC-rich gel phase than do any of the other sphingolipids examined (almost 15-fold higher than that measured for sphingomyelin). In control experiments the partition coefficients determined for fluorescent ceramide, sphingomyelin, lactocerebroside, ceramide trihexoside and GM1 were found to be unchanged when the level of these species was reduced from the normal 2 mol% to 1 mol% (data not shown). The result is particularly important for ganglioside, whose affinity for the gel phase has been reported to decrease as the ganglioside level in the bilayer rises above a relatively low threshold concentration (Rock et al., 1991).
Also shown in Table 1 are the results of measurements of the partitioning of selected fluorescent lipids in bilayers composed of DOPC and N-(18-bromostearoyl)-sphingomyelin (BrSphM) at 20°C. The gel/liquid-crystalline phase partition coefficient determined for 1-myristoyl-2-(18-indolylstearoyl)-PC is essentially identical to that measured for the same fluorescent lipid in BrDPPC/DOPC/cholesterol bilayers. By contrast, the partition coefficients measured for the different sphingolipid species examined are all 1.6- to 1.8-fold greater in the sphingomyelin-containing system. These results suggest that preferential sphingolipid-sphingolipid interactions enhance partitioning of the fluorescent sphingolipids into the gel-phase domains in the latter system.
Partitioning of fluorescent lipids in cholesterol-containing bilayers
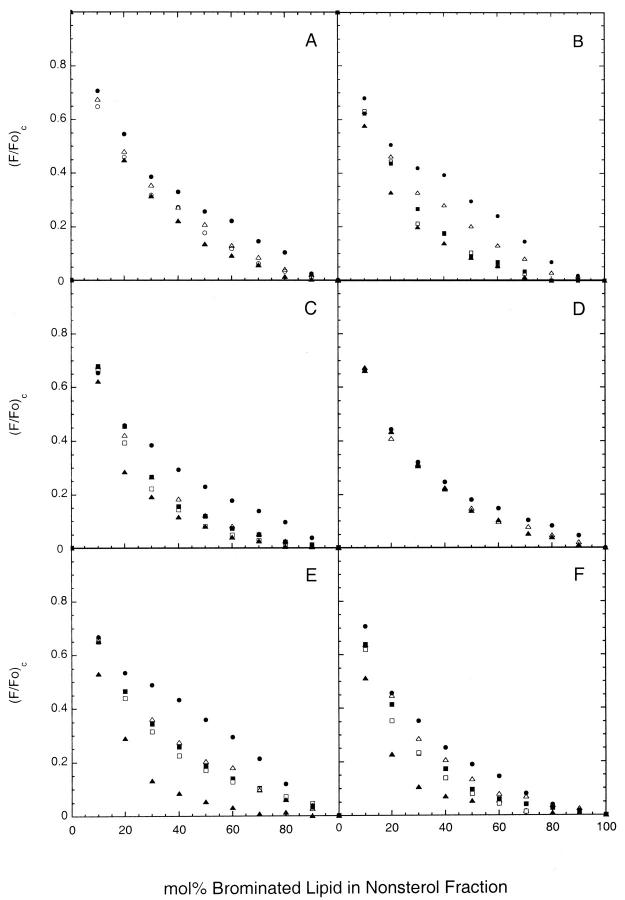
To compare the affinities of different lipid species for partitioning into liquid-ordered domains in cholesterol-containing bilayers, we next determined the quenching curves for various phosphatidylcholine and sphingolipid probes in bilayers combining cholesterol (33 mol%) and DOPC with either BrDPPC or BrSphM at 20°C. These systems, similarly to analogous mixtures combining DOPC and cholesterol with unbrominated DPPC or sphingomyelin, form laterally segregated liquid-ordered (BrDPPC- or BrSphM-enriched) and liquid-disordered (DOPC-enriched) domains in this temperature range (Schroeder et al., 1994; Ahmed et al., 1997; Dietrich et al., 2001; Samsonov et al., 2001; Wang and Silvius, 2001). As shown in Fig. 4, A and B, the quenching curves measured for different fluorescent lipids in these systems diverge substantially over a broad range of compositions, reflecting the differential partitioning of the various fluorescent lipids between coexisting liquid-ordered and liquid-disordered domains. In both systems the polyunsaturated 1-linoleoyl-2-(18-indolylstearoyl)-PC shows substantially weaker quenching than do saturated phospho- and sphingolipid probes, reflecting the preferential partitioning of the former species into liquid-disordered domains (Wang and Silvius, 2001). The quenching curves obtained for the different labeled sphingolipids reveal stronger partitioning into liquid-ordered domains than is observed for the chain-matched 1-myristoyl-2-(18-indolylstearoyl)-phosphatidylcholine. This latter difference is still more pronounced in BrSphM/DOPC/cholesterol bilayers (Fig. 4 B). Similar behavior was observed in bilayers in which 1-palmitoyl-2-linoleoyl PC replaced DOPC as the unsaturated lipid component (illustrated in Wang and Silvius, 2001). However, DOPC was routinely used in the present experiments to minimize the possibility of lipid peroxidation during extended sample incubations. The quenching curves obtained in the above systems for fluorescent sphingomyelin, ceramide trihexoside, and ganglioside GM1 were unchanged when these species were present at 1 mol% rather than the normal 2 mol% (not shown).
FIGURE 4.
Representative quenching curves determined for fluorescent phospho- and sphingolipids in (A) BrDPPC/DOPC/(33 mol%) cholesterol bilayers at 20°C, (B) BrSphM/DOPC/(33 mol% cholesterol) bilayers at 20°C, (C) (BrSphM + BrGalCer)/DOPC/(33 mol% cholesterol) bilayers at 20°C, (D) (BrSphM + BrGalCer)/DOPC/(33 mol% cholesterol) bilayers at 37°C, (E) BrDSPC/DOPC/(33 mol% cholesterol) bilayers at 20°C and (F) (BrSphM + BrLacCer)/DOPC/(33 mol% cholesterol) bilayers at 20°C. Symbols used for different fluorescent lipids are as follows; (•) 1-linoleoyl-2-(18-indolylstearoyl)-PC, (▵) 1-myristoyl-2-(18-indolylstearoyl)-PC, (▪) N-(18-indolylstearoyl)-sphingomyelin, (□) N-(18-indolylstearoyl)-galactosylceramide, (▴) N-(18-indolylstearoyl)-ceramide and (○) (N-(18-indolylstearoyl)-lactosylceramide (panel A only). For clarity, data are shown in Panel (D) only for 1-linoleoyl-2-(18-indolylstearoyl)-PC, 1-myristoyl-2-(18-indolylstearoyl)-PC, and N-(18-indolylstearoyl)-ceramide and in panel (A), only for these three species plus N-(18-indolylstearoyl)-lactosylceramide. Quenching curves were determined as described in the text.
The results of a quantitative analysis of the above data are presented in Table 2, where we compare the estimated extents of partitioning of different fluorescent lipids into liquid-ordered domains in bilayers combining equimolar proportions of cholesterol, DOPC, and either BrDPPC or BrSphM. 1-Myristoyl-2-(18-indolylstearoyl)-PC partitions to a similar extent into liquid-ordered domains in the two cholesterol-containing systems. By contrast, the various fluorescent sphingolipids partition into liquid-ordered domains to a significantly greater degree in BrSphM/DOPC/cholesterol than in BrDPPC/DOPC/cholesterol bilayers, recalling the enhanced partitioning of fluorescent sphingolipids into ordered domains in BrSphM/DOPC as compared to BrDPPC/DOPC bilayers (Table 1). In both cholesterol-containing lipid mixtures the majority of each sphingolipid probe is associated with liquid-ordered domains. In these cholesterol-containing systems, as was observed in the BrDPPC/DOPC system, the partitioning of different glycosphingolipids into the liquid-ordered domains does not vary in any simply predictable manner with the size of the attached glycosyl residue.
TABLE 2.
Estimated extents of partitioning of fluorescent sphingolipids into liquid-ordered domains in bilayers composed of equimolar proportions of DOPC, cholesterol and saturated (brominated) phospho- or sphingolipids
| % in liquid-ordered domains at 20°C in bilayers of composition: | |||||
|---|---|---|---|---|---|
| Fluorescent lipid | 1:1:1 BrDPPC/DOPC/Ch | 1:1:1 BrSM/DOPC/Ch | 1:1:2:2 BrSphM/BrGalCer/DOPC/Ch | 1:1:1 BrDSPC/DOPC/Ch | 1:1:2:2 BrSphM/BrLacCer/DOPC/Ch |
| 14:0/(18Indolyl18:0)-PC | 59 ± 4 | 54 ± 5 | 70 ± 4 | 48 ± 4 | 42 ± 2 |
| 18:0/(18Indolyl18:0)-PC | 72 ± 6 | ||||
| N-(18Indolyl18:0)-SM | 75 ± 6 | 93 ± 3 | 85 ± 1 | 56 ± 2 | 62 ± 4 |
| N-(18Indolyl18:0)-sulfatide | 75 ± 1 | 76 ± 4 | 58 ± 3 | 52 ± 4 | |
| N-(18Indolyl18:0)-GalCer | 74 ± 2 | 100 ± 5 | 89 ± 6 | 69 ± 2 | 65 ± 3 |
| N-(18Indolyl18:0)-GlcCer | 76 ± 5 | 61 ± 2 | |||
| N-(18Indolyl18:0)-LacCer | 68 ± 3 | 95 ± 2 | 94 ± 2 | 67 ± 3 | 65 ± 4 |
| N-(18Indolyl18:0)-Ceramide trihexoside | 86 ± 1 | 93 ± 4 | 75 ± 4 | 62 ± 3 | |
| N-(18Indolyl18:0)-Ganglioside GM1 | 76 ± 2 | 79 ± 4 | 60 ± 3 | 60 ± 5 |
Results similar to those observed in the BrSphM/DOPC/cholesterol system were obtained using bilayers in which the BrSphM component was replaced by an equimolar mixture of BrSphM and N-(18-bromostearoyl)-galactosylceramide (Fig. 4 C and Table 2). Because this bilayer composition fairly closely mimics that found in the outer leaflet of mammalian cell plasma membranes, we also determined the quenching curves for various fluorescent lipids in this system at 37°C (Fig. 4 D). The quenching curves for saturated and unsaturated fluorescent lipids diverge even at the latter temperature. However, the absolute magnitude of this divergence at 37°C was too small, relative to the background scatter in the fluorescence readings, to quantitate accurately the extents of partitioning of various saturated phospho- and sphingolipids into liquid-ordered domains.
To assess more sensitively the relative affinities of different sphingolipids for liquid-ordered domains, we identified two cholesterol-containing systems in which the quenching curves determined for the various fluorescent sphingolipids showed greater divergence than in those discussed above. The first such system comprised bilayers composed of 1-stearoyl-2-(18-bromostearoyl)-PC (BrDSPC), DOPC, and 33 mol% cholesterol at 20°C, which like the systems discussed above forms segregated liquid-ordered and liquid-disordered domains (Samsonov et al., 2001). The second system examined combined DOPC, cholesterol (33 mol%), and varying proportions of a 1:1 mixture of BrSphM and N-(18-bromostearoyl)-lactosylceramide (BrLacCer). The phase behavior of this system has not previously been characterized in detail. However, given the reported favorable interaction of N-stearoyl-lactosylceramide with cholesterol (Slotte et al., 1993), this system is also expected to form liquid-ordered domains in the presence of cholesterol.
As illustrated in Fig. 4 E, the quenching curves obtained for different labeled phospho- and sphingolipids in the BrDSPC/DOPC/cholesterol system differ strongly, approaching the degree of divergence observed in the cholesterol-free systems described earlier. Most strikingly, the quenching curve for the fluorescent ceramide shows considerably greater partitioning into liquid-ordered domains than is observed for other fluorescent sphingolipids. Similar results are observed using the (BrSphM + BrLacCer)/DOPC/cholesterol system (Fig. 4 F). The quenching curves obtained for the fluorescent ceramide in these two lipid systems were essentially unchanged when the concentration of labeled ceramide was reduced from the standard 2 mol% to 1 mol% (results not shown). Likewise, the addition of 2 mol% of unlabeled N-stearoylceramide had no significant effect on the quenching curves determined for either N-(18-indolylstearoyl)-ceramide or N-(18-indolylstearoyl)-glucosylceramide in these systems (not shown). These results indicate that the markedly divergent quenching curves observed for the fluorescent ceramide in these systems reflect a uniquely high affinity of this species for liquid-ordered domains and not a tendency (at the molar concentrations examined here) to increase significantly the extent of formation of such domains.
Using the reasonable assumptions that in 1:1:1 (molar proportions) BrDSPC/DOPC/cholesterol bilayers the scaled fluorescence of the fluorescent ceramide in liquid-ordered domains is no less than that observed in pure BrDSPC/cholesterol bilayers, and that the scaled fluorescence for this species in liquid-disordered domains is no less than that measured for 1-linoleoyl-2-(18-indolylstearoyl)-PC in bilayers of the same composition, we can calculate (using a slight modification of Eq. 3 in Materials and Methods) that a minimum of 88% of the fluorescent ceramide is partitioned into liquid-ordered domains in bilayers containing equimolar BrDSPC, DOPC, and cholesterol. (The equation used for this calculation was
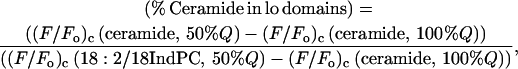 |
(4) |
|---|
where the two terms in the numerator represent the scaled fluorescence measured for the fluorescent ceramide in bilayers composed of 1:1:1 BrDSPC/DOPC/cholesterol or of 1:1 BrDSPC/cholesterol, respectively, and the first term in the denominator represents the scaled fluorescence measured for 1-linoleoyl-2-(18-indolylstearoyl)-PC in 1:1:1 BrDSPC/DOPC/cholesterol bilayers). Assuming that in bilayers of this composition roughly equal proportions of liquid-ordered and liquid-disordered domains are present, we can estimate that labeled ceramide thus partitions with at least seven- to eightfold greater avidity into liquid-ordered than into liquid-disordered domains in these bilayers. It is important to note that any errors in the assumptions just noted are expected to lead to underestimation of the true extent of partitioning of the fluorescent ceramide into the BrDSPC-enriched domains. These results demonstrate that the fluorescent ceramide partitions very favorably into ordered domains in cholesterol-containing as well as cholesterol-free systems.
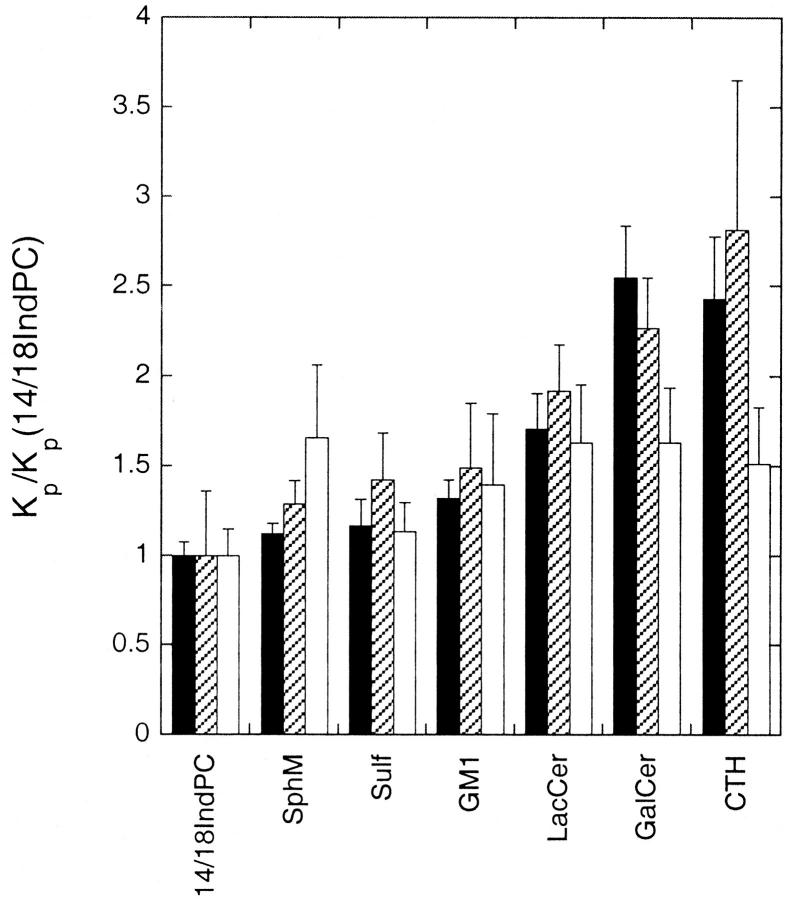
In Table 2 we summarize the estimated extents of partitioning of different fluorescent lipids into liquid-ordered domains in lipid mixtures composed of either 1:1:1 (molar proportions) BrDSPC/DOPC/cholesterol or 1:1:2:2 BrSphM/BrLacCer/DOPC/cholesterol, calculated from the experimental quenching curves as for the other systems discussed earlier. These results suggest that for these cholesterol-containing systems, in bilayers of a given composition, different sphingolipids with the same N-acyl chain (with the prominent exception of ceramide) partition with only moderately differing affinities into liquid-ordered domains. To assess this point more quantitatively, we analyzed the quenching curves obtained for different fluorescent phospho- and sphingolipids in these two systems to determine the relative values of the (liquid-ordered/liquid-disordered) domain partition coefficients (_K_p(_l_o/_l_d)) for different fluorescent lipids, using a previously described analysis as outlined in Materials and Methods. The results of these analyses are summarized in Fig. 5, where we have plotted the values of _K_p(_l_o/_l_d), expressed as a ratio to the corresponding value determined for 1-myristoyl-2-(18-indolylstearoyl)-PC, in the BrDSPC/DOPC/cholesterol, (BrLacCer + BrSphM)/DOPC/cholesterol and (for comparison) BrDPPC/DOPC systems at 20°C. The pattern of relative affinities for ordered (gel or liquid-ordered) domains is very similar in the BrDPPC/DOPC and BrDSPC/DOPC/cholesterol systems. A similar pattern of results was also obtained by analyzing similarly the fluorescence-quenching data for the BrDPPC/DOPC/cholesterol system, although in this case the standard errors of estimation were considerably larger (results not shown). These results suggest that the presence of cholesterol per se does not greatly alter the relative affinities of different saturated phospho- and sphingolipids for ordered lipid domains. In the (BrLacCer + BrSphM)/DOPC/cholesterol system the (liquid-ordered/liquid-disordered) domain partition coefficients for different fluorescent sphingolipids vary over a somewhat narrower range (less than twofold) and are less well correlated with the gel/fluid partition partition coefficients determined in BrDPPC/DOPC bilayers.
FIGURE 5.
Relative values of the (ordered/disordered) domain partition coefficients determined for different sphingolipids at 20°C in bilayers composed of 1:1 BrDPPC/DOPC (dark bars), 1:1:1 BrDSPC/DOPC/cholesterol (hatched bars) or 1:1:2:2 BrSphM/BrLacCer/DOPC/cholesterol (white bars). In each case the partition coefficients for different fluorescent lipids are expressed as a ratio to that determined for 1-myristoyl-2-(indolylstearoyl)-PC in bilayers of the same composition. Partition coefficients for fluorescent lipids in BrDPPC/DOPC bilayers were determined as illustrated in Fig. 2. Relative partition coefficients for different fluorescent species in the cholesterol-containing systems were determined by curve-fitting analysis of the quenching curves as described in the text. Calculations were based on quenching curves averaged from three or more independent experiments for each fluorescent lipid.
DISCUSSION
The results presented in this study lead to three principal conclusions. First, our findings confirm that sphingolipids with a variety of headgroups will partition favorably into gel or liquid-ordered domains in bilayers where such domains coexist with liquid-disordered domains. Second, we find that although sphingolipids with different polar headgroups can exhibit significant differences in affinity for ordered lipid domains, in most cases these differences are relatively modest and do not vary in a systematic manner with the size or other simple structural features of the lipid headgroup. As a result, in a given bilayer where ordered (gel or liquid-ordered) and disordered domains coexist, the distributions of different sphingolipids between the coexisting domains are generally expected to differ only modestly. Finally, however, we observe that in diverse lipid systems, ceramide shows a strikingly higher affinity for ordered phases than do other sphingolipids, a fact that may be relevant to its proposed role in compartmentalized signaling in rafts and related domains in biological membranes.
The fluorescent species used in this study were chosen to approach as closely as possible the structures and properties of naturally occurring phospho- and sphingolipids, and in particular to minimize interference of the fluorescent acyl chain with headgroup-dependent interactions between the fluorescent lipid and surrounding lipid molecules. Two quantitative comparisons suggest that lipids bearing an 18-indolylstearoyl chain partition into ordered lipid phases with affinities similar to (or slightly weaker than) those observed for the corresponding lipid species bearing a palmitoyl chain. First, our fluorescence-quenching data indicate that for BrDPPC/DOPC bilayers at 20°C, the region of phase separation extends from roughly 20 mol% to 95 mol% BrDPPC. This indicates that within the region of phase separation the brominated DPPC exhibits a partition coefficient of roughly (0.95/0.20) or about fivefold in favor of the gel-phase regions of the bilayer. Similar values for the partition coefficient (_K_p = ∼4 and 5, respectively) can be estimated for unbrominated DPPC in DPPC/POPC or DPPC/dielaidoyl PC bilayers at 20°C, using the phase diagrams reported for the latter systems (Grant et al., 1974; Curatolo et al., 1985). These values are roughly twofold higher than the partition coefficient determined here for 1-palmitoyl-2-(18-indolylstearoyl)-PC in BrDPPC/DOPC bilayers at 20°C (2.4 ± 0.3). Second, from data reported by Palestini et al. (1995) we can calculate that N-palmitoyl ganglioside GM1 exhibits a partition coefficient of roughly 2.5 in favor of gel-state regions in phase-separated DPPC/dielaidoyl PC bilayers at 20°C. This value is very similar to that found here for the N-(18-indolylstearoyl)-GM1 in BrDPPC/DOPC bilayers (2.49 ± 0.20). Taken together, these results suggest that the (18-indolylstearoyl)-labeled lipids studied here partition into ordered lipid domains with affinities comparable to or slightly lower than those expected for the corresponding palmitoyl-substituted lipids. Naturally occurring membrane sphingolipids typically include large amounts of molecular species bearing saturated C18 and longer acyl chains (Estep et al.,1979; Rock et al., 1991), which partition into gel- and liquid-ordered domains with significantly (typically two- to threefold) higher affinity than do the equivalent palmitoyl-substituted species (Palestini et al., 1995; Wang et al., 2000). It thus appears that regardless of their polar headgroup structure, naturally occurring sphingolipids will typically be substantially (at least severalfold) enriched in liquid-ordered over liquid-disordered membrane domains.
Our present findings indicate that with the prominent exception of ceramide, sphingolipids with different headgroups show only modest differences in their partitioning between gel and liquid-crystalline phases. Moreover, these differences in partitioning are not enhanced, and in some cases may even be somewhat diminished, in systems exhibiting segregation of liquid-ordered and liquid-disordered domains. Monoglycosylceramides exhibit significantly higher affinities for ordered lipid domains than does sphingomyelin bearing the same N-acyl chain, as we also concluded previously from a more limited study using different fluorescent analogs of these species (Wang and Silvius, 2000). However, the affinity of various glycosphingolipids for ordered phases does not vary in a consistent manner with headgroup size and in fact is very similar for, e.g., sphingomyelin and ganglioside GM1. In this regard our findings agree with the conclusion of Thompson and co-workers (Rock et al., 1991; Palestini et al., 1995) that the affinity of naturally occurring GM1 for ordered (gel-state) lipid domains rests primarily on the nature of its ceramide moiety (which for brain GM1 consists largely of N-stearoyl species (Rock et al., 1991)) rather than on special features of the GM1 headgroup. These workers have reported a similar conclusion for Forssman antigen, a family of complex neutral glycosphingolipids (Rock et al., 1991). Our results suggest that simpler glycosphingolipids, which generally carry similar ceramide groups, can partition into gel or liquid-ordered lipid domains at least as avidly as do more complex glycosphingolipid species.
Various reports have suggested that complex glycosphingolipids may segregate laterally to form clusters in fluid as well as ordered lipid domains and even at low concentrations in the bilayer (Vié et al., 1998; Yuan and Johnston, 2001; Yuan et al., 2002 and references therein), although other studies have reached the opposite conclusion (Thompson et al., 1985; Mehlhorn et al., 1988; Khalil et al., 2000 and references therein). Our present findings do not provide any clear indication for such clustering in the systems examined here, either through any distinctive properties of the quenching curves for the more complex fluorescent glycosphingolipids examined or through any evident concentration dependence of their interdomain partitioning. If however clusters of fluorescent complex sphingolipids are in fact present in our experimental systems, it is clear from our results that such clusters must incorporate a very high proportion of host matrix lipids, because the quenching curves obtained for such sphingolipids (e.g., ganglioside GM1) in various lipid systems closely resemble those observed for other, simpler fluorescent sphingolipids (e.g., sphingomyelin and sulfatide) in the same systems.
Recent reports (Iwabuchi et al., 1998; Chigorno et al., 2000; Gómez-Mouton et al., 2001) have suggested that membranes may exhibit different populations of lipid rafts and raft-related domains, which differ strongly in their content of particular sphingolipids. In principle, such heterogeneity in raft composition could arise at least in part from differential interactions between different sphingolipid species. However, for two reasons we conclude that our results provide little direct support for this proposal, at least for the sphingolipid species and bilayer compositions examined here. First, as already noted, in none of the different cholesterol-containing lipid mixtures examined here do we observe dramatic differences in the partitioning of different fluorescent sphingolipids into liquid-ordered domains. Second, the pattern of relative affinities of different sphingolipids for such domains varies only modestly in systems of widely varying composition (Table 2 and Fig. 5). These modest differences do not appear to be sufficiently large to suggest that different raft populations could segregate based on differential interactions among different sphingolipid species. We suggest that differences in the interactions of particular sphingolipids (e.g., gangliosides) with raft-localized membrane proteins may play a greater role in creating heterogeneity in raft sphingolipid compositions.
The very high affinity that we observe for partitioning of ceramide into both gel and liquid-ordered domains is consistent with previous reports that this lipid species promotes the formation of such domains (Carrer and Maggio, 1999; Holopainen et al., 2000; Massey, 2001; Xu et al., 2001). A previous study from our laboratory (Wang and Silvius, 2000), using N-(diphenylhexatrienyl)propionyl-labeled (DPH3:0-) fluorescent sphingolipids, did not detect the strikingly higher affinity of ceramide for liquid-ordered lipid domains, compared to other sphingolipids, that we observe in the present experiments. This may reflect in part the fact that several of the lipid mixtures used in the present study afford much better discrimination of the partitioning of different sphingolipids into ordered lipid domains than do those used previously. Our present findings may also reflect the fact that the N-(18-indolylstearoyl)-sphingolipid probes used here have an acyl chain structure that matches much more closely that of natural sphingolipids, particularly in the region near the site of attachment to the sphingosine backbone. Our results suggest that ceramide produced by cleavage of sphingomyelin in the plasma membrane will show a strong preferential enrichment in rafts and related liquid-ordered lipid domains. The fluorescent ceramide examined in this study shows a 30- to 45-fold enrichment in gel- over fluid-phase domains in bilayers combining an unsaturated lipid with a saturated phosphatidylcholine or sphingomyelin. The relative enrichment of the fluorescent ceramide in liquid-ordered domains cannot be determined with equal precision for the ternary BrDSCP/DOPC/cholesterol system but is at least seven- to eightfold, and likely considerably higher. Recalling that the major molecular species found for most membrane sphingolipids, including ceramides, are expected to partition more avidly into ordered lipid domains than do the corresponding N-(18-indolylstearoyl) species studied here, we can conclude that naturally occurring ceramides will become greatly enriched in rafts and raft-related domains (e.g., caveolae) compared to coexisting liquid-disordered regions of the membrane. This property may underly the report of Liu and Anderson (1995) that ceramide signaling in the plasma membrane occurs in a compartmentalized manner within raft-like domains.
Acknowledgments
This research was supported by the Canadian Institutes of Health Research (MOP-7776) to J.R.S., and by a Studentship award from the Canadian Institutes of Health Research to T.W.
References
- Abrams, F. S., and E. London. 1992. Calibration of the parallax fluorescence quenching method for determination of membrane penetration depth: refinement and comparison of quenching by spin-labeled and brominated lipids. Biochemistry. 31:5312–5322. [DOI] [PubMed] [Google Scholar]
- Ahmed, S. N., D. A. Brown, and E. London. 1997. On the origin of sphingolipid/cholesterol-rich detergent-insoluble cell membranes: physiological concentrations of cholesterol and sphingolipid induce formation of a detergent-insoluble, liquid-ordered lipid phase in model membranes. Biochemistry. 36:10944–10953. [DOI] [PubMed] [Google Scholar]
- Brown, D. A., and E. London. 1998a. Structure and origin of ordered lipid domains in biological membranes. J. Membr. Biol. 164:103–114. [DOI] [PubMed] [Google Scholar]
- Brown, D. A., and E. London. 1998b. Functions of lipid rafts in biological membranes. Annu. Rev. Cell Dev. Biol. 14:111–136 [DOI] [PubMed] [Google Scholar]
- Brown, R. E. 1998. Sphingolipid organization in biomembranes: what physical studies of model membranes reveal. J. Cell Sci. 111:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrer, D. C., and B. Maggio. 1999. Phase behavior and molecular interactions in mixtures of ceramide with dipalmitoylphsphatidylcholine. J. Lipid Res. 40:1978–1989. [PubMed] [Google Scholar]
- Chattopadhyay, A., and E. London. 1987. Parallax method for direct measurement of membrane penetration depth utilizing fluorescence quenching by spin-labeled phospholipids. Biochemistry. 26:39–45. [DOI] [PubMed] [Google Scholar]
- Chigorno, V., P. Palestini, M. Sciannamblo, V. Dolo, A. Pavan, G. Tettamanti, and S. Sonnino. 2000. Evidence that ganglioside enriched domains are distinct from caveolae in MDCK II and human fibroblast cells in culture. Eur. J. Biochem. 267:4187–4197. [DOI] [PubMed] [Google Scholar]
- Curatolo, W., B. Sears, and L. J. Neuringer. 1985. A calorimetry and deuterium NMR study of mixed model membranes of 1-palmitoyl-2-oleylphosphatidylcholine and saturated phosphatidylcholines. Biochim. Biophys. Acta. 817:261–270. [DOI] [PubMed] [Google Scholar]
- Curatolo, W. 1986. The interactions of 1-palmitoyl-2-oleoylphosphatidylcholine and bovine brain cerebroside. Biochim. Biophys. Acta. 861:373–376. [DOI] [PubMed] [Google Scholar]
- Dietrich, C., L. A. Bagatolli, Z. N. Volovyk, N. L. Thompson, M. Levi, K. Jacobson, and E. Gratton. 2001. Lipid rafts reconstituted in model membranes. Biophys. J. 80:1417–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estep, T. N., D. Mountcastle, Y. Barenoholz, R. L. Biltonen, and T. E. Thompson. 1979. Thermal behavior of synthetic sphingomyelin-cholesterol dispersions. Biochemistry. 18:2112–2117. [DOI] [PubMed] [Google Scholar]
- Gómez-Mouton, C., J. L. Abad, E. Mira, R. A. Lacalle, E. Gallardo, S. Jimenez-Baranda, I. Illa, A. Bernad, S. Manes, and C. Martinez-A. 2001. Segregation of leading-edge and uropod components into specific lipid rafts during T cell polarization. Proc. Natl. Acad. Sci. USA. 98:9642–9647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant, C. W. M., S. H. Wu, and H. M. McConnell. 1974. Lateral phase separations in binary lipid mixtures: correlation between spin label and freeze-fracture electron microscopic studies. Biochim. Biophys. Acta. 363:151–158. [DOI] [PubMed] [Google Scholar]
- Holopainen, J. M., J. Lemmich, F. Richter, O. G. Mouritsen, G. Rapp, and P. Kinnunen. 2000. Dimyristoylphosphatidylcholine/C16:0-ceramide binary liposomes studied by differential scanning calorimetry and wide- and small-angle X-ray scattering. Biophys. J. 78:2459–2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, N. N., K. Florine-Casteel, G. W. Feigenson, and C. Spink. 1988. Effect of fluorophore linkage position of n-(9-anthroyloxy) fatty acids on probe distribution between coexisting gel and fluid phospholipid phases. Biochim. Biophys. Acta. 939:124–130. [DOI] [PubMed] [Google Scholar]
- Iwabuchi, K., K. Handa, and S. Hakomori. 1998. Separation of ‘glycosphingolipid signaling domain’ from caveolin-containing membranes in mouse melanoma B16 cells and its role in cell adhesion coupled with signaling. J. Biol. Chem. 273:33766–33773. [DOI] [PubMed] [Google Scholar]
- Khalil, M. B., M. Kates, and D. Carrier. 2000. FTIR study of the monosialoganglioside GM1 in perdeuterated dimyristoylcholine (DMPCd54) multilamellar bilayers: spectroscopic evidence of a significant interaction between Ca2+ ions and the sialic acid moiety of GM1. Biochemistry. 39:2980–2988. [DOI] [PubMed] [Google Scholar]
- Kishimoto, Y. 1975. A facile synthesis of ceramides. Chem. Phys. Lipids. 15:33–36. [DOI] [PubMed] [Google Scholar]
- Koshy, K. M., and J. M. Boggs. 1983. Partial synthesis and physical properties of cerebroside sulfate containing palmitic acid or alpha-hydroxy palmitic acid. Chem. Phys. Lipids. 34:41–53. [DOI] [PubMed] [Google Scholar]
- Liu, P., and R. G. Anderson. 1995. Compartmentalized production of ceramide at the cell surface. J. Biol. Chem. 270:27179–27185. [DOI] [PubMed] [Google Scholar]
- London, E., and G. W. Feigenson. 1981. Fluorescence quenching in model membranes. An analysis of the local phospholipid environments of diphenylhexatriene and gramicidin A'. Biochim. Biophys. Acta. 649:89–97. [Google Scholar]
- Mason, J. T., A. V. Broccoli, and C. Huang. 1981. A method for the synthesis of isomerically pure saturated mixed-chain phosphatidylcholines. Anal. Biochem. 113:96–101 [DOI] [PubMed] [Google Scholar]
- Masserini, M., and D. Ravasi. 2001. Role of sphingolipids in the biogenesis of membrane domains. Biochim. Biophys. Acta. 1532:149–161. [DOI] [PubMed] [Google Scholar]
- Massey, J. B. 2001. Interaction of ceramides with phosphatidylcholine, sphingomyelin and sphingomyelin/cholesterol bilayers. Biochim. Biophys. Acta. 1510:167–184. [DOI] [PubMed] [Google Scholar]
- Mehlhorn, I. E., K. R. Barber, and C. M. W. Grant. 1988. Globoside with spin-labeled fatty acid: bilayer lateral distribution and immune recognition. Biochim. Biophys. Acta. 943:389–404. [DOI] [PubMed] [Google Scholar]
- Miyashita, N., A. Yoshikoshi, and P. A. Grieco. 1977. Pyridinium p-toluenesulfonate. A mild and efficient catalyst for the tetrahydropyranylation of alcohols. J. Org. Chem. 42:3772–3774. [Google Scholar]
- Palestini, P., M. Allietta, S. Sonnino, G. Tettamanti, T. E. Thompson, and T. W. Tillack. 1995. Gel phase preference of ganglioside GM1 at low concentration in two-component, two-phase phosphatidylcholine bilayers depends on the ceramide moiety. Biochim. Biophys. Acta. 1235:221–230. [DOI] [PubMed] [Google Scholar]
- Rietveld, A., and K. Simons. 1998. The differential miscibility of lipids as the basis for the formation of functional membrane rafts. Biochim. Biophys. Acta. 1376:467–479. [DOI] [PubMed] [Google Scholar]
- Rock, P., M. Allietta, W. W. Young, Jr., T. E. Thompson, and T. W. Tillack. 1991. Ganglioside GM1 and asialo-GM1 at low concentration are preferentially incorporated into the gel phase in two-component, two-phase phosphatidylcholine bilayers. Biochemistry. 30:19–25. [DOI] [PubMed] [Google Scholar]
- Samsonov, A. V., I. Mihalyov, and F. S. Cohen. 2001. Characterization of cholesterol-sphingomyelin domains and their dynamics in bilayer membranes. Biophys. J. 81:1486–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder, R., E. London, and D. Brown. 1994. Interactions between saturated acyl chains confer detergent resistance on lipids and glycosylphosphatidylinositol (GPI)-anchored proteins: GPI-anchored proteins in liposomes and cells show similar behavior. Proc. Natl. Acad. Sci. USA. 91:12130–12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzmann, G., and K. K. Sandhoff. 1987. Lysogangliosides: synthesis and use in preparing labeled gangliosides. Methods Enzymol. 138:319–341 [DOI] [PubMed] [Google Scholar]
- Silvius, J. R. 1992. Cholesterol modulation of lipid intermixing in phospholipid and glycosphingolipid mixtures. Evaluation using fluorescent lipid probes and brominated lipid quenchers. Biochemistry. 31:3398–3408. [DOI] [PubMed] [Google Scholar]
- Silvius, J. R., D. del Giudice, and M. Lafleur. 1996. Cholesterol at different bilayer concentrations can promote or antagonize lateral segregation of phospholipids of differing acyl chain length. Biochemistry. 35:15198–15208. [DOI] [PubMed] [Google Scholar]
- Simons, K., and E. Ikonen. 1997. Functional rafts in cell membranes. Nature. 387:569–572. [DOI] [PubMed] [Google Scholar]
- Slotte, J. P., A.-L. Östman, E. R. Kumar, and R. Bittman. 1993. Cholesterol interacts with lactosyl and maltosyl cerebrosides but not with glucosyl or galactosyl cerebrosides in mixed monolayers. Biochemistry. 32:7886–7892. [DOI] [PubMed] [Google Scholar]
- Thompson, T. E., and T. W. Tillack. 1985. Organization of glycosphingolipids in bilayers and plasma membranes of mammalian cells. Annu. Rev. Biophys. Biophys. Chem. 14:361–386. [DOI] [PubMed] [Google Scholar]
- Thompson, T. E., M. Allietta, R. E. Brown, M. L. Johnson, and T. W. Tillack. 1985. Organization of ganglioside GM1 in phosphatidylcholine bilayers. Biochim. Biophys. Acta. 817:229–237. [DOI] [PubMed] [Google Scholar]
- Vié, V., N. Van Mau, E. Lesniewska, J. P. Goudonnet, F. Heitz, and C. Le Grimellec. 1998. Distribution of ganglioside GM1 between two-component, two-phase phosphatidylcholine monolayes. Langmuir. 14:4574–4583. [Google Scholar]
- Wang, T.-Y., and J. R. Silvius. 2000. Different sphingolipids show differential partitioning into sphingolipid/cholesterol-rich domains in lipid bilayers. Biophys. J. 79:1478–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, T.-Y., and J. R. Silvius. 2001. Cholesterol does not induce segregation of liquid-ordered domains in bilayers modeling the inner leaflet of the plasma membrane. Biophys. J. 81:2762–2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, T.-Y., R. Leventis, and J. R. Silvius. 2000. Fluorescence-based evaluation of the partitioning of lipids and lipidated peptides into liquid-ordered lipid microdomains: a model for molecular partitioning into “lipid rafts”. Biophys. J. 79:919–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, T.-Y., R. Leventis, and J. R. Silvius. 2001. Partitioning of lipidated peptide sequences into liquid-ordered domains in model and biological membranes. Biochemistry. 40:13031–13040. [DOI] [PubMed] [Google Scholar]
- Xu, X., R. Bittman, G. Duportail, D. Heissler, C. Vilchezel, and E. London. 2001. Effect of the structure of natural sterols and sphingolipids on the formation of ordered sphingolipid/sterol domains (rafts). Comparison of cholesterol to plant, fungal and disease-associated sterols and comparison of sphingomyelin, cerebrosides and ceramide. J. Biol. Chem. 276:33540–33546. [DOI] [PubMed] [Google Scholar]
- Yuan, C., and L. J. Johnston. 2001. Atomic force microscopy studies of ganglioside GM1 domains in phosphatidylcholine and phosphatidylcholine/cholesterol bilayers. Biophys. J. 81:1059–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, C., J. Furlong, P. Burgos, and L. J. Johnston. 2002. The size of lipid rafts: an atomic force microscopy study of ganglioside GM1 domains in sphingomyelin/DOPC/cholesterol membranes. Biophys. J. 82:2526–2535. [DOI] [PMC free article] [PubMed] [Google Scholar]